Abstract
AKT phosphorylation following peripheral nerve injury or inflammation may play a role in somatic pain processes and visceral inflammation. To examine such a role in micturition reflexes with bladder inflammation, we induced bladder inflammation in adult female Wistar rats (200–300 g) by injecting cyclophosphamide (CYP) intraperitoneally at acute (150 mg/kg; 4 h), intermediate (150 mg/kg; 48 h), and chronic (75 mg/kg; every third day for 10 days) time points. Western blot analyses of whole urinary bladders showed significant increases (P ≤ 0.01) in phosphorylated (p) AKT at all time points; however, the magnitude of AKT phosphorylation varied with duration of CYP treatment. Immunohistochemical analyses of pAKT immunoreactivity (pAKT-IR) in cryostat bladder sections demonstrated duration-dependent, significant (P ≤ 0.01) increases in pAKT-IR in both the urothelium and detrusor smooth muscle of CYP-inflamed bladders. Additionally, a suburothelial population of pAKT-IR macrophages (CD68-, MAC2-, and F4/80-positive) was present in chronic CYP-treated bladders. The functional role of pAKT in micturition was evaluated using open, conscious cystometry with continuous instillation of saline in conjunction with administration of an inhibitor of AKT phosphorylation, deguelin (1.0 μg/10 μl), or vehicle (1% DMSO in saline) in control (no inflammation) and CYP (48 h)-treated rats. Bladder capacity, void volume, and intercontraction void interval increased significantly (P ≤ 0.05) following intravesical instillation of deguelin in CYP (48 h)-treated rats. These results demonstrate increased AKT phosphorylation in the urinary bladder with urinary bladder inflammation and that blockade of AKT phosphorylation in the urothelium improves overall bladder function.
Keywords: urothelium, cystometry, inflammation, deguelin
the serine-threonine protein kinase AKT is involved in cellular survival and protection from apoptosis in a number of tissues and organ systems (6, 18, 25). Recent studies have demonstrated additional roles for activated (phosphorylated; p) AKT in the initiation and maintenance of neuropathic pain and visceral inflammation (53, 59, 60, 71). Immunohistochemical techniques demonstrate AKT activation in pain transducing C-fiber primary afferents and dorsal horn neurons following peripheral neuronal injury or tissue inflammation (26, 58, 59, 60, 71). In visceral inflammation, pAKT levels increase in the spinal cord dorsal horn following chemically induced colitis (53). Upstream regulators of pAKT include growth factors such as platelet-derived growth factor, epidermal growth factor, insulin, thrombin, nerve growth factor (NGF), and brain-derived neurotrophic factor (BDNF) as well as other physiological stimuli (6, 25, 68).
Diverse growth factors activate AKT (6, 31, 68), and growth factors are increased in the urinary bladder with inflammation (12, 47, 48, 66, 70). Specifically, recent studies by Chung et al. (16) demonstrated increases in pAKT levels with cyclophosphamide (CYP)-induced bladder inflammation and determined that inflammation-induced AKT phosphorylation as well as phosphorylation of ERK1/2 and JNK were dependent on the presence of NGF. A number of additional studies have demonstrated the presence of NGF and associated receptors (TrkA, p75NTR) as well as functional roles of NGF-receptor interactions in micturition reflex pathways with and without inflammation (15, 20, 41, 46, 54, 57, 70, 72, 75). Recent studies have also demonstrated a functional role for BDNF-receptor interactions in mediating urinary bladder dysfunction following urinary bladder inflammation (52). Thus the inflammatory milieu of the urinary bladder following CYP-induced cystitis may contain a number of upstream mediators of AKT; however, the contribution of activated AKT to urinary bladder reflex function has not been previously addressed. We hypothesize that pAKT contributes to urinary bladder dysfunction induced by urinary bladder inflammation.
AKT, ERK1/2, and JNK are all potential signaling pathways downstream of NGF/receptor activation in the urinary bladder in the context of urinary bladder inflammation (12, 16, 17, 22a). CYP-induced cystitis increased ERK phosphorylation in the urinary bladder (16, 17), and Corrow and Vizzard (17) demonstrated a functional role for pERK in micturition reflexes using an upstream inhibitor of ERK phosphorylation to significantly decrease CYP-induced bladder hyperreflexia in rats. Expression (16) as well as functional roles for JNK activation (Vizzard et al., unpublished observations) in the urinary bladder following cystitis have also been demonstrated. The current study addresses the contribution of AKT phosphorylation to micturition reflex function in a CYP model of urinary bladder inflammation.
In this study, we determined 1) AKT activation in the urinary bladder following CYP treatment of varying duration using Western blot analyses, immunohistochemistry, and semiquantitative image analyses; and 2) the functional effects of intravesical instillation of an upstream inhibitor of AKT phosphorylation (deguelin) on bladder function in control (noninflamed) and CYP-treated rats using open-void, continuous cystometry in conscious rats.
MATERIALS AND METHODS
Animals
Adult female Wistar rats (200–300 g), purchased from Charles River Canada (St. Constant, PQ), were housed two per cage and maintained in standard laboratory conditions with free access to food and water. In these studies, the estrous cycle status of female rats was not monitored. The University of Vermont Institutional Animal Care and Use Committee (IACUC) approved all animal use procedures (protocol 08-085). Animal experimentation was carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
CYP-Induced Cystitis in Female Rats
Rats were anesthetized with isoflurane (2%) and received intraperitoneal (ip) injection(s) of CYP (Sigma-Aldrich, St. Louis, MO) to produce urinary bladder inflammation. To induce chronic bladder inflammation, CYP was injected (75 mg/kg ip) every third day for 8 days, with euthanasia occurring on the eighth day (3, 17, 39). To induce acute bladder inflammation, CYP was injected (150 mg/kg ip), with euthanasia occurring 4 or 48 h after injection (3, 17, 39). Control rats received no treatment. For conscious cystometry studies, rats received CYP as described, with bladder function testing occurring 48 h after injection.
Western Blotting for pAKT Expression in Whole Urinary Bladder From Control and CYP-Treated Rats
The whole urinary bladder was dissected from control and CYP-treated groups (n = 16; 4/group) and homogenized separately in tissue protein extraction agent (T-PER; Roche, Indianapolis, IN), a mild zwitterionic dialyzable detergent in 25 mM bicine, 150 mM sodium chloride (pH 7.6) supplemented with a protease inhibitor mix (16 μg/ml benzamidine, 2 μg/ml leupeptin, 50 μg/ml lima bean trypsin inhibitor, and 2 μg/ml pepstatin A, Sigma-Aldrich) and a phosphatase inhibitor cocktail (1:100; Sigma-Aldrich), and aliquots were removed for protein assay. Samples (25 μg) were suspended in sample buffer for fractionation on gels and subjected to SDS-PAGE. Proteins were transferred to nitrocellulose membranes, and the efficiency of transfer was evaluated. Membranes were blocked for 1 h in a solution of 5% milk in Tris-buffered saline with 0.1% Tween for 4 h at room temperature. Membranes were incubated in anti-pAKT (1:1,000; 736Ell, Cell Signaling Technology, Danvers, MA) in 5% bovine serum albumin in Tris-buffered saline with 0.1% Tween. Washed membranes were incubated in a species-specific secondary antibody (1:2,000; goat anti-rabbit horseradish peroxidase) in 5% milk in Tris-buffered saline with 0.1% Tween for 2 h at room temperature for enhanced chemiluminescence detection (Pierce, Rockford, IL). Blots were exposed to Biomax film (Kodak, Rochester, NY) and developed. The intensity of each band was analyzed, and background intensities were subtracted using Un-Scan It software (Silk Scientific, Orem, UT). Western blot analysis of AKT (1:1,000; Cell Signaling Technology) in samples was used as a loading control.
