Abstract
Regulator of G protein signaling (RGS) proteins, and notably members of the RGS-R4 subfamily, control vasocontractility by accelerating the inactivation of Gα-dependent signaling. RGS5 is the most highly and differently expressed RGS-R4 subfamily member in arterial smooth muscle. Expression of RGS5 first appears in pericytes during development of the afferent vascular tree, suggesting that RGS5 is a good candidate for a regulator of arterial contractility and, perhaps, for determining the mass of the smooth muscle coats required to regulate blood flow in the branches of the arterial tree. Consistent with this hypothesis, using cultured vascular smooth muscle cells (VSMCs), we demonstrate RGS5 overexpression inhibits G protein-coupled receptor (GPCR)-mediated hypertrophic responses. The next objective was to determine which physiological agonists directly control RGS5 expression in VSMCs. GPCR agonists failed to directly regulate RGS5 mRNA expression; however, platelet-derived growth factor (PDGF) acutely represses expression. Downregulation of RGS5 results in the induction of migration and the activation of the GPCR-mediated signaling pathways. This stimulation leads to the activation of mitogen-activated protein kinases directly downstream of receptor stimulation, and ultimately VSMC hypertrophy. These results demonstrate that RGS5 expression is a critical mediator of both VSMC contraction and potentially, arterial remodeling.
Keywords: regulator of G protein signaling, platelet-derived growth factor, G protein-coupled receptor, vasoactive agonists, cardiovascular signaling
angiotensin, endothelin, thrombin, acetylcholine, and catcholamines are major regulators of both smooth muscle contraction and arterial wall mass. All of these agonists transmit their signals through G protein-coupled receptors (GPCRs), a family of genes that comprise ∼1% of the mammalian genome (11). GPCR-mediated signaling has many implications for vascular disease. The characterization of the receptor-agonist interaction should be and has been an important therapeutic target for both systemic and pulmonary hypertension. An equally important target may be the complex of regulatory molecules that determine the extent and duration of GPCR signaling within the vascular smooth muscle cell (VSMC). Expression of different regulators may determine very different functions for the same GPCR in different cells. One such group of regulatory protein, the regulator of G protein signaling (RGS) proteins, has been implicated in controlling the function of vasoactive GPCRs.
Members of the RGS protein family determine the signaling pathways downstream of activation of G proteins (5, 20, 32, 37, 58, 82, 83). Modulation of GPCR signaling by RGS proteins depends on the function of RGS proteins as activators of GTPase activity for GTP-bound Gα large G proteins (9, 25). Because of this activity, members of the RGS-R4 subfamily appear to be critical to cardiovascular function and pathology (5, 62). For example, cardiac-directed overexpression of RGS5 and RGS4 results in the failure to efficiently remodel in response to pressure overload (41, 59, 60). RGS2 has been linked to blood pressure regulation, presumably via modulation of vasocontractility (31, 38, 56, 66, 70). We have demonstrated RGS5 is preferentially expressed in aortic SMCs, relative to venous SMCs (1, 2). Developmental studies and studies of tumor angiogenesis suggest RGS5 may be critical to vascular stability in newly formed vascular beds (7, 34, 51). Finally, we (55) and others (18, 22, 30) determined that RGS5 is also linked to blood pressure regulation.
The present study is aimed at determining which physiological agonists control RGS5 expression in vascular SMCs. Very little is known about regulation of expression in this gene family other than the observation that ANG II upregulates RGS2 expression in vitro, both at the transcript and protein level (43, 61), whereas sphingosine-1-phosphate (S1P) upregulates RGS2 and RGS16 expression (36). In contrast, we found that RGS5 expression is not directly regulated by any of the seven candidate GPCR agonists assayed following either 2, 6, or 24 h of stimulation. We previously demonstrated RGS5 expression is downregulated in response to aortic constriction (79). Perhaps explaining this in vivo response, RGS5 expression is downregulated in response to platelet-derived growth factor (PDGF) treatment. This results in increased migration and hypertrophic signaling in VSMCs. These results suggest that the role of PDGF in the vascular response to injury may be mediated by cross-talk between the growth factor and GPCR signaling pathways.
MATERIALS AND METHODS
Cell Culture
All cells lines were derived from the rat aorta. The RGS5− VSMC line is described in Wang et al. (79) and was cultured in DMEM (GIBCO) and supplemented with 10% FBS (Hyclone) and penicillin-streptomycin. The RGS5+ VSMC line was kindly provided by Dr. Gary Owens [University of Virginia (68)] and was cultured in DMEM/F12 (GIBCO), supplemented with 10% FBS (Hyclone) and penicillin-streptomycin. All cells are grown at 37°C and 5% CO2. Importantly, RGS5+ VSMCs express members of the RGS-R4 subfamily at endogenous levels (see Fig. 2).
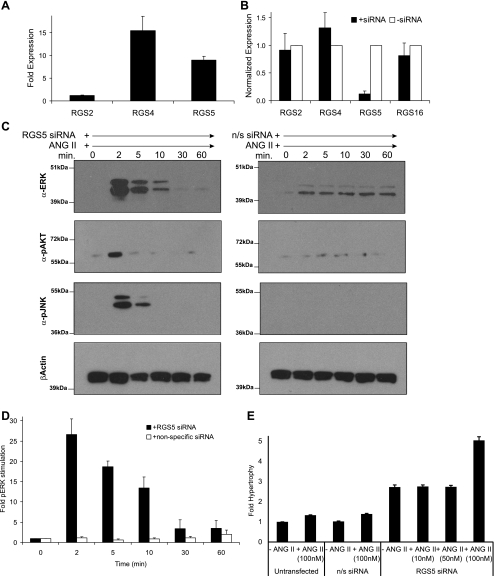
Fig. 2.
RGS5 expression regulates ANG II-stimulated signaling pathways. A: relative expression of RGS-R4 subfamily members was assessed in rat aortic VSMCs by quantitative Real-Time RT-PCR (qPCR). In multiple rat aortic VSMC isolates, the expression of RGS2, RGS4, and RGS5 was determined and normalized to the relative expression of RGS2 (2−ΔΔCt). Error bars are means ± SE; n = 4. B: specificity of the RGS5 small interfering RNA (siRNA) was assessed by qPCR. As shown, the siRNA specifically downregulates RGS5, independently from other RGS-R4 subfamily members (error bars are means ± SE; n = 3). C: knockdown of RGS5 expression stimulates ANG II-stimulated phosphorylation cascades. VSMCs were stimulated with ANG II (100 nM) in the presence of either RGS5 siRNA (left) or nonspecific siRNA (right). Whole cell extracts were prepared and immunoblotted. The phosphorylation of ERK, AKT, and JNK is stimulated when RGS5 is specifically knocked down. β-Actin is shown as a control for equivalent protein loading. D: quantitation of immunoblots (error bars are means ± SE; n = 3) demonstrating RGS5 knockdown stimulates ANG II-mediated phosphorylation of ERK. E: RGS5 knockdown induces ANG II-stimulated hypertrophy. VSMCs were transfected with either RGS5 siRNA or nonspecific siRNA, stimulated with ANG II (0, 10, 50, and 100 nM) for 24 h, pulsed with 1 μCi [3H]leucine for 5 h, and the amount of [3H]leucine incorporated into new protein was quantitated to assess hypertrophic growth. Error bars are means ± SE; n = 3.
