Abstract
Previous studies suggest β-epithelial Na+ channel protein (β-ENaC) may mediate myogenic constriction, a mechanism of blood flow autoregulation. A recent study demonstrated that mice with reduced levels of β-ENaC (β-ENaC m/m) have delayed correction of whole kidney blood flow responses, suggesting defective myogenic autoregulatory capacity. Reduced renal autoregulatory capacity is linked to renal inflammation, injury, and hypertension. However, it is unknown whether β-ENaC m/m mice have any complications associated with reductions in autoregulatory capacity such as renal inflammation, injury, or hypertension. To determine whether the previously observed altered autoregulatory control was associated with indicators of renal injury, we evaluated β-ENaC m/m mice for signs of renal inflammation and tissue remodeling using marker expression. We found that inflammatory and remodeling markers, such as IL-1β, IL-6, TNF-α, collagen III and transforming growth factor-β, were significantly upregulated in β-ENaC m/m mice. To determine whether renal changes were associated with changes in long-term control of blood pressure, we used radiotelemetry and found that 5-day mean arterial blood pressure (MAP) was significantly elevated in β-ENaC m/m (120 ± 3 vs. 105 ± 2 mmHg, P = 0.016). Our findings suggest loss of β-ENaC is associated with early signs of renal injury and increased MAP.
Keywords: epithelial Na+ channel, ion channel, degenerin, renal autoregulation, myogenic constriction
myogenic constriction is a mechanism of blood flow autoregulation, which describes the ability of a vascular bed to maintain a constant flow despite variations in the level of arterial pressure by regulating vascular resistance. With increasing pressure, flow is maintained by vasoconstriction; with decreasing pressure, flow is maintained by vasodilation. The myogenic mechanism is an inherent mechano-dependent property of vascular smooth muscle cells (VSMCs) in resistance arteries and arterioles and is initiated by pressure-induced vessel stretch. Vascular stretch is thought to activate a mechano-dependent signaling mechanism leading to vasoconstriction (5, 17, 20, 21).
Several candidates have been considered as transducers of vascular stretch into intracellular signaling, including integrins, transient receptor potential channels, and epithelial Na+ channel (ENaC) proteins (16). Our laboratory has considered ENaC proteins as candidates for mechanotransducers because ENaC proteins have an evolutionary link to mechanotransduction (33). ENaC proteins are related to a family of proteins that form the ion channel pore of a mechanosensor in the nematode Caenorhabditis elegans. In addition, several lines of evidence suggest a specific ENaC protein, β-ENaC, is essential to transduction of myogenic constriction in vitro (19, 35). First, β-ENaC is expressed in VSMCs and transient gene silencing using small interfering (si) RNA or dominant-negative constructs demonstrates silencing of β-ENaC alone is sufficient to nearly abolish myogenic constriction in mouse renal interlobar arteries (19). Second, myogenic constriction in isolated middle cerebral arteries is abolished in a mouse model with reduced levels of β-ENaC (β-ENaC m/m) (35). Third, β-ENaC m/m mice have delayed correction of renal blood flow (RBF) following a step increase in renal perfusion pressure, suggestive of an altered myogenic autoregulatory capacity (13).
Since the autoregulatory response prevents transmission of damaging systemic pressure to delicate microvessels, it is not surprising that the loss of renal autoregulatory capacity is associated with renal injury. If the injury is significant, hypertension may result (9, 11). Several studies indicate loss of renal autoregulation leads to injury, which can be quantified by infiltration of inflammatory cells, activation of growth factors, and changes in the glomerular structure (3, 7, 9, 21, 24, 27, 32, 34, 36). The fast nature of the myogenic response suggests it may play a particularly important role in preventing transient blood pressure fluctuations from reaching delicate renal microvessels (9, 20, 23). Although β-ENaC m/m mice have reduced myogenic responsiveness, it is unknown whether they have any indications of renal inflammation, injury, or elevated blood pressure. Therefore, the goal of this study was to determine whether loss of β-ENaC protein leads to renal inflammation/injury, or possibly, hypertension. Results of this study suggest that mice with reduced levels of β-ENaC have signs of renal inflammation and injury, and elevated blood pressure.
METHODS
Animals.
