Abstract
Studies were performed to determine the unknown status of PKC and RhoA/ROCK in the phorbol 12,13-dibutyrate (PDBu)-stimulated state in the human internal anal sphincter (IAS) smooth muscle cells (SMCs). We determined the effects of PDBu (10−7 M), the PKC activator, on PKCα and RhoA and ROCK II translocation in the human IAS SMCs. We used immunocytochemistry and fluorescence microcopy in the basal state, following PDBu, and before and after PKC inhibitor calphostin C (10−6 M), cell-permeable RhoA inhibitor C3 exoenzyme (2.5 μg/ml), and ROCK inhibitor Y 27632 (10−6 M). We also determined changes in the SMC lengths via computerized digital micrometry. In the basal state PKCα was distributed almost uniformly throughout the cell, whereas RhoA and ROCK II were located in the higher intensities toward the periphery. PDBu caused significant translocation of PKCα, RhoA, and ROCK II. PDBu-induced translocation of PKCα was attenuated by calphostin C and not by C3 exoenzyme and Y 27632. However, PDBu-induced translocation of RhoA was blocked by C3 exoenzyme, and that of ROCK II was attenuated by both C3 exoenzyme and Y 27632. Contraction of the human IAS SMCs caused by PDBu in parallel with RhoA/ROCK II translocation was attenuated by C3 exoenzyme and Y 27632 but not by calphostin C. In human IAS SMCs RhoA/ROCK compared with PKC are constitutively active, and contractility by PDBu is associated with RhoA/ROCK activation rather than PKC. The relative contribution of RhoA/ROCK vs. PKC in the pathophysiology and potential therapy for the IAS dysfunction remains to be determined.
Keywords: membrane translocation; protein kinase C; RhoA/Rho kinase; smooth muscle tone; phorbol 12,13-dibutyrate
phorbol esters, especially phorbol 12,13-dibutyrate (PDBu), have been routinely used as PKC activators in different systems including the gastrointestinal smooth muscles (17, 21, 26). PDBu binds to the phorbol ester binding domain of PKC, causing direct activation of PKC, and has been used to determine the effect of PKC activation on the contraction of smooth muscle cells (SMCs) via Ca2+ sensitization pathways (PKC and RhoA/ROCK). However, the effect of PDBu in the human internal anal sphincter (IAS) SMCs has not been examined. It is well known that an increase in the smooth muscle contraction initiated by Ca2+-calmodulin/myosin light chain kinase is maintained by the inhibition of myosin light chain phosphatase (MLCP) via phosphorylation of myosin-binding subunit of MLCP (p-MYPT1) and via phosphorylation of endogenous inhibitory protein of MLCP (p-CPI-17) (16). The latter is mostly regulated by the PKC activation and is also known as protein kinase C-potentiated inhibitor. In addition, it has been shown that PKCα is an important PKC isozyme in the agonist (especially PDBu)-induced sustained contraction of the smooth muscles (8, 16, 30). It is also well known that calphostin C is a wide spectrum and potent inhibitor PKC isozymes (in the category of conventional and novel PKCs) of which PKCα is part of (13, 27). Consequently, some studies have referred to PDBu and calphostin C as the PKCα activator and inhibitor, respectively (3).
The effects of PDBu in the intact spontaneously tonic smooth muscles are controversial and poorly understood. In the opossum IAS (4), it has been shown that PDBu produces relaxation of the smooth muscle. Similar observations were subsequently made in the rat stomach fundus (14). Variable effects of PDBu, either contraction, relaxation, or bimodal effects (15), have also been reported depending on the basal tonic state of the smooth muscles. The proposed inhibitory mechanisms in the smooth muscle contractility are attenuation of Ca2+ influx (4, 14) and inhibition of Na+-K+-ATPase (28).
Besides the PKC, a number of studies using intact smooth muscles have demonstrated that RhoA/ROCK pathway also plays an important role in the sustained contraction and the basal tone via Ca2+ sensitization (10, 16, 23, 25, 31). Recent studies using intact spontaneously tonic smooth muscles of rat IAS (25) and human (29) and cat lower esophageal sphincter (19) via the use of selective inhibitors (while tracking down the enzymatic activities and the corresponding signal transduction pathway) have shown that RhoA/ROCK rather than PKC pathway is the major determinant of the basal tone. It has been shown repeatedly that substances like C3 exoenzyme (9) and Y 27632 (11) serve as selective RhoA and ROCK inhibitors, respectively. According to these studies, RhoA/ROCK and the related signal transduction machinery are upregulated. A number of studies have determined the higher expression levels of RhoA/ROCK and PKC at the transcriptional and translational levels. Besides this, the status of expression of these signals transduction proteins and their corresponding activities can also be tracked by determining their immunofluorescence intensities. Higher immunofluorescence intensity in the membrane or periphery of the SMCs vs. the interior of the cell or the cytosol is considered to be an important gage for the PKC and RhoA/ROCK activities (5). The effects of most commonly used PKC activator PDBu in these spontaneously tonic preparations at either the tissue or the cellular level have not been examined.