Immunohistochemical Localization of AKT and pAKT in Cryostat Sections of Urinary Bladder From Control and CYP-Treated Rats
The bladders were rapidly dissected from control and CYP-treated rats (n = 16; 4/group), placed in 4% paraformaldehyde followed by overnight incubation in 30% sucrose in 0.1 M PBS for cryoprotection. Tissue was frozen in optimal cutting temperature compound, sectioned (20 μm) on a freezing cryostat, and mounted directly onto gelled (0.5%) microscope slides (3, 14, 17). Sections were incubated overnight at room temperature in rabbit anti-pAKT (1:500; D9, Cell Signaling Technology) or rabbit anti-AKT (1:1,000, Cell Signaling Technology) diluted in 1% goat serum and 0.1 M phosphate buffer. After overnight incubation, sections were washed (3 × 10 min) with 0.1 M PBS (pH 7.4). Sections were then incubated with goat anti-rabbit Cy3-conjugated secondary antibody (1:500; Jackson ImmunoResearch Laboratories, West Grove, PA) for 2 h at room temperature followed by washes (3 × 10 min) with PBS and coverslipping with antifade medium (Citifluor, Fisher Scientific, Pittsburgh, PA). Immunohistochemical controls included incubation of tissue sections with 1% goat serum and 0.1 M phosphate buffer alone (no primary antibody) or rabbit isotype control (1:500 and 1:1,000; Cell Signaling Technology), followed by normal washing and incubation with secondary antibodies to evaluate background staining levels. In the absence of primary antibody or in the presence of the isotype control, no positive immunostaining that was above background levels was observed.
To determine the cellular identity of pAKT-immunoreactive inflammatory cell infiltrates, some bladder sections were also immunostained with markers of macrophages including CD68 (ED1 rat homolog; 1:750, AbD Serotec, Raleigh, NC), MAC-2 (1:200, Cedarlane Laboratories), or F4/80 (1:200, AbD Serotec). Urinary bladder sections were incubated in cocktails of primary antibodies against pAKT and a macrophage marker followed by incubation in species-specific secondary antibodies (1:200; Cy2-conjugated antibody for macrophage markers; Jackson ImmunoResearch Laboratories) and processed as described above.
Visualization and Semiquantitative Analyses of pAKT in Urothelium and Detrusor Smooth Muscle
pAKT immunoreactivity (pAKT-IR) in bladder sections was visualized, and images were captured using an Olympus fluorescence photomicroscope (Optical Analysis, Nashua, NH). The filter was set to an excitation range of 560–569 nm and an emission range of 610–655 nm for visualization of Cy3. Images were captured, acquired in tagged image file format, and imported into image-analysis software (Meta Morph, version 4.5r4; University Imaging, Downingtown, PA) (3, 14, 17). The free-hand drawing tool was used to select the urothelium, and the urothelium was measured in total pixels area (3, 14, 17). To evaluate staining in the detrusor smooth muscle, with care taken to avoid nondetrusor structures (e.g., blood vessels, inflammatory cells), a rectangle of fixed dimension (125 × 125 pixels) was placed on the section according to random x and y coordinates. This process was repeated five times for each image without overlap (14, 38). A threshold encompassing an intensity range of 100–250 grayscale values was applied to the region of interest in the least brightly stained condition first. The threshold was adjusted for each experimental series using concomitantly processed negative controls as a guide for setting background fluorescence. The same threshold was subsequently used for all images. Immunoreactivity was considered to be positive only when the staining for pAKT exceeded the established threshold. Percent pAKT-IR above threshold in the total area selected was calculated. For the detrusor smooth muscle, the percent pAKT-IR above threshold was calculated by averaging the data collected from all rectangles placed on the image (14, 38).
Assessment of Immunohistochemical Staining in Urinary Bladder Regions
Immunohistochemistry and subsequent evaluation of AKT- or pAKT-IR in bladder sections or whole mount preparations were performed in control and experimental tissues simultaneously to reduce the incidence of staining variation that can occur between tissues processed on different days. Staining in experimental tissue was compared with that in experiment-matched negative controls. Urinary bladder sections or whole mounts exhibiting immunoreactivity that was greater than background level in experiment-matched negative controls were considered positively stained.
Immunohistochemical Localization of pAKT in Suburothelial Nerve Plexus in Urinary Bladder Whole Mounts
The urinary bladder was dissected rapidly and placed into oxygenated (95% O2 and 5% CO2) physiological saline solution (119.0 mmol NaCl, 4.7 mmol KCl, 24.0 mmol NaHCO3, 1.2 mmol KH2PO4, 1.2 mmol MgSO4-7 H2O, 11.0 mmol glucose) (14, 39, 73). Starting at the urethra, a midline incision was made through the bladder, then it was pinned flat onto a silicon-coated plate (Sylgard, Dow Corning, Midland, MI), maximally stretched, and then fixed in 2% paraformaldehyde+0.2% picric acid for 1.5 h. After fixation, the urothelium was separated from the detrusor layer using fine-tip forceps, iris scissors, and a dissecting microscope as previously described (14, 39, 73). Notches were made in the region of the bladder neck to track orientation and assess regional immunoreactivity of the bladder. Urothelium and bladder musculature were processed for pAKT-IR as described previously. In some whole mounts processed for pAKT-IR, nerve fibers in the suburothelial nerve plexus were also stained with the pan-neuronal marker protein gene product (PGP9.5, 1:3,000; AbD Serotec) to determine potential expression of pAKT in suburothelial nerve fibers and visualized with Cy2-conjugated species-specific secondary antibodies (1:200; Jackson ImmunoResearch Laboratories).
Visualization of pAKT-IR in Suburothelial Plexus in Bladder Whole Mounts
Whole mount tissues from control and experimental groups (n = 4 for control and CYP treatment groups) were examined using an Olympus fluorescence photomicroscope (Optical Analysis) with a multiband filter set for simultaneous visualization of the Cy3 and Cy2 fluorophores. Cy2 was viewed using a filter with an excitation range of 447–501 nm and an emission range from 510 to 540 nm.
Intravesical Catheter Implant
A lower midline abdominal incision was performed under general anesthesia with 2–3% isoflurane using aseptic surgical techniques (3, 13, 34, 38). The end of polyethylene tubing (PE-50, Clay Adams, Parsippany, NJ) was flared with heat, inserted into the dome of the bladder, and secured in place with a 6-0 nylon purse-string suture (3, 13, 34, 38). The distal end of the tubing was sealed and tunneled subcutaneously to the back of the neck where it was externalized, out of the animal's reach (3, 13, 34, 38). Rats received buprenorphine (0.05 mg/kg sc) starting at the time of surgery and then every 8–12 h postoperatively for a total of four doses. Animals were maintained for 96 h after surgery before conscious cystometry was initiated to ensure complete recovery and clearance of postoperative analgesics.
Conscious Cystometry with Continuous Intravesical Infusion of Saline and Blockade of AKT Phosphorylation
The effects of pAKT on bladder function in control (no inflammation) and CYP-treated rats (48 h) were evaluated with an upstream inhibitor of AKT phosphorylation, deguelin (1.0 μg/10 μl; EMD4Biosciences, EMD Chemicals) using conscious cystometry with continuous intravesical infusion of saline. During cystometry, unrestrained and conscious rats were placed in a recording cage over a scale and pan to collect and measure voided urine. To elicit repetitive bladder contractions, room temperature saline was infused at a constant rate (10 ml/h). At least six reproducible micturition cycles were recorded after an initial stabilization period (15–30 min). Intravesical pressure changes were recorded using a Small Animal Cystometry System (Med Associates, St. Albans, VT) (3, 13, 17, 38). Filling pressure, pressure threshold for voiding, maximal voiding pressure, and intercontraction interval were evaluated. Nonvoiding bladder contractions, defined as rhythmic intravesical pressure increases 7 cmH2O above baseline without the release of fluid from the urethra, were also determined per voiding cycle. Bladder capacity was measured as the amount of saline infused into the bladder at the time when micturition commenced (10, 32).
To evaluate the effects of pAKT on bladder function, rats were anesthetized (1–2% isoflurane) and vehicle [1% DMSO (Sigma-Aldrich) in saline] or deguelin (1.0 μg/10 μl; EMD4Biosciences, EMD Chemicals), the upstream inhibitor of AKT phosphorylation, was intravesically infused for 30 min. The concentration selected for evaluation was based upon previous studies (71). Before intravesical drug infusion, the bladder was manually emptied using the Credé maneuver. Bladders were then infused with ∼1 ml (less than bladder capacity to not elicit a bladder contraction and expulsion of instillate) of vehicle (1% DMSO in saline; Sigma-Aldrich), or deguelin (1.0 μg/10 μl; EMD4Biosciences, EMD Chemicals) according to prior published studies (3, 13, 38). Rats remained anesthetized (1–2% isoflurane) during infusion (30 min) to subdue the micturition reflex and prevent expulsion of the drug from the bladder. To avoid potential variation resulting from circadian rhythms, experiments were conducted at similar times of the day (21). At the conclusion of the study, rats were euthanized as described above. Micturition function before and after vehicle or deguelin intravesical instillation was evaluated in the same rats (control and 48 h CYP-induced cystitis).