Viral Overexpression of RGS5
The construction and production of RGS5 retrovirus are described in Wang et al. (79).
siRNA Knockdown of RGS5 Expression
RGS5 was knocked down in RGS5+ VSMCs using a specific small interfering RNA (siRNA) from Invitrogen (5′-AAUUCUCACAGGCAACCCAGAACUC-3′). VSMCs were transfected by electroporation with the human AoSMC nuclefector kit (Amaxa Biosystems) following manufacturers specifications. Briefly, 6 × 106 cells were transfected with either RGS5-specific siRNA (40 nM) or nonspecific siRNA (40 nM; Invitrogen) and plated at a final density of 1 × 106 cells/100 mm dish (for protein isolation) or 1 × 105 cells/6-well dish (for RNA isolation) and grown in complete growth media. After 24 h, the cells were changed to serum-free media and starved for 24 h. Cells were subsequently treated with the following agonists for 24 h: ANG II (100 nM; Sigma), endothelin-1 (ET-1; 100 nM; Sigma), phenylepherine (PE; 10 μM; Sigma), isoproterenol (Iso; 10 μM; Sigma), serotonin (5-HT; 5 μM; Sigma), norepinephrine (NE; 10 μM; Sigma), sphingosine-1-phosphate (S1P, 1 μM; Cayman), PDGF (10 ng/ml; R&D systems), vascular endothelial growth factor (VEGF; 10 ng/ml; R&D systems), and epidermal growth factor (EGF; 10 ng/ml; R&D systems).
Quantitative Real-Time RT-PCR
RNA was isolated from VSMCs with or without agonist treatment using the RNAEasy RNA isolation mini kit (Qiagen). After determination of RNA quantity and quality, cDNA was prepared by reverse transcription (Reverse Transcription cDNA Synthesis kit; Applied Biosystems), using random hexamer primers. Real-time PCR was performed by mixing 20 ng cDNA with 20× primer-probe mixes and 2× Taqman PCR Master Mix (Applied Biosystems), and the quantity of each product was determined on the 7900HT Real Time PCR machine (Applied Biosystems). Thermal cycling for PCR was as follows: 2 min at 50°C, 10 min at 95°C, followed by 40 cycles of 15 s at 95°C for denaturation and 1 min at 60°C for annealing and extension. The amount of each target molecule mRNA was calculated using a comparative CT method (2^-ΔΔCT; Applied Biosystems, Relative Quantitation Of Gene Expression, ABI PRISM 7700 Sequence Detection System: User Bulletin no. 2), after normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (48). The rat genes assayed were the following: RGS2 (Rn00584932_m1; Applied Biosystems), RGS4 (Rn00568067_m1; Applied Biosystems), RGS5 (Rn00571047_m1; Applied Biosystems), and GAPDH (Rn99999916_s1; Applied Biosystems).
Immunoblot
Protein cell lysates were prepared from VSMCs following 0, 2, 5, 10, 30, and 60 min of agonist treatment. Briefly, cells were washed and scraped into 1× PBS. Whole cell extracts were prepared by resuspending the cell pellet in lysis buffer [50 mM Tris·HCl (pH 8.0), 120 mM NaCl, 0.5% Igepal, 1 mM EDTA, and protease inhibitor cocktail (Calbiochem)]. After protein quantitation, 16 μg of each protein extract was separated on 10% bis-Tris gels. Proteins were transferred to PVDF and blocked with 5% nonfat dry milk (NFDM) in TBS-T (0.1% Tween). Membranes were incubated with the following primary antibodies overnight at 4°C, diluted in 5% NFDM in TBS-T: 1:1,000 phospho-p42/44 (Thr202/Tyr204; pERK) (Cell Signaling); 1:5,000 total p42/44 (ERK) [kindly provided by Dr. Jean Campbell (65)]; 1:1,000 phospho-Akt (Ser437) (Cell Signaling), 1:1,000 phospho-SAPK/JNK (Thr183/Tyr185) (Cell Signaling), 1:1,000 phospho-JNK2/3 (Cell Signaling); and 1:20,000 βActin (Abcam). After 4× washes with TBS-T, membranes were incubated with the following secondary antibodies at room temperature for 1 h, diluted in 5% NFDM in TBS-T: 1:8,000 goat α-rabbit IgG HRP conjugate (Bio-Rad); 1:8,000 goat α-mouse IgG HRP (Bio-Rad). After 4× washes with TBS-T, blots were incubated in ECL reagent (Super Signal West Pico, Pierce) and exposed to autoradiographic film.
Hypertrophy Assay
After agonist stimulation for 24 h, cells were incubated with 1 μCi [3H]leucine for 5 h. Cells were washed 2× with cold PBS, followed by washing 2× with 10% TCA. To precipitate proteins, the cells were incubated with 1 ml 0.5 N NaOH for 15 min at room temperature. Cell lysates were transferred to scintillation vials and mixed with scintillation fluid, and the radioactivity was quantified with a scintillation counter.
Migration Assay
Transwell plates (Costar) were pretreated with 0.1% collagen (type 1) (Sigma) overnight at 4°C. RGS5+ VSMCs were trypsinized, counted, and resuspended in migration media [DMEM/F12 supplemented with 0.2% BSA (Sigma)] at a final concentration of 1 × 106 cells/ml. After removal of collagen and being washed with serum-free media, 1 × 105 cells were plated in the upper chamber of each well. Migration media containing PDGF (0, 0.3, 1, or 3 ng/ml) or VEGF (0 or 1 ng/ml) was added to the lower chamber after incubation of the cells for 30 min at 37°C. After incubation for 7 h, the cells are fixed (MeOH for 15 min, 70% EtOH overnight at 4°C). The migrated cells are stained with hematoxylin and Scotts blue. The membrane was excised and mounted on glass slides, and the number of cells/40× field was counted.
RESULTS
Overexpression of RGS5 Inhibits the Hypertrophic Response in Rat Aortic VSMCs
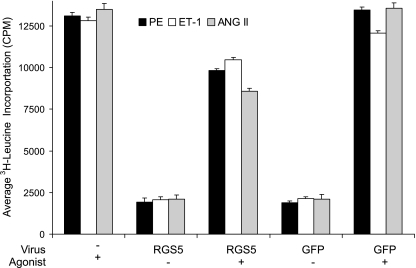
Multiple studies have implicated members of the RGS-R4 subfamily in the control of cardiovascular function [reviewed in Wieland and Mittman (83), Bansal et al. (5), Riddle et al. (58), and Gu et al. (32)]. RGS-R4 subfamily proteins interact specifically with the Gαq and Gαi large G proteins (9, 25, 69). Therefore, it is expected that RGS-R4 subfamily members will regulate signals of common vasoactive agonists, which signal through Gαq- and Gαi-dependent GPCRs. Among others, RGS2 has been shown to interact with the α1A-adrenergic receptor (33, 80), whereas RGS4 interacts with the acetylcholine receptor (63) and the opioid receptor (29). Here, we demonstrate that viral overexpression of RGS5 results in a downregulation of hypertrophic responses to GPCR agonists (Fig. 1). Using [3H]leucine incorporation as a measure of hypertrophic protein production, we demonstrate PE, ET-1, and ANG II-induced hypertrophy is inhibited by approximately 25% on average (25%, 18%, and 36%, respectively). Therefore, in VSMCs, RGS5 interacts with Gαi and Gαq and effectively inhibits GPCR-mediated hypertrophy.
Fig. 1.
Regulator of G protein signaling 5 (RGS5) overexpression inhibits hypertrophy in vascular smooth muscle cells (VSMCs). Rat aortic VSMCs were infected with retrovirus overexpressing either RGS5 or green fluorescent protein (GFP) (control). After stimulation with the G protein-coupled receptor (GPCR) agonists [10 μM phenlyepinephrine (PE), 100 nM endothelin-1 (ET-1), 100 nM ANG II] for 24 h and pulsed with 1 μCi [3H]leucine for 5 h, the amount of [3H]leucine incorporated into new protein was quantitated to assess hypertrophic growth. Each GPCR agonist assayed stimulated hypertrophic growth of VSMCs. When RGS5 was overexpressed, but not when GFP was overexpressed, hypertrophic growth was inhibited. Error bars are means ± SE; n = 3.
Characterization of an In Vitro Model System: Expression of RGS5 in a Cultured VSMC Line
Rat aortic VSMCs express RGS-R4 subfamily members.
