Abstract
The androgen receptor (AR) is critical in the normal development and function of the prostate, as well as in prostate carcinogenesis. Androgen deprivation therapy is the mainstay in the treatment of advanced prostate cancer, however, after an initial response, the disease inevitably progresses to castration-resistant prostate cancer (CRPC). Recent evidence suggests that continued AR activation, sometimes in a ligand-independent manner, is commonly associated with the development of CRPC. Thus, novel agents targeting the AR are urgently needed as a strategic step in developing new therapies for this disease state. In this study, we investigated the effect of berberine on AR signaling in prostate cancer. We report that berberine decreased the transcriptional activity of AR. Berberine did not affect AR mRNA expression, but induced AR protein degradation. Several ligand-binding domain truncated AR splice variants have been identified and these variants are believed to promote the development of CRPC in patients. Interestingly, we found that these variants were more susceptible to berberine-induced degradation than the full-length AR. Furthermore, the growth of LNCaP xenografts in nude mice was inhibited by berberine and AR expression was reduced in the tumors, whereas the morphology and AR expression in normal prostates were not affected. This report is the first to show that berberine suppresses AR signaling and suggests that berberine or its derivatives is a promising agent for the prevention and/or treatment of prostate cancer.
Keywords: berberine, androgen receptor, prostate cancer, xenograft
Introduction
Prostate cancer is the second most common cancer and a leading cause of cancer mortality in men in the United States (1). Androgen deprivation therapy (ADT), which aims to reduce the level of circulating androgens or to block the binding of androgens to their receptor, is a mainstay treatment for advanced prostate cancer. However, after a moderate and short-term response, the disease eventually progresses to castration-resistant prostate cancer (CRPC), which is a lethal and incurable. Therefore, preventing the transition to CRPC and treating CRPC effectively have become critical challenges for prostate cancer management.
The androgen receptor (AR), a member of the steroid nuclear receptor family, is activated upon binding to androgens. The AR signaling axis not only plays a crucial role in the normal growth of the prostate, but also promotes prostate cancer initiation and progression. Recent studies have shown that AR reactivation is the driving force of the development of CRPC. Several mechanisms have been proposed to explain the sustained AR activation in a low androgen environment. First, AR amplification and AR overexpression, which were seen in 20% tumors from CRPC patients by gene expression analysis and immunohistochemistry assay (2,3), could sensitize the receptor to low concentrations of androgens (4). Laboratory studies indicate that AR overexpression is necessary and sufficient to induce CRPC in a xenograft model (5). Second, AR mutations could alter its ligand-binding specificity and allow it to be activated by nonandrogen steroids or increase its binding affinity for androgens (5–8). Third, tyrosine kinases could activate AR directly by phosphorylation (9,10). Fourth, recent studies indicate that CRPC cells acquire the ability of intracellular synthesis of testosterone and dihydrotestosterone (DHT) from weak adrenal androgens (11) or from cholesterol (12–15). Recently, several AR alternative splicing variants that lack the ligand-binding domain (LBD) but retain the DNA-binding domain have been identified in both xenografts and human tissues (16–19). These variants, referred to as ARΔLBDs hereafter, have been shown to be transcriptionally active in the absence of androgens and drive androgen-independent growth of prostate cancer cells (17–19). Collectively, these studies provide new insights into the development of CRPC and underscore the importance of AR as a direct therapeutic target in all stages of prostate cancer.
Berberine (2,3-methylenedioxy-9,10-dimenthoxyprotoberberine chloride, BBR) is an isoquinoline alkaloid (Figure 1A) with a long history of use in traditional medicine. It can be isolated from several plants of the genera Berberis and Coptis, including goldenseal, Oregon grape, and barberry. Recent studies suggested that BBR has anticancer activities against several types of tumors (20,21), including prostate cancer (22,23). In this study, we tested the hypothesis that BBR suppresses the AR signaling pathway, which has not been reported previously.
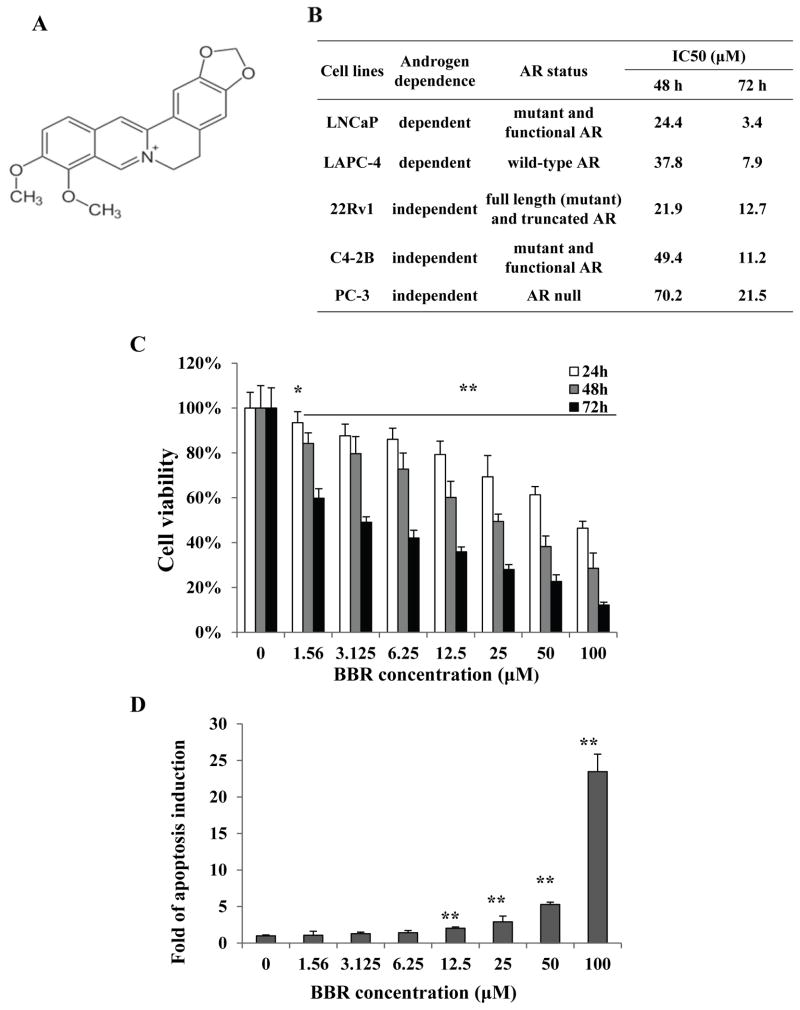
Figure 1.
