Abstract
Chronic hypoxia (CH) activates the Ca2+-dependent transcription factor nuclear factor of activated T cells isoform c3 (NFATc3) in mouse pulmonary arteries. However, the mechanism of this response has not been explored. Since we have demonstrated that NFATc3 is required for CH-induced pulmonary arterial remodeling, establishing how CH activates NFATc3 is physiologically significant. The goal of this study was to test the hypothesis that endothelin-1 (ET-1) contributes to CH-induced NFATc3 activation. We propose that this mechanism requires increased pulmonary arterial smooth muscle cell (PASMC) intracellular Ca2+ concentration ([Ca2+]i) and stimulation of RhoA/Rho kinase (ROK), leading to calcineurin activation and actin cytoskeleton polymerization, respectively. We found that: 1) CH increases pulmonary arterial pre-pro-ET-1 mRNA expression and lung RhoA activity; 2) inhibition of ET receptors, calcineurin, L-type Ca2+ channels, and ROK blunts CH-induced NFATc3 activation in isolated intrapulmonary arteries from NFAT-luciferase reporter mice; and 3) both ET-1-induced NFATc3 activation in isolated mouse pulmonary arteries ex vivo and ET-1-induced NFATc3-green fluorescence protein nuclear import in human PASMC depend on ROK and actin polymerization. This study suggests that CH increases ET-1 expression, thereby elevating PASMC [Ca2+]i and RhoA/ROK activity. As previously demonstrated, elevated [Ca2+]i is required to activate calcineurin, which dephosphorylates NFATc3, allowing its nuclear import. Here, we demonstrate that ROK increases actin polymerization, thus providing structural support for NFATc3 nuclear transport.
Keywords: nuclear factor of activated T cells isoform c3; Rho kinase; cytoskeletal dynamics, pulmonary, smooth muscle
in rodents, long-term exposure to chronic hypoxia (CH) (more than 1 wk) causes pulmonary hypertension due to pulmonary vasoconstriction, arterial remodeling, and polycythemia, which ultimately results in right ventricular hypertrophy and often heart failure (64). CH and associated pulmonary hypertension also occur in patients with chronic bronchitis, emphysema, cystic fibrosis, and severe restrictive lung diseases or in high-altitude residents (43).
We have previously demonstrated that as little as 2 days of hypoxic exposure maximally activates the Ca2+-dependent transcription factor nuclear factor of activated T cells isoform c3 (NFATc3) in mouse pulmonary arterial smooth muscle cells (PASMC) (19, 20). This observation suggests that NFATc3 may act as an early response transcription factor. NFATc3 belongs to a family of four transcription factors (NFATc1, NFATc2, NFATc3, and NFATc4) that share the property of Ca2+/calcineurin-dependent nuclear translocation (reviewed in Ref. 41). In addition, we have demonstrated that NFATc3 is required for CH-induced pulmonary arterial remodeling and pulmonary hypertension in mice (19, 20). However, the mechanisms that lead to CH-induced NFATc3 activation are unknown.
Bonnet et al. (9) have demonstrated that NFATc2 is required for PASMC proliferation in patients with primary pulmonary hypertension and in monocrotaline-pulmonary hypertensive rats. In mice, the only NFAT isoform that is activated by CH is NFATc3 and mediates CH-induced PASMC hypertrophy (20).
In smooth muscle, NFATc3 nuclear accumulation and transcriptional activity are increased upon stimulation of Gq/11-coupled receptors (35, 36, 71). Increases in intracellular calcium concentration [Ca2+]i lead to calcineurin activation, which dephosphorylates NFATc3 and exposes nuclear localization signals that allow for NFATc3 nuclear translocation. NFATc3 nuclear accumulation is also controlled by a series of Ser/Thr kinases such as GSK3β, CaM kinase II, and JNK1/2 (34, 36).
Interestingly, the expression of endothelin 1 (ET-1), which activates Gq/11-coupled receptors, is increased in both humans and animals with pulmonary hypertension (8, 27, 81). Furthermore, oral administration of bosentan (dual ETA and ETB antagonist) is currently indicated for patients with pulmonary hypertension (32) while selective ETA receptor (ETAR) blockers prevent CH-induced pulmonary hypertension in rats (8, 83). However, little is known about the role of NFAT in ET-1 signaling.
During CH there is an increase in ET-1-mediated vasoconstriction in isolated pulmonary arteries, which involves increased PASMC [Ca2+]i through inhibition of voltage-gated potassium (KV) channels, membrane depolarization, and influx of Ca2+ through L-type Ca2+ channels (52). In addition, ET-1 increases Ca2+ sensitivity (increased muscle tension under constant-Ca2+ conditions) (79). RhoA is a member of a subfamily of the Ras superfamily of monomeric G proteins that activates Rho kinase (ROK), mediating ET-1-induced Ca2+ sensitization (79) and cytoskeleton reorganization (stress-fiber formation) (40, 42, 72). The cytoskeleton of smooth muscle cells is a filamentous network consisting largely of filamentous actin (F-actin), and to a lesser extent, monomeric actin (G-actin). RhoA/ROK is a signaling pathway that also appears to regulate NFAT activity (80). We have shown that ROK inhibition prevents intermittent hypoxia-induced NFATc3 activation in mouse mesenteric arteries both in vivo and ex vivo (17). However, the mechanism by which RhoA/ROK activates NFATc3 is still unclear.
Therefore, we hypothesized that ET-1 contributes to CH-induced NFATc3 activation through a mechanism that requires increased PASMC [Ca2+]i and stimulation of RhoA/ROK leading to calcineurin activation and actin cytoskeleton polymerization, respectively. Consistent with this hypothesis, we found that: 1) CH increases pulmonary arterial pre-pro-ET-1 mRNA expression and lung RhoA activity; 2) inhibition of ETAR, calcineurin, L-type Ca2+ channels, and ROK blunts CH-induced activation of NFATc3; and 3) ET-1-induced NFATc3 nuclear translocation in isolated mouse pulmonary arteries and human PASMC (HPASMC) depends on ROK and actin polymerization.
METHODS
All protocols employed in this study were reviewed and approved by the Institutional Animal Care and Use Committee of the University of New Mexico, School of Medicine (Albuquerque, NM).
Animals.
Adult male 9x-NFAT-luciferase reporter (NFAT-luc), NFATc3 knockout (KO), and BalB/C wild-type (WT) mice (20–25 g) were used. NFAT-luc mice were provided by Dr. Jeffery D. Molkentin (Department of Pediatrics, Children's Hospital Medical Center, Cincinnati, Ohio) (11). NFATc3 KO mice were a gift from Dr. Laurie Glimcher (Harvard University) (63). Heterozygous mice were bred to obtain age-matched WT (Balb/C) and KO.
Chronic hypoxia exposure.
Animals designated for exposure to CH were housed in a hypobaric chamber with barometric pressure maintained at ∼380 Torr for 2, 7, or 21 days. Control animals were housed at ambient barometric pressure (normoxia, N, ∼630 Torr). All animals were maintained on a 12:12-h light-dark cycle.
Animal treatments.
All treatments were started 1 day before CH exposure, followed by 2 days during CH. NFAT-luc mice were treated as follows: 1) bosentan (dual ET receptor antagonist; 30 mg·kg−1·day−1; gift from Actelion Pharmaceuticals, Switzerland) was administered in the drinking water; 2) cyclosporine A (CsA, calcineurin inhibitor; 25 mg·kg−1·day−1; Calbiochem) or Cremophor EL (vehicle, Sigma) was injected subcutaneously; 3) PD155080 (selective ETAR antagonist; 50 μg·kg−1·day−1; College of Pharmacy, University of New Mexico) or placebo was administered in oral dough pellets (17, 84); 4) diltiazem (L-type Ca2+ channel inhibitor; 100 mg·kg−1·day−1; Sigma) or saline (vehicle) was administered subcutaneously via osmotic pumps (Alzet); or 5) the ROK inhibitors fasudil (30 mg·kg−1·day−1), HA 1152 (1 mg·kg−1·day−1) (both from LC Laboratories), or saline (vehicle) was administered subcutaneously via osmotic pumps.
