Abstract
The availability of oxygen (O2) is a critical parameter affecting vascular tube formation. In this study, we hypothesize that dissolved oxygen (DO) levels in collagen gels change during the three-dimensional (3D) culture of human umbilical vein endothelial cells (HUVECs) in atmospheric conditions and that such changes affect the kinetics of tube formation through the production of reactive oxygen species (ROS). We demonstrate a decrease in O2 tension during 3D cultures of HUVECs. Noninvasive measurements of DO levels during culture under atmospheric conditions revealed a profound decrease that reached as low as 2% O2 at the end of 24 h. After media replacement, DO levels rose rapidly and equilibrated at ∼15% O2, creating a reoxygenated environment. To accurately estimate DO gradients in 3D collagen gels, we developed a 3D mathematical model and determined the Michaelis-Menten parameters, Vmax and Km, of HUVECs in collagen gels. We detected an increase in ROS levels throughout the culture period. Using diphenyliodonium to inhibit ROS production resulted in the complete inhibition of tube formation. Interference RNA studies further showed that hypoxia-inducible factors (HIFs)-1α and -2α are not involved in the formation of 3D tubes in collagen gels. We conclude that ROS affect the tubulogenesis process through HIFα-independent pathways, where the levels of ROS are influenced by the uncontrolled variations in DO levels. This study is the first demonstration of the critical and unexpected role of O2 during 3D in vitro culture models of tubulogenesis in atmospheric conditions.
Keywords: microvasculature, reactive oxygen species, hypoxia-inducible factors
an understanding of the mechanisms controlling how blood vessels form from endothelial cells (ECs) is crucial for developmental biology and vascular tissue engineering. In recent decades, our understanding of the molecular regulation underlying vascular development has vastly expanded, primarily because of newly available, well-defined in vitro models. One common model is the culturing of ECs in gels made of the extracellular matrix component collagen, which has enabled us to investigate the effects of many factors on tubulogenesis (24, 38, 39).
The availability of oxygen (O2) is a critical parameter affecting how vascular cells form tubes. In vitro models have demonstrated that hypoxic conditions enhance tube formation, as do hypoxic regions formed by the O2 consumption of the cells in three-dimensional (3D) structures (3, 19, 32). Research has shown that the rate of O2 consumption of cells follows the Michaelis-Menten kinetics and depends on the cell type, cell density (15), and dissolved oxygen (DO) levels (1). Variations in DO levels strongly affect angiogenesis (19, 48) through different vascular cell responses, such as viability, differentiation, migration, and remodeling of the extracellular matrix (4, 27, 40).
Hypoxia-inducible factors (HIFs) and reactive oxygen species (ROS) are among the effectors responsible for cellular responses to changing DO levels. HIF1α and HIF2α, two isoforms of HIFα, regulate the expression of many angiogenic genes (29, 45), while they may also exhibit a distinct regulation selectivity over angiogenic genes in ECs (14). For instance, it was demonstrated that HIF1α, but not HIF2α, is responsible for the cord formation of human umbilical vein endothelial cells (HUVECs) through basic fibroblast growth factor (7), whereas only HIF2α promotes the expression of vascular endothelial growth factor (VEGF) receptor-2 (22). Also, a growing body of evidence suggests that the two isoforms of HIFα differ in terms of their stabilization dependence on the available DO (6, 44, 52). HIF1α, considered the master regulator of angiogenesis under hypoxic conditions, only stabilizes at very low levels of oxygen (<1%) in ECs—at levels lower than in other cell types (1, 34). By contrast, HIF2α can be stabilized under normoxia or mild hypoxia (1 to 3% O2) in different cell types, including ECs (44, 52). Nonetheless, under hypoxic conditions, both isoforms are highly accumulated and colocalized into the nucleus to induce angiogenic responses.
ROS generation in ECs is strongly regulated through the mitochondrial electron transport chain (41) and nicotinamide adenine dinucleotide phosphate (NADPH)-oxidases (NOX) (16) in response to the fluctuations in available DO. The direction of the change in ROS production due to changes in DO levels depends on both the cell type and the exposure time to various DO levels. However, the vast majority of the results obtained on ECs agree on the fact that hypoxia (34) and reoxygenation (46, 47) increase the levels of intracellular ROS. Previous studies have shown that an optimal level of NOX-derived ROS is required for EC proliferation, migration, and capillary formation (2, 36). Research has demonstrated the involvement of ROS in angiogenesis through HIFα-dependent, via inhibition of prolyl hydroxylases (33), and -independent mechanisms (5, 10, 21, 33, 49). Interestingly, one study suggested that increased ROS levels in ECs, unlike in other cell types (9, 33), did not affect the stabilization of HIF1α (34).
We have previously shown that DO levels decrease during the two-dimensional (2D) cultures of ECs, suggesting that these decreases could become even more dramatic in 3D environments, which require higher cell-seeding concentrations, resulting in a higher rate of oxygen consumption (1). Here, we hypothesize that DO levels in collagen gels change during the 3D culture of ECs and that such changes affect the kinetics of tube formation through ROS production independent of HIFα activation. We demonstrate that an uncontrolled drop in DO levels occurs during the course of tubulogenesis of HUVECs in collagen gels in atmospheric conditions, while the frequency of media replacement affects the oxygenation of the cells. In addition, we show that these DO fluctuations influence ROS production of HUVECs and that tube formation in collagen gels requires NOX-derived ROS. Finally, our results reveal the involvement of ROS in the tubulogenesis process through HIFα-independent pathways, where the levels of ROS are influenced by the uncontrolled variations in DO levels.
METHODS
Cell culture.
HUVECs (passages 1:5, PromoCell, Hiedelberg, Germany) were cultured in humidified incubators at 37°C with atmospheric air maintained at 5% CO2. We cultured the cells in endothelial growth medium (EGM) supplemented with endothelial growth supplemental mix (PromoCell). The cells were passaged every 4 to 5 days with 0.05% trypsin, whereas the media were changed every other day.
Preparation of collagen gels and 3D culture conditions.
