Abstract
Statins are 3-hydroxyl-3-methyglutaryl-CoA reductase inhibitors that are commonly used to inhibit cholesterol biosynthesis. Emerging data have suggested that they also have “pleotropic effects,” including modulating actin cytoskeleton reorganization. Here, we report an effect of simvastatin on the trafficking of aquaporin-2 (AQP2). Specifically, simvastatin induced the membrane accumulation of AQP2 in cell cultures and kidneys in situ. The effect of simvastatin was independent of protein kinase A activation and phosphorylation at AQP2-Ser256, a critical event involved in vasopressin (VP)-regulated AQP2 trafficking. Further investigation showed that simvastatin inhibited endocytosis in parallel with downregulation of RhoA activity. Overexpression of active RhoA attenuated simvastatin's effect, suggesting the involvement of this small GTPase in simvastatin-mediated AQP2 trafficking. Finally, the effect of simvastatin on urinary concentration was investigated in VP-deficient Brattleboro rats. Simvastatin acutely (3–6 h) increased urinary concentration and decreased urine output in these animals. In summary, simvastatin regulates AQP2 trafficking in vitro and urinary concentration in vivo via events involving downregulation of Rho GTPase activity and inhibition of endocytosis. Our study provides an alternative mechanism to regulate AQP2 trafficking, bypassing the VP-vasopressin receptor signaling pathway.
Keywords: aquaporin 2, simvastatin, vasopressin, endocytosis, Brattleboro rats
aquaporin-2 (AQP2) is an essential water channel mediating water reabsorption and maintaining water homeostasis in mammals. Under physiological conditions, AQP2 trafficking is regulated mainly by vasopressin (VP) in response to plasma osmolarity and volume status (8, 22, 32). The impairment of AQP2 signaling results in water retention seen in various edematous states as well as a concentrating defect seen in diabetes insipidus (DI) (9, 33). The classic view of AQP2-regulated trafficking is that, in the presence of VP, the G protein-coupled vasopressin type 2 receptor (V2R) is activated, which induces Gsα dissociation, adenylyl cyclase activation, and subsequently an elevation of intracellular cAMP. The rise in cAMP activates protein kinase type A (PKA) and leads to the phosphorylation of AQP2 at serine 256 and other residues in the AQP2 COOH terminus (7, 14, 15, 27). Phosphorylation of AQP2 at Ser256 promotes the membrane accumulation of AQP2 by dual effects on AQP2 exocytosis and endocytosis (10, 19, 26, 30, 35).
Considerable progress has been made in elucidating signaling events that result in AQP2 translocation not only in response to VP but also to other stimuli that are independent of VP and cAMP in vitro. Some of these alternative stimuli are promising potential therapies for bypassing the defective VP-V2R pathway that contributes to 90% of the cases of congenital nephrogenic DI. Indeed, cumulative data have shown that, besides VP and cAMP, alternative pathways exist to modulate AQP2 trafficking. For example, phosphodiesterase inhibitors, sodium nitroprusside, and l-arginine regulate AQP2 trafficking via a V2R-independent nitric oxide/cGMP pathway and cyclooxygenase (Cox) 2 inhibitors via the COX/prostaglandin E2 pathway (3, 6, 20, 46, 57).
Interestingly, several reports have shown that modulating the actin cytoskeleton network can also affect AQP2 trafficking in vitro (31, 34, 51, 54). Rho GTPases have been shown to play a critical role in cytoskeletal organization. Together with their regulating proteins, including Rho GTPase-activating proteins, guanine nucleotide exchange factors, and GDP dissociation inhibitors (GDI), they serve as molecular switches to regulate multiple cellular processes, including growth, migration, and vesicle/protein trafficking (1, 2, 18, 41, 48). Among many reported functions of Rho GTPases, the involvement of RhoA in endocytosis and intracellular vesicle trafficking are of potential relevance to studies on AQP2 trafficking (53). It has been reported that depolymerization of the actin network and Rho inhibition result in an increase of membrane accumulation of AQP2, while blockade of VP-induced AQP2 translocation in response to Rho activation was shown to be associated with increased actin polymerization (21, 50, 52, 54, 57). Precisely how Rho GTPases and their family members modulate AQP2 trafficking and membrane accumulation is, however, largely unknown.
Recent studies have shown that statins, a family of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, appear to be important modulators of Rho GTPases and their binding proteins (17, 36). Statins were originally developed as effective therapeutic agents for reducing cholesterol and improving cardiovascular outcome. However, newer studies have revealed a broad spectrum of “pleiotropic” effects of statins, including the activation of endothelial nitric oxide synthesis and anti-inflammation through modulating Rho activity in neutrophils (28, 43). More interestingly, studies in vitro have also suggested that statins reduced endocytosis of fluorescein isothiocyanate (FITC)-labeled albumin in cultured kidney proximal tubular cells possibly by inhibiting isoprenylation of Rho GTPase, which leads to actin cytoskeletal reorganization during protein trafficking (47). Therefore, we hypothesized that statins may play a similar important role in regulating AQP2 trafficking in collecting duct (CD) principal cells in vitro and in vivo (4).
MATERIALS AND METHODS
Reagents and chemicals.
Polyclonal anti-AQP2 antibody was from Santa Cruz Biotechnology (Santa Cruz, CA), monoclonal RhoA was from Cell Signaling (Danvers, MA), the RhoA activation kit was from Cytoskeleton (Denver, CO), and secondary FITC or Cy3-conjugated antibodies were from the Jackson Laboratory (Bar Harbor, ME). FITC-dextran (10,000 kDa) and tetramethylrhodamine-transferrin conjugate (TrfRh) were from Molecular Probes (Invitrogen, Carlsbad, CA). The cAMP assay kit was from GE Health Care (Piscataway, NJ). Sulfo-NHS-LC-Biotin was purchased from Pierce and streptavidin-coupled Dynabeads from Invitrogen. PKA inhibitors H-89 and myristoylated PKA inhibitor 14–22 amide (mPKI) were from Calbiochem/EMD (Gibbstown, NJ). VP, methyl-β-cyclodextrin (mβCD), and simvastatin were purchased from Sigma (St. Louis, MO). Simvastatin was dissolved in ethanol (EtOH) to a concentration of 20 mM as a stock solution and was subsequently diluted in culture medium or saline. Constitutively active RhoA G14V and dominant-negative RhoA T19N constructs were kindly provided by Dr. Silvio Gutkind (National Institutes of Health/National Institute of Dental and Craniofacial Research) and Emily Lecuona (Northwestern University).
Cell culture.
LLC-PK1 cells or stable cell lines expressing wild-type AQP2 and an AQP2-S256A mutant mimicking the dephosphorylated state of AQP2 were established and maintained in DMEM, 10% FBS at 37°C as previously described (19, 25). In some experiments, cells were pretreated with H-89 (1 μM) or mPKI (1 μM) for 1 h before adding simvastatin or VP. In experiments in which cells were transfected with RhoA constructs, cells were treated with simvastatin or VP as mentioned above 48 h after transfection.
Kidney slice incubations in vitro.