Luciferase activity.
Intrapulmonary arteries were isolated from NFAT-luc mice and lysed (Promega buffer). Luciferase activity was measured using a Luciferase Assay System kit (Promega), and light was detected with a luminometer (TD20/20, Turner). Protein content was determined by the Bradford method (Bio-Rad) or bicinchoninic acid protein assay reagent (Pierce) and used to normalize luciferase activity.
Quantitative real-time polymerase chain reaction.
Isolated intrapulmonary arteries from NFAT-luc (exposed to normoxia, 2, 7, and 21 days of CH), NFATc3 WT and KO (exposed to normoxia and 2 days CH) mice were stored in RNAlater (Ambion). Total RNA was isolated using the RNeasy Mini Kit (Qiagen) and reverse transcribed to cDNA using a high capacity reverse transcription kit (Applied Biosystems). For real time detection of pre-pro-ET-1 transcripts, SYBR Green Master Mix (Applied Biosystems) was used as previously described (18). The normalized gene expression method (2−ΔΔCT) for relative quantification of gene expression was used (53).
RhoA activity.
RhoA activity was determined in whole lung extracts using the G-LISA Small G-protein Activation Assay from Cytoskeleton. RhoA activity could not be detected with this assay in isolated intrapulmonary arteries due to limited sensitivity of the assay. An alternative is the GTP-bound RhoA pull-down assay (cytoskeleton); however, this assay requires more protein that could be extracted from these small arteries.
NFATc3 nuclear import.
HPASMC (Invitrogen) were grown on polylysine-coated plates in Media 231 with smooth muscle growth supplement (Invitrogen) at 37°C in 5% CO2 with controlled humidity. Cells were used up to passage 5. HPASMC were electroporated with NFATc3-green fluorescent protein (GFP) expression vector using Nucleofector (Lonza). The vector was created by Dr. F. McKeon (Harvard University, Cambridge, MA) and kindly provided by Dr. L. F. Santana (Washington State University, Seattle, WA).
NFATc3-GFP expressing HPASMC were seeded on microscope coverslips and cultured for at least 48 h on Media 231 with smooth muscle differentiation supplement (Invitrogen). At the time of experimentation, the coverslip was mounted on a heating plate chamber and maintained in HEPES-PSS at ∼37°C during the experiment. Nuclear GFP fluorescence was monitored using a Nikon Diaphot 300 at ×200 magnification. Individual HPASMC were imaged once every 30 s for 30 min. All cells were incubated for 5 min with a myosin light chain peptide inhibitor (Peptide 18, 10−6 M, Calbiochem) to reduce cell shrinkage in response to constrictors. Cells were treated with the CRM1 exportin inhibitor leptomycin B (4 × 10−8 M, LC Laboratories) to prevent NFATc3-GFP nuclear export. Immediately, cells were treated with vehicle or ET-1 (10−8 M, Sigma) in the presence or absence of the ROK inhibitor fasudil (10−6 M, LC Laboratories); the actin polymerization inhibitor cytochalasin B (10−6 M, Sigma), or the well-established actin stabilizing agent (14) jasplakinolide (0.05 μM, Sigma). Images were captured using Andor IQ 1.9 software. Nuclear fluorescence (F) was background corrected and expressed as fold change from baseline nuclear fluorescence (F/F0) (Metamorph Universal Imaging software).
[Ca2+]i measurement.
HPASMC were loaded with a mix of fura-2 AM (2 μM)-pluronic acid (0.02%) in HEPES PSS solution for 15 min at room temperature, followed by 20 min wash with HEPES PSS at 37°C. The ratio of 340/380 nm fura-2 AM fluorescence was monitored every 300 ms for 17 min using a NIKON Diaphot 300 at ×200 magnification. Images were captured using Andor IQ 1.9 software. Cells were treated with ET-1 in the presence or absence of leptomycin B, fasudil, or cytochalasin B. Fluorescence at 340 and 380 nm was background corrected, and the ratio was expressed as percent fold change from baseline.
Immunofluorescence confocal microscopy.
NFATc3-GFP expressing HPASMC were incubated with vehicle or ET-1 for 5 min at 37°C in the presence or absence of fasudil or cytochalasin B. Cells were formaldehyde fixed (4% in phosphate-buffered saline, PBS), permeabilized, and blocked for nonspecific binding. Primary antibody (rabbit polyclonal anti-NFATc3, 1:100; Santa Cruz) was prepared in 0.2% gelatin in PBS and applied overnight at 4°C. Secondary antibody (anti-rabbit Cy5; Jackson Immunoresearch Laboratories) was prepared in 0.2% gelatin in PBS and applied for 1 h together with 6.6 × 10−6 M Alexa Fluor 488 phallodine (Invitrogen) and 0.64 × 10−6 M Alexa Fluor 594 DNase I (Invitrogen) at room temperature. Nuclei were stained using Hoechst 33342 (1:2,000 in PBS; Invitrogen). Cells were imaged with a ×63 objective on a META Zeiss 510 laser scanning confocal microscope. Specificity of immune staining was confirmed by the absence of fluorescence in cells incubated with primary or secondary antibodies alone. NFATc3-F-actin (phallodine) colocalization was determined using Image J software (NIH). The software was programmed so that individual pixels appear white if F-actin (green) stain and NFATc3 (red) stain are colocalized, and the number of white pixels was determined as reported previously (29). F-actin and G-actin (DNase I) integrated intensity (arbitrary units, AU) were measured in the threshold individual channels using Metamorph and used to calculate F/G actin content. The same threshold was applied to every image.
Statistics.
Results are expressed as means ± SE. Statistical significance was tested at the 95% (P < 0.05) confidence level using one- or two-way repeated measures ANOVA. NFATc3-GFP nuclear import curves were obtained by nonlinear regression fit to a one-phase association equation.
RESULTS
CH-induced NFATc3 activation requires ET-1 and ROK.
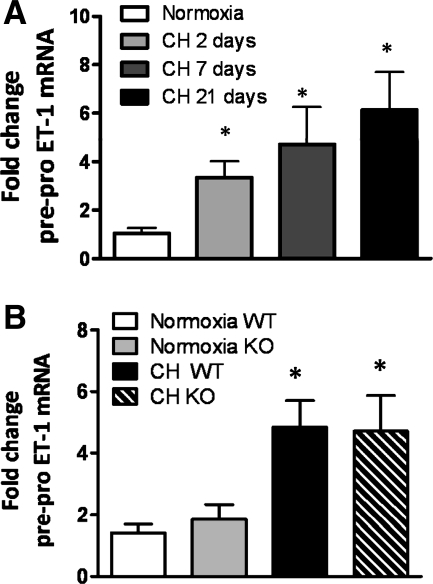
Pre-pro-ET-1 mRNA expression was significantly increased in pulmonary arteries from mice exposed to CH from 2 to 21 days (Fig. 1A). In addition, pre-pro-ET-1 mRNA levels were similarly increased in NFATc3 WT and KO mice at 2 days of CH (Fig. 1B) demonstrating that CH-induced increases in pre-pro-ET-1 mRNA are independent of NFATc3.
Fig. 1.
Chronic hypoxia (CH) increases intrapulmonary arterial pre-pro-endothelin-1 (ET)-1 mRNA independently of nuclear factor of activated T cells isoform c3 (NFATc3). Pre-pro-ET-1 transcripts were measured in isolated intrapulmonary arteries from mice exposed to normoxia, CH (2, 7, or 21 days) (A), and from NFATc3 wild-type (WT) and knockout (KO) mice exposed to normoxia and 2 days of CH (B). The results were expressed as fold change from normoxia. Data are means ± SE; n = 6 animals/group. *P < 0.05 vs. normoxia.