Because of the concern with the physiological relevance of overexpression of a regulator of signal transduction, we explored the physiological role of RGS-R4 proteins in an in vitro rat aortic cell line that expresses RGS5 and other members of the RGS-R4 subfamily, at endogenous levels. Although RGS5 is the most abundant arterial RGS-R4 subfamily member (see below), VSMC cell lines derived from rodents rapidly downregulate RGS5 expression when placed in culture. After screening a number of available cell lines, we obtained and characterized a rat aortic VSMC cell line that maintains expression of the RGS-R4 subfamily members.
Relative quantitative real-time RT-PCR analysis demonstrates RGS5 and RGS4 are approximately equally expressed, whereas RGS2 is expressed at lower levels (Fig. 2A). This closely relates to additional analyses from our laboratory demonstrating the differential expression pattern for individual members of the RGS-R4 subfamily in the rat and the mouse aorta in vivo: RGS5 > RGS4 > RGS2 > RGS16 (data not shown). While an absence of sensitive antibodies makes protein quantification of the RGS-R4 subfamily members difficult, we propose the modulation of RGS mRNA expression and the subsequent measurement of GPCR signaling activity is the best approach to study the functional effect of RGS-R4 expression changes.
Knockdown of RGS5 by siRNA is specific to RGS5 relative to other closely related RGS-R4 subfamily members.
RGS proteins have highly conserved structures and functions throughout evolution. This is of particular importance when comparing members of the RGS-R4 subfamily, since this family is structurally described as having short NH2- and COOH-terminal domains and the catalytic RGS domain, which comprises approximately two-thirds of the protein (25, 69). Therefore, when specifically targeting one member of the family with a siRNA, it is necessary to determine whether the expression of additional members is accidently modified. As demonstrated in Fig. 2B, relative to RGS2, RGS4, and RGS16, we have specifically targeted RGS5 by siRNA knockdown. Furthermore, we analyzed RGS5 expression 24 h following knockdown with two additional siRNAs (see supplemental Figs. S1 and S2B online at the AJP-Cell Physiol website) and confirmed that multiple independent siRNAs have equivalent effects upon RGS5 expression. Therefore, in subsequent studies, we are confident we have knocked down RGS5, and we can attribute the functional response in these cells to RGS5 expression (or loss thereof).
RGS5 Expression Regulates the Functional Response in VSMCs
Knockdown of RGS5 activates endogenous GPCR-mediate signaling in VSMCs.
RGS proteins, as GTPase activating proteins (GAPs) (24, 27, 62, 87), function to inhibit GPCR signaling through GαGTP-dependent pathways. Since overexpression of RGS5 affected the ANG II-dependent hypertrophic response, we focused on the effects of RGS5 knockdown on this signaling pathway. ANG II has been shown to activate multiple signaling pathways (74, 84). Importantly, phosphorylation of mitogen-activated protein kinases (MAPKs) has been implicated in the contractile, mitogenic, and trophic responses of arterial VSMCs (16, 42, 44, 47, 67, 86). Therefore, to determine whether RGS5 expression regulates ANG II-dependent signaling, the effect of specific knock down of RGS5 upon MAPK stimulation was analyzed in vitro.
Figure 2C demonstrates that targeted knockdown of RGS5 results in the potentiation of ANG II-mediated activation of multiple downstream MAPKs. Twenty-four hours after siRNA treatment, VSMCs were stimulated with 100 nM ANG II. Relative to the VSMCs treated with nonspecific siRNA, phosphorylated ERK (p42/p44) was markedly activated in the VSMCs treated with RGS5 siRNA. A similar response was observed for the two additional RGS5-specific siRNAs assayed (see online supplemental Fig. S2). This response is quantified in Fig. 2D. This induction occurred within 2 min after ANG II treatment and diminished after 10 min of ANG II treatment. Phosphorylation of two additional kinases, Akt and JNK, was similarly activated within 2 min of stimulation, although the phosphorylation was not as sustained as observed for pERK. Similarly, RGS5-dependent responses were observed for additional GPCR agonists studied (ET-1, PE, Iso, S1P, NE, 5-HT; see supplemental Fig. S3, A–F). In summary, RGS5 expression clearly controls the GPCR-mediated rapid and transient increase in kinase phosphorylation in VSMCs.
RGS5 inhibition activates the hypertrophic response in VSMCs.
To confirm the activation of functional cascades downstream of ANG II stimulation, the analysis of hypertrophic incorporation of [3H]leucine was analyzed in the presence and absence of RGS5-specific siRNA. As expected, knockdown of RGS5 resulted in an approximately fivefold increase in hypertrophic protein production (Fig. 2E). Interestingly, knockdown of RGS5 expression does not sensitize VSMCs to the ANG II-mediated hypertrophic response. Specifically, an induction of hypertrophy is only observed when VSMCs are stimulated with 100 nM ANG II but not at lesser ANG II concentrations (either 10 or 50 nM). As above, similar RGS5-dependent hypertrophic responses were observed for additional GPCR agonists studied (ET-1, PE, Iso, S1P, NE, 5-HT; see Supplemental Fig. S3G). Taken together, these data indicate that in VSMCs, RGS5 expression controls both the transient signaling events (MAPK activation is largely complete 10 min after ANG II stimulation; Fig. 2, C and D) and the more long-term physiologicalal effects in response to GPCR stimulation (hypertrophic growth is assayed 29 h following ANG II stimulation; Fig. 2E), and specifically, the signaling pathways stimulated by ANG II treatment.
Agonist-Dependent Regulation of RGS5 Expression
GPCR agonists do not directly activate RGS5 expression in VSMCs.
Expression of some members of the RGS-R4 subfamily is directly controlled by GPCR agonist stimulation. For example, RGS2 expression is upregulated in response to ANG II treatment both in cultured VSMCs (43) and in the adrenocortical carcinoma cell line (61). To determine whether RGS5 expression is similarly induced in response to GPCR stimulation, VSMCs were treated with ANG II, ET-1, PE, Iso, 5-HT, NE, and S1P, and the expression of RGS5 was determined by quantitative real-time RT-PCR. In contrast to the described induction of RGS2 in response to ANG II stimulation, none of the GPCR agonists studied significantly affected RGS5 expression levels following 2, 6, or 24 h of stimulation (Fig. 3A). As demonstrated in Fig. 2, C and D, when RGS5 is knocked down, ANG II-mediated phosphorylation of ERK, Akt, and JNK occurs rapidly. Therefore, to investigate whether short-term GPCR stimulation had any affect upon RGS5 expression, we assayed a few candidate GPCR agonists (ANG II, PE, ET-1) for changes in RGS5 mRNA levels. As expected from the more extended stimulation assays, acute stimulation by these three candidate agonists had no significant effect upon RGS5 expression (Fig. 3B). Taken together, this indicates an alternative mechanism(s) is controlling expression of RGS5, and potentially additional candidate RGS-R4 subfamily members, in cultured VSMCs.
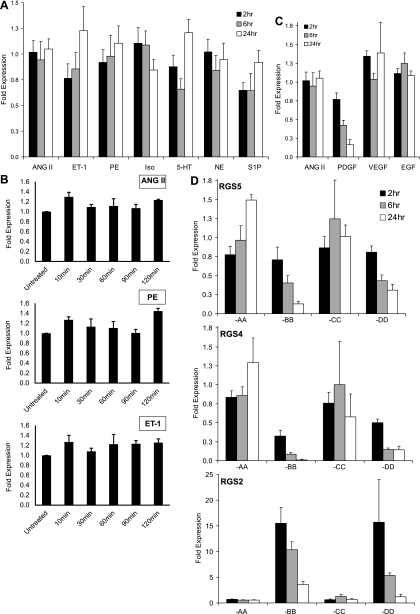
Fig. 3.