The effect of simvastatin on AQP2 trafficking was further studied using thin slices of kidney as previously described (3). Briefly, kidneys were rapidly dissected out, and thin transversal kidney sections (0.5 mm) were cut immediately. After incubation in equilibrated Hanks' balanced salt solution (HBSS) at 37°C for 15 min, simvastatin or VP was added and incubated for various times. Next, kidney slices were fixed by immersion in periodate-lysine paraformaldehyde fixative and processed as described previously (5).
Immunostaining of cells and kidney tissue, and immunoblotting.
Immunostaining of cells grown on cover slips and of tissue sections was performed as reported previously, and immunoblotting was also performed as previously reported (26). Apical AQP2 fluorescence was quantified as follows. All images were collected using the same exposure time and analyzed using IPLab Spectrum software. A segmentation function was used to define the area occupied by AQP2 at the apical pole of principal cells. Background intensity levels were defined as the mean pixel intensity of the nucleus, and staining was defined as pixel intensities greater than two times the background. The total area of the highlighted pixels was quantified for each cell. On average, 30 measurements were taken for each sample, and samples were taken from at least 3 different experiments. Results are expressed as means ± SE of the pixel area occupied by the fluorescence signal. Statistical analyses were made using the Student's t-test. Differences were considered significant at P values at least <0.05.
Cell surface biotinylation.
Cell surface biotinylation was performed as previously described (25). Briefly, after treatment with simvastatin or VP, cells were incubated with sulfo-NHS-LC-Biotin (1 mg/ml) at 4°C for 30 min. After being washed, cells were lysed in RIPA buffer and subjected to pull down by streptavidin-coupled dynabeads following the manufacturer's instructions (Invitrogen). Samples from streptavidin pull down experiments were subjected to SDS-PAGE and immunoblotting using an anti-AQP2 antibody to detect AQP2 signal in the surface biotinylated membrane protein pool. Protein signal intensity from the immunoblots was measured and quantified using IPlab software as described previously (26).
cAMP assays.
Measurements of intracellular cAMP levels in the presence of simvastatin and VP were performed using a cAMP Biotrak Enzymeimmunoassay according to the manufacturer's instructions (GE HealthCare). Each cAMP assay was performed in triplicate.
Exocytosis and endocytosis assays.
The fluorescence exocytosis assay was performed as previously described (35). Briefly, LLC-AQP2 cells stably expressing a soluble, secreted yellow fluorescent protein (LLC-AQP2-ssYFP) were grown to confluence on 24-well cell culture plates. After being washed with HBSS, cells were incubated with 200 μl of HBSS in the presence or absence of simvastatin for 1 h. Next, 100 μl of medium were transferred from each well to a black half-area 96-well plate (Corning). The ssYFP fluorescence in each well was read immediately after collection using a multimode plate reader (model DTX880; Beckman-Coulter). Background fluorescence values were obtained from empty wells. The fluorescence value for each sample was reported as relative fluorescence intensity after subtracting the background signal. Control experiments were set up with and without the solvent EtOH. The values represent at least three independent experiments, measured in triplicate.
The endocytosis assay was performed on stable LLC-AQP2 cells as described previously (25). Briefly, cells were seeded on 24-well cell culture plates and treated with simvastatin for 1 h, and then cells were incubated with 0.5 mg/ml FITC-dextran (10,000 kDa) in HBSS buffer for 30 min at 37°C. After being washed with cold PBS buffer, cells were lysed in 200 μl of lysis buffer (20 mM Tris·HCl, pH 7.4, 5 mM EDTA, 5 mM EGTA, 30 mM NaF, 30 mM Na4P2PO7, 2 mM Na3Vo4, 1% Triton X-100, and 0.1% SDS). Fluorescence released in the medium was detected using the multimode plate reader as mentioned above. The values represent at least three independent experiments, measured in triplicate. In addition, some experiments were performed using TrfRh to monitor clathrin-mediated endocytosis as reported previously (26). Briefly, cells grown on cover slips were treated with simvastatin for 1 h, followed by incubation with 3 × 10−7 M TrfRh at 37°C for 30 min. After being washed with cold PBS, cells were fixed with 4% paraformaldehyde/PBS, and then cover slips were mounted and viewed using our fluorescence microscope.
Animal experiments.
Animal experiments were approved by the MGH Institutional Committee on Research Animal Care in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Before the experiment, adult Brattleboro rats were allowed to accustom to metabolic cages for 24 h, and their urine was collected. Simvastatin (75 μl) at 20 mM was mixed with 75 μl of saline and injected intraperitoneally to animals to give an estimated serum concentration of 200 μM. An equal volume of EtOH (solvent) was injected in control animals. Urine from each animal was collected every hour after injection for 6 h and then every 12 h for 3 days. Body weight and food and water consumption were monitored and recorded. Similar experiments were repeated a second time after over one month of drug washout in each group. Similar urinary results were obtained from both the first and second rounds of experiments. Animals were killed 6 h after the second time of treatment with simvastatin. Kidney tissues were processed as described previously, and blood samples were collected before the animals were killed. The chemistry profiles of serum and urine were analyzed at the MGH Clinical Pathology Laboratory facility. Urine volume and osmolarity were analyzed using a vapor pressure osmometer (Vapro 5520; Wescor, Logan, UT).
Data analysis.
Quantification of fluorescence intensity in immunofluorescently stained cells and tissues was performed using IPlab software as mentioned in the individual experiments above. Data are expressed as means ± SE. Statistical analyses were done using the Student's t-test. Differences were considered to be significant at P values <0.05. As presented in individual histograms, the error bars indicate SE, and the asterisks indicate P < 0.05.
RESULTS
Simvastatin causes membrane accumulation of AQP2 in cultured cells and kidney slices in vitro.
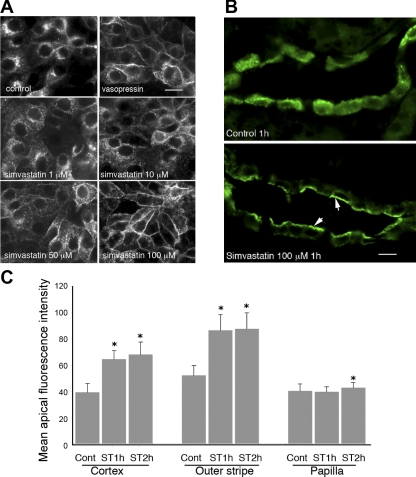
The effect of simvastatin on AQP2 trafficking was investigated in cells and kidney slices in vitro. Our data showed that simvastatin treatment resulted in membrane accumulation of AQP2 in LLC-AQP2 cells in a dose-dependent manner. Specifically, a partial membrane accumulation of AQP2 was detectable after treatment with 10 μM simvastatin for 1 h and became more evident with increasing concentrations up to 200 μM. The membrane accumulation peaked at concentrations around 100 μM (Fig. 1A). There was no significant toxicity detected in cells treated with simvastatin at 200 μM for 2 h by morphological examination and trypan blue staining (data not shown). However, profound and diffuse intracellular vacuolization was seen in cells treated with the two higher concentrations of simvastatin for 12 h or more. To confirm that this effect on AQP2 was not simply a cell culture phenomenon, we applied simvastatin to cultured kidney slices (Fig. 1B). Kidney slices were obtained from freshly dissected Brattleboro rat kidneys and were immediately processed and treated with simvastatin at a concentration of 100 μM for 1, 2, and 4 h. Our results showed that, similarly to the observations in cell cultures, 100 μM simvastatin caused significant membrane accumulation of AQP2 in the principal cells of the CD in cultured kidney slices after 1 and 2 h of treatment. Quantification revealed that simvastatin-induced membrane accumulation of AQP2 was significant in CD from the cortex and outer medulla and was less evident in the inner medulla/papilla (p < 0.01, Fig. 1C).