Effects of Intravesical Infusion of Deguelin or Vehicle on AKT Phosphorylation in Urinary Bladder
To examine the effects of intravesical instillation of deguelin or vehicle on AKT phosphorylation in the urinary bladder, the bladder was infused with ∼1 ml (less than bladder capacity to not elicit a bladder contraction and expulsion of instillate) of vehicle (1% DMSO in saline; n = 4), or deguelin (1.0 μg/10 μl, n = 4; EMD4Biosciences, EMD Chemicals). Rats remained (30 min) anesthetized (1–2% isoflurane) to subdue the micturition reflex and prevent expulsion of the drug from the urinary bladder. After 30 min, rats were euthanized as described above, and the urinary bladder was harvested for immunohistochemical detection and quantitation of pAKT levels in the urothelium or detrusor smooth muscle using methodology previously described.
Exclusion Criteria
Rats were removed from the study when adverse events occurred that included a ≥20% reduction in body weight postsurgery, a significant postoperative event, lethargy, pain, or distress not relieved by our IACUC-approved regimen of postoperative analgesics or hematuria in control rodents (3, 13, 38). In the present study, no rats were excluded from the study or from analysis due to any of these exclusion criteria. In addition, behavioral movements such as grooming, standing, walking, and defecation rendered bladder pressure recordings unusable during these events.
Materials
Deguelin was prepared as a stock solution in DMSO, aliquoted, and stored at −20°C until use. Aliquots were diluted with saline to achieve final concentration.
Figure Preparation
Digital images were obtained using a charge-coupled device camera (MagnaFire SP, Optronics; Optical Analysis) and an LG-3 frame grabber attached to an Olympus fluorescence photomicroscope (Optical Analysis). Exposure times were held constant when images were acquired from control and experimental animals processed and analyzed on the same day. Images were imported into a graphics editing program (Adobe Photoshop, version 8.0, Adobe Systems, San Jose, CA) where groups of images were assembled and labeled.
Statistical Analyses
All values represent means ± SE. Data from Western blot studies were compared with ANOVA. Percent data from image analysis were arcsin transformed to meet the requirements of this statistical test. Cystometry data were compared using repeated measures ANOVA, where each animal served as its own control. Animals, processed and analyzed on the same day, were tested as a block in the ANOVA. When F-ratios exceeded the critical value (P ≤ 0.05), the Newman-Keuls or Dunnett's post hoc tests were used to compare group means.
RESULTS
pAKT Protein Levels in Whole Rat Urinary Bladder and Effects of CYP-Induced Cystitis
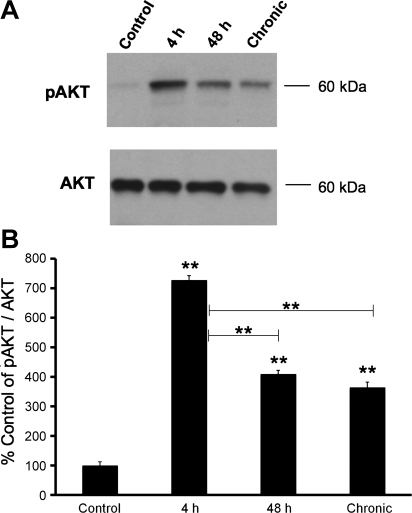
pAKT protein levels increased significantly (P ≤ 0.01) following 4-h (5.7-fold), 48-h (3.4-fold), and chronic (2.9-fold) CYP-treatment as determined with Western blot analyses (Fig. 1). Four-hour CYP treatment induced the greatest increase in phosphorylation of AKT that was significantly (P ≤ 0.01) greater than pAKT levels after 48-h (1.7-fold) and chronic (2.0-fold) CYP treatment (Fig. 1).
Fig. 1.
A: representative Western blot of phosphorylated (p) AKT expression in whole urinary bladders (25 μg) from control rats and those treated with cyclophosphamide (CYP) for varying duration. Total AKT expression was used as a loading control. B: summary histogram of relative pAKT band density in each group normalized to total AKT expression expressed as a percentage (%) of control in the same samples. Four-hour, 48-h, and chronic CYP-treatment significantly (P ≤ 0.01) increased pAKT expression compared with control urinary bladder. pAKT expression was significantly increased following 4-h CYP treatment compared with 48-h and chronic CYP treatment. Statistical analyses were performed on raw data using ANOVA as described in Statistical Analyses. Values are means ± SE. **P ≤ 0.01; n = 4 for each time point and control.
AKT- and pAKT-IR in Urothelium and Detrusor in Control (No CYP Treatment) and CYP-Treated Rats
Urothelium.
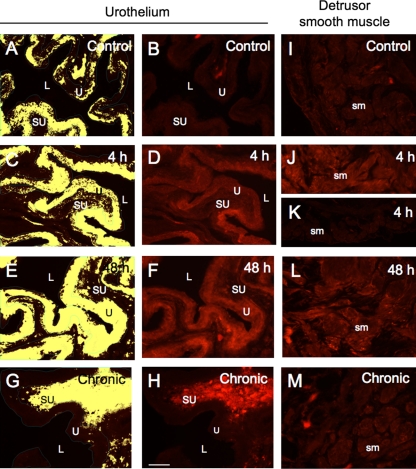
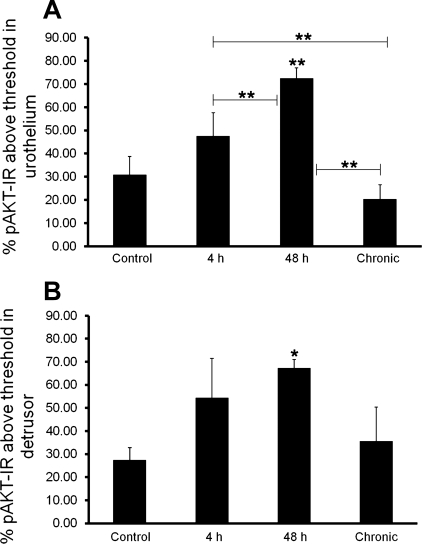
In control (no CYP treatment) rats, the urothelium exhibited weak AKT-IR (data not shown). No change in AKT-IR was observed in the urothelium after CYP treatment (4 h, 48 h, or chronic; data not shown), consistent with the demonstration of no change in AKT expression from the whole urinary bladder using Western blotting techniques (Fig. 1A). Patchy, low-intensity pAKT-IR was present in the urothelium of control (no CYP treatment) rat bladders (Fig. 2, A and B). CYP treatment increased urothelial pAKT-IR significantly (P ≤ 0.01) at the 48-h (2.3-fold) time point, but not 4 h or chronic time points examined (Figs. 2, A–H, and 3A). pAKT-IR at the 48-h time point was also significantly (P ≤ 0.01) greater than 4-h (1.5-fold) and chronic (3.6-fold) CYP treatment (Figs. 2, C–H, and 3A). Although 4-h CYP-treatment did not significantly increase pAKT-IR vs. control, pAKT levels did increase significantly (P ≤ 0.01) compared with chronic (2.3-fold) CYP treatment (Figs. 2, C, D, G, and H, and 3A). Double-labeling experiments demonstrated that the pAKT-IR suburothelial inflammatory cells present in chronic CYP-treated bladders (Fig. 2H) also exhibited immunoreactivity for markers of macrophages including CD68 (ED1 rat homolog), MAC-2, or F4/80 (data not shown).
Fig. 2.
pAKT expression in the urothelium and detrusor smooth muscle of control rats and those treated with CYP. A, C, E, and G: the urothelium was outlined in green and measured in total pixels per area. To define positive staining, a threshold intensity value was determined using the darkest image and the same threshold was subsequently applied to all images. All pixels above threshold are depicted in yellow. B, D, F, and H: corresponding fluorescence images of pAKT expression in control (B) or after 4-h (D), 48-h (F), or chronic (H) CYP-treatment. I–M: pAKT expression in the detrusor smooth muscle of control (I) rats and following 4-h (J and K), 48-h (L), and chronic (M) CYP treatment. The variability of pAKT expression in detrusor smooth muscle with 4-h CYP treatment is demonstrated in J and K. For all images, exposure times were held constant, and all tissues were processed simultaneously. sm, Detrusor smooth muscle; U, urothelium; SU, suburothelium; L, lumen. Calibration bar = 50 μm.
