Abstract
The indirect flight muscle (IFM) of insects is characterized by a near crystalline myofilament lattice structure that likely evolved to achieve high power output. In Drosophila IFM, the myosin rod binding protein flightin plays a crucial role in thick filament organization and sarcomere integrity. Here we investigate the extent to which the COOH terminus of flightin contributes to IFM structure and mechanical performance using transgenic Drosophila expressing a truncated flightin lacking the 44 COOH-terminal amino acids (flnΔC44). Electron microscopy and X-ray diffraction measurements show decreased myofilament lattice order in the flnΔC44 line compared with control, a transgenic flightin-null rescued line (fln+). flnΔC44 fibers produced roughly 1/3 the oscillatory work and power of fln+, with reduced frequencies of maximum work (123 Hz vs. 154 Hz) and power (139 Hz vs. 187 Hz) output, indicating slower myosin cycling kinetics. These reductions in work and power stem from a slower rate of cross-bridge recruitment and decreased cross-bridge binding in flnΔC44 fibers, although the mean duration of cross-bridge attachment was not different between both lines. The decreases in lattice order and myosin kinetics resulted in flnΔC44 flies being unable to beat their wings. These results indicate that the COOH terminus of flightin is necessary for normal myofilament lattice organization, thereby facilitating the cross-bridge binding required to achieve high power output for flight.
Keywords: fiber mechanics, cross-bridge kinetics, thick filaments
in muscle, the thick and thin filament lattice provides the structural and mechanical foundation for transmitting contractile forces throughout the cell. The highly ordered indirect flight muscle (IFM) of Drosophila melanogaster is an attractive model system to study the relationship between lattice structure and muscle function, because its in vivo lattice organization can be measured via X-ray diffraction in living flies (15) and its function can be measured from the whole fly to the molecule (14, 20, 30). In addition, the means for producing genetic alterations of specific proteins in D. melanogaster are well established, permitting precise manipulation of thick and thin filament proteins. In this study, we combine these approaches to define the role of flightin, specifically the COOH terminus, in lattice organization and its effects on cross-bridge cycling kinetics and overall muscle performance.
In Drosophila, flightin is a ∼20-kDa (182 amino acids) protein that is expressed exclusively in the IFM (33). Flightin binds the light meromyosin region of myosin, ∼2/3 of the way down the rod, because substituting aspartic acid 1554 for lysine abolishes flightin's interaction in vitro (1) and accumulation in vivo (18). Immunolocalization studies in Drosophila and Lethocerus IFM indicate that flightin is associated with the thick filament backbone (25, 26), consistent with studies that show flightin is absent in IFM lacking thick filaments (29).
Studies using Drosophila mutants demonstrate that flightin plays several important roles in maintaining muscle integrity. Flightin is required for normal thick filament assembly and for establishing or maintaining in vivo filament length and flexural rigidity (8, 26). Thick filaments assembled in the absence of flightin are, on average, >30% longer and are 30–45% more flexible than normal filaments (8). Thick filaments lacking flightin are also more prone to in vivo fragmentation, indicating that flightin is essential for their structural integrity (26). Notably, genetic ablation of flightin expression results in complete loss of flight due to structurally and mechanically compromised flight muscles (13, 26). Sarcomere degradation and fiber hypercontraction are often extreme in the absence of flightin, suggesting that flightin fulfills a crucial role in maintaining normal myofilament lattice integrity of the IFM. Thus, flightin's influence spans from the sarcomere to the fiber, both of which are routinely disrupted by contractile forces when flightin is absent or present in reduced amounts (13, 18, 23, 26).
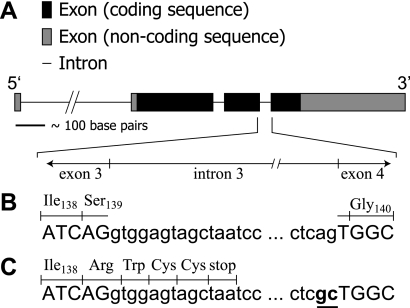
The remarkable and distinct phenotypes manifested by flightin mutants (3, 8, 13, 23, 26, 31) and mutants that affect flightin expression (13, 18, 23) raise important questions about the functional roles and molecular properties of this unique protein. However, predicting flightin sequences that may fulfill important functional roles is difficult because flightin's amino acid sequence is not similar to any known protein domains. Therefore, we are left with the alternative approach of identifying flightin homologues and making predictions about protein function based on regions of sequence conservation across many species. A comparison of flightin sequences from 12 Drosophila species reveal a tripartite organization (Fig. 1). The three distinct regions appear to be under different evolutionary constraints, raising the possibility that these regions define separate functional domains. In this study, we focus on the COOH-terminal region (amino acids 137 to 182) whose sequence shows an intermediate conservation profile, compared with the poorly conserved NH2-terminal region and the highly conserved midregion. High conservation suggests that the midregion contains critical sequences for flightin's interaction with the filament lattice, and we expect that genetic manipulation of this region would produce a phenotype similar to the flightin null. In contrast, the COOH-terminal region shows strong patterns of conservation only among closely related species (e.g., D. persimilis and D. pseudoobscura), suggesting that its function may be taxon-specific. To investigate the structural and functional influences of flightin's COOH terminus, we generated a new mutant allele by removing 44 COOH-terminal amino acids (Fig. 2). We find that the COOH-terminal truncated flightin associates with thick filaments but that the absence of the COOH terminus reduces myofilament lattice order and sarcomere regularity. Structural changes in IFM fibers with the COOH-terminal truncated flightin lead to decreased cross-bridge binding and reduced power output, which results in flies that are unable to beat their wings.
Fig. 1.
ClustalW alignment of flightin amino acid sequences from 12 Drosophila species. Identities are marked by asterisks (bottom line), and the heavy underline indicates the region of highest conservation (i.e., the midsection of the flightin sequence). The region immediately COOH-terminal to the midsection was deleted in this study.
Fig. 2.
A schematic illustration of the flightin gene (A), with the 3′-junction of exon 3 to the 5′-junction of exon 4 expanded. Selected exon (uppercase) and intron (lowercase) sequences corresponding with this expanded region are listed for the flightin rescue (fln+) gene (B) and the COOH-terminal truncated flightin (flnΔC44) gene (C). The codon for Ser139 is normally split by intron 3. The truncation results from two mutated nucleotides at the end of intron 3 (underlined and bold in C) that abolish intron splicing. As a result, translational read through intron 3 changes Ser139 to Arg and incorporates three additional ectopic amino acids (Trp,Cys,Cys).