The purpose of the present investigation was to determine immunocytochemically the effects of PDBu on PKCα and RhoA/ROCK II in the spontaneously tonic SMC of the human IAS. Studies with PDBu-induced contraction of the IAS SMCs while tracking down PKC and RhoA/ROCK II would be important to resolve the controversial effects of PDBu in the gastrointestinal smooth muscles.
MATERIAL AND METHODS
Human IAS smooth muscle tissue samples were obtained from five subjects undergoing total removal of anorectum because of conditions other than the rectoanal motility disorders, via the departments of Surgery and Pathology of Thomas Jefferson University. The studies were approved by the institutional review board of Thomas Jefferson University. Tissue samples were kept in oxygenated, 0.22 μm-filtered Krebs physiological solution (KPS) at 4°C before isolation of the SMCs. The composition of KPS was as follows (in mM): 118.07 NaCl, 4.69 KCl, 2.52 CaCl2, 1.16 MgSO4, 1.01 NaH2PO4, 25 NaHCO3, and 11.10 glucose.
Isolation of SMCs.
The serosal adventitia with blood vessels, mucosal, submucosal, and longitudinal smooth muscle layers were carefully removed from the IAS tissues, by sharp dissection, preserving the circular smooth muscle layer. Throughout the dissection, tissues were kept submerged in oxygenated KPS. SMCs from the IAS were isolated as described previously (24). Briefly, the circular IAS smooth muscle was cut into small pieces (∼1-mm cubes) and incubated in oxygenated KPS containing 0.1% collagenase and 0.01% soybean trypsin inhibitor at 37°C for 1 h. Solution was replaced with fresh KPS containing 0.1% collagenase and 0.01% soybean trypsin inhibitor after brief centrifugation. The tissues were incubated for a few minutes to 1 h depending on the dispersal of SMCs. The dispersal of the SMCs was continuously monitored with 10 μl of sample solution from the tube, under the microscope. The cell suspension was then filtered through a 500-μm Nitex mesh. The tissue trapped on the mesh was rinsed with 25 ml (5 × 5 ml) of collagenase-free KPS. At this stage, the tissue was incubated in collagenase-free KPS at 37°C (for a few minutes to 1 h), and dispersion of the cells was monitored periodically by microscopic examination of a 10-μl aliquot of the mixture.
The SMCs were then harvested by filtration through the Nitex mesh. The filtrate containing the cells was centrifuged at 350 g for 10 min at room temperature. The cells in the pellet were resuspended in DMEM growth medium with 5% fetal bovine serum, 5% penicillin-streptomycin, 50 μg/ml gentamicin, and 2 μg/ml amphotericin B on Lab-Tek II chamber slides (Nulge Nunc International, Naperville, IL) at 37°C and 5% CO2 in an incubator with regulated humidity. The cell-attached medium was then replaced with serum-free medium for 24 h before treatment of the SMCs with PDBu (10−7 M for 20 min) before and after calphostin C 10−6 M, C3 exoenzyme 2.5 μg/ml, or Y 27632 10−6 M for 1 h. For controls, the cells were left untreated with any agent but otherwise under the similar conditions as the treated cells. (Cell passage was carefully recorded and the cells were not used beyond second passage to eliminate the concern of the SMC dedifferentiation in culture medium after a number of passages.)
Immunofluorescence staining and confocal microscopy.
Following the above protocol for treatment with different agents, the SMCs were quickly rinsed with Dulbecco's phosphate-buffered saline (DPBS) and fixed with chilled acetone for 10 min at −20°C. SMCs were then washed three times with DPBS and incubated overnight at room temperature in a humid environment with 1:100 dilution of PKCα (mouse), RhoA (mouse), ROCK II (rabbit), pThr696-MYPT1 (rabbit), pThr18/Ser19-MLC20 (rabbit), and monoclonal α-actin (raised in mouse) primary antibodies (Sigma Chemical, St. Louis, MO) in DPBS containing 0.2% Triton X-100 and 0.5% bovine serum albumin. SMCs were washed three times with DPBS and incubated with Texas red-conjugated secondary antibodies raised in donkey (1:200) depending on the source of primary antibody in DPBS with 0.3% Triton X-100 and 2% donkey serum for 1 h. SMCs were then washed three times with DPBS and stained with 4′,6-diamidino-2-phenylindole (DAPI) nucleic acid staining dye in DPBS for 3 min in chambered slides. The slides were then air dried and coverslipped with Prolong Gold mounting medium (Invitrogen, Carlsbad, CA). Slides were kept overnight at 4°C for appropriate polymerization of the mounting medium and then sealed with clear nail polish. The confocal microscopic images were acquired via a Carl Zeiss LSM 510 UV META inverted confocal microscope (Carl Zeiss Microimaging, Thornwood, NY) with a Plan-Apo ×40 oil immersion lens at room temperature and Zeiss AIM 4.2 SP1 software. Images were analyzed by using MetaMorph v7.6.5 (Bioimaging Facility of the Kimmel Cancer Center, Thomas Jefferson University).
