Abstract
Upon ligand binding, epidermal growth factor (EGF) receptor (R) autophosphorylates on COOH-terminal tyrosines, generating docking sites for signaling partners that stimulate proliferation, restitution, and chemotaxis. Specificity for individual EGFR tyrosines in cellular responses has been hypothesized but not well documented. Here we tested the requirement for particular tyrosines, and associated downstream pathways, in mouse colon epithelial cell chemotactic migration. We compared these requirements to those for the phenotypically distinct restitution (wound healing) migration. Wild-type, Y992/1173F, Y1045F, Y1068F, and Y1086F EGFR constructs were expressed in EGFR−/− cells; EGF-induced chemotaxis or restitution were determined by Boyden chamber or modified scratch wound assay, respectively. Pharmacological inhibitors of p38, phospholipase C (PLC), Src, MEK, JNK/SAPK, phosphatidylinositol 3-kinase (PI 3-kinase), and protein kinase C (PKC) were used to block EGF-stimulated signaling. Pathway activation was determined by immunoblot analysis. Unlike wild-type EGFR, Y992/1173F and Y1086F EGFR did not stimulate colon epithelial cell chemotaxis toward EGF; Y1045F and Y1068F EGFR partially stimulated chemotaxis. Only wild-type EGFR promoted colonocyte restitution. Inhibition of p38, PLC, and Src, or Grb2 knockdown, blocked chemotaxis; JNK, PI 3-kinase, and PKC inhibitors or c-Cbl knockdown blocked restitution but not chemotaxis. All four EGFR mutants stimulated downstream signaling in response to EGF, but Y992/1173F EGFR was partially defective in PLCγ activation whereas both Y1068F and Y1086F EGFR failed to activate Src. We conclude that specific EGFR tyrosines play key roles in determining cellular responses to ligand. Chemotaxis and restitution, which have different migration phenotypes and physiological consequences, have overlapping but not identical EGFR signaling requirements.
Keywords: ErbB receptors, colon epithelial cell, phosphotyrosines, intestinal repair
cell migration is required for the physiological processes of wound repair, resolution of inflammation, and organogenesis, but is also involved in the pathology of tumor invasion (34). Thus investigation of the regulatory mechanisms of cell migration is important for both understanding basic intestinal biology and identifying potential therapeutic targets for disorders such as inflammatory bowel diseases and colon cancer. Chemotaxis (movement of individual cells along a chemical gradient) and restitution (coordinated wound closure by the cell monolayer) represent two phenotypically distinct forms of migration with different roles in tissue homeostasis and disease. However, the specific intracellular signaling events that distinguish these two cellular responses from each other are not well understood.
Epidermal growth factor (EGF) receptor (R) is a 170-kDa receptor tyrosine kinase that plays key roles in cellular migration, proliferation, and differentiation. Also known as ErbB1/HER1, EGFR is the prototypic member of the ErbB family of growth factor receptors. It binds a panel of ligands including EGF, TGF-α, amphiregulin, betacellulin, heparin-binding EGF, and epiregulin (21). Upon ligand binding, EGFR forms homodimers and heterodimers with other ErbBs (4), which activates the intrinsic protein tyrosine kinase activities of the receptor and leads to transautophosphorylation of specific tyrosine residues within the cytoplasmic domain (9, 20).
The six autophosphorylation sites in the COOH-terminal tail of EGFR are tyrosines 992, 1045, 1068, 1086, 1148, and 1173. Once phosphorylated, these residues serve as docking sites for intracellular signaling proteins containing Src homology 2 (SH2) or phosphotyrosine binding (PTB) domains and provide a connection between the external stimulation and specific internal signal transduction pathways (30). Phospho-Y992 and -Y1173 recruit and bind to the SH2 domain of phospholipase (PL) Cγ1, resulting in phosphorylation of PLCγ1 on tyrosines 771 and 1254 by EGFR (33, 36, 38) and increased phospholipase activity, which is required for EGF-stimulated cell motility (44). Y1045 phosphorylation creates a docking site for c-Cbl, which mediates EGFR ubiquitinylation and degradation (40). Phosphorylation of EGFR on Y1068 and Y1086 creates binding sites for Grb2 (2, 26), leading to activation of the MAPK/ERK cascade, and a binding site for Gab1, which recruits the p85 subunit of phosphatidylinositol 3-kinase (PI 3-kinase), leading to AKT activation (25). Finally, when phosphorylated, Y1148 and Y1173 serve as docking sites for the adaptor protein Shc, which also increases activity of the MAPK/ERK cascade (28).
Numerous studies have demonstrated a role for EGFR in stimulating either chemotaxis or restitution, and a variety of intracellular signaling pathways such as PLCγ1 (6, 23), Src (18), p38 MAPK (12), ERK MAPK (44), and PI 3-kinase (23) have been implicated in EGF-induced cell motility. However, it is not clear whether any selectivity for chemotaxis vs. restitution exists in EGFR-initiated signaling. Whether specific EGFR autophosphorylation sites and their associated signaling cascades provide the opportunity for a cell to choose between chemotaxis and restitution as a migration phenotype is as yet unknown.
We have previously described a number of the signaling cascades required for EGF-stimulated restitution (11, 23) or chemotactic movement (24) of small intestinal and colonic epithelial cells. The goal of the present study was to determine whether there is a differential requirement for individual EGF-stimulated pathways in chemotaxis and restitution and to test the role of specific EGFR phosphotyrosines in these responses. We established tyrosine to phenylalanine mutations in the EGFR autophosphorylation sites Y992/1173, Y1045, Y1068, and Y1086 and determined the roles of these residues in EGF-stimulated migration using modified Boyden chamber and scratch wound assays. Our results demonstrate that chemotaxis and restitution have overlapping but not identical EGFR signaling requirements, via selective activation of downstream signaling pathways.
MATERIALS AND METHODS
Growth factors, antibodies, and inhibitors.