MATERIALS AND METHODS
Fly strains.
Drosophila melanogaster w1118 (an otherwise wild-type strain except for the white eye color mutation) and w*;T(2;3) apXa, apXa/CyO; TM3, Sb1 were obtained from the Bloomington Stock Center (Bloomington, IN). w1118 was used as host for generating the transgenic lines. The control strain, fln+, is a transgenic strain expressing the wild-type flightin gene in a flightin-null (fln0) background (2). All fly lines were maintained in a constant temperature (21 ± 1°C) environmental chamber on a 12:12-h light-dark cycle. Female flies (1.5–3 days old) were used for all experiments.
Sequence analysis.
The genomes of eleven Drosophila species available in GenBank (D. simulans, D. sechellia, D. yakuba, D. erecta, D. ananassae, D. pseudoobscura, D. persimilis, D. willistoni, D. virilis, D. mojavensis, D. grimshawi) were searched for flightin by BLAST using the flightin cDNA sequence from D. melanogaster [GenBank accession no. AY060802; (33)]. Amino acid sequences were aligned using ClustalW.
Construction of the transformation vector.
The COOH-terminal deletion was engineered in a pCaSpeR transformation vector containing the full-length flightin gene and the actin Act88F promoter (2). The last two nucleotides of the third intron 3′-splice site were changed from AG to GC by PCR using primers with the altered sequence, as previously described (3).
Solutions.
Solutions were prepared according to a computer program that solves the ionic equilibria (11). Unless listed otherwise, chemicals were purchased from Sigma-Aldrich (St. Louis, MO) and concentrations are in mmol/l (mM). Relaxing solution (pCa 8.0, pCa = −log10[Ca2+]) consisted of 20 N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid (BES), 20 creatine phosphate (CP), 600 U/ml creatine phosphokinase (CPK), 1 DTT, 5 EGTA, 1 Mg2+, 12 MgATP, ionic strength of 200 meq adjusted with sodium methane sulfate, and pH 7.0. Activating solution was like relaxing solution, except at pCa 4.0. Rigor solution was like activating solution, without CP, CPK, and MgATP. Storage solution (pCa 8) was 20 BES, 10 DTT, 5 EGTA, 1 Mg2+, 5 MgATP, 0.25 Pi, a protease inhibitor cocktail (Roche, Indianapolis, IN), ionic strength of 175 meq adjusted with sodium methane sulfate, and pH 7.0 with 50% wt/vol glycerol added. Skinning solution was like storage solution, except with 0.5% Triton X-100.
Generation of the P{flnΔC44} strain.
Transformation and generation of transgenic lines was done as previously described (2). Linkage group was determined by standard crosses to w*;T(2;3) apXa, apXa/CyO; TM3, Sb1. A line with a second chromosome insertion was crossed into the flightin-null background (fln0) (26) to generate a homozygous transgenic line with no endogenous flightin expression. The genotype for this line is w1118; P{w+, flnΔC44}; fln0, e and herein will be referred to as flnΔC44. Expression of the transgene in this line was confirmed by RT-PCR analysis via RNA isolated from 20 two-day old flies, using the primers 5′-TCCAGATAAACAACTGCC-3′ and 5′-ATTTAGGTGCGCTTACTA-3′.
Gel electrophoresis and Western blot analysis.
One (1DE)- and two (2DE)-dimensional gel electrophoresis and Western blot analysis were done as previously described (2), with the following modifications. For 1DE analysis, IFM fibers were dissected and incubated in skinning solution for 2 h at room temperature, precipitated by spinning on a tabletop microfuge, drained of solution, rinsed for 5 min twice in washing solution, and dissolved in SDS gel sample buffer. All solutions contained protease inhibitor cocktail (Sigma-Aldrich). Samples were separated by 12% SDS-PAGE and transferred to nitrocellulose membrane (0.45 μm pore size) using a Tris-glycine buffer (National Diagnostics, Atlanta, GA) at 30 V for ∼14 h. Western blots were blocked using a 1:1 Aquablock-PBS solution (East Coast Biologics, North Berwick, ME) and incubated with a 1:3,000 dilution of anti-flightin polyclonal antibody (26), then a 1:2,000 dilution of Alexa Fluor 680 goat anti-rabbit Ig (Invitrogen, Carlsbad, CA). Blots were scanned in an Odyssey Imaging System (LI-COR Biosciences, Lincoln, NE) to detect flightin.
Quantitative Western blot analysis of 1DE gels was used to determine the relative flightin abundance in flnΔC44 and fln+ as described previously (2), with the following modifications. Fly thoraces were homogenized in SDS gel sample buffer containing 8 M urea without bromophenol blue, and total protein concentration was determined by Nanodrop (Thermo Scientific, Wilmington, DE). Sample concentration was adjusted to 8 mg/ml, 4 mg/ml, and 2 mg/ml, and equal amounts of protein from fln+ and flnΔC44 thoraces were combined, mixed with bromophenol blue, and loaded on a 12% SDS-PAGE gel. After electrophoresis, gels were blotted and processed for Western blot fluorescence detection as described (2) and analyzed with Phoretix 1D software (Nonlinear Dynamics, Durham, NC).
For 2DE analysis, whole thoraces were directly homogenized in a buffer containing 8 M urea, 4% CHAPS, 100 mM DTT, and 0.2% Biolytes 4/6. The sample (a 1:1 mixture of fln+ and flnΔC44 flies, ∼100 μg total protein each) was spun for 3 min at 10,000 g and the supernatant was loaded onto an IEF pH 4–7 strip and allowed to rehydrate for 18 h. Isoelectric separation occurred via a linear volt ramp at 250 V (20 min), a rapid volt ramp at 8,000 V (2.5 h), and another rapid volt ramp at 8,000 V to reach 50,000 V h. Strips were first equilibrated in a solution containing 6 M urea, 2% SDS, 0.375 M Tris·HCl [pH 6.8], 20% glycerol, and 130 mM DTT. A second equilibration was carried out in the same buffer, but substituting 135 mM iodoacetamide for DTT. The strips were loaded on a 12% SDS gel and the proteins separated by electrophoresis at 85 V for 45 min followed by 150 V for 5 h. The gels were blotted onto nitrocellulose and probed with a rabbit anti-flightin polyclonal antibody as described previously (26).