Berberine inhibits prostate cancer cell growth and induces apoptosis. A, structure of berberine. B, characteristics of the cell lines and IC50 values of berberine. C, BBR inhibited the growth of LNCaP. Cells were treated with various concentrations of BBR for 24, 48, or 72 h. MTT assay was performed to determine cell viability. The viability of control cells were set at 100%. The data plotted are mean ± SD, n=8. D, BBR induced apoptosis in LNCaP. Apoptosis was quantitated by Cell Death ELISA in LNCaP cells treated with indicated concentrations of BBR for 24 h. The data plotted are mean± SD, n=3. *, P<0.05; **, P<0.01.
Materials and Methods
Reagents
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), sulforhodamine B (SRB), cycloheximide, 17-(Allylamino)-17-demethoxygeldanamycin (17-AAG), and cycloheximide were purchased from Sigma-Aldrich (St. Louis, MO). R1881 and dihydrotestosterone (DHT) were from Steraloids (Newport, RI), Lipofectamine™ and Plus™ from Invitrogen (Carlsbad, CA), MG132 from American Peptide Company (Vista, CA), and the pan-caspase inhibitor z-VAD-fmk from BD Pharmingen (San Jose, CA). Berberine (Purity > 99%) was acquired from Northeast Pharmaceutical Group (Shenyang, China) and from Sigma-Aldrich.
Cell culture and MTT assay
The LNCaP, 22Rv1, and PC-3 cell lines were obtained from American Type Culture Collection at Passage 4. The LAPC-4 cell line was provided by Dr. Charles L. Sawyers (24). C4-2B cells were provided by Dr. Shahriar Koochekpour at Louisiana State University Health Sciences Center at New Orleans. All cells used in this study were within 20 passages after receipt or resuscitation (~3 months of non-continuous culturing). The requirement of androgen for growth was tested intermittently and the expression of AR was tested by Western analysis during the study. LNCaP, LAPC-4, 22Rv1, C4-2B and PC-3 prostate cancer cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. In experiments required androgen stimulation, LNCaP and 22Rv1 cells were cultured in phenol red-free RPMI 1640 supplemented with 10% charcoal-stripped FBS (cs-FBS). MTT assay was performed in 96-well plates in octuplicate. Cells were seeded at a density of 3×103 cells/well overnight, and treated with BBR for 24, 48, or 72 h. IC50 values of BBR were calculated using the software Origin 6.0.
Apoptosis assay
LNCaP cells were plated onto 96-well plates at a density of 6×103 cells/well in RPMI 1640 with 10% FBS overnight, and treated with BBR for 24 h. Apoptosis was detected by the Cell Death Detection ELISA kit (Roche). Cell number was determined in parallel by SRB assay and was used to normalize the apoptosis result. The data were expressed as fold of apoptosis induction over control.
Transient transfection and reporter gene assay
LNCaP and 22Rv1 cells were seeded in 10-cm dishes at a density to become 80–90% confluent 24 h later. Transient transfection was performed by using the Lipofectamine and Plus reagents following the manufacturer’s instructions. Cells were transfected with 4 μg of the ARR3 plasmid (single luciferase assay) or co-transfected with 4 μg ARR3 plasmid and 200 ng pRL-TK plasmid (dual luciferase assay). After incubating with the transfection mixture for 4 h, cells were trypsinized and re-plated in triplicate onto 24-well plates at a density of 6×104 cells/well in RPMI 1640 containing 10% cs-FBS. Cells were allowed to recover overnight before treated with 0, 25, 50, or 100 μM BBR in the presence or absence of 1 nM DHT. For single luciferase assay, LNCaP cells were lysed with 1X Reporter Lysis Buffer (Promega) at 6 or 24 h post-treatment and luciferase activity was assayed by using the Luciferase Assay System (Promega). Protein concentration was determined by using the BCA Protein Assay kit (Pierce). The luciferase activity was normalized by the protein concentration of the same sample. Dual-luciferase assay was conducted when there was a need to compare results from different cell lines. The assay performed at 24 h post treatment using the Dual-luciferase Reporter Assay System (Promega). The Renilla luciferase activity was used to normalize that of firefly luciferase.
Quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
qRT-PCR was performed as described previously (25). cDNA was synthesized from 1.5 μg of total RNA by the SuperScript III reverse transcriptase (Invitrogen). The mRNAs for AR, prostate specific antigen (PSA), and 24-dehydrocholesterol reductase (DHCR24) were analyzed by the relative gene expression protocol, and β-actin was used as the internal control.
Western blotting and immunoprecipitation
Cells were washed with ice-cold phosphate-buffered saline (PBS) and lysed with 2X Cell Lysis Buffer (Cell Signaling) containing a phosphatase inhibitor and the protease inhibitor cocktail (Sigma) by incubating on ice for 30 min. Lysates were collected by centrifugation and protein concentrations were determined by the BCA method. For immunoprecipitation, cells were lysed with the lysis buffer (50 mM Tris-HCl, pH 8.0, 125 mM NaCl, 5 mM EDTA, 0.5% NP-40) and 1.2 mg total protein were incubated with the anti-HSP90α/β antibody or the mouse IgG (negative control) overnight at 4°C. Protein-G agarose (Invitrogen) was added and the mixture was incubated for 1 h at 4°C. The immunocomplex was precipitated by a magnetic rack, washed four times with the lysis buffer, and resuspended in the SDS-loading buffer. The samples were separated on 10% SDS-polyacrylamide gels and transferred onto polyvinylidene fluoride membranes. After blocking in TBS buffer (150 mM NaCl, 10 mM Tris, pH 7.4) containing 5% nonfat milk, the blots were incubated with a primary antibody overnight at 4°C and a fluorescent-labeled secondary antibody for 1 h at room temperature. The fluorescent signals were obtained by the Odyssey Infrared Imaging System (LI-COR Bioscience). The primary antibodies used were as follows: anti-AR (Millipore), anti-PSA, anti-HSP90α/β (Santa Cruz), anti-DHCR24 (Cell Signaling), and anti-GAPDH (Chemicon).
Immunocytofluorescence imaging
LNCaP cells were cultured on poly-D-lysine-coated cover slides in phenol red-free RPMI 1640 plus 10% cs-FBS overnight and treated with BBR for 2 h. R1881, a synthetic androgen, was added to a final concentration of 1 nM. After 2.5 h, cells were fixed, permeabilized, and incubated with an anti-AR antibody and a fluorescein isothiocynate (FITC)-conjugated secondary antibody. Nuclei were counterstained with 4′, 6-diamidino-2-phenylindole (DAPI). Images were captured at 63× magnification using a Leica TCS SP2 confocal microscope.