Human recombinant EGF was provided by Carlos George-Nascimento (Chiron, Emeryville, CA). Human recombinant HGF, rat tail collagen type I, mouse collagen type IV, mouse laminin, and ITS+ culture medium supplement were from BD Biosciences (Bedford, MA). Recombinant murine interferon (IFN)-γ and human fibronectin were from Intergen (Norcross, GA); other medium additives were purchased from Mediatech (Herndon, VA). Antibodies were sourced as follows: anti-active ERK1/ERK2 MAPK, Promega (Madison, WI); rabbit anti-ERK1/ERK2, phospho-Akt (Ser-473), Akt, phospho-p38, phospho-SAPK/JNK and phospho-EGFR (Y1045, Y1068 and Y1173), Cell Signaling Technology (Beverly, MA); rabbit anti-EGFR, HER2, phospho-HER2 (Y1248) and phospho-PLCγ1 (Y783), Upstate (Charlottesville, VA); mouse monoclonal anti-EGFR conjugated to phycoerythrin [EGFR (528) PE], Santa Cruz Biotechnology (Santa Cruz, CA). AG1478 and SB202190 were from Calbiochem (San Diego, CA); U73122, U73343, D-609, L-108, PP1, and PP2 were from Biomol (Plymouth Meeting, PA). LY294002 was purchased from Sigma Chemical (St. Louis, MO). All pharmacological inhibitors and agonists were dissolved in DMSO and added to the medium with the final concentration of DMSO <0.1%.
Cell culture.
The conditionally immortalized young adult mouse colon (YAMC) and EGFR-null (EGFR−/−) mouse colonic epithelial (MCE) cells were generously provided by Dr. Robert Whitehead (Vanderbilt University, Nashville, TN). These cells express a heat-labile simian virus 40 large T antigen with an IFN-γ inducible promoter (35, 43). Cells were grown on culture dishes coated with rat tail collagen type I (5 μg/cm2) in RPMI 1640 supplemented with 5% FBS, 0.1% ITS+, 100,000 IU/l penicillin, 100 mg/l streptomycin, and 5 U/ml murine IFN-γ (Intergen, Norcross, GA). Cells were cultured under immortalizing (permissive) conditions at 33°C in the presence of IFN-γ until confluent, and then the medium was removed. The cells were immediately washed twice with PBS and cultured under nonpermissive conditions at 37°C with 0.5% FBS in the absence of IFN-γ and ITS+ until ready for use.
Chemotaxis assays.
Directed migration assays were performed by using a modified 48-well Boyden chamber apparatus (Neuroprobe, Cabin John, MD). Poretics polycarbonate membranes with 8-μm pores (Osmonics, Livermore, CA) were coated with 1 μg/cm2 of human fibronectin in carbonate buffer (pH 9.4) overnight before use as previously described (24). In some experiments, mouse laminin, mouse collagen type IV, or rat tail collagen type I was used at 1 μg/cm2 to coat the membrane. Growth factors were diluted in RPMI 1640 and placed into the lower wells of the chamber in triplicate. The wells were then covered with the matrix-coated polycarbonate membrane. These assays were performed following 16 h of culture under nonpermissive conditions. Cells were detached with 0.05% trypsin-EDTA for 1 min, neutralized with 10% FBS-RPMI 1640 for 10 min, washed twice with RPMI 1640 without FBS, and then pretreated with inhibitors for 45 min at 37°C. Cells were placed into the top chamber at 50,000 cells/well, and the chamber was incubated for 2 h at 37°C in an atmosphere of 95% air and 5% CO2. At the end of the incubation time, the chamber was disassembled and the cells were scraped off the top of the membrane. The remaining cells were fixed in 100% methanol (10 min), washed in water, stained with hematoxylin (10 min), and rinsed with water. The membrane was placed on a slide and coverslipped with Aqua Polymount (Polysciences, Warrington, PA). Three (×40) images were obtained of each well and counted by an investigator blind to experimental conditions. An example of a chemotaxis assay image is shown in Fig. 1A. Chemotaxis was calculated as the difference between the number of migrated cells in the presence of a gradient and in the absence (growth factor added to both the top and bottom wells) of a gradient.
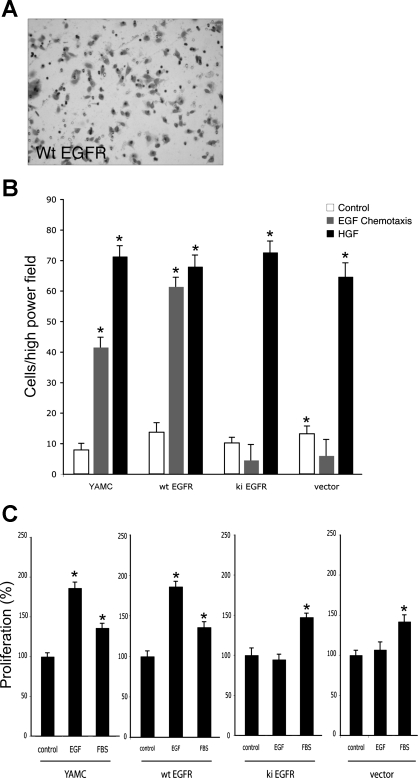
Fig. 1.
EGF receptor (EGFR) kinase activity is required for EGF-induced chemotaxis and proliferation of mouse colon epithelial (MCE) cells. A: representative image of chemotaxis assay using MCE cells, expressing wild-type (wt) EGFR, migrating toward EGF. B: young adult MCE (YAMC) cells or EGFR−/− MCE cells expressing wt EGFR, kinase inactive (ki) EGFR, or vector alone plated on fibronectin-coated polycarbonate membranes in a Boyden chamber and subjected to an EGF (10 ng/ml)-directed chemotaxis assay for 2 h. HGF (50 ng/ml) was used as a positive control for EGFR-independent migration. C: cells were exposed to EGF for 24 h and counted via a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2 h-tetrazolium (MTS)-based colorimetric assay. FBS was used as a positive control. *P < 0.001 vs. control.
Plasmid generation and cellular transfection.
The Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) was employed for generation of tyrosine-to-phenylalanine mutations in pcDNA 3.1(−)-wild-type (wt) EGFR. For transfections, EGFR−/− MCE were plated at 350,000 cells per well in a six-well plate. After 16-h incubation at permissive condition, transfection was performed by using the Lipofectamine 2000 (Invitrogen) reagent using 2 μg of DNA/well following the manufacturer's instructions. At 48 h after transfection, cells were selected with 200 ng/ml Zeocin (Invitrogen). Zeocin-selected pools of cells were stained with anti-EGFR 528-PE antibody (50 μl per 5×106 cells). PE (EGFR)-positive cells were sorted at the Veterans Affairs Medical Center Flow Cytometry Special Resource Center (Nashville, TN) by using a Becton-Dickinson FACSAria. Stable EGFR-expressing pools were maintained in 200 μg/ml Zeocin.