Heterozygote β-ENaC +/m mating pairs on a mixed genetic background were generously provided (E. Hummler and B. Rossier, University of Lausanne, Lausanne, Switzerland). Animals were provided standard rodent chow containing 0.4% Na+ (Teklad) and water ad libitum. The University of Mississippi Medical Center's Institutional Animal Care and Use Committee approved all animal protocols. Male and female mice were used for all studies. Wild-type littermates were used as controls for all studies. For blood pressure studies, mice were housed individually in isolets. Animals were exposed to 12:12-h light (06:00–18:00)-dark (18:00–06:00) cycles.
Genotyping.
Offspring of heterozygote mating pairs were genotyped at 3 wk of age using DNA isolated (DirectTail PCR, Viagen) from tail samples and reconfirmed following phenotypic analysis using liver samples as previously described (35).
Assessment of renal inflammation and injury.
In male mice, we used renal macrophage infiltration as one of several methods to assess renal inflammation. Mouse kidneys (12–16 wk of age) were perfusion-fixed with 10% formalin, and 5-μm paraffin sections were prepared for analysis. Kidney sections were stained for the presence of monocytes/macrophages using an F4/80 antibody (1:75, AbD Serotec, Raleigh, NC) and counterstained with hematoxylin and eosin. Macrophage infiltration labeling was quantified using a point counting method with a ×40-objective lens. Twenty fields, obtained from six to nine tissue sections from four to five animals, were analyzed for each histological section. Results are expressed as the number of cells positive for F4/80 per tissue section.
We used Western blotting to determine whether a reduction in β-ENaC expression is associated with renal injury in a separate group of male mice. For assessment of renal inflammation/injury, mice at 12–16 wk and 1 yr were used. Whole kidney protein lysates were separated on 12.5% SDS-PAGE gels and transferred to nitrocellulose membranes. Membranes were incubated with antibodies to inflammatory markers including 1) mouse anti-target of antiproliferative, a leukocyte marker (TAPA-1, 1:1,000, Abcam, Cambridge, MA); 2) mouse anti-TNF-α (1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA), an inflammatory cytokine; 3) mouse anti-IL-1β; 1:1,000, Abcam); and 4) mouse anti-IL-6 (1:1,000, Abcam). Membranes were also incubated with markers of injury/remodeling including 1) mouse anti-collagen III (1:500, Abcam) and 2) mouse anti-transforming growth factor β1, a cytokine associated with renal fibrosis (TGF-β1, 1:1,000 Abcam). Rabbit anti-β-actin antibody (1:5,000, Abcam) was used as a loading control. Membranes were incubated with donkey anti-rabbit IR800 (1:2,000, Rockland Immunologicals, Rockland, IL) and donkey anti-mouse IR700 (1:2,000, Rockland Immunologicals) then visualized using the Odyssey Infrared Imaging System (LiCor, Lincoln, Nebraska). To quantify marker expression, we used Odyssey quantitation software. Pixel intensity was normalized to the β-actin loading control for each sample.
Blood pressure and locomotor activity measurement by telemetry in conscious mice.
Chronic arterial pressure and locomotor activity were measured by telemetry in separate β-ENaC wild-type (+/+) and homozygous mutant (m/m) mice. The radiotelemetry system (Data Sciences International, St. Paul, MN) components have been previously described elsewhere (2). For implantation of telemetry units, mice (12–16 wk of age) were anesthetized with isoflurane and pressure-sensing catheters were implanted into the left common carotid and a transmitter placed subcutaneously along the left flank. Mice were allowed at least 7 days of recovery from surgery after which time heart rate (HR), systolic (SAP), diastolic (DAP), mean arterial pressure (MAP), and activity were continuously recorded (10-s sampling period at 15-min intervals) for 5 days. MAP data were obtained in β-ENaC +/+ (n = 7, 3F/4M at 16 ± 1 wk of age) and m/m (n = 8, 3F/5M at 18 ± 2 wk of age) littermates. Data were collected and stored using the Dataquest ART data-acquisition system (Data Sciences International, St. Paul, MN) and exported into Microsoft Excel for analysis.
Statistical analysis.
All data are presented as means ± SE. Data were analyzed using ANOVA (Sigma Stat or InStat), followed by the Student-Newman-Keuls post hoc test, or independent/dependent t-tests where appropriate. Statistical significance was determined as P ≤ 0.05.