Fig. 1.
Simvastatin-induced membrane accumulation of aquaporin-2 (AQP2) in AQP2-expressing LLCPK1 cells (LLC-AQP2) and in principal cells of the collecting duct (CD) from kidney slices in vitro. A: LLC-AQP2 cells treated with ethanol (EtOH, control), vasopressin (VP, 10−8 M) for 30 min, and with simvastatin at various concentrations for 1 h. Membrane accumulation of AQP2 is seen in cells treated with VP and with simvastatin in a dose-dependent manner. Specifically, AQP2 membrane accumulation is detectable at 10 μM of simvastatin and peaks at 100 μM of simvastatin. Simvastatin-induced membrane accumulation of AQP2 was further investigated in Brattleboro rat kidney slices in vitro as shown in B. Similar to results in cell culture, treatment with simvastatin (100 μM) for 1 h causes significant membrane accumulation of AQP2 in principal cells of the CD in kidney slices (indicated by arrows), whereas in the control unstimulated state, AQP2 distributes throughout the cytoplasm. C: quantification of apical accumulation of AQP2 in kidney slices treated with simvastatin (ST). A strong and significant apical accumulation of AQP2 is seen in CD located in the cortex and outer medulla, whereas a weaker apical staining is seen in the inner medulla. Cont, control. Error bars represent means ± SE. *P < 0.01. Data were obtained from 3 experiments. Bar = 10 μm in A and B.
Membrane accumulation of AQP2 induced by simvastatin is independent of cAMP/PKA activation and the phosphorylation of AQP2 at Ser256.
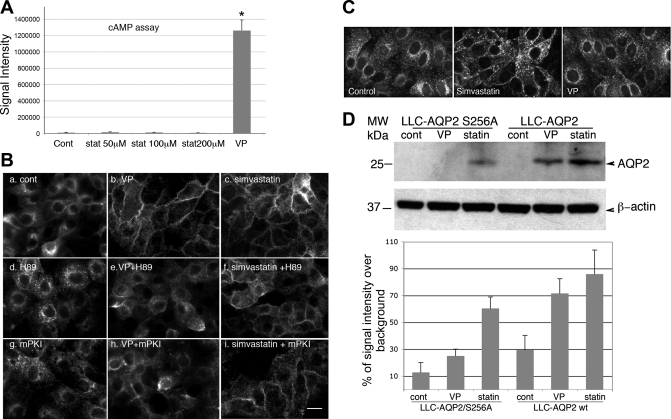
Because cAMP-mediated AQP2 trafficking is the predominant mechanism of regulated trafficking of AQP2 by VP under physiological conditions, the involvement of cAMP/PKA activation in simvastatin-mediated membrane accumulation of AQP2 was investigated (Fig. 2). First we determined the level of intracellular cAMP in cells treated with simvastatin (Fig. 2A). In contrast to cells treated with VP, which caused a greater than sixfold increase of intracellular cAMP compared with unstimulated cells, simvastatin did not cause significant elevation of intracellular cAMP. To further confirm the lack of involvement of PKA/cAMP signaling in simvastatin-induced AQP2 trafficking, cells were treated with PKA inhibitors before exposure to simvastatin (Fig. 2B). Inhibiting PKA activation by H-89 or the PKA peptide inhibitor mPKI did not affect the AQP2 membrane accumulation induced by simvastatin (Fig. 2B, f and i). In contrast, VP-induced AQP2 membrane accumulation was greatly reduced by PKA inhibition (Fig. 2B, e and h).
Fig. 2.
Simvastatin-induced membrane accumulation of AQP2 is not mediated through cAMP/protein kinase type A (PKA) activation and is independent of phosphorylation of AQP2 at Ser256. The level of cAMP inside cells (LLC-AQP2) after treatment with VP or simvastatin was measured (A). There is no significant elevation of intracellular cAMP in response to treatment with simvastatin, even at 200 μM. In contrast, intracellular cAMP is elevated >6-fold in cells treated with VP (10−8 M). Simvastatin-induced membrane accumulation was next examined in the presence of PKA inhibitors, H-89 and myristoylated PKA inhibitor (mPKI) (B). LLC-AQP2 cells were pretreated with 1 μM H-89 or 1 μM mPKI for 1 h before addition of VP or simvastatin. H-89 or mPKI alone do not cause any change of baseline AQP2 distribution (d and g), nor do they affect simvastatin-induced membrane accumulation of AQP2 (c, f, and i). In contrast, the VP-induced membrane accumulation of AQP2 is markedly inhibited by blocking PKA activity both with H-89 or mPKI (b, e, and h). The effect of simvastatin on AQP2 trafficking was also investigated in LLC-AQP2-S256A mutant cells expressing Ser256A, mimicking unphosphorylated AQP2 at Ser256 using both immunofluorescence staining (C) and cell surface biotinylation (D). Even though to a lesser degree compared with LLC-AQP2 cells [expressing wild-type (wt) AQP2], simvastatin induces membrane accumulation of AQP2 in cells expressing AQP2-S256A while VP does not (C and D). The histogram in D represents quantification of surface-biotinylated AQP2 signal from 3 independent experiments (percentage increase of signal intensity over background). stat, Statin; MW, molecular weight. Error bars represent means ± SE. *P < 0.01 in B. Scale bar = 10 μm in B and C.
Next, we investigated the requirement of phosphorylation of Ser256 (an essential event in VP-mediated AQP2 trafficking) in simvastatin-induced membrane accumulation of AQP2. As previously reported (6, 19, 27), in LLCPK1 cells expressing AQP2-S256A, mimicking the dephosphorylated state of AQP2 at Ser256, AQP2 failed to accumulate on the plasma membrane upon treatment with VP. However, in the presence of simvastatin, AQP2 S256A was capable of accumulating in the plasma membrane, even though to a lesser degree compared with wild-type AQP2 (Fig. 2, C and D). To confirm this observation, we performed cell surface biotinylation to show that indeed simvastatin was able to cause membrane accumulation of AQP2 S256A in cells, but again to a lesser degree compared with cells expressing wild-type AQP2 (Fig. 2D). Therefore, both immunofluorescence staining and cell surface biotinylation indicate that the membrane accumulation of AQP2 induced by simvastatin is independent of PKA activation and is most likely independent of phosphorylation of AQP2 at Ser256.
Simvastatin-induced membrane accumulation of AQP2 is mainly due to reduced endocytosis rather than increased exocytosis.