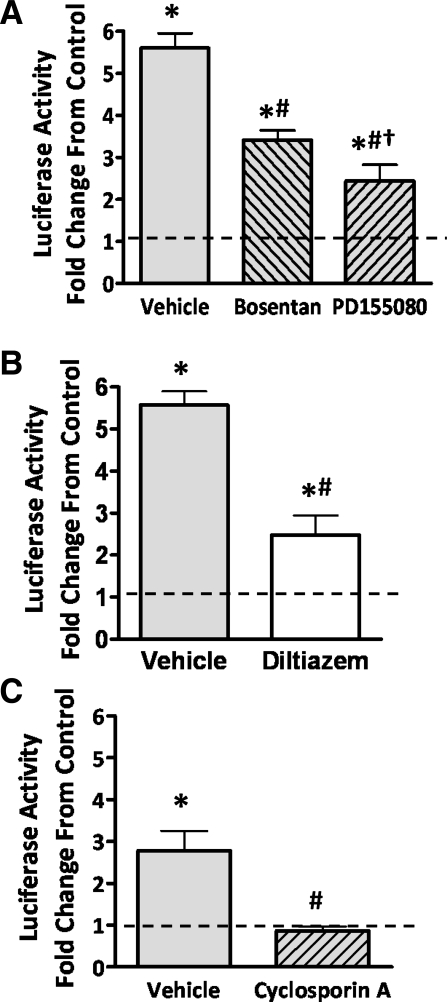
To determine whether the increased expression of ET-1 leads to increased intrapulmonary arterial NFATc3 transcriptional activity in CH (20), the dual ET-1 receptor blocker bosentan or the selective ETAR blocker PD155080 was administered to NFAT-luc mice while exposed to CH for 2 days. Both bosentan and PD155080 significantly attenuated CH-induced NFATc3 transcriptional activation in isolated intrapulmonary arteries, shown as luciferase reporter activity, with PD155080 being significantly more effective (Fig. 2A).
Fig. 2.
Mechanisms of NFAT activation by CH. Bosentan or PD155080 (A), diltiazem (B), and cyclosporin A (C) were administered to mice exposed to CH for 2 days, and luciferase activity normalized to total protein was measured in isolated intrapulmonary arteries. The results were expressed as fold change from normoxia (dotted line). Data are means ± SE; n = 6 animals/group. *P < 0.05 vs. normoxia; #P < 0.05 vs. CH vehicle; †P < 0.05 vs. CH bosentan.
Increased [Ca2+]i is required for NFATc3 dephosphorylation and activation by calcineurin (34–36, 41). Accordingly, we found that administration of the L-type Ca2+ channel inhibitor diltiazem significantly attenuated CH-induced NFATc3 activation (Fig. 2B).
As expected, calcineurin inhibition with CsA prevented NFATc3 activation by CH (Fig. 2C). Administration of Cremophor EL, the vehicle for CsA, significantly reduced the effect of CH on NFATc3 activation compared with the vehicle for diltiazem and bosentan (saline in osmotic pump and drinking water, respectively) (Fig. 2, A–C). Regardless of the effect of Cremophor EL, CsA completely inhibited the effect of CH on NFATc3 activation.
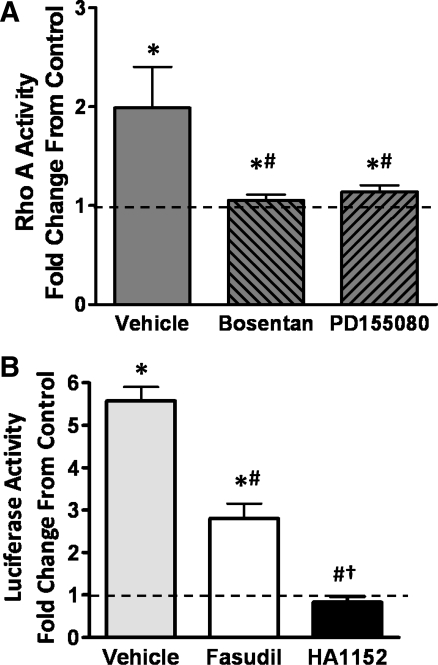
The RhoA/ROK pathway has been shown to be upregulated in CH (46, 79). Since we hypothesized that this pathway is implicated in ET-1-induced NFATc3 nuclear translocation, RhoA activity was compared between normoxia and CH exposed mice. Figure 3A shows that RhoA activity was increased in lungs from CH-exposed mice. To determine whether RhoA activation is downstream of ETAR stimulation, mice were treated with bosentan or PD155080 during CH exposure. Indeed, ETAR activation was required for CH-induced increases in RhoA activity as shown in Fig. 3A. One limitation of our study is that we were not able to measure RhoA activity in isolated pulmonary arteries due to the limited sensitivity of the assay, which is consistent with the lack of reports measuring RhoA activity in isolated mouse small arteries.
Fig. 3.
CH-induced NFATc3 activation in a RhoA/ROK-dependent manner. A: RhoA activity was measured in whole lung lysate from mice exposed to normoxia or 2 days of CH and treated with bosentan or PD155080; B: fasudil and HA 1152 were administered to mice exposed to CH for 2 days, and luciferase activity normalized by total protein was measured in isolated intrapulmonary arteries. The results were expressed as fold change from normoxia (dotted line). Data are means ± SE; n = 6 animals/group. *P < 0.06 vs. normoxia; #P < 0.05 vs. CH vehicle; †P < 0.05 vs. CH fasudil.
Also consistent with our hypothesis, we found that administration of the ROK inhibitor fasudil [IC50 1.9 × 10−6 M (66)], significantly attenuated CH-induced NFATc3 activation in intrapulmonary arteries from NFAT-luc mice (Fig. 3B). In addition, a more potent ROK inhibitor HA 1152 [IC50 7.9 × 10−8 M (66)] prevented NFATc3 activation by CH (Fig. 3B). Together, these results suggest that CH increases ET-1 levels leading to activation of NFATc3 in intrapulmonary arteries via increased [Ca2+]i, calcineurin, and RhoA/ROK activity.
ET-1-induced NFATc3 nuclear import in HPASMC requires ROK and actin polymerization.
To determine the mechanism by which ROK mediates ET-1-induced NFATc3 activation, we performed studies in cultured HPASMC.
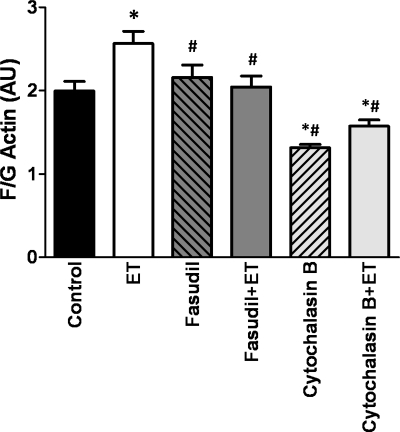
Figure 4 shows that 5 min treatment with ET-1 significantly increased the F/G actin ratio in HPASMC. This increase was prevented by the ROK inhibitor fasudil. As expected, cytochalasin B, which blocks addition of actin monomer (G-actin) to filamentous actin (F-actin), significantly reduced the F/G actin ratio and prevented ET-1-induced increases in F-actin content (Fig. 4).
Fig. 4.
ET-1 increases F-actin through ROK. Human pulmonary arterial smooth muscle cells (HPASMC) were treated with ET-1 ± fasudil or cytochalasin B, and F/G actin content was determined by inmunofluorescence confocal microscopy. The results were expressed as F/G actin fluorescence intensity (AU). Data are means ± SE; n = 7 cells/group. *P < 0.05 vs. control, #P < 0.05 vs. ET.