The effect of GPCR- and receptor tyrosine kinase (RTK)-stimulation on RGS-R4 subfamily expression. A: VSMCs were stimulated for 2, 6, and 24 h, with GPCR agonists: ANG II (100 nM), ET-1 (100 nM), PE (10 μM), isoproterenol (Iso, 10 μM), serotonin (5-HT, 5 μM), norepinephrine (NE, 10 μM), and sphingosine-1-phosphate (S1P, 1 μM). The expression of RGS5 was quantitated by qPCR and normalized to the relative expression of RGS5 in untreated cells. B: VSMCs were stimulated for 10, 30, 60, 90, and 120 min with candidate GPCR agonists: ANG II (100 nM), PE (10 mM), and ET-1 (100 nM). The expression of RGS5 was quantitated by qPCR and normalized to the relative expression of RGS5 in untreated cells. C: VSMCs were stimulated for 2, 6, and 24 h, with RTK agonists: platelet-derived growth factor (PDGF, 10 ng/ml), vascular endothelial growth factor (VEGF, 10 ng/ml), and epidermal growth factor (EGF, 10 ng/ml). The expression of RGS5 was quantitated by qPCR and normalized to the relative expression of RGS5 in untreated cells. D: VSMCs were stimulated for 2, 6, and 24 h with each PDGF isoform: PDGF-AA, -BB, -CC, -DD. The relative expression of RGS5 (top), RGS4 (middle), and RGS2 (bottom) was quantitated by qPCR and normalized to the relative expression of RGS5, RGS4, and RGS2 in untreated cells, respectively. Error bars are means ± SE; n = 4.
PDGF-BB stimulation directly represses RGS5 expression in VSMCs.
We are interested in the possibility that PDGF might regulate RGS5 expression because RGS5 and the PDGF-Rβ are expressed in pericytes, which function to stabilize neovasculature (7, 12, 13, 21, 53). The effect of PDGF upon a GPCR-mediated pathway is not unexpected because there is growing evidence of cross-talk between GPCRs and receptor tyrosine kinases (RTKs) (40, 54, 78, 81). Treatment of VSMCs with PDGF-BB resulted in the immediate and sustained downregulation of RGS5 expression (Fig. 3C). As shown, RGS5 expression is downregulated by 25%, 50%, and 80% following 2, 6, and 24 h of PDGF stimulation, respectively. Importantly, this effect is specific to PDGF, whereas additional RTK stimulants (VEGF and EGF) failed to alter RGS5 expression.
PDGF isoforms have different effects on RGS-R4 subfamily member expression.
Figure 3C clearly demonstrates that PDGF-BB treatment downregulates RGS5 expression. Unfortunately, PDGF-BB binds all conformations of the PDGF receptor in vitro: the PDGFRα homodimer, the PDGFβ homodimer, and the PDGFRα/β heterodimer (3, 71). Therefore, to determine which receptor is responsible for the downregulation of RGS5, VSMCs were treated with each PDGF isoform (PDGF-AA, -BB, -CC, and -DD). In addition to RGS5 expression, the expression of both RGS4 and RGS2 was also determined by quantitative real-time RT-PCR in response to each of the PDGF isoforms.
In VSMCs, the PDGF-AA and PDGF-CC isoforms did not significantly affect RGS2, RGS4, or RGS5 expression following stimulation for 2, 6, and 24 h (Fig. 3D). Conversely, the PDGF-BB and PDGF-DD isoforms regulated expression of these RGS-R4 subfamily members. RGS5 and RGS4 were downregulated by both PDGF-BB and PDGF-DD throughout the stimulation time course. Interestingly, RGS2 was acutely induced by PDGF-BB and PDGF-DD stimulation; however, expression decreases in a similar pattern to both RGS5 and RGS4 following 6 and 24 h of stimulation. As with the published activation of RGS2 by ANG II (43, 61), our results provide another example of different regulatory mechanisms controlling the expression of these different RGS-R4 subfamily members. Finally, since an equivalent effect on RGS5 and RGS4 expression is observed following PDGF-BB and PDGF-DD treatment, and PDGF-BB has been shown to only signal through the PDGFRβ in vivo (3, 49, 73), the PDGF-mediated effect in VSMCs is most likely through PDGFRβ.
RGS5 Expression Controls Physiological Responses of VSMCs
Inhibition of RGS5 expression enhances PDGF-mediated VSMC migration.
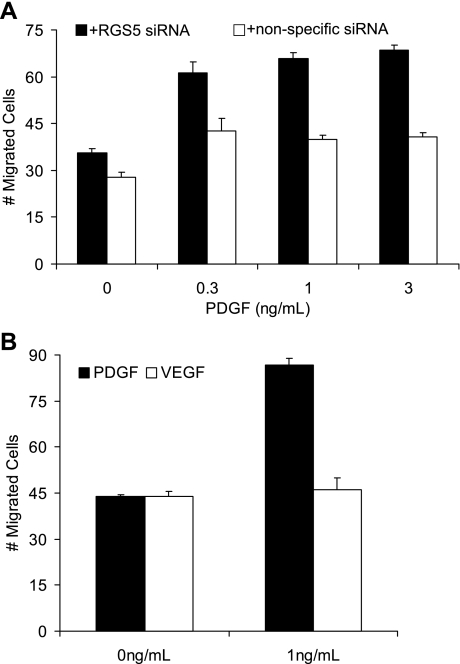
Classically, PDGF-BB functions to induce the migration and proliferation of VSMCs (15, 28, 57). To determine whether RGS5 expression regulates a cell's migratory capability, VSMCs were treated with RGS5-specific siRNA and exposed to increasing concentrations of PDGF-BB. As shown in Fig. 4A, PDGF-dependent migration is induced ∼1.5-fold upon knockdown of RGS5 expression. Interestingly, there is not a concentration dependence of this migratory phenotype, as an equal number of cells migrate at all concentrations of PDGF-BB assayed when RGS5 is knocked down. To confirm that this effect was dependent on PDGF, cells were treated with VEGF and the number of migrated cells was quantitated (Fig. 4B). As expected for this negative control treatment, VEGF failed to induce VSMC migration when RGS5 was knocked down.
Fig. 4.
Knockdown of RGS5 expression by siRNA induces PDGF-induced VSMC migration. A: a migration assay was performed in the presence of either RGS5 siRNA or nonspecific siRNA. At each concentration of PDGF-BB (0.3, 1, and 3 ng/ml), VSMCs are more migratory when RGS5 has been specifically knocked down. B: a migration assay was performed in the presence of PDGF-BB or VEGF in VSMCs, where RGS5 is specifically knocked down. Only PDGF-BB stimulates migration in VSMCs. Error bars are means ± SE; n = 3.
PDGF-dependent inhibition of RGS5 expression activates ANG II-dependent signaling in VSMCs.
