Abstract
The proper targeting of ion channels to specialized domains is crucial for cell function. Kir4.1, the inwardly rectifying potassium channel, and aquaporin-4 (AQP4), the type 4 water-permeable channel, are localized at the basolateral domain of polarized epithelial cells; however, the mechanisms involved in their localization have yet to be determined. In this study, we investigated the role of the extracellular matrix in the localization of these channels in polarized Madin-Darby canine kidney (MDCK) cells. MDCK cells expressing green fluorescent protein-Kir4.1 or -AQP4 were cultured on laminin-1 or fibronectin and examined by confocal microscopy and cell surface biotinylation to assess plasma membrane expression of Kir4.1 and AQP4. Our data show that laminin-1 and fibronectin induce a significant increase in cell surface expression of both channels at the basolateral domain. Using fluorescence recovery after photobleaching, we demonstrate that laminin-1 and fibronectin reduce the diffusion rates of these channels. Finally, we show that the laminin receptor dystroglycan is important for cell surface expression of Kir4.1 but not AQP4. However, laminin-1 increases cell surface expression of both channels in cells deficient for dystroglycan, indicating that other receptors are involved. Indeed, RGD-containing peptides, which inhibit fibronectin binding to certain integrins, prevent the fibronectin-induced increase in Kir4.1 and AQP4 cell surface expression and reverse the laminin- and fibronectin-induced reduction in both channels' diffusion rates. Similarly, the αvβ3-integrin function-blocking antibody alters the reduction of AQP4 diffusion rates induced by both laminin and fibronectin, suggesting that αvβ3-integrin plays a role in the stabilization of APQ4 at the basolateral domain of epithelial cells.
Keywords: dystroglycan, laminin-1, fibronectin, FRAP, polarized distribution
the dystrophin-associated protein (DAP) complex is a multiprotein complex expressed in neuronal, astrocytic, epithelial, and muscle cells (2, 3, 6, 9, 20, 23, 29, 31, 44). Central to the DAP complex is dystroglycan (DG), a molecule composed of a transmembrane protein, β-DG, and an extracellular protein, α-DG. α-DG interacts with the extracellular matrix (ECM) components laminin, agrin, perlecan, and neurexin (4, 48, 46), whereas β-DG interacts with the cytoplasmic protein dystrophin, which in turn binds to the actin cytoskeleton. In muscle, the DAP complex and DG have a role in maintaining the structural integrity of the sarcolemma (42, 49). In the brain, alterations in DG cause a discontinuity in the glia limitans and abnormal neuronal migration (28, 30). DG is also expressed in a variety of epithelia, including those of the kidney, lung, and testis (1, 19, 24, 40, 41). In the kidney, inhibition of laminin binding to α-DG causes the disruption of epithelial cell differentiation, indicating that the DAP complex plays an important role in epithelial morphogenesis (7). Furthermore, numerous studies have demonstrated a role for DG in the orientation of polarity. Indeed, DG deletion in Drosophila results in the loss of the apicobasal polarity of epithelial cells as well as the anteroposterior polarity of the oocyte (5, 45). In mammary cells, DG deletion results in the disruption of laminin assembly in the ECM and a subsequent loss of cell polarity (52). The large family of heteromeric transmembrane proteins, integrins, has extensively been studied for its role in epithelial cell polarity via their interaction with the ECM. To date, 18 α- and 8 β-subunits have been identified, and these can form 24 distinct integrin dimers (15). Epithelial cells express several integrins including α1β1, α2β1, α6β1, α3β1, α6β4, α5β1, and αvβ3 (27). It has been shown that epithelial Madin-Darby canine kidney (MDCK) cells adhere to the ECM via the laminin and collagen receptor α2β1, the laminin, collagen, and fibronectin receptor α3β1, and the vitronectin and fibronectin receptor αvβ3 (16). In addition, MDCK cells also express α6β4-integrin, but, unlike in other cell types, this receptor does not appear to possess affinity for laminin. Nonetheless, it has been demonstrated that β1-containing integrins orient the apical domain of polarized MDCK cysts through Rac1 activation and laminin assembly in the basement membrane (38, 53).
In astrocytes of the mammalian brain and Muller cells of the retina, both the inward rectifying potassium channel 4.1 (Kir4.1) and aquaporin-4 (AQP4) aggregate at discrete membrane domains that abut blood vessels and the vitreous body (32, 33). This polarized distribution is critically dependent on the interaction between laminin and the DAP complex via DG (10, 12, 28, 34, 36, 43) and plays a major role in astrocyte- and Muller cell-mediated potassium buffering (21).
In vivo, epithelial cells possess well-defined basolateral and apical membrane domains, each with a distinct complement of proteins. In the case of the epithelial cells of the tubules of the kidney, the basolateral enrichment of Kir4.1 and AQP4 is critical as it allows the renal system to maintain systemic osmostasis (47). AQP4, which functions in tandem with the vasopressin-regulated AQP2 channel to concentrate the urine and therefore minimize the amounts of water lost via this route, is expressed basolaterally in the cells of the collecting duct (51). Kir4.1 is found in the distal convoluted tubules of the kidney, where it serves to regulate K+ excretion (17, 50). The basolateral localization of these channels is thus clearly of great importance given the asymmetric transepithelial transport processes that they mediate. However, the specific factors that influence their localization remain to be elucidated. In the present study, we hypothesize that proper distribution of Kir4.1 and AQP4 is dependent on receptor-ECM interactions. To address this question, we used MDCK epithelial cells as a model of renal epithelia to investigate the impact of the ECM on the cell surface expression and stability of Kir4.1 and AQP4 channels. Via immunofluorescence, we first determined that these channels are localized basolaterally in both the mammalian kidney and MDCK cells and that they codistribute with the laminin receptor DG, the DAP component syntrophin, and E-cadherin. We also found that the ECM components fibronectin and laminin-1 both induce an increase in the amounts of either channel at the basolateral domain of MDCK cells. Using fluorescence recovery after photobleaching (FRAP), we demonstrated that fibronectin and laminin-1 stabilize these channels by reducing their diffusion rates within the plasma membrane. Finally, we show that while DG is required to maintain proper expression of Kir4.1, integrin receptors may play a compensatory role in cells deficient for DG. This is further evidenced by our data showing that RGD-containing peptides known to interact with integrins reverse the reduction of the Kir4.1 diffusion rate induced by laminin, indicating that in epithelial cells, laminin can stabilize Kir4.1 not only via DG, but also via the integrin family of cell surface receptors. Moreover, both RGD peptides and the αvβ3-integrin function-blocking antibody inhibit the reduction of AQP4 diffusion rates, suggesting that αvβ3-integrin plays a role in the cell surface stabilization of APQ4 in epithelial cells.
MATERIALS AND METHODS
Antibodies.
Antibodies used in this study include mouse anti-β-DG, 43DAG1/8D5, against 15 of the last 16 amino acids at the C terminus of the human DG sequence (Novocastra Laboratories, Newcastle-upon-tyne, UK); mouse anti-syntrophin, SYN1351, against Torpedo syntrophin (ABR-Affinity Bioreagents, Golden, CO); mouse anti-E-cadherin against amino acids 735–883 of the human E-cadherin (BD Transduction Laboratories); mouse anti-αvβ3 integrin, CD51/61, against purified adhesion receptor from the human melanoma cell line M21 (Millipore); rabbit anti-AQP4 against rat GST AQP4 corresponding to residues 249–323 (Alomone Laboratories, Jerusalem, Israel); and rabbit anti-Kir4.1 against amino acids 356–375 of the intracellular C-terminal region of rat Kir4.1 (Alomone Laboratories).
Cell culture.