Cellular proliferation assays.
Cellular proliferation was measured by a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2 h-tetrazolium (MTS)-based colorimetric proliferation assay kit (Promega) as previously described (46). Cells were plated in 96-well culture dishes (5,000 cells/well) and incubated at nonpermissive condition in the presence and absence of EGF for 24 h. The produced formazan was dissolved and absorbance was measured at 490 nm. Reported values reflect averages of at least 12 replicate wells.
Restitution assays.
Wound healing of colon epithelial cell monolayers was performed as previously described (11). Briefly, cells were plated on fibronectin, grown to confluence, and shifted to nonpermissive conditions overnight. Multiple circular wounds were made in the monolayer cultures using a drill press-mounted rotating silicone rod. Wounds were photographed over time and % closure at 8 h was determined.
Cellular lysate preparation and Western blot analysis.
Cells were washed twice with ice-cold PBS, scraped into 1% Nonidet P-40 buffer [1% Nonidet P-40, 120 mM NaCl, and 50 mM Tris·HCl, pH 7.4, protease inhibitor cocktail (Sigma), phosphatase inhibitor I and II (Sigma)], and incubated in an ice bath for 20 min. Lysates were cleared by centrifugation and subjected to SDS-PAGE analysis as previously described (10).
siRNA knockdown experiments.
Nontargeting small interfering RNA (siRNA) pools and pools specific for PLCγ1, GRB2, and c-Cbl were purchased from Dharmacon (Lafayette, CO). Mouse colon epithelial cells were transfected with 20 nM siRNA pools by using the Turbofect siRNA cationic polymer agent (Fermentas, Glen Burnie, MD) following the manufacturer's instructions. At 48 h after transfection, cells were shifted to nonpermissive conditions for 24 h and subjected to Boyden chamber or scratch wound assays. Knockdown was monitored by Western blot analysis of lysates from parallel cultures.
Statistical analysis.
All data are representative of at least three independent experiments. Statistical analyses were performed by use of Prism software (GraphPad, La Jolla, CA). Statistical significance of differences from controls was assessed by ANOVA analysis with Tukey posttest. Error bars indicate SE.
RESULTS
EGFR tyrosine kinase activity is required for chemotactic migration of colon epithelial cells toward EGF.
We and others have shown a requirement for EGFR tyrosine kinase activity in intestinal cell migration (1, 19, 23). The majority of these studies have used pharmacological inhibitors of EGFR signaling, which could have off-target effects or show activity against other ErbBs. Therefore, to confirm the specificity of these results and establish a system for reexpressing EGFR on an EGFR-null background to test the effects of specific EGFR Y>F mutations, stable EGFR−/− MCE cells expressing vector, wt EGFR, or kinase inactive (ki; K721R mutation that blocks ATP binding) EGFR were established as described in materials and methods. EGF-directed chemotaxis of these cells was studied in modified 48-well Boyden chambers. Chemotaxis toward EGF was observed in YAMC cells (which express endogenous EGFR) and wt EGFR- but not vector- or ki EGFR-expressing EGFR−/− MCE cells (Fig. 1B). Untransfected control EGFR−/− MCE cells showed an identical response to vector (data not shown). Further validation of the cell system was provided by the observation that, in parallel restitution and proliferation assays, EGF-stimulated cell wound closure (see Fig. 4) and proliferation (Fig. 1C) in EGFR−/− MCE cells are also dependent on reexpression of wt EGFR.
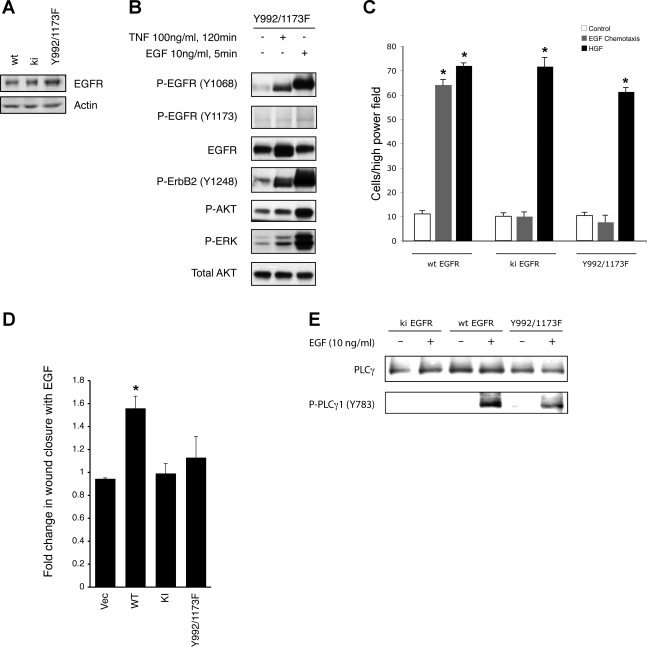
Fig. 4.
Y992/1173F mutation on EGFR blocks EGF-stimulated colon epithelial cell migration. A: wt, ki, and Y992/1173F EGFR were expressed in EGFR−/− MCE cells and relative expression was determined by Western blot analysis. B: EGFR−/− MCE cells expressing Y992/1173F EGFR were stimulated with TNF to transactivate, or EGF to directly activate, EGFR. Western blot analysis of whole cell lysates was performed to assess EGFR activation and phosphorylation of ErbB2, AKT, and ERK. C: wt, ki, and Y992/1173F EGFR-expressing cells were subjected to EGF-directed chemotaxis assays. D: cell monolayers were subjected to EGF-stimulated restitution assays. *P < 0.01 vs. control. Vec, vector. E: cells were stimulated with EGF for 5 min; PLCγ phosphorylation was determined by Western blot analysis using phosphospecific antibody. All blots are representative of at least 4 independent experiments.
p38 MAPK, PLCγ, and Src are required for EGF-stimulated colon epithelial chemotaxis.