Fig. 3.
Summary histogram of pAKT immunoreactivity (pAKT-IR) in the urothelium (A) and detrusor (B) of the urinary bladder of control and CYP-treated rats. Percent pAKT-IR above threshold in the urothelium following 48-h CYP treatment was significantly (**P ≤ 0.01) greater than control, 4-h, and chronic CYP treatment (A). Four-hour CYP treatment significantly increased percent pAKT-IR in the urothelium compared with chronic CYP treatment (A). B: percent pAKT-IR above threshold in the detrusor smooth muscle was significantly (*P ≤ 0.05) increased with 48-h CYP-treatment. Values are means ± SE (n = 4/group).
Detrusor.
In control (no CYP treatment) rats, the detrusor smooth muscle exhibited weak AKT-IR (data not shown). No change in AKT-IR was observed in the detrusor after CYP treatment (4 h, 48 h or chronic) (data not shown) consistent with the demonstration of no change in AKT expression from the whole urinary bladder using Western blotting techniques (Fig. 1A). The expression of pAKT-IR was very weak to absent in the detrusor smooth muscle of control rat bladders (Fig. 2I). Forty-eight-hour CYP treatment significantly (P ≤ 0.05; 2.5-fold) increased detrusor pAKT-IR (Figs. 2L and 3B). There was no difference in pAKT levels in detrusor smooth muscle among control (Fig. 2I), 4-h (Fig. 2, J and K), or chronic (Fig. 2M) CYP treatment groups (Fig. 3B).
Bladder whole mount preparations.
pAKT-IR was not observed in the suburothelial nerve plexus in control or CYP-treated whole mount bladder preparations. The suburothelial nerve plexus did exhibit PGP9.5-IR; however, colocalization with pAKT-IR was not observed with indirect immunofluorescence techniques (data not shown). Whole mount preparations exhibited pAKT staining in the urothelial and detrusor smooth muscle in control and CYP treatment groups, consistent with staining observed in cryostat bladder sections from the same groups described above (data not shown).
Effects of Intravesical Deguelin Instillation on AKT Phosphorylation in Urinary Bladder with CYP Treatment
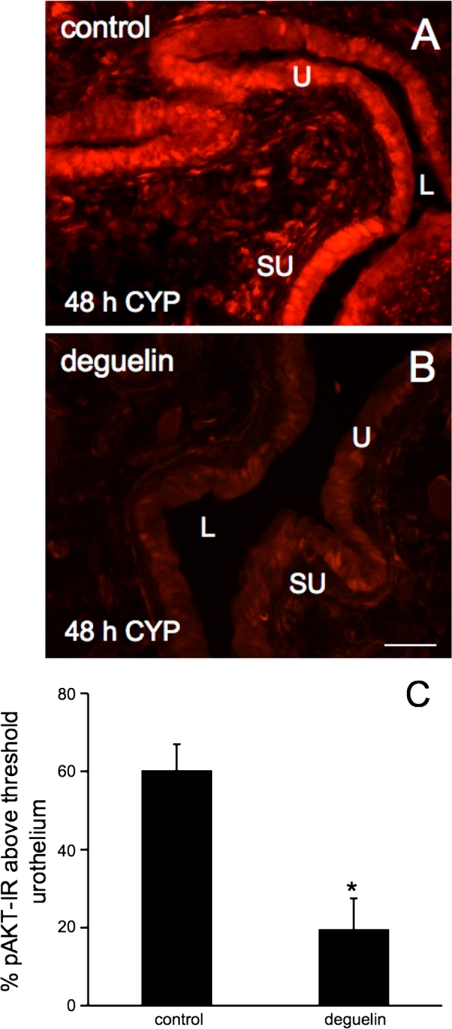
Intravesical instillation (30 min) of deguelin (1.0 μg/10 μl), an upstream inhibitor of AKT phosphorylation, significantly (P ≤ 0.05) decreased (3.1-fold) urothelial pAKT-IR in rats with 48-h CYP treatment compared with rats with instillation of vehicle (1% DMSO in saline) (Fig. 4, A–C). In contrast, no changes in pAKT-IR were observed in the detrusor smooth muscle of CYP-treated rats after deguelin instillation compared with rats with vehicle treatment (48 h; data not shown).
Fig. 4.
Reduction of AKT phosphorylation in urothelium of urinary bladder by intravesical deguelin (1.0 μg/10 μl) infusion. Percent pAKT-IR above threshold in the urothelium significantly (*P ≤ 0.05) decreased following intravesical instillation of deguelin (B) compared with vehicle (DMSO) treatment (A) in CYP (48 h)-treated rats. pAKT-IR was quantified as described in methods and Fig. 2. Values are means ± SE. (n = 4/group). Calibration bar = 50 μm.
Effect of Blockade of AKT Phosphorylation on Bladder Function
Conscious, open outlet cystometry with continuous intravesical infusion of saline was performed in control and CYP-treated (48 h) rats to establish baseline voiding frequency, bladder capacity, and void volume (Figs. 5A and 6A). After baseline bladder function was established, deguelin (1.0 μg/10 μl) or 1% DMSO in saline (vehicle) was intravesically instilled for 30 min with rats anesthetized (2% isoflurane). The same rats were subsequently evaluated with conscious cystometry an additional time to determine the effects of blocking AKT phosphorylation with deguelin or vehicle in control or CYP-treated rats.
Fig. 5.
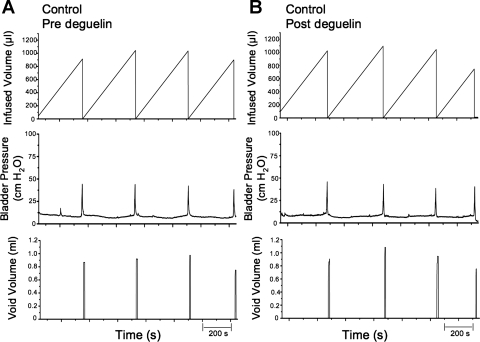
Representative cystometrogram recordings using continuous intravesical infusion of saline in conscious rats with an open outlet from a control (no CYP treatment) rat before and after blocking of AKT phosphorylation with intravesical deguelin instillation (1.0 μg/10 μl). A and B: bladder function before deguelin treatment (pre deguelin; A) and after deguelin treatment (post deguelin; B) in a control rat with continuous intravesical infusion of saline. Bladder function recordings in A and B are from the same rat. Infused volume (μl, top), bladder pressure (cmH2O, middle), and void volume (μl, bottom) before (A) and after (B) deguelin treatment are shown. s, Seconds.
Fig. 6.
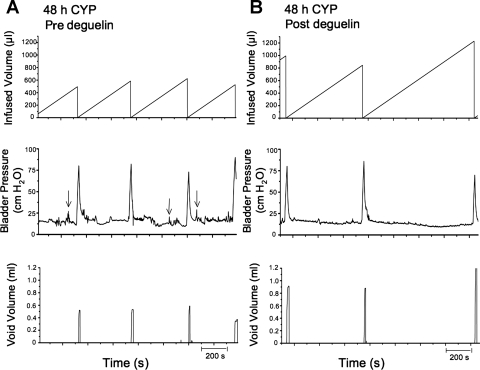
Representative cystometrogram recordings using continuous intravesical infusion of saline in conscious rats with an open outlet from a CYP-treated (48 h) rat before and after blocking upstream AKT phosphorylation with deguelin. A and B: before deguelin (pre deguelin; A) and after deguelin treatment (post deguelin; B) in a 48-h CYP-treated rat with continuous intravesical infusion of saline. Bladder function recordings in A and B are from the same rat. Arrows (A, bladder pressure trace) indicate some examples of nonvoiding contractions observed in CYP-treated rats as defined in methods. Infused volume (μl, top), bladder pressure (cmH2O, middle), and void volume (ml, bottom) before (A) and after (B) deguelin treatment are shown.