We used Collagen Type I (8.2 mg/ml, BD Biosciences) stock solution to prepare collagen gels at a density of 2.5 mg/ml, as previously described in the literature. Gel formation was achieved by simultaneously decreasing the pH of the solution and increasing the temperature to 37°C. To prepare 1 ml of 2.5 mg/ml collagen gel, we added solution 1 [39 μl M199 (10×), 408.9 μl M199 (1×), and 2.1 μl NaOH (5 N)] to solution 2 [61 μl acetic acid (0.1%) and 288.8 μl Collagen Type I] and mixed them thoroughly on ice. HUVECs resuspended in 200 μl medium 199 (1×) were then mixed with the gel solution for a final concentration of 2 million cells/ml. We transferred an appropriate volume of gel solution into either 24- or 96-well plates to obtain a gel thickness of 1.7 mm [as used in other studies (24)] and incubated the gels for 30 min at 37°C. Finally, the EGM, supplemented with 50 ng/ml VEGF, was added at a ratio of 1.78 ml media to 103 cells (24). We changed the media either every day or every other day.
DO measurements and cell viability.
We measured DO levels noninvasively and continuously using commercially available sensor patches and dish readers (Presens, Regensburg, Germany), as we have previously described (1). We added the collagen solution (with or without cells) at the top of the O2 sensor patches and allowed it to polymerize for 30 min at 37°C. After the gel formation, we added cell medium and measured DO levels at the bottom of the gel every 15 min for 48 h.
To determine cell viability, the gels were first degraded in collagenase solution (1 mg/ml) for 20 min at 37°C and then broken mechanically with pipette tips. The cells were collected and incubated with Trypan blue for 2 min at room temperature and counted under a light microscope.
Small interfering RNA transfection.
HUVECs were transfected with siGENOME SMARTpool human HIF1α and HIF2α (Dharmacon, Lafayette, CO), using the manufacturer's protocol as previously described (17). Briefly, we prepared the RNA interference (RNAi) transfection solution by mixing an antibiotic- and serum-free EGM (PromoCell) with DharmaFECT4 RNAi transfection reagent (Dharmacon). HUVECs were seeded on a 24-well plate (NUNC, Roskilde, Denmark) with a cell-seeding density of 1.2 × 105 cells/well. For transfection, we replaced growth medium with 400 μl of antibiotic-free EGM (PromoCell) and 100 μl transfection solution in each well to achieve a final RNAi concentration of 100 nmol/l. We incubated transfected cells at 37 μL C and replaced the medium with a fresh antibiotic-free EGM medium after 24 h. We performed RNA analyses after 24 h, whereas we performed protein analyses after 48 h. Confirmed transfected HUVECs were used for experiments after 48 h.
ROS inhibition and detection.
The production of ROS in HUVECs was inhibited by adding diphenyleneiodonium (DPI) (Sigma-Aldrich, St. Louis, MO), a NOX inhibitor, to the growth medium at a concentration of 10 μM (28). To visualize intracellular ROS, we replaced the media with a dye solution consisting of 10 μM 2′,7′-dichlorodihydrofluorescein diacetate (H2-DCFDA) (Invitrogen, Carlsbad, CA) in phosphate-buffered saline (PBS); we incubated the cells for 30 min at 37°C. The gel was then rinsed with PBS three times prior to imaging using a fluorescent microscope with a ×10 objective lens (Axiovert, Zeiss, Thornwood, NY), at multiple time points during 2 days of HUVEC cultures in 3D collagen gels. We analyzed six image fields per gel from three distinct experiments (n = 3) performed in duplicate. We quantified total fluorescence intensity using MetaMorph software and normalized the averaged values by the percentage of viable cells.
Fluorescent imaging and tube formation quantification.
We analyzed tube formation of cells using fluorescent imaging of both live and fixed cells. The cells were fixed in the gel by incubating with a 3.7% paraformaldehyde solution for 45 min at room temperature. After permeabilizing the cells with 0.1% Triton X-100 and incubating them in 1% BSA blocking solution for 1 h, we stained them with phalloidin (1:40) and 4′,6-diamidino-2-phenylindole (DAPI) (1:1,000) to visualize the cytoplasm and nuclei, respectively.
We performed live staining using Calcein (Invitrogen), which can penetrate through the cell walls and produce fluorescence in viable cells as result of its hydrolysis by intracellular esterases. We replaced the growth media with Calcein dye solution (2 μM) and incubated the cells for 30 min at 37°C. The mean tube length and thickness and percentage of tube coverage were quantified, as we previously described (17, 50). Briefly, we analyzed 18 images (×10 magnification) taken at six different regions on the gel surface and three different positions in the gel depth (duplicates of n = 3) using the Angiogenesis tool of MetaMorph software 6.1 (Universal Imaging, Downingtown, PA).
Real-time quantitative RT-PCR.
We performed two-step RT-PCR on HUVECs that were transfected with HIF1α small interfering RNA (siRNA), HIF2α siRNA, and luciferase for 24 h at two different transfection reagent concentrations. Total RNA was extracted by using TRIzol (Invitrogen) and quantified using an ultraviolet spectrophotometer according to the manufacturer's instructions. We used a Moloney murine leukemiavirus (M-MLV) reverse transcriptase and oligo(dT) primers (both from Promega, Madison, WI) to transcribe 1 μg RNA (per sample). We used the TaqMan Universal PCR Master Mix and Gene Expression Assay (Applied Biosystems, Foster City, CA) for the TaqMan PCR step performed with an Applied Biosystems StepOne real-time PCR system (Applied Biosystems). The relative expressions of HIF1α and HIF2α were normalized to the amount of β-actin. We employed the comparative computerized tomography method (Applied Biosystems) to calculate the amplification differences between the different samples. The triplicate values for fold change in expression were averaged and graphed with standard deviations.
Statistical analysis.
We performed all oxygen measurements on triplicate samples (n = 3), with triplicate readings of each sample for each data point. Real-time RT-PCR was also performed on triplicate samples (n = 3) with triplicate readings. We plotted graphs for DO in atmospheric O2 with standard deviation (SD), using DO data points every 15 min throughout the experiment. Unpaired two-tailed t-tests were performed for tube formation quantification and RNAi suppression data, using GraphPad Prism 4.02 (GraphPad Software, La Jolla, CA). P values <0.05 were considered statistically significant.