RESULTS
Evidence of renal inflammation and mild injury in β-ENaC m/m mice.
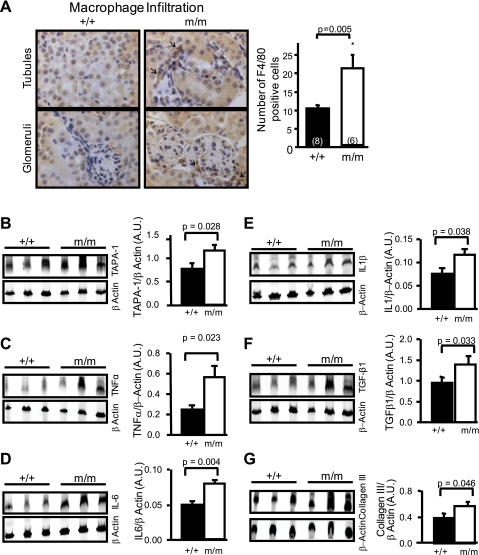
To determine whether loss of myogenic constriction in β-ENaC m/m mice is associated with signs of renal inflammation or injury, we assessed macrophage infiltration by immunohistochemistry and expression of several proinflammatory and injury markers by Western blotting. Representative images of macrophage labeling (F4/80 antibody labeling) in glomerular and tubular regions from a +/+ and m/m β-ENaC mouse (12–16 wk of age) are shown in Fig. 1A (left), and group data are shown in Fig. 1A (right). The number of cells that stained positive for F4/80 was twofold higher in β-ENaC m/m mice. Further evidence of inflammation is the increased expression of the leukocyte marker TAPA-1, and cytokines TNF-α, IL-1β, and IL-6 (Fig. 1, B–E). Additionally, indicators of renal injury such as TGF-β1 and collagen III were upregulated in β-ENaC m/m mice (Fig. 1, F–H). Data shown are for 1-yr-old littermates; however, similar results were obtained for mice at 12–16 wk of age. Although the expression of certain inflammatory markers is increased, there are no changes observed in renal ultrastructure (data not shown, i.e., glomerulosclerosis, matrix expansion, tubular atrophy, or casts). Thus there is no evidence of overt renal injury in β-ENaC m/m mice at the age studied; however, there is evidence of inflammation and mild injury.
Fig. 1.
Indications of renal inflammation and signs of subtle renal injury in a mouse model of reduced β-epithelial Na channel (ENaC). A, left: representative images of renal cortical sections stained with the F4/80 antibody to identify macrophage infiltration (arrows). The sections were counterstained with hematoxylin-and-eosin. Right: group data showing that kidneys from mutant mice (m/m, n = 6) contain 2-fold more F4/80-positive cells than wild-type (+/+, n = 8). Shown is Western blotting detection of leukocyte marker target of an antiproliferative antibody (TAPA-1; B) and inflammatory cytokines TNF-α (C), IL-6 (D), IL-1β (E), as well as transforming growth factor (TGF)-β1 (F), a growth factor associated with proliferation and expansion of matrix, and collagen III (G), a marker of extracellular matrix. Protein samples were obtained from whole kidney lysates in wild-type (+/+, n = 3) and mutant mice (m/m, n = 3). β-Actin loading control is shown below the corresponding blot. Quantitative data are shown on the right. Values are means ± SE. P values for analysis with t-test are provided. *Significantly different from control, P < 0.05.
Twenty-four-hour blood pressure, heart rate, and locomotor activity in β-ENaC +/+ and m/m mice.