Type II MDCK cells were transfected with the cDNA coding for AQP4 (25) and Kir4.1 (48) inserted into the enhanced green fluorescent protein expression plasmids EGFP-C3 (Clontech Laboratories) and EGFP-C1, respectively. Cells were grown in high-glucose DMEM containing 10% fetal bovine serum (Medicorp, Montreal, QC), 1% penicillin/streptomycin, and 1% l-glutamine (GIBCO-BRL) in a humidified incubator at 37°C with 5% CO2-95% air and transfected 1 day after plating using the Effectene reagent following the supplier's instructions (Qiagen, Mississauga, ON). Individual colonies of MDCK cells stably expressing either GFP-AQP4 or GFP-Kir4.1 were selected using 500 μg/ml geneticin-containing DMEM and grown at a density of 3 × 103 cells/ml for 72 h. For confocal microscopic analysis, FRAP, and cell surface biotinylation, the cells were grown on 12-mm coverslips, eight-well Ibidi μ-slides (Ibidi, Martinsried, Munich, Germany), and 25-mm PET track-etched filters with 0.4-μm pores (BD Biosciences, Mississauga, ON), respectively. These were coated for 18 h with 100 μg/ml collagen IV, 5.0 or 50.0 μg/ml fibronectin, or 9.6 or 96.0 μg/ml mouse Engelbreth-Holm-Swarm (EHS) Sarcoma laminin-1 (Sigma-Aldrich) prepared in DMEM. For the fibronectin-competition experiment, the specific fibronectin-competing peptides RGD (17.5 μg/ml) and GRGDSPK (35 μg/ml; Sigma-Aldrich) were applied to the cells in suspension for 5–10 min before plating. To ensure saturation of all potential binding sites, GFP-Kir4.1- and GFP-AQP4-expressing MDCK cells in suspension were exposed to these peptides in 900-fold molar excess (compared with fibronectin) before their plating on 50 μg/ml fibronectin-coated filters, as well as for the entire duration of the culture period. The FRAP experiments pertaining to blocking the interaction between αvβ3-integrin and the ECM were performed first by incubating the cells for 30 min at room temperature with 10 μg/ml of the αvβ3-integrin function-blocking antibody and then by plating them on substrates containing 50 μg/ml exogenous fibronectin or 96 μg/ml laminin-1.
Cell surface biotinylation.
MDCK cells grown on 25-mm PET track-etched filters were placed on ice and washed with cold PBS containing 1.0 mM CaCl2 and 0.5 mM MgCl2 (DPBS) three times for 5 min each, and then their basolateral membrane was labeled for 30 min at 4°C using 0.5 mg/ml EZ-Link Sulfo-NHS-LC-Biotin (Pierce Biotechnology, Rockford, IL). The biotin solution was removed, and the reaction was quenched with 50 mM NH4Cl in DPBS for 10 min. The cells were extensively washed and incubated for 1 h with extraction buffer (25 mM Tris, pH 7.4, 25 mM glycine, 150 mM NaCl, and 5 mM EDTA) containing 1% Triton X-100 and 1× complete protease inhibitor cocktail (Roche, Laval, QC, Canada). The biotinylated proteins were precipitated using streptavidin covalently attached to agarose beads (Pierce Biotechnology).
The apicobasal expression of Kir4.1 and AQP4 was determined by cell surface biotinylation on confluent MDCK cells grown on PET track-etched filters. The biotin was applied on the apical or basolateral domains, and subsequent sample collection and analysis steps were carried out using the method described above.
Immunoblotting.
The biotinylated proteins were denatured by boiling at 95°C for 9 min in reducing sample buffer, then separated on 10% SDS-PAGE, and electrotransferred to nitrocellulose membranes (Bio-Rad, Mississauga, ON). The blots were first blocked with a solution of 5% nonfat milk dissolved in TBST (20 mM Tris, pH 7.5, 150 mM NaCl, and 0.1% Tween 20) and then probed using antibodies against AQP4 (1:1,000), Kir4.1 (1:750), β-DG (1:500), syntrophin (1:500), and E-cadherin (1:500). The bound antibodies were detected using horseradish peroxidase-conjugated goat anti-rabbit IgG or goat anti-mouse IgG (1:2,000; Pierce), and signals were visualized on Bioflex Econofilm (Interscience, Markham, ON) using chemiluminescence (Amersham Biosciences, Buckinghamshire, UK).
Immunofluorescence.
For immunofluorescence, rat cryostat kidney sections and cells grown on 12-mm glass coverslips were washed three times with PBS for 5 min each. The sections were fixed using 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer, and the cells were fixed using either 2% PFA or methanol-acetone (80–20%) for 20 min. After three additional washes with PBS, the sections and cells were blocked and permeabilized at room temperature (20–22°C) with a solution containing 2% BSA and 0.3% Triton X-100 in PBS. They were then incubated at room temperature for 1 h with primary antibodies against β-DG (1:50), E-cadherin (1:100), or syntrophin (1:100) and rinsed with PBS (3 × 15 min). Subsequently, they were incubated with Alexa Fluor 488 goat anti-rabbit IgG and Alexa Fluor 568 goat anti-rabbit IgG for 1 h (1:700; Molecular Probes). After several washes with PBS, kidney sections and cells were mounted on glass coverslips and slides, respectively, using Prolong Gold Antifade reagent (Invitrogen, Burlington, ON). To confirm the specificity of the labeling, control sections and cells were treated equivalently in the absence of primary antibodies. Fluorescent labeling was visualized using a confocal microscope (Fluoview 1,000; Olympus) and an Uplan Apochromat 1.35 NA ×60 objective (Olympus).
FRAP.
MDCK cells stably expressing either GFP-Kir4.1 or GFP-AQP4 were grown to confluence for 48 h in 8-well ibidi μ-slides (Ibidi), and images were acquired on a confocal microscope (FV1000; Olympus) using an Uplan Apochromat 1.35 NA ×60 objective and fully opened pinhole. Areas of interest encompassing the coincident plasma membranes of two adjacent GFP-AQP4 or GFP-Kir4.1 cells were photobleached over 15 frames using a 405-nm simultaneous scanner set to 60% power. All images were captured at a resolution of 512 × 512 using a scan rate of 2 μs/pixel, a frame rate of 1.1 s/frame over a period of ∼45 s. Fluorescence recovery was analyzed, and “best-fit” curves derived, using the built-in nonlinear regression function of Prism 4.00 (Graphpad Software, La Jolla, CA). FRAP analysis was also conducted on Kir2.1 in MDCK cells transiently transfected with Kir2.1 cDNA inserted into the pEGFP-C1 expression plasmid (48).
Small interference RNA transfection.
Adherent MDCK cells cultured for 1 day to near-confluence were transfected with small interference (si) RNAs specific for canine Dag1 (ON-TARGETplus SMARTpool siRNA reagents, Dharmacon; 100 pmol for cells grown on 12-mm coverslips, 250 pmol for cells grown on 25-mm PET track-etched membranes) using Lipofectamine 2000. Controls for nonspecific siRNA activity were performed using equivalent amounts of ON-TARGETplus Non-targeting siRNA.
Quantitative and statistical analysis.
Fluorescence intensity analysis from three different experiments was performed using ImagePro Plus (Media Cybernetics). Six fields containing an average of 100 cells were analyzed for each experiment. Images of individual optical sections at the level of the basolateral surface from the same experiment were acquired and quantified under identical settings. In the case of Western blotting experiments, the quantifications were performed by densitometric analysis. All statistical analyses were performed using Prism 4.00 software. For the FRAP assay, fluorescence values at each time point were first normalized to prebleach values using Microsoft Excel, then plotted and analyzed with GraphPad Prism.
As previously described in opossum kidney cells (48), Kir4.1 fluorescence was seen at the basolateral plasma membrane as well as in the Golgi complex of MDCK cells (Supplemental Fig. S2F; all supplementary material for this article is available online at the journal web site). To quantify the basolateral pool of Kir4.1 fluorescence, the total Kir4.1 fluorescence was subtracted from that of basolateral E-cadherin, giving rise to the fluorescence intensity of intracellular Kir4.1. This value was subsequently subtracted from the total Kir4.1 fluorescence intensity, enabling the determination of Kir4.1 fluorescence intensity associated with the basolateral plasma membrane.