Flight performance.
Flight tests and wing-beat frequency analysis were performed on 10 flies from each line as previously described (31).
Electron microscopy.
Fly thoraces were bisected, fixed, embedded, sectioned, and imaged as previously described (2).
X-ray diffraction experiments.
Live fly preparation and X-ray diffraction measurements were done as previously described (15). X-ray diffraction patterns from resting flies were obtained using the small-angle instrument on the Biophysics Collaborative Access Team (BioCAT) beam line 18ID at the Advanced Photon Source (Argonne, IL). These patterns were analyzed to extract the intensities, widths, and separations of the 1,0 and 2,0 equatorial reflections, conferring information about the structure and heterogeneity of the filament lattice (14). We calculated inter-thick filament spacing as 2/√3 multiplied by the d1,0 lattice spacing value (d1,0 is the distance between the 1,0 lattice planes). We also calculated the ratio of the 2,0 and 1,0 equatorial reflection intensities (I2,0/I1,0), and the relative heterogeneity in inter-filament spacing among myofibrils (σd).
Single fiber mechanics.
Fiber preparation and mechanical measurements were performed as previously described (21). Briefly, flies were anesthetized with CO2 and single dorsolongitudinal muscle fibers were split lengthwise to make their diameter ∼100 μm. The fibers were demembranated in skinning solution, clipped with aluminum T-clips at both ends ∼300 μm apart, and mounted between a piezoelectric motor (model P-841.10, Physik Instrumente, Auburn, MA) driven by an amplifier (model E662, Physik Instrumente) and a strain gauge (model AE801, Kronex, Walnut Creek, CA). Fibers were mounted in relaxing solution (pCa 8.0), stretched until just taut, and then stretched an additional 5% in 1% increments to the initial fiber length (L0). Fibers were activated (pCa 5.0) and stretched in increments of 3% L0 until oscillatory work production reached a stable maximum as measured by sinusoidal analysis. Fibers were returned to relaxing solution and placed directly into rigor solution or progressively calcium-activated (to measure isometric tension versus pCa) and placed into rigor solution. Following tension stabilization at each condition, isometric tension and sinusoidal analysis measurements were recorded (15°C). Individual recordings of normalized isometric tension versus pCa were fit to a 3-parameter Hill equation to assess calcium sensitivity (pCa50) and the Hill coefficient (nH). Small-amplitude sinusoidal length changes (0.125% fiber length) were applied at 50 discrete frequencies (0.5–650 Hz), with data sampled at 5 kHz.
A digital fast Fourier transform (FFT) was applied to the strain (the change in muscle length divided by the original length) and stress (force divided by cross-sectional area) signals, and the complex modulus [Y(ω), where ω is angular frequency] was calculated from the quotient of these FFTs (stress/strain). Elastic and viscous moduli are separated via the in-phase (real part) and out-of-phase (imaginary part) portions of complex modulus. Fitting Eq. 1 to these data provides estimates for six model parameters: A, k, B, b, C, and c.
| (1) |
The magnitudes provided by A, B, and C are related to stress produced by the fiber (expressed in kN/m2), while the characteristic frequencies b and c (expressed in Hz) are related to cross-bridge cycling rates. k is a unitless exponent (range, 0 to 1) describing the degree to which the measured viscoelastic behavior correlates with a purely elastic (k = 0) versus a purely viscous (k = 1) mechanical response.
The A-process reflects viscoelastic properties of structural elements within the fiber and holds no enzymatic dependence (22). Enzymatic cross-bridge cycling behavior produces a frequency dependence in the measured viscous and elastic modulus during Ca2+-activated contraction. This enzymatic behavior is represented by the B- and C-processes, which characterize work-producing (cross-bridge recruitment) and -absorbing (cross-bridge distortion) processes, respectively (6, 16, 24). The characteristic frequency b is correlated with the observed rate of myosin force production and scales proportionally with shifts in the frequency of maximal oscillatory work production (17, 35), while c is related to the mean duration of cross-bridge attachment (24).
Statistics.
All data are means ± SE. Nonlinear least squares fitting of Eq. 1 to recorded complex moduli was performed using a Levenberg-Marquardt routine (IDL 7.0, ITT Visual Information Solutions, Boulder, CO). Student's t-tests were used to examine differences between fln+ and flnΔC44 for most variables, except elastic and viscous moduli. Because these moduli data were measured across different sinusoidal oscillation frequencies, we applied a repeated-measures analysis with frequency as a repeated measure, followed by Bonferroni-adjusted pairwise comparisons between the two groups at each frequency. All analyses were conducted with SPSS software (version 16; SPSS, Chicago, IL).
RESULTS
Comparison of flightin sequences among Drosophila species.
To identify sequences important for flightin function, we conducted a BLAST search of 11 Drosophila genomes using the flightin sequence from D. melanogaster (33). Alignment of the 12 sequences revealed a tripartite organization (Fig. 1). The NH2-terminal region extending to amino acid 65 (D. melanogaster numbering) shows very low conservation (<15% identity). In contrast, the midsection extending from residue 66 to residue 136 shows very high conservation (93% identity) while the COOH-terminal region (residues 137 to 182) shows intermediate conservation (60% identity). Thus, different regions of flightin appear to be evolving independently and may represent separate functional domains.
Sarcomeres in flnΔC44 flies incorporate COOH-terminal truncated flightin.
To elucidate the contribution of the COOH-terminal sequence of flightin to flight muscle function, we investigated transgenic flies expressing a truncated flightin in a background void of endogenous full-length flightin. This was achieved by mutating two nucleotides in the 3′-splice site of intron 3, preventing normal removal of intron 3 that results in a translation read-through of the boundary of exon 3 and intron 3 until encountering a cryptic stop codon within intron 3 (Fig. 2). The truncated protein lacks the final 44 amino acids of the native flightin sequence (i.e., the COOH-terminal semiconserved region), with serine 139 replaced by arginine followed by three ectopic amino acids. This truncated protein has a predicted molecular mass of 16,230 Da and predicted pI of 4.90, compared with 20,656 Da and 5.30 for the full-length, unmodified native flightin.