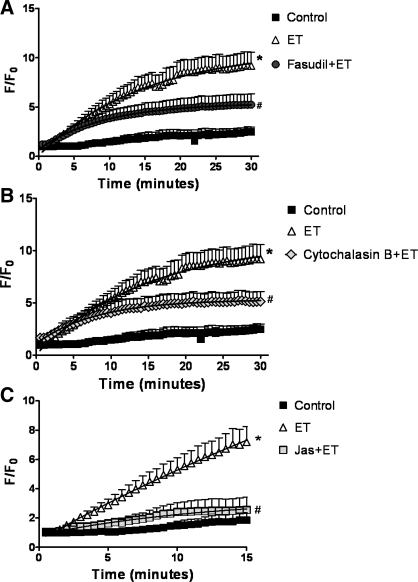
GFP nuclear fluorescence accumulation was tracked in NFATc3-GFP-expressing HPASMC after stimulation with ET-1 in the presence or absence of the ROK inhibitor fasudil. Figure 5A shows that fasudil significantly attenuated ET-1-induced increases in NFATc3-GFP nuclear import. In addition, cytochalasin B, which decreases F-actin content, inhibited ET-1-induced NFATc3 nuclear import (Fig. 5B). Interestingly, jasplakinolide, an actin stabilizing agent (14), also inhibited the effect of ET-1 on NFATc3 nuclear import (Fig. 5C). Fasudil, cytochalasin B, and jasplakinolide had no effect when applied without ET-1 (data not shown).
Fig. 5.
ET-1-induced NFATc3 nuclear import is inhibited by actin cytoskeleton depolymerization and stabilization. HPASMC were treated with ET-1 ± fasudil (A), cytochalasin B (B), or ± jasplakinolide (C). NFATc3-green fluorescent protein nuclear fluorescence was monitored over time. After background subtraction, nuclear fluorescence (F) was divided by basal fluorescence at time 0 (F0). Data are means ± SE; n = 7 cells/group. *P < 0.05 vs. control, #P < 0.05 vs. ET.
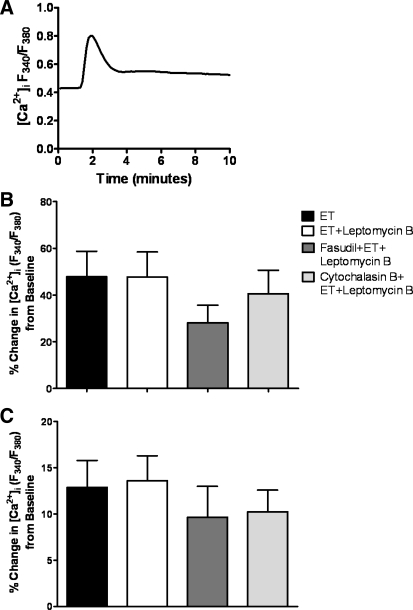
To demonstrate that ROK inhibition and derangement of actin cytoskeleton do not reduce ET-1-induced increases in [Ca2+]i, the effect of each inhibitor was measured on HPASMC loaded with the Ca2+ indicator fura-2 AM loaded. As expected, ET-1 significantly increased [Ca2+]i, showing an initial peak followed by a plateau (Fig. 6A). Neither fasudil, cytochalasin B, leptomycin B, nor jasplakinolide significantly affected ET-1-induced increases in [Ca2+]i (Fig. 6, B and C).
Fig. 6.
ET-1-induced increases in intracellular Ca2+ concentration ([Ca2+]i) are not affected by ROK inhibition or actin cytoskeleton depolymerization. Fura-2 AM-loaded HPASMC were treated with ET-1 ± leptomycin B, fasudil, or cytochalasin B. Ratio of 340/380 fluorescence was background subtracted and expressed as percent change from baseline. Representative trace (A), average peak (B), and plateau of ET-1-induced [Ca2+]i increase (C) are shown. Data are means ± SE; n = 7 cells/group. No significant difference was observed between groups.
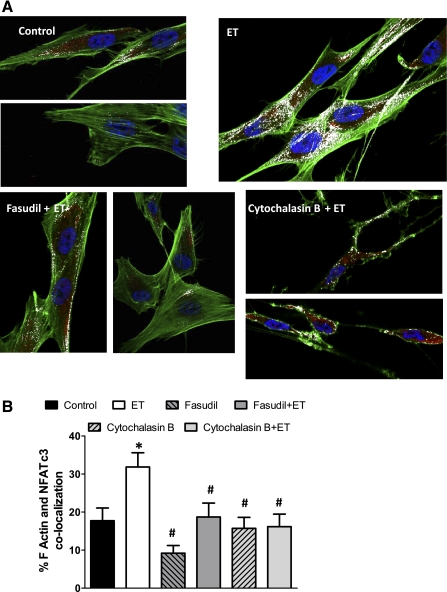
To determine how ET-1-induced F-actin polymerization increases NFATc3 nuclear import, immunofluorescence colocalization of F-actin and NFATc3 was performed in HPASMC treated with ET-1 in the presence or absence of fasudil and cytochalasin B. Figure 7, A and B, shows that ET-1 increased the colocalization of F-actin and NFATc3, which was prevented by fasudil and cytochalasin B. Neither fasudil nor cytochalasin B had any significant effect when applied without ET-1.
Fig. 7.
ET-1 increases F-actin and NFATc3 colocalization. HPASMC were treated with ET-1 ± fasudil or cytochalasin B, fixed and F-actin and NFATc3 were fluorescently labeled. A: representative confocal images of F-actin (green) and NFATc3 (red). White pixels indicate F-actin and NFATc3 colocalization. B: summary of results showing percent F-actin and NFATc3 colocalization. Data are means ± SE; n = 15 cells/group. *P < 0.05 vs. control, #P < 0.05 vs. ET.
ET-1-mediated NFATc3 transcriptional activation in isolated mouse pulmonary arteries requires ROK and actin polymerization.
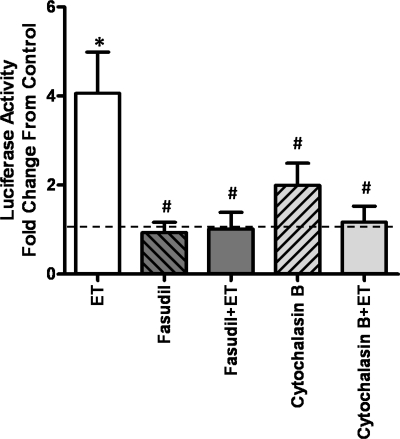
To test whether ET-1 activates NFATc3 ex vivo in the absence of hypoxia or transmural pressure, isolated intrapulmonary arteries from NFAT-luc mice were incubated with ET-1. Figure 8 shows that ET-1 induced a fourfold increase in luciferase activity; i.e., NFATc3 transcriptional activity, over basal. Pretreatment with the ROK inhibitor fasudil and the actin polymerization inhibitor cytochalasin B prevented ET-1-induced NFATc3 activation (Fig. 8). Neither fasudil nor cytochalasin B had any significant effect when applied without ET-1.
Fig. 8.
ET-1-induced increases in NFAT-dependent transcription in pulmonary arteries depend on ROK activation and actin cytoskeleton polymerization. Intrapulmonary arteries were isolated from NFAT-luciferase mice and treated with ET-1 ± fasudil or cytochalasin B. Luciferase activity was measured, normalized to total protein, and expressed as fold change from control (dotted line). Data are means ± SE; n = 6 arteries from 6 mice/group. *P < 0.05 vs. control, #P < 0.05 vs. ET.
DISCUSSION
In this study, we hypothesized that CH activates NFATc3 in PASMC through ET-1-mediated Ca2+ mobilization and stimulation of RhoA/ROK, leading to calcineurin activation and F-actin polymerization. In agreement with this hypothesis, we found that: 1) CH increases pulmonary arterial pre-pro-ET-1 mRNA expression and lung RhoA activity; 2) inhibition of ETAR, calcineurin, L-type Ca2+ channels, and ROK blunts CH-induced increase in NFATc3 transcriptional activity; and 3) ET-1-induced NFATc3 nuclear translocation in HPASMC and isolated mouse pulmonary arteries depends on ROK-mediated F-actin polymerization.
CH-induced NFATc3 activation requires ET-1 and ROK.