In vivo tumor growth in a xenograft model
LNCaP cells (4×106) were collected in 70 μL PBS and mixed with 70 μL Matrigel Matrix (BD Biosciences). The mixture was injected subcutaneously to one side of the dorsal flank of 6- to 7-week old male Nu/Nu mice (NCI, Frederick, MD). When tumor volume reached 100 mm3, mice were randomized into two groups (n=6) and treated with vehicle (DMSO, 1 mL/kg) or BBR (5 mg/kg, dissolved in DMSO), respectively, by daily i.p. injection. Tumor volumes were measured every three days and calculated using the formula: r1×r22×0.52, where r1>r2. Mice were sacrificed 15 days after treatment.
Immunohistochemistry staining
Tumor tissues were fixed in 10% buffered formalin and embedded in paraffin. The sections were de-waxed and rehydrated in graded ethanol. Antigen retrieval was performed by soaking the sections in 10 mM sodium citrate buffer (pH 6.0) and heating in a high-power microwave oven for 20 min. The sections were incubated with a mouse anti-AR monoclonal antibody (BioGenex, San Ramon, CA) for 30 min at room temperature. After washing, the secondary antibody was added and incubated for 30 min. Reactive products were visualized by staining with 3,3′-diaminobenzidene (DAB) and counterstained with hematoxylin for 30 seconds. Images covering the entire slide were captured with the same optical parameters. All images, excluding areas near the edges or with necrosis, were analyzed using the ImageJ software (National Insitute of Health). Cells with strong, medium, weak, or negative staining for AR were counted and the percentage of cells in each category was calculated.
Statistical analysis
All results were presented as mean±standard deviation (SD) unless otherwise stated. Statistical significance was determined with Student’s t- test (two-tailed). P<0.05 was considered statistically significant.
Results
BBR inhibits cell growth and induces apoptosis in prostate cancer
We first used the MTT assay to investigate the effect of BBR on cell viability in several prostate cancer lines, including LNCaP, LAPC-4, 22Rv1, C4-2B, and PC-3. The AR-expression and androgen-dependence status of these lines are described in Fig. 1B. Cells were treated with increasing concentrations of BBR (from 1.56 to 100 μM in 2-fold increments) for 24, 48, or 72 h. The MTT results show that BBR reduced the viability of all cell lines tested in a dose- and time-dependent manner (Fig. 1C for LNCaP, remaining data not shown). The IC50 values indicate that AR-positive cell lines are more sensitive to BBR than the AR-negative, androgen-independent PC-3 cells. These results suggest BBR may exert its growth inhibitory effect by disrupting the AR signaling pathway.
We also performed apoptosis assay in LNCaP cells following a 24 h BBR treatment. As shown in Fig. 1D, a dose-dependent induction of apoptosis was detected, starting at 12.5 μM. This result confirms previous reports showing that BBR is a potent inducer of apoptosis in prostate cancer cells (23).
BBR inhibits ligand-dependent and -independent AR transactivation
Clued by the MTT results, we set out to examine the influence of BBR on the transcriptional activity of AR. LNCaP cells were transiently transfected with an androgen response element (ARE)-luciferase reporter construct and cells were cultured in the presence or absence of androgen. The basal activity of AR was very low in LNCaP without androgen stimulation, but the activity was induced significantly (>120 fold) by adding 1 nM DHT (Fig. 2A). After 6 h of treatment, BBR inhibited androgen-stimulated AR transactivation in a dose-dependent manner. AR activity was further induced by DHT at 24 h, and BBR inhibited AR activity in a similar manner (data not shown). To assess effect of BBR on ligand-independent AR activity, 22Rv1 and LNCaP cells were transfected with the reporter construct and cultured in an androgen-depleted condition and treated with BBR for 24 h. Consistent with being an androgen-independent cell line, the basal activity of AR was 10 times higher in 22Rv1 than that in LNCaP (Fig. 2B). BBR suppressed AR activity by more than 90% in 22Rv1 cells, suggesting that BBR suppresses the constitutive AR activity.
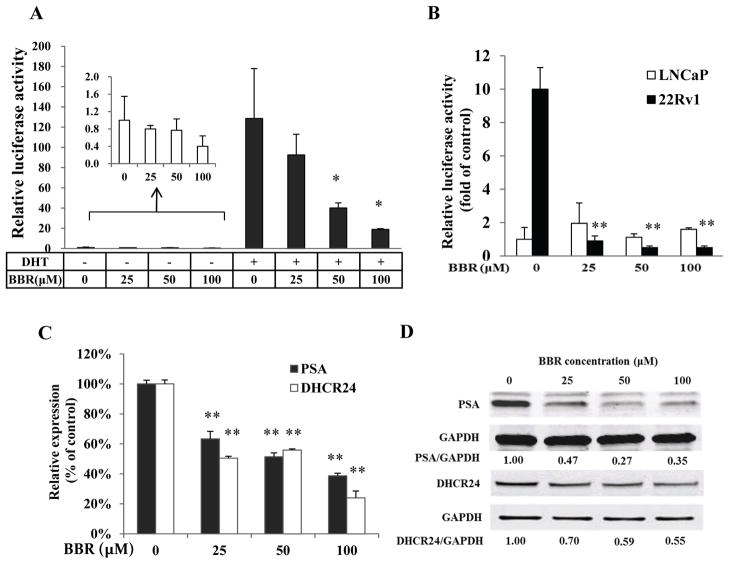
Figure 2.
BBR inhibits the transcriptional activity of AR. A, BBR inhibited the ligand-dependent AR transactivation. LNCaP cells were transfected with the pARR3-luc construct in bulk and divided into equal aliquots in medium containing 10% cs-FBS. Cells were stimulated with 1 nM DHT and treated with 25, 50, and 100 μM BBR for 6 h. The luciferase activity was normalized by total protein concentration. B, BBR inhibited the ligand-independent AR transactivation. LNCaP and 22Rv1 cells were co-transfected with the pARR3-luciferase and pRL-TK constructs. Cells were cultured in medium containing 10% cs-FBS and treated with BBR for 24 h. Dual-luciferase assay was performed and the firefly luciferase activity was normalized by the renilla luciferase activity. C&D, BBR down-regulated the expression of AR-regulated genes. LNCaP cells were cultured in medium containing 10% FBS and the expression of PSA and DHCR24 was analyzed by qRT-PCR (C) and Western blotting (D) following a 24 h treatment. *, P<0.05; **, P<0.01.
In addition to the ARE-luciferase assay, we analyzed the influence of BBR on the expression of AR-regulated genes. PSA is a well characterized target gene of AR. DHCR24 has been recently identified as an AR-regulated gene (25,26). Figs. 2C and 2D showed that at the 24 h time point, the mRNA and protein levels of both genes were reduced by BBR in LNCaP. This is consistent with the results from the ARE-luciferase assay. BBR also decreased PSA expression in C4-2B cells (Supplementary Fig. S1).
