Abstract
Fibroblast growth factor receptors (Fgfrs) consist of four signaling family members and one nonsignaling “decoy” receptor, Fgfr-like 1 (Fgfrl1), all of which are expressed in the developing kidney. Several studies have shown that exogenous fibroblast growth factors (Fgfs) affect growth and maturation of the metanephric mesenchyme (MM) and ureteric bud (UB) in cultured tissues. Transgenic and conditional knockout approaches in whole animals have shown that Fgfr1 and Fgfr2 (predominantly the IIIc isoform) in kidney mesenchyme are critical for early MM and UB formation. Conditional deletion of the ligand, Fgf8, in nephron precursors or global deletion of Fgfrl1 interrupts nephron formation. Fgfr2 (likely the IIIb isoform signaling downstream of Fgf7 and Fgf10) is critical for ureteric morphogenesis. Moreover, Fgfr2 appears to act independently of Frs2α (the major signaling adapter for Fgfrs) in regulating UB branching. Loss of Fgfr2 in the MM leads to many kidney and urinary tract anomalies, including vesicoureteral reflux. Thus Fgfr signaling is critical for patterning of virtually all renal lineages at early and later stages of development.
Keywords: metanephric mesenchyme, ureteric bud, renal organogenesis, conditional knockout
Background on Kidney Development and Fibroblast Growth Factor Receptors
the metanephric kidney arises primarily from two tissues, the nephrogenic cord and the Wolffian (nephric) duct, that in turn give rise to the metanephric mesenchyme (MM) and ureteric bud (UB), respectively (10). At embryonic day 10.5 (E10.5) in the mouse and week 5 of gestation in humans, the MM induces the UB to grow out from the Wolffian duct into the MM (10). Stromal mesenchyme between the Wolffian duct and the MM restricts the UB to its proper position and prevents ectopic budding (21). Ongoing signaling from the MM stimulates the UB to elongate and repeatedly branch, leading to formation of the collecting ducts, pelvis, and ureter. Shortly after making contact with the UB, the MM divides into a nephrogenic lineage lying adjacent to the bud, and a surrounding renal cortical stromal lineage (10, 16). Each terminal tip of the UB induces local areas of nephrogenic mesenchyme to differentiate into nephron epithelia, progressing from renal vesicles, to comma-shaped bodies, to S-shaped bodies, and then to immature nephrons (10). The renal cortical stroma provides a framework and likely a niche for the other renal lineages (and the vasculature), and ultimately differentiates into interstitial and other supportive cells within the kidney.
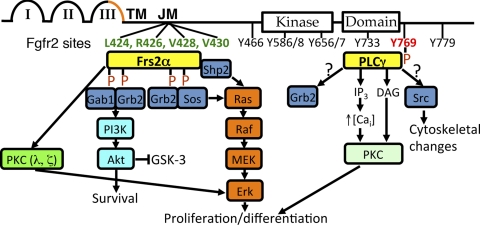
Fibroblast growth factor receptors (Fgfrs) are receptor tyrosine kinases (RTKs) with four signaling family members that have 22 known Fgf ligands in mammals (31). Fgfrs have three external immunoglobulin-like (Ig-like) domains, a transmembrane (TM) domain, and an intracellular kinase domain (31) (Fig. 1). Alternate splicing of C-terminal portions of the third Ig-like domains of Fgfrs1–3 results in IIIb or IIIc isoforms with different ligand specificities (Fgfr4 only has a IIIc isoform); in the context of embryonic development, Fgfr IIIb and IIIc isoforms have generally been felt to be expressed in and active in epithelium and mesenchyme, respectively (31) (Fig. 1). Molecules, such as fibroblast growth factor receptor substrate 2α (Frs2α) and phospholipase Cγ (PLCγ), transmit Fgfr signals via binding sites that are conserved across different receptor family members (31). Frs2α constitutively binds Fgfrs in the juxtamembrane region and upon receptor activation becomes phosphorylated, then activating Erk, Akt, and protein kinase C (PKC)-λ and -ξ (Fig. 1). PLCγ binds to a phosphorylated tyrosine on activated Fgfrs that activates PKC (and possibly Src and Grb2) (Fig. 1). Given that the receptor isoforms use the same intracellular signaling molecules, much of the specificity of the pathway is likely dictated by the ligands. In addition to the four signaling Fgfrs, fibroblast growth factor receptor-like 1 (Fgfrl1, formerly Fgfr5) is a molecule with an extracellular domain highly homologous to Fgfrs that can bind Fgf ligands, but it lacks a tyrosine kinase domain for intracellular signaling (14); recent data suggest that Fgfrl1 may act as a decoy receptor, competing with signaling Fgfrs for ligands (43).
Fig. 1.