In the case of the biotinylation assays, densitometric analyses were performed to quantitate the fibronectin- and laminin-induced changes in the amounts of Kir4.1 and AQP4 at the basolateral membrane domain. These were first normalized against E-cadherin and were then normalized against the signal seen in the control untreated cells.
RESULTS
Kir4.1 and AQP4 are localized at the basolateral plasma membrane domain in kidney epithelial cells.
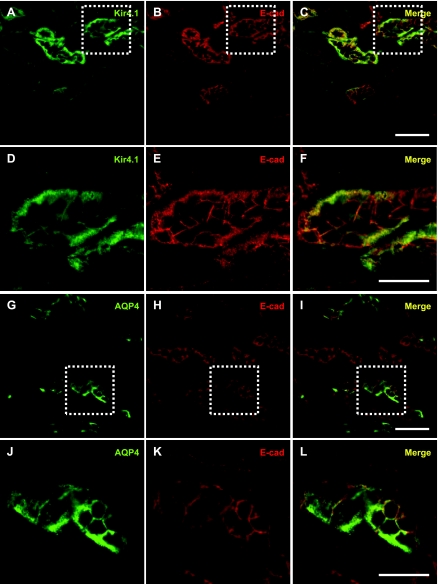
To examine the distribution of Kir4.1 and AQP4 in the renal cortex and medulla, cryostat sections of rat kidneys were double-immunolabeled for Kir4.1 or AQP4 and E-cadherin. Consistent with previous observations (17, 51), labeling for Kir4.1 and AQP4 was observed at the basolateral domain of epithelial cells where it colocalizes with E-cadherin (Fig. 1, A–C and G–I).
Fig. 1.
Kir4.1 and aquaporin-4 (AQP4) are localized basolaterally in kidney epithelial cells. Coronal kidney sections were double immunolabeled for Kir4.1 (A) or AQP4 (G) and the basolateral marker E-cadherin (B and H). The merged images show that Kir4.1 and AQP4 codistribute with E-cadherin (C and I) in kidney epithelial cells. Zoomed images of the boxed areas in A–C and G–I are shown in D–F and J–L, respectively. Scale bars = 50 μm for A–C and G–I and 25 μm for D–F and J–L.
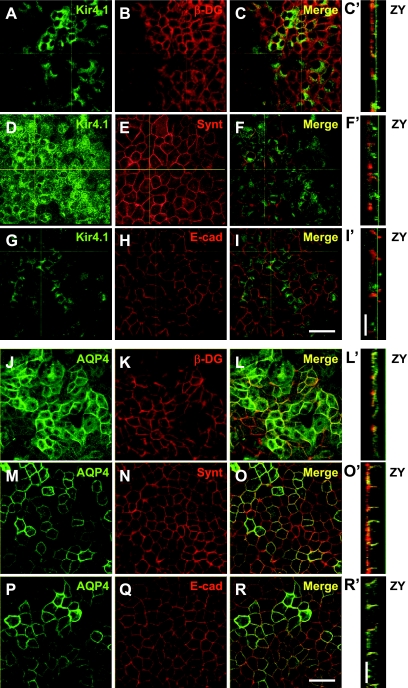
We next examined whether Kir4.1 and AQP4 were codistributed with β-DG, syntrophin, and E-cadherin in confluent epithelial MDCK cells stably expressing GFP-Kir4.1 or GFP-AQP4. AQP4 is mainly localized at the plasma membrane whereas Ki4.1 is also associated with the Golgi apparatus as has been previously described in opossum kidney cells (48). In addition, we found that GFP-Kir4.1 and GFP-AQP4 codistribute with syntrophin (Fig. 2, D–F, F′, M–O, and O′) and E-cadherin (Fig. 2, G–I, I′, P–R, and R′) at the basolateral domain with a more obvious codistribution at the lateral domain. While the 2% PFA fixation necessitated by the use of the antibody against β-DG caused substantial flattening of the epithelial monolayer (Fig. 2, C′ and L′), lateral codistribution of Kir4.1 and AQP4 with β-DG is still evident (Fig. 2, A–C, C′, J–L, and L′).
Fig. 2.
Kir4.1 and AQP4 are expressed basolaterally in Madin-Darby canine kidney (MDCK) cells. Confluent MDCK cells expressing either green fluorescent protein (GFP)-Kir4.1 (A, D, and G) or GFP-AQP4 (J, M, and P) were immunolabeled for β-dystroglycan (DG; B and K), syntrophin (E and N), or E-cadherin (H and Q). The merged images of XY (C, F, I, L, O, R) and ZY views (C′, F′, I′, L′, O′, R′) show that Kir4.1 and AQP4 codistribute with components of the dystrophin-associated protein complex as well as with E-cadherin at the basolateral membrane domains of MDCK cells. Scale bars = 50 μm.
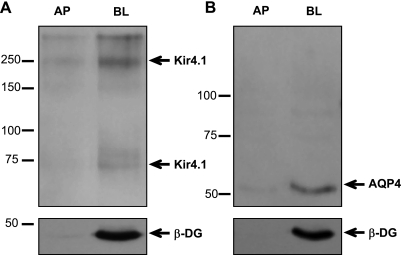
To further demonstrate that both Kir4.1 and AQP4 are expressed predominantly at the basolateral membrane of MDCK cells in culture, we performed biotinylation studies of the apical and basolateral domains of cells expressing these channels (Fig. 3). We found that both Kir4.1, detected in its monomeric and tetrameric forms (∼67 and ∼268 kDa) (Fig. 3A), and AQP4 (Fig. 3B) are predominantly expressed at the basolateral domain. β-DG is also localized to the same domain.
Fig. 3.
Kir4.1 and AQP4 are expressed predominantly within the basolateral membrane domain of MDCK cells. Biotinylated cell surface fractions from the apical (AP) and basolateral (BL) domains of confluent MDCK cells expressing either GFP-Kir4.1 or GFP-AQP4 were immunoblotted for Kir4.1 (A; arrow points at the tetramer and monomer, respectively) and AQP4 (B) as well as β-DG (A and B, bottom).
Fibronectin and laminin increase expression and reduce lateral mobility of Kir4.1 and AQP4 at the basolateral plasma membrane domain of MDCK cells.
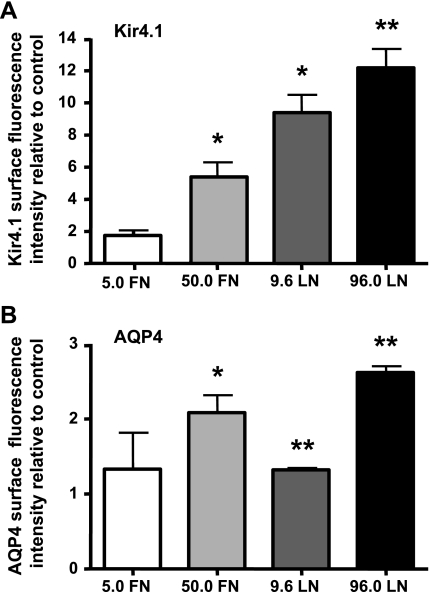
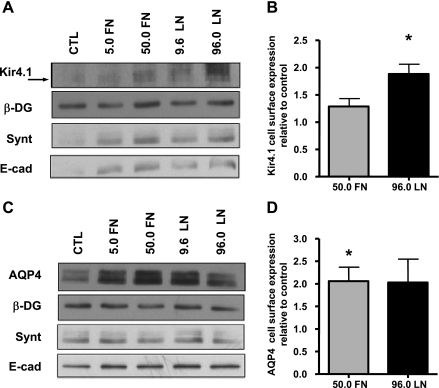
We next sought to determine whether the interaction of the DAP complex with the ECM plays a role in the basolateral localization of Kir4.1 and AQP4. MDCK cells expressing GFP-Kir4.1 and GFP-AQP4 were grown for 72 h on coverslips coated with either fibronectin or laminin-1 (5.0 FN = 5.0 μg/ml fibronectin; 50.0 FN = 50.0 μg/ml fibronectin; 9.6 LN = 9.6 μg/ml laminin; 96.0 LN = 96.0 μg/ml laminin) (Fig. 4). The amounts of Kir4.1 and AQP4 localized at the lateral domain of MDCK cells were then determined via ImagePro analysis of confocal micrographs using E-cadherin staining as a marker of the basolateral domain. We found that the cells cultured on 50.0 μg/ml fibronectin, 9.6 μg/ml laminin, or 96.0 μg/ml laminin presented significantly greater fluorescence intensity for both Kir4.1 and AQP4 compared with control cells cultured on uncoated coverslips (Fig. 4, A and B). Collagen IV produced no change in the fluorescence intensity of either channel (data not shown). In addition, cell surface biotinylation of the basolateral domain of MDCK cells cultured on permeable supports coated with low and high concentrations of either fibronectin or laminin revealed an increase in the cell surface expression of both Kir4.1 and AQP4 (Fig. 5, A and C). It is important to point out that the total expression levels of either one of the channels remained unchanged when the cells were treated with fibronectin or laminin (Supplemental Fig. S4). Densitometric analysis showed that fibronectin and laminin-1 resulted in a significant 1.3- and 1.9-fold increase in Kir4.1 cell surface expression, respectively (Fig. 5B). AQP4 cell surface expression is also increased approximately twofold in the presence of fibronectin and laminin-1 (Fig. 5D).