Images were also taken by Nikon Eclipse 80i microscope, and fluorescence intensity was analyzed by Nikon imaging software (NIS-elements 3.1) (Nikon Instruments, Melville, NY) (Center for Translational Medicine, Department of Medicine, Thomas Jefferson University).
Fluorescence intensity calculation.
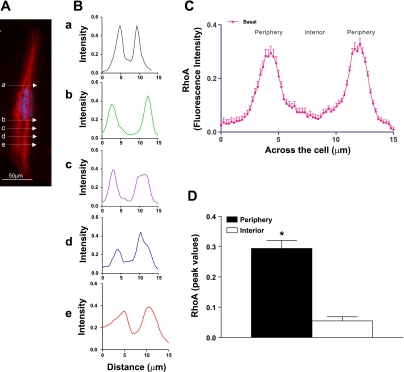
The integrity of the SMCs used for the fluorescence intensity studies was first confirmed by extensive staining of the microfilaments with α-actin. The fluorescence intensities were calculated by the method previously described by Chiba et al. (5) and later adapted in our laboratory (7). Briefly, using Image-Pro Plus version 4.0 software (MediaCybernetics, Silver Spring, MD), we calculated the pixel intensities (grayscale, 0–250) along the width of the cells by line graph method. For this, a line of 1-μm width was drawn across the width of the cell at five different sites at random, along the length of the cells (Fig. 1A). These values were averaged and used as n = 1. The fluorescence intensity under that line was plotted by normalizing the maximum intensity, i.e., 250, as 1.0 and black background as 0 (Fig. 1B). Nuclear area was not included in calculating the peripheral (membrane) vs. the interior (cytosolic) fluorescence intensity of different proteins. The outer 15% area of the cell was considered as membrane and inner 70% as cytosol (Fig. 1C). The above procedure was repeated in 10 cells at random and calculated as means ± SE of n = 10. The peak intensities along the periphery and interior of the cells were also graphed for direct comparison (Fig. 1D).
Fig. 1.
A: example of a single smooth muscle cell (SMC) showing RhoA immunofluorescence in the basal state. B: line graph showing scans of the fluorescence intensity at 5 different sites across the cell designated as a, b, c, d, and e. The scanning analysis reveals the peak intensities (higher in the periphery and lower levels toward the interior of the cell). C: peak RhoA immunofluorescence intensity represented by bar graph shows significantly higher values in the periphery (membrane) vs. the interior (cytosol) (*P < 0.05; n = 10) of the SMCs. The n value here and throughout the article denotes multiple scanning of each cell (5 times) in 10 SMCs under each experimental protocol. D: bar graph showing that in the basal state the peak intensity values of RhoA are significantly (*P < 0.05) higher in the periphery compared with those in the interior of the internal anal sphincter (IAS) SMCs.
Western blot analysis.
Levels of pThr696-MYPT1 and pThr18/Ser19-MLC20 were determined via Western blot analysis of human IAS SMCs, under control and following pretreatment with PDBu (10−7 M), before and after calphostin C (10−6 M), C3 exoenzyme (2.5 μg/ml), and Y 27632 (10−6 M) conditions. The cells were rinsed with PBS and lysed with 500 μl lysis buffer (1% SDS, 1.0 mM sodium orthovanadate, and 10 mM Tris, pH 7.4) containing protease and phosphatase inhibitor cocktail (Pierce Protein Research Products, a part of Thermo Fisher Scientific, Rockford, IL) was added to inactivate proteases and phosphatases. Cell lysate was centrifuged at 14,000 rpm for 5 min and supernatant was transferred into different tubes and protein quantification was carried out by using BCA kit from Pierce. Twenty micrograms of protein in 20 μl of lysates were mixed with 2× Laemmli sample buffer (with final concentrations of 62.5 mM Tris, 1% SDS, 15% glycerol, 0.005% bromophenol blue, and 2% β-mercaptoethanol) and placed in a boiling water bath for 5 min. Samples were loaded on 15% polyacrylamide gel. The separated proteins were electrophoretically transferred onto a 0.2 μm Immun-Blot polyvinylidene fluoride membrane (Bio-Rad, Hercules, CA). The membranes were kept in Odyssey blocking buffer (LI-COR Biotechnology, Lincoln, NE) for 1 h and stained overnight with the primary antibody (raised in goat) of pThr696-MYPT1 pThr18/Ser19-MLC20 in Odyssey blocking buffer containing 0.2% Tween. The membranes were washed thrice for 10 min each with PBS with 0.2% Tween and incubated with anti-goat infrared dye (IRdye800)-conjugated secondary antibody for 1 h and the membranes were scanned with Odyssey infrared scanner. Western blot band intensities were calculated with Image J 1.41o (National Institutes of Health; means ± SE), and graphs were plotted as the intensity ratios of pThr696-MYPT1 and pThr18/Ser19-MLC20, vs. nonphosphorylated forms of MYPT1 and MLC20.