MATHEMATICAL MODEL
To estimate DO gradients throughout the gel depth, we developed a mathematical model. In a single culture well, O2 transport takes place in three different layers: 1) air (top layer), 2) growth medium (middle layer), and 3) gel (bottom layer) (Supplemental Fig. S1A; Supplemental Material for this article is available online at the Journal website). The mass transfer only occurs through diffusion, since all three layers remain stagnant during the process. The spatial change in the O2 concentration at a quasi-steady state can be described for each layer by the species continuity equations below:
| (1) |
| (2) |
| (3) |
where CO2 is the concentration of oxygen as a function of time (t) and position; and DO2Air, DO2Media, and DO2Gel are the diffusion coefficients of O2 in the air, media, and gel, respectively. At equilibrium, O2 flux remains continuous at the interfaces, and we can relate the amount of DO per unit volume of media linearly to the partial pressure of O2 in air (PO2Air) by the solubility coefficient of O2 in media (SO2Media 1.19 nmol·cm−3·mmHg−1) given in Eq. 4.
| (4) |
We can expect the permeability of O2 in collagen with cells to be lower than its permeability in media as described by the well-known obstruction theory (43), owing to the fact that collagen is composed of fibers that can hinder O2 transport (35) and that the oxygen diffusivity of cells suspended in the gel is less than that in medium (37). However, for the relatively low collagen gel density of 2.4 mg/ml and cell density of 2 million cells/ml used in our study, the O2 permeability of gel containing cells can be considered as the O2 permeability in medium according to Prager's obstruction theory. Therefore, we assumed that O2 transport in collagen gels occurred the same as in the medium.
The oxygen consumption rate of cells (R), given in Eq. 3, depends on the spatial cell density (ρcell) and oxygen uptake rate (OUR) of cells, which is assumed to follow Michaelis-Menten kinetics.
| (5) |
where Vmax is the maximum OUR and Km is the O2 concentration at which the OUR is half-maximal. The cells were assumed to be uniformly distributed in the 3D gel; therefore, ρcell is only a function of time, which is determined by viability measurements. To the best of our knowledge, previous studies reported the values of Vmax and Km parameters of HUVECs at various cell-seeding densities (15, 42). However, all of these estimations were carried out in 2D cultures. The literature currently lacks studies investigating whether Vmax and Km can differ when the HUVECs are encapsulated in 3D gels.
We used the model developed in this study to determine the Michaelis-Menten parameters of HUVECs in 3D low-density collagen gels, altering the parameter values to give the best fit between the measured and predicted DO levels at the bottom of the gel at various gel thicknesses. For this purpose, we prepared the gels in 24-well OxoDishes (Presens, Regensburg, Germany) at six different thicknesses, keeping the cell density constant. We measured the steady-state DO levels and found that they differed for every gel thickness. Simulations were then run for each thickness using the cell viability values measured at steady-state points and altering the Vmax and Km parameters. The residual sum of squares (RSS) method was used to determine the parameters giving the best fit between the measured and predicted DO values at the bottom of the well.
We also considered the resistance of the culture plate lid to the O2 transfer from the outside environment into the wells (see appendix). For this purpose, we added culture media to the wells without cells and placed the plates, with and without lids, in a hypoxic chamber equilibrated at 1% O2. Then, we removed the plates from the chamber to atmospheric O2 and determined equilibration rates, comparing DO level changes in plates with and without lids (Supplemental Fig. S1B).
Because the oxygen consumption rate follows the Michaelis-Menten kinetics and is nonlinear, we first used finite element methods to solve the model equations with a commercial software package, Comsol Multiphysics (Comsol, Los Angeles, CA), using the built-in 2D diffusion tool with a relative tolerance value of 1 × 10−6 and predefined normal mesh size uniformly distributed over the solution geometry. A simplified analytical solution (see appendix) was then developed, for CO2 >> Km, and used to verify the consistency of the numerical solution at high oxygen concentrations.
RESULTS
Dissolved oxygen levels and cell viability decrease in collagen gels during tube formation of HUVECs.
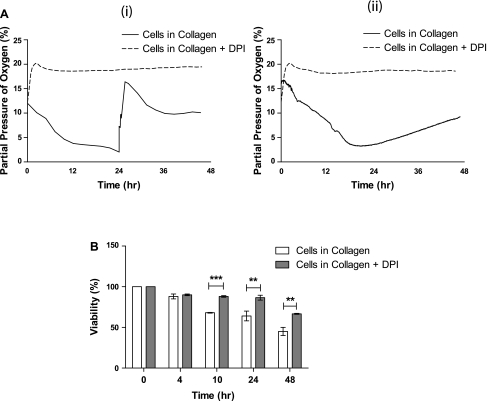
Using 24-well plates at 21% O2 tension, we measured DO at the bottom of each collagen gel with or without HUVECs. We changed the media either after 24 h or left it undisturbed for the 48-h culture period. We found that DO levels decreased rapidly to 2% within 24 h; after media replacement, they increased immediately to 15% and finally equilibrated at ∼10% (Fig. 1Ai), which we could not observe in our control measurements of collagen gel in media alone (data not shown). When we added DPI to the culture media to determine whether ROS were involved in the process of tube formation, DO levels at the bottom of the cultures containing DPI remained at 20% throughout the 48 h, suggesting that adding DPI limited the O2 consumption and DO fluctuations of HUVECs (Fig. 1Ai). When we did not replace media after 24 h, O2 levels reached a steady state at 2% for a short period of time and then increased continuously, reaching 10% at the end of 48 h (Fig. 1Aii).
Fig. 1.
Dissolved oxygen (DO) levels and cell viability during three-dimensional (3D) culture of human umbilical vein endothelial cells (HUVECs) in collagen gels. A: measured DO levels during 48 h of HUVEC culture in 3D collagen gels in atmospheric conditions, with and without reactive oxygen species (ROS) inhibitor diphenyleneiodonium (DPI), with media replacement after 24 h (Ai) and no media replacement (Aii). B: percentage of viable HUVECs at multiple time points during the culture period in untreated and DPI-treated 3D cultured HUVECs. **P < 0.01, ***P < 0.001; n = 3.