BBR down-regulates AR protein expression
To investigate how BBR exerts its inhibitory effect on AR transactivation, we investigated whether BBR modulated the expression of AR. The qRT-PCR results showed that the AR mRNA level was not decreased by BBR after a 24 h treatment (Fig. 3A). Instead, AR transcript was increased by the 25 and 50 μM concentrations. In contrast, AR protein was down-regulated by BBR in a concentration- and time-dependent manner (Fig. 3B). A reduction of AR protein was first detected after 16 h of treatment with 100 μM BBR. After 24 h, all three concentrations of BBR decreased AR protein. These results indicate that BBR exerts inhibitory effect on AR expression through mechanisms beyond the mRNA level.
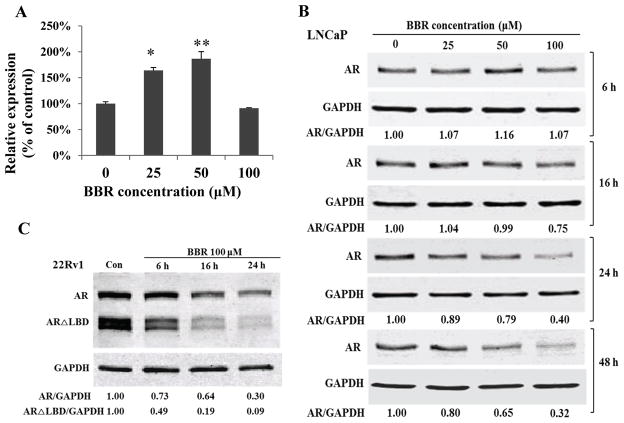
Figure 3.
BBR down-regulates AR protein expression. Cells were cultured in RPMI 1640 containing 10% FBS. A, qRT-PCR analysis of AR mRNA in LNCaP following BBR treatment for 24 h. *, P<0.05; **, P<0.01. B&C, AR immunoblotting in LNCaP cells (B) and 22Rv1 cells (C) treated with 100 μM BBR.
In addition to LNCaP, we found BBR decreased AR expression in other AR-positive lines, including 22Rv1 (Fig. 3C), LAPC-4, and C4-2B (Supplementary Fig. S2). This demonstrates that BBR down-regulation of AR protein is universal in prostate cancer cells. It is noteworthy that we detected a high abundance of AR isoforms in the range of 75~80 KDa in 22RV1 cells. These AR isoforms have been shown to be alternative splicing products and lack the LBD (16–19). These variants have been found to be enriched in CRPCs (27) and strongly implicated in promoting the progression to CRPC (17,19). Interestingly, we found that these ARΔLBDs were more susceptible to BBR than the full-length AR in 22Rv1 cells (Fig. 3C).
BBR induces AR degradation
The above data led us to speculate that BBR may affect AR protein levels by reducing the half-life of AR. To test this possibility, LNCaP cells were pre-treated with 50 μg/ml cycloheximide for 30 min to stop protein synthesis and then treated with 100 μM BBR. Cells were lysed at different intervals for Western blotting. Normalized AR protein levels were analyzed by linear regression to determine the half-life. As shown in Fig. 4A, AR half-life was 19.2 h in control cells comparing to 10.8 h in cells treated with BBR, suggesting that BBR induces AR protein degradation.
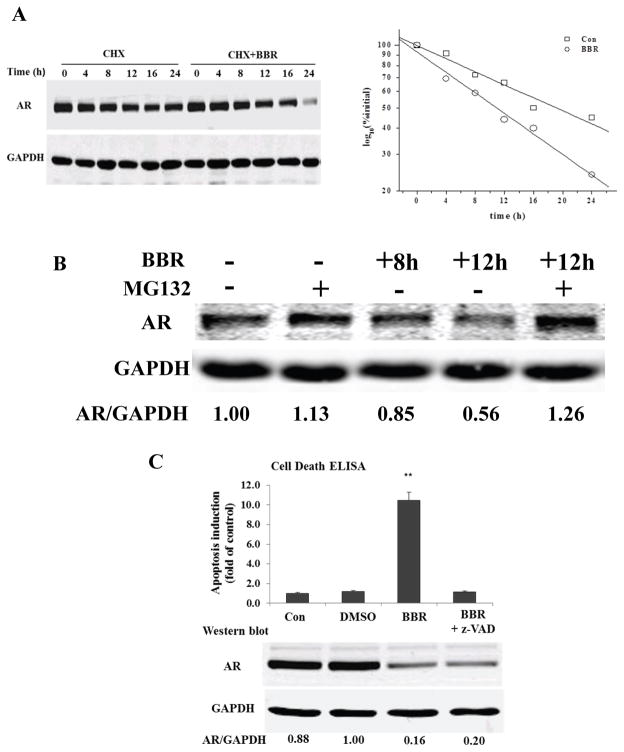
Figure 4.
BBR induces AR protein degradation. A, LNCaP was treated with 50 μg/ml cycloheximide for 30 min before 100 μM BBR was added. AR protein was analyzed by immunoblotti and densitometry. Normalized AR intensities (by GAPDH) were analyzed by linear regression to determine AR half-life. B, effect of MG132 on berberine-induced AR degradation. LNCaP was treated with 100 μM BBR for 8 h before MG-132 was added for another 4 h. C, apoptosis assay and AR protein expression after pre-treatment with z-VAD-fmk (pan-caspase inhibitor) for 2 h, and berberine for 24 h. **, P<0.01.
It has been shown that AR could be degraded by two pathways, one is mediated by the proteasome (28) and the other by caspase-3 (29). To test the involvement of the proteasome pathway, LNCaP cells were treated with 100 μM BBR for 8 h before the proteasome inhibitor MG132 was added. Consistent with a previous report (30), MG132 increased AR level in the absence of BBR (Fig. 4B, lane 2). In cells treated with BBR, the presence of MG132 prevented further AR degradation (Fig. 4B, lanes 4 and 5).
To determine the involvement of a caspase-mediated pathway, we pre-treated LNCaP cells with a pan-caspase inhibitor for 2 h. Cells were then treated with BBR for 24 h before lysed for apoptosis assay and Western blotting. As shown in Fig. 4C, treatment with the inhibitor totally blocked apoptosis induction by BBR, suggesting that apoptosis induced by BBR is caspase-dependent. AR protein was reduced by BBR to similar extents in the presence or absence of the inhibitor, indicating that BBR-induced AR degradation was not mediated by a caspase-dependent pathway. This also suggests that AR degradation is a direct effect of BBR, rather than a bystander effect of apoptosis. Taken together, these results suggest that BBR-induced AR protein degradation is mediated predominantly through the proteasome pathway.