Western blot results (Fig. 3A) illustrate the presence of flightin within skinned fiber preparations, indicating that thick filaments of flnΔC44 flies incorporate the truncated flightin protein analogous to thick filament incorporation of flightin in fln+ flies. Blots show that COOH-terminal truncated flightin from flnΔC44 migrates slightly further than full-length flightin in fln+. Quantitative Western blot analysis was performed on 1DE blots where protein extracts from flnΔC44 and fln+ thoraces were mixed (Fig. 3B), showing that the average band intensity of the truncated flightin is 25% less than full-length flightin. Normalizing intensity units to protein load (8 mg) produced values of 104 ± 15 and 78 ± 8 for fln+ and flnΔC44, respectively (means ± SE, n = 8). Because the truncated protein has 24% less sequence than the full-length version, the 25% reduction in band intensity most likely results from fewer epitopes on the truncated flightin molecule, assuming epitopes are uniformly distributed along the length of flightin. Shifts in pI are shown on the 2DE blots where protein extracts from flnΔC44 and fln+ thoraces were mixed (Fig. 3C). The COOH-terminal truncation reduced the number of spots to 8, compared with 11. This reduced phosphorylation profile, which normally includes at least 9 phosphovariants (32), agrees with previous studies where putative phosphorylation sites within the truncated COOH terminus were mutated to alanines (3).
Fig. 3.
A: Western blot of indirect flight muscle (IFM) skinned fiber separated by one-dimensional (1DE) gel electrophoresis for the control (fln+) and COOH-terminal truncated flightin (flnΔC44) lines shows that the protein band from flnΔC44 migrates further than fln+. Note that the fln+ band is broader, reflecting a higher level of phosphorylation (33). B: relative flightin content was quantified from Western blots of whole thorax proteins from fln+ flies (lane 1) and a 1:1 mix of fln+ and flnΔC44 flies (lane 2) separated by 1DE gel electrophoresis. C: Western blot of whole thorax proteins separated by two-dimensional (2DE) gel electrophoresis for a mixture of flnΔC44 and fln+ flies, with the isoelectric focusing (pI) gradient running basic to acidic from left to right. The shifts in mobilities along the pI and mass axes are consistent with predictions based on amino acid sequence. Blots were probed with an anti-flightin polyclonal antibody.
flnΔC44 flies cannot beat their wings.
The parental transgenic line expressing flnΔC44 in a w1118 background (i.e., with endogenous flightin) showed normal behavior and flight ability, indicating that the truncated flightin insertion does not interfere with function. flnΔC44 flies were flightless (flight score = 0) and unable to produce any sustained, consistent wing movement emulating wing beats, although sporadic, small-amplitude wing motions were observed. fln+ flies were flight capable, with a flight score of 4.5 ± 0.1 and a wing-beat frequency of 186 ± 1 Hz, consistent with previous results (2).
flnΔC44 flies display disorganized sarcomeric structure.
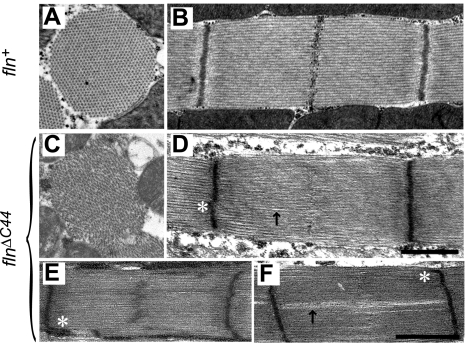
Previous characterization of flightin-null flies (fln0) showed that flightin's absence increases sarcomere length and severely compromises sarcomere integrity in adult IFM (26). The sarcomeric defects of fln0 are completely reversed in fln+ flies by reintroducing the wild-type flightin gene into the fln0 strain by genetic transformation (2) (Fig. 4, A and B). However, flnΔC44 expression in a flightin-null background only partially rescued the sarcomeric defects observed in fln0, with flnΔC44 sarcomeres appearing (Fig. 4, C–F) more disorganized than the control, fln+ (Fig. 4, A and B). Although flnΔC44 showed cylindrical myofibril cross sections (Fig. 4C) and sarcomeres with well-defined Z-lines (Fig. 4D), the filament lattice appeared disordered (Fig. 4C) and M-lines were often absent or showed reduced intensity (Fig. 4D). In some flnΔC44 sarcomeres the M-line appeared wavy or zigzagged with electron dense material streaming into the A-band overlap zone (Fig. 4E). Irregularities in Z-band structure were also observed (asterisks in Fig. 4, D–F). Another common feature of flnΔC44 sarcomeres were gaps along the A-band (arrow in Fig. 4D), which occasionally ran the length of the sarcomere (Fig. 4F). These longitudinal gaps may be consistent with separations of the lattice that appear in the cross-sectioned images (Fig. 4C), disrupting the nearly crystalline hexagonal lattice found in the control IFM. These gaps are reminiscent of features seen in fln0 sarcomeres (26), though much less extreme. Sarcomere length in flnΔC44 was slightly shorter than fln+, at 3.12 ± 0.02 μm (n = 212) versus 3.42 ± 0.11 μm (n = 225).
Fig. 4.
Electron microscopy images of dorsolongitudinal flight muscles from fln+ (A and B) and flnΔC44 (C–F) transgenic fly lines. Cross-sectionally, fln+ myofibrils show the cylindrical shape and double hexagonal myofilament array characteristic of wild-type IFM myofibrils (A). In contrast, flnΔC44 myofibrils show a less regular shape and frequent breaks in the myofilament lattice (C). These breaks are also evident on longitudinal sections and can vary in width and length (arrows in D and F). Compared with fln+ (B), M-lines in flnΔC44 sarcomeres are absent (D) or dispersed (E). Spreading of Z-band density in mutant sarcomeres is indicated by asterisks. All negatives were magnified at ×6,000, and scale bar represents 1 μm for A–D and E and F.
flnΔC44 flies exhibit disordered myofilament lattice.
The flnΔC44 flies had a 0.4 nm (∼1%) decrease in inter-thick filament spacing and an increase in σd, a relative measure of the heterogeneity among myofibrils (Table 1). The large increase in equatorial intensity ratio (I2,0/I1,0) for flnΔC44 indicates a mass shift away from the thick filament toward the thin filament (Table 1). An increased I2,0/I1,0 is normally interpreted to result from repositioning of the myosin further from the thick filament backbone toward the thin filaments, thereby increasing the probability of binding (7, 9). However, the increased I2,0/I1,0 could also result from the COOH-terminal truncated flightin decreasing thick filament mass, relative to thin filament mass. Given that the mass of the thick filament is ∼90% myosin (the heavy chain dimer dominating with ∼4,000 aa) and a flightin-to-myosin ratio of approximately 1:2, we estimate that flightin (182 aa) contributes no more than 2% of the total thick filament mass [≈(182/4,000) × 45%]. Because the missing COOH-terminal sequence represents ∼25% of the flightin mass, the reduction in thick filament mass due to a truncated flightin would be no more than 0.5% of total thick filament mass. Thus, the minimal mass decrease is unlikely to account for the large increase in I2,0/I1,0 from flnΔC44 flies compared with fln+.