Drugs and chemicals.
PDBu, calphostin C, and monoclonal α-actin primary antibody were obtained from Sigma; MLC20 primary antibody was from Abcam (Cambridge, MA); RhoA, PKCα, ROCK II, pThr696-MYPT1, pThr18/Ser19-MLC20, MYPT1, horseradish peroxidase-conjugated secondary antibodies, and Texas red- and FITC-conjugated secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Cell-permeable C3 exoenzyme was purchased from Cytoskeleton (Denver, CO), and Y 27632 was purchased from Biomol (Plymouth Meeting, PA).
Data analysis.
Results were expressed as means ± SE and graphed by using GraphPad Prism 5.0 (Graph Pad Software, San Diego, CA). Cell lengths were calculated by automated computerized micrometry analysis of the stored images taken under the microscope, immediately at the end of each experimental protocols. The controls to determine the effects of different treatments were either the cells without any treatment or PDBu alone. Fluorescence intensities were calculated by Image-Pro Plus 4.0 software (MediaCybernetics), using line and bar graph analyses. Statistical significance was tested by the one-way analysis of variance (ANOVA) followed by Dunnett's post hoc test when three or more different groups were compared. The unpaired Student's t-test was used to compare only two different groups. A P value less than 0.05 was considered statistically significant.
RESULTS
Reproducibility of fluorescence intensity in the human IAS SMC.
The fluorescence intensity was used to monitor the location and translocation of PKCα, RhoA, and ROCK II in the basal state vs. following stimulation with PDBu before and after different treatments. To determine the reproducibility of these determinations, we used RhoA fluorescence intensity as prototype in the human IAS SMC. RhoA fluorescence intensity was found to be concentrated predominantly toward the periphery of IAS SMC (Fig. 1A). The DAPI staining (blue) in center of the cell denotes the nucleus of the cell. The predominance of RhoA toward the periphery suggests that RhoA is constitutively active in the basal state of the human IAS SMC. Figure 1B demonstrates the reproducibility of line graph analyses across five sites of the cell selected at random (marked by a, b, c, d, and e arrows in Fig. 1A), calculated automatically by computer. The corresponding raw line graph data were accumulatively calculated as means ± SE as spatial distributions of RhoA across the cells (calculated as mean of five means from 10 different cells; n = 10; Fig. 1C). Data reproducibly show distinctly higher levels in the periphery of the cell compared with the interior. These data were further represented as the peak fluorescence intensities in the periphery vs. the interior of the cells (*P < 0.05; means ± SE; n = 10), in the form of bar graph analysis (Fig. 1D).
Effect of PDBu on PKCα translocation in human IAS SMC: influence of calphostin C, C3 exoenzyme, and Y 27632.
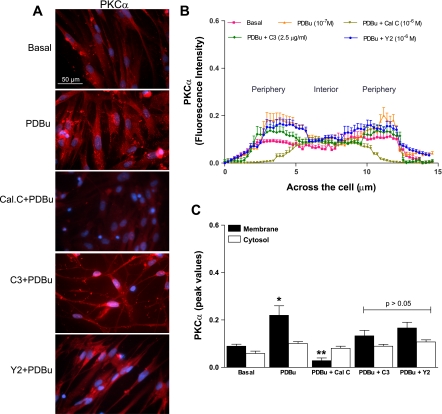
Figure 2A shows typical examples of PKCα fluorescence intensity of the human IAS SMC in the basal state, and following pretreatment with PDBu before and after calphostin C, C3 exoenzyme, and Y 27632. Fluorescence intensity analyses reveal 1) the detailed spatial distribution of PKCα across the cells, the periphery (membrane) vs. the interior (cytosol) (Fig. 2B), as explained above, and 2) the peak values (means ± SE of n = 10) representing the highest and lowest intensities in the membrane vs. the cytosol, shown by the bar graphs in Fig. 2C, under different experimental protocols. Data show that, in contrast with RhoA, demarcation for the distribution of PKCα fluorescence intensity in the membrane vs. the cytosol was less marked. This suggests almost uniform distribution of PKCα across the cell. PDBu (10−7 M) pretreatment caused significant translocation of PKCα toward the membrane (*P < 0.05; n = 10) that was blocked significantly (**P < 0.05; n = 10 different SMC; Fig. 2C) by calphostin C but not by C3 exoenzyme and Y 27632 (P > 0.05).