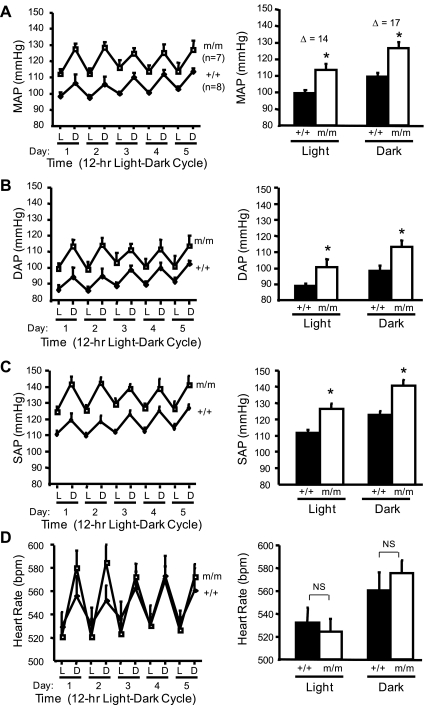
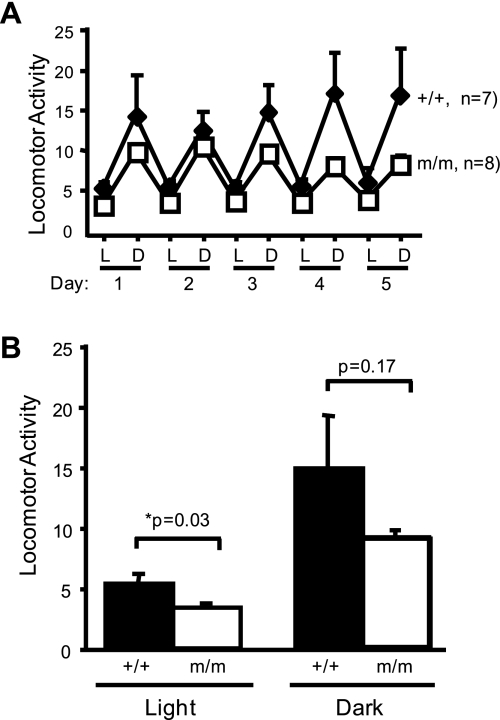
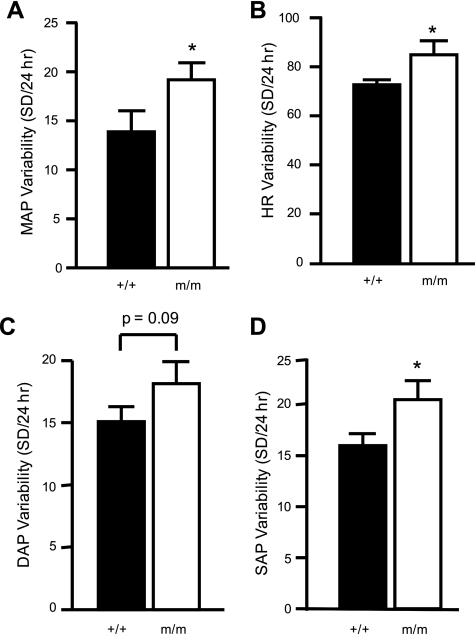
Average 12:12-h light-dark cycle MAP, SAP, DAP, and HR over the 5-day recording period are shown in Fig. 2 (left). The 24-h average MAP was significantly greater in the β-ENaC m/m mice (105 ± 2 vs. 120 ± 3 mmHg). As expected, MAP, SAP, DAP, and HR were increased during the dark cycle (Fig. 2, right). The 5-day average MAP, SAP, and DAP, but not HR, during the 12:12-h light and dark cycles was significantly greater in the m/m mice (Fig. 2, right). No differences were found between male and female mice. The average locomotor activity during the 12-h light cycle was reduced in m/m mice; however, activity was similar in m/m and +/+ mice during the 12-h dark cycle (Fig. 3, A and B). MAP, SAP, and HR variability during the last 24 h of telemetry recording during normal salt intake was significantly greater in the β-ENaC m/m mice (Fig. 4). Consistent with the increased blood pressure, we found the cardiac mass [heart wt (mg)/body wt (g)] was significantly elevated in β-ENaC m/m mice (4.87 ± 0.07 vs. 4.58 ± 0.16, P = 0.05).
Fig. 2.
Blood pressure and heart rate (HR) in normal salt (0.4% Na+)-fed animals. Mean arterial pressure (MAP), diastolic arterial pressure (DAP), systolic arterial pressure (SAP), and heart rate (HR) are shown in panels A, B, C, and D, respectively, in wild-type (+/+, filled bars, n = 7) and homozygous β-ENaC mutant mice (m/m, open bars, n = 8). Pressures for 12-h light (L) and dark (D) cycles for each of 5 days are shown (left). Values are means ± SE. *Significantly different from wild-type (+/+) control animals, P < 0.05.