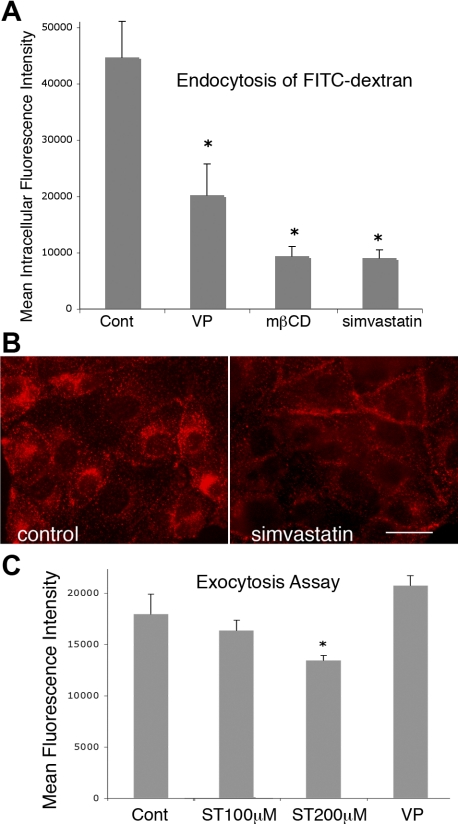
Because membrane accumulation of AQP2 seen in the presence of simvastatin could be attributed to either reduced endocytosis of AQP2, facilitated exocytosis, or both, these trafficking events were investigated in the presence of simvastatin. Our data showed that simvastatin caused significant inhibition of endocytosis in cells (Figs. 3, A and B). Figure 3A shows the quantification of endocytosed FITC-dextran (a fluid phase marker) by cells; there was a significant up to 75% reduction of overall endocytosis of FITC-dextran (10 kDa) by cells in the presence of simvastatin (100 μM) (Fig. 3A). Both VP and mβCD also caused significant inhibition of endocytosis of this fluid phase marker. Our studies and those of other groups have shown that AQP2 is endocytosed mainly via clathrin-mediated endocytosis (25, 49). Therefore, the effect of simvastatin on clathrin-mediated endocytosis was investigated using TrfRh (Fig. 3B). There was a clear blockade of uptake of TrfRh by cells that were treated with simvastatin, suggesting an inhibition of the clathrin-mediated endocytotic process in those cells (Fig. 3B). Untreated cells internalized most of the TrfRh into a perinuclear compartment (Fig. 3B, left), whereas a considerable amount of TrfRh remained at the cell surface in simvastatin-treated cells (Fig. 3B, right). In addition, the effect of simvastatin on exocytosis was also investigated using our recently developed fluorimetric ssYFP exocytosis assay (35) (Fig. 3C). In fact, we quantified a small inhibition of exocytosis (∼10%) in simvastatin-treated cells at a concentration of 200 μM and no significant inhibition nor stimulation of exocytosis at 100 μM, a concentration that induces significant membrane accumulation of AQP2 in cells and kidney slices. Despite this reduction in exocytosis, AQP2 accumulated at the cell surface in response to simvastatin treatment, indicating that this effect was mainly due to a more quantitatively important inhibition of endocytosis.
Fig. 3.
Simvastatin inhibits endocytosis but has little effect on exocytosis. Endocytosis of the fluid phase marker fluorescein isothiocyanate (FITC)-dextran (10 kDa) in cells treated with simvastatin is significantly reduced by acute treatment with simvastatin in LLC-AQP2 cells (A). There is a significant 75% reduction of the uptake of FITC-dextran by cells treated with 100 μM simvastatin. Similarly, VP and methyl-β-cyclodextrin (mβCD) also cause significant reduction of the endocytosis of FITC-dextran (*P < 0.01). The specific effect of simvastatin on clathrin-medicated endocytosis was also investigated using rhodamine-labeled transferrin (TrfRh), which is endocytosed via the clathrin-mediated pathway (B). Simvastatin greatly reduces the endocytosis of rhodamine-transferrin compared with the untreated control. Much of the rhodamine-transferrin remains on the cell surface without being endocytosed in cells treated with 100 μM simvastatin. In contrast to its dramatic effect on endocytosis, simvastatin causes a minor (∼10%, *P < 0.05) reduction of overall exocytosis in our soluble, secreted yellow fluorescent protein fluorimetric exocytosis assay even at the highest concentration of 200 μM, but not at 100 μM, whereas VP treatment increases overall exocytosis (C), which is consistent with our previous report (9). Error bars represent means ± SE. Data were obtained from 3 independent experiments. Scale bar = 10 μm in B.
Simvastatin downregulates rho GTPase activity in LLC-AQP2 cells.
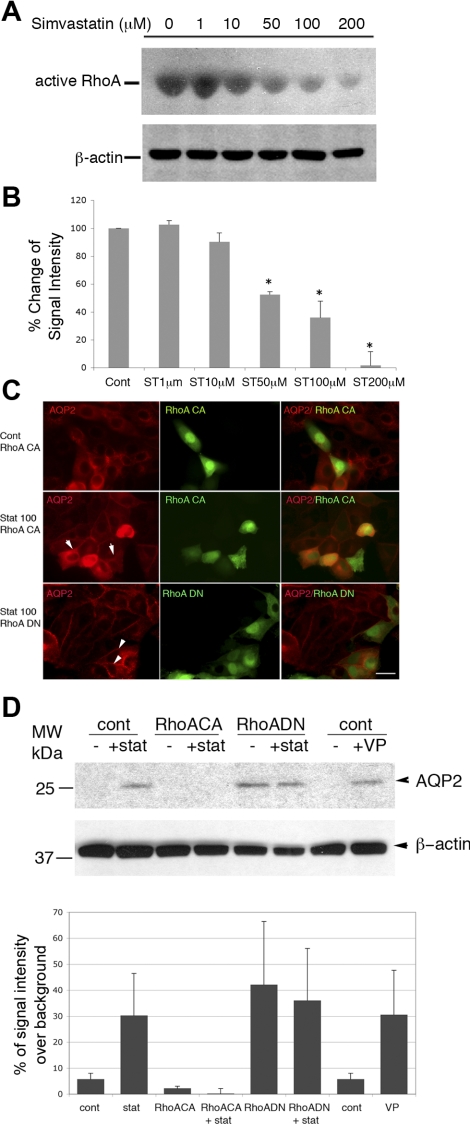
The mechanism underlying simvastatin's effect on AQP2 trafficking was investigated further by examining Rho GTPase activity in cells treated with simvastatin (Fig. 4). Immunoblot analysis showed that simvastatin downregulated RhoA activity in AQP2-expressing cells in a dose-dependent manner using a RhoA activation assay kit (Biochem Kit from Cytoskeleton) to detect the Rhotekin-RBD-RhoA-GTP complex (Fig. 4A). Simvastatin already had a small effect on the level of active RhoA at a concentration as low as 10 μM, and this effect became more significant at concentrations of 50 μM and above. The downregulation of RhoA activity by simvastatin was quantified in Fig. 4B. The percentage reduction of RhoA activity in relation to the control condition was plotted. Our data show that simvastatin significantly downregulates RhoA activity (Fig. 4B). The level of total RhoA in simvastatin-treated cells was not significantly or consistently altered in multiple experiments (data not shown). To further investigate the involvement of RhoA in simvastatin-induced membrane trafficking of AQP2, cells were transfected with the constitutively active RhoA construct RhoA G14V or the dominant-negative construct RhoA T19N and then treated with simvastatin (Fig. 4, C and D). In transfected cells, expression of constitutively active RhoA had no effect on baseline distribution of AQP2 (Fig. 4C) but appeared to greatly attenuate simvastatin-induced membrane accumulation of AQP2 (Fig. 4C). Expressing dominant-negative RhoA (RhoA T19N) had, as expected, no significant impact on simvastatin-induced AQP2 trafficking (Fig. 4B). This was further confirmed by cell surface biotinylation experiments (Fig. 4D). Again, expression of constitutive active RhoA (RhoAG14V) attenuated statin-induced membrane accumulation of AQP2 (quantification in Fig. 4D histogram). Overexpression of dominant-negative RhoA was by itself sufficient to cause membrane accumulation of AQP2, which is inconsistent with previous reports (21). Therefore, our data show that RhoGTPase, specifically RhoA, is involved in simvastatin-mediated membrane trafficking of AQP2.