Fig. 4.
Fibronectin (FN) and laminin-1 (LN) increase Kir4.1 and AQP4 levels at the basolateral domains of MDCK cells. MDCK cells expressing GFP-Kir4.1 (A) or GFP-AQP4 (B) were grown on coverslips coated with FN (5.0 or 50.0 μg/ml) or LN (9.6 or 96.0 μg/ml) for 72 h. Histograms represent the means GFP fluorescence intensity normalized to that of the respective untreated controls ± SE of 3 experiments. The asterisks represent statistically significant increases in the fluorescence intensity compared with control cells as determined by a 2-tailed Student's t-test (*P < 0.05, **P < 0.005).
Fig. 5.
FN and LN increase cell surface expression of Kir4.1 and AQP4 at the basolateral domains of MDCK cells. Biotinylated cell surface fractions from control cells (CTL) and cells grown on either FN (5.0 or 50.0 μg/ml) or LN (9.6 or 50.0 μg/ml) for 72 h were immunoblotted for Kir4.1 (A), AQP4 (C), β-DG (A and C), syntrophin (A and C), and E-cadherin (A and C). Histograms of the densitometric analysis represent the means pixel intensity ± SE relative to control of 3 experiments (B and D). The asterisks represent statistically significant increases in Kir4.1 and AQP4 cell surface expression compared with control cells as determined by a 2-tailed Student's t-test (*P < 0.05).
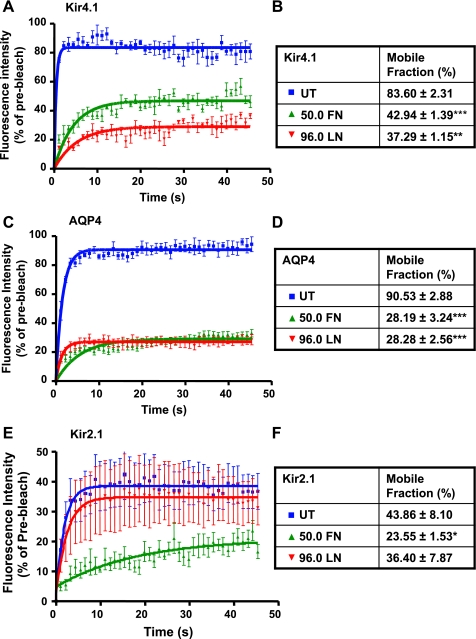
To determine the mechanisms underlying the increased channel expression at the cell surface, we next investigated whether fibronectin and laminin alter the lateral mobilities of Kir4.1 and AQP4 within the plasma membrane. The cells were cultured to confluence in eight-well μ-slides coated with either 50.0 μg/ml fibronectin or 96.0 μg/ml laminin, and 2-μm sections of lateral membranes were photobleached so that <50% of initial fluorescence remained. The fluorescence intensity within the bleached areas was then measured every 1.1 s over 45 s. Both fibronectin and laminin caused a dramatic reduction in the rate of recovery and mobile fractions of GFP-Kir4.1 (Fig. 6, A and B) and GFP-AQP4 (Fig. 6, C and D). These data reveal a role for both fibronectin and laminin in the regulation of Kir4.1 and AQP4 diffusion rates at the plasma membrane of epithelial MDCK cells. To determine whether the effect of fibronectin and laminin is specific to Kir4.1, we tested their effect on the diffusion rates of GFP-Kir2.1 by FRAP analysis using parameters identical to those used for GFP-Kir4.1. We found that fibronectin induces a significant reduction in the fluorescence recovery of Kir2.1, whereas laminin has no effect compared with control untreated cells (Fig. 6, E and F).
Fig. 6.
FN and LN reduce the membrane diffusion of Kir4.1 and AQP4 in MDCK cells. MDCK cells expressing GFP-Kir4.1 (A), GFP-AQP4 (C), or GFP-Kir2.1 (D) grown in 8-well μ-slides coated with FN (50.0) or LN (96.0) for 48–72 h were analyzed by fluorescence recovery after photobleaching (FRAP). Data points represent averaged percent fluorescence intensity ± SE of GFP-Kir4.1, GFP-AQP4, and GFP-Kir2.1 recovered in the bleached area and normalized to the intensity observed before the bleaching in the same cell (n = 8 cells/condition). The tables summarize the percent mobile fraction of GFP-Kir4.1 (B), GFP-AQP4 (D), and GFP-Kir2.1 (E) in control cells and cells grown on FN or LN (***P < 0.0001, **P = 0.0042, *P = 0.0384).
DG is involved in Kir4.1 and AQP4 basolateral distribution regulated by fibronectin and laminin.
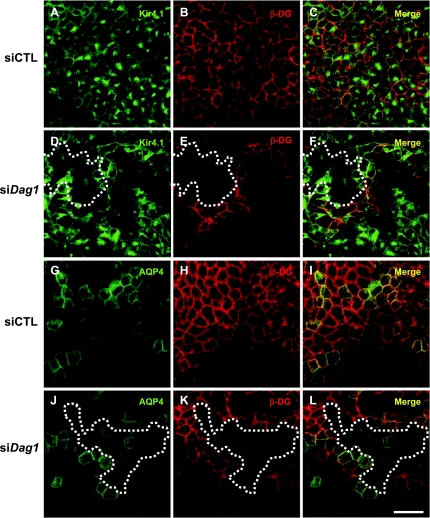
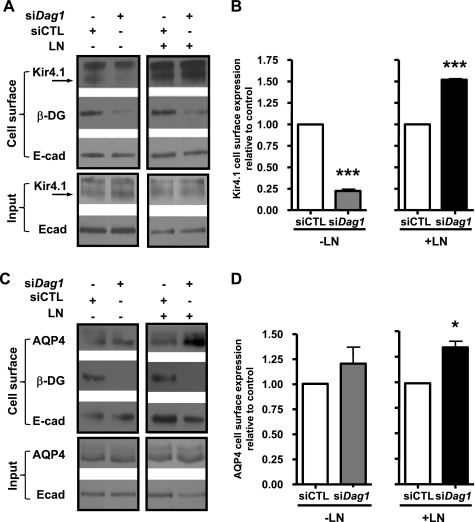
Our previous studies in astrocytes and Müller glia showed that laminin-induced redistribution of Kir4.1 and AQP4 is mediated via DG (12, 36, 37). Here, we used siRNA targeted against canine DG (si-Dag1) to silence its expression and determined whether DG is involved in the laminin-induced increase in basolateral expression of Kir4.1 and AQP4 by both fluorescence and cell surface biotinylation (Figs. 7 and 8). We found that patches of cells were effectively knocked down for DG, and we estimated these patches to constitute ∼50% of the cell population. Cells deficient for DG express reduced levels of Kir4.1 fluorescence (compare Fig. 7, D–F to A–C). However, AQP4 fluorescence is comparable to that seen in control cells (compare Fig. 7, J–L to G–I). Knockdown levels between the GFP-Kir4.1 and GFP-AQP4 cells were comparable (Supplemental Fig. S5). These results are supported by the cell surface biotinylation data, showing that DG deficiency significantly reduces Kir4.1 cell surface expression (Fig. 8, A and B) but does not alter that of AQP4 (Fig. 8, C and D).