Our present results show that CH increases ET-1 expression in mouse pulmonary arteries are consistent with the reported role for ET-1 as an important mediator of CH-induced pulmonary hypertension in both animals and humans (8, 27, 32, 81, 83). In addition, we have demonstrated that CH-induced upregulation of ET-1 is independent of NFATc3. It is not likely that other NFATc isoforms contribute to CH-induced ET-1 expression because we have previously demonstrated that NFATc3 is the only isoform activated by CH in mouse pulmonary arteries (20). This previous finding also supports our current focus on the study on NFATc3.
This is the first study to demonstrate that in vivo inhibition of ETAR attenuates CH-induced NFATc3 activation in intrapulmonary arteries. These findings are in agreement with previous reports showing that ET-1 is a potent activator of NFAT (17, 48, 61).
ET-1 causes release of Ca2+ from intracellular stores, protein kinase C-dependent inhibition of voltage-gated K+ (Kv) channels, and subsequent membrane depolarization and Ca2+ influx through L-type Ca2+ channels (69, 70). In addition, other Ca2+ entry pathways have been shown to contribute to global changes in Ca2+ in pulmonary hypertension (1). Consistently, administration of the L-type Ca2+ channel inhibitor diltiazem significantly attenuated but did not abolish CH-induced NFAT activation. Although, it is well established that increased [Ca2+]i via influx through L-type Ca2+ channels and Ca2+ release from intracellular stores are required for calcineurin activation followed by NFAT dephosphorylation (34–36, 41), it has not been previously demonstrated in vivo. This study demonstrates a role for Ca2+ in CH-induced NFATc3 activation in vivo. In addition, our results are consistent with the demonstrated central role of calcineurin in NFAT regulation since CsA abrogated CH-induced NFATc3 activation.
It is well described that ET-1 binding to ETAR activates RhoA/ROK pathway (7, 33, 55). Therefore, we explored the role of RhoA/ROK signaling as an additional pathway that could participate in CH-induced NFATc3 activation. Our results showing elevated RhoA levels in lungs from CH mice are in accordance with previous reports demonstrating that RhoA/ROK signaling is upregulated by hypoxia (13, 45, 46) and the implication of this pathway in the development of pulmonary hypertension (2, 3, 12, 25, 31, 56, 57, 79).
Our results also suggest that ETAR are activated in CH due to the elevated ET-1 levels leading to increased RhoA activity because both a dual ET receptor and a selective ETAR antagonist prevent CH-induced increases in RhoA activity. Subsequent elevations in PASMC [Ca2+]i and RhoA lead to NFATc3 nuclear translocation and activation. This conclusion is supported by our data showing that ET receptor, L-type Ca2+ channels, calcineurin, and ROK inhibitors attenuate and/or prevent CH-induced increases in NFATc3 transcriptional activity. However, additional factors besides ET-1 might be contributing to CH-induced NFATc3 activation because the ETAR receptor antagonist completely prevented CH-induced RhoA activation but did not completely prevent NFATc3 activation; it caused ∼75% attenuation. Therefore, our study does not exclude the contribution of other factors such as serotonin, angiotensin II, or reactive oxygen species (ROS), which have been shown to be elevated by CH (4, 15, 23, 24, 28, 37, 46, 68) and are known activators of NFAT (5, 30, 37, 47, 49, 60, 73, 76, 78). Of interest, several studies have shown cross talk between ET-1 and angiotensin II. In particular, administration of an angiotensin converting enzyme inhibitor reduces elevated plasma concentrations of ET-1 (54). In addition, angiotensin II increases ET-1 synthesis via elevated ROS in human arteries in culture, and there is a significant association between elevated angiotensin II levels, increased oxidative stress, and increased ET-1 concentrations (50). Recently, it has been shown that 20-wk-old mice with SM22α-targeted overexpression of the serotonin transporter are pulmonary hypertensive (37). NFATc2 is activated in PASMC from these mice. However, calcineurin/NFAT inhibition with CsA administration does not reverse the pulmonary hypertension (37). Thus the potential role of angiotensin II, serotonin, and ROS in CH-induced NFATc3 activation remains to be explored.
CH causes pulmonary vasoconstriction leading to elevated pulmonary pressure. It is possible to argue that in vivo administration of inhibitors of ET receptors, L-type Ca2+ channels, and calcineurin prevents CH-induced vasoconstriction and increases in pulmonary pressure since the effectiveness of these drugs in lowering pulmonary pressure in rodents and humans has been previously demonstrated, and the site of action is primarily the vasculature (10, 17, 18, 21, 22, 44, 51, 75, 82). Elevated intrapulmonary pressure could be a mechanical stimulus that contributes to increased PASMC [Ca2+]i leading to NFATc3 activation (36, 58). However, ex vivo application of ET-1 to isolated intrapulmonary arteries in the absence of intravascular pressure also increased NFATc3 activity through ROK activation. Therefore, upregulation of ET-1 (and possibly other vasoactive factors), rather than elevated pulmonary arterial pressure, is most likely the stimulus that leads to NFATc3 activation in response to CH.
ET-1-induced RhoA/ROK-mediated actin polymerization leads to NFATc3 nuclear translocation.
RhoA/ROK signaling has been previously implicated in NFAT activation by our laboratory and others (17, 80). The mechanism has not been explored; however, it has been shown that RhoG, a RhoA-related small GTPase, potentiates NFAT-dependent transcription correlating with its capacity to increase actin polymerization (77). This finding supports the possibility that NFAT-dependent transcription is an actin-dependent process. Along these lines, ET-1 via RhoA/ROK increases F-actin content (i.e., induces cytoskeleton actin polymerization) in airway smooth muscle cells and preadipocytes (42, 72). Consistent with these reported findings, we found that in HPASMC, ET-1 increased F-actin content and NFATc3 nuclear import, both of which were ROK dependent.
The increase in the proportion of F-actin that occurs in response to the stimulation of smooth muscle cells and the essential role of stimulus-induced actin polymerization and cytoskeletal dynamics in the generation of mechanical tension has been convincingly documented in vascular smooth muscle (reviewed in Refs. 39, 72, 74). In addition, actin cytoskeleton polymerization has been implicated in the transport of transcription factors to the nucleus (26). Actin cytoskeleton dynamics are regulated by many signaling pathways. In general, receptor activation and/or integrin ligation activates protein kinases and/or small GTPases, which in turn regulate the functional status of the actin regulatory proteins and eventually cause actin filament assembly or structural reorganization (reviewed in Ref. 74). In particular, it has been reported that nuclear import of nuclear factor κB (NF-κB; which, along with NFAT, is a member of the Rel family of transcription factors) requires activation of RhoA/ROK (26). Stimulation of RhoA/ROK enhances LIM kinase activity, leading to phosphorylation and inactivation of cofilin (actin regulatory protein) (6, 26). When cofilin is phosphorylated, it no longer binds to actin, thus facilitating actin polymerization (6) and NF-κB transport to the nucleus (26). A recent study demonstrates that ET-1, in a ROK-dependent manner, increases phospho-cofilin-to-cofilin ratio in pulmonary artery rings and cultured PASMC (16). Hypoxia also has been shown to increase phophocofilin levels in PASMC (59). Our present study shows that both destabilization and stabilization of the actin cytoskeleton inhibit NFATc3 nuclear transport. This finding suggests that NFATc3 nuclear transport depends on dynamic reorganization of the actin cytoskeleton and is similar to the mechanism of NF-κB nuclear transport (26). Contrary to our findings in HPASMC, disruption of actin polymerization in T cells results in prolonged [Ca2+]i elevation and persistent NFAT nuclear accumulation due to decreased Ca2+ export (67).
In conclusion, this study demonstrates that CH increases ET-1 expression leading to ETAR activation, elevated PASMC [Ca2+]i, and stimulation of RhoA/ROK activity. As previously demonstrated, elevated [Ca2+]i activates calcineurin, which dephosphorylates NFATc3, exposing nuclear localization signals (reviewed in Refs. 41 and 65). In addition, ROK increases actin polymerization providing the structural support for NFATc3 nuclear transport.