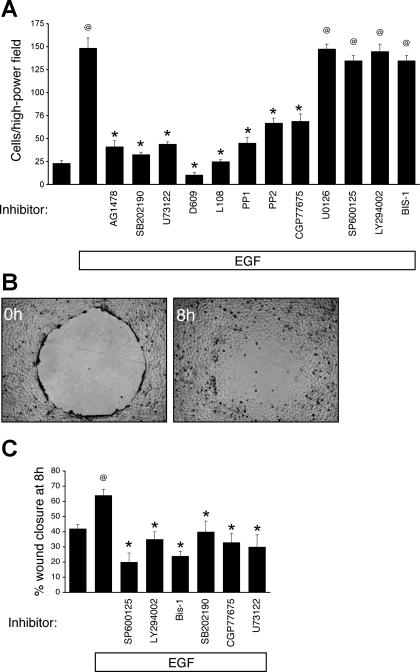
EGF-stimulated wound closure of a cell monolayer involves a variety of downstream signaling cascades, including ERK MAPK (16), JNK/SAPK (15), p38 MAPK (12), PI 3-kinase (10), PLCγ (24), Src (12), and PKC (23). However, it is not clear that pathways required for restitution are necessarily involved in directed migration of individual cells along a gradient. To determine which of these pathways are required for the chemotactic response, we assessed MCE cell movement toward EGF in a Boyden chamber in the presence of pharmacological inhibitors. Blockade of p38 (with SB202190), PLC (with U73122, D609, L108), or Src (with PP1, PP2, CGP77675) inhibited colon epithelial cell chemotaxis toward EGF (Fig. 2A). Chemotaxis was not blocked by inhibitors to MEK (U0126; blocks ERK activation), JNK (SP600125), PI 3-kinase (LY294002), or PKC (bisindolylmaleimide-1) (Fig. 2A). In contrast, restitution of cell monolayers over 8 h, which models the coordinated cell sheet migration seen in wound healing, is sensitive to the JNK, PI 3-kinase, and PKC inhibitors (Fig. 2B) as well as to p38, Src, or PLC blockade (Fig. 2B and Refs. 10, 12, 23). These results indicate that EGF-directed chemotaxis and EGF-stimulated restitution, which are phenotypically distinct at the cellular level, have signaling requirements that only partially overlap.
Fig. 2.
EGF-induced chemotaxis is sensitive to p38, PLC, or Src inhibition. A: wt EGFR-expressing EGFR−/− MCE cells were subjected to EGF-directed chemotaxis assays in the presence of inhibitors to EGFR (AG1478, 150 nM), p38 (SB202190, 10 μM), PLC (U73122, 1 μM; D609, 100 μM; and L108, 10 μM), Src (PP1, 10 μM; PP2, 10 μM; and CGP77675, 2 μM), MEK (U0126, 10 μM), JNK/SAPK (SP600125, 10 μM), phosphatidylinositol 3-kinase (PI 3-kinase) (LY294002, 5 μM), and PKC [bisindolylmaleimide-1 (BIS-1), 100 nM]. B: representative images of MCE cells subjected to restitution assay in the presence of EGF. C: cell monolayers were subjected to EGF-stimulated restitution assays in the presence of inhibitors. @P < 0.001 vs. control; *P < 0.01 vs. EGF.
PLCγ1 is the PLC isoform required for chemotaxis multiple PLC isoforms are expressed in mammalian cells.
To clarify which isoform is required for directed migration toward EGF, cells were transfected with siRNA for PLCγ1 and subjected to chemotaxis assays. PLCγ1 knockdown blocked EGF-stimulated chemotaxis (Fig. 3).
Fig. 3.
PLCγ1 is required for colon epithelial cell chemotaxis toward EGF. A: EGFR−/− MCE cells expressing wt EGFR were transfected with nontargeting (NT) or PLCγ1-specific small interfering RNA (siRNA), and cell chemotaxis toward EGF and HGF was determined. *P < 0.01 vs. no stimulation; @P < 0.01 for comparison of indicated pairs. B: PLCγ1 knockdown was determined by Western blot analysis (representative blots of 3 independent experiments shown).
Mutation of the primary PLCγ interaction sites on EGFR blocks EGF-directed migration.
Multiple tyrosines in the COOH-terminal portion of EGFR are phosphorylated upon ligand binding and receptor activation, but little is known about the relative contributions of individual sites to cellular responses. To begin to address this question in the context of cellular motility we expressed receptor with Y>F mutations at Y992 and Y1173 [the interaction sites between EGFR and PLCγ (5, 27)] in EGFR−/− MCE cells. The Y992F/Y1173F mutant was expressed (Fig. 4A) and became phosphorylated and stimulated downstream signaling in response to EGF (Fig. 4B) or tumor necrosis factor [which promotes EGFR transactivation in colon epithelial cells (46)], but the Y>F substitutions attenuated EGF-stimulated chemotaxis, similar to ki EGFR (Fig. 4C). This construct was also deficient in EGF-stimulated restitution when compared with wt EGFR (Fig. 4D). Surprisingly, however, EGF-stimulated PLCγ phosphorylation was only partially attenuated in these cells (Fig. 4E); densitometric analysis of four independent experiments indicates that EGF-stimulated PLCγ phosphorylation in Y992F/Y1173F EGFR-expressing cells is 56.3 ± 6.4% of that in wt EGFR-expressing cells. This response, possibly a result of Y992F/Y1173F EGFR heterodimerization with other ErbB family members such as ErbB2, suggests that either a high threshold level of PLCγ activity is required for chemotaxis and restitution, or additional cooperative signaling cascades affected by the Y992F/Y1173F mutant are also required.