Control (no CYP treatment).
Treatment with deguelin (1.0 μg/10 μl) had no effects on intercontraction interval, bladder capacity, or void volume compared with baseline recordings (no treatment) in the same rats (control, no CYP treatment) (Fig. 5B). There were no changes in filling, threshold, or peak micturition pressures following intravesical instillation of deguelin or vehicle (Table 1) in control (no CYP treatment) rats. Residual volume in control rats before or after deguelin treatment was minimal (≤10 μl).
Table 1.
Mean bladder pressures during conscious cystometry
| Filling Pressure, cmH2O | Threshold Pressure, cmH2O | Peak Micturition Pressure, cmH2O | |
|---|---|---|---|
| Control | |||
| Pre-DMSO | 11.7 ± 1.6 | 12.6 ± 1.4 | 43.4 ± 3.7 |
| Post-DMSO | 12.9 ± 2.2 | 15.2 ± 1.0 | 49.1 ± 6.7 |
| Pre-deguelin | 10.5 ± 0.8 | 11.7 ± 1.4 | 45.0 ± 5.0 |
| Post-deguelin | 9.0 ± 0.8 | 11.4 ± 1.4 | 50.5 ± 5.0 |
| 48-h CYP | |||
| Pre-DMSO | 21.3 ± 1.9* | 21.3 ± 2.5* | 59.4 ± 4.2 |
| Post-DMSO | 22.7 ± 3.4* | 22.0 ± 4.2 | 59.1 ± 2.3 |
| Pre-deguelin | 15.8 ± 3.0 | 16.4 ± 2.0 | 61.8 ± 7.5 |
| Post-deguelin | 13.0 ± 2.5 | 16.6 ± 2.7 | 60.1 ± 5.9 |
Values are means ± SE; n = 4/group. Filling, threshold, and peak micturition pressures (cmH2O) during conscious cystometry for control and cylclophosphamide (CYP)-treated (48 h) rats before and after intravesical vehicle (1% DMSO in saline) or deguelin (1.0 μg/10 μl) treatment are shown.
P ≤ 0.05 vs. control and 48-h CYP treatment groups.
CYP treatment.
The 48-h time point of CYP-induced cystitis was evaluated because Western blot and immunohistochemical techniques both demonstrated significant increases in pAKT levels in the urinary bladder with this duration of CYP treatment.
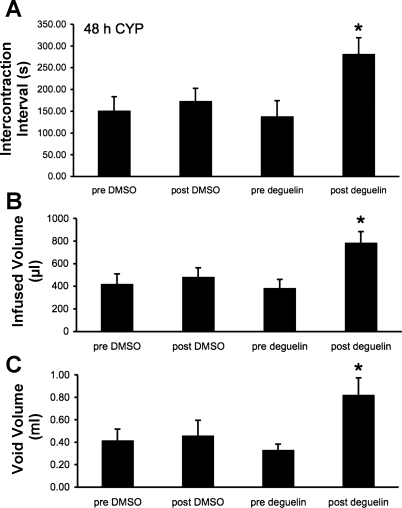
As previously demonstrated (3, 13, 34, 38), and confirmed here, CYP treatment (48 h) increased void frequency and decreased bladder capacity, intercontraction interval, and void volume compared with control rats (no CYP treatment) (Figs. 5A and 6A). Additionally, CYP treatment significantly (P ≤ 0.05) increased filling, threshold, and micturition pressures (Table 1). Deguelin treatment (1.0 μg/10 μl) (Figs. 6B and 7) significantly (P ≤ 0.05) increased the intercontraction interval (i.e., decreased voiding frequency) (Fig. 7A; 2.0-fold), increased bladder capacity (Fig. 7B; 2.0-fold), and increased void volume (Fig. 7C; 2.2-fold) compared with baseline recordings (no treatment). Deguelin treatment increased bladder capacity to 70% of control rats (no CYP treatment) (Figs. 5A, 6B, and 7). Effects of deguelin on bladder function in CYP-treated rats persisted for at least 2 h, and recovery to baseline recordings (no treatment) was not observed during the time course of the current studies. Residual volume in CYP (48 h)-treated rats before or after deguelin treatment was minimal and similar to that observed in control (no CYP treatment) (≤10 μl).
Fig. 7.
Summary histograms of the effects of vehicle (1% DMSO in saline) and deguelin (1.0 μg/10 μl) treatments on intercontraction interval (s), infused volume (μl), and void volume (ml) in CYP-treated (48 h) rats. Intravesical instillation of deguelin significantly (*P ≤ 0.05) increased intercontraction interval (A), infused volume (B), and void volume (C) in CYP-treated (48 h) rats compared with pre deguelin treatment. Values are means ± SE (n = 4/group).
DISCUSSION
These studies demonstrate basal activation (phosphorylation) of AKT in the urinary bladder, inflammation-enhanced phosphorylation of AKT with a CYP model of cystitis, and a functional role for AKT signaling in female rat micturition pathways in the context of urinary bladder inflammation. Studies have previously demonstrated changes in AKT activation levels in primary sensory neurons, dorsal horn neurons, and the urinary bladder (16, 53, 59, 60, 71) following tissue inflammation. The present studies extend previous findings (16) by demonstrating a functional significance for AKT signaling in bladder reflex pathways. pAKT may be a functional mediator of inflammation-induced bladder dysfunction because pAKT 1) is present at low levels in the urothelium of control urinary bladders; 2) increases in the urinary bladder (urothelium and detrusor smooth muscle) after CYP treatment of varying duration; 3) is present in suburothelial inflammatory cells with chronic CYP treatment; and 4) blockade of AKT activation with deguelin increases (2.0-fold) bladder capacity in rats with CYP-induced cystitis. Therefore, the AKT signaling pathway may represent a novel target for pharmaceutical intervention and improving bladder function in the context of urinary bladder inflammation.
pAKT is classically known as a putative cellular survival signal (18). More recently, studies have demonstrated novel roles for AKT signaling in altered sensory/pain processing. For example, basal AKT phosphorylation is present in rodent lumbosacral dorsal root ganglia (DRG) (58, 59) and superficial laminae of the spinal cord (26, 60) and significantly increases following peripheral nerve injury or inflammation (26, 58, 59, 60, 71). Additionally, colocalization of pAKT with markers of unmyelinated, pain-transducing C-fibers such as IB4, TrkA, calcitonin gene-related peptide (CGRP), and transient potential vanilloid-1 channel (TRPV1) (58, 59, 71, 74) has been demonstrated. Specific inhibitors of AKT signaling including deguelin, AKT Inhibitor IV, SH-6, or NL-71–101 can attenuate mechanical hyperalgesia resulting from intradermal hindpaw capsaicin injection (59, 60) or sciatic nerve ligation (71), suggesting a nociceptive role for AKT signaling in peripheral inflammation or injury. In addition to playing a role in mediating somatic pain processes, phosphoinositide 3-kinase (PI3-K)/AKT signaling may also mediate visceral inflammation. For example, basal AKT phosphorylation in the superficial dorsal horn of lumbosacral spinal cord increases significantly following trinitrobenzenesulfonic acid-induced colitis (53). This demonstrated regulation of AKT activation (53) together with previous demonstrations of AKT phosphorylation in the urinary bladder of the mouse (65) and rat (16) are suggestive of a functional role for AKT signaling in other pelvic viscera including the urinary bladder following inflammation. Studies by Chung et al. (16) demonstrate a different time course of AKT activation in a rat model of CYP-induced cystitis compared with the present studies. In the present study, CYP-induced cystitis resulted in peak AKT activation in the urinary bladder following 4-h CYP treatment. Forty-eight-hour and chronic CYP-induced cystitis also resulted in significant increases in AKT activation in the urinary bladder but were significantly less than that observed with 4-h CYP treatment. In contrast, Chung et al. demonstrated significant AKT activation in the bladder following 8-h CYP treatment that was reduced to basal expression by at least 48 h following CYP administration. Differences in the time course of AKT activation may reflect different antibodies used for pAKT detection as well as differences in gender and strain of rats used. The results of our study suggest an involvement of pAKT in both acute and chronic bladder inflammation.