To determine viability, we released cells from collagen gels at specific time intervals during the 2-day culture period and counted viable cells. We found that the viability of HUVECs decreased during the culture, reaching ∼65% at the end of day 1, whereas 50% of the cells were found to die after 48 h in both cases of replaced media (Fig. 1B) and undisturbed media (data not shown). The significant decrease in the number of viable cells after 24 h agrees well with the observed increase in steady-state DO levels after 24 h, shown in Fig. 1Ai. Similarly, when the media were left undisturbed for 48 h, the continuous increase in DO levels after 24 h (Fig. 1Aii) can be attributed to a significant decrease in the total O2 consumption as a result of lower number of viable cells. Cells cultured in growth medium containing 10 μm DPI demonstrated significantly higher viability than cells cultured without DPI; 70% of the cells cultured with DPI were still viable after 48 h (Fig. 1B). These data suggest that the constant DO levels in 3D cultures of HUVECs with DPI occur because HUVECs consume oxygen at a considerably lower rate, not because of cell death. It should be noted that we continued this study focusing on the case of media replacement due to the better tube formation observed.
Estimating the Michaelis-Menten's parameters for HUVECs and the DO gradients in 3D collagen gels.
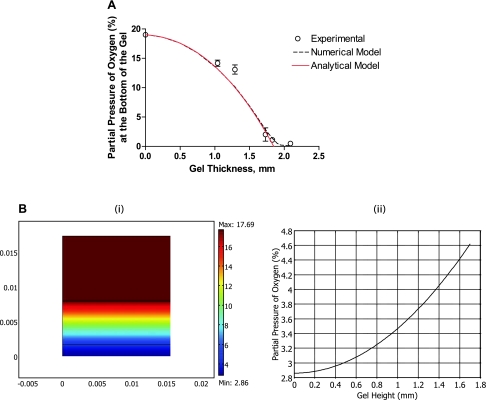
To accurately estimate DO gradients in 3D collagen gel, we first determined the Vmax and Km parameters of HUVECs for the given conditions. Steady-state DO levels were measured for five different gel thicknesses at a constant cell-seeding density of 2 million cells/ml. In addition, we measured cell viability when DO reached an equilibrium and used this as an input for the simulations. Figure 2A shows that the gel thickness strongly affected the steady-state DO levels in the gel; consequently, the oxygenation of the cells and DO levels approached zero for a gel thickness of 2.1 mm. We performed the simulations for each thickness value while varying the Vmax and Km parameters to obtain the best fit to the experimental values according to the RSS method. We determined that the Vmax and Km of HUVECs in 3D collagen gel with a density of 2.5 mg/ml and at a cell-seeding density of 2 million cells/ml were 24 × 10−18 mol/s and 0.5 μM, respectively. Both Vmax and Km values were comparable to previously reported values for HUVECs in 2D cultures at a cell density of 1 million cells/cm2 (15). Furthermore, the analytical and numerical solutions were found to be consistent with each other at high oxygen concentrations indicating the reliability of the numerical solution. Expectedly, the analytical solution deviated from the numerical solution at lower oxygen concentrations highlighting the role of the Km parameter and thus the lowered oxygen consumption rates of cells with decreasing DO levels.
Fig. 2.
Model prediction of DO levels and gradients. A: steady-state DO at the bottom of collagen gels at different gel thicknesses. We fitted model numerical predictions (dashed black line) and analytical prediction (solid red line) to the experimental values (○) to determine Vmax and Km using the residual sum of squares method. B: model predictions for oxygen gradient after 24 h of culture in the three-layer model (Bi) and across the gel (Bii).
Next, using the Michaelis-Menten parameters determined for the given conditions, we estimated the DO gradients throughout the gel depth. The simulation results, illustrated in Fig. 2B, show that, after 24 h, HUVECs are exposed to a gradient of oxygen ranging from 2.8% O2 at the bottom up to 4.6% O2 at the top layer of the gel.
Tubulogenesis requires an increase in intracellular ROS levels during 3D HUVEC cultures in atmospheric conditions.
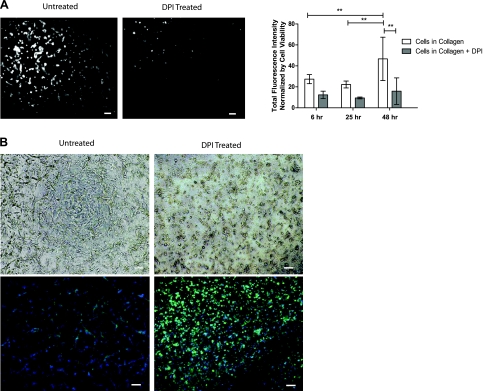
Previous studies have demonstrated the effect of hypoxia (34) and reoxygenation (46, 47) on the ROS production of ECs. Therefore, we investigated the effects of varying DO levels on intracellular ROS levels during two-day cultures of HUVEC in 3D collagen gel using H2-DCFDA dye (10, 36). We found that ROS levels continuously increased during the 48-h culture period. To examine whether tubulogenesis requires this increase, we inhibited ROS production by adding DPI to the culture medium. Adding DPI to the culture medium significantly reduced ROS levels at each time point compared with the media without DPI (Fig. 3A). We found that treating HUVEC cultures with DPI completely inhibited the tube formation observed with untreated cells (Fig. 3B). These results imply that increased intracellular ROS levels are required for the tubulogenesis of HUVECs in 3D collagen gel under atmospheric conditions.
Fig. 3.
Intracellular ROS generation during 3D culture of HUVECs in collagen gels. A: fluorescence images of intracellular ROS in untreated and DPI-treated 3D HUVEC cultures after 48 h (left) and quantification of the ROS fluorescence intensity at multiple points, normalized by cell viability (right). **P < 0.01. B: tube formation of untreated and DPI-treated HUVECs after 48 h using light microscope images (top) and fluorescence microscope images (bottom; phalloidin in green, nuclei in blue). Scale bars, 100 μm.