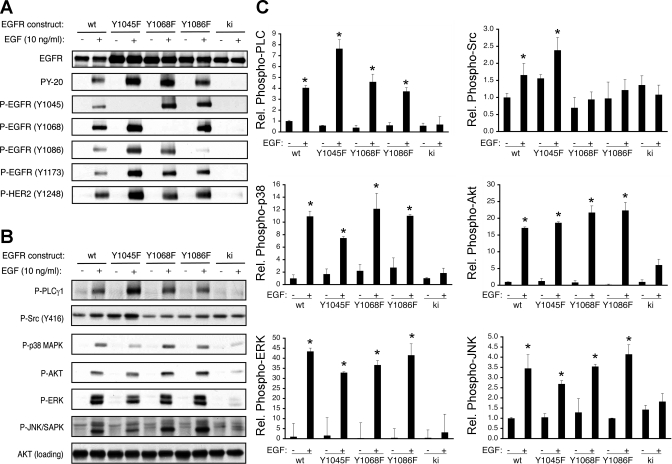
Phenylalanine substitutions on EGFR Y1045, Y1068, and Y1086 inhibit EGF-stimulated chemotaxis and restitution.
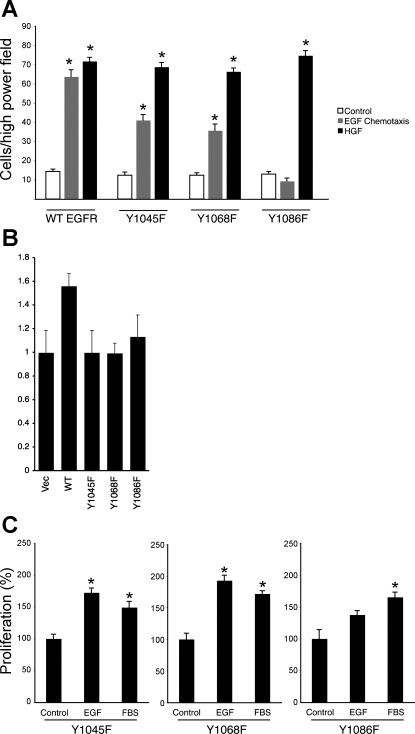
Phenylalanine substitution at Y1045, which is the primary Cbl binding site on EGFR and is a key feedback target of p38 signaling (11), partially blocked chemotactic migration toward EGF (Fig. 5A). Similarly, mutation of Y1068, one of the sites for Grb2 or Gab1 binding, attenuated but did not completely block chemotaxis. In contrast, phenylalanine mutation of the second Grb2 docking site, Y1086, completely blocked EGF-directed chemotactic movement of these cells. Interestingly, all three of these mutants, Y1045F, Y1068F, and Y1086F, were unable to support EGF-stimulated wound healing (Fig. 5B), but Y1045F and Y1068F mutants did not impair EGF-stimulated cell proliferation (Fig. 5C). Thus specific EGFR tyrosine residues have differential impact on chemotaxis, restitution, and proliferation.
Fig. 5.
Phosphorylation of Y1045, Y1068, and Y1086 on EGFR promotes colonocyte chemotaxis toward EGF. A: EGF-directed chemotaxis assays were performed on wt, Y1045F, Y1068F, and Y1086F EGFR-expressing cells. B: cell monolayers were subjected to EGF-stimulated restitution assays. C: cells were exposed to EGF for 24 h and counted via an MTS-based colorimetric assay. FBS was used as a positive control. *P < 0.001 vs. control.
To examine the signaling linked to these tyrosine residues, we prepared whole cell lysates from EGF-treated MCE cells expressing Y1045F, Y1068F, or Y1086F EGFR compared with wt and ki EGFR and performed Western blot analysis by using phosphospecific antibodies. All of the Y>F mutant constructs were activated by EGF, with no substantial alteration in individual phosphosite modification except on the expected mutant sites (Fig. 6A). Similarly, wt (but not ki) EGFR and all three Y>F mutant constructs promoted phosphorylation of ErbB2, a key heterodimer partner in colon epithelial cells.
Fig. 6.
Signaling stimulated by EGF in Y1045F, Y1068F, and Y1086F EGFR-expressing colon epithelial cells. Cells expressing wt, Y1045F, Y1068F, 1086F, or ki EGFR were exposed to EGF for 5 min and whole cell lysates were prepared. A: Western blot analysis was performed with use of EGFR and ErbB2 phosphospecific antibodies. B: activation of PLC, Src, p38, AKT, ERK, and JNK/SAPK was determined by Western blot analysis with phosphospecific antibodies. All blots are representative of at least 3 independent experiments. C: densitometric analysis of results from experiments performed for B. *P < 0.05 vs. unstimulated from same cell line. Rel., relative.
PLCγ1, Src, AKT, ERK, and p38 were all phosphorylated in response to EGF in Y1045F EGFR-expressing cells (Fig. 6, B and C), indicating that decreased chemotaxis with this mutant may not be due to a simple defect in activating these targets. We have previously shown that failure of restitution in Y1045F EGFR-expressing cells is associated with a loss of sensitivity to p38-induced feedback through Cbl, with no change in p38 activation (11). The defect in Y1045F chemotaxis and the inhibition by SB202190 in the present study may represent a similar mechanism.
In Y1068F and Y1086F EGFR-expressing cells compared with wt, we observed decreased Src phosphorylation in response to EGF (Fig. 6, B and C). Thus these tyrosines are key for signaling through Src family members. As Src inhibition blocked chemotaxis in wt EGFR-expressing cells (Fig. 2) and EGF-stimulated PLCγ1, AKT, ERK, and p38 activation were unchanged in the Y1068F and Y1086F lines, the decreased Src activation is likely to play a role in reduced chemotaxis.
Grb2 and c-Cbl knockdown attenuate chemotaxis and restitution.
The effects of EGFR Y1068F, Y1086F, and Y1045F on chemotaxis and restitution suggest that the signaling adaptor Grb2 and the ubiquitin ligase c-Cbl may be important for these cellular responses. Therefore we performed chemotaxis and restitution assays in wt EGFR-expressing MCE cells depleted of Grb2 or c-Cbl by siRNA transfection (Fig. 7A). Chemotaxis toward EGF was inhibited by Grb2 knockdown, but not by loss of c-Cbl (Fig. 7B). In contrast, EGF-stimulated restitution was decreased by loss of either Grb2 or c-Cbl (Fig. 7C).
Fig. 7.
Grb2 and c-Cbl regulate colon epithelial cell migration. MCE cells expressing wt EGFR were transfected with NT siRNA (si) or siRNA directed against Grb2 or c-Cbl. A: knockdown of Cbl and Grb2 was determined by Western blot analysis. Blots are representative of 3 independent experiments. Transfected cells were subjected to assays for chemotactic migration (A) and restitution/wound healing migration (B). *P < 0.05 vs. NT control. n.s., Not significant.