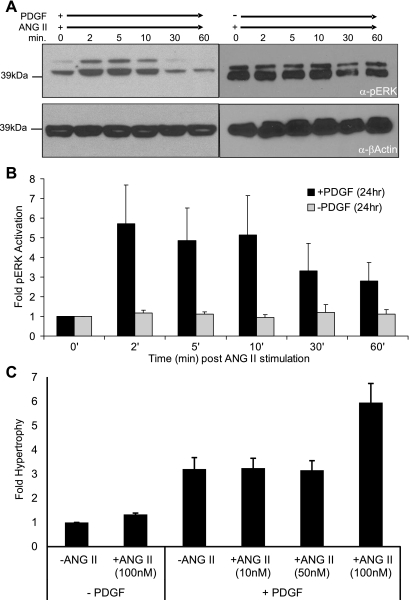
Beyond VSMC migration, we determined whether PDGF-BB treatment performed the same physiological role as knocking down RGS5 expression by siRNA in VSMCs. Specifically, we determined whether the signaling and hypertrophic events characterized in Fig. 2 could be recapitulated though the pharmacological knockdown of RGS5 expression by PDGF-BB. As shown in Fig. 5, treatment with PDGF-BB for 24 h, followed by a short time course of ANG II stimulation, resulted in an equivalent, yet transient, increase in phosphorylated MAPK (Fig. 5A and Supplemental Fig. S5). Conversely, when cells were not treated with PDGF-BB for 24 h, ANG II-dependent pERK stimulation is not observed in these RGS5+ VSMCs. The combined effect of PDGF-BB and ANG II on pERK stimulation is quantitated in Fig. 5B. This indicates the GPCR-mediated activation of pERK is dependent on first knocking down RGS5 expression by PDGF-BB treatment. Importantly, not only are the signaling pathways downstream of ANG II stimulated, but the hypertrophic response in VSMCs is also activated by the combination of PDGF-BB and ANG II treatment. Similar to the effect in VSMCs treated with ANG II and RGS5-specific siRNA, VSMCs treated with PDGF-BB for 24 h followed by ANG II treatment exhibited a sixfold increase in hypertrophic protein production (Fig. 5C). Also, as observed following specific knockdown of RGS5 by siRNA (Fig. 2E), PDGF-mediated knockdown of RGS5 does not sensitize VSMCs to the ANG II-mediated hypertrophic response. As predicted by the siRNA experiment, hypertrophic growth is only observed when VSMCs are stimulated with 100 nM ANG II but not at the lesser ANG II concentrations. To confirm RGS5 expression remained inhibited following PDGF-BB treatment, we assayed RGS5 mRNA expression at each time point of potential agonist addition during the hypertrophy assay. As shown in Supplemental Fig. S4, once RGS5 expression is inhibited, it remains repressed throughout the entire 53-h experimental time course. Combined, these data provide the first evidence of RTK activation directly regulating the expression of a mediator of Gα signaling, RGS5, leading to increased cell migration and enhanced hypertrophic signaling.
Fig. 5.
PDGF-BB stimulates RGS5 knockdown, induces ANG II-mediated ERK phosphorylation, and induces ANG II mediated hypertrophy. A: VSMCs were stimulated with ANG II (100 nM) for the indicated times, following 24 h treatment of PDGF-BB (10 ng/ml; left) or without PDGF-BB treatment (right). Whole cell extracts were prepared and immunoblotted for the expression of phosphorylated ERK. β-Actin is shown as a control for equivalent protein loading. Note: for comparison, the pERK blot is overexposed relative to the + nonspecific siRNA blot (Fig. 2E). B: quantitation of immunoblots (n = 4) demonstrating PDGF-BB treatment stimulates ANG II-mediated phosphorylation of ERK. C: PDGF-BB induces ANG II-stimulated hypertrophy. VSMCs were treated with or without PDGF-BB for 24 h, stimulated with ANG II (0, 10, 50, and 100 nM) for 24 h, and pulsed with 1 μCi [3H]leucine for 5 h, and the amount of [3H]leucine incorporated into new protein was quantitated to assess hypertrophic growth. Error bars are means ± SE; n = 3.
DISCUSSION
Beyond its role in controlling blood pressure (18, 22, 30, 55), RGS5 has been implicated in the control of vessel branching in normal development (7, 53), as well as in cancer (34, 51). The observations presented here suggest a mechanism by which the PDGF signaling cascade directly regulates GPCR-mediated signaling via RGS5 downregulation, thereby linking vascular remodeling to the intricate control of RGS protein expression and the downstream physiological responses (i.e., VSMC migration and hypertrophy).
As expected, we found that RGS5 overexpression inhibits GPCR-mediated hypertrophy in VSMCs (Fig. 1). This might lead one to expect a feedback regulation of RGS5 expression, as has been reported for ANG II-mediated induction of RGS2 in both VSMCs (43) and adrenocortical cells (61). However, we found that expression of RGS5 is neither stimulated nor repressed in response to stimulation with GPCR agonists (Fig. 3, A and B). In contrast, when VSMCs are treated with PDGF, RGS5 expression is inhibited (Fig. 3, C and D). When RGS5 is inhibited, either by siRNA (Fig. 2B) or pharmacologically (Fig. 3, C and D), VSMCs become more migratory (Fig. 4) and GPCR-mediated signaling is induced (Figs. 2 and 5). It is interesting to note that the hypertrophic response is stimulated when RGS5 is knocked down, even in the absence of exogenous GPCR agonists (Fig. 2E and Supplemental Fig. S3G). This implies that RGS5 functions to inhibit GPCR-mediated signaling by endogenous agonists present in the culture media. Therefore, by simply knocking out RGS5, signaling, and ultimately hypertrophic growth, is induced. However, when cells are stimulated by additional GPCR agonists, hypertrophic growth is further stimulated in the absence of RGS5. Therefore, RGS5 functions to maintain the “steady-state” of the SMC.
While RGS5 identifies the pericyte and its expression is downregulated in PDGF-BB and PDGFRβ knockout mice, RGS5-null animals do not have a deficiency in pericyte coverage (12, 13, 35, 55). Pericytes are SMC-like cells that function to stabilize newly formed capillaries during angiogenesis, including tumor angiogenesis and development (4, 76). The pericyte is believed to be derived by PDGF-mediated migration of adventitial mesenchymal cells along the axis of newly formed endothelial branches. The process is dependent on endothelial cell-derived PDGF-BB and PDGFRβ (6, 10, 35, 45). PDGF-BB and PDGFRβ knockout mice are characterized by “leaky” and unstable vessels because of the lack of pericytes. Our data suggest that at this stage of pericyte formation, RGS5 expression would be downregulated. RGS5 expression would be upregulated once neovessels are fully encoated by perictyes and are therefore functionally capable of distributing blood flow, as suggested by Mitchell et al. (53). One possible function of RGS5 is the stabilization or organization of the newly formed vessels, perhaps accounting for the observation that RGS5 controls blood flow through experimental tumors in RGS5 knockout mice (34) and the observation of RGS5 as a tumor progression marker (7, 17, 19, 51).
This hypothesis is strengthened by our observation that RGS5 also opposes PDGF function. This may open up a new and unsuspected variation on the idea of crosstalk between GPCRs and RTKs (14, 52, 54). Usually this has been thought of in terms of activation of the growth factor receptors following GPCR stimulation (23, 40, 75). Recently, Wang et al. (78) demonstrated signaling through the angiotensin type I receptor (AT1R) activates the PDGFRβ-dependent signaling cascade. However, all of these studies imply a one-way activation of RTK signaling cascades following GPCR stimulation. This mechanism has been referred to as the triple-membrane-passing-signaling model (77, 81). Our data showing PDGFRβ stimulation functions to downregulate RGS5 expression establishes a link between the RTK and GPCR signaling cascade, independent of the GPCR-to-RTK connection established previously.
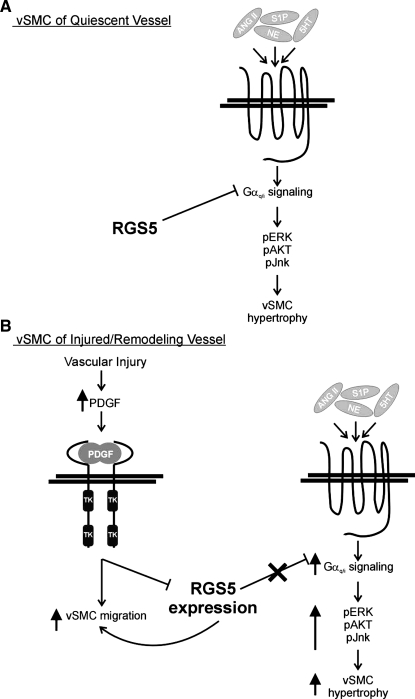
Taken together, our results have led us to propose the following model (Fig. 6). In the quiescent arterial wall, differentiated VSMCs express RGS5, resulting in the inhibition of the GPCR-mediated signaling cascade. Therefore, Gαq/i signaling is inhibited and cells do not migrate or undergo hypertrophy, even in the presence of circulating/local GPCR agonists (Fig. 6A). However, upon vascular injury, the local concentration of PDGF-BB increases, through release from platelets, activated endothelial cells, and invading macrophages (50, 64, 72). As a result, RGS5 expression is inhibited and VSMCs become more migratory and proliferative. In addition, the inhibition of RGS5 expression enables signaling through the relevant GPCRs. The specific inhibition of RGS5 results in the phosphorylation-mediated activation of MAPKs downstream of Gαq/i, and ultimately, VSMC hypertrophy/remodeling (Fig. 6B). Once the injury response is complete, the platelets and macrophage dissipate and the local concentration of PDGF-BB is diminished. As a result, RGS5 expression returns to endogenous levels, and GPCR-mediated signaling is consequently inhibited.