Diagram of fibroblast growth factor receptor (Fgfr) 2 and its signaling through Fgfr substrate 2α (Frs2α) and PLCγ. Fgfr2 (like other signaling Fgfrs) contains 3 Ig-like domains (I–III), a transmembrane domain (TM), and a kinase domain. Alternate splicing of C-terminal portions of the third Ig-like domains of Fgfrs1–3 results in IIIb or IIIc isoforms (orange). Frs2α binds constitutively to the juxtamembrane (JM) region of Fgfrs at conserved sites (critical amino acids on Fgfr2 are in green); upon receptor activation, Frs2α becomes phosphorylated, leading to a cascade of signaling, activating Erk, Akt, and PKC (λ, ξ). PLCγ binds to a phosphorylated tyrosine on activated Fgfrs (tyrosine on Fgfr2 is in orange) that activates PKC [and possibly Src and growth factor receptor-bound protein 2 (Grb2)]. Gab1, growth factor receptor-bound protein 2-associated protein 1; Sos, son of sevenless homolog; Src, sarcoma viral oncogene homolog; Shp2, Src homology region 2-containing protein tyrosine phosphatase-2; PI3K, phosphoinositide (PI) 3-kinase; MEK, MAP kinase kinase; IP3, inositol trisphosphate; DAG, diacylglycerol; Cai, intracellular calcium.
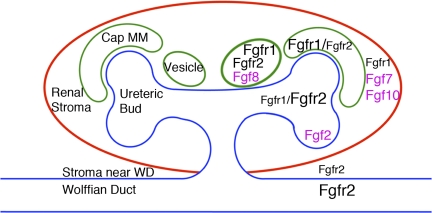
Although all Fgfrs have been detected in embryonic kidneys (5, 13, 19), animal studies have shown that Fgfr1, Fgfr2, and Fgfrl1 are the most relevant to renal development (see below). Fgfr1 is expressed most prominently in MM lineages (early MM, cap mesenchyme and developing nephrons beginning with vesicles) but is present at lower levels in the ureteric lineage and in renal cortical stroma (11, 24, 28, 30, 51) (Fig. 2). Fgfr2 is strongly expressed in the Wolffian duct and the UB tree (tips and trunks) and differentiating nephrons (beginning with vesicles) but is present at lower levels in early MM and stromal mesenchyme adjacent to the Wolffian duct (5, 11, 24, 28, 30, 51) (Fig. 2). Fgfrl1 is present in renal vesicles (14). Ligands with suspected or proven roles in kidney development include Fgf2, Fgf7, Fgf8, and Fgf10 (2, 15, 23, 27, 34) (Fig. 2).
Fig. 2.
Diagram showing expression of Fgf ligands (pink) and receptors (black) in the developing kidney. Schematic diagram of a developing mouse kidney that has secondary ureteric branching is shown. Cap metanephric mesenchyme (Cap MM) is only shown around the dorsal ureteric tips, and only 1 of 2 vesicles is shown ventral to each dorsal Cap MM. Relative receptor expression levels in particular lineages are indicated by the sizes of the fonts. WD, Wolffian duct.
Roles of Fgfrs in Patterning the Early Kidney
Fgfrs were first shown to have a role in early renal development when transgenic mice with a dominant negative Fgfr fragment developed renal aplasia or severe dysplasia (6). However, global gene-targeting studies in mice failed to elucidate which endogenous receptors were required for early kidney development. Deletion of Fgfr3 or Fgfr4 (alone and in combination) resulted in no structural kidney defects (Refs. 8 and 47, unpublished observations). While Fgfr1 and/or Fgfr2 were thus implicated as critical for early renal development, deletion of Fgfr1 or Fgfr2 resulted in early embryonic lethality, before the onset of renal development (1, 9, 48, 49).
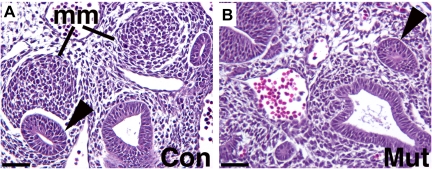
To circumvent the early lethality of global knockouts, conditional knockout approaches have been used including utilization of a Pax3cre transgenic line that drives deletion of genes in the MM (30). While deletion of any three of four alleles of Fgfr1 and Fgfr2 generally resulted in robust kidney formation, deletion of all four alleles (Fgfr1/2Mes−/−) resulted in severe renal dysgenesis with no recognizable MM at E10.5 (Table 1) (Fig. 3). MM markers Eya1 and Six1 were present in a very restricted domain near the UB, but downstream markers Six2, Sall1, and Pax2 were absent in kidney mesenchyme. In the ureteric lineage, E10.5 Fgfr1/2Mes−/− embryos had ureteric outgrowth (and occasionally multiple buds) due to expression of glial cell line-derived neurotrophic factor (Gdnf) in the MM; however, by E11.5 Gdnf was no longer present, leading to an absence of ureteric elongation or branching. Thus Fgfr1 and Fgfr2 signaling (together) in the MM are critical for formation of the MM and early development of the UB.
Table 1.