ROS mediates tubulogenesis through HIFα-independent pathways.
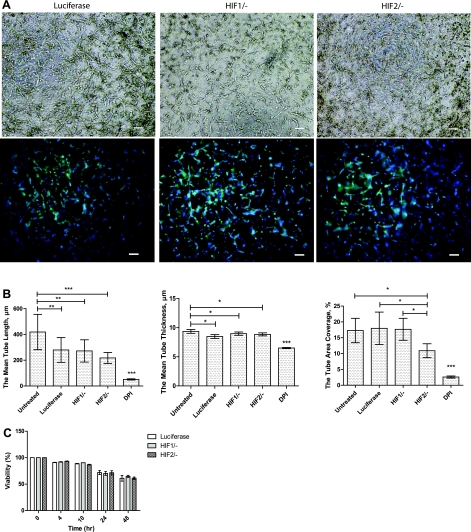
The influence of ROS could occur through their contribution to HIFα stabilization (5, 33) and/or HIFα-independent pathways, such as interacting with proteins involved in angiogenesis (11, 49). To examine the involvement of HIF1α and HIF2α in the formation of HUVEC tubes under atmospheric conditions, we used an RNAi suppression approach. We encapsulated HIF1α and HIF2α siRNA-treated HUVECs in 3D collagen gels after confirming the suppression (Supplemental Fig. S2). We investigated the kinetics of tube formation after 48 h (Fig. 4A). Quantification of tubular structures revealed that the mean length, mean thickness, and tube area coverage of the tubelike structures were 419 μm, 9.3 μm, and 17.2%, respectively, for untreated HUVECs, whereas all transfected cells exhibited slightly shorter and thinner structures (Fig. 4B). The mean length, mean thickness, and tube area coverage of the HIF1α-knocked-down HUVECs were 272 μm, 8.9 μm, and 17.6%, respectively, i.e., similar to luciferase-transfected HUVECs. Similarly, the suppression of HIF2α did not result in a significant decrease in tube formation parameters compared with empty transfection. Additionally, we exposed the DPI-treated cells to hypoxic conditions (2% DO) for 2 days and to conditions that mimic the reoxygenation dynamics to exclude the possibility that DPI treatment affects HIFα stabilization by affecting DO levels. DPI treatment of both cases resulted in no tube formation, which was similar to what we observed in DPI-treated cells under atmospheric conditions (Supplemental Fig. S3). Moreover, we examined the viability of HIFα-silenced HUVECs during the culture period, which we found to be higher than the untreated and luciferase-transfected cells (Fig. 4C), ruling out the possible effect of cell death on the inhibition of tube formation. Altogether, these results, along with the complete inhibition of tube formation by DPI treatment (Fig. 3C), suggest that intracellular ROS contribute to tubulogenesis via a HIFα-independent mechanism.
Fig. 4.
Small interfering RNA (siRNA) studies of hypoxia-inducible factor-α (HIFα). A: light microscope images (top) and fluorescence microscope images (bottom; phalloidin in green, nuclei in blue) of 3D collagen culture of HUVECs transfected with siRNA of luciferase, HIF1α, and HIF2α after 48 h (scale bars, 100 μm). B: tube length, thickness, and area coverage of untreated cells, DPI-treated cells, and HIF1α- and HIF2α-silenced cells. *P < 0.05, **P < 0.01, ***P < 0.001. C: viability of HUVECs at multiple time points as a comparison of luciferase-transfected cells to HIF1α and HIF2α siRNA-treated cells.
DISCUSSION
The vast majority of ongoing research investigating tubulogenesis of vascular cells in 3D hydrogels focuses primarily on the effects of various parameters, such as growth factors (26, 53), integrins, metalloproteases (12, 13, 25), gel stiffness (54), and scaffold ultrastructure. Most tubulogenesis assays have been performed conventionally in multiwell culture plates in atmospheric conditions without monitoring DO levels inside the 3D gel. In the first part of our study, we demonstrated the uncontrolled drop of O2 tension during 3D cultures of HUVECs in 2.4 mg/ml collagen gel at a cell-seeding density of 2 million cells/ml. Noninvasive measurements of DO levels at the bottom of the gel during culture under atmospheric conditions showed that DO levels decreased, reaching as low as 2% O2 at the end of 24 h. After changing the media on day 1, the DO levels increased rapidly, equilibrating at ∼15% O2. We suggest that the contribution of these DO level oscillations should be taken into consideration when investigating the effect of various factors during tubulogenesis of HUVECs in 3D collagen gels in atmospheric conditions. Furthermore, in assays of 3D tube formation performed in higher cell-seeding densities (39) or collagen concentrations (13), more rapid and dramatic decreases in DO levels are expected leading to severe hypoxia and anoxia early in the culture period.
The frequency of media change has always been considered to be a factor regulating cellular responses through the concentration of soluble factors and toxic cytokines produced by the cells. However, here we demonstrated that changing the media could also cause fluctuations in DO levels; for instance, media replacement after 24 h reoxygenated the cells, which was previously shown to have crucial effects on cellular responses, including angiogenesis (30, 47). Previous studies have reported the values of Vmax and Km of HUVECs at various cell-seeding densities (15, 42). For example, the Vmax and Km of HUVECs were found to be 22.05 ± 1.92 (pmol·s−1·10−6 cells) and 0.55 ± 0.02 (μM), respectively, for a seeding density of 1 × 106 cells/ml. However, all of these studies were carried out in 2D cultures. The literature currently lacks studies investigating whether Vmax and Km differ when 3D gels encapsulate the cells. Noninvasive measurement of DO at the bottom of the gel allowed us to estimate, for the first time, the Vmax and Km of HUVECs in a 3D culture setting. We found that both Vmax and Km values were similar to the values reported in the literature for HUVECs in 2D cultures. Model predictions using these Vmax and Km values demonstrated that HUVECs in the collagen gel are exposed to O2 tension in a range from 2.8 to 4.6%. Either way, this DO gradient found in 3D gels could critically affect the uniformity of cellular responses. Therefore, the formation of DO gradients should also be taken into account when analyzing tubulogenesis within collagen gels.