Considering that NFATc3 is required for CH-induced pulmonary hypertension in mice (20), future studies should address whether the signaling pathway demonstrated in this study for NFATc3 activation applies to other animal models of pulmonary hypertension, primary pulmonary hypertension, and/or to other NFATc isoforms. Arguing against a role for the ET-1/ETR/RhoA/ROK pathway in NFATc2 activation, however, is evidence that NFATc2, but not NFATc3, is activated in PASMC from primary pulmonary hypertensive patients (9) treated with ET receptor antagonists. Nevertheless, much evidence suggests that RhoA/ROK signaling plays an important role in the pathogenesis of various experimental models of pulmonary hypertension, including those resulting from CH, monocrotaline, bleomycin, shunt, and vascular endothelial growth factor receptor inhibition combined with CH exposure (2, 3, 38, 57, 62, 85), as well as in patients with pulmonary arterial hypertension (31). The role of NFATc3 activation in the pathogenesis of pulmonary hypertension in patients with chronic bronchitis, emphysema, cystic fibrosis, and severe restrictive lung diseases or in high-altitude residents should be explored. Therefore, our study highlights the importance of exploring whether NFATc3 pathway inhibition could have potential clinical relevance to prevent and/or reverse the development of pulmonary hypertension and its associated vascular pathology.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01-HL-088151 and supplement from American Recovery And Reinvestment Act of 2009.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Aaronson PI, Robertson TP, Knock GA, Becker S, Lewis TH, Snetkov V, Ward JPT. Hypoxic pulmonary vasoconstriction: mechanisms and controversies. J Physiol Online 570: 53–58, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abe K, Tawara S, Oi K, Hizume T, Uwatoku T, Fukumoto Y, Kaibuchi K, Shimokawa H. Long-term inhibition of Rho-kinase ameliorates hypoxia-induced pulmonary hypertension in mice. J Cardiovasc Pharmacol 48: 280–285, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Abe K, Shimokawa H, Morikawa K, Uwatoku T, Oi K, Matsumoto Y, Hattori T, Nakashima Y, Kaibuchi K, Sueishi K, Takeshit A. Long-term treatment with a Rho-kinase inhibitor improves monocrotaline-induced fatal pulmonary hypertension in rats. Circ Res 94: 385–393, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Aguirre JI, Morrell NW, Long L, Clift P, Upton PD, Polak JM, Wilkins MR. Vascular remodeling and ET-1 expression in rat strains with different responses to chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 278: L981–L987, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Amberg GC, Rossow CF, Navedo MF, Santana LF. NFATc3 regulates Kv2.1 expression in arterial smooth muscle. J Biol Chem 279: 47326–47334, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Bamburg JR, McGough A, Ono S. Putting a new twist on actin: ADF/cofilins modulate actin dynamics. Trends Cell Biol 9: 364–370, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Barman SA. Vasoconstrictor effect of endothelin-1 on hypertensive pulmonary arterial smooth muscle involves Rho-kinase and protein kinase C. Am J Physiol Lung Cell Mol Physiol 293: L472–L479, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Blumberg FC, Wolf K, Arzt M, Lorenz C, Riegger GAJ, Pfeifer M. Effects of ET-A receptor blockade on eNOS gene expression in chronic hypoxic rat lungs. J Appl Physiol 94: 446–452, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Bonnet S, Rochefort G, Sutendra G, Archer SL, Haromy A, Webster L, Hashimoto K, Bonnet SN, Michelakis ED. The nuclear factor of activated T cells in pulmonary arterial hypertension can be therapeutically targeted. PNAS 104: 11418–11423, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bonvallet ST, Zamora MR, Hasunuma K, Sato K, Hanasato N, Anderson D, Sato K, Stelzner TJ. BQ123, an ETA-receptor antagonist, attenuates hypoxic pulmonary hypertension in rats. Am J Physiol Heart Circ Physiol 266: H1327–H1331, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Braz JC, Bueno OF, Liang Q, Wilkins BJ, Dai YS, Parsons S, Braunwart J, Glascock BJ, Klevitsky R, Kimball TF, Hewett TE, Molkentin JD. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J Clin Invest 111: 1475–1486, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Broughton BR, Walker BR, Resta TC. Chronic hypoxia induces Rho kinase-dependent myogenic tone in small pulmonary arteries. Am J Physiol Lung Cell Mol Physiol 294: L797–L806, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Broughton BRS, Jernigan NL, Norton CE, Walker BR, Resta TC. Chronic hypoxia augments depolarization-induced Ca2+ sensitization in pulmonary vascular smooth muscle through superoxide-dependent stimulation of RhoA. Am J Physiol Lung Cell Mol Physiol 298: L232–L242, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bubb MR, Senderowicz AM, Sausville EA, Duncan KL, Korn ED. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J Biol Chem 269: 14869–14871, 1994 [PubMed] [Google Scholar]
- 15. Chi AY, Waypa GB, Mungai PT, Schumacker PT. Prolonged hypoxia increases ROS signaling and RhoA activation in pulmonary artery smooth muscle and endothelial cells. Antioxid Redox Signal 12: 603–610, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dai YP, Bongalon S, Mutafova-Yambolieva VN, Yamboliev IA. Distinct effects of contraction agonists on the phosphorylation state of cofilin in pulmonary artery smooth muscle. Adv Pharmacol Sci 2008: 362741, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Frutos S, Caldwell E, Nitta CH, Kanagy NL, Wang J, Wang W, Walker MK, Gonzalez Bosc LV. NFATc3 contributes to intermittent hypoxia-induced arterial remodeling in mice. Am J Physiol Heart Circ Physiol 299: H356–H363, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Frutos S, Duling L, Alo D, Berry T, Jackson-Weaver O, Walker M, Kanagy N, Gonzalez Bosc L. NFATc3 is required for intermittent hypoxia-induced hypertension. Am J Physiol Heart Circ Physiol 294: H2382–H2390, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Frutos S, Nitta CH, Caldwell E, Friedman J, Gonzalez Bosc LV. Regulation of soluble guanylyl cyclase-α1 expression in chronic hypoxia-induced pulmonary hypertension: role of NFATc3 and HuR. Am J Physiol Lung Cell Mol Physiol 297: L475–L486, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Frutos S, Spangler R, Alo D, Gonzalez Bosc LV. NFATc3 mediates chronic hypoxia-induced pulmonary arterial remodeling with α-actin up-regulation. J Biol Chem 282: 15081–15089, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dicarlo VS, Chen SJ, Meng QC, Durand J, Yano M, Chen YF, Oparil S. ETA-receptor antagonist prevents and reverses chronic hypoxia-induced pulmonary hypertension in rat. Am J Physiol Lung Cell Mol Physiol 269: L690–L697, 1995 [DOI] [PubMed] [Google Scholar]
- 22. Eddahibi S, Raffestin B, Braquet P, Chabrier PE, Adnot S. Pulmonary vascular reactivity to endothelin-1 in normal and chronically pulmonary hypertensive rats. J Cardiovasc Pharmacol 17, Suppl 7: S358–S361, 1991 [DOI] [PubMed] [Google Scholar]