Targeted deletions of Fgf ligands, receptors, and signaling adapter proteins resulting in renal developmental anomalies in mice
| Targeted Gene | If Conditional, Lineage Targeted | Reported Results | Reference(s) |
|---|---|---|---|
| Fgf7* | Small kidneys with fewer UBs and nephrons | 34 | |
| Fgf10* | Small kidneys with fewer UBs | 23 | |
| Fgfr2IIIb* | Small kidneys with fewer nephrons | 35 | |
| Fgfrl1 | Severe renal dysgenesis | 14 | |
| Fgfr2 | UB | Small kidneys with aberrant UB branching, fewer nephrons and stromal mesenchymal patterning defects | 51 |
| Frs2α | UB | Mild renal hypoplasia with less ureteric branching and fewer nephrons | 40 |
| Fgfr2/ Frs2α | UB | Small, cystic dysplastic kidneys and severe UB branching defects | 42 |
| Fgf8 | MM | Small kidneys with interrupted nephron formation | 15, 27 |
| Fgfr1/Fgfr2 | MM | Aplasia with poorly formed MM and unbranched UBs | 30 |
| Fgfr1/Fgfr2IIIc | MM† | Similar to Fgfr1/Fgfr2 MM deletion except MM formation is partially rescued | 41 |
| Fgfr2 | MM | Partially penetrant abnormalities including duplex ureters, duplex kidneys, obstructive hydroureter, renal aplasia, anterior shift of UB, vesicoureteral reflux | 17, 18 |
UB, ureteric bud; MM, metanephric mesenchyme.
Fgf7 and Fgf10 are the ligands for Fgfr2IIIb.
Fgfr1 is conditionally deleted in MM, and Fgfr2IIIc is globally deleted.
Fig. 3.
Comparison of the embryonic day 10.5 (E10.5) metanephric mesenchyme (MM) and ureteric bud (UB) in control and Fgfr1/2Mes−/− mice. A and B: hematoxylin and eosin stains of transverse sections through caudal intermediate mesoderm demonstrate that control embryos (A) possess initial UBs (arrowheads) and compacted metanephric mesenchymal (mm) tissues; Fgfr1/2Mes−/− mice (B) appear to possess initial UBs (arrowheads) but no obvious MM. Scale bar = 200 μm. [Reprinted from Fig. 7 in Ref. 30 with permission from Elsevier].
To determine which Fgfr isoforms are critical in mediating early kidney patterning, mice were generated with global deletion of Fgfr2IIIc (Fgfr2IIIc−/−) alone and, in combination with Pax3cre, conditional deletion of Fgfr1 in MM (Fgfr1Mes−/−Fgfr2IIIc−/−) (Table 1) (41). Fgfr2IIIc−/− mice had normal kidneys (based on histology, explant analysis, and size measurements) and normal ureteric induction. At E10.5, Fgfr1Mes−/−Fgfr2IIIc−/− mice had small but clearly identifiable MM that expressed many normal markers including Eya1, Six2, and Pax2 (unlike Fgfr1/2Mes−/− mice that had no obvious MM and expressed only Eya1). By E11.5, Fgfr1Mes−/−Fgfr2IIIc−/− mice had only rudimentary MM tissues that expressed Eya1 but not Six2 or Pax2. In situ hybridization revealed the presence of Fgfr2IIIb in the MM in controls and Fgfr1Mes−/−Fgfr2IIIc−/− mutants, which may be acting to partially rescue the early mesenchymal defects in the mutants. In the ureteric lineage, E10.5 Fgfr1Mes−/−Fgfr2IIIc −/− embryos had ureteric outgrowth (and occasionally multiple buds) secondary to Gdnf expression in the MM; however, by E11.5 Gdnf was no longer present, leading to an absence of ureteric elongation and branching (similar to Fgfr1/2Mes−/− mice). Beyond E11.5, Fgfr1Mes−/−Fgfr2IIIc−/− mice had no recognizable renal tissue. Thus Fgfr2IIIc and Fgfr1 in kidney mesenchyme (together) are critical for normal early renal development. The absence of renal abnormalities in Fgfr2IIIc−/−, the less severe anomalies in Fgfr1Mes−/−Fgfr2IIIc−/− mice (compared with Fgfr1/2Mes−/−), and the presence of Fgfr2IIIb in MM strongly suggest that Fgfr2IIIb has some activity in the MM (challenging the thoughts that only IIIc Fgfr isoforms are active in developing mesenchymal tissues).
Roles of Fgfrs in Nephron Development
Early studies in rodent and Xenopus laevis explants showed that exogenous Fgf2 (alone or in combination with other growth factors) was able to sustain mesenchymal tissue growth and in some cases induce formation of epithelial nephrons (2–4, 26, 29). More definitive evidence that Fgfr signaling is critical for nephron formation came from conditional Fgf8 knockout studies (Table 1). Given that Fgf8 is expressed in early MM nephrogenic condensates, it was an attractive candidate ligand for regulating nephron development; however, Fgf8−/− mice died early in embryogenesis (44). To circumvent the early lethality of Fgf8 null mice, two groups of investigators used conditional knockout approaches with Pax3cre transgenic mice or Brachyury (T) cre transgenic mice (that express cre in all mesodermal tissues) (15, 27). The resulting mice that had no functional Fgf8 in the MM (Fgf8Mes−/−) survived until birth, but had small kidneys with an interruption in nephron formation after the epithelial vesicle stage. Deletion of Fgf8 in the nephron lineages led to downregulation of Wnt4 and Lim1, two molecules that are critical for nephron development. The receptor(s) that bind to and mediate the effects of Fgf8 in the nephron lineages are currently unknown. Interestingly, global deletion of Fgfrl1 (the apparent “decoy” Fgfr) also resulted in blockade of nephron differentiation (14); thus nephron formation may depend on a proper balance of positive and negative signaling through Fgf receptors.