Fig. 4.
Simvastatin downregulates Rho GTPase in cells, and expression of constitutively active RhoA attenuates the effect of simvastatin-induced membrane accumulation of AQP2. LLC-AQP2 cells were treated with various concentrations of simvastatin for 1 h. The activity of RhoA inside cells was evaluated using a RhoA activity assay kit (Cytoskeleton) to detect the Rhotekin-RBD-RhoA-GTP complex. There is a significant, dose-dependent downregulation of RhoA activity in simvastatin-treated cells (A). Simvastatin causes significant downregulation of RhoA activity at a concentration of 50 μM and more dramatically at 200 μM. Total RhoA in simvastatin-treated and control cells was unchanged by simvastatin (data not shown). A, bottom: actin loading control. B: quantification of the percentage of change of RhoA activity from simvastatin-treated samples vs. control. Data were obtained from 3 independent experiments. Error bars represent means ± SE. *P <0.05. The involvement of RhoA in simvastatin-induced AQP2 trafficking was further investigated in cells by immunofluorescence staining and cell surface biotinylation (C and D). Expression of constitutively active RhoA [RhoA CA in C, indicated by coexpression of green fluorescent protein (GFP)] attenuates membrane accumulation of AQP2 induced by simvastatin (arrows in C, middle), whereas expression of dominant-negative RhoA (RhoA DN) has no obvious effect on simvastatin-induced AQP2 trafficking (arrowheads in C, bottom). This is clearly seen in surface biotinylation experiments (D). While there is no significant effect on the level of surface AQP2 at baseline, expression of the constitutively active RhoA attenuates simvastatin-induced surface accumulation of AQP2 (biotinylated AQP2). Expression of the dominant-negative RhoA results in membrane accumulation of AQP2, even without any further stimulation. D: quantification of surface-biotinylated AQP2 signal from 3 independent experiments. Error bars represent means ± SE. Scale bar = 10 μm in C.
Simvastatin causes acute urinary concentration and membrane accumulation of AQP2 in principal cells in CD in brattleboro rat kidney.
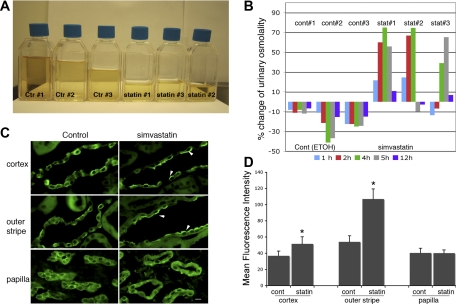
The in vivo effect of simvastatin on urinary concentration was investigated in Brattleboro rats, which lack endogenous VP because of a spontaneous mutation of the VP gene (Fig. 5) (24). DI in these animals is readily correctable by exogenous VP administration (23). After intraperitoneal injection of simvastatin to give a final calculated plasma concentration of 200 μM, the urine volume and osmolality of individual animals at each hour after intraperitoneal injection was recorded for the first 6 h and then every 12 h after. In simvastatin-treated animals, decreased urinary volume with simultaneously increased urinary osmolality was clearly detected in each treated animal within 5–6 h after a single injection (Fig. 5, A and B). The total volume of the first 6-h urine collection from each animal is shown in Fig. 5A. Reduction of urinary volume was associated with the increase of urinary osmolality in treated animals. For example, urine osmolarity increased from 122 to a peak concentration of 210 mosmol/kgH2O at 4 h postinjection in animal 4 (statin 1), from 142 to a peak value of 248 at 4 h for animal 5 (statin 2), and from 118 to peak value of 195 at 5 h postinjection in animal 6 (statin 3). Given the variations of baseline urinary osmolality, time of response, and duration of response of individual animals, the percentage change of the urinary osmolality compared with the individual baseline value was calculated for individual animals (Fig. 5B). Despite variations in the onset and offset of the urinary concentrating effect of simvastatin in individually treated animals, there was a significant increase of urinary osmolality in the treated group compared with untreated controls. Interestingly, animals in the control group that received the EtOH (an equal amount as the simvastatin group) showed mildly reduced urinary osmolality after intraperitoneal injection. For example, urine osmolarity was reduced from 135 to 119 mosmol/kgH2O in control 1, from 136 to 78 in control 2, and from 168 to 123 in control 3. Despite this diuretic effect of the solvent (EtOH), treatment with simvastatin still caused a significant urinary concentrating effect. However, the initial, potent urinary concentrating effect of simvastatin diminished rapidly at times >6 h after injection (Fig. 5B). A second round of experiments was performed in these animals after >1 mo of drug washout. Similar urinary results were obtained from both the first and second rounds of experiments. Urinary data presented here represent the results from the first round of experimentation.
Fig. 5.
Simvastatin increases urinary concentration and induces membrane accumulation of AQP2 in the CD of kidneys from Brattleboro (BB) rats. After treatment, the urine output from each animal (3 animals in the control group and 3 animals in the treated group) was collected and measured every hour during the first 6 h and then every 12 h after injection. The total urine volume of pooled 6-h urine from individual animals was measured and photographed (A). Clearly, the simvastatin-treated group produces less than half of the urine volume compared with the control animals. An increase in urinary osmolality is detectable as soon as 1 h after injection (B). The percentage increase or decrease of hourly urinary osmolality in individual animals compared with its own baseline after injection is shown in B. There is a clear stimulatory effect on urinary osmolality in treated animals (statin#) compared with the controls (cont#) within 6 h postinjection. AQP2 distribution in CD of treated and control BB animals was examined by immunofluorescence staining of kidneys (C). In rats treated with simvastatin for 6 h, there is a significant membrane accumulation of AQP2 in CD principal cells in the cortex and medullary outer stripe but not in the papilla. The fluorescence signal resulting from apical AQP2 in principal cells was acquired and quantified using IPlab software as detailed in the text (D). There is a significant increase of apical AQP2 staining in CD from cortex and outer medulla in treated animals. Error bars represent means ± SE. *P < 0.01. Scale bar = 10 μm in C. Arrows indicate membrane accumulation of AQP2.
Immunofluorescence staining revealed significantly increased apical membrane staining of AQP2 in the principal cells of the CD in the cortex and outer medulla of the kidney from simvastatin-treated animals (Fig. 5C). This simvastatin-induced membrane accumulation of AQP2 was not seen in principal cells from the inner medulla/papilla region in treated animals, consistent with data observed in experiments using kidney slices in vitro. The signal intensity of membrane-accumulated AQP2 was measured and quantified (Fig. 5D). Again, there was a significant membrane accumulation of AQP2 in the CD of the cortex and outer stripe from kidneys in the treated group. Interestingly, we did not detect any significant alteration in the subcellular distribution of the A and B subunits of the V-ATPase (another protein that shows extensive membrane recycling) in intercalated cells from simvastatin-treated animals [Supplemental Fig. 1 (Supplemental data for this article may be found on the American Journal of Physiology: Renal Physiology website)], but it is certainly possible that simvastatin may modulate the trafficking and cell surface expression of other membrane channels that we have not examined here.