Fig. 3.
Locomotor activity during light and dark cycles. A: mean locomotor activity for 12-h L and D cycles for each of 5 days. B: mean locomotor activity for L and D cycles over 5-day period. Values are means ± SE. *Significantly different from wild-type (+/+) control animals, P < 0.05.
Fig. 4.
Blood pressure and HR variability. Pressure and HR variability during the last 24 h of telemetry recording are shown for MAP (A), HR (B), DAP (C), and SAP (D). *Significantly different from wild-type (+/+) control animals, P < 0.05.
DISCUSSION
As a consequence of blood flow control, autoregulatory responses also prevent transmission of systemic pressures to the microvasculature, thereby protecting delicate microvessels from pressure-dependent injury (1, 9, 10, 22). In the kidney, at least two mechanisms promote autoregulatory adjustments to vascular resistance: myogenic constriction and tubuloglomerular feedback (TGF) (26). The TGF mechanism is mediated by increases in sodium chloride delivery to the macula densa in the early distal tubule, which leads to vasoconstriction of the afferent arteriole (31). TGF operates at a low frequency (<0.05 Hz) and requires ∼20–25 s for TGF signaling to regulate vascular resistance. The myogenic mechanism operates at a higher frequency (0.2–0.3 Hz) and adjusts vascular resistance approximately within 5–10 s and thus has an earlier onset than TGF (5, 17, 20, 21). The fast nature of the myogenic response has led several investigators to hypothesize it is the key protective mechanism (9, 20, 23). However, few animal models with reduced myogenic responsiveness are available to test this hypothesis (34). Our laboratory has identified a mouse model, β-ENaC m/m, with a delayed correction of RBF following a step increase in pressure, suggesting the model has altered renal myogenic autoregulation (13). However, it is unclear whether reduced myogenic renal autoregulatory capacity is associated with renal inflammation/injury or increased blood pressure. Therefore, the goal of this study was to determine whether renal inflammation, injury, remodeling, or increased blood pressure is present in β-ENaC m/m mouse. The major findings of this study indicate β-ENaC m/m mice demonstrate signs of renal inflammation and mild injury and increased blood pressure.
Mouse model.
In the present study, we used a mouse model with reduced β-ENaC levels. The animal model was generated using standard gene-targeting approaches in the course of generating a model of Liddle's syndrome (increased β-ENaC) by inserting a premature stop codon in the C-terminus coding region (30). However, the presence of the neomycin selection marker disrupts the β-ENaC gene locus, resulting in reduced β-ENaC expression. Thus a mouse model that under-, rather than overexpresses, β-ENaC was generated. Mice homozygous for the mutation (m/m) express very low levels of β-ENaC transcripts and protein in the lung and kidney as well as reduced β-ENaC protein in cerebral VSMCs (13, 30, 35). A prior study from our laboratory demonstrated β-ENaC m/m mice had a delay in recovery of RBF following a step increase in renal perfusion pressure when TGF was intact (13). In the prior study, we found RBF returned to control levels within 5 s in β-ENaC +/+, but took closer to 20 s in β-ENaC m/m mice. By 2 min, there was no difference in steady-state RBF between +/+ and m/m mice when TGF was active. Thus β-ENaC m/m mice have delays in RBF correction in a time frame associated with the myogenic response; however, steady-state autoregulatory responses are intact.
Link between myogenic constriction and renal injury.
Several lines of evidence suggest that the myogenic mechanism of autoregulation plays a significant role in protecting the kidney from pressure-related injury by preventing transmission of swings in systemic pressure to microvessels. First, loss of autoregulation in the kidney ablation model is associated with severe renal injury (1, 9–11). Second, animal models with impaired myogenic responsiveness are associated with an increased susceptibility to pressure-induced glomerular injury, while models with exaggerated myogenic responsiveness are protected from renal injury (4, 14, 15, 18). Third, patients with chronic renal disease, diabetes, and atherosclerosis (reduced renal autoregulatory capacity) have an increased risk of pressure-related renal injury (1, 9, 10, 12, 27). Fourth, under conditions of reduced autoregulatory capacity, pressure-dependent injury can still occur within the normotensive range (11). These findings indicate autoregulation prevents increases in systemic pressure from being transmitted to the glomerulus and protects against renal injury. However, whether a loss of β-ENaC-dependent myogenic autoregulation is associated with renal injury has not been addressed previously.