Effects of Fgfr Signaling on Ureteric Branching
Several investigators have clearly demonstrated roles for Fgf ligands in regulating ureteric morphogenesis. Early studies in isolated rat UB cultures and/or in intact rat embryonic kidney explants showed that Fgf1, Fgf2, Fgf7, and Fgf10 stimulated growth and differentially affected ureteric branching (33, 34). In addition, overexpression of FGF2 or FGF7 in developing rodent kidneys in vivo led to cystic dilation of collecting ducts (20, 22). Global deletion of Fgf7 (produced by cortical stromal cells) resulted in viable mice with a reduction in ureteric branch number and as a secondary consequence, fewer nephrons (Table 1) (34). Global targeting of Fgf10, another ligand expressed in the MM, led to perinatal lethality likely from severe dysgenesis/agenesis of lungs and limbs; similar to Fgf7−/− mice, Fgf10−/− mice had smaller kidneys and fewer collecting ducts (Table 1) (23).
Genetic approaches have also begun to determine which receptors are critical for normal ureteric branching morphogenesis. As might be expected, global targeting of Fgfr2IIIb, the receptor isoform for both Fgf7 and Fgf10, results in smaller kidneys with fewer nephrons than normal (similar to the Fgf7 and Fgf10 knockouts) (Table 1) (35). Also, deletion of Fgfrl1 (present in renal vesicles) led to decreased ureteric branching, although it is unclear whether the ureteric defects are secondary to the interruption in nephrogenesis or whether there is a direct affect of Fgfrl1 on ureteric branching (Table 1) (14).
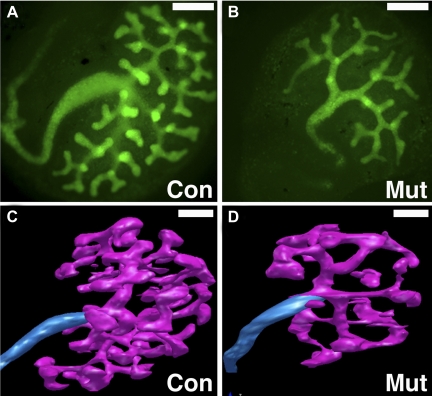
To determine which signaling Fgfrs were critical for ureteric morphogenesis, Hoxb7creEGFP transgenic mice (expressing cre recombinase and a green fluorescent protein in the nephric duct and the UB) were used to drive ureteric deletion of the receptors (FgfrUB−/−) (Table 1) (39, 51). While the mice were viable, Fgfr2UB−/− mutants had small kidneys with ureteric defects, including longer branches and significantly fewer ampullary tips (Fig. 4). Fgfr2UB−/− mice also had thickened cortical stroma with no interdigitations and ∼70% fewer nephrons than controls, likely from fewer UB tips. Although there was a normal distribution of developing nephron types (vesicles through immature glomeruli), mutants had larger developing nephrons (as a group and as glomeruli) compared with controls. Fgfr1UB−/− mice had no defects and Fgfr1/2UB−/− were indistinguishable from Fgfr2UB−/−, strongly suggesting that Fgfr2 is the major receptor family member driving ureteric morphogenesis.
Fig. 4.
Comparison of control and Fgfr2UB−/− UB branching in explants and by 3-dimensional (3D) reconstructive imaging. A and B: fluorescent micrographs of E11.5 explants after 3 days' growth demonstrating that compared with controls (A) Fgfr2UB−/− explants (B) have long ureteric trunks and fewer UB tips with thin ampullae (×40 magnification). Scale bar = 50 μm. C and D: 3D reconstructive imaging of E13.5 kidneys confirm that compared with controls (C), Fgfr2UB−/− mutant ureteric epithelium (D) occupies less relative volume and has fewer but longer ureteric segments. Scale bars = 100 μm.
Recently, there has been much attention on signaling downstream of Fgfrs in the UB. For example, one report showed that β1-integrin is necessary for Fgfr activation of intracellular signaling pathways in the UB (50). We have focused on the relationship between Fgfr2 and Frs2α, a key docking protein that transmits Fgfr signaling, in regulating UB morphogenesis. First, mice were generated with ureteric deletion of Frs2α with the Hoxb7cre line (Frs2aUB−/−) (Table 1) (40). Frs2aUB−/− mice had only mild renal hypoplasia characterized by decreased ureteric branching, but with fairly normal branching architecture and normal cortical stromal development (which was surprising since Frs2α transmits signals for both Fgfrs and Ret, another RTK critical for UB development) (36). The mild renal abnormalities in Frs2aUB−/− mice suggested that Fgfr2 might not utilize Frs2α to signal in the UB.