Table 1.
Small angle X-ray diffraction of the IFM from live flies
| Line | Inter-Thick Filament Spacing, nm | Intensity Ratio (I2,0/I1,0) | Lattice Disorder (σd) |
|---|---|---|---|
| fln+ | 56.2 ± 0.1 (11) | 0.82 ± 0.03 (8) | 1.5 ± 0.2 (8) |
| flnΔC44 | 55.8 ± 0.1* (12) | 1.37 ± 0.08* (8) | 4.7 ± 0.4* (8) |
Values are means ± SE (number of flies analyzed is shown in parentheses).
IFM, indirect flight muscle; σd, related to the lattice spacing distribution among myofibrils and represents lattice heterogeneity.
Significant difference (P < 0.01) from fln+ control.
Altogether, the in vivo X-ray diffraction results show that the COOH-terminal truncated flightin produces slightly smaller lattice structure, more heterogeneity in the myofilament lattice spacing, and repositioning of the myosin heads away from the thick filament backbone. X-ray diffraction results agree well with electron microscopy observations, both indicating that ultrastructural organization of the IFM is reduced in the flnΔC44 flies compared with fln+ flies.
Muscle fibers from flnΔC44 flies show decreased cross-bridge cycling kinetics.
Relaxed steady-state isometric tension increased in flnΔC44 fibers (Table 2), although relaxed tensions at L0 were similar for both lines (1.2 ± 0.1 kN/m2 for fln+ vs. 1.4 ± 0.2 kN/m2 for flnΔC44). Notably, flnΔC44 fibers required twice the stretch from L0 to reach stable, maximum work production, compared with the fln+ (16 ± 4% vs. 8 ± 3%). These results suggest that the greater relaxed isometric tension for flnΔC44 fibers likely stems from greater connecting filament strain due to the increased stretching required to achieve maximal oscillatory work (34).
Table 2.
Isometric tension measurements from demembranated IFM fibers
| Tension, kN/m2 |
Hill Parameters |
||||
|---|---|---|---|---|---|
| Line | Relaxed | Net Active | Net Rigor | pCa50 | nH |
| fln+ | 1.1 ± 0.1 (14) | 0.8 ± 0.1 (14) | 1.5 ± 0.2 (11) | 5.81 ± 0.06 (6) | 2.6 ± 0.5 (6) |
| flnΔC44 | 1.9 ± 0.2* (14) | 1.0 ± 0.1 (14) | 1.8 ± 0.3 (11) | 5.76 ± 0.03 (6) | 2.4 ± 0.5 (6) |
Values are means ± SE (number of flies analyzed is shown in parentheses). Net active (pCa 5) or net rigor values represent tension increase from relaxed (pCa 8).
Significant difference (P < 0.01) from fln+ control.
There were no differences in net active or net rigor tension between fln+ and flnΔC44 fibers. The isometric tension-pCa relationship was similar for fln+ and flnΔC44, quantified by calcium sensitivity (pCa50) and cooperativity (nH) (Table 2). These results indicate that cross bridges are capable of binding actin and generating similar steady-state force values in fln+ and flnΔC44 lines.
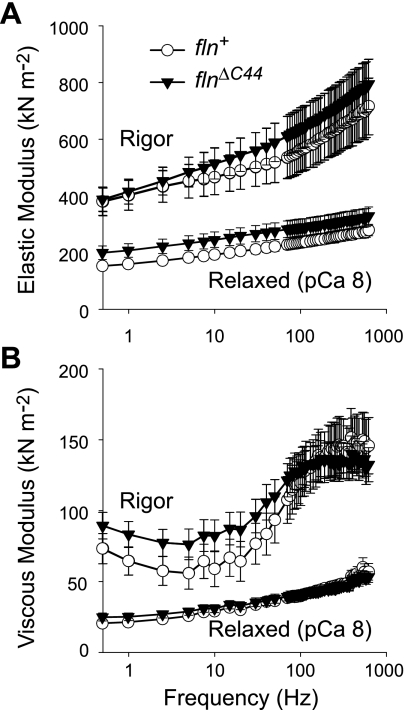
Elastic and viscous moduli were similar at each individual frequency under relaxed and rigor conditions for flnΔC44 and fln+ (Fig. 5). By eliminating cross-bridge cycling, relaxed (without strong cross-bridge binding) and rigor (without ATP so strongly bound cross bridges cannot detach from actin) measurements highlight the structural elements contributing to the fiber's viscoelastic properties. The rigor measurements include a contribution from the strongly bound cross bridges, in addition to the passive elements measured under relaxed conditions. These results show no obvious differences between the fln+ and flnΔC44 fibers, indicating similar viscoelastic mechanical properties for filaments and cross bridges between both lines.
Fig. 5.
Elastic (A) and viscous (B) moduli versus frequency for relaxed and rigor conditions for demembranated IFM fibers from flnΔC44 and fln+ lines. Relaxed or rigor values did not differ at any frequency between the two lines.
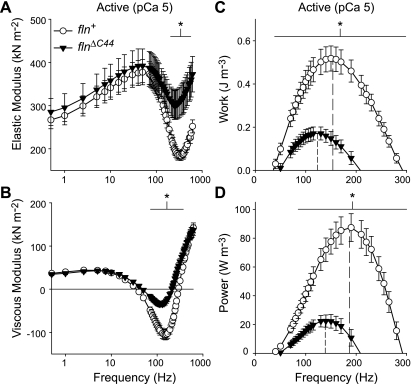
At maximal calcium activation (pCa 5.0), flnΔC44 fibers had a greater elastic moduli from 210–615 Hz and smaller viscous moduli from 70–365 Hz (Fig. 6, A and B). Maximal oscillatory work and power output were less for flnΔC44 than fln+ fibers (Fig. 6, C and D). The decreases in maximal work and power for flnΔC44 were accompanied by lower frequencies of maximal work and power. These measurements demonstrate that diminished organization of flnΔC44 IFM compromises the cross-bridge recruitment required to achieve flight.