Fig. 7.
DG is essential for the proper localization of Kir4.1 but not AQP4. MDCK cells expressing GFP-Kir4.1 (A and D) and GFP-AQP4 (G and J) were transfected with Dag1 small interfering RNA (siDag1; D–F and J–L) and immunolabeled for β-DG (B, E, H, and K). siCTL-transfected cells were used as control (A–C and G–I). The dotted lines show fields containing cells deficient in DG. Scale bar = 50 μm.
Fig. 8.
DG is not the only LN receptor involved in the LN-induced increase in cell surface expression of Kir4.1 and AQP4 in MDCK cells. Biotinylated cell surface fractions from control (siCTL) and Dag1 siRNA-transfected (siDag1) GFP-Kir4.1 and GFP-AQP4-expressing cells grown in the absence (−LN) or the presence of 96 μg/ml LN (+LN; A and B) for 72 h were immunoblotted for Kir4.1 (A), AQP4 (C), β-DG, and E-cadherin (A and C). Histograms of the densitometric analysis represent the means pixel intensity ± SE relative to control of 3 experiments (B and D). The asterisks represent statistically significant increases in Kir4.1 and AQP4 cell surface expression compared with control cells as determined by a 2-tailed Student's t-test (*P < 0.05, ***P < 0.001).
To determine whether the laminin-induced increase in cell surface expression of Kir4.1 and AQP4 is due to the interaction of laminin with cell surface receptors other than α-DG, GFP-Kir4.1- and GFP-AQP4-expressing MDCK cells transfected with si-Dag1 were grown in the presence of 96 μg/ml laminin-1 for 72 h, and the membrane expression of Kir4.1 and AQP4 was determined by cell surface biotinylation. We found that laminin-1 induces an increase in both Kir4.1 and AQP4 cell surface expression (Fig. 8). Of particular interest, this laminin-induced increase is more robust in si-Dag1-transfected cells than in control cells (Fig. 8, A and C). These results show that, in the presence of exogenous laminin, DG deficiency in MDCK cells does not impair the ability of laminin-1 to alter cell surface expression of these channels and suggest that other laminin receptors may also be involved.
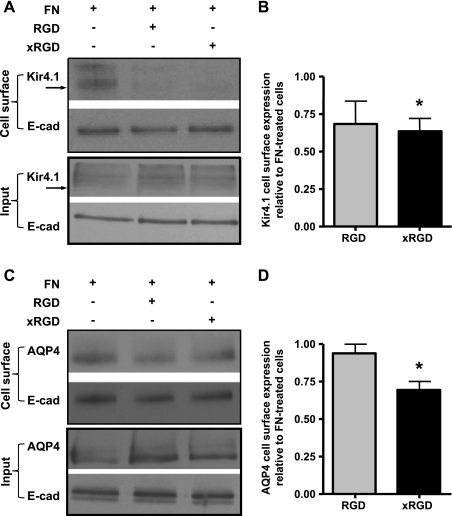
To investigate further the effect of fibronectin on Kir4.1 and AQP4 cell surface expression, we used the two RGD motif-containing disintegrin peptides Arg-Gly-Asp (RGD) and Gly-Arg-Gly-Asp-Ser-Pro-Lys (GRGDSPK) known to block fibronectin binding to integrins. To ensure saturation of all potential binding sites, GFP-Kir4.1- and GFP-AQP4-expressing MDCK cells in suspension were exposed to 900-fold molar excess (compared with fibronectin) of these peptides before plating on fibronectin-coated filters as well as during the entire culture period. Via cell surface biotinylation, we found that GRGDSPK (xRGD) reduces significantly the effect of fibronectin on the cell surface expression of both Kir4.1 (Fig. 9, A and B) and AQP4 (Fig. 9, C and D).
Fig. 9.
RGD and xRGD peptides inhibit the FN-induced increase in cell surface expression of Kir4.1 and AQP4. Control MDCK cells, and cells incubated with Arg-Gly-Asp (RGD) or Gly-Arg-Gly-Asp-Ser-Pro-Lys (GRGDSPK = xRGD) peptides, were cultured to confluence over 72 h on FN-coated cell culture filters and then subjected to cell surface biotinylation of the basolateral domain. Cell surface fractions were immunoblotted for Kir4.1 (A) or AQP4 (C), and for E-cadherin (A and C). Histograms of the densitometric analysis represent the means pixel intensity ± SE relative to fibronectin-treated cells of 3 experiments (B and D). The asterisks represent statistically significant decreases in Kir4.1 and AQP4 cell surface expression compared with FN-treated cells as determined by a 2-tailed Student's t-test (*P < 0.05).
RGD-containing peptides and αvβ3-integrin antibody alter AQP4 and Kir4.1 mobility on fibronectin and laminin.
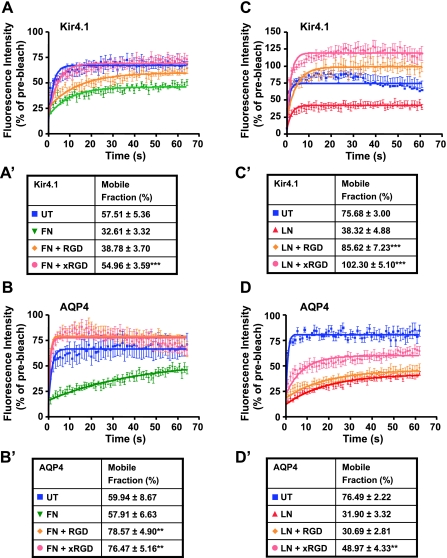
Having demonstrated that the rate of recovery and mobile fractions of Kir4.1 and AQP4 are significantly decreased in the presence of fibronectin and that integrin-blocking peptides inhibit the fibronectin-induced increase in Kir4.1 and AQP4 cell surface expression, we next sought to determine the effect of fibronectin and these peptides on the rate of recovery of both channels using FRAP analysis. We found that both RGD and GRGDSPK (xRGD) significantly inhibit the effect of fibronectin, resulting in a faster rate of recovery and higher mobile fractions than those of control cells (Fig. 10, A, A′ B, and B′). When applied in conjunction with laminin, both peptides restore significantly the diffusion rate of Kir4.1 (Fig. 10, C and C′). However, only GRGDSPK is effective in reversing the effect of laminin on the mobility of AQP4 (Fig. 10, D and D′). These data reveal a differential effect of RGD-containing peptides on Kir4.1 and AQP4 stability at the plasma membrane. Although both Kir4.1 and AQP4 are regulated by laminin-1 and fibronectin, Kir4.1 is more responsive than AQP4 to RGD-containing peptides in the presence of laminin. Conversely, AQP4 is more responsive than Kir4.1 to these peptides in the presence of fibronectin.
Fig. 10.