To more definitively determine whether Fgfr2 signals through Frs2α in the UB, mice were generated with point mutations in the Frs2α binding site on Fgfr2 (termed Fgfr2LR based on the amino acids that were mutated to alanine: these mutations abrogate Frs2α-Fgfr2 binding, while not affecting signaling via other adapters) (12). Fgfr2LR/LR mice had normal kidneys based on histology, explant analysis, and size measurements (40). To avoid rescue by intact Fgfr1 monomers forming heterodimers with Fgfr2LR, Hoxb7creTg/+Fgfr1Lox/LoxFgfr2LR/LR mice were generated but also found to have normal kidneys, by size, histology, and three-dimensional (3D) imaging (42).
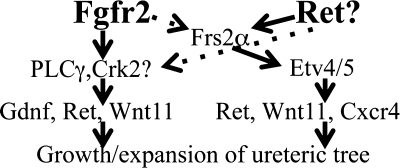
Mice with Hoxb7cre-driven Frs2a/Fgfr2 deletion (Fgfr2/Frs2aUB−/−) were also generated (the rationale being that if Frs2α adapts signals only from Fgfr2, then combined mutants should mimic Fgfr2UB−/−). While E13.5 Fgfr2/Frs2aUB−/− and Fgfr2UB−/− mice appear similar, at later ages, Fgfr2/Frs2aUB−/− mice have small, cystic dysplastic kidneys and significant UB branching defects that were clearly more severe than in Fgfr2UB−/− mice (Table 1) (42). Analysis of downstream targets supports independent actions of Fgfr2 and Frs2α; quantitative real-time PCR showed that compared with controls, E13.5 Fgfr2/Frs2αUB−/− kidneys have decreases in Ret, Gdnf, and Wnt11 (all P < 0.05) similar to Fgfr2UB−/−, whereas compound mutants had decreases in Etv4, Etv5, and Cxcr4 (all P < 0.05) similar to Frs2αUB−/−. Thus, while Fgfr2 and Frs2α have critical roles in the ureteric lineage, they appear to act separately and additively. Furthermore, it is likely that other intracellular molecules such as PLCγ transmit signaling for Fgfr2 in the UB. A diagram of proposed RTK and adapter signaling in the ureteric epithelium is shown in Fig. 5.
Fig. 5.
Diagram of proposed receptor tyrosine kinase and adapter protein signaling in the ureteric epithelium. The 2 receptor tyrosine kinases (RTKs) that appear to regulate ureteric morphogenesis, Fgfr2 and Ret, are in bold. Adapter proteins and their downstream targets are in smaller size font.
Effects of Fgfr Signaling on Ureteric Induction
Since Fgfr1/2Mes−/− mice (and other Pax3cre mutants including Fgfr1Mes−/−Fgfr2IIIc−/−) sometimes had multiple UBs per Wolffian duct, mice with single Pax3cre-driven Fgfr deletions were examined for ureteric induction defects (17). While Fgfr1Mes−/− mice were normal, 67% of E10.5–11.5 Fgfr2Mes−/− mice had two UBs per nephric duct (that often later fused into one UB) (Table 1). The ureteric induction abnormalities resulted in structural defects in ∼25% of older embryos, including duplex kidneys/collecting systems (partial and complete), renal aplasia, and obstructive hydroureter (17). Key molecules known to regulate the UB induction site, including Gdnf, Robo2, bone morphogenetic protein 4, and Sprouty1, were expressed normally by in situ hybridization in mutants; thus, mechanisms causing UB induction defects were unclear. Knowing that stromal mesenchyme around the nephric duct constrains the UB to its proper site, mutants were examined for expression of Fgfr2 in this region. Whole mount and section in situ hybridization revealed that Fgfr2 was deleted in these cells, possibly meaning that Fgfr2 acts in stromal tissue to constrain the UB induction site. To test this further, we have begun studies to conditionally delete Fgfr2 using a Tbx18creTg mouse line (a gift from Fen Chen) (46) that drives cre expression in the stromal tissues but not the kidney mesenchyme.
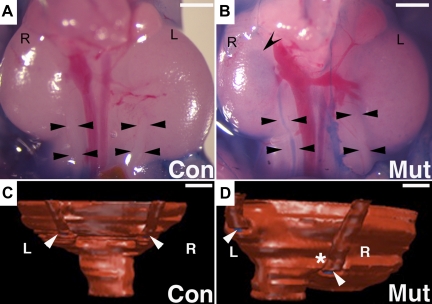
Given that abnormally positioned UBs are postulated to lead to vesicoureteral reflux (VUR) postnatally, Pax3cre-driven Fgfr2Mes−/− mutants (including those with single UBs per nephric duct) were assessed for displaced UBs and predisposition to VUR. Compared with controls, Fgfr2Mes−/− embryos with single UBs per nephric duct had increased common nephric duct lengths, indicating cranial displacement of the UBs along the Wolffian duct. Postnatal days 2–3 Fgfr2 mutants also had high rates of VUR compared with controls (40 vs. 3.8%, P = 0.0003) (Fig. 6). 3D reconstructive imaging of mutants with unilateral reflux revealed that the refluxing ureters were inserted much closer to the bladder neck than nonrefluxing ureters (Fig. 6). Moreover, the external insertional angles of the ureter at the outer wall of the bladder (formed by the ureteral insertion points and bladder neck) were much greater in the mutant refluxing ureters compared with contralateral nonrefluxing ureters as well as control ureters. Thus Pax3cre-mediated deletion of Fgfr2 causes abnormal positioning of the UB, which is associated with abnormal ureteral insertion in the bladder and subsequent VUR. If Tbx18cre-mediated deletion of Fgfr2 results in ureteric induction abnormalities (see above), we will assess these mice for the presence of VUR as well.