Fig. 6.
Elastic modulus (A), viscous modulus (B), work (C), and power (D) versus frequency for active IFM fibers from flnΔC44 and fln+ lines. Vertical dashed lines in C and D represent frequency of maximum work and power, occurring at 123 ± 3 and 135 ± 5 Hz for flnΔC44 versus 154 ± 4 and 187 ± 4 Hz for fln+. Lines below the asterisks denote frequency ranges where measured values are significantly different between flnΔC44 and fln+ (P < 0.05).
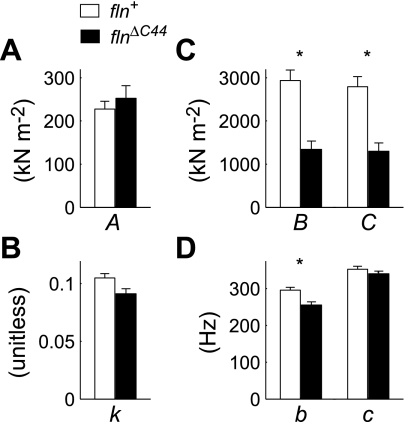
The model parameters A and k were similar between flnΔC44 and fln+, indicating likeness between the passive mechanical elements of their muscle fibers (i.e., M-lines, Z-lines, thick, thin, and connecting filaments; Fig. 7, A and B). The flnΔC44 fibers had reduced B and C values, roughly half those observed for fln+ (Fig. 7C), indicating decreases in the number of strongly bound cross bridges or cross-bridge stiffness. Because similar values among passive and rigor moduli for both lines (Fig. 5) imply comparable cross-bridge stiffness values, the decreases in B and C signify less cross-bridge binding during active contraction in flnΔC44 flies. The values for c were not different between lines (Fig. 7D), indicating no variation in mean cross-bridge attachment duration [ton = (2πc)−1] (24). However, the value of b decreased in the flnΔC44 line (Fig. 7D), suggesting a slower rate of cross-bridge recruitment that correlates with decreased frequencies of maximal work and power (Fig. 6, C and D).
Fig. 7.
Fitted model parameters (Eq. 1) describing the A-process (A and B), and B- and C-processes (C and D) for active fiber measurements from flnΔC44 and fln+ lines (Fig. 6, A and B). *Significant differences for any single parameter between flnΔC44 and fln+ (P < 0.05).
DISCUSSION
This study further defines the critical role that thick filament-associated proteins play in maintaining structural integrity of sarcomeres to promote functional muscle contraction. This study demonstrates that the COOH terminus of flightin, a myosin rod binding protein, is required for normal myofilament lattice organization, which in turn coordinates cross-bridge cycling kinetics to generate high power output of Drosophila IFM to accommodate flight. These findings exemplify complex behavior in a biological system, where rather subtle differences in myofilament lattice structure cascade into moderate differences in cellular function that underlie severe differences in organismal behavior.
In a flightin-null background, the IFM fibers expressed and incorporated the COOH-terminal truncated flightin into the sarcomere (Fig. 3). Although muscle ultrastructure is less ordered for flnΔC44 than fln+ (Fig. 4), the COOH-terminal truncated flightin has greatly improved muscle integrity compared with similarly aged fln0 flies (1.5–3 days old). The fln0 flies had severely disrupted sarcomeres, with breakdown of Z-bands and nearly complete loss of M-lines (26). Although the four ectopic amino acids incorporated into the truncated gene construct could potentially interfere with the function of the truncated flightin, we expect these influences to be small because the COOH terminus of flightin does not appear to be essential for flightin expression, stability, or incorporation into the thick filament (Figs. 3 and 4). These results also indicate that the flnΔC44 preserves critical amino acid sequences for binding the myosin rod and maintaining sarcomere stability (Fig. 4).
Quantitative Western blot analysis showed the expected 25% intensity reduction for the truncated flightin band compared with control (Fig. 3B). We interpret that the reduced intensity is due to ∼24% of the protein missing, meaning the COOH-terminal truncation does not significantly alter flightin expression. This interpretation is based on the assumption that the anti-flightin polyclonal antibody recognizes epitopes uniformly along the length of the polypeptide chain. However, it is possible the 25% intensity reduction is due to a decrease in expression of the truncated flightin. Supporting evidence that the truncated flightin expression is not reduced comes from comparing flnΔC44 fibers to fibers from a flightin deficiency heterozygote mutant [Df(3L)fln1], which showed a 20% decrease in flightin expression due to haploidy (31). Indices of muscle and flight performance were considerably different between these two lines, with flnΔC44 fibers showing reduced peak power output and decreased frequency of maximal power output while Df(3L)fln1 fibers showed no change in peak power output and increased frequency of maximal power output. The slowed cross-bridge kinetics for flnΔC44 are opposite the accelerated kinetics for Df(3L)fln1, resulting in the flnΔC44 line being incapable of beating their wings while Df(3L)fln1 are flight capable. Importantly, both lines show a similar increase in sarcomere disorder compared with controls, which indicates that altered myofilament structure is not causing the differences between the flnΔC44 and Df(3L)fln1 lines. If the COOH-terminal flightin truncation phenotype resulted from reduced expression rather than the truncation per se, the flnΔC44 phenotype should have resembled that of Df(3L)fln1, but that is not the case. Together with the quantitative Western blots, this comparison indicates that the COOH-terminal truncation does not alter flightin expression compared with controls.
We observe 8 of the 11 expected isoelectric variants in flnΔC44 compared with fln+ (Fig. 3C), 9 of which have been attributed to phosphorylation (32). A reduction in the number of phosphorylated spots was expected given prior studies suggesting the presence of five putative phosphorylation sites on the COOH-terminal region of flightin (3). More importantly, the multiple spots observed in the 2DE gels from flnΔC44 fibers suggest that many other phosphorylation sites are retained in the truncated protein. Combining this result with the expected shifts in pI due to fewer amino acids (Fig. 3), we speculate that the truncation does not lead to a different phosphorylation profile for the remaining phosphorylation sites, in contrast with findings for COOH-terminally truncated myosin binding protein C (10). Similarities among the expected phosphorylation profiles for flnΔC44 and fln+ suggest that the truncated protein folds properly, at least to the extent it can be recognized by protein kinases, and is stable in the adult muscle.