DISCUSSION
In this study, we report that p38 MAPK, Src, and PLCγ1 activity are required for colon epithelial cell chemotactic migration along an EGF gradient (Fig. 2) as well as for restitution/wound healing migration. Furthermore, either chemotaxis or restitution in response to EGF was inhibited by blockade of phosphorylation on EGFR Y992/Y1173, Y1045, Y1068, or Y1086 (Figs. 4 and 5). In Y992F/Y1173F EGFR-expressing cells, this correlated with reduced PLCγ activation, whereas decreased Src phosphorylation was observed with Y1068F and Y1086F EGFR constructs (Fig. 6). However, the phosphosite-specific responses and downstream signaling requirements for chemotaxis were not identical with the signals necessary for EGF-stimulated restitution. For example, Y1045F and Y1068F mutants completely blocked restitution in response to EGF while showing only a partial effect on chemotaxis, and restitution but not chemotaxis was sensitive to JNK, PI 3-kinase, and PKC inhibition. Furthermore, loss of Grb2, which binds Y1068/1086, decreased both forms of migration, whereas in contrast c-Cbl knockdown only affected restitution (Fig. 7). Thus, although the differences are subtle, EGF-driven chemotaxis and restitution responses are biochemically separable. Given that the former phenotype models cancer cell invasion whereas the latter represents a beneficial repair response, further investigation of these differences is warranted.
Following ligand-EGFR binding, rapid signal transduction to a number of downstream cascades including Ras/Raf/MEK/ERK, PLC/PKC, PI 3-kinase/AKT, and Src/p38MAPK results in regulated outcomes including proliferation, migration, and survival. The mechanisms by which a cell determines its behavior in response to stimulation are not well understood, though differential regulation of heterodimerization with other ErbBs and context-dependent interaction with downstream signaling molecules such as Src, PLC, and p38 are likely to play a major role. For example, expression of ErbB2 is critical for the corneal epithelial wound healing response to EGFR ligand (45), and proliferation vs. chemotaxis of melanoma cell lines in response to growth factor is dependent on the suite of ErbBs available (13). Additionally, we have previously shown that p38 activation and its ability to promote a feedback regulatory loop on EGFR, involving Cbl activation and intracellular receptor trafficking, is a key determinant of the proliferation/restitution switch in EGF-treated colonocytes (11). Interestingly, the observation that Y1045 is required for maximal chemotaxis (Fig. 5) but c-Cbl is not (Fig. 7) suggests the involvement of an additional EGFR binding partner that docks at Y1045.
It should be noted that the specific signaling cascades necessary for chemotaxis and restitution may be somewhat context specific. For example, whereas we observe no role for ERK in EGF-driven motility of colon epithelial cells (Ref. 12 and Fig. 2), ERK is required for chemotaxis of PC12 cells (17) or keratinocytes (41) in response to EGF. Furthermore, in many cell types the response is stimulus specific; for example, in BEAS-2B bronchial epithelial cells ERK is unnecessary for EGF- but required for TFF2-stimulated chemotaxis (7). Whether these differences represent tissue-specific signaling or reflect the particulars of different in vitro model systems remains an open, and important, question.
Previous studies using EGFR mutant constructs to identify potential site-specific responses have yielded mixed results. For example, the Carpenter laboratory found that in NIH3T3 cells expressing point-mutant human receptors, there was no strict requirement for individual phosphosites to mediate binding to downstream targets (31). The same group reported that Y992 is critical for EGFR activation and signaling only in the context of a truncated, but not full-length, receptor (32), suggesting that binding at some tyrosines can compensate for loss of others. On the other hand, Gotoh and colleagues (14) found that single autophosphorylation site mutants were additive in their effects on mitogenesis, indicating that each site makes a separable contribution. Similar to the discussion above, responses to EGFR mutation may well arise from cell- or tissue-specific context, and in particular the ErbB heterodimer partners available. This possibility is underscored by the observation that kinase-dead K721M EGFR is able to heterodimerize with ErbB2, but not ErbB3 or ErbB4, to promote activation of ERK and Akt and drive 32D cell survival (8). The requirement for phosphorylation on specific EGFR tyrosines for downstream signaling events might similarly depend on the panel of other ErbBs present in a given cell.
One signaling arm required for EGF-directed chemotaxis in this study was through Y992/Y1173-dependent activation of PLCγ1 (Fig. 4). In prior reports, PLC blockade inhibited cell migration independently of MAPK- and PI 3-kinase-driven proliferation (29, 44). Signaling through this cascade promotes cytoskeletal reorganization that enables cellular polarization (39, 42). Interestingly, in our results Y992F/Y1173F EGFR double mutation only partially inhibited the activation of PLCγ in response to EGF (Fig. 4E), although both chemotaxis and restitution were fully impaired by this mutant. As a number of cellular receptors, including the EGFR heterodimer partner ErbB2, can stimulate PLCγ (22), it may be that the residual activity in response to EGF in Y992F/Y1173F cells is a response to ErbB2 activation that, although insufficient for cell motility, is responsible for other cellular responses.
In this report we studied cross-communication between specific EGFR autophosphorylation sites with p38 MAPK, Grb2, Src kinase, and PLCγ signaling. A recent study of individual phosphosite activation in non-small cell lung cancer demonstrated a bias toward increased EGFR Y1068 and Y1148 phosphorylation in tumors (37); it is not yet clear whether there are ligand/receptor combinations that lead to differential phosphorylation of EGFR tyrosines in nonpathological conditions. Identification of PLC and Src as possible targets of phosphosite-specific signaling (Figs. 4 and 6) raises interesting possibilities for pathway-directed therapies. For example, the production of small peptides mimicking the binding site at EGFR phospho-Y1086 might be used to influence Grb2 binding and Src activation downstream of EGFR, as has been demonstrated for MUC1 interactions with β-catenin and EGFR (3). Such an approach could potentially selectively modulate chemotaxis or tumor cell invasion.