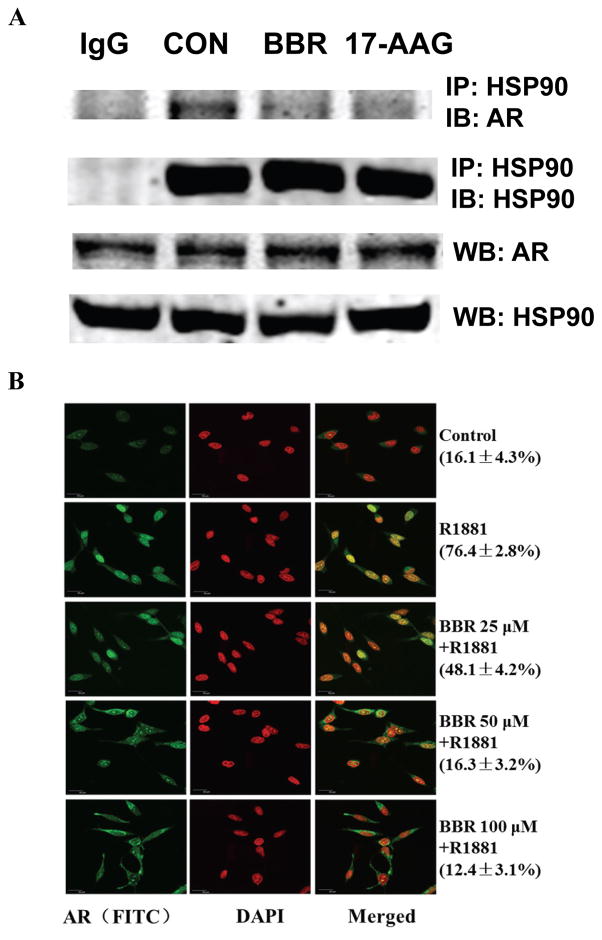
BBR disrupts AR-Hsp90 interaction
Similar to other steroid nuclear receptors, unliganded AR resides in the cytoplasm and interacts with heat shock protein 90 (Hsp90), a major chaperone protein in the cytoplasm (31). This interaction stabilizes AR (32) and maintains the proper conformation of AR for high-affinity ligand binding (33). Upon ligand stimulation, AR undergoes conformational changes which lead to its dissociation with Hsp90, and subsequently translocates to the nucleus to function as a transcription factor. Disruption of the AR-Hsp90 interaction will not only render AR susceptible to protein degradation (32,34), but also interfere with nuclear translocation (35,36). We next examined the interaction between AR and Hsp90 by co-immunoprecipitation. LNCaP was cultured in a low androgen condition for 48 h, and then treated with 100 μM BBR for 2 h. Whole-cell lysates were immunoprecipitated by an anti-Hsp90 antibody. Fig. 5A showed that the AR-Hsp90 association was significantly disrupted by BBR. Western blotting using input cell lysates showed that neither AR nor Hsp90 level was decreased by BBR under this condition.
Figure 5.
BBR disrupts the AR/Hsp90 interaction and inhibits AR nuclear translocation. A, LNCaP cells were cultured in medium containing with 5% cs-FBS for 48 h. Co-immunoprecipitation was performed with an anti-Hsp90α/β antibody following treatment with 100 μM BBR or 500 nM 17-AAG for 2 h. Western analysis was performed with the anti-Hsp90 and anti-AR antibodies. B, LNCaP cells were cultured in medium containing 10% cs-FBS overnight and treated with BBR for 2h. Immunocytofluorescence analysis for AR was performed following stimulation with 1nM R1881 for another 2.5 h. Numbers in the parentheses indicate percentage ± SD of cells displayed greater AR staining in the nucleus than in the cytoplasm.
BBR inhibits AR nuclear translocation
The effect of BBR on AR subcellular distributed was examined by immunocytofluorescence staining. As shown in Fig. 5B, in the absence of androgen, AR staining was predominantly cytoplasmic. Following stimulation with R1881, the nuclear staining of AR was increased dramatically. AR staining in the nucleus was reduced in a dose-dependent manner in cells pre-treated by BBR, accompanied by increased staining in the peri-nuclear cytoplasm. These results suggest that BBR inhibits AR nuclear translocation and provide an explanation to our observation that BBR inhibited AR activity before AR protein level was reduced.
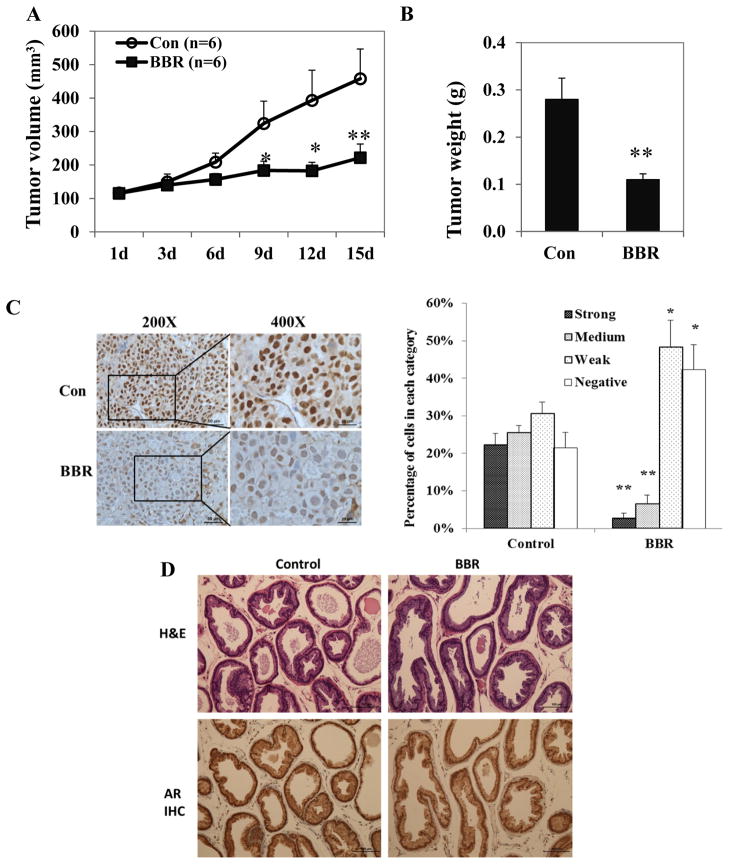
BBR inhibits tumor growth and suppresses AR in vivo
To test the in vivo efficacy of BBR, we first established LNCaP xenografts in nude mice. BBR treatment started when tumors reach 100 mm3 at a dose of 5 mg/kg/day. Fig. 6A shows that treatment with BBR significantly inhibited the growth of the tumor xeongrafts, and this result was confirmed by the final tumor weights (Fig. 6B). No toxicity was observed in mice treated with BBR, as body weight changed similarly in both groups (Supplementary Fig. S3). The expression of AR in tumors was analyzed by immunohistochemical staining of formalin-fixed tissues. AR staining was found predominantly in the nucleus, both in the control and treatment samples (Fig. 6C). A decrease in AR staining intensity was visually apparent in samples from the treatment group. Quantitative analysis showed that BBR reduced the percentages of cells with strong or medium staining of AR, and increased the percentages of cells stained weakly or negatively for AR. These results confirmed our cell culture data and showed BBR is effective in suppressing AR in vivo. Furthermore, the growth inhibitory and AR-downregulating effects of BBR seems to be limited to the tumors, as BBR treatment does not affect the histology or AR expression in normal prostate glands of the mice (Fig. 6D).