RGD peptides reverse the FN- and LN-induced reduction in channel mobility. Cells expressing either GFP-tagged Kir4.1 or AQP4 were incubated with RGD or GRGDSPK (xRGD) peptides, plated on substrates containing 50 μg/ml exogenous FN or 96 μg/ml LN, and then subjected to FRAP analysis. Although FN potently retards the lateral diffusion of Kir4.1, xRGD completely abolished this effect, restoring channel mobility level to that seen for untreated cells (A and A′; ***P < 0.0001). RGD and xRGD are also effective in significantly increasing AQP4 diffusion rates in the presence of FN (B and B′; **P = 0.0015 and 0.0036, respectively). While both RGD and xRGD peptides are capable of significantly reversing the effect of LN, raising Kir4.1 mobility to levels even beyond those of control cells (C and C′; ***P < 0.0001), only xRGD is capable of partially reversing the effect of LN on AQP4 mobility (D and D′; **P = 0.0074).
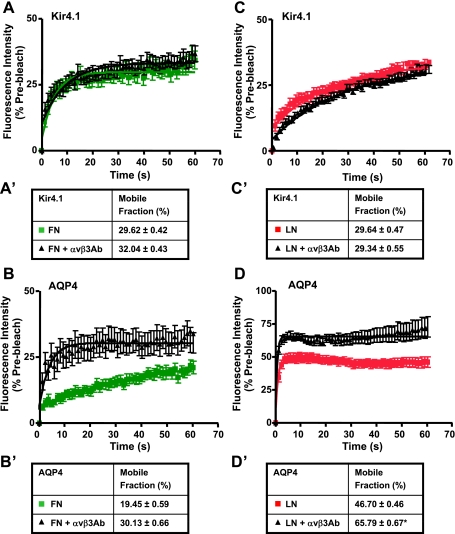
Given that αvβ3-integrin interacts with ECM proteins including fibronectin via its RGD binding motif (13) and that RGD-containing peptides significantly reverse the decreased channel diffusion induced by fibronectin (Fig. 10, A, A′, B, and B′) and laminin (Fig. 10, C, C′, D, and D′), we hypothesized that this reversal occurs via blockage of the interaction between the ECM and αvβ3-integrin. We therefore assessed Kir4.1 and AQP4 diffusion rates in cells treated with either 50 μg/ml fibronectin or 96 μg/ml laminin plus 10 μg/ml of αvβ3-integrin function-blocking antibody by FRAP (Fig. 11). Here, the cells were incubated with the αvβ3-integrin antibody for 30 min at room temperature before being plated on a substrate containing either fibronectin or laminin and in culture media containing the αvβ3-integrin antibody. First, we showed that the αvβ3-integrin antibody effectively blocks adhesion of GFP-Kir4.1- and GFP-AQP4-expressing cells to fibronectin (Supplemental Fig. S6). Second, we performed FRAP analysis and the data show that the decreased diffusion rates of AQP4 induced by either laminin or fibronectin are reversed by the αvβ3-integrin antibody (Fig. 11, B, B′, D, and D′); however, those of Kir4.1 remain unchanged (Fig. 11, A, A′, C, and C′). These data suggest that αvβ3-integrin plays a role in the ECM-induced stabilization of AQP4 at the basolateral domain.
Fig. 11.
αvβ3-Integrin plays a role in the regulation of the FN- and LN-induced stability of AQP4 at the plasma membrane. Cells expressing either GFP-tagged Kir4.1 or AQP4 were incubated for 30 min at room temperature with 10 μg/ml of αvβ3-integrin antibody, plated on substrates containing 50 μg/ml exogenous FN or 96 μg/ml LN, and then subjected to FRAP analysis. The αvβ3-integrin antibody does not significantly affect the lateral mobility of Kir4.1 in MDCK cells grown in the presence of either FN or LN (A, A′, C, and C′); however, it inhibits that of AQP4 in MDCK cells grown on laminin (D and D′; *P = 0.04). Note that while the predicted recovery of AQP4 is not significantly different between cells treated with FN and cells treated with FN plus the αvβ3-integrin antibody (B′), the initial recovery (up to 15 s) is accelerated in cells treated with FN plus the αvβ3-integrin antibody.
DISCUSSION
Laminin-DG regulation of basolateral expression of Kir4.1 and AQP4.
The ECM is a critical determinant of epithelial morphology and organization. Several studies have demonstrated that laminin assembly plays a crucial role in the polarization of MDCK cells (38, 54) and that interactions between laminin and β1-integrin, relayed via a Rac1-dependent mechanism, are necessary for the proper polarization of three-dimensional MDCK cysts (38). While previous studies have detailed the importance of DG in mediating epithelial and oocyte polarity in Drosophila (5), the role of this high-affinity laminin receptor (11) in the distribution of polarized proteins in epithelial cells remains to be determined.
Our previous findings have described a role for DG in the laminin-dependent localization of Kir4.1 and AQP4 in glial cells (12, 36, 43). In astrocyte cultures, we have shown that laminin-1 requires DG for the formation of macrodomains containing laminin, the DG complex, and both Kir4.1 and AQP4 (12, 37). In the intact mouse brain, α-DG, Kir4.1, and AQP4 are concentrated at perivascular domains of astrocytes; however, this distribution pattern is lost in the Largemyd mouse, presenting a hypoglycosylation of α-DG and a subsequent loss of laminin binding to α-DG (43). These findings strongly suggest that DG-laminin interaction is important in the polarized distribution of Kir4.1 and AQP4 in vivo. To determine whether this type of interaction plays also a role in regulating the basolateral distribution of Kir4.1 and AQP4 in epithelial cells, we used MDCK epithelial cells as a model of renal epithelia. In the present study, we show that laminin-1 and fibronectin induce a significant increase in the levels of both Kir4.1 and AQP4 at the basolateral plasma membrane domain of MDCK cells, indicating that they are important determinants of polarized channel expression in the renal system. These data suggest that water permeability and potassium conductance of the plasma membrane of renal epithelial cells may be regulated by the ECM. DG and syntrophin are localized basolaterally in MDCK cells where they codistribute with Kir4.1 and AQP4, supporting a role for the DAP complex in the regulation of the polarized distribution of these channels. Indeed, siRNA-mediated knockdown of DG resulted in a decrease in basolateral Kir4.1 expression in the absence of exogenous laminin, indicating that DG is crucial for the polarized distribution of this channel in epithelial cells. However, in the presence of exogenous laminin increased basolateral expression of both Kir4.1 and AQP4 occurs in MDCK cells deficient for DG. This suggests that regulation of the polarity of these channels by laminin in epithelial cells involves DG but also other laminin receptors such as those of the integrin family. Indeed, numerous studies have described synergistic interactions between DG and receptors of the integrin family (14, 35, 39).
DG and integrins contribute to the basolateral stabilization of Kir4.1 and AQP4 by laminin and fibronectin.
To address the mechanisms underlying the increase in channel expression in the presence of fibronectin and laminin, we performed FRAP analysis on the lateral membrane of cells expressing GFP-tagged Kir4.1 or AQP4 channels cultured on either ECM molecule. We found that both caused significant reductions in the mobilities of the channels in a dose-dependent manner. These results show that the ECM regulates the stabilization of both channels at the membrane, thereby contributing to their polarized distribution at the basolateral domain of epithelial cells. This ECM-mediated stabilization of the channels may result from a direct mechanism whereby fibronectin and laminin have access to the lateral domains due to the partial polarization of the cells grown on glass coverslips. Alternatively, it may be due to an indirect mechanism involving cell signaling resulting from the interaction of the ECM with the basal cell domains. Reduced endocytosis may participate in the increased ECM-induced stabilization of the channels; however, we believe that to be unlikely as the FRAP analysis was conducted over short time periods (45–60 s).
We have also investigated the effect of the ECM on the stability of Kir2.1 at the plasma membrane of MDCK cells by FRAP. Our results show a decrease in Kir2.1 diffusion rate in the presence of fibronectin but not laminin. Although both Kir4.1 and Kir2.1 contain a PDZ-binding motif at the C terminal that enables their interaction with the DAP complex and therefore with laminin in the ECM (22), Kir2.1 stabilization at the membrane does not seem to be regulated by laminin. This is likely due to the fact that Kir2.1 interacts with the DAP complex with a lower affinity than Kir4.1 (22). These data argue that laminin-induced stabilization of Kir4.1 is mediated by the DAP complex and that inwardly rectifying potassium channels containing a PDZ-binding motif are differentially regulated by laminin.