In summary, we have shown a requirement for p38 MAPK, Grb2, Src, and PLCγ1 activities for EGF-stimulated chemotactic migration and restitution in MCE cells. In contrast, inhibition of PI 3-kinase, PKC, and JNK/SAPK affected only restitution, whereas ERK MAPK blockade affected neither type of migration studied. These findings suggest that a limited set of EGFR downstream signal pathways are capable of regulating EGF-stimulated cell movement and are consistent with decreased chemotaxis and restitution in cells expressing EGFR with nonphosphorylatable mutations on Y992/1173, Y1086, Y1045, and Y1068, with restitution being more sensitive to substitution on the latter two sites. Further study of the interaction between individual EGFR autophosphorylation sites and cellular behavior may lead to the development of new experimental and therapeutic tools to manipulate the cell's response to EGFR activation.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01DK056008 and R01DK54993 (to D. B. Polk), and K01DK077956 (to M. R. Frey).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank the members of the Polk and Frey laboratories for thoughtful discussion and helpful advice.
REFERENCES
- 1. Basson MD, Modlin IM, Flynn SD, Jena BP, Madri JA. Independent modulation of enterocyte migration and proliferation by growth factors, matrix proteins, and pharmacologic agents in an in vitro model of mucosal healing. Surgery 112: 299–307; discussion 307–298, 1992 [PubMed] [Google Scholar]
- 2. Batzer AG, Rotin D, Urena JM, Skolnik EY, Schlessinger J. Hierarchy of binding sites for Grb2 and Shc on the epidermal growth factor receptor. Mol Cell Biol 14: 5192–5201, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bitler BG, Menzl I, Huerta CL, Sands B, Knowlton W, Chang A, Schroeder JA. Intracellular MUC1 peptides inhibit cancer progression. Clin Cancer Res 15: 100–109, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boni-Schnetzler M, Pilch PF. Mechanism of epidermal growth factor receptor autophosphorylation and high-affinity binding. Proc Natl Acad Sci USA 84: 7832–7836, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chattopadhyay A, Vecchi M, Ji Q, Mernaugh R, Carpenter G. The role of individual SH2 domains in mediating association of phospholipase C-gamma1 with the activated EGF receptor. J Biol Chem 274: 26091–26097, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Chen P, Xie H, Sekar MC, Gupta K, Wells A. Epidermal growth factor receptor-mediated cell motility: phospholipase C activity is required, but mitogen-activated protein kinase activity is not sufficient for induced cell movement. J Cell Biol 127: 847–857, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chwieralski CE, Schnurra I, Thim L, Hoffmann W. Epidermal growth factor and trefoil factor family 2 synergistically trigger chemotaxis on BEAS-2B cells via different signaling cascades. Am J Respir Cell Mol Biol 31: 528–537, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Deb TB, Su L, Wong L, Bonvini E, Wells A, David M, Johnson GR. Epidermal growth factor (EGF) receptor kinase-independent signaling by EGF. J Biol Chem 276: 15554–15560, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Downward J, Parker P, Waterfield MD. Autophosphorylation sites on the epidermal growth factor receptor. Nature 311: 483–485, 1984 [DOI] [PubMed] [Google Scholar]
- 10. El-Assal ON, Besner GE. HB-EGF enhances restitution after intestinal ischemia/reperfusion via PI3K/Akt and MEK/ERK1/2 activation. Gastroenterology 129: 609–625, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Frey MR, Dise RS, Edelblum KL, Polk DB. p38 kinase regulates epidermal growth factor receptor downregulation and cellular migration. EMBO J 25: 5683–5692, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frey MR, Golovin A, Polk DB. Epidermal growth factor-stimulated intestinal epithelial cell migration requires Src family kinase-dependent p38 MAPK signaling. J Biol Chem 279: 44513–44521, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Gordon-Thomson C, Jones J, Mason RS, Moore GP. ErbB receptors mediate both migratory and proliferative activities in human melanocytes and melanoma cells. Melanoma Res 15: 21–28, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Gotoh N, Tojo A, Muroya K, Hashimoto Y, Hattori S, Nakamura S, Takenawa T, Yazaki Y, Shibuya M. Epidermal growth factor-receptor mutant lacking the autophosphorylation sites induces phosphorylation of Shc protein and Shc-Grb2/ASH association and retains mitogenic activity. Proc Natl Acad Sci USA 91: 167–171, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hauck CR, Sieg DJ, Hsia DA, Loftus JC, Gaarde WA, Monia BP, Schlaepfer DD. Inhibition of focal adhesion kinase expression or activity disrupts epidermal growth factor-stimulated signaling promoting the migration of invasive human carcinoma cells. Cancer Res 61: 7079–7090, 2001 [PubMed] [Google Scholar]
- 16. Ho W, Uniyal S, Meakin SO, Morris VL, Chan BM. A differential role of extracellular signal-regulated kinase in stimulated PC12 pheochromocytoma cell movement. Exp Cell Res 263: 254–264, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Ho WC, Uniyal S, Zhou H, Morris VL, Chan BM. Threshold levels of ERK activation for chemotactic migration differ for NGF and EGF in rat pheochromocytoma PC12 cells. Mol Cell Biochem 271: 29–41, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Krymskaya VP, Goncharova EA, Ammit AJ, Lim PN, Goncharov DA, Eszterhas A, Panettieri RA., Jr Src is necessary and sufficient for human airway smooth muscle cell proliferation and migration. FASEB J 19: 428–430, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Leeb SN, Vogl D, Falk W, Scholmerich J, Rogler G, Gelbmann CM. Regulation of migration of human colonic myofibroblasts. Growth Factors 20: 81–91, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Margolis BL, Lax I, Kris R, Dombalagian M, Honegger AM, Howk R, Givol D, Ullrich A, Schlessinger J. All autophosphorylation sites of epidermal growth factor (EGF) receptor and HER2/neu are located in their carboxyl-terminal tails. Identification of a novel site in EGF receptor. J Biol Chem 264: 10667–10671, 1989 [PubMed] [Google Scholar]