Potential simvastatin-associated side effects in animals were also examined. We did not observe any changes in hemodynamics, including blood pressure and heart rate, or glomerular filtration rate (GFR) in animals treated with simvastatin (data not shown). There was no significant difference in blood chemistries, including blood urea nitrogen, creatinine, and Na, or urinary chemistries, including Na and creatinine, and no change of liver function tests in treated animals compared with controls (data not shown). Water consumption in each animal was measured and recorded and corresponded as expected to the urine output of each animal. No significant change of food consumption or body weight was seen among animals from each group during the course of experiments.
To summarize, the membrane accumulation of AQP2 induced by acute treatment with simvastatin probably accounts for the increase of urinary concentration (osmolality) and reduced urine volume seen in treated Brattleboro rats.
DISCUSSION
The work presented here using in vitro cell cultures, kidney slices in vitro, and Brattleboro rats shows that simvastatin induces AQP2 membrane accumulation, which results in increased water reabsorption and urinary concentration in vivo. This effect was independent of cAMP/PKA activation and Ser256 phosphorylation of AQP2, the classical pathway that has been associated with VP-regulated AQP2 trafficking. Membrane accumulation of AQP2 induced by simvastatin was mainly due to the inhibition of AQP2 endocytosis and not to an increase in AQP2 exocytosis. This effect could result from a significant downregulation of the activity of the GTPase RhoA in simvastatin-treated cells.
Statins have long been known to be strong inhibitors of cholesterol synthesis and, therefore, eventually deplete the cholesterol content of cells. We previously showed that reducing plasma membrane cholesterol using mβCD also results in a rapid accumulation of AQP2 at the plasma membrane of renal epithelial cells in culture (25) and in principal cells in the intact perfused kidney (44). This effect is due to a reduction of clathrin-mediated endocytosis of AQP2 that results from membrane cholesterol depletion (12, 13, 25, 49, 55). Furthermore, it has recently been shown that treatment of cultured mouse cortical CD cells with statin for 3 days resulted in increased membrane expression of AQP2 (40). In this latter study, the statin effect was attributed to a reduction in plasma membrane cholesterol that occurred after chronic statin treatment (40). In general, it requires at least 35 h or more for statins to deplete 50% of the membrane cholesterol to exert an effect on protein/vesicular trafficking (12, 39). However, in our experiments, a rapid acute effect of statins on AQP2 membrane expression was detected after only 60 min of treatment, at a time when there is little or no reduction in plasma membrane cholesterol content after statin treatment, especially when cells are grown in the presence of serum, which is 10% FBS in our case (42). Thus it is unlikely that this acute effect of statins on AQP2 membrane accumulation is due to cholesterol depletion. Instead, we believe that this result reflects a rapid effect of statins on the actin cytoskeleton reorganization through modulating Rho GTPase activity.
Statins are known to have multiple pleiotropic effects. Through inhibiting isoprenylation, statins affect modifications of a variety of proteins, such as the Rho family proteins, leading to their enhanced interaction with membranes and defining their localization to specific membrane compartments (56). Prenylation of multiple Rho GTPases, including RhoA, can be affected by statins. This in turn affects actin cytoskeleton organization, which is important in multiple membrane trafficking and intracellular transport processes, including AQP2 trafficking in this case. We found that acute simvastatin exposure of renal epithelial cells resulted in significant downregulation of RhoA activity, suggesting that this is a potential mechanism underlying the acute effect of statins on AQP2 trafficking. Multiple Rho GTPases, such as RhoA, Rac1, and Cdc42, have all been reported to be involved in exocytosis, endocytosis, and vesicle trafficking in cells (11, 41). The involvement of other Rho GTPases such as Rac1 and Cdc42 in statin-mediated AQP2 trafficking cannot be ruled out, however.
In addition to prenylation, the activity and localization of some of the Rho family proteins are also regulated by phosphorylation. For example, RhoA can be phosphorylated by PKA and results in reduced membrane association (active form of RhoA) and increased association with GDI (23). It was also reported that cAMP-induced AQP2 translocation is associated with RhoA inhibition through RhoA phosphorylation with RhoGDI (52). We have shown in a previous study that, besides regulated trafficking through VP/PKA/cAMP, AQP2 constitutively recycles independently of the phosphorylation of Ser256 (25). Simply blocking endocytosis by mβCD or a dominant-negative dynamin mutant causes rapid and significant membrane accumulation of AQP2. The differential trafficking of the constitutively recycling pool and the “regulatable” pool of AQP2 (if they are indeed different pools) has not been well understood. Because statin-induced membrane translocation of AQP2 seems to be independent of cAMP/PKA activation, RhoA phosphorylation by PKA may not be a major modifier in our experimental conditions, or for this specific pool of AQP2.
The urinary concentrating effect in animals could also be caused through altering hemodynamic and/or modulating the production/secretion of multiple hormones. Our data showed that there were no significant changes of blood pressure in simvastatin-treated animals. The overall GFR and blood and urinary chemistry were not altered either, which is in agreement with recent studies in animals (37, 38). Even though we have not measured the concentrations of multiple hormones in our animals, such as atrial natriuretic peptide, brain natriuretic peptide, aldosterone, vasopressin (AVP), angiotensin II, and renin, recent studies in humans have shown that neither acute nor chronic statin treatment affects plasma concentrations of these hormones (37). Furthermore, our work was performed using VP-deficient Brattleboro rats, eliminating the possibility that this antidiuretic hormone could be responsible for the observed effect of simvastatin in vivo.
The urinary concentrating effect exerted by simvastatin appeared to be short acting and rapidly disappeared 5–6 h after a single injection. It is possibly due to the highly lipophilic and high protein-binding nature of the drug, which leads to rapid tissue redistribution and reduction of plasma levels. According to several reports, the estimated half-life for various statins ranges from only a couple of hours to ∼14 h (45). Continuous delivery of an adequate amount of simvastatin has been challenging because of limitations of the delivery capacity of the osmotic pump. Development of a less lipophilic version of statins or induction of a structural modification to prolong their half-life in the serum may provide a better outcome (29).
As we have observed in both the kidney slice cultures and animal experiments, simvastatin caused significant membrane accumulation of AQP2 in principal cells of the CD in kidney cortex and the outer medullary region, with a much lower or no effect in the inner medullary/papilla region. The region-specific factors that contribute to this differential response to simvastatin in various segments of animal kidney remain to be identified. Despite the absence of a detectable response to simvastatin in the papillary region, we nevertheless observed a significant >50% increase of urinary concentration in animals, presumably because of the substantial contribution of cortical and outer medullary CD to regulated water reabsorption.