Fig. 6.
The role of RGS5 in regulating VSMC signaling. A: RGS5 normally represses GPCR signaling. B: in response to vascular injury, local concentrations of PDGF increase, thereby repressing RGS5 expression. Upon inhibition of RGS5 expression, VSMC migration and GPCR-stimulated signaling cascades are activated, leading to enhanced VSMC hypertrophy and vascular remodeling.
These data correlate with our previously described expression pattern for RGS5 in vivo, in the remodeling response to thoracic aortic banding in the rat (79). Upon vascular injury, the expression of PDGF is induced, along with the expression of Krupple-like factor 4 (KLF4), which results in a downregulation genes expressed in differentiated VSMCs, including smooth muscle myosin heavy chain and SM22α/transgelin (26, 46, 85). Acutely, injured arteries also go into spasm, perhaps due to the combined contractile effect of PDGF and the loss of expression of RGS5 (8). In addition to stimulating contraction, PDGF also induces VSMCs to dedifferentiate and become more proliferative and migratory (39), resulting in the classic vascular response to injury.
The data presented here provide a mechanism by which the combined effects of vasoactive GPCR stimulants and the circulating PDGF account for the classic vascular response to injury. Furthermore, our results establish a central role for RGS5, and potentially additional members of the RGS-R4 subfamily, in cardiovascular remodeling.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute Grants HL-007312 and HL-087513.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGEMENTS
We thank Dr. Mark Majesky (Seattle Children's Research Institute) for useful discussions and for critically reading this manuscript.
REFERENCES
- 1. Adams LD, Geary RL, Li J, Rossini A, Schwartz SM. Expression profiling identifies smooth muscle cell diversity within human intima and plaque fibrous cap: loss of RGS5 distinguishes the cap. Arterioscler Thromb Vasc Biol 26: 319–325, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Adams LD, Geary RL, McManus B, Schwartz SM. A comparison of aorta and vena cava medial message expression by cDNA array analysis identifies a set of 68 consistently differentially expressed genes, all in aortic media. Circ Res 87: 623–631, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev 22: 1276–1312, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. az-Flores L, Gutierrez R, Madrid JF, Varela H, Valladares F, Acosta E, Martin-Vasallo P, az-Flores L., Jr Pericytes Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol 24: 909–969, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Bansal G, Druey KM, Xie Z. R4 RGS proteins: regulation of G-protein signaling and beyond. Pharmacol Ther 116: 473–495, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development 125: 1591–1598, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Berger M, Bergers G, Arnold B, Hammerling GJ, Ganss R. Regulator of G-protein signaling-5 induction in pericytes coincides with active vessel remodeling during neovascularization. Blood 105: 1094–1101, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Berk BC, Alexander RW, Brock TA, Gimbrone MA, Jr, Webb RC. Vasoconstriction: a new activity for platelet-derived growth factor. Science 232: 87–90, 1986 [DOI] [PubMed] [Google Scholar]
- 9. Berman DM, Wilkie TM, Gilman AG. GAIP and RGS4 are GTPase-activating proteins for the Gi subfamily of G protein alpha subunits. Cell 86: 445–452, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Betsholtz C, Lindblom P, Gerhardt H. Role of pericytes in vascular morphogenesis. EXS: 115–125, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Bockaert J, Pin JP. Molecular tinkering of G protein-coupled receptors: an evolutionary success. EMBO J 18: 1723–1729, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bondjers C, He L, Takemoto M, Norlin J, Asker N, Hellstrom M, Lindahl P, Betsholtz C. Microarray analysis of blood microvessels from PDGF-B and PDGF-Rbeta mutant mice identifies novel markers for brain pericytes. FASEB J 20: 1703–1705, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Bondjers C, Kalen M, Hellstrom M, Scheidl SJ, Abramsson A, Renner O, Lindahl P, Cho H, Kehrl J, Betsholtz C. Transcription profiling of platelet-derived growth factor-B-deficient mouse embryos identifies RGS5 as a novel marker for pericytes and vascular smooth muscle cells. Am J Pathol 162: 721–729, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Booz GW, Baker KM. Molecular signalling mechanisms controlling growth and function of cardiac fibroblasts. Cardiovasc Res 30: 537–543, 1995 [PubMed] [Google Scholar]
- 15. Bornfeldt KE, Graves LM, Raines EW, Igarashi Y, Wayman G, Yamamura S, Yatomi Y, Sidhu JS, Krebs EG, Hakomori S. Sphingosine-1-phosphate inhibits PDGF-induced chemotaxis of human arterial smooth muscle cells: spatial and temporal modulation of PDGF chemotactic signal transduction. J Cell Biol 130: 193–206, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bornfeldt KE, Krebs EG. Crosstalk between protein kinase A and growth factor receptor signaling pathways in arterial smooth muscle. Cell Signal 11: 465–477, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Boss CN, Grunebach F, Brauer K, Hantschel M, Mirakaj V, Weinschenk T, Stevanovic S, Rammensee HG, Brossart P. Identification and characterization of T-cell epitopes deduced from RGS5, a novel broadly expressed tumor antigen. Clin Cancer Res 13: 3347–3355, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Chang YP, Liu X, Kim JD, Ikeda MA, Layton MR, Weder AB, Cooper RS, Kardia SL, Rao DC, Hunt SC, Luke A, Boerwinkle E, Chakravarti A. Multiple genes for essential-hypertension susceptibility on chromosome 1q. Am J Hum Genet 80: 253–264, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen X, Higgins J, Cheung ST, Li R, Mason V, Montgomery K, Fan ST, van de RM, So S. Novel endothelial cell markers in hepatocellular carcinoma. Mod Pathol 17: 1198–1210, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Cho H, Harrison K, Kehrl JH. Regulators of G protein signaling: potential drug targets for controlling cardiovascular and immune function. Curr Drug Targets Immune Endocr Metabol Disord 4: 107–118, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Cho H, Kozasa T, Bondjers C, Betsholtz C, Kehrl JH. Pericyte-specific expression of Rgs5: implications for PDGF and EDG receptor signaling during vascular maturation. FASEB J 17: 440–442, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Cho H, Park C, Hwang IY, Han SB, Schimel D, Despres D, Kehrl JH. Rgs5 targeting leads to chronic low blood pressure and a lean body habitus. Mol Cell Biol 28: 2590–2597, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Daub H, Weiss FU, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature 379: 557–560, 1996 [DOI] [PubMed] [Google Scholar]
- 24. De Vries L, Gist FM. RGS proteins: more than just GAPs for heterotrimeric G proteins. Trends Cell Biol 9: 138–144, 1999 [DOI] [PubMed] [Google Scholar]