Fig. 2.
A: examples of typical microscopic images of cells showing PKCα immunofluorescence in the basal state, and following phorbol 12,13-dibutyrate (PDBu; 10−7 M) before and after PKC and RhoA/ROCK inhibitors calphostin C (Cal.C; 10−6 M), C3 exoenzyme (C3; 2.5 μg/ml), and Y 27632 (Y2; 10−6 M), respectively. B: line graph showing the fluorescence intensity of cells calculated as means of means of multiple scans in each cell repeated in 10 cells. Data show lower intensities levels of PKCα and absence of its discrete distribution in the membrane vs. the cytosol, in the basal state, compared with the levels of RhoA. C: bar graph data showing the peak fluorescence intensities both in the membrane vs. the cytosol of cells in the basal state and following PDBu before and after different inhibitors. Data show that PDBu causes significant translocation of PKCα toward the membrane (*P < 0.05), which is reversed by calphostin C (**P < 0.05), but not by C3 exoenzyme and Y 27632 (P > 0.05).
Effect of PDBu on RhoA translocation in the human IAS SMC: influence of calphostin C, C3 exoenzyme, and Y 27632.
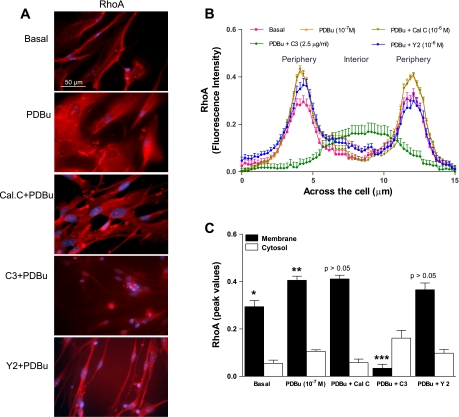
As shown in Fig. 1, RhoA's fluorescence intensity was significantly higher toward the membrane than the cytosol in the basal state (*P < 0.05; n = 10; Fig. 3, A and B). PDBu caused significant increase in the translocation of RhoA toward the membrane (0.41 ± 0.04 vs. the control of 0.29 ± 0.03; **P < 0.05; n = 10 different SMCs; Fig. 3, B and C). This increase in the RhoA translocation caused by PDBu was significantly reversed by C3 exoenzyme (2.5 μg/ml) easily distinguishable as peak intensities (***P > 0.05; n = 10; Fig. 3C), but not by calphostin C and Y 27632 (10−6 M) (P > 0.05). These data suggest that PDBu causes membrane translocation of RhoA and therefore its activation.
Fig. 3.
A: examples of typical microscopic images of cells showing the RhoA immunofluorescence in the basal state and following PDBu (10−7 M) before and after PKC and RhoA/ROCK inhibitors calphostin C (10−6 M), C3 exoenzyme (2.5 μg/ml), and Y 27632 (10−6 M), respectively. B: line graph analysis of scans across the SMCs shows a distinct pattern of cellular distribution of RhoA, significantly higher intensities in the periphery vs. the interior (*P < 0.05; n = 10). The line graph (B) plus the bar graph (C) analyses show that the higher distribution of RhoA intensity toward the membrane is further intensified by PDBu (**P < 0.05) that is significantly reversed by RhoA inhibitor C3 exoenzyme (***P < 0.05), but not by calphostin C and Y 27632 (P > 0.05).
Effect of PDBu on ROCK II translocation in the human IAS SMC: influence of calphostin C, C3 exoenzyme, and Y 27632.
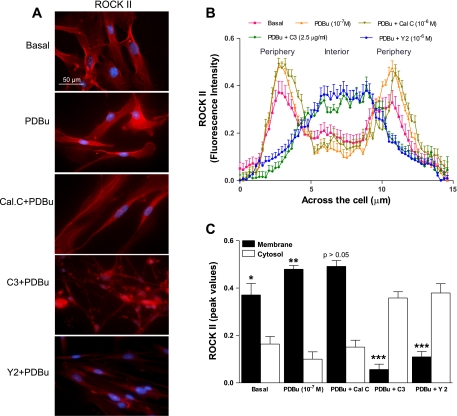
In resemblance with RhoA distribution, ROCK II was also significantly higher on the membrane than the cytosol in the basal state (*P < 0.05; n = 10; Fig. 4, A and B). Data from peak intensities of ROCK II in the basal state and following PDBu pretreatment before and after different inhibitors has been summarized in Fig. 4C. Data show that PDBu caused further and significant increase in the fluorescence intensity of the basal fluorescence intensity of ROCK II in the membrane (from 0.37 ± 0.04 in control to 0.48 ± 0.01; **P < 0.05; n = 10; Fig. 4C). This increased translocation of ROCK II to the membrane was reversed significantly by both C3 exoenzyme and Y 27632 (***P < 0.05; n = 10) but not by calphostin C (P > 0.05).