Results of the current investigation suggest the loss of β-ENaC-dependent myogenic autoregulation does not lead to overt renal injury in β-ENaC m/m mice up to 1 yr of age. However, we did find signs of inflammation and mild injury in the β-ENaC m/m mice including 1) infiltration of macrophages (F4/80) and other inflammatory cells (TAPA-1); 2) upregulation of TGF-β1 and collagen III, markers associated with expansion of the extracellular matrix; and 3) upregulation of inflammatory cytokines IL-1β, IL-6, and TNF-α (3, 24, 32). While steady-state autoregulation is intact in the β-ENaC m/m mice, we speculate weakened or slowed myogenic constriction leads to increased opportunities for transmission of pressure fluctuations to delicate microvessels. Cumulative exposure of microvessels to pressure fluctations over a lifetime may lead to indications of injury and inflammation. Although we did not observe glomerulosclerosis, matrix expansion, tubular necrosis, or casts by histological examination in β-ENaC m/m mice, it is possible that loss of myogenic constriction could increase their susceptibility to injury or accelerate injury if a secondary stimulus, such as diabetes, end-stage renal disease, or greater increases in arterial pressure were superimposed. Furthermore, the genetic background of the β-ENaC m/m model may protect the kidney from injury. Understanding the importance of secondary factors and genetic background on the protective ability of the myogenic response is an exciting area for future investigation.
Loss of myogenic autoregulation and renal inflammation is associated with increased blood pressure.
In the current study, we found the 24-h, 5-day average MAP was significantly increased 15 mmHg in β-ENaC m/m mice. The increase in MAP is not likely due to an increase in locomotor activity since activity levels were reduced or equivalent to +/+ controls. In a previous investigation, Pradervand et al. (30) reported MAP of ∼130–140 mmHg in β-ENaC +/+ and m/m mice of similar age. The most likely explanation for the higher pressure found by Pradervand et al. is the acute method of blood pressure measurement via an intracarotid catheter 4 h following recovery from anesthesia. Surgical stress may have masked pressure differences between +/+ and m/m mice. Furthermore, the increase in cardiac hypertrophy in the β-ENaC m/m mice in the current study is consistent with the increased blood pressure.
Because the β-ENaC m/m mouse was generated using homologous recombination, reduced levels of β-ENaC would be expected in all tissues, including renal tubular cells. It is not likely that defects in tubular salt and water transport contribute to hypertension since they would be expected to be associated with reduced or normal blood pressure (with compensatory upregulation of sodium-retaining hormones). Thus, at first consideration, our finding that blood pressure is elevated in the β-ENaC m/m mice seems counterintuitive. However, when the elevated blood pressure data are taken in context with loss of myogenic autoregulation and the presence of renal inflammation in the β-ENaC m/m mice, the possibility of renal injury-related increases in blood pressure becomes a plausible explanation.
The increase in blood pressure variability suggests that fluctuations in blood pressure are either more frequent or larger in the β-ENaC m/m mice compared with their wild-type littermates. The underlying reason for increased variability is not clear but may reflect the importance of ENaC proteins in arterial baroreception (6). However, in the face of reduced myogenic capacity, the increased frequency and/or magnitude of upward swings in blood pressure translates into more opportunities for transmission of damaging systemic pressure to the delicate renal microvasculature, and consequently, the increased likelihood for renal injury. By itself, reduced myogenic autoregulation may not be sufficient to cause outright injury; however, in conjunction with a “second hit,” it may lead to increased susceptibility to renal injury. Thus understanding whether additional insults increase susceptibility to renal injury in the β-ENaC m/m model remains to be determined.
Another factor contributing to the elevated blood pressure in the β-ENaC m/m mice may be related to compensatory hormonal responses needed to maintain blood volume/pressure status associated with decreases tubular salt and water transport. One potential factor is aldosterone, which is elevated twofold in this model (30). Both clinical and experimental studies demonstrate a link between aldosterone and end-organ injury (8, 25, 28, 29). Thus the compensatory upregulation of aldosterone may also contribute to organ injury and result in hypertension. It is possible that alterations in other volume-regulatory hormones, such as the renin-angiotensin system or endothelin, also contribute to the hypertension in this model.