An important consistency between flnΔC44 and fln+ fibers results from the similarities in their elastic and viscous moduli under relaxed conditions, indicating no differences in the mechanical properties of passive structural elements constituting their fibers (Fig. 5). The agreement between rigor moduli (Fig. 5), which measures passive structural elements as well as strongly bound cross bridges (4, 22), demonstrates no differences in cross-bridge-dependent stiffness (calculated as the difference between rigor and passive moduli). The likeness of rigor measurements also indicates that myosin cross bridges are equivalently capable of binding actin in COOH-terminal truncated flightin fibers as in control fibers. Active moduli data also demonstrate similarities in passive mechanical elements of the myofilament lattice between the two lines, evident though comparable values for A and k (Fig. 7, A and B). These results show that the COOH terminus of flightin is not required for coordinating thick filament assembly, stabilizing mechanical properties of thick filaments, or forming strongly bound cross bridges (given enough time for rigor bridges to form).
Structural results from electron microscopy (Fig. 4) and X-ray diffraction measurements (Table 1) show that the absence of flightin's COOH terminus leads to disorder at the sarcomere, filament, and cross-bridge level. This flightin truncation led to inconsistent appearances of M-lines combined with abnormal Z-bands, as well as longitudinal or radial breaks in sarcomeric structure (Fig. 4, D–F). These changes were accompanied by a slightly compacted myofilament lattice and less homogenous thick filament spacing (Table 1). The greater I2,0/I1,0 in the flnΔC44 flies shows that myosin heads are positioned further away from the thick filament backbone, although the reduced mechanical performance suggests that fewer heads are well oriented for binding actin.
flnΔC44 fibers generated reduced work and power output at lower frequencies of maximal work and power, indicating slower cross-bridge cycling kinetics compared with fln+ (Fig. 6, C and D). Slower cross-bridge kinetics in flnΔC44 fibers likely result from a reduced rate of cross-bridge recruitment, evident by the reduced value for b (17, 35) (Fig. 7D). A slower recruitment rate for flnΔC44 fibers without any change in [ton = (2πc)−1, Fig. 7D] would lead to fewer strongly bound, high-force bearing cross bridges compared with fln+. The observed decreases in model parameters B and C (Fig. 7C) agree with diminished cross-bridge binding for flnΔC44 compared with fln+. Despite the reduced cross-bridge binding in flnΔC44 fibers, active tension is similar for flnΔC44 and fln+ fibers (Table 2), which is not surprising given that observed isometric tension changes in the IFM (21, 28) generally require larger changes in cross-bridge cycling kinetics (>50%) than observed here (∼20%). Altogether, the decrease in cross-bridge recruitment reduces the number of strongly bound cross bridges, resulting in diminished maximal work and power output in the IFM, leaving flnΔC44 flies unable to beat their wings.
As discussed above, measured viscoelastic moduli (Figs. 5 and 6) indicate no differences in thick filament or cross-bridge stiffness between flnΔC44 and fln+. However, previous fln0 measurements demonstrated that reduced filament stiffness can slow the rate of force development (8, 13), consistent with computational modeling predictions (5, 19). Specifically, previous studies showed that flightin is responsible for 30–45% of the thick filament's flexural rigidity (8), which correlated with a ∼35% reduction in the frequency of maximal work reported for the more compliant fln0 fibers (13). Because thick filaments are estimated to be 17 times stiffer than connecting filaments (12), passive measurements largely describe properties of the more easily stretched connecting filaments (34). Because the passive properties are similar between flnΔC44 and fln+, variations in thick filament stiffness would be most evident from the rigor measurements, but no rigor differences were found (Fig. 5). While subtle changes in thick filament stiffness may not be discernible in the rigor data, there is no evidence indicating that the reduced cross-bridge cycling kinetics in flnΔC44 fibers stem from large increases in thick filament compliance.
We hypothesize that increased lattice disorder leads to diminished contractile function in the flnΔC44 fibers. This increased lattice disorder compromises the ability of myosins to readily find their actin binding sites, reducing the rate of cross-bridge recruitment and suppressing the number of bound cross bridges, although cross-bridge stiffness and mean cross-bridge attachment duration remain unchanged once binding occurs. These measurements suggest that removing the COOH terminus of flightin does not significantly affect flightin's integration into the sarcomere or mechanical properties of thick filaments, indicating that the COOH terminus of flightin is not solely responsible for the large changes in filament length and flexural rigidity found in flightin-null flies (8). In summary, the COOH terminus of flightin promotes normal myofilament lattice order and sarcomeric structure, enabling the exceptionally fast cross-bridge kinetics that power flight (27).