Fig. 4.
A: typical examples of microscopic images of cells showing ROCK II immunofluorescence in the basal state and following PDBu (10−7 M) before and after PKC and RhoA/ROCK inhibitors calphostin C (10−6 M), C3 exoenzyme (2.5 μg/ml), and Y 27632 (10−6 M), respectively. B: line graph analysis demonstrating scanning across the SMCs shows a distinct pattern of cellular distribution of ROCK II almost similar to that of RhoA, significantly higher intensities in the periphery vs. the interior (*P < 0.05; n = 10). The line graph (B) plus the bar graph (C) analyses show that the higher distribution of the intensity toward the membrane is further intensified by PDBu (**P < 0.05) that is significantly reversed by C3 exoenzyme and Y 27632 (***P < 0.05), but not by calphostin C (P > 0.05).
The above data suggest activation of RhoA/ROCK following PKC activation with PDBu pretreatment.
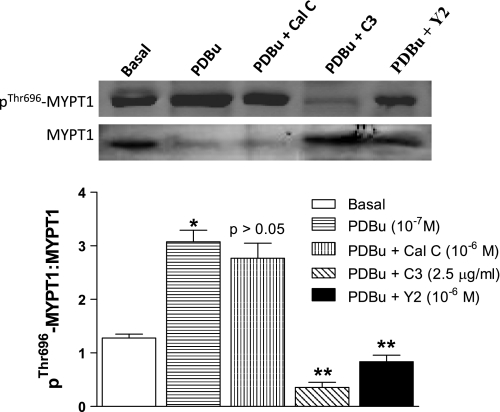
Effect of PDBu on pThr696-MYPT1 levels in human IAS SMC.
Western blot analyses of pThr696-MYPT1 (a down stream effector of ROCK II) show significant increase in the basal levels of pThr696-MYPT1 by 10−7 M PDBu (*P < 0.05) than the basal state. C3 exoenzyme and Y 27632 caused significant inhibition of these increases in pThr696-MYPT1 in human IAS SMCs (**P < 0.05; n = 5; Fig. 5). Conversely, calphostin C (10−6 M) had no significant effect on PDBu-induced increases in these phosphorylation levels (P > 0.05).
Fig. 5.
Top: Western blot analysis of pThr696-MYPT1 in the basal state and following PDBu (10−7 M) before and after PKC and RhoA/ROCK inhibitors calphostin C (10−6 M), C3 exoenzyme 2.5 μg/ml, and Y 27632 (10−6 M), respectively. Bottom: bar graphs data show that PDBu caused a significant increase in the levels of pThr696-MYPT1 (*P < 0.05) in the basal state of the human IAS SMCs, that were significantly (**P < 0.05) attenuated by C3 exoenzyme and Y 27632, but not by calphostin C (P > 0.05).
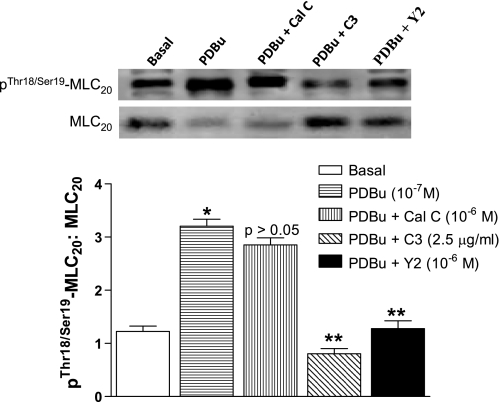
Effect of PDBu on pThr18/Ser19-MLC20 levels in human IAS SMC.
pThr18/Ser19-MLC20 plays a crucial role in the smooth muscle contraction via actin-myosin complex formation. In the basal state of human IAS SMCs, the levels of this phosphoprotein were significantly higher. PDBu 10−7 M caused further significant increase in the pThr18/Ser19-MLC20 (*P < 0.05; Fig. 6). This increase of pThr18/Ser19-MLC20 caused by PDBu was significantly decreased by C3 exoenzyme and Y 27632 (**P < 0.05; n = 5) but not by calphostin C (P > 0.05; Fig. 6).
Fig. 6.
Top: Western blot analysis of pThr18/Ser19-MLC20 in the basal state and following PDBu (10−7 M) before and after calphostin C, C3 exoenzyme, and Y 27632, respectively. Bottom: bar graphs show that in resemblance with the effect on pThr696-MYPT1, PDBu had similar effect in the human IAS SMCs on the expression levels of pThr18/Ser19-MLC20. PDBu caused a significant increase in pThr696-MYPT1 in the basal state (*P < 0.05) that was significantly inhibited by C3 exoenzyme and Y 27632 (**P < 0.05), but not by calphostin C (P > 0.05).