The expression of several fibronectin-binding integrins (46) and the stabilization of both Kir4.1 and AQP4 in MDCK cells in the presence of fibronectin, prompted us to investigate the possible implication of integrins in this effect. The application of fibronectin-integrin-blocking peptides Arg-Gly-Asp (RGD) and Gly-Arg-Gly-Asp-Ser-Pro-Lys (GRGDSPK) in tandem with fibronectin to MDCK cells followed by cell surface biotinylation shows that GRGDSPK reduces significantly the effect of fibronectin on Kir4.1 and AQP4 expression at the basolateral domain (Fig. 9, B and D). In addition, FRAP analysis shows that RGD peptides inhibit the effect of fibronectin, resulting in a faster rate of recovery and higher mobile fractions of Kir4.1 and AQP4. These data suggest that fibronectin receptors, most likely integrins, play a role in limiting the rate of lateral diffusion within the plasma membrane of both Kir4.1 and AQP4. We then determined the effect of these disintegrin peptides on laminin-induced retardation of channel diffusion and found that GRGDSPK enhances the rate of lateral diffusion of Kir4.1 and AQP4 over cells treated with laminin alone, suggesting that laminin's effect involves the contribution of fibronectin and fibronectin-binding receptors as well. Addition of exogenous laminin-1 to MDCK cells leads to the accumulation of endogenous fibronectin, suggesting that the laminin-induced effects may be indirect in that they involve fibronectin (Supplemental Fig. S3). Furthermore, the inhibition of fibronectin binding to αvβ3-integrin using a function-blocking antibody (Fig. 11) indicates that αvβ3-integrin plays a role in the ECM-mediated stabilization of AQP4 but not Kir4.1 at the basolateral domain of MDCK cells. The reversal of the fibronectin-induced decreases in Kir4.1 diffusion rate by RGD-containing peptides and the inability of the αvβ3-integrin antibody to prevent this effect suggest that the ECM-mediated stabilization of Kir4.1 may be regulated by α5β1-integrin (16).
To date, evidence from the literature has focused on the role of the interactions of DG and integrins with laminins in the morphogenesis and wound healing in the kidney, salivary gland, and lung (4, 8, 18, 26). Our findings reveal a new role for DG and integrins and show that the interaction of these receptors with the ECM plays an important role in the polarized distribution of Kir4.1 and AQP4 at the basolateral domain of kidney epithelial cells and therefore is critical to the maintenance of osmoregulation in the renal system.
GRANTS
This study was supported by a grant from the Natural Sciences and Engineering Research Council of Canada (NSERC) to H. Moukhles.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Drs. N. Klocker and J. Merot for providing the constructs for the GFP-Kir4.1 and VSV-AQP4 channels, Susan Goto for generating the GFP-AQP4 construct as well as the GFP-Kir4.1 and GFP-AQP4 stable MDCK cell lines, and Yadan Qi for helping with the immunohistochemistry on kidney sections.
REFERENCES
- 1. Adams ME, Butler MH, Dwyer TM, Peters MF, Murnane AA, Froehner SC. Two forms of mouse syntrophin, a 58 kD dystrophin-associated protein, differ in primary structure and tissue distribution. Neuron 11: 531–540, 1993 [DOI] [PubMed] [Google Scholar]
- 2. Ahn AH, Kunkel LM. The structural and functional diversity of dystrophin. Nat Genet 3: 283–291, 1993 [DOI] [PubMed] [Google Scholar]
- 3. Blake DJ, Hawkes R, Benson MA, Beesley PW. Different dystrophin-like complexes are expressed in neurons and glia. J Cell Biol 147: 645–658, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen D, Roberts R, Pohl M, Nigam S, Kreidberg J, Wang Z, Heino J, Ivaska J, Coffa S, Harris RC, Pozzi A, Zent R. Differential expression of collagen- and laminin-binding integrins mediates ureteric bud and inner medullary collecting duct cell tubulogenesis. Am J Physiol Renal Physiol 287: F602–F611, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Deng WM, Schneider M, Frock R, Castillejo-Lopez C, Gaman EA, Baumgartner S, Ruohola-Baker H. Dystroglycan is required for polarizing the epithelial cells and the oocyte in Drosophila. Development 130: 173–184, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Durbeej M, Henry MD, Ferletta M, Campbell KP, Ekblom P. Distribution of dystroglycan in normal adult mouse tissues. J Histochem Cytochem 46: 449–457, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Durbeej M, Larsson E, Ibraghimov-Beskrovnaya O, Roberds SL, Campbell KP, Ekblom P. Non-muscle alpha-dystroglycan is involved in epithelial development. J Cell Biol 130: 79–91, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Durbeej M, Talts JF, Henry MD, Yurchenco PD, Campbell KP, Ekblom P. Dystroglycan binding to laminin alpha1LG4 module influences epithelial morphogenesis of salivary gland and lung in vitro. Differentiation 69: 121–134, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Ervasti JM, Campbell KP. Dystrophin and the membrane skeleton. Curr Opin Cell Biol 5: 82–87, 1993 [DOI] [PubMed] [Google Scholar]
- 10. Frigeri A, Nicchia GP, Nico B, Quondamatteo F, Herken R, Roncali L, Svelto M. Aquaporin-4 deficiency in skeletal muscle and brain of dystrophic mdx mice. FASEB J 15: 90–98, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Gee SH, Blacher RW, Douville PJ, Provost PR, Yurchenco PD, Carbonetto S. Laminin-binding protein 120 from brain is closely related to the dystrophin-associated glycoprotein, dystroglycan, and binds with high affinity to the major heparin binding domain of laminin. J Biol Chem 268: 14972–14980, 1993 [PubMed] [Google Scholar]
- 12. Guadagno E, Moukhles H. Laminin-induced aggregation of the inwardly rectifying potassium channel, Kir41, and the water-permeable channel, AQP4, via a dystroglycan-containing complex in astrocytes. Glia 47: 138–149, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Horton MA. The alpha v beta 3 integrin “vitronectin receptor.” Int J Biochem Cell Biol 29: 721–725, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Hozumi K, Suzuki N, Nielsen PK, Nomizu M, Yamada Y. Laminin alpha1 chain LG4 module promotes cell attachment through syndecans and cell spreading through integrin alpha2beta1. J Biol Chem 281: 32929–32940, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 110: 673–687, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69: 11–25, 1992 [DOI] [PubMed] [Google Scholar]
- 17. Ito M, Inanobe A, Horio Y, Hibino H, Isomoto S, Ito H, Mori K, Tonosaki A, Tomoike H, Kurachi Y. Immunolocalization of an inwardly rectifying K+ channel, K(AB)-2 (Kir4.1), in the basolateral membrane of renal distal tubular epithelia. FEBS Lett 388: 11–15, 1996 [DOI] [PubMed] [Google Scholar]
- 18. Jiang ST, Chiu SJ, Chen HC, Chuang WJ, Tang MJ. Role of alpha3beta1 integrin in tubulogenesis of Madin-Darby canine kidney cells. Kidney Int 59: 1770–1778, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Khurana TS, Hoffman EP, Kunkel LM. Identification of a chromosome 6-encoded dystrophin-related protein. J Biol Chem 265: 16717–16720, 1990 [PubMed] [Google Scholar]
- 20. Kim TW, Wu K, Xu JL, Black IB. Detection of dystrophin in the postsynaptic density of rat brain and deficiency in a mouse model of Duchenne muscular dystrophy. Proc Natl Acad Sci USA 89: 11642–11644, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kofuji P, Newman EA. Potassium buffering in the central nervous system. Neuroscience 129: 1045–1056, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leonoudakis D, Conti LR, Anderson S, Radeke CM, McGuire LM, Adams ME, Froehner SC, Yates JR, 3rd, Vandenberg CA. Protein trafficking and anchoring complexes revealed by proteomic analysis of inward rectifier potassium channel (Kir2x)-associated proteins. J Biol Chem 279: 22331–22346, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Lidov HG, Byers TJ, Watkins SC, Kunkel LM. Localization of dystrophin to postsynaptic regions of central nervous system cortical neurons. Nature 348: 725–728, 1990 [DOI] [PubMed] [Google Scholar]