After demonstrating the uncontrolled fluctuations in DO levels during 3D tube formation of HUVECs in collagen gels, we sought to determine whether these variations in DO levels affected tube formation. HIFα and ROS were previously shown to be major effectors responsible for cellular responses, including the upregulation and activation of angiogenic factors and receptors, to changes in DO levels (10, 29, 45). Therefore, we examined the possible roles of HIF1α, HIF2α, and ROS in the process. We found that ROS levels increased during the culture period. Using DPI to inhibit ROS production in HUVECs precluded the rise of intracellular ROS levels observed after 48 h and resulted in the complete inhibition of the tube formation. A cell viability assay proved that the cells remain viable during their culture in media containing DPI, suggesting that the inhibition of tubulogenesis is not due to cell death, which agrees with other studies demonstrating the effects of DPI on angiogenesis (34, 45). Treatment with DPI was also previously shown to inhibit mitochondrial respiration (28), which may in turn increase the DO levels available in the gel. Similarly, our measurements of DO levels during DPI treatment also showed that DPI inhibited O2 consumption, resulting in equilibration with atmospheric O2. Thus, the profound difference in tube formation we observed between untreated cells and DPI-treated cells could be due to three independent factors: 1) the changes in ROS levels, which can directly or indirectly (through HIFα) affect tubulogenesis; 2) the inhibition of mitochondrial respiration, which may influence ATP generation; and 3) the equilibration of DO levels under atmospheric conditions, which keeps the intracellular O2 concentration at higher levels. However, previous research showed that anaerobic glycolysis was the major mechanism involved in ATP generation by HUVECs, suggesting that blocking mitochondrial respiration does not reduce ATP generation in the cells (34). On the other hand, we did not observe any structure formation when collagen gels were cultured in hypoxic conditions in media containing DPI. Therefore, we conclude that the inhibition of tube formation by DPI treatment could only occur through the inhibition of ROS accumulation.
Intracellular ROS were shown to play various roles in the tubulogenesis of HUVECs. Some studies demonstrated ROS acting indirectly by contributing to HIFα stabilization, which, in turn, promotes the expression of many angiogenic growth factors, including VEGF (5, 21, 33, 49). On the other hand, other studies suggested that ROS act independently in the process, such as through autophosphorylation of the VEGF-A receptor (10), activation of the Toll-like receptor-2 (51), or upregulation of activating transcription factor-3 (31). Therefore, we further examined whether intracellular ROS levels have a direct effect on tubulogenesis of HUVECs in collagen gel. We did not observe any significant inhibition of tube formation when HIF1α or HIF2α were silenced, suggesting that these transcription factors are not involved in the process and that ROS have an independent effect on tube formation.
Cell viability measurements revealed less cellular viability in control cultures without DPI, where tube formation was observed. Others (39) have also previously observed this correlation between cell viability and tube formation, suggesting that tube formation requires programmed cell death. Related to this, ROS were also linked to programmed cell death in HUVECs (20, 23). Thus, one possible direct effect of ROS levels may occur by contributing to the programmed cell death required for the formation of tubes in HUVECs. However, the pathways of this direct effect need further investigation.
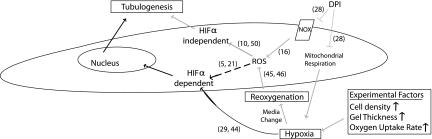
Figure 5 summarizes the suggested mechanisms and the experimental factors that occur during tube formation in 3D collagen gels. Even though decreased DO levels did not lead to severe hypoxia in our conditions, it is worth emphasizing that the effect of the variations in DO levels will be more pronounced with higher cell densities and gel thicknesses, as well as when using such other cell types as human embryonic stem cells and induced pluripotent stem cells, which have higher oxygen consumption rates and greater sensitivity to O2 changes (1, 8). Thus, in those cases, HIF1α and HIF2α could play substantial roles, affecting tube formation kinetics in experiments conducted under atmospheric conditions.
Fig. 5.
Possible mechanisms involved in tubulogenesis of HUVECs in 3D collagen gel in atmospheric conditions. Varying experimental conditions, such as cell-seeding density, collagen gel and media thickness, and oxygen uptake rate, lower the available DO levels and determine the severity of hypoxia, which may lead to HIFα stabilization. Subsequent changes of media result in the reoxygenation of the cells and promote intracellular ROS generation, which, in turn, mediates tubulogenesis through both HIFα-dependent and -independent pathways. Moreover, adding DPI to the culture medium precludes the rise of ROS both by inhibiting NOX-derived ROS and by the formation of hypoxic conditions via the blocking of mitochondrial respiration and therefore ROS generation derived by reoxygenation. The gray and black solid lines denote the evidence shown in this study and in others (shown in brackets), respectively, whereas dashed black lines denote results still under debate.
Overall, our study revealed that ROS affect the tubulogenesis process through HIFα-independent pathways, where uncontrolled variations in DO levels influence the levels of ROS. These results highlight the critical and unexpected role of O2 during 3D in vitro models of tubulogenesis under atmospheric conditions.
APPENDIX
Because the mass transfer of oxygen takes place only across the gel depth, Eqs. 1–3 can be reduced into one-dimensional forms. Moreover, the nonlinear oxygen consumption rate term in Eq. 3 can be simplified for high concentrations of oxygen (CO2 >> Km), and converted into a linear equation as below:
| (6) |
In addition, the oxygen mass flux (NO2) between the culture well and the outside environment depends on the additional resistance of the culture plate lid and can be defined as a boundary condition with the Eq. 7, where mass transfer coefficient, Klid, is determined to be 5 × 10−6 m/s (Supplemental Fig. S1B).
| (7) |
Oxygen concentration profiles at each individual layer can be obtained by solving the Eqs. 1–3, 6, and 7, as below:
Air:
| (8) |
Media:
| (9) |
Gel:
| (10) |
where δair, δmedia, and δgel are the thicknesses of the air, media, and gel layers, respectively.