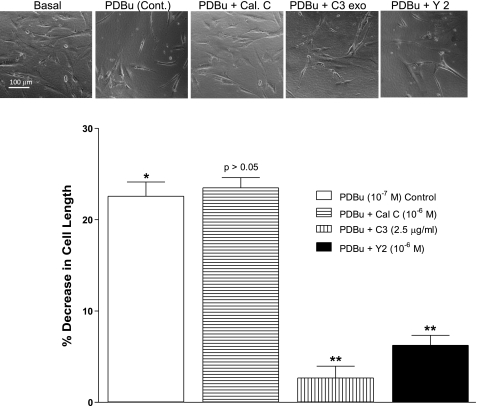
Effect of PDBu before and after the PKC, RhoA, and ROCK inhibitors on the human IAS SMC lengths.
The phase-contrast images in the basal state and following PDBu before and after different inhibitors (Fig. 7, top) were used to calculate the SMCs length under these experimental conditions. Data reveal that PDBu caused significant shortening of the SMCs (*P < 0.05; n = 50) that was significantly blocked by C3 exoenzyme and Y 27632 (**P < 0.05; Fig. 7, bottom) but not by calphostin C (P > 0.05). These data suggest that PDBu causes contraction of the human IAS SMC via RhoA/ROCK activation.
Fig. 7.
Top: phase-contrast images of human IAS SMC in the basal state following PDBu before and after calphostin C, C3 exoenzyme, and Y 27632. Bottom: bar graph data show that decrease in the cell lengths caused by PDBu (10−7 M) is significantly attenuated by C3 exoenzyme (2.5 μg/ml) and Y 27632 (10−6 M) (*P < 0.05; n = 50), but not by calphostin C (10−6 M) (P > 0.05).
DISCUSSION
Present studies for the first time show that the commonly used PKC activator phorbol ester PDBu causes contraction of the human IAS SMC associated with RhoA/ROCK activation rather than the PKC activation. These studies provide immunocytochemical evidence that the PKC activator causes the translocation of RhoA and ROCK II that is selectively blocked by their respective inhibitors in relation to the contraction of the SMCs.
In a separate set of studies we have observed that 10−7 M of PDBu (used in the present studies) is maximally effective in contracting the IAS SMC (data not shown). In addition, the present data show that this effect of PDBu is attenuated significantly by both RhoA (C3 exoenzyme) and ROCK (Y 27632) inhibitors, but not by the PKC inhibitor calphostin C. These results suggest that the contractile effect of PDBu may be independent of PKC activation and perhaps via activation of other kinases since PDBu can bind to many other proteins. In the final analysis, however, the contractile effect of PDBu in tonic smooth muscle of the IAS appears to be dependent on RhoA/ROCK activation. The precise role of PKC vs. RhoA/ROCK pathways following PDBu in the SMCs of the phasic tissues of the rectum and colon, however, remains to be determined.
To track down the location and translocation of PKC within the SMCs, we focused on PKCα immunofluorescence, based on a number of studies in the gastrointestinal tissues and the SMC (8, 16, 30). Accordingly, PKCα rather than other isozymes of PKC (especially in response to PDBu) plays a major role in the sustained contraction of the SMC, via Ca2+ sensitization. Recent studies in the human IAS SMCs also suggest a predominant role of PKCα in the PKC-mediated SMC contraction (31). The role of other PKC isozymes in the PDBu-induced contraction of the human IAS SMC, however, if involved, could be determined by the effect of wide-spectrum PKC inhibitor calphostin C. The latter studies show that calphostin C has no effect on PDBu-induced SMC contraction, thus negating the role of other PKC isozymes as well.
It is noteworthy that the human IAS SMCs are in a state of tonic contraction in the basal state. The size of the IAS SMC is significantly shorter than those from the immediately flanking region of the rectum. In addition, it has been shown that the original smooth muscle where these cells were obtained from is spontaneously in a state of myogenic tone (2, 18). Considering the involvement of PKC and RhoA/ROCK pathways in the IAS tone (1, 8, 16, 19, 25, 29, 30), it is conceivable that the human IAS SMC similar to those from the other species were characterized with the elevated levels of PKCα and RhoA/ROCK II in the periphery of the cells. It is well known that these molecular proteins translocate to the periphery or the cell membrane following their activation with different contractile agonists (21). The presence of these molecular proteins in the higher levels in the basal state toward the periphery of the IAS SMC allows one to monitor their movement during the contractile as well as the relaxant responses. The agonists that produce contraction are expected to cause further translocation of these proteins to the periphery. Conversely, the agonists or stimuli that produce relaxation may cause their movements to the interior of the cells.