Fig. 6.
Representative cystograms and 3D reconstructions through the ureterovesical junction (UVJ) of newborn Fgfr2Mes−/− and control mice. A and B: gravity-driven methylene blue cystograms show a control mouse (A) with no dye in either ureter (arrowheads) and a mutant mouse (b) with dye in the right ureter (arrowheads) and the pelvis of the right kidney (concave arrowhead). Scale bars = 1 mm. C and D: posterior views of 3D reconstructions of the UVJ following cystograms show that insertion of the ureters (arrowheads) is at the same level in controls with no reflux (C); in the mutant (D) the right ureter (side of reflux, asterisk) inserts lower than the left (nonrefluxing) ureter. R, right side; L, left side. Scale bars = 200 μm. [Reprinted from Figs. 4 and 5 in Ref. 18 with permission from Elsevier].
Human Correlates
Structural kidney disease is the leading cause of chronic kidney disease in children. Over the last several years, some of the genetic mutations responsible for structural kidney disease have been elucidated, including mutations in Fgfrs. For example, activating mutations of FGFR1 and/or 2 cause syndromes such as Apert's syndrome, Antley-Bixler syndrome, Pfeiffer syndrome, and Beare-Stevenson syndrome, which are sometimes associated with urogenital anomalies such as hydroureter, solitary kidney, and VUR (7, 25, 37, 38). Loss of function mutations in FGFR1 have also been associated with some variants of Kallman syndrome that can be associated with unilateral renal aplasia. Rarely, activating mutations in FGFR3 leading to thanatophoric dysplasia are associated with renal hypoplasia or cystic dysplasia (32, 45). Thus a spectrum of structural kidney anomalies in humans is associated with mutations in FGFRs. It is also quite possible that other genetic alterations such as copy number variations in FGF or FGFR loci may be responsible for sporadic causes of structural kidney disease.
Conclusions
In conclusion, many Fgfs and all of their receptors are expressed in the developing kidney. In vitro and in vivo studies have shown that exogenous Fgfs affect both renal mesenchymal and UB development. Follow-up transgenic and gene knockout studies have shown that Fgfr signaling is required for patterning of all renal lineages, including the UB, nephrogenic mesenchyme, and renal stromal mesenchyme. Furthermore, Fgfr activity is critical at early stages as well as later stages of kidney development. Also, there appears to be redundancy between Fgfr1 and Fgfr2 in the kidney mesenchyme. In humans, activating and loss-of-function mutations in FGFRs cause syndromes that are sometimes associated with urogenital anomalies (7, 25, 32, 37, 38, 45). Perturbations in fibroblast growth factor receptor signaling may also be responsible for sporadic cases of human renal congenital malformations and VUR.
GRANTS
Some of the work presented was supported by National Institute of Diabetes and Digestive and Kidney Disease Grants R01 DK070030 and R01 DK081128 (C. Bates).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The author thanks Elsevier for permission to reprint some of the figures used in this publication.
REFERENCES
- 1. Arman E, Haffner-Krausz R, Chen Y, Heath JK, Lonai P. Targeted disruption of fibroblast growth factor (FGF) receptor 2 suggests a role for FGF signaling in pregastrulation mammalian development. Proc Natl Acad Sci USA 95: 5082–5087, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barasch J, Qiao J, McWilliams G, Chen D, Oliver JA, Herzlinger D. Ureteric bud cells secrete multiple factors, including bFGF, which rescue renal progenitors from apoptosis. Am J Physiol Renal Physiol 273: F757–F767, 1997 [DOI] [PubMed] [Google Scholar]
- 3. Barasch J, Yang J, Ware CB, Taga T, Yoshida K, Erdjument-Bromage H, Tempst P, Parravicini E, Malach S, Aranoff T, Oliver JA. Mesenchymal to epithelial conversion in rat metanephros is induced by LIF. Cell 99: 377–386, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Brennan HC, Nijjar S, Jones EA. The specification and growth factor inducibility of the pronephric glomus in Xenopus laevis. Development 126: 5847–5856, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Cancilla B, Ford-Perriss MD, Bertram JF. Expression and localization of fibroblast growth factors and fibroblast growth factor receptors in the developing rat kidney. Kidney Int 56: 2025–2039, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Celli G, LaRochelle WJ, Mackem S, Sharp R, Merlino G. Soluble dominant-negative receptor uncovers essential roles for fibroblast growth factors in multi-organ induction and patterning. EMBO J 17: 1642–1655, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cohen MM, Jr, Kreiborg S. Visceral anomalies in the Apert syndrome. Am J Med Genet 45: 758–760, 1993 [DOI] [PubMed] [Google Scholar]
- 8. Colvin JS, Bohne BA, Harding GW, McEwen DG, Ornitz DM. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nature Genet 12: 390–397, 1996 [DOI] [PubMed] [Google Scholar]