Figure 6.
BBR suppresses AR expression in vivo. LNCaP xenografts were established in nude mice and BBR was given at a dose of 5 mg/kg when tumor size reached 100 mm3. Control mice received the DMSO. A, serial measurements of tumor volume. B, final tumor weight. C, left panel, immunohistochemistry of representative sections of LNCaP xenografts stained for AR. Right panel, quantitation of the IHC results. D, H&E and AR staining in fixed mouse dorsalateral prostates. High-resolution images for AR staining are available in Supplementary Figure S6. All data presented are mean ± standard error of mean (SEM). *, P<0.05; **, P<0.01.
Discussion
The development of CRPC following androgen deprivation is the most critical challenge in the clinical management of prostate cancer. Despite the recent development of new therapeutic options, treatment of patients with CRPC remains a significant clinical challenge. For example, sipuleucel-T (PROVENGE), the first therapeutic cancer vaccine approved by the Food and Drug Administration, has been shown by clinical trials to prolong the median survival of patients with metastatic CRPC by 4.1 months (37). Abiraterone, an inhibitor of the rate-limiting enzyme CYP17 in androgen biosynthesis, has shown promises in clinical trials involving patients with metastatic CRPC (38,39). However, its efficacy against CRPC driven by the newly discovered AR splice variants has not been established. The lack of LBD could render these AR variants resistant to current androgen blockade strategies. Therefore, suppressing the expression of all forms of AR appears to be a provocative and plausible strategy against CRPC.
Bioactive natural products provide a rich source for pharmacological discovery. Berberine has been shown to induce apoptosis in prostate cancer cells by inducing reactive oxygen species (ROS) and oxidative stress (40). Our discovery that BBR induces AR degradation appears to be a novel mechanism that is independent of these actions of BBR. First, as shown in Fig. 4C, treatment with a pan-caspase inhibitor blocked apoptosis induction by BBR but had no impact on AR degradation, suggesting that AR degradation is not a result of destruction of cellular proteins during apoptosis. Second, AR degradation by BBR was not affected by treatment with a ROS scavenger, N-acetyl-L-cysteine (Supplementary Fig. S4). In LNCaP cells, it has been shown that BBR induced apoptosis by activating p53 (22). Therefore, at least in LNCaP cells, BBR seems to modulate two important signaling pathways in prostate cancer in a highly coordinated manner, i.e. suppressing the pro-survival AR pathway and inducing the pro-apoptotic p53 pathway.
Our observation that BBR inhibited AR transactivation before the decrease in AR protein could be explained by the disruption of AR-Hsp90 interaction and reduction of AR nuclear translocation by BBR. These events were observed as early as 2 h and 4 h after treatment, respectively. The reduced AR-Hsp90 interaction is likely a result of decreased chaperone activity of Hsp90, as the level of Hsp90 protein was not affected by BBR at this time point. The activity of Hsp90 is regulated by the acetylation status, which in turn is regulated by histone deacetylase 6 (HDAC6). HDAC6 deacetylates the lysine residues of Hsp90 and plays a critical role in maintaining the chaperone function of Hsp90 (41). HDAC6 inhibition inactivates Hsp90 by interfering with ATP binding and association with client proteins (42). The molecular effects of BBR we have observed, including down-regulation of the expression, transcriptional activity and nuclear localization of AR, and disruption of the AR/Hsp90 interaction, are similar to those achieved by HDAC6 inhibit (41,43). Therefore, it is possible that BBR exerts its inhibitory action on AR through affecting the HDAC6/Hsp90 pathway.
The client proteins of Hsp90 include steroid hormone receptors, including the estrogen receptor (ER), glucocorticoid receptor, and progesterone receptor. The possibility that BBR inhibits Hsp90 activity implies that BBR could have similar effects on these receptors. Indeed, we found that BBR reduced the protein level of ERα in MCF-7 breast cancer cells (Supplementary Fig. S5). Consistent with our finding, it has been shown that BBR enhances the anticancer efficacies of ER antagonists in ER-positive MCF-7 cells, but not in ER-negative MDA-MB-231 cells (44). The effect of BBR on the ER signaling pathway warrants further investigation.
Consistent with a previous report (22), our animal study demonstrated that BBR is very effective in inhibiting the growth of prostate cancer in a xenograft model. The 5 mg/kg dose used in this experiment is well below the LD50 of BBR via the same route of injection, which is about 50 mg/kg (45). It is important to point out that despite that this dose was expected to achieve a blood concentration of BBR 2 orders of magnitude lower than the doses used in the cell culture experiments, our in vitro findings that BBR reduced AR protein and the expression of AR-regulated genes were confirmed by the animal experiment. Similar blood concentration of BBR can be achieved in human taking oral doses of BBR (46). Therefore, the findings described in this report are potentially clinically relevant.
In 22Rv1 cells, the basal activity of AR is 10 times of that in LNCaP cells (Fig. 2B). Since 22Rv1 cells express high levels of ARΔLBDs (Fig. 3C), this ligand-independent AR transactivation is presumably driven by the splice variants. The data in Fig. 2B show that BBR is particularly effective in shutting down the ligand-independent AR activity in 22Rv1 cells, which is further supported by the data in Fig. 3C that BBR decreased the expression of ARΔLBDs more effectively than the full-length AR. In addition to BBR, several other natural or synthetic compounds have been shown to have similar inhibitory effects on AR slice variants (46–49). The discovery of these compounds should generate excitement since there is no drug currently in clinical trials to target castration-resistant prostate cancer progression driven by the AR splice variants. Our study suggests BBR should be further tested and developed in the treatment of CRPC.