- 23. Egermayer P, Town GI, Peacock AJ. Role of serotonin in the pathogenesis of acute and chronic pulmonary hypertension. Thorax 54: 161–168, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Esteve J, Launay JM, Kellermann O, Maroteaux L. Functions of serotonin in hypoxic pulmonary vascular remodeling. Cell Biochem Biophysics 47: 33–43, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Fagan KA, Oka M, Bauer NR, Gebb SA, Ivy DD, Morris KG, McMurtry IF. Attenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of Rho-kinase. Am J Physiol Lung Cell Mol Physiol 287: L656–L664, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Fazal F, Bijli KM, Minhajuddin M, Rein T, Finkelstein JN, Rahman A. Essential role of cofilin-1 in regulating thrombin-induced RelA/p65 nuclear translocation and intercellular adhesion molecule 1 (ICAM-1) expression in endothelial cells. J Biol Chem 284: 21047–21056, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ferri C, Bellini C, De Angelis C, De Siati L, Perrone A, Properzi G, Santucci A. Circulating endothelin-1 concentrations in patients with chronic hypoxia. J Clin Pathol 48: 519–524, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fike CD, Slaughter JC, Kaplowitz MR, Zhang Y, Aschner JL. Reactive oxygen species from NADPH oxidase contribute to altered pulmonary vascular responses in piglets with chronic hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 295: L881–L888, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Filosa JA, Nelson MT, Gonzalez Bosc LV. Activity-dependent NFAT3 nuclear accumulation in pericytes from cortical parenchymal microvessel. Am J Physiol Cell Physiol 293: C1797–C1805, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Fujii T, Onohara N, Maruyama Y, Tanabe S, Kobayashi H, Fukutomi M, Nagamatsu Y, Nishihara N, Inoue R, Sumimoto H, Shibasaki F, Nagao T, Nishida M, Kurose H. Gα12/13-mediated Production of Reactive Oxygen Species Is Critical for Angiotensin Receptor-induced NFAT Activation in Cardiac Fibroblasts. J Biol Chem 280: 23041–23047, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Fujita H, Fukumoto Y, Saji K, Sugimura K, Demachi J, Nawata J, Shimokawa H. Acute vasodilator effects of inhaled fasudil, a specific Rho-kinase inhibitor, in patients with pulmonary arterial hypertension. Heart Vessels 25: 144–149, 2010 [DOI] [PubMed] [Google Scholar]
- 32. Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, Kimura S, Masaki T, Duguid WP, Stewart DJ. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med 328: 1732–1739, 1993 [DOI] [PubMed] [Google Scholar]
- 33. Gohla A, Schultz G, Offermanns S. Role for G(12)/G(13) in agonist-induced vascular smooth muscle cell contraction. Circ Res 87: 221–227, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Gomez MF, Gonzalez Bosc LV, Stevenson AS, Wilkerson MK, Hill-Eubanks DC, Nelson MT. Constitutively elevated nuclear export activity opposes Ca2+-dependent NFATc3 nuclear accumulation in vascular smooth muscle: role of JNK2 And Crm-1. J Biol Chem 278: 46847–46853, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Gomez MF, Stevenson AS, Bonev AD, Hill-Eubanks DC, Nelson MT. Opposing actions of inositol 1,4,5-trisphosphate and ryanodine receptors on nuclear factor of activated T-cells regulation in smooth muscle. J Biol Chem 277: 37756–37764, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Gonzalez Bosc LV, Wilkerson MK, Bradley KN, Eckman DM, Hill-Eubanks DC, Nelson MT. Intraluminal pressure is a stimulus for NFATc3 nuclear accumulation - Role of calcium, endothelium-derived nitric oxide, and cGMP-dependent protein kinase. J Biol Chem 279: 10702–10709, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Guignabert C, Izikki M, Tu LI, Li Z, Zadigue P, Barlier-Mur AM, Hanoun N, Rodman D, Hamon M, Adnot S, Eddahibi S. Transgenic mice overexpressing the 5-hydroxytryptamine transporter gene in smooth muscle develop pulmonary hypertension. Circ Res 98: 1323–1330, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Guilluy C, Sauzeau V, Rolli-Derkinderen M, Guerin P, Sagan C, Pacaud P, Loirand G. Inhibition of RhoA/Rho kinase pathway is involved in the beneficial effect of sildenafil on pulmonary hypertension. Br J Pharmacol 146: 1010–1018, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gunst SJ, Zhang W. Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am J Physiol Cell Physiol 295: C576–C587, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol 10: 31–54, 1994 [DOI] [PubMed] [Google Scholar]
- 41. Hill-Eubanks DC, Gomez MF, Stevenson AS, Nelson MT. NFAT regulation in smooth muscle. Trends Cardiovasc Med 13: 56–62, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Hirshman CA, Emala CW. Actin reorganization in airway smooth muscle cells involves Gq and Gi-2 activation of Rho. Am J Physiol Lung Cell Mol Physiol 277: L653–L661, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Hopkins N, McLoughlin P. The structural basis of pulmonary hypertension in chronic lung disease: remodelling, rarefaction or angiogenesis? J Anat 201: 335–348, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Iqbal J, Sanghia R, Das SK. Endothelin receptor antagonists: an overview of their synthesis and structure-activity relationship. Mini Rev Med Chem 5: 381–408, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Jernigan NL, Walker BR, Resta TC. Chronic hypoxia augments protein kinase G-mediated Ca2+ desensitization in pulmonary vascular smooth muscle through inhibition of RhoA/Rho kinase signaling. Am J Physiol Lung Cell Mol Physiol 287: L1220–L1229, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Jernigan NL, Walker BR, Resta TC. Reactive oxygen species mediate RhoA/Rho kinase-induced Ca2+ sensitization in pulmonary vascular smooth muscle following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 295: L515–L529, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kalivendi SV, Konorev EA, Cunningham S, Vanamala SK, Kaji EH, Joseph J, Kalyanaraman B. Doxorubicin activates nuclear factor of activated T-lymphocytes and Fas ligand transcription: role of mitochondrial reactive oxygen species and calcium. Biochem J 389: 527–539, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kawamura T, Ono K, Morimoto T, Akao M, Iwai-Kanai E, Wada H, Sowa N, Kita T, Hasegawa K. Endothelin-1-dependent nuclear factor of activated T lymphocyte signaling associates with transcriptional coactivator p300 in the activation of the B cell leukemia-2 promoter in cardiac myocytes. Circ Res 94: 1492–1499, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Ke Q, Li J, Ding J, Ding M, Wang L, Liu B, Costa M, Huang C. Essential role of ROS-mediated NFAT activation in TNF-α induction by crystalline silica exposure. Am J Physiol Lung Cell Mol Physiol 291: L257–L264, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Knappe D, Sill B, Tharun B, Koester R, Baldus S, Muenzel T, Meinertz T, Kahler J. Endothelin-1 in humans is increased by oxygen-derived radicals ex vivo and in vivo. J Investig Med 55: 306–314, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Landsberg JW, Yuan JXJ. Calcium and TRP channels in pulmonary vascular smooth muscle cell proliferation. News Physiol Sci 19: 44–50, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Li KX, Fouty B, McMurtry IF, Rodman DM. Enhanced ETA-receptor-mediated inhibition of Kv channels in hypoxic hypertensive rat pulmonary artery myocytes. Am J Physiol Heart Circ Physiol 277: H363–H370, 1999 [DOI] [PubMed] [Google Scholar]
- 53. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-[Delta][Delta]CT method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 54. Lund AK, Goens MB, Kanagy NL, Walker MK. Cardiac hypertrophy in Aryl hydrocarbon receptor (AhR) null mice is correlated with elevated angiotensin II, endothelin-1 and mean arterial blood pressure. Hypertension 193: 177–187, 2003 [DOI] [PubMed] [Google Scholar]