- 25. De Vries L, Zheng B, Fischer T, Elenko E, Farquhar MG. The regulator of G protein signaling family. Annu Rev Pharmacol Toxicol 40: 235–271, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Deaton RA, Gan Q, Owens GK. Sp1-dependent activation of KLF4 is required for PDGF-BB-induced phenotypic modulation of smooth muscle. Am J Physiol Heart Circ Physiol 296: H1027–H1037, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Druey KM. Bridging with GAPs: receptor communication through RGS proteins. Sci STKE 2001: RE14, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Ferns GA, Sprugel KH, Seifert RA, Bowen-Pope DF, Kelly JD, Murray M, Raines EW, Ross R. Relative platelet-derived growth factor receptor subunit expression determines cell migration to different dimeric forms of PDGF. Growth Factors 3: 315–324, 1990 [DOI] [PubMed] [Google Scholar]
- 29. Georgoussi Z, Leontiadis L, Mazarakou G, Merkouris M, Hyde K, Hamm H. Selective interactions between G protein subunits and RGS4 with the C-terminal domains of the mu- and delta-opioid receptors regulate opioid receptor signaling. Cell Signal 18: 771–782, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Grayson TH, Ohms SJ, Brackenbury TD, Meaney KR, Peng K, Pittelkow YE, Wilson SR, Sandow SL, Hill CE. Vascular microarray profiling in two models of hypertension identifies Cav-1, Rgs2 and Rgs5 as antihypertensive targets. BMC Genomics 8: 404, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gross V, Tank J, Obst M, Plehm R, Blumer KJ, Diedrich A, Jordan J, Luft FC. Autonomic nervous system and blood pressure regulation in RGS2-deficient mice. Am J Physiol Regul Integr Comp Physiol 288: R1134–R1142, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Gu S, Cifelli C, Wang S, Heximer SP. RGS proteins: identifying new GAPs in the understanding of blood pressure regulation and cardiovascular function. Clin Sci (Lond) 116: 391–399, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Hague C, Bernstein LS, Ramineni S, Chen Z, Minneman KP, Hepler JR. Selective inhibition of alpha1A-adrenergic receptor signaling by RGS2 association with the receptor third intracellular loop. J Biol Chem 280: 27289–27295, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Hamzah J, Jugold M, Kiessling F, Rigby P, Manzur M, Marti HH, Rabie T, Kaden S, Grone HJ, Hammerling GJ, Arnold B, Ganss R. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature 453: 410–414, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126: 3047–3055, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Hendriks-Balk M, van Loenen P, Hajji N, Michel M, Peters S, Alewijnse A. S1P receptor signalling and RGS proteins: expression and function in vascular smooth muscle cells and transfected CHO cells. Eur J Pharmacol 600: 1–9, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Hendriks-Balk MC, Peters SL, Michel MC, Alewijnse AE. Regulation of G protein-coupled receptor signalling: focus on the cardiovascular system and regulator of G protein signalling proteins. Eur J Pharmacol 585: 278–291, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Heximer SP, Knutsen RH, Sun X, Kaltenbronn KM, Rhee MH, Peng N, Oliveira-Dos-Santos A, Penninger JM, Muslin AJ, Steinberg TH, Wyss JM, Mecham RP, Blumer KJ. Hypertension and prolonged vasoconstrictor signaling in RGS2-deficient mice. J Clin Invest 111: 445–452, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Holycross BJ, Blank RS, Thompson MM, Peach MJ, Owens GK. Platelet-derived growth factor-BB-induced suppression of smooth muscle cell differentiation. Circ Res 71: 1525–1532, 1992 [DOI] [PubMed] [Google Scholar]
- 40. Kalmes A, Daum G, Clowes AW. EGFR transactivation in the regulation of SMC function. Ann NY Acad Sci 947: 42–54, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Li H, He C, Feng J, Zhang Y, Tang Q, Bian Z, Bai X, Zhou H, Jiang H, Heximer SP, Qin M, Huang H, Liu PP, Huang C. Regulator of G protein signaling 5 protects against cardiac hypertrophy and fibrosis during biomechanical stress of pressure overload. Proc Natl Acad Sci USA 107: 13818–13823, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li S, Moon JJ, Miao H, Jin G, Chen BP, Yuan S, Hu Y, Usami S, Chien S. Signal transduction in matrix contraction and the migration of vascular smooth muscle cells in three-dimensional matrix. J Vasc Res 40: 378–388, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Li Y, Hashim S, Anand-Srivastava MB. Angiotensin II-evoked enhanced expression of RGS2 attenuates Gi-mediated adenylyl cyclase signaling in A10 cells. Cardiovasc Res 66: 503–511, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Liang KW, Ting CT, Yin SC, Chen YT, Lin SJ, Liao JK, Hsu SL. Berberine suppresses MEK/ERK-dependent Egr-1 signaling pathway and inhibits vascular smooth muscle cell regrowth after in vitro mechanical injury. Biochem Pharmacol 71: 806–817, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lindblom P, Gerhardt H, Liebner S, Abramsson A, Enge M, Hellstrom M, Backstrom G, Fredriksson S, Landegren U, Nystrom HC, Bergstrom G, Dejana E, Ostman A, Lindahl P, Betsholtz C. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev 17: 1835–1840, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu Y, Sinha S, McDonald OG, Shang Y, Hoofnagle MH, Owens GK. Kruppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. J Biol Chem 280: 9719–9727, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Liu Y, Suzuki YJ, Day RM, Fanburg BL. Rho kinase-induced nuclear translocation of ERK1/ERK2 in smooth muscle cell mitogenesis caused by serotonin. Circ Res 95: 579–586, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Majack RA, Grieshaber NA, Cook CL, Weiser MC, McFall RC, Grieshaber SS, Reidy MA, Reilly CF. Smooth muscle cells isolated from the neointima after vascular injury exhibit altered responses to platelet-derived growth factor and other stimuli. J Cell Physiol 167: 106–112, 1996 [DOI] [PubMed] [Google Scholar]
- 50. Majesky MW. Neointima formation after acute vascular injury. Role of counteradhesive extracellular matrix proteins. Tex Heart Inst J 21: 78–85, 1994 [PMC free article] [PubMed] [Google Scholar]
- 51. Manzur M, Ganss R. Regulator of g protein signaling 5: a new player in vascular remodeling. Trends Cardiovasc Med 19: 26–30, 2009 [DOI] [PubMed] [Google Scholar]
- 52. Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 292: C82–C97, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Mitchell TS, Bradley J, Robinson GS, Shima DT, Ng YS. RGS5 expression is a quantitative measure of pericyte coverage of blood vessels. Angiogenesis 11: 141–151, 2008 [DOI] [PubMed] [Google Scholar]
- 54. Natarajan K, Berk BC. Crosstalk coregulation mechanisms of G protein-coupled receptors and receptor tyrosine kinases. Methods Mol Biol 332: 51–77, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Nisancioglu MH, Mahoney WM, Jr, Kimmel DD, Schwartz SM, Betsholtz C, Genove G. Generation and characterization of rgs5 mutant mice. Mol Cell Biol 28: 2324–2331, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Obst M, Tank J, Plehm R, Blumer KJ, Diedrich A, Jordan J, Luft FC, Gross V. NO-dependent blood pressure regulation in RGS2-deficient mice. Am J Physiol Regul Integr Comp Physiol 290: R1012–R1019, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Raines EW. PDGF and cardiovascular disease. Cytokine Growth Factor Rev 15: 237–254, 2004 [DOI] [PubMed] [Google Scholar]