GRANTS
B. C. W. Tanner was supported by a National Institutes of Health (NIH) postdoctoral fellowship (T32 HL007647) and a National Science Foundation (NSF) Postdoctoral Fellowship in Biology (DBI-0905830). This work was supported by NSF IOS-0718417 to J. O. Vigoreaux and NIH K01 Mentored Research Grant to M. S. Miller (AG-031303). Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Basic Energy Sciences, Office of Science, under contract no. W-31-109-ENG-38. Biophysics Collaborative Access Team (BioCAT) is an NIH-supported Research Center (RR-08630). The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Center for Research Resources or the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Ayer G, Vigoreaux JO. Flightin is a myosin rod binding protein. Cell Biochem Biophys 38: 1483–1499, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Barton B, Ayer G, Heymann N, Maughan DW, Lehmann FO, Vigoreaux JO. Flight muscle properties and aerodynamic performance of Drosophila expressing a flightin transgene. J Exp Biol 208: 549–560, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Barton B, Ayer G, Maughan DW, Vigoreaux JO. Site directed mutagenesis of Drosophila flightin disrupts phosphorylation and impairs flight muscle structure and mechanics. J Muscle Res Cell Motil 28: 219–230, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Bullard B, Burkart C, Labeit S, Leonard K. The function of elastic proteins in the oscillatory contraction of insect flight muscle. J Muscle Res Cell Motil 26: 479–485, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Campbell K. Filament compliance effects can explain tension overshoots during force development. Biophys J 91: 4102–4109, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Campbell K, Chandra M, Kirkpatrick R, Slinker B, Hunter W. Interpreting cardiac muscle force-length dynamics using a novel functional model. Am J Physiol Heart Circ Physiol 286: H1535–H1545, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Colson BA, Locher MR, Bekyarova T, Patel JR, Fitzsimons DP, Irving TC, Moss RL. Differential roles of regulatory light chain and myosin binding protein-C phosphorylations in the modulation of cardiac force development. J Physiol 588: 981–993, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Contompasis JL, Nyland LR, Maughan DW, Vigoreaux JO. Flightin is necessary for length determination, structural integrity, and large bending stiffness of insect flight muscle thick filaments. J Mol Biol 395: 340–348, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Farman G, Miller MS, Reedy M, Soto-Adames F, Vigoreaux J, Maughan D, Irving T. Phosphorylation and the N-terminal extension of the regulatory light chain help orient and align the myosin heads in Drosophila flight muscle. J Struct Biol 168: 240–249, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ge Y, Rybakova IN, Xu Q, Moss RL. Top-down high-resolution mass spectrometry of cardiac myosin binding protein C revealed that truncation alters protein phosphorylation state. Proc Natl Acad Sci USA 106: 12658–12663, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Godt RE, Lindley BD. Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog. J Gen Physiol 80: 279–297, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hao Y, Miller MS, Swank DM, Liu H, Bernstein SI, Maughan D, Pollack GH. Passive stiffness in Drosophila indirect flight muscle reduced by disrupting paramyosin phosphorylation, but not by embryonic myosin S2 hinge substitution. Biophys J 91: 4500–4506, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Henkin JA, Maughan DW, Vigoreaux JO. Mutations that affect flightin expression in Drosophila alter the viscoelastic properties of flight muscle fibers. Am J Physiol Cell Physiol 286: C65–C72, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Irving TC. X-ray diffraction of indirect flight muscle from Drosophila in vivo. In: Nature's Versatile Engine: Insect Flight Muscle Inside and Out, edited by Vigoreaux JO. Georgetown, TX: Landes Bioscience, 2006 [Google Scholar]
- 15. Irving TC, Maughan DW. In vivo x-ray diffraction of indirect flight muscle from Drosophila melanogaster. Biophys J 78: 2511–2515, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kawai M, Brandt P. Sinusoidal analysis: a high resolution method for correlating biochemical reactions with physiological processes in activated skeletal muscles of rabbit, frog and crayfish. J Muscle Res Cell Motil 1: 279–303, 1980 [DOI] [PubMed] [Google Scholar]
- 17. Kawai M, Halvorson H. Two step mechanism of phosphate release and the mechanisms of force generation in chemically skinned fibers of rabbit psoas muscle. Biophys J 59: 329–342, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kronert WA, O'Donnel PT, Fieck A, Lawn A, Vigoreaux JO, Sparrow JC, Bernstein SI. Defects in the Drosophila myosin rod permit sarcomere assembly but cause flight muscle degeneration. J Mol Biol 249: 111–125, 1995 [DOI] [PubMed] [Google Scholar]
- 19. Luo Y, Cooke R, Pate E. A model of stress relaxation in cross-bridge systems: effect of a series elastic element. Am J Physiol Cell Physiol 265: C279–C288, 1993 [DOI] [PubMed] [Google Scholar]
- 20. Maughan DW, Vigoreaux JO. An integrated view of insect flight muscle: genes, motor molecules, and motion. News Physiol Sci 14: 87–92, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Miller MS, Dambacher CM, Knowles AF, Braddock JM, Farman GP, Irving TC, Swank DM, Bernstein SI, Maughan DW. Alternative S2 hinge regions of the myosin rod affect myofibrillar structure and myosin kinetics. Biophys J 96: 4132–4143, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mulieri LA, Barnes WD, Leavett BJ, Ittleman F, LeWinter MM, Alpert NR, Maughan DW. Alterations of myocardial dynamic stiffness implicating abnormal crossbridge function in human mitral regurgitation heart failure. Circ Res 90: 66–72, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Nongthomba U, Cummins M, Clark S, Vigoreaux JO, Sparrow JC. Suppression of muscle hypercontraction by mutations in the myosin heavy chain gene of Drosophila melanogaster. Genetics 164: 209–222, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palmer B, Suzuki T, Wang Y, Barnes W, Miller M, Maughan D. Two-state model of acto-myosin attachment-detachment predicts C-process of sinusoidal analysis. Biophys J 93: 760–769, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qiu F, Brendel S, Cunha PM, Astola N, Song B, Furlong EE, Leonard KR, Bullard B. Myofilin, a protein in the thick filaments of insect muscle. J Cell Sci 118: 1527–1536, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Reedy MC, Bullard B, Vigoreaux JO. Flightin is essential for thick filament assembly and sarcomere stability in Drosophila flight muscle. J Cell Biol 151: 1483–1499, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Swank D, Vishnudas V, Maughan D. An exceptionally fast actomyosin reaction powers insect flight muscle. Proc Natl Acad Sci USA 103: 17543–17547, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Swank DM, Knowles AF, Suggs JA, Sarsoza F, Lee A, Maughan DW, Bernstein SI. The myosin converter domain modulates muscle performance. Nat Cell Biol 4: 312–316, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Vigoreaux JO. Alterations in flightin phosphorylation in Drosophila flight muscles are associated with myofibrillar defects engendered by actin and myosin heavy-chain mutant alleles. Biochem Genet 32: 301–314, 1994 [DOI] [PubMed] [Google Scholar]
- 30. Vigoreaux JO. Genetics of the Drosophila flight muscle myofibril: a window into the biology of complex systems. Bioessays 23: 1047–1063, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Vigoreaux JO, Hernandez C, Moore J, Ayer G, Maughan DW. A genetic deficiency that spans the flightin gene of Drosophila melanogaster affects the ultrastructure and function of the flight muscles. J Exp Biol 201: 2033–2044, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Vigoreaux JO, Perry L. Multiple isoelectric variants of flightin in Drosophila stretch-activated muscles are generated by temporally regulated phosphorylations. J Muscle Res Cell Motil 15: 607–616, 1994 [DOI] [PubMed] [Google Scholar]
- 33. Vigoreaux JO, Saide JD, Valgeirsdottir K, Pardue ML. Flightin, a novel myofibrillar protein of Drosophila stretch-activated muscles. J Cell Biol 121: 587–191, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. White DC. The elasticity of relaxed insect fibrillar flight muscle. J Physiol 343: 31–57, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao Y, Kawai M. The effect of the lattice spacing change on cross-bridge kinetics in chemically skinned rabbit psoas muscle fibers. II. Elementary steps affected by the spacing change. Biophys J 64: 197–210, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]