- 55. Miao L, Dai Y, Zhang J. Mechanism of RhoA/Rho kinase activation in endothelin-1- induced contraction in rabbit basilar artery. Am J Physiol Heart Circ Physiol 283: H983–H989, 2002 [DOI] [PubMed] [Google Scholar]
- 56. Nagaoka T, Fagan KA, Gebb SA, Morris KG, Suzuki T, Shimokawa H, McMurtry IF, Oka M. Inhaled Rho kinase inhibitors are potent and selective vasodilators in rat pulmonary hypertension. Am J Respir Crit Care Med 171: 494–499, 2005 [DOI] [PubMed] [Google Scholar]
- 57. Nagaoka T, Gebb SA, Karoor V, Homma N, Morris KG, McMurtry IF, Oka M. Involvement of RhoA/Rho kinase signaling in pulmonary hypertension of the fawn-hooded rat. J Appl Physiol 100: 996–1002, 2006 [DOI] [PubMed] [Google Scholar]
- 58. Naik JS, Earley S, Resta TC, Walker BR. Pressure-induced smooth muscle cell depolarization in pulmonary arteries from control and chronically hypoxic rats does not cause myogenic vasoconstriction. J Appl Physiol 98: 1119–1124, 2005 [DOI] [PubMed] [Google Scholar]
- 59. Negash S, Narasimhan SR, Zhou W, Liu J, Wei FL, Tian J, Raj JU. Role of cGMP-dependent protein kinase in regulation of pulmonary vascular smooth muscle cell adhesion and migration: effect of hypoxia. Am J Physiol Heart Circ Physiol 297: H304–H312, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nieves-Cintron M, Amberg GC, Nichols CB, Molkentin JD, Santana LF. Activation of NFATC3 down-regulates the beta 1 subunit of large conductance, calcium-activated K+ channels in arterial smooth muscle and contributes to hypertension. J Biol Chem 282: 3231–3240, 2006 [DOI] [PubMed] [Google Scholar]
- 61. Nilsson LM, Sun ZW, Nilsson J, Nordstrom I, Chen YW, Molkentin JD, Wide-Swensson D, Hellstrand P, Lydrup ML, Gomez MF. Novel blocker of NFAT activation inhibits IL-6 production in human myometrial arteries and reduces vascular smooth muscle cell proliferation. Am J Physiol Cell Physiol 292: C1167–C1178, 2007 [DOI] [PubMed] [Google Scholar]
- 62. Oka M, Fagan KA, Jones PL, McMurtry IF. Therapeutic potential of RhoA/Rho kinase inhibitors in pulmonary hypertension. Br J Pharmacol 155: 444–454, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Oukka M, Ho IC, de la Brousse FC, Hoey T, Grusby MJ, Glimcher LH. The transcription factor NFAT4 is involved in the generation and survival of T cells. Immunity 9: 295–304, 1998 [DOI] [PubMed] [Google Scholar]
- 64. Rabinovitch M, Gamble W, Nadas AS, Miettinen OS, Reid L. Rat pulmonary circulation after chronic hypoxia: hemodynamic and structural features. Am J Physiol Heart Circ Physiol 236: H818–H827, 1979 [DOI] [PubMed] [Google Scholar]
- 65. Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol 15: 707–747, 1997 [DOI] [PubMed] [Google Scholar]
- 66. Rattan S, Patel CA. Selectivity of ROCK inhibitors in the spontaneously tonic smooth muscle. Am J Physiol Gastrointest Liver Physiol 294: G687–G693, 2008 [DOI] [PubMed] [Google Scholar]
- 67. Rivas FV, O'Keefe JP, Alegre ML, Gajewski TF. Actin cytoskeleton regulates calcium dynamics and NFAT nuclear duration. Mol Cell Biol 24: 1628–1639, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shimoda LA, Sham JS, Sylvester JT. Altered pulmonary vasoreactivity in the chronically hypoxic lung. Physiol Res 49: 549–560, 2000 [PubMed] [Google Scholar]
- 69. Shimoda LA, Sylvester JT, Sham JS. Mobilization of intracellular Ca2+ by endothelin-1 in rat intrapulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 278: L157–L164, 2000 [DOI] [PubMed] [Google Scholar]
- 70. Shimoda LA, Sylvester JT, Sham JS. Inhibition of voltage-gated K+ current in rat intrapulmonary arterial myocytes by endothelin-1. Am J Physiol Lung Cell Mol Physiol 274: L842–L853, 1998 [DOI] [PubMed] [Google Scholar]
- 71. Stevenson AS, Gomez MF, Hill-Eubanks DC, Nelson MT. NFAT4 movement in native smooth muscle. A role for differential Ca(2+) signaling. J Biol Chem 276: 15018–15024, 2001 [DOI] [PubMed] [Google Scholar]
- 72. Strawbridge AB, Elmendorf JS. Phosphatidylinositol 4,5-bisphosphate reverses endothelin-1-induced insulin resistance via an actin-dependent mechanism. Diabetes 54: 1698–1705, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Suzuki E, Nishimatsu H, Satonaka H, Walsh K, Goto A, Omata M, Fujita T, Nagai R, Hirata Y. Angiotensin II induces myocyte enhancer factor 2- and calcineurin/nuclear factor of activated T Cell-dependent transcriptional activation in vascular myocytes. Circ Res 90: 1004–1011, 2002 [DOI] [PubMed] [Google Scholar]
- 74. Tang DD, Anfinogenova Y. Physiologic properties and regulation of the actin cytoskeleton in vascular smooth muscle. J Cardiovasc Pharmacol Therap 13: 130–140, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tjen AL, Ekman R, Osborn J, Keith I. Pulmonary vascular pressure effects by endothelin-1 in normoxia and chronic hypoxia: a longitudinal study. Am J Physiol Heart Circ Physiol 271: H2246–H2253, 1996 [DOI] [PubMed] [Google Scholar]
- 76. Vega Lde L, Munóz E, Calzado MA, Lieb K, Candelario-Jalil E, Gschaidmeir H, Färber L, Mueller W, Stratz T, Fiebich BL. The 5-HT3 receptor antagonist tropisetron inhibits T cell activation by targeting the calcineurin pathway. Biochem Pharmacol 70: 369–380, 2005 [DOI] [PubMed] [Google Scholar]
- 77. Vigorito E, Billadeu DD, Savoy D, McAdam S, Doody G, Fort P, Turner M. RhoG regulates gene expression and the actin cytoskeleton in lymphocytes. Oncogene 22: 330–342, 2003 [DOI] [PubMed] [Google Scholar]
- 78. Vindis C, D'Angelo R, Mucher E, NΦgre-Salvayre A, Parini A, Mialet-Perez J. Essential role of TRPC1 channels in cardiomyoblasts hypertrophy mediated by 5-HT2A serotonin receptors. Biochem Biophys Res Commun 391: 979–983, 2010 [DOI] [PubMed] [Google Scholar]
- 79. Weigand L, Sylvester JT, Shimoda LA. Mechanisms of endothelin-1-induced contraction in pulmonary arteries from chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 290: L284–L290, 2006 [DOI] [PubMed] [Google Scholar]
- 80. Yaghi A, Sims SM. Constrictor-induced translocation of NFAT3 in human and rat pulmonary artery smooth muscle. Am J Physiol Lung Cell Mol Physiol 289: L1061–L1074, 2005 [DOI] [PubMed] [Google Scholar]
- 81. Yang X, Chen W, Chen J. Change of level and expression of endothelin-1 in the lungs of rats with hypoxic pulmonary hypertension [published erratum appears in Chin Med J (Engl) 1997 Mar;110(3):186]. Chin Med J (Engl ) 110: 104–108, 1997 [PubMed] [Google Scholar]
- 82. Yang Y, Gao M, Guo Y, Qiao J. Calcium antagonists, diltiazem and nifedipine, protect broilers against low temperature-induced pulmonary hypertension and pulmonary vascular remodeling. Anim Sci J 81: 494–500, 2010 [DOI] [PubMed] [Google Scholar]
- 83. Yuyama H, Fujimori A, Sanagi M, Koakutsu A, Noguchi Y, Sudoh K, Sasamata M, Miyata K. A novel and selective endothelin ETA receptor antagonistYM598 prevents the development of chronic hypoxia-induced pulmonary hypertension in rats. Vasc Pharmacol 43: 40–46, 2005 [DOI] [PubMed] [Google Scholar]
- 84. Zhang N, Agbor LN, Scott JA, Zalobowski T, Elased KM, Trujillo A, Duke MS, Wolf V, Walsh MT, Born JL, Felton LA, Wang J, Wang W, Kanagy NL, Walker MK. An activated renin-angiotensin system maintains normal blood pressure in aryl hydrocarbon receptor heterozygous mice but not in null mice. Biochem Pharmacol 80: 197–204, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ziino AJA, Ivanovska J, Belcastro R, Kantores C, Xu EZ, Lau M, McNamara PJ, Tanswell AK, Jankov RP. Effects of Rho-kinase inhibition on pulmonary hypertension, lung growth, and structure in neonatal rats chronically exposed to hypoxia. Pediatr Res 67: 177–182, 2010 [DOI] [PubMed] [Google Scholar]