Significance.
In summary, our data support the hypothesis that loss of β-ENaC-mediated myogenic constriction is associated with indications of renal inflammation and mild renal injury. This is the first study to address long-term control of blood pressure in a mouse model of reduced β-ENaC. The presence of increased chronic blood pressure in a mouse model of reduced β-ENaC is very intriguing because it raises the possibility that there may be a human population harboring β-ENaC loss-of-function mutations with increased blood pressure.
GRANTS
National Institutes of Health Grants HL086996 (H. Drummond), HL088421 (D. E. Stec), and HL51971 (J. Hall) supported this work.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Drs. Bernard Rossier and Edith Hummler at the University of Lausanne for generously providing our laboratory with the β-ENaC m/m mouse model. The authors also thank Stephanie Evans for assistance with macrophage labeling and histological analysis of kidney sections and Dr. Christine Maric for assistance in examining kidney sections for injury.
REFERENCES
- 1. Bidani AK, Schwartz MM, Lewis EJ. Renal autoregulation and vulnerability to hypertensive injury in remnant kidney. Am J Physiol Renal Fluid Electrolyte Physiol 252: F1003–F1010, 1987 [DOI] [PubMed] [Google Scholar]
- 2. Butz GM, Davisson RL. Long-term telemetric measurement of cardiovascular parameters in awake mice: a physiological genomics tool. Physiol Genomics 5: 89–97, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Cheng H, Zhang M, Moeckel GW, Zhao Y, Wang S, Qi Z, Breyer MD, Harris RC. Expression of mediators of renal injury in the remnant kidney of ROP mice is attenuated by cyclooxygenase-2 inhibition. Nephron Exp Nephrol 101: e75–e85, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Churchill PC, Churchill MC, Bidani AK, Griffin KA, Picken M, Pravenec M, Kren V, St, Lezin E, Wang JM, Wang N, Kurtz TW. Genetic susceptibility to hypertension-induced renal damage in the rat. Evidence based on kidney-specific genome transfer. J Clin Invest 100: 1373–1382, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cupples WA, Novak P, Novak V, Salevsky FC. Spontaneous blood pressure fluctuations and renal blood flow dynamics. Am J Physiol Renal Fluid Electrolyte Physiol 270: F82–F89, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Drummond HA, Price MP, Welsh MJ, Abboud FM. A molecular component of the arterial baroreceptor mechanotransducer. Neuron 21: 1435–1441, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Elmarakby AA, Quigley JE, Imig JD, Pollock JS, Pollock DM. TNF-α inhibition reduces renal injury in DOCA-salt hypertensive rats. Am J Physiol Regul Integr Comp Physiol 294: R76–R83, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fritsch Neves M, Schiffrin EL. Aldosterone: a risk factor for vascular disease. Curr Hypertens Rep 5: 59–65, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Griffin KA, Bidani AK. Hypertensive renal damage: insights from animal models and clinical relevance. Curr Hypertens Rep 6: 145–153, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Griffin KA, Picken MM, Bidani AK. Blood pressure lability and glomerulosclerosis after normotensive 5/6 renal mass reduction in the rat. Kidney Int 65: 209–218, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Griffin KA, Picken MM, Bidani AK. Deleterious effects of calcium channel blockade on pressure transmission and glomerular injury in rat remnant kidneys. J Clin Invest 96: 793–800, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Griffin KA, Picken MM, Churchill M, Churchill P, Bidani AK. Functional and structural correlates of glomerulosclerosis after renal mass reduction in the rat. J Am Soc Nephrol 11: 497–506, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Grifoni SC, Chiposi R, McKey SE, Ryan MJ, Drummond HA. Altered whole kidney blood flow autoregulation in a mouse model of reduced β-ENaC. Am J Physiol Renal Physiol 298: F285–F292, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hayashi K, Epstein M, Loutzenhiser R. Enhanced myogenic responsiveness of renal interlobular arteries in spontaneously hypertensive rats. Hypertension 19: 153–160, 1992 [DOI] [PubMed] [Google Scholar]