In summary, this work is the first report that BBR suppresses the AR signaling pathway in prostate cancer. BBR decreased AR transcriptional activity and reduced the expression of AR-regulated genes in both androgen-dependent and CRPC cells. AR protein was degraded by BBR, which was predominantly mediated by a proteasome pathway. Moreover, the disruption of AR-Hsp90 interaction and the inhibition of AR nuclear translocation by BBR could contribute to the depressed AR transcriptional activity and AR protein degradation. Interestingly, BBR induced degradation of not only the full-length AR, but also the ARΔLBDs. The animal data showed the BBR exerted the growth inhibitory and AR downregulating effects specifically in the tumor tissues. Our study identifies a novel mechanism for the anticancer effect of BBR and provides support for BBR as a potential preventive and therapeutic agent in prostate cancer, especially for AR-mediated CRPC growth.
Supplementary Material
Acknowledgments
Grant Support: This work was supported by start-up funds from the Louisiana Cancer Research Consortium, a Tulane Cancer Center Matching Fund, a Tulane University School of Medicine Pilot Fund, a Jilin Provincial Scholarship from Jilin, China (H. Zhang), ACS grant No. RSG-07-218-01-TBE, NIH grant No. K01CA114252 (Y. Dong).
We would like to extend our sincere gratitude to Dr. Charles L. Sawyers at the UCLA Jonsson Comprehensive Cancer Center for LAPC-4 cells, to Dr. Shahriar Koochekpour at Louisiana State University Health Sciences Center for providing the C4-2B cells, to Dr. Guoyong Wang at Department of Structural and Cellular Biology, Tulane University School of Medicine for assistance with confocal microscopy, and to Dr. Sudesh Srivastav at Department of Biostatistics, Tulane University School of Public Health and Tropical Medicine for help with statistical analysis.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Holzbeierlein J, Lal P, LaTulippe E, Smith A, Satagopan J, Zhang L, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164:217–27. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linja MJ, Savinainen KJ, Saramaki OR, Tammela TL, Vessella RL, Visakorpi T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001;61:3550–5. [PubMed] [Google Scholar]
- 4.Waltering KK, Helenius MA, Sahu B, Manni V, Linja MJ, Janne OA, et al. Increased expression of androgen receptor sensitizes prostate cancer cells to low levels of androgens. Cancer Res. 2009;69:8141–9. doi: 10.1158/0008-5472.CAN-09-0919. [DOI] [PubMed] [Google Scholar]
- 5.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 6.Taplin ME, Bubley GJ, Shuster TD, Frantz ME, Spooner AE, Ogata GK, et al. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med. 1995;332:1393–8. doi: 10.1056/NEJM199505253322101. [DOI] [PubMed] [Google Scholar]
- 7.Taplin ME, Bubley GJ, Ko YJ, Small EJ, Upton M, Rajeshkumar B, et al. Selection for androgen receptor mutations in prostate cancers treated with androgen antagonist. Cancer Res. 1999;59:2511–5. [PubMed] [Google Scholar]
- 8.Zhao XY, Malloy PJ, Krishnan AV, Swami S, Navone NM, Peehl DM, et al. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat Med. 2000;6:703–6. doi: 10.1038/76287. [DOI] [PubMed] [Google Scholar]
- 9.Guo Z, Dai B, Jiang T, Xu K, Xie Y, Kim O, et al. Regulation of androgen receptor activity by tyrosine phosphorylation. Cancer Cell. 2006;10:309–19. doi: 10.1016/j.ccr.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 10.Kraus S, Gioeli D, Vomastek T, Gordon V, Weber MJ. Receptor for activated C kinase 1 (RACK1) and Src regulate the tyrosine phosphorylation and function of the androgen receptor. Cancer Res. 2006;66:11047–54. doi: 10.1158/0008-5472.CAN-06-0596. [DOI] [PubMed] [Google Scholar]
- 11.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–25. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 12.Dillard PR, Lin MF, Khan SA. Androgen-independent prostate cancer cells acquire the complete steroidogenic potential of synthesizing testosterone from cholesterol. Mol Cell Endocrinol. 2008;295:115–20. doi: 10.1016/j.mce.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leon CG, Locke JA, Adomat HH, Etinger SL, Twiddy AL, Neumann RD, et al. Alterations in cholesterol regulation contribute to the production of intratumoral androgens during progression to castration-resistant prostate cancer in a mouse xenograft model. Prostate. 2010;70:390–400. doi: 10.1002/pros.21072. [DOI] [PubMed] [Google Scholar]
- 14.Locke JA, Guns ES, Lubik AA, Adomat HH, Hendy SC, Wood CA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–15. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 15.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–54. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68:5469–77. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69:2305–13. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69:16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun S, Sprenger CC, Vessella RL, Haugk K, Soriano K, Mostaghel EA, et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010;120:2715–30. doi: 10.1172/JCI41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu WH, Hsieh YS, Kuo HC, Teng CY, Huang HI, Wang CJ, et al. Berberine induces apoptosis in SW620 human colonic carcinoma cells through generation of reactive oxygen species and activation of JNK/p38 MAPK and FasL. Arch Toxicol. 2007;81:719–28. doi: 10.1007/s00204-006-0169-y. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z, Liu Q, Xu B, Wu J, Guo C, Zhu F, et al. Berberine induces p53-dependent cell cycle arrest and apoptosis of human osteosarcoma cells by inflicting DNA damage. Mutat Res. 2009;662:75–83. doi: 10.1016/j.mrfmmm.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Choi MS, Oh JH, Kim SM, Jung HY, Yoo HS, Lee YM, et al. Berberine inhibits p53-dependent cell growth through induction of apoptosis of prostate cancer cells. Int J Oncol. 2009;34:1221–30. [PubMed] [Google Scholar]
- 23.Mantena SK, Sharma SD, Katiyar SK. Berberine, a natural product, induces G1-phase cell cycle arrest and caspase-3-dependent apoptosis in human prostate carcinoma cells. Mol Cancer Ther. 2006;5:296–308. doi: 10.1158/1535-7163.MCT-05-0448. [DOI] [PubMed] [Google Scholar]
- 24.Klein KA, Reiter RE, Redula J, Moradi H, Zhu XL, Brothman AR, et al. Progression of metastatic human prostate cancer to androgen independence in immunodeficient SCID mice. Nat Med. 1997;3:402–8. doi: 10.1038/nm0497-402. [DOI] [PubMed] [Google Scholar]