GRANTS
This research was funded by an American Heart Association-Scientist Development Grant, a March of Dimes Basil O'Connor Starter Scholar Award (to S. Gerecht), National Institutes of Health Grant U54CA143868, and National Science Foundation Grant 1054415.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. German Drazer from the Johns Hopkins University Department of Chemical and Biomolecular Engineering for critical comments on the mathematical model. We thank Sezgi Nuzumlali for technical assistance in tissue culture and cell staining.
REFERENCES
- 1. Abaci HE, Truitt R, Luong E, Drazer G, Gerecht S. Adaptation to oxygen deprivation in cultures of human pluripotent stem cells, endothelial progenitor cells, and umbilical vein endothelial cells. Am J Physiol Cell Physiol 298: C1527–C1537, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abid MR, Kachra Z, Spokes KC, Aird WC. NADPH oxidase activity is required for endothelial cell proliferation and migration. FEBS Lett 486: 252–256, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Akimoto T, Liapis H, Hammerman MR. Microvessel formation from mouse embryonic aortic explants is oxygen and VEGF dependent. Am J Physiol Regul Integr Comp Physiol 283: R487–R495, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Ben-Yosef Y, Miller A, Shapiro S, Lahat N. Hypoxia of endothelial cells leads to MMP-2-dependent survival and death. Am J Physiol Cell Physiol 289: C1321–C1331, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Block K, Gorin Y, Hoover P, Williams P, Chelmicki T, Clark RA, Yoneda T, Abboud HE. NAD(P)H oxidases regulate HIF-2alpha protein expression. J Biol Chem 282: 8019–8026, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Brown ST, Nurse CA. Induction of HIF-2α is dependent on mitochondrial O2 consumption in an O2-sensitive adrenomedullary chromaffin cell line. Am J Physiol Cell Physiol 294: C1305–C1312, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Calvani M, Rapisarda A, Uranchimeg B, Shoemaker RH, Melillo G. Hypoxic induction of an HIF-1alpha-dependent bFGF autocrine loop drives angiogenesis in human endothelial cells. Blood 107: 2705–2712, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cameron CM, Harding F, Hu WS, Kaufman DS. Activation of hypoxic response in human embryonic stem cell-derived embryoid bodies. Exp Biol Med (Maywood) 233: 1044–1057, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem 275: 25130–25138, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Chen JX, Zeng H, Lawrence ML, Blackwell TS, Meyrick B. Angiopoietin-1-induced angiogenesis is modulated by endothelial NADPH oxidase. Am J Physiol Heart Circ Physiol 291: H1563–H1572, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Colavitti R, Pani G, Bedogni B, Anzevino R, Borrello S, Waltenberger J, Galeotti T. Reactive oxygen species as downstream mediators of angiogenic signaling by vascular endothelial growth factor receptor-2/KDR. J Biol Chem 277: 3101–3108, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Davis GE, Black SM, Bayless KJ. Capillary morphogenesis during human endothelial cell invasion of three-dimensional collagen matrices. In Vitro Cell Dev Biol Anim 36: 513–519, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Davis GE, Pintar Allen KA, Salazar R, Maxwell SA. Matrix metalloproteinase-1 and -9 activation by plasmin regulates a novel endothelial cell-mediated mechanism of collagen gel contraction and capillary tube regression in three-dimensional collagen matrices. J Cell Sci 114: 917–930, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Fong GH. Regulation of angiogenesis by oxygen sensing mechanisms. J Mol Med 87: 549–560, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Garedew A, Kammerer U, Singer D. Respiratory response of malignant and placental cells to changes in oxygen concentration. Respir Physiol Neurobiol 165: 154–160, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 86: 494–501, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Hanjaya-Putra D, Yee J, Ceci D, Truitt R, Yee D, Gerecht S. Vascular endothelial growth factor and substrate mechanics regulate in vitro tubulogenesis of endothelial progenitor cells. J Cell Mol Med 14: 2436–2447, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Helmlinger G, Endo M, Ferrara N, Hlatky L, Jain RK. Formation of endothelial cell networks. Nature 405: 139–141, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Ho FM, Liu SH, Lin WW, Liau CS. Opposite effects of high glucose on MMP-2 and TIMP-2 in human endothelial cells. J Cell Biochem 101: 442–450, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Ikeda S, Ushio-Fukai M, Zuo L, Tojo T, Dikalov S, Patrushev NA, Alexander RW. Novel role of ARF6 in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res 96: 467–475, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Kappel A, Ronicke V, Damert A, Flamme I, Risau W, Breier G. Identification of vascular endothelial growth factor (VEGF) receptor-2 (Flk-1) promoter/enhancer sequences sufficient for angioblast and endothelial cell-specific transcription in transgenic mice. Blood 93: 4284–4292, 1999 [PubMed] [Google Scholar]
- 23. Kobayashi S, Nojima Y, Shibuya M, Maru Y. Nox1 regulates apoptosis and potentially stimulates branching morphogenesis in sinusoidal endothelial cells. Exp Cell Res 300: 455–462, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Koh W, Stratman AN, Sacharidou A, Davis GE. In vitro three dimensional collagen matrix models of endothelial lumen formation during vasculogenesis and angiogenesis. Methods Enzymol 443: 83–101, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Lafleur MA, Handsley MM, Knauper V, Murphy G, Edwards DR. Endothelial tubulogenesis within fibrin gels specifically requires the activity of membrane-type-matrix metalloproteinases (MT-MMPs). J Cell Sci 115: 3427–3438, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Leslie-Barbick JE, Moon JJ, West JL. Covalently-immobilized vascular endothelial growth factor promotes endothelial cell tubulogenesis in poly(ethylene glycol) diacrylate hydrogels. J Biomater Sci Polym Ed 20: 1763–1779, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Li C, Issa R, Kumar P, Hampson IN, Lopez-Novoa JM, Bernabeu C, Kumar S. CD105 prevents apoptosis in hypoxic endothelial cells. J Cell Sci 116: 2677–2685, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Li Y, Trush MA. Diphenyleneiodonium, an NAD(P)H oxidase inhibitor, also potently inhibits mitochondrial reactive oxygen species production. Biochem Biophys Res Commun 253: 295–299, 1998 [DOI] [PubMed] [Google Scholar]