- 9. Deng CX, Wynshaw-Boris A, Shen MM, Daugherty C, Ornitz DM, Leder P. Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev 8: 3045–3057, 1994 [DOI] [PubMed] [Google Scholar]
- 10. Dressler GR. The cellular basis of kidney development. Annu Rev Cell Dev Biol 22: 509–529, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Dudley AT, Godin RE, Robertson EJ. Interaction between FGF and BMP signaling pathways regulates development of metanephric mesenchyme. Genes Dev 13: 1601–1613, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eswarakumar VP, Ozcan F, Lew ED, Bae JH, Tome F, Booth CJ, Adams DJ, Lax I, Schlessinger J. Attenuation of signaling pathways stimulated by pathologically activated FGF-receptor 2 mutants prevents craniosynostosis. Proc Natl Acad Sci USA 103: 18603–18608, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fuhrmann V, Kinkl N, Leveillard T, Sahel J, Hicks D. Fibroblast growth factor receptor 4 (FGFR4) is expressed in adult rat and human retinal photoreceptors and neurons. J Mol Neurosci 13: 187–197, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Gerber SD, Steinberg F, Beyeler M, Villiger PM, Trueb B. The murine Fgfrl1 receptor is essential for the development of the metanephric kidney. Dev Biol 335: 106–119, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Grieshammer U, Cebrian C, Ilagan R, Meyers E, Herzlinger D, Martin GR. FGF8 is required for cell survival at distinct stages of nephrogenesis and for regulation of gene expression in nascent nephrons. Development 132: 3847–3857, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Guillaume R, Bressan M, Herzlinger D. Paraxial mesoderm contributes stromal cells to the developing kidney. Dev Biol 329: 169–175, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hains D, Sims-Lucas S, Kish K, Saha M, McHugh K, Bates CM. Role of fibroblast growth factor receptor 2 in kidney mesenchyme. Pediatr Res 64: 592–598, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hains DS, Sims-Lucas S, Carpenter A, Saha M, Murawski I, Kish K, Gupta I, McHugh K, Bates CM. High incidence of vesicoureteral reflux in mice with Fgfr2 deletion in kidney mesenchyma. J Urol 183: 2077–2084, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Korhonen J, Partanen J, Alitalo K. Expression of FGFR-4 mRNA in developing mouse tissues. Int J Dev Biol 36: 323–329, 1992 [PubMed] [Google Scholar]
- 20. Li Z, Jerebtsova M, Liu XH, Tang P, Ray PE. Novel cystogenic role of basic fibroblast growth factor in developing rodent kidneys. Am J Physiol Renal Physiol 291: F289–F296, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Miyazaki Y, Oshima K, Fogo A, Hogan BL, Ichikawa I. Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J Clin Invest 105: 863–873, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nguyen HQ, Danilenko DM, Bucay N, DeRose ML, Van GY, Thomason A, Simonet WS. Expression of keratinocyte growth factor in embryonic liver of transgenic mice causes changes in epithelial growth and differentiation resulting in polycystic kidneys and other organ malformations. Oncogene 12: 2109–2119, 1996 [PubMed] [Google Scholar]
- 23. Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, Itoh N. FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem Biophys Res Commun 277: 643–649, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Orr-Urtreger A, Givol D, Yayon A, Yarden Y, Lonai P. Developmental expression of two murine fibroblast growth factor receptors, flg and bek. Development 113: 1419–1434, 1991 [DOI] [PubMed] [Google Scholar]
- 25. Passos-Bueno MR, Wilcox WR, Jabs EW, Sertie AL, Alonso LG, Kitoh H. Clinical spectrum of fibroblast growth factor receptor mutations. Hum Mutat 14: 115–125, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Perantoni AO, Dove LF, Karavanova I. Basic fibroblast growth factor can mediate the early inductive events in renal development. Proc Natl Acad Sci USA 92: 4696–4700, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perantoni AO, Timofeeva O, Naillat F, Richman C, Pajni-Underwood S, Wilson C, Vainio S, Dove LF, Lewandoski M. Inactivation of FGF8 in early mesoderm reveals an essential role in kidney development. Development 132: 3859–3871, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Peters KG, Werner S, Chen G, Williams LT. Two FGF receptor genes are differentially expressed in epithelial and mesenchymal tissues during limb formation and organogenesis in the mouse. Development 114: 233–243, 1992 [DOI] [PubMed] [Google Scholar]