In conclusion, we have shown that simvastatin regulates AQP2 trafficking in vitro and induces acute urinary concentration in vivo, by inhibiting AQP2 endocytosis. This effect is probably associated with the downregulation of Rho GTPase, specifically RhoA activity. More importantly, our data reveal that simvastatin-induced AQP2 membrane accumulation is largely independent of cAMP/PKA activation and, therefore, provides an alternative strategy to modulate AQP2 trafficking in vitro and in vivo bypassing the classic VP-V2R signal transduction pathway. Even though the total reduction of urine output may seem to be modest with simvastatin-treated animals, it would be significant for patients with DI who may produce up to 10–15 liters of urine each day. Despite the promising effect of statins on urinary concentration shown by our animal experiments, the well-known side effects associated with statins, such as myopathy and hepatotoxicity, indicate that the effect and utility of statins as an alternative therapy for DI in human subjects have to be cautiously evaluated before clinical application (16).
GRANTS
We thank Dr. Silvio Gutkind [National Institutes of Health (NIH)/National Institute of Dental and Craniofacial Research] and Emily Lecuona (Northwestern University) for providing important reagents. Dr. H. A. J. Lu is supported by an NIH KO8 Grant DK-075940 and a Gottschalk research grant from the American Society of Nephrology. Additional support was from NIH Grant DK-38452 (D. Brown). R. Bouley received a Young Investigator Award from the National Kidney Foundation. U. Hasler was supported by a Swiss Fondation Suisee de Bourse en Médicine et Biologie Fellowship and an Executive Committee on Research Fellowship from Massachusetts General Hospital. The Microscopy Core Facility of the Program in Membrane Biology receives additional support from the Boston Area Diabetes and Endocrinology Research Center (NIH DK-57521) and from the Center for the Study of Inflammatory Bowel Disease (NIH DK-43351).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
REFERENCES
- 1. Aspenstrom P, Ruusala A, Pacholsky D. Taking Rho GTPases to the next level: the cellular functions of atypical Rho GTPases. Exp Cell Res 313: 3673–3679, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Beckers CM, van Hinsbergh VW, van Nieuw Amerongen GP. Driving Rho GTPase activity in endothelial cells regulates barrier integrity. Thromb Haemost 103: 40–55, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Bouley R, Breton S, Sun T, McLaughlin M, Nsumu NN, Lin HY, Ausiello DA, Brown D. Nitric oxide and atrial natriuretic factor stimulate cGMP-dependent membrane insertion of aquaporin 2 in renal epithelial cells. J Clin Invest 106: 1115–1126, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bouley R, Hasler U, Lu HA, Nunes P, Brown D. Bypassing vasopressin receptor signaling pathways in nephrogenic diabetes insipidus. Semin Nephrol 28: 266–278, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bouley R, Pastor-Soler N, Cohen O, McLaughlin M, Breton S, Brown D. Stimulation of AQP2 membrane insertion in renal epithelial cells in vitro and in vivo by the cGMP phosphodiesterase inhibitor sildenafil citrate (Viagra). Am J Physiol Renal Physiol 288: F1103–F1112, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Brown D. The ins, and outs of aquaporin-2 trafficking. Am J Physiol Renal Physiol 284: F893–F901, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Brown D, Hasler U, Nunes P, Bouley R, Lu HA. Phosphorylation events and the modulation of aquaporin 2 cell surface expression. Curr Opin Nephrol Hypertens 17: 491–498, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown D, Katsura T, Gustafson CE. Cellular mechanisms of aquaporin trafficking. Am J Physiol Renal Physiol 275: F328–F331, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Deen PM, Dahl N, Caplan MJ. The aquaporin-2 water channel in autosomal dominant primary nocturnal enuresis. J Urol 167: 1447–1450, 2002 [PubMed] [Google Scholar]
- 10. Fushimi K, Sasaki S, Marumo F. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J Biol Chem 272: 14800–14804, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Hall A. Rho GTPases, and the control of cell behaviour. Biochem Soc Trans 33: 891–895, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Hansen GH, Niels-Christiansen LL, Thorsen E, Immerdal L, Danielsen EM. Cholesterol depletion of enterocytes. Effect on the Golgi complex and apical membrane trafficking. J Biol Chem 275: 5136–5142, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Heiniger HJ, Kandutsch AA, Chen HW. Depletion of L cell sterol depresses endocytosis. Nature 263: 515–517, 1976 [DOI] [PubMed] [Google Scholar]
- 14. Hoffert JD, Nielsen J, Yu MJ, Pisitkun T, Schleicher SM, Nielsen S, Knepper MA. Dynamics of aquaporin-2 serine-261 phosphorylation in response to short-term vasopressin treatment in collecting duct. Am J Physiol Renal Physiol 292: F691–F700, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Hoffert JD, Pisitkun T, Wang G, Shen RF, Knepper MA. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: regulation of aquaporin-2 phosphorylation at two sites. Proc Natl Acad Sci USA 103: 7159–7164, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Igel M, Sudhop T, von Bergmann K. Metabolism and drug interactions of 3-hydroxy-3-methylglutaryl coenzyme A.-reductase inhibitors (statins). Eur J Clin Pharmacol 57: 357–364, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Jacobson JR, Dudek SM, Birukov KG, Ye SQ, Grigoryev DN, Girgis RE, Garcia JG. Cytoskeletal activation and altered gene expression in endothelial barrier regulation by simvastatin. Am J Respir Cell Mol Biol 30: 662–670, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Karlsson R, Pedersen ED, Wang Z, Brakebusch C. Rho GTPase function in tumorigenesis. Biochim Biophys Acta 1796: 91–98, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Katsura T, Gustafson CE, Ausiello DA, Brown D. Protein kinase A phosphorylation is involved in regulated exocytosis of aquaporin-2 in transfected LLC-PK1 cells. Am J Physiol Renal Physiol 272: F817–F822, 1997 [PubMed] [Google Scholar]
- 20. Kim SW, Kim JW, Choi KC, Ma SK, Oh Y, Jung JY, Kim J, Lee J. Indomethacin enhances shuttling of aquaporin-2 despite decreased abundance in rat kidney. J Am Soc Nephrol 15: 2998–3005, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Klussmann E, Tamma G, Lorenz D, Wiesner B, Maric K, Hofmann F, Aktories K, Valenti G, Rosenthal W. An inhibitory role of Rho in the vasopressin-mediated translocation of aquaporin-2 into cell membranes of renal principal cells. J Biol Chem 276: 20451–20457, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Knepper MA, Wade JB, Terris J, Ecelbarger CA, Marples D, Mandon B, Chou CL, Kishore BK, Nielsen S. Renal aquaporins. Kidney Int 49: 1712–1717, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Krukoff TL, Calaresu FR. Exogenous vasopressin reverses hyperactivity in the hypothalamus of Brattleboro rats. Am J Physiol Regul Integr Comp Physiol 247: R932–R935, 1984 [DOI] [PubMed] [Google Scholar]