- 29. Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JG, Semenza GL. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 105: 659–669, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Maulik N, Das DK. Redox signaling in vascular angiogenesis. Free Radic Biol Med 33: 1047–1060, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Okamoto A, Iwamoto Y, Maru Y. Oxidative stress-responsive transcription factor ATF3 potentially mediates diabetic angiopathy. Mol Cell Biol 26: 1087–1097, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ottino P, Finley J, Rojo E, Ottlecz A, Lambrou GN, Bazan HE, Bazan NG. Hypoxia activates matrix metalloproteinase expression and the VEGF system in monkey choroid-retinal endothelial cells: involvement of cytosolic phospholipase A2 activity. Mol Vis 10: 341–350, 2004 [PubMed] [Google Scholar]
- 33. Pan Y, Mansfield KD, Bertozzi CC, Rudenko V, Chan DA, Giaccia AJ, Simon MC. Multiple factors affecting cellular redox status and energy metabolism modulate hypoxia-inducible factor prolyl hydroxylase activity in vivo and in vitro. Mol Cell Biol 27: 912–925, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Quintero M, Colombo SL, Godfrey A, Moncada S. Mitochondria as signaling organelles in the vascular endothelium. Proc Natl Acad Sci USA 103: 5379–5384, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ramanujan S, Pluen A, McKee TD, Brown EB, Boucher Y, Jain RK. Diffusion and convection in collagen gels: implications for transport in the tumor interstitium. Biophys J 83: 1650–1660, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rocic P, Kolz C, Reed R, Potter B, Chilian WM. Optimal reactive oxygen species concentration and p38 MAP kinase are required for coronary collateral growth. Am J Physiol Heart Circ Physiol 292: H2729–H2736, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Rumsey WL, Schlosser C, Nuutinen EM, Robiolio M, Wilson DF. Cellular energetics and the oxygen dependence of respiration in cardiac myocytes isolated from adult rat. J Biol Chem 265: 15392–15402, 1990 [PubMed] [Google Scholar]
- 38. Saunders WB, Bayless KJ, Davis GE. MMP-1 activation by serine proteases and MMP-10 induces human capillary tubular network collapse and regression in 3D collagen matrices. J Cell Sci 118: 2325–2340, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Segura I, Serrano A, De Buitrago GG, Gonzalez MA, Abad JL, Claveria C, Gomez L, Bernad A, Martinez AC, Riese HH. Inhibition of programmed cell death impairs in vitro vascular-like structure formation and reduces in vivo angiogenesis. FASEB J 16: 833–841, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Semenza GL. Vascular responses to hypoxia and ischemia. Arterioscler Thromb Vasc Biol 30: 648–652, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem 277: 44784–44790, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Steinlechner-Maran R, Eberl T, Kunc M, Margreiter R, Gnaiger E. Oxygen dependence of respiration in coupled and uncoupled endothelial cells. Am J Physiol Cell Physiol 271: C2053–C2061, 1996 [DOI] [PubMed] [Google Scholar]
- 43. Stroeve P. Diffusion of gases in protein solutions. Ind Eng Chem Fundam 14: 140–141, 1975 [Google Scholar]
- 44. Takahashi R, Kobayashi C, Kondo Y, Nakatani Y, Kudo I, Kunimoto M, Imura N, Hara S. Subcellular localization and regulation of hypoxia-inducible factor-2alpha in vascular endothelial cells. Biochem Biophys Res Commun 317: 84–91, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Takeda N, Maemura K, Imai Y, Harada T, Kawanami D, Nojiri T, Manabe I, Nagai R. Endothelial PAS domain protein 1 gene promotes angiogenesis through the transactivation of both vascular endothelial growth factor and its receptor, Flt-1. Circ Res 95: 146–153, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Therade-Matharan S, Laemmel E, Carpentier S, Obata Y, Levade T, Duranteau J, Vicaut E. Reactive oxygen species production by mitochondria in endothelial cells exposed to reoxygenation after hypoxia and glucose depletion is mediated by ceramide. Am J Physiol Regul Integr Comp Physiol 289: R1756–R1762, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Therade-Matharan S, Laemmel E, Duranteau J, Vicaut E. Reoxygenation after hypoxia and glucose depletion causes reactive oxygen species production by mitochondria in HUVEC. Am J Physiol Regul Integr Comp Physiol 287: R1037–R1043, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Ushio-Fukai M, Alexander RW. Reactive oxygen species as mediators of angiogenesis signaling: role of NAD(P)H oxidase. Mol Cell Biochem 264: 85–97, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Ushio-Fukai M, Tang Y, Fukai T, Dikalov SI, Ma Y, Fujimoto M, Quinn MT, Pagano PJ, Johnson C, Alexander RW. Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res 91: 1160–1167, 2002 [DOI] [PubMed] [Google Scholar]
- 50. Vo E, Hanjaya-Putra D, Zha Y, Kusuma S, Gerecht S. Smooth-muscle-like cells derived from human embryonic stem cells support and augment cord-like structures in vitro. Stem Cell Rev 6: 237–247, 2010 [DOI] [PubMed] [Google Scholar]
- 51. West XZ, Malinin NL, Merkulova AA, Tischenko M, Kerr BA, Borden EC, Podrez EA, Salomon RG, Byzova TV. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature 467: 972–976, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wiesener MS, Turley H, Allen WE, Willam C, Eckardt KU, Talks KL, Wood SM, Gatter KC, Harris AL, Pugh CW, Ratcliffe PJ, Maxwell PH. Induction of endothelial PAS domain protein-1 by hypoxia: characterization and comparison with hypoxia-inducible factor-1alpha. Blood 92: 2260–2268, 1998 [PubMed] [Google Scholar]
- 53. Xin X, Yang S, Ingle G, Zlot C, Rangell L, Kowalski J, Schwall R, Ferrara N, Gerritsen ME. Hepatocyte growth factor enhances vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. Am J Pathol 158: 1111–1120, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yamamura N, Sudo R, Ikeda M, Tanishita K. Effects of the mechanical properties of collagen gel on the in vitro formation of microvessel networks by endothelial cells. Tissue Eng 13: 1443–1453, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.