- 29. Plisov SY, Yoshino K, Dove LF, Higinbotham KG, Rubin JS, Perantoni AO. TGF beta 2, LIF and FGF2 cooperate to induce nephrogenesis. Development 128: 1045–1057, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Poladia DP, Kish K, Kutay B, Hains D, Kegg H, Zhao H, Bates CM. Role of fibroblast growth factor receptors 1 and 2 in the metanephric mesenchyme. Dev Biol 291: 325–339, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Powers CJ, McLeskey SW, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer 7: 165–197, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Prontera P, Sensi A, Pilu G, Baldi M, Baffico M, Bonasoni R, Calzolari E. FGFR3 mutation in thanatophoric dysplasia type 1 with bilateral cystic renal dysplasia: coincidence or a new association? Genet Couns 17: 407–412, 2006 [PubMed] [Google Scholar]
- 33. Qiao J, Bush KT, Steer DL, Stuart RO, Sakurai H, Wachsman W, Nigam SK. Multiple fibroblast growth factors support growth of the ureteric bud but have different effects on branching morphogenesis. Mech Dev 109: 123–135, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Qiao J, Uzzo R, Obara-Ishihara T, Degenstein L, Fuchs E, Herzlinger D. FGF-7 modulates ureteric bud growth and nephron number in the developing kidney. Development 126: 547–554, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Revest JM, Spencer-Dene B, Kerr K, De Moerlooze L, Rosewell I, Dickson C. Fibroblast growth factor receptor 2-IIIb acts upstream of Shh and Fgf4 and is required for limb bud maintenance but not for the induction of Fgf8, Fgf10, Msx1, or Bmp4. Dev Biol 231: 47–62, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Schuchardt A, D'Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret [see comments]. Nature 367: 380–383, 1994 [DOI] [PubMed] [Google Scholar]
- 37. Sergi C, Stein H, Heep JG, Otto HF. A 19-week-old fetus with craniosynostosis, renal agenesis and gastroschisis: case report and differential diagnosis. Pathol Res Pract 193: 579–585, 1997 [DOI] [PubMed] [Google Scholar]
- 38. Seyedzadeh A, Kompani F, Esmailie E, Samadzadeh S, Farshchi B. High-grade vesicoureteral reflux in Pfeiffer syndrome. Urol J 5: 200–202, 2008 [PubMed] [Google Scholar]
- 39. Sims-Lucas S, Argyropoulos C, Kish K, McHugh K, Bertram JF, Quigley R, Bates CM. Three-dimensional imaging reveals ureteric and mesenchymal defects in Fgfr2-mutant kidneys. J Am Soc Nephrol 20: 2525–2533, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sims-Lucas S, Cullen-McEwen L, Eswarakumar VP, Hains D, Kish K, Becknell B, Zhang J, Bertram JF, Wang F, Bates CM. Deletion of Frs2alpha from the ureteric epithelium causes renal hypoplasia. Am J Physiol Renal Physiol 297: F1208–F1219, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sims-Lucas S, Cusack B, Baust J, Eswarakumar VP, Masatoshi H, Takeuchi A, Bates CM. Fgfr1 and the IIIc isoform of Fgfr2 play critical roles in the metanephric mesenchyme mediating early inductive events in kidney development. Dev Dyn 240: 240–249, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sims-Lucas S, Cusack B, Eswarakumar VP, Zhang J, Wang F, Bates CM. Independent roles of Fgfr2 and Frs2α in ureteric epithelium. Development 138: 1275–1280, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Steinberg F, Zhuang L, Beyeler M, Kalin RE, Mullis PE, Brandli AW, Trueb B. The FGFRL1 receptor is shed from cell membranes, binds fibroblast growth factors (FGFs), and antagonizes FGF signaling in Xenopus embryos. J Biol Chem 285: 2193–2202, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sun X, Meyers EN, Lewandoski M, Martin GR. Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes Dev 13: 1834–1846, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tohya T, Miura K, Nagata N. A case of thanatophoric dwarfism with renal hypoplasia. Pediatr Int 28: 232–237, 1986 [Google Scholar]
- 46. Wang Y, Tripathi P, Guo Q, Coussens M, Ma L, Chen F. Cre/lox recombination in the lower urinary tract. Genesis, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weinstein M, Xu X, Ohyama K, Deng CX. FGFR-3 and FGFR-4 function cooperatively to direct alveogenesis in the murine lung. Development 125: 3615–3623, 1998 [DOI] [PubMed] [Google Scholar]
- 48. Xu X, Weinstein M, Li C, Naski M, Cohen RI, Ornitz DM, Leder P, Deng C. Fibroblast growth factor receptor 2 (FGFR2)-mediated reciprocal regulation loop between FGF8 and FGF10 is essential for limb induction. Development 125: 753–765, 1998 [DOI] [PubMed] [Google Scholar]
- 49. Yamaguchi TP, Harpal K, Henkemeyer M, Rossant J. fgfr-1 is required for embryonic growth and mesodermal patterning during mouse gastrulation. Genes Dev 8: 3032–3044, 1994 [DOI] [PubMed] [Google Scholar]
- 50. Zhang X, Mernaugh G, Yang DH, Gewin L, Srichai MB, Harris RC, Iturregui JM, Nelson RD, Kohan DE, Abrahamson D, Fassler R, Yurchenco P, Pozzi A, Zent R. Beta1 integrin is necessary for ureteric bud branching morphogenesis and maintenance of collecting duct structural integrity. Development 136: 3357–3366, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhao H, Kegg H, Grady S, Truong HT, Robinson ML, Baum M, Bates CM. Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev Biol 276: 403–415, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]