- 21. Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J 19: 3159–3167, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peles E, Levy RB, Or E, Ullrich A, Yarden Y. Oncogenic forms of the neu/HER2 tyrosine kinase are permanently coupled to phospholipase C gamma. EMBO J 10: 2077–2086, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Polk DB. Epidermal growth factor receptor-stimulated intestinal epithelial cell migration requires phospholipase C activity. Gastroenterology 114: 493–502, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Polk DB, Tong W. Epidermal and hepatocyte growth factors stimulate chemotaxis in an intestinal epithelial cell line. Am J Physiol Cell Physiol 277: C1149–C1159, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Rodrigues GA, Falasca M, Zhang Z, Ong SH, Schlessinger J. A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling. Mol Cell Biol 20: 1448–1459, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rojas M, Yao S, Lin YZ. Controlling epidermal growth factor (EGF)-stimulated Ras activation in intact cells by a cell-permeable peptide mimicking phosphorylated EGF receptor. J Biol Chem 271: 27456–27461, 1996 [DOI] [PubMed] [Google Scholar]
- 27. Rotin D, Honegger AM, Margolis BL, Ullrich A, Schlessinger J. Presence of SH2 domains of phospholipase C gamma 1 enhances substrate phosphorylation by increasing the affinity toward the epidermal growth factor receptor. J Biol Chem 267: 9678–9683, 1992 [PubMed] [Google Scholar]
- 28. Sakaguchi K, Okabayashi Y, Kido Y, Kimura S, Matsumura Y, Inushima K, Kasuga M. Shc phosphotyrosine-binding domain dominantly interacts with epidermal growth factor receptors and mediates Ras activation in intact cells. Mol Endocrinol 12: 536–543, 1998 [DOI] [PubMed] [Google Scholar]
- 29. Sewell JM, Smyth JF, Langdon SP. Role of TGF alpha stimulation of the ERK, PI3 kinase and PLC gamma pathways in ovarian cancer growth and migration. Exp Cell Res 304: 305–316, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Shoelson SE. SH2 and PTB domain interactions in tyrosine kinase signal transduction. Curr Opin Chem Biol 1: 227–234, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Soler C, Beguinot L, Carpenter G. Individual epidermal growth factor receptor autophosphorylation sites do not stringently define association motifs for several SH2-containing proteins. J Biol Chem 269: 12320–12324, 1994 [PubMed] [Google Scholar]
- 32. Sorkin A, Helin K, Waters CM, Carpenter G, Beguinot L. Multiple autophosphorylation sites of the epidermal growth factor receptor are essential for receptor kinase activity and internalization. Contrasting significance of tyrosine 992 in the native and truncated receptors. J Biol Chem 267: 8672–8678, 1992 [PubMed] [Google Scholar]
- 33. Spivak-Kroizman T, Rotin D, Pinchasi D, Ullrich A, Schlessinger J, Lax I. Heterodimerization of c-erbB2 with different epidermal growth factor receptor mutants elicits stimulatory or inhibitory responses. J Biol Chem 267: 8056–8063, 1992 [PubMed] [Google Scholar]
- 34. Stossel TP. On the crawling of animal cells. Science 260: 1086–1094, 1993 [DOI] [PubMed] [Google Scholar]
- 35. Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris RC, Barnard JA, Yuspa SH, Coffey RJ, Magnuson T. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science 269: 230–234, 1995 [DOI] [PubMed] [Google Scholar]
- 36. Tvorogov D, Carpenter G. EGF-dependent association of phospholipase C-gamma1 with c-Cbl. Exp Cell Res 277: 86–94, 2002 [DOI] [PubMed] [Google Scholar]
- 37. VanMeter AJ, Rodriguez AS, Bowman ED, Jen J, Harris CC, Deng J, Calvert VS, Silvestri A, Fredolini C, Chandhoke V, Petricoin EF, 3rd, Liotta LA, Espina V. Laser capture microdissection and protein microarray analysis of human non-small cell lung cancer: differential epidermal growth factor receptor (EGPR) phosphorylation events associated with mutated EGFR compared with wild type. Mol Cell Proteomics 7: 1902–1924, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wahl MI, Nishibe S, Kim JW, Kim H, Rhee SG, Carpenter G. Identification of two epidermal growth factor-sensitive tyrosine phosphorylation sites of phospholipase C-gamma in intact HSC-1 cells. J Biol Chem 265: 3944–3948, 1990 [PubMed] [Google Scholar]
- 39. Ware MF, Wells A, Lauffenburger DA. Epidermal growth factor alters fibroblast migration speed and directional persistence reciprocally and in a matrix-dependent manner. J Cell Sci 111: 2423–2432, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Waterman H, Katz M, Rubin C, Shtiegman K, Lavi S, Elson A, Jovin T, Yarden Y. A mutant EGF-receptor defective in ubiquitylation and endocytosis unveils a role for Grb2 in negative signaling. EMBO J 21: 303–313, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Watson A, Morris VL, Chan BM. Coordinated integrin and growth factor regulation of primary keratinocyte migration mediated through extracellular signal regulated kinase and phosphoinositide 3-kinase. Arch Dermatol Res 301: 307–317, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Wells A, Ware MF, Allen FD, Lauffenburger DA. Shaping up for shipping out: PLCgamma signaling of morphology changes in EGF-stimulated fibroblast migration. Cell Motil Cytoskeleton 44: 227–233, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Whitehead RH, VanEeden PE, Noble MD, Ataliotis P, Jat PS. Establishment of conditionally immortalized epithelial cell lines from both colon and small intestine of adult H-2Kb-tsA58 transgenic mice. Proc Natl Acad Sci USA 90: 587–591, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xie H, Pallero MA, Gupta K, Chang P, Ware MF, Witke W, Kwiatkowski DJ, Lauffenburger DA, Murphy-Ullrich JE, Wells A. EGF receptor regulation of cell motility: EGF induces disassembly of focal adhesions independently of the motility-associated PLCgamma signaling pathway. J Cell Sci 111: 615–624, 1998 [DOI] [PubMed] [Google Scholar]
- 45. Xu KP, Riggs A, Ding Y, Yu FS. Role of ErbB2 in corneal epithelial wound healing. Invest Ophthalmol Vis Sci 45: 4277–4283, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yamaoka T, Yan F, Cao H, Hobbs SS, Dise RS, Tong W, Polk DB. Transactivation of EGF receptor and ErbB2 protects intestinal epithelial cells from TNF-induced apoptosis. Proc Natl Acad Sci USA 105: 11772–11777, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]