The present studies demonstrate regulation of pAKT in urinary bladder following inflammation as well as a functional role of AKT signaling in altered micturition reflexes induced by CYP treatment. Intravesical instillation of deguelin, an upstream inhibitor of AKT phosphorylation, decreased voiding frequency and increased bladder capacity and void volume following CYP-induced bladder hyperreflexia. Previous studies have demonstrated increased somatic sensitivity in rodents treated with CYP (13, 27, 28), but effects of deguelin on somatic (i.e., hindpaw, pelvic) sensitivity were not determined in the present study. The laparotomy performed to access the urinary bladder for cannulation and drug infusion is a potential confounding factor. Studies addressing effects of deguelin on somatic sensitivity induced by CYP treatment should be pursued in rats with intravesical instillation of deguelin using a transurethral route.
Although the focus of the current studies was on the expression and regulation of pAKT in the urinary bladder, other components of the micturition reflex pathways may also contribute to AKT signaling in the context of urinary bladder inflammation. Numerous studies have demonstrated activation of AKT in DRG and spinal cord neurons following peripheral nerve injury or inflammation (26, 58, 59, 60, 71). In the present study, neither AKT nor pAKT was observed in the suburothelial nerve plexus of control or CYP-treated bladder whole mounts. However, CYP-induced cystitis in female rats increases AKT activation in lumbosacral DRG cell bodies and spinal cord (Vizzard et al., unpublished observations), and basal AKT is present in these same tissues in control (no inflammation) rats. Lack of detection of AKT or pAKT in the suburothelial nerve plexus may be due to low-level expression of the proteins in the peripheral (i.e., target) innervation or lack of transport distally. Intrathecal delivery of inhibitors of AKT phoshorylation in combination with cystometric and somatic sensitivity studies are needed to determine a functional significance to AKT activation in these elements (e.g., DRG, spinal cord) of the micturition reflex.
Bladder pain syndrome (BPS)/interstitial cystitis (IC) is viewed as one type of chronic pain syndrome characterized by pain, pressure, or discomfort perceived to be bladder related with at least one urinary symptom such as urinary frequency (29, 30). The CYP rat model is one way to examine the contribution of inflammatory and immune mediators and signaling pathways to urinary bladder dysfunction and referred somatic sensitivity exhibited in the human syndrome. The CYP model permits a controlled analysis of some aspects of this chronic pain syndrome as a means of identifying therapeutic targets and early drug development that are not feasible to address in humans. Although the etiology and pathogenesis of IC/BPS are unknown, numerous theories, including infection, autoimmune disorder, toxic urinary components, deficiency in bladder wall lining, and neurogenic causes have been proposed (22, 35, 36, 49, 50, 56). We hypothesized that pain associated with BPS/IC involves alteration of visceral sensation/bladder sensory physiology. Altered visceral sensations from the bladder (i.e., pain at low or moderate bladder filling) that accompany BPS/IC may be mediated by many factors including changes in the properties of peripheral bladder afferent pathways such that bladder afferent neurons respond in an exaggerated manner to normally innocuous stimuli (allodynia). These changes may be mediated, in part, by inflammatory changes in the urinary bladder. Potential mediators of bladder inflammation are numerous and include cytokines (23, 40, 43), neuropeptides (11, 69), neuroactive compounds (9), chemokines (3, 55, 67, 73) and growth factors (70, 72, 75) and signaling molecules such as pERK1/2 (16, 17), pJNK (16, Vizzard et al., unpublished observations), and pAKT (4, 16).
Numerous growth factors including platelet-derived growth factor, epidermal growth factor, insulin, thrombin, NGF, and BDNF (6, 25, 68) are potential upstream activators of AKT. NGF is a bladder inflammatory mediator of particular interest because NGF protein levels are increased in the urine and urothelium of patients with BPS/IC (42, 48), and NGF is an important mediator of both somatic and visceral inflammation (1, 15, 24, 44, 45, 51). NGF interactions with the high-affinity receptor TrkA are known to activate the PI3-K/AKT, ERK1/2, and JNK signaling pathways. Chung et al. (16) demonstrate that AKT and ERK1/2 phosphorylation in the urinary bladder with CYP-induced bladder inflammation is dependent on the presence of NGF, suggesting that NGF constitutes a major upstream activator of AKT phosphorylation in bladder inflammation; however, the involvement of NGF/TrkA and/or NGF/p75NTR interactions was not identified. Numerous rodent studies demonstrate the physiological relevance of NGF signaling in the urinary bladder (15, 20, 34, 41, 57, 72, 75). Previous (17) and present studies now demonstrate that blockade of potential downstream NGF signaling targets, ERK1/2, or AKT activation, reduces urinary bladder hyperreflexia following CYP-induced cystitis. Whether cross talk exists between these two pathways in the context of urinary bladder inflammation (16) or whether further improvement in bladder function can be achieved by blocking both AKT and ERK1/2 activation remains to be determined.
In the absence of CYP-induced bladder inflammation, intravesical instillation of deguelin was without effect on bladder function. Thus AKT signaling may not play a major role in bladder function in the absence of inflammation. However, in this study only a single concentration of deguelin was evaluated so potential effects with higher concentrations are not known. The concentration of deguelin used in this study was identical to that used to reduce pain behavior after peripheral nerve injury (71) although the route of administration was different (intravesical vs. intrathecal). Additionally, Sun et al. (59) and Xu et al. (71) suggest that PI3-K/AKT signaling does not influence basal thermal or mechanical sensitivity before peripheral inflammatory or mechanical insult. These observations are consistent with the present studies where effects of blockade of AKT phosphorylation were only observed on bladder function in the context of urinary bladder inflammation. The mechanisms by which deguelin exerts its effects in other systems include downregulation of cyclooxygenase-2 (COX-2) expression, effects on NF-κB, as well as effects on AKT signaling pathways (19). Future studies should address the underlying mechanisms of deguelin action on bladder function following CYP-induced cystitis as both COX-2 (33) and NF-κB (37) have demonstrated roles in the inflamed urinary bladder.
Considering the robust urothelial activation of AKT in the inflamed bladder, AKT signaling may contribute to functional bladder sensory physiology via urothelium-mediated mechanisms. The urothelium, once thought to provide an impermeable barrier only, is now suggested to have “neuron-like” properties such as plasticity and sensory, transducive, and signaling capabilities, especially in the context of bladder inflammation (2, 5, 7, 8, 61–64). Urothelial cells share a number of similarities with sensory neurons, including the expression of receptors such as purinergic, norepinephrine, acetylcholine, neuropeptide- and protease-activated receptors, acid-sensing ion channels, neurotrophin receptors, and transient receptor potential channels (2, 5, 7, 8, 61–64). Because of functional receptor expression and secretion capabilities, urothelial-mediated communication with the detrusor smooth muscle, suburothelial plexus, and/or interstitial cells has been suggested (2, 7). Therefore, inflammatory signaling cascades may activate AKT signaling in the urothelium and thus facilitate the release of urothelial-derived mediators such as ATP, nitric oxide (NO), or chemokines that may then influence the suburothelial nerve plexus to affect micturition function via urothelium-to-nerve communication (2, 3, 5, 7, 8). Consistent with this hypothesis, AKT phosphorylation in the urothelium of inflamed bladders was significantly decreased with intravesical deguelin administration and further suggests the involvement of the urothelium in AKT signaling in urinary bladder dysfunction induced by CYP. Alone, or in combination with urothelium-mediated mechanisms, AKT phosphorylation in the detrusor smooth muscle as well as urothelium-mesenchyme interactions may contribute to CYP-induced bladder hyperreflexia. An alternative role for the phosphorylation of AKT in detrusor smooth muscle following cystitis may involve NGF-induced increases in type I collagen and resultant bladder hypertrophy via activation of AKT (16). Future studies may address NGF-induced release of ATP or NO via AKT signaling using cultured rat urothelial cells and pharmacological blockade of AKT phosphorylation.
Conclusions
These studies demonstrate increased activation of AKT in the rat urinary bladder after CYP-induced cystitis. Importantly, a functional role for the phosphorylation of AKT in bladder function in the context of urinary bladder inflammation induced by CYP has also been demonstrated. Blockade of AKT activation in the urothelium may be a potential target for a pharmacological intervention aimed at improving bladder dysfunction resulting from bladder inflammation.