- 25.Dong Y, Zhang H, Gao AC, Marshall JR, Ip C. Androgen receptor signaling intensity is a key factor in determining the sensitivity of prostate cancer cells to selenium inhibition of growth and cancer-specific biomarkers. Mol Cancer Ther. 2005;4:1047–55. doi: 10.1158/1535-7163.MCT-05-0124. [DOI] [PubMed] [Google Scholar]
- 26.Bonaccorsi L, Luciani P, Nesi G, Mannucci E, Deledda C, Dichiara F, et al. Androgen receptor regulation of the seladin-1/DHCR24 gene: altered expression in prostate cancer. Lab Invest. 2008;88:1049–56. doi: 10.1038/labinvest.2008.80. [DOI] [PubMed] [Google Scholar]
- 27.Tepper CG, Boucher DL, Ryan PE, Ma AH, Xia L, Lee LF, et al. Characterization of a novel androgen receptor mutation in a relapsed CWR22 prostate cancer xenograft and cell line. Cancer Res. 2002;62:6606–14. [PubMed] [Google Scholar]
- 28.Sheflin L, Keegan B, Zhang W, Spaulding SW. Inhibiting proteasomes in human HepG2 and LNCaP cells increases endogenous androgen receptor levels. Biochem Biophys Res Commun. 2000;276:144–50. doi: 10.1006/bbrc.2000.3424. [DOI] [PubMed] [Google Scholar]
- 29.Lin HK, Hu YC, Lee DK, Chang C. Regulation of androgen receptor signaling by PTEN (phosphatase and tensin homolog deleted on chromosome 10) tumor suppressor through distinct mechanisms in prostate cancer cells. Mol Endocrinol. 2004;18:2409–23. doi: 10.1210/me.2004-0117. [DOI] [PubMed] [Google Scholar]
- 30.Deep G, Oberlies NH, Kroll DJ, Agarwal R. Isosilybin B causes androgen receptor degradation in human prostate carcinoma cells via PI3K-Akt-Mdm2-mediated pathway. Oncogene. 2008;27:3986–98. doi: 10.1038/onc.2008.45. [DOI] [PubMed] [Google Scholar]
- 31.Pratt WB, Galigniana MD, Morishima Y, Murphy PJ. Role of molecular chaperones in steroid receptor action. Essays Biochem. 2004;40:41–58. doi: 10.1042/bse0400041. [DOI] [PubMed] [Google Scholar]
- 32.Solit DB, Zheng FF, Drobnjak M, Munster PN, Higgins B, Verbel D, et al. 17- Allylamino-17-demethoxygeldanamycin induces the degradation of androgen receptor and HER-2/neu and inhibits the growth of prostate cancer xenografts. Clin Cancer Res. 2002;8:986–93. [PubMed] [Google Scholar]
- 33.Fang Y, Fliss AE, Robins DM, Caplan AJ. Hsp90 regulates androgen receptor hormone binding affinity in vivo. J Biol Chem. 1996;271:28697–702. doi: 10.1074/jbc.271.45.28697. [DOI] [PubMed] [Google Scholar]
- 34.Vanaja DK, Mitchell SH, Toft DO, Young CY. Effect of geldanamycin on androgen receptor function and stability. Cell Stress Chaperones. 2002;7:55–64. doi: 10.1379/1466-1268(2002)007<0055:eogoar>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ai J, Wang Y, Dar JA, Liu J, Liu L, Nelson JB, et al. HDAC6 regulates androgen receptor hypersensitivity and nuclear localization via modulating Hsp90 acetylation in castration-resistant prostate cancer. Mol Endocrinol. 2009;23:1963–72. doi: 10.1210/me.2009-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saporita AJ, Ai J, Wang Z. The Hsp90 inhibitor, 17-AAG, prevents the ligand-independent nuclear localization of androgen receptor in refractory prostate cancer cells. Prostate. 2007;67:509–20. doi: 10.1002/pros.20541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheever MA, Higano C. PROVENGE (Sipuleucel-T) in Prostate Cancer: The First FDA Approved Therapeutic Cancer Vaccine. Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- 38.Danila DC, Morris MJ, de Bono JS, Ryan CJ, Denmeade SR, Smith MR, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010;28:1496–501. doi: 10.1200/JCO.2009.25.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reid AH, Attard G, Danila DC, Oommen NB, Olmos D, Fong PC, et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. 2010;28:1489–95. doi: 10.1200/JCO.2009.24.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meeran SM, Katiyar S, Katiyar SK. Berberine-induced apoptosis in human prostate cancer cells is initiated by reactive oxygen species generation. Toxicol Appl Pharmacol. 2008;229:33–43. doi: 10.1016/j.taap.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 41.Kovacs JJ, Murphy PJ, Gaillard S, Zhao X, Wu JT, Nicchitta CV, et al. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell. 2005;18:601–7. doi: 10.1016/j.molcel.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 42.Bali P, Pranpat M, Bradner J, Balasis M, Fiskus W, Guo F, et al. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J Biol Chem. 2005;280:26729–34. doi: 10.1074/jbc.C500186200. [DOI] [PubMed] [Google Scholar]
- 43.Basak S, Pookot D, Noonan EJ, Dahiya R. Genistein down-regulates androgen receptor by modulating HDAC6-Hsp90 chaperone function. Mol Cancer Ther. 2008;7:3195–202. doi: 10.1158/1535-7163.MCT-08-0617. [DOI] [PubMed] [Google Scholar]
- 44.Liu J, He C, Zhou K, Wang J, Kang JX. Coptis extracts enhance the anticancer effect of estrogen receptor antagonists on human breast cancer cells. Biochem Biophys Res Commun. 2009;378:174–8. doi: 10.1016/j.bbrc.2008.10.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kheir MM, Wang Y, Hua L, Hu J, Li L, Lei F, et al. Acute toxicity of berberine and its correlation with the blood concentration in mice. Food Chem Toxicol. 2010;48:1105–10. doi: 10.1016/j.fct.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 46.Zeng X, Zeng X. Relationship between the clinical effects of berberine on severe congestive heart failure and its concentration in plasma studied by HPLC. Biomed Chromatogr. 1999;13:442–4. doi: 10.1002/(SICI)1099-0801(199911)13:7<442::AID-BMC908>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 47.Mashima T, Okabe S, Seimiya H. Pharmacological targeting of constitutively active truncated androgen receptor by nigericin and suppression of hormone-refractory prostate cancer cell growth. Mol Pharmacol. 2010;78:846–54. doi: 10.1124/mol.110.064790. [DOI] [PubMed] [Google Scholar]
- 48.Narizhneva NV, Tararova ND, Ryabokon P, Shyshynova I, Prokvolit A, Komarov PG, et al. Small molecule screening reveals a transcription-independent pro-survival function of androgen receptor in castration-resistant prostate cancer. Cell Cycle. 2009;8:4155–67. doi: 10.4161/cc.8.24.10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andersen RJ, Mawji NR, Wang J, Wang G, Haile S, Myung JK, et al. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell. 2010;17:535–46. doi: 10.1016/j.ccr.2010.04.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.