- 24. Love DR, Morris GE, Ellis JM, Fairbrother U, Marsden RF, Bloomfield JF, Edwards YH, Slater CP, Parry DJ, Davies KE. Tissue distribution of the dystrophin-related gene product and expression in the mdx and dy mouse. Proc Natl Acad Sci USA 88: 3243–3247, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Madrid R, Le Maout S, Barrault MB, Janvier K, Benichou S, Merot J. Polarized trafficking and surface expression of the AQP4 water channel are coordinated by serial and regulated interactions with different clathrin-adaptor complexes. EMBO J 20: 7008–7021, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mak GZ, Kavanaugh GM, Buschmann MM, Stickley SM, Koch M, Goss KH, Waechter H, Zuk A, Matlin KS. Regulated synthesis and functions of laminin 5 in polarized Madin-Darby canine kidney epithelial cells. Mol Biol Cell 17: 3664–3677, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matlin KS, Haus B, Zuk A. Integrins in epithelial cell polarity: using antibodies to analyze adhesive function and morphogenesis. Methods 30: 235–246, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Michele DE, Barresi R, Kanagawa M, Saito F, Cohn RD, Satz JS, Dollar J, Nishino I, Kelley RI, Somer H, Straub V, Mathews KD, Moore SA, Campbell KP. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature 418: 417–422, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Montanaro F, Carbonetto S, Campbell KP, Lindenbaum M. Dystroglycan expression in the wild type and mdx mouse neural retina: synaptic colocalization with dystrophin, dystrophin-related protein but not laminin. J Neurosci Res 42: 528–538, 1995 [DOI] [PubMed] [Google Scholar]
- 30. Moore SA, Saito F, Chen J, Michele DE, Henry MD, Messing A, Cohn RD, Ross-Barta SE, Westra S, Williamson RA, Hoshi T, Campbell KP. Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature 418: 422–425, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Moukhles H, Carbonetto S. Dystroglycan contributes to the formation of multiple dystrophin-like complexes in brain. J Neurochem 78: 824–834, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Nagelhus EA, Horio Y, Inanobe A, Fujita A, Haug FM, Nielsen S, Kurachi Y, Ottersen OP. Immunogold evidence suggests that coupling of K+ siphoning and water transport in rat retinal Muller cells is mediated by a coenrichment of Kir4.1 and AQP4 in specific membrane domains. Glia 26: 47–54, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Nagelhus EA, Veruki ML, Torp R, Haug FM, Laake JH, Nielsen S, Agre P, Ottersen OP. Aquaporin-4 water channel protein in the rat retina and optic nerve: polarized expression in Muller cells and fibrous astrocytes. J Neurosci 18: 2506–2519, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nico B, Frigeri A, Nicchia GP, Corsi P, Ribatti D, Quondamatteo F, Herken R, Girolamo F, Marzullo A, Svelto M, Roncali L. Severe alterations of endothelial and glial cells in the blood-brain barrier of dystrophic mdx mice. Glia 42: 235–251, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Nodari A, Previtali SC, Dati G, Occhi S, Court FA, Colombelli C, Zambroni D, Dina G, Del Carro U, Campbell KP, Quattrini A, Wrabetz L, Feltri ML. Alpha6beta4 integrin and dystroglycan cooperate to stabilize the myelin sheath. J Neurosci 28: 6714–6719, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Noel G, Belda M, Guadagno E, Micoud J, Klocker N, Moukhles H. Dystroglycan Kir4.1 coclustering in retinal Muller glia is regulated by laminin-1 and requires the PDZ-ligand domain of Kir4.1. J Neurochem 94: 691–702, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Noel G, Tham DK, Moukhles H. Interdependence of laminin-mediated clustering of lipid rafts and the dystrophin complex in astrocytes. J Biol Chem 284: 19694–19704, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O'Brien LE, Jou TS, Pollack AL, Zhang Q, Hansen SH, Yurchenco P, Mostov KE. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol 3: 831–838, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Peng H, Shah W, Holland P, Carbonetto S. Integrins and dystroglycan regulate astrocyte wound healing: the integrin beta1 subunit is necessary for process extension and orienting the microtubular network. Dev Neurobiol 68: 559–574, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Peters MF, Adams ME, Froehner SC. Differential association of syntrophin pairs with the dystrophin complex. J Cell Biol 138: 81–93, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Peters MF, O'Brien KF, Sadoulet-Puccio HM, Kunkel LM, Adams ME, Froehner SC. β-Dystrobrevin, a new member of the dystrophin family. Identification, cloning, and protein associations. J Biol Chem 272: 31561–31569, 1997 [DOI] [PubMed] [Google Scholar]
- 42. Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA 90: 3710–3714, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rurak J, Noel G, Lui L, Joshi B, Moukhles H. Distribution of potassium ion and water permeable channels at perivascular glia in brain and retina of the Large(myd) mouse. J Neurochem 103: 1940–1953, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Schmitz F, Holbach M, Drenckhahn D. Colocalization of retinal dystrophin and actin in postsynaptic dendrites of rod and cone photoreceptor synapses. Histochemistry 100: 473–479, 1993 [DOI] [PubMed] [Google Scholar]
- 45. Schneider M, Khalil AA, Poulton J, Castillejo-Lopez C, Egger-Adam D, Wodarz A, Deng WM, Baumgartner S. Perlecan and dystroglycan act at the basal side of the Drosophila follicular epithelium to maintain epithelial organization. Development 133: 3805–3815, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schoenenberger CA, Zuk A, Zinkl GM, Kendall D, Matlin KS. Integrin expression and localization in normal MDCK cells and transformed MDCK cells lacking apical polarity. J Cell Sci 107: 527–541, 1994 [DOI] [PubMed] [Google Scholar]
- 47. Schrier RW, Cadnapaphornchai MA. Renal aquaporin water channels: from molecules to human disease. Prog Biophys Mol Biol 81: 117–131, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Stockklausner C, Klocker N. Surface expression of inward rectifier potassium channels is controlled by selective Golgi export. J Biol Chem 278: 17000–17005, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Sunada Y, Campbell KP. Dystrophin-glycoprotein complex: molecular organization and critical roles in skeletal muscle. Curr Opin Neurol 8: 379–384, 1995 [PubMed] [Google Scholar]
- 50. Tanemoto M, Abe T, Onogawa T, Ito S. PDZ binding motif-dependent localization of K+ channel on the basolateral side in distal tubules. Am J Physiol Renal Physiol 287: F1148–F1153, 2004 [DOI] [PubMed] [Google Scholar]
- 51. Terris J, Ecelbarger CA, Marples D, Knepper MA, Nielsen S. Distribution of aquaporin-4 water channel expression within rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 269: F775–F785, 1995 [DOI] [PubMed] [Google Scholar]
- 52. Weir ML, Oppizzi ML, Henry MD, Onishi A, Campbell KP, Bissell MJ, Muschler JL. Dystroglycan loss disrupts polarity and beta-casein induction in mammary epithelial cells by perturbing laminin anchoring. J Cell Sci 119: 4047–4058, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yu W, Datta A, Leroy P, O'Brien LE, Mak G, Jou TS, Matlin KS, Mostov KE, Zegers MM. Beta1-integrin orients epithelial polarity via Rac1 and laminin. Mol Biol Cell 16: 433–445, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zinkl GM, Zuk A, van der Bijl P, van Meer G, Matlin KS. An antiglycolipid antibody inhibits Madin-Darby canine kidney cell adhesion to laminin and interferes with basolateral polarization and tight junction formation. J Cell Biol 133: 695–708, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]