GRANTS
This work was funded by National Institutes of Health (NIH) grants DK051369, DK065989, and DK060481. NIH Grant P20 RR-16435 from the Centers of Biomedical Research Excellence Program of the National Center also supported the project for research resources.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Allen SJ, Dawbarn D. Clinical relevance of the neurotrophins and their receptors. Clin Sci (Lond) 110: 175–191, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Apodaca G, Balestreire E, Birder LA. The uroepithelial-associated sensory web. Kidney Int 72: 1057–1064, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Arms L, Girard BM, Vizzard MA. Expression and function of CXCL12/CXCR4 in rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol 298: F589–F600, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arms L, Vizzard MA. Expression and function of phosphorylated AKT in rat urinary bladder after cyclophosphamide (CYP)-induced cystitis. Program No. 795.15/EEE10.2010 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience, 2010. Online [Google Scholar]
- 5. Arms L, Girard BM, Vizzard MA. Role of the bladder urothelium in voiding dysfunction. Current Bladder Dysfunct Rep 4: 227–233, 2009 [Google Scholar]
- 6. Beier F, Loeser RF. Biology and pathology of Rho GTPase, PI-3 kinase-Akt, and MAP kinase signaling pathways in chondrocytes. J Cell Biochem 110: 573–580, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Birder LA. Urinary bladder urothelium: molecular sensors of chemical/thermal/mechanical stimuli. Vascul Pharmacol 45: 221–226, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Birder LA, de Groat WC. Mechanisms of disease: involvement of the urothelium in bladder dysfunction. Nat Clin Prac 4: 46–54, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Birder LA, Wolf-Johnston A, Buffington CA, Roppolo JR, de Groat WC, Kanai AJ. Altered inducible nitric oxide synthase expression and nitric oxide production in the bladder of cats with feline interstitial cystitis. J Urol 173: 625–629, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, De Groat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci 5: 856–860, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Braas KM, May V, Zvara P, Nausch B, Kliment J, Dunleavy JD, Nelson MT, Vizzard MA. Role for pituitary adenylate cyclase activating polypeptide in cystitis-induced plasticity of micturition reflexes. Am J Physiol Regul Integr Comp Physiol 290: R951–R962, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen MW, Levin RM, Buttyan R. Peptide growth factors in normal and hypertrophied bladder. World J Urol 13: 344–348, 1995 [DOI] [PubMed] [Google Scholar]
- 13. Cheppudira BP, Girard BM, Malley SE, Dattilio A, Schutz KC, May V, Vizzard MA. Involvement of JAK-STAT signaling/function after cyclophosphamide-induced bladder inflammation in female rats. Am J Physiol Renal Physiol 297: F1038–F1044, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheppudira BP, Girard BM, Malley SE, Schutz KC, May V, Vizzard MA. Upregulation of vascular endothelial growth factor isoform VEGF-164 and receptors (VEGFR-2, Npn-1, and Npn-2) in rats with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol 295: F826–F836, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chuang YC, Fraser MO, Yu Y, Chancellor MB, de Groat WC, Yoshimura N. The role of bladder afferent pathways in bladder hyperactivity induced by the intravesical administration of nerve growth factor. J Urol 165: 975–979, 2001 [PubMed] [Google Scholar]
- 16. Chung CW, Zhang QL, Qiao LY. Endogenous nerve growth factor regulates collagen expression and bladder hypertrophy through Akt and MAPK pathways during cystitis. J Biol Chem 285: 4206–4212, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Corrow KA, Vizzard MA. Phosphorylation of extracellular signal-regulated kinases in urinary bladder in rats with cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol 293: R125–R134, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev 13: 2905–2927, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Dell'Eva R, Ambrosini C, Minghelli S, Noonan DM, Albini A, Ferrari N. The Akt inhibitor deguelin, is an angiopreventive agent also acting on the NF-kappaB pathway. Carcinogenesis 28: 404–413, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Dmitrieva N, Shelton D, Rice AS, McMahon SB. The role of nerve growth factor in a model of visceral inflammation. Neuroscience 78: 449–459, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Dorr W. Cystometry in mice—influence of bladder filling rate and circadian variations in bladder compliance. J Urol 148: 183–187, 1992 [DOI] [PubMed] [Google Scholar]
- 22. Driscoll A, Teichman JM. How do patients with interstitial cystitis present? J Urol 166: 2118–2120, 2001 [PubMed] [Google Scholar]
- 22a. Dugan C, Corrow K, Malley S, Arms L, Vizzard MA. Role of c-jun N-terminal Kinase (JNK) activation in micturition reflexes in cyclophosphamide (CYP)-induced cystitis in female rats. 2011 Neuroscience Meeting Planner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Erickson DR, Xie SX, Bhavanandan VP, Wheeler MA, Hurst RE, Demers LM, Kushner L, Keay SK. A comparison of multiple urine markers for interstitial cystitis. J Urol 167: 2461–2469, 2002 [PubMed] [Google Scholar]
- 24. Frossard N, Freund V, Advenier C. Nerve growth factor and its receptors in asthma and inflammation. Eur J Pharmacol 500: 453–465, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Frost RA, Lang CH. Protein kinase B/Akt: a nexus of growth factor and cytokine signaling in determining muscle mass. J Appl Physiol 103: 378–387, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Guedes RP, Araujo AS, Janner D, Bello-Klein A, Ribeiro MF, Partata WA. Increase in reactive oxygen species and activation of Akt signaling pathway in neuropathic pain. Cell Mol Neurobiol 28: 1049–1056, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guerios SD, Wang ZY, Boldon K, Bushman W, Bjorling DE. Blockade of NGF and trk receptors inhibits increased peripheral mechanical sensitivity accompanying cystitis in rats. Am J Physiol Regul Integr Comp Physiol 295: R111–R122, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guerios SD, Wang ZY, Bjorling DE. Nerve growth factor mediates peripheral mechanical hypersensitivity that accompanies experimental cystitis in mice. Neurosci Lett 392: 193–197, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Hanno P, Lin A, Nordling J, Nyberg L, van Ophoven A, Ueda T, Wein A. Bladder Pain Syndrome Committee of the International Consultation on Incontinence. Neurourol Urodyn 29: 191–198, 2011 [DOI] [PubMed] [Google Scholar]
- 30. Hanno P, Nordling J, Fall M. Bladder pain syndrome. Med Clin North Am 95: 55–73, 2011 [DOI] [PubMed] [Google Scholar]
- 31. Hemmings BA. Akt signaling: linking membrane events to life and death decisions. Science 275: 628–630, 1997 [DOI] [PubMed] [Google Scholar]
- 32. Herrera GM, Pozo MJ, Zvara P, Petkov GV, Bond CT, Adelman JP, Nelson MT. Urinary bladder instability induced by selective suppression of the murine small conductance calcium-activated potassium (SK3) channel. J Physiol 551: 893–903, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hu VY, Malley S, Dattilio A, Folsom JB, Zvara P, Vizzard MA. COX-2 and prostanoid expression in micturition pathways after cyclophosphamide-induced cystitis in the rat. Am J Physiol Regul Integr Comp Physiol 284: R574–R585, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Hu VY, Zvara P, Dattilio A, Redman TL, Allen SJ, Dawbarn D, Stroemer RP, Vizzard MA. Decrease in bladder overactivity with REN1820 in rats with cyclophosphamide induced cystitis. J Urol 173: 1016–1021, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Hurst RE, Moldwin RM, Mulholland SG. Bladder defense molecules, urothelial differentiation, urinary biomarkers, and interstitial cystitis. Urology 69: 17–23, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Johansson S, Ogawa K, Fall M. Interstitial cystitis. In: The Pathology of Interstitial Cystitis, edited by Sant GR. Philadelphia, PA: Lippincott-Raven, 1997, p. 143–152 [Google Scholar]