- 24. Laycock JF, Lee J, Lewis AF. The effect of chlorpropamide on water balance in pitressin-treated Brattleboro rats. Br J Pharmacol 52: 253–263, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lu H, Sun TX, Bouley R, Blackburn K, McLaughlin M, Brown D. Inhibition of endocytosis causes phosphorylation (S256)-independent plasma membrane accumulation of AQP2. Am J Physiol Renal Physiol 286: F233–F243, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Lu HA, Sun TX, Matsuzaki T, Yi XH, Eswara J, Bouley R, McKee M, Brown D. Heat shock protein 70 interacts with aquaporin-2 and regulates its trafficking. J Biol Chem 282: 28721–28732, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Lu HJ, Matsuzaki T, Bouley R, Hasler U, Qin QH, Brown D. The phosphorylation state of serine 256 is dominant over that of serine 261 in the regulation of AQP2 trafficking in renal epithelial cells. Am J Physiol Renal Physiol 295: F290–F294, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maher BM, Dhonnchu TN, Burke JP, Soo A, Wood AE, Watson RW. Statins alter neutrophil migration by modulating cellular Rho activity–a potential mechanism for statins-mediated pleotropic effects? J Leukoc Biol 85: 186–193, 2009 [DOI] [PubMed] [Google Scholar]
- 29. McKenney JM. Pharmacologic characteristics of statins. Clin Cardiol 26: III32–II38, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moeller HB, Praetorius J, Rutzler MR, Fenton RA. Phosphorylation of aquaporin-2 regulates its endocytosis and protein-protein interactions. Proc Natl Acad Sci USA 107: 424–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nedvetsky PI, Tamma G, Beulshausen S, Valenti G, Rosenthal W, Klussmann E. Regulation of aquaporin-2 trafficking. Handb Exp Pharmacol 22: 33–157, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Nielsen S, Frokiaer J, Marples D, Kwon TH, Agre P, Knepper MA. Aquaporins in the kidney: from molecules to medicine. Physiol Rev 82: 205–244, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Nielsen S, Kwon TH, Christensen BM, Promeneur D, Frokiaer J, Marples D. Physiology and pathophysiology of renal aquaporins. J Am Soc Nephrol 10: 647–663, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Noda Y, Horikawa S, Katayama Y, Sasaki S. Identification of a multiprotein “motor” complex binding to water channel aquaporin-2. Biochem Biophys Res Commun 330: 1041–1047, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Nunes P, Hasler U, McKee M, Lu HA, Bouley R, Brown D. A fluorimetry-based ssYFP secretion assay to monitor vasopressin-induced exocytosis in LLC-PK1 cells expressing aquaporin-2 (AQP2). Am J Physiol Cell Physiol 295: C1476–C1487, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Park HJ, Kong D, Iruela-Arispe L, Begley U, Tang D, Galper JB. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors interfere with angiogenesis by inhibiting the geranylgeranylation of RhoA. Circ Res 91: 143–150, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Paulsen L, Holm C, Bech JN, Starklint J, Pedersen EB. Effects of statins on renal sodium and water handling: acute and short-term effects of atorvastatin on renal haemodynamics, tubular function, vasoactive hormones, blood pressure and pulse rate in healthy, normocholesterolemic humans. Nephrol Dial Transplant 23: 1556–1561, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Paulsen L, Holst LM, Bech JN, Starklint J, Pedersen EB. Glomerular filtration rate and blood pressure are unchanged by increased sodium intake in atorvastatin-treated healthy men. Scand J Clin Lab Invest 69: 323–329, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Pediconi MF, Gallegos CE, De Los Santos EB, Barrantes FJ. Metabolic cholesterol depletion hinders cell-surface trafficking of the nicotinic acetylcholine receptor. Neuroscience 128: 239–249, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Procino G, Barbieri C, Carmosino M, Rizzo F, Valenti G, Svelto M. Lovastatin-induced cholesterol depletion affects both apical sorting and endocytosis of aquaporin-2 in renal cells. Am J Physiol Renal Physiol 298: F266–F278, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Ridley AJ. Rho GTPases, and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol 16: 522–529, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Ridsdale A, Denis M, Gougeon PY, Ngsee JK, Presley JF, Zha X. Cholesterol is required for efficient endoplasmic reticulum-to-Golgi transport of secretory membrane proteins. Mol Biol Cell 17: 1593–1605, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rikitake Y, Liao JK. Rho GTPases, statins, and nitric oxide. Circ Res 97: 1232–1235, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Russo LM, McKee M, Brown D. Methyl-beta-cyclodextrin induces vasopressin-independent apical accumulation of aquaporin-2 in the isolated, perfused rat kidney. Am J Physiol Renal Physiol 291: F246–F253, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Schachter M. Chemical, pharmacokinetic, and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol 19: 117–125, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Shaw S, Marples D. Nethylmaleimide causes aquaporin-2 trafficking in the renal inner medullary collecting duct by direct activation of protein kinase A. Am J Physiol Renal Physiol 288: F832–F839, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Sidaway JE, Davidson RG, McTaggart F, Orton TC, Scott RC, Smith GJ, Brunskill NJ. Inhibitors of 3-hydroxy-3-methylglutaryl-CoA reductase reduce receptor-mediated endocytosis in opossum kidney cells. J Am Soc Nephrol 15: 2258–2265, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Siderovski DP, Willard FS. The GAPs, GEFs, and GDIs of heterotrimeric G.-protein alpha subunits. Int J Biol Sci 1: 51–66, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sun TX, Van Hoek A, Huang Y, Bouley R, McLaughlin M, Brown D. Aquaporin-2 localization in clathrin-coated pits: inhibition of endocytosis by dominant-negative dynamin. Am J Physiol Renal Physiol 282: F998–F1011, 2002 [DOI] [PubMed] [Google Scholar]
- 50. Tamma G, Klussmann E, Maric K, Aktories K, Svelto M, Rosenthal W, Valenti G. Rho inhibits cAMP-induced translocation of aquaporin-2 into the apical membrane of renal cells. Am J Physiol Renal Physiol 281: F1092–F1101, 2001 [DOI] [PubMed] [Google Scholar]
- 51. Tamma G, Klussmann E, Oehlke J, Krause E, Rosenthal W, Svelto M, Valenti G. Actin remodeling requires ERM function to facilitate AQP2 apical targeting. J Cell Sci 118: 3623–3630, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Tamma G, Klussmann E, Procino G, Svelto M, Rosenthal W, Valenti G. cAMP-induced AQP2 translocation is associated with RhoA inhibition through RhoA phosphorylation and interaction with RhoGDI. J Cell Sci 116: 1519–1525, 2003 [DOI] [PubMed] [Google Scholar]
- 53. Trifaro JM, Gasman S, Gutierrez LM. Cytoskeletal control of vesicle transport and exocytosis in chromaffin cells. Acta Physiol (Oxf) 192: 165–172, 2008 [DOI] [PubMed] [Google Scholar]
- 54. Valenti G, Procino G, Tamma G, Carmosino M, Svelto M. Minireview: aquaporin 2 trafficking. Endocrinology 146: 5063–5070, 2005 [DOI] [PubMed] [Google Scholar]
- 55. Wang Y, Thiele C, Huttner WB. Cholesterol is required for the formation of regulated and constitutive secretory vesicles from the trans-Golgi network. Traffic 1: 952–962, 2000 [DOI] [PubMed] [Google Scholar]
- 56. Wennerberg K, Der CJ. Rho-family GTPases: it's not only Rac and Rho (and I. like it). J Cell Sci 117: 1301–1312, 2004 [DOI] [PubMed] [Google Scholar]
- 57. Zelenina M, Christensen BM, Palmer J, Nairn AC, Nielsen S, Aperia A. Prostaglandin E2 interaction with AVP: effects on AQP2. phosphorylation and distribution. Am J Physiol Renal Physiol 278: F388–F394, 2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.