- 58. Riddle EL, Schwartzman RA, Bond M, Insel PA. Multi-tasking RGS proteins in the heart: the next therapeutic target? Circ Res 96: 401–411, 2005 [DOI] [PubMed] [Google Scholar]
- 59. Rogers JH, Tamirisa P, Kovacs A, Weinheimer C, Courtois M, Blumer KJ, Kelly DP, Muslin AJ. RGS4 causes increased mortality and reduced cardiac hypertrophy in response to pressure overload. J Clin Invest 104: 567–576, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rogers JH, Tsirka A, Kovacs A, Blumer KJ, Dorn GW, Muslin AJ. RGS4 reduces contractile dysfunction and hypertrophic gene induction in Galpha q overexpressing mice. J Mol Cell Cardiol 33: 209–218, 2001 [DOI] [PubMed] [Google Scholar]
- 61. Romero DG, Plonczynski MW, Gomez-Sanchez EP, Yanes LL, Gomez-Sanchez CE. RGS2 is regulated by angiotensin II and functions as a negative feedback of aldosterone production in H295R human adrenocortical cells. Endocrinology 147: 3889–3897, 2006 [DOI] [PubMed] [Google Scholar]
- 62. Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem 69: 795–827, 2000 [DOI] [PubMed] [Google Scholar]
- 63. Roy AA, Lemberg KE, Chidiac P. Recruitment of RGS2 and RGS4 to the plasma membrane by G proteins and receptors reflects functional interactions. Mol Pharmacol 64: 587–593, 2003 [DOI] [PubMed] [Google Scholar]
- 64. Sapienza P, di Marzo L, Cucina A, Corvino V, Mingoli A, Giustiniani Q, Ziparo E, Cavallaro A. Release of PDGF-BB and bFGF by human endothelial cells seeded on expanded polytetrafluoroethylene vascular grafts. J Surg Res 75: 24–29, 1998 [DOI] [PubMed] [Google Scholar]
- 65. Seger R, Seger D, Reszka AA, Munar ES, Eldar-Finkelman H, Dobrowolska G, Jensen AM, Campbell JS, Fischer EH, Krebs EG. Overexpression of mitogen-activated protein kinase kinase (MAPKK) and its mutants in NIH 3T3 cells. Evidence that MAPKK involvement in cellular proliferation is regulated by phosphorylation of serine residues in its kinase subdomains VII and VIII. J Biol Chem 269: 25699–25709, 1994 [PubMed] [Google Scholar]
- 66. Semplicini A, Lenzini L, Sartori M, Papparella I, Calo LA, Pagnin E, Strapazzon G, Benna C, Costa R, Avogaro A, Ceolotto G, Pessina AC. Reduced expression of regulator of G-protein signaling 2 (RGS2) in hypertensive patients increases calcium mobilization and ERK1/2 phosphorylation induced by angiotensin II. J Hypertens 24: 1115–1124, 2006 [DOI] [PubMed] [Google Scholar]
- 67. Servant MJ, Giasson E, Meloche S. Inhibition of growth factor-induced protein synthesis by a selective MEK inhibitor in aortic smooth muscle cells. J Biol Chem 271: 16047–16052, 1996 [DOI] [PubMed] [Google Scholar]
- 68. Shimizu RT, Blank RS, Jervis R, Lawrenz-Smith SC, Owens GK. The smooth muscle alpha-actin gene promoter is differentially regulated in smooth muscle versus non-smooth muscle cells. J Biol Chem 270: 7631–7643, 1995 [DOI] [PubMed] [Google Scholar]
- 69. Sierra DA, Gilbert DJ, Householder D, Grishin NV, Yu K, Ukidwe P, Barker SA, He W, Wensel TG, Otero G, Brown G, Copeland NG, Jenkins NA, Wilkie TM. Evolution of the regulators of G-protein signaling multigene family in mouse and human. Genomics 79: 177–185, 2002 [DOI] [PubMed] [Google Scholar]
- 70. Sun X, Kaltenbronn KM, Steinberg TH, Blumer KJ. RGS2 is a mediator of nitric oxide action on blood pressure and vasoconstrictor signaling. Mol Pharmacol 67: 631–639, 2005 [DOI] [PubMed] [Google Scholar]
- 71. Tallquist M, Kazlauskas A. PDGF signaling in cells and mice. Cytokine Growth Factor Rev 15: 205–213, 2004 [DOI] [PubMed] [Google Scholar]
- 72. Tani K, Ogushi F, Takahashi H, Kawano T, Endo T, Sone S. Thrombin stimulates platelet-derived growth factor release by alveolar macrophages in rats–significance in bleomycin-induced pulmonary fibrosis. J Med Invest 44: 59–65, 1997 [PubMed] [Google Scholar]
- 73. Thomas JA, Deaton RA, Hastings NE, Shang Y, Moehle CW, Eriksson U, Topouzis S, Wamhoff BR, Blackman BR, Owens GK. PDGF-DD, a novel mediator of smooth muscle cell phenotypic modulation, is upregulated in endothelial cells exposed to atherosclerosis-prone flow patterns. Am J Physiol Heart Circ Physiol 296: H442–H452, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev 52: 639–672, 2000 [PubMed] [Google Scholar]
- 75. Ushio-Fukai M, Griendling KK, Becker PL, Hilenski L, Halleran S, Alexander RW. Epidermal growth factor receptor transactivation by angiotensin II requires reactive oxygen species in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 21: 489–495, 2001 [DOI] [PubMed] [Google Scholar]
- 76. von TD, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res 312: 623–629, 2006 [DOI] [PubMed] [Google Scholar]
- 77. Wallasch C, Crabtree JE, Bevec D, Robinson PA, Wagner H, Ullrich A. Helicobacter pylori-stimulated EGF receptor transactivation requires metalloprotease cleavage of HB-EGF. Biochem Biophys Res Commun 295: 695–701, 2002 [DOI] [PubMed] [Google Scholar]
- 78. Wang C, Wu LL, Liu J, Zhang ZG, Fan D, Li L. Crosstalk between angiotensin II and platelet derived growth factor-BB mediated signal pathways in cardiomyocytes. Chin Med J (Engl) 121: 236–240, 2008 [PubMed] [Google Scholar]
- 79. Wang X, Adams LD, Pabon LM, Mahoney WM, Jr, Beaudry D, Gunaje J, Geary RL, deBlois D, Schwartz SM. RGS5, RGS4, and RGS2 expression and aortic contractibility are dynamically co-regulated during aortic banding-induced hypertrophy. J Mol Cell Cardiol 44: 539–550, 2008 [DOI] [PubMed] [Google Scholar]
- 80. Wang X, Zeng W, Soyombo AA, Tang W, Ross EM, Barnes AP, Milgram SL, Penninger JM, Allen PB, Greengard P, Muallem S. Spinophilin regulates Ca2+ signalling by binding the N-terminal domain of RGS2 and the third intracellular loop of G-protein-coupled receptors. Nat Cell Biol 7: 405–411, 2005 [DOI] [PubMed] [Google Scholar]
- 81. Wetzker R, Bohmer FD. Transactivation joins multiple tracks to the ERK/MAPK cascade. Nat Rev Mol Cell Biol 4: 651–657, 2003 [DOI] [PubMed] [Google Scholar]
- 82. Wieland T, Lutz S, Chidiac P. Regulators of G protein signalling: a spotlight on emerging functions in the cardiovascular system. Curr Opin Pharmacol 7: 201–207, 2007 [DOI] [PubMed] [Google Scholar]
- 83. Wieland T, Mittmann C. Regulators of G-protein signalling: multifunctional proteins with impact on signalling in the cardiovascular system. Pharmacol Ther 97: 95–115, 2003 [DOI] [PubMed] [Google Scholar]
- 84. Yin G, Yan C, Berk BC. Angiotensin II signaling pathways mediated by tyrosine kinases. Int J Biochem Cell Biol 35: 780–783, 2003 [DOI] [PubMed] [Google Scholar]
- 85. Yoshida T, Kaestner KH, Owens GK. Conditional deletion of Kruppel-like factor 4 delays downregulation of smooth muscle cell differentiation markers but accelerates neointimal formation following vascular injury. Circ Res 102: 1548–1557, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhang H, Chalothorn D, Jackson LF, Lee DC, Faber JE. Transactivation of epidermal growth factor receptor mediates catecholamine-induced growth of vascular smooth muscle. Circ Res 95: 989–997, 2004 [DOI] [PubMed] [Google Scholar]
- 87. Zhou J, Moroi K, Nishiyama M, Usui H, Seki N, Ishida J, Fukamizu A, Kimura S. Characterization of RGS5 in regulation of G protein-coupled receptor signaling. Life Sci 68: 1457–1469, 2001 [DOI] [PubMed] [Google Scholar]