- 37. Kiuchi H, Takao T, Yamamoto K, Nakayama J, Miyagawa Y, Tsujimura A, Nonomura N, Okuyama A. Sesquiterpene lactone parthenolide ameliorates bladder inflammation and bladder overactivity in cyclophosphamide induced rat cystitis model by inhibiting nuclear factor-kappaB phosphorylation. J Urol 181: 2339–2348, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Klinger MB, Vizzard MA. Role of p75NTR in female rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol 295: F1778–F1789, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Klinger MB, Dattilio A, Vizzard MA. Expression of cyclooxygenase-2 in urinary bladder in rats with cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol 293: R677–R685, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Lamale LM, Lutgendorf SK, Zimmerman MB, Kreder KJ. Interleukin-6, histamine, and methylhistamine as diagnostic markers for interstitial cystitis. Urology 68: 702–706, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Lamb K, Gebhart GF, Bielefeldt K. Increased nerve growth factor expression triggers bladder overactivity. J Pain 5: 150–156, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Lowe EM, Anand P, Terenghi G, Williams-Chestnut RE, Sinicropi DV, Osborne JL. Increased nerve growth factor levels in the urinary bladder of women with idiopathic sensory urgency and interstitial cystitis. Br J Urol 79: 572–577, 1997 [DOI] [PubMed] [Google Scholar]
- 43. Malley SE, Vizzard MA. Changes in urinary bladder cytokine mRNA and protein after cyclophosphamide-induced cystitis. Physiol Genomics 9: 5–13, 2002 [DOI] [PubMed] [Google Scholar]
- 44. McMahon SB, Dmitrieva N, Koltzenburg M. Visceral pain. Br J Anaesth 75: 132–144, 1995 [DOI] [PubMed] [Google Scholar]
- 45. Molliver DC, Lindsay J, Albers KM, Davis BM. Overexpression of NGF or GDNF alters transcriptional plasticity evoked by inflammation. Pain 113: 277–284, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Murray E, Malley SE, Qiao LY, Hu VY, Vizzard MA. Cyclophosphamide induced cystitis alters neurotrophin and receptor tyrosine kinase expression in pelvic ganglia and bladder. J Urol 172: 2434–2439, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Oddiah D, Anand P, McMahon SB, Rattray M. Rapid increase of NGF, BDNF and NT-3 mRNAs in inflamed bladder. Neuroreport 9: 1455–1458, 1998 [DOI] [PubMed] [Google Scholar]
- 48. Okragly AJ, Niles AL, Saban R, Schmidt D, Hoffman RL, Warner TF, Moon TD, Uehling DT, Haak-Frendscho M. Elevated tryptase, nerve growth factor, neurotrophin-3 and glial cell line-derived neurotrophic factor levels in the urine of interstitial cystitis and bladder cancer patients. J Urol 161: 438–441, 1999 [PubMed] [Google Scholar]
- 49. Parsons CL. The role of the urinary epithelium in the pathogenesis of interstitial cystitis/prostatitis/urethritis. Urology 69: 9–16, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Petrone R, Agha A, Roy J, Hurst R. Urodynamic findings in patients with interstitial cystitis (Abstract). J Urol 153: 290A, 1995 [Google Scholar]
- 51. Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci 29: 507–538, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Pinto R, Frias B, Allen S, Dawbarn D, McMahon SB, Cruz F, Cruz CD. Sequestration of brain derived nerve factor by intravenous delivery of TrkB-Ig2 reduces bladder overactivity and noxious input in animals with chronic cystitis. Neuroscience 166: 907–916, 2010 [DOI] [PubMed] [Google Scholar]
- 53. Qiao LY, Grider JR. Colitis induces calcitonin gene-related peptide expression and Akt activation in rat primary afferent pathways. Exp Neurol 219: 93–103, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Qiao LY, Vizzard MA. Cystitis-induced upregulation of tyrosine kinase (TrkA, TrkB) receptor expression and phosphorylation in rat micturition pathways. J Comp Neurol 454: 200–211, 2002 [DOI] [PubMed] [Google Scholar]
- 55. Sakthivel SK, Singh UP, Singh S, Taub DD, Novakovic KR, Lillard JW., Jr CXCL10 blockade protects mice from cyclophosphamide-induced cystitis. J Immune Based Ther Vaccines 6: 6, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sant GR, Hanno PM. Interstitial cystitis: current issues and controversies in diagnosis. Urology 57: 82–88, 2001 [DOI] [PubMed] [Google Scholar]
- 57. Schnegelsberg B, Sun TT, Cain G, Bhattacharya A, Nunn PA, Ford AP, Vizzard MA, Cockayne DA. Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function. Am J Physiol Regul Integr Comp Physiol 298: R534–R547, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shi TJ, Huang P, Mulder J, Ceccatelli S, Hokfelt T. Expression of p-Akt in sensory neurons and spinal cord after peripheral nerve injury. Neurosignals 17: 203–212, 2009 [DOI] [PubMed] [Google Scholar]
- 59. Sun R, Yan J, Willis WD. Activation of protein kinase B/Akt in the periphery contributes to pain behavior induced by capsaicin in rats. Neuroscience 144: 286–294, 2007 [DOI] [PubMed] [Google Scholar]
- 60. Sun RQ, Tu YJ, Yan JY, Willis WD. Activation of protein kinase B/Akt signaling pathway contributes to mechanical hypersensitivity induced by capsaicin. Pain 120: 86–96, 2006 [DOI] [PubMed] [Google Scholar]
- 61. Sun Y, Chai TC. Augmented extracellular ATP signaling in bladder urothelial cells from patients with interstitial cystitis. Am J Physiol Cell Physiol 290: C27–C34, 2006 [DOI] [PubMed] [Google Scholar]
- 62. Sun Y, Chai TC. Up-regulation of P2X3 receptor during stretch of bladder urothelial cells from patients with interstitial cystitis. J Urol 171: 448–452, 2004 [DOI] [PubMed] [Google Scholar]
- 63. Sun Y, Keay S, Lehrfeld TJ, Chai TC. Changes in adenosine triphosphate-stimulated ATP release suggest association between cytokine and purinergic signaling in bladder urothelial cells. Urology 74: 1163–1168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sun Y, Keay S, De Deyne PG, Chai TC. Augmented stretch activated adenosine triphosphate release from bladder uroepithelial cells in patients with interstitial cystitis. J Urol 166: 1951–1956, 2001 [PubMed] [Google Scholar]
- 65. Tamarkin FJ, Kang WS, Cohen JJ, Wheeler MA, Weiss RM. A role for Akt in the rapid regulation of inflammatory and apoptotic pathways in mouse bladder. Naunyn-Schmiedeberg's Arch Pharmacol 373: 349–359, 2006 [DOI] [PubMed] [Google Scholar]
- 66. Tuttle JB, Steers WD, Albo M, Nataluk E. Neural input regulates tissue NGF and growth of the adult rat urinary bladder. J Auton Nerv Syst 49: 147–158, 1994 [DOI] [PubMed] [Google Scholar]
- 67. Vera PL, Iczkowski KA, Wang X, Meyer-Siegler KL. Cyclophosphamide-induced cystitis increases bladder CXCR4 expression and CXCR4-macrophage migration inhibitory factor association. PLoS ONE 3: e3898, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Viglietto G, Amodio N, Malanga D, Scrima M, De Marco C. Contribution of PKB/AKT signaling to thyroid cancer. Front Biosci 16: 1461–1487, 2011 [DOI] [PubMed] [Google Scholar]
- 69. Vizzard MA. Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J Chem Neuroanat 21: 125–138, 2001 [DOI] [PubMed] [Google Scholar]
- 70. Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol 161: 273–284, 2000 [DOI] [PubMed] [Google Scholar]
- 71. Xu JT, Tu HY, Xin WJ, Liu XG, Zhang GH, Zhai CH. Activation of phosphatidylinositol 3-kinase and protein kinase B/Akt in dorsal root ganglia and spinal cord contributes to the neuropathic pain induced by spinal nerve ligation in rats. Exp Neurol 206: 269–279, 2007 [DOI] [PubMed] [Google Scholar]
- 72. Yoshimura N, Bennett NE, Hayashi Y, Ogawa T, Nishizawa O, Chancellor MB, de Groat WC, Seki S. Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. J Neurosci 26: 10847–10855, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yuridullah R, Corrow KA, Malley SE, Vizzard MA. Expression of fractalkine and fractalkine receptor in urinary bladder after cyclophosphamide (CYP)-induced cystitis. Auton Neurosci 126–127: 380–389, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhuang ZY, Xu H, Clapham DE, Ji RR. Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J Neurosci 24: 8300–8309, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zvara P, Vizzard MA. Exogenous overexpression of nerve growth factor in the urinary bladder produces bladder overactivity and altered micturition circuitry in the lumbosacral spinal cord. BMC Physiol 7: 9, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]