- 15. Hayashi K, Epstein M, Saruta T. Altered myogenic responsiveness of the renal microvasculature in experimental hypertension. J Hypertens 14: 1387–1401, 1996 [DOI] [PubMed] [Google Scholar]
- 16. Hill MA, Davis MJ, Meininger GA, Potocnik SJ, Murphy TV. Arteriolar myogenic signalling mechanisms: Implications for local vascular function. Clin Hemorheol Microcirc 34: 67–79, 2006 [PubMed] [Google Scholar]
- 17. Holstein-Rathlou NH, Wagner AJ, Marsh DJ. Dynamics of renal blood flow autoregulation in rats. Kidney Int Suppl 32: S98–S101, 1991 [PubMed] [Google Scholar]
- 18. Imig JD, Falck JR, Gebremedhin D, Harder DR, Roman RJ. Elevated renovascular tone in young spontaneously hypertensive rats. Role of cytochrome P-450. Hypertension 22: 357–364, 1993 [DOI] [PubMed] [Google Scholar]
- 19. Jernigan NL, Drummond HA. Myogenic vasoconstriction in mouse renal interlobar arteries: role of endogenous β- and γ-ENaC. Am J Physiol Renal Physiol 291: F1184–F1191, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Loutzenhiser R, Bidani A, Chilton L. Renal myogenic response: kinetic attributes and physiological role. Circ Res 90: 1316–1324, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Loutzenhiser R, Bidani AK, Wang X. Systolic pressure and the myogenic response of the renal afferent arteriole. Acta Physiol Scand 181: 407–413, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Loutzenhiser R, Griffin K, Williamson G, Bidani A. Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol 290: R1153–R1167, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Loutzenhiser R, Griffin KA, Bidani AK. Systolic blood pressure as the trigger for the renal myogenic response: protective or autoregulatory? Curr Opin Nephrol Hypertens 15: 41–49, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Maecker HT, Todd SC, Kim EC, Levy S. Differential expression of murine CD81 highlighted by new anti-mouse CD81 monoclonal antibodies. Hybridoma 19: 15–22, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Marney AM, Brown NJ. Aldosterone and end-organ damage. Clin Sci (Lond) 113: 267–278, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Navar LG, Marsh DJ, Blantz RC, Hall J, Ploth DW, Nasjletti A. Intrinsic control of renal hemodynamics. Fed Proc 41: 3022–3030, 1982 [PubMed] [Google Scholar]
- 27. Palmer BF. Impaired renal autoregulation: implications for the genesis of hypertension and hypertension-induced renal injury. Am J Med Sci 321: 388–400, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 348: 1309–1321, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 341: 709–717, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Pradervand S, Barker PM, Wang Q, Ernst SA, Beermann F, Grubb BR, Burnier M, Schmidt A, Bindels RJ, Gatzy JT, Rossier BC, Hummler E. Salt restriction induces pseudohypoaldosteronism type 1 in mice expressing low levels of the beta-subunit of the amiloride-sensitive epithelial sodium channel. Proc Natl Acad Sci USA 96: 1732–1737, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schnermann J, Traynor T, Yang T, Arend L, Huang YG, Smart A, Briggs JP. Tubuloglomerular feedback: new concepts and developments. Kidney Int Suppl 67: S40–S45, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Sharma K, Cook A, Smith M, Valancius C, Inscho EW. TGF-β impairs renal autoregulation via generation of ROS. Am J Physiol Renal Physiol 288: F1069–F1077, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Syntichaki P, Tavernarakis N. Genetic models of mechanotransduction: the nematode Caenorhabditis elegans. Physiol Rev 84: 1097–1153, 2004 [DOI] [PubMed] [Google Scholar]
- 34. van Dokkum RP, Sun CW, Provoost AP, Jacob HJ, Roman RJ. Altered renal hemodynamics and impaired myogenic responses in the fawn-hooded rat. Am J Physiol Regul Integr Comp Physiol 276: R855–R863, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Van Landingham LG, Gannon KP, Drummond HA. Pressure-induced constriction is inhibited in a mouse model of reduced β-ENaC. Am J Physiol Regul Integr Comp Physiol 297: R723–R728, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang X, Ajikobi DO, Salevsky FC, Cupples WA. Impaired myogenic autoregulation in kidneys of Brown Norway rats. Am J Physiol Renal Physiol 278: F962–F969, 2000 [DOI] [PubMed] [Google Scholar]