Following the above logic, the present studies reveal that PDBu causes translocation of PKCα to the periphery, which is reversed by the PKC inhibitor calphostin C. We selected this PKC inhibitor (10−6 M) because it has been shown to be a wide-spectrum and yet selective inhibitor of different PKCs (including PKCα) (4, 13, 31). In addition, calphostin C, in contrast with C3 exoenzyme and Y 27632, has no significant effect on the basal cell lengths and the morphology of the IAS SMC. An increase in the recruitment of PKCα toward the periphery of the cells following PDBu and its reversal by calphostin C further validates the use of these tools and the concentrations used in the human IAS SMC. Unexpectedly, however, PDBu-induced contraction of the SMC does not appear to be mediated via PKC activation since it is not modified by the PKC inhibitor calphostin C. In a separate set of studies we have shown that calphostin C in the concentrations used here almost obliterates the basal PKC activity in the human IAS SMCs (data not shown).
Immunocytochemical studies provide evidence for the activation of RhoA/ROCK by their translocation to the periphery of the SMCs by PDBu. The increased translocation of RhoA caused by PDBu was selectively blocked by RhoA inhibitor C3 exoenzyme. In addition, PDBu-induced ROCK II translocation was selectively reversed by C3 exoenzyme and Y 27632. These observations are consistent with a number of recent studies showing the involvement of RhoA/ROCK activation in PDBu-induced actions in different systems (12, 32). In addition, earlier studies in the rat IAS have shown that contraction of the SMC via direct activation by PKC is dependent on RhoA/ROCK activation (20). This was shown via the determination the effect of PKC and RhoA/ROCK inhibitors on the PKC-induced contraction of the SMC. In the human IAS SMCs, however, it appears that RhoA/ROCK activation via PDBu appears to be independent of PKC activation. In the present studies immunocytochemical evidence combined with the functional data suggest that PDBu-induced contraction of the human IAS SMC utilize RhoA/ROCK pathways rather than the PKC.
The exact mechanism of RhoA/ROCK activation following PDBu that is independent on PKC activation is not presently known. It is well known that PDBu can also bind to the proteins other than PKC which has C1 domain (DAG binding domain). These include GTPase-activating proteins for Rac, Ras guanyl-releasing proteins (RasGRPs), and myotonic dystrophy kinase-related Cdc42 binding kinase (MRCK), a member of Rho family G proteins (6, 22). The fate of PDBu-activated PKC that does not participate in RhoA/ROCK stimulation is not presently known. In addition, whether overactivation of PKC by PDBu leads to its own downregulation or disintegration or whether it activates other unknown intermediary kinase/s for RhoA/ROCK activation remains to be determined.
We conclude that PDBu-induced contraction of the human IAS SMC appears to be associated with RhoA/ROCK activation. The relative contribution of PKC vs. RhoA/ROCK pathways in the basal IAS tone in the human IAS remains to be determined.
GRANTS
The work was supported by National Institutes of Diabetes and Digestive and Kidney Diseases Grant DK-35385 and an institutional grant from Thomas Jefferson University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Bitar KN, Ibitayo A, Patil SB. HSP27 modulates agonist-induced association of translocated RhoA and PKC-α in muscle cells of the colon. J Appl Physiol 92: 41–49, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Burleigh DE, D'Mello A. Neural and pharmacological factors affecting motility of the internal anal sphincter. Gastroenterology 84: 409–417, 1983 [PubMed] [Google Scholar]
- 3. Byers HR, Boissel SJ, Tu C, Park HY. RNAi-mediated knockdown of protein kinase C-alpha inhibits cell migration in MM-RU human metastatic melanoma cell line. Melanoma Res 20: 171–178, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Chakder S, Sarma D, Rattan S. Mechanism of internal anal sphincter smooth muscle relaxation by phorbol 12,13-dibutyrate. Am J Physiol Gastrointest Liver Physiol 280: G1341–G1350, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Chiba Y, Uchida T, Sakai S, Oku T, Itoh S, Tsuji T, Misawa M. Acetylcholine-induced translocation of RhoA in freshly isolated single smooth muscle cells of rat bronchi. J Pharm Sci 95: 479–482, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Choi SH, Czifra G, Kedei N, Lewin NE, Lazar Z, Pu Y, Marquez VE, Blumberg PM. Characterization of the interaction of phorbol esters with the C1 domain MRCK (myotonic dystrophy kinase-related Cdc42 binding kinase) αβ. J Biol Chem 16: 10543–10549, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Godoy MAF, Rattan S. Translocation of AT1- and AT2-receptors by higher concentrations of angiotensin II in the smooth muscle cells of rat internal anal sphincter. J Pharmacol Exp Ther 319: 1088–1095, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Eto M, Kitazawa T, Yazawa A, Mukai H, Ono Y, Brautigan DL. Histamine-induced vasoconstriction involves phosphorylation of a specific inhibitor protein for myosin phosphatase by protein kinase C α and δ isoforms. J Biol Chem 276: 29072–29078, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Genth H, Gerhard R, Maeda A, Amano M, Kaibuchi K, Aktories K, Just I. Entrapment of Rho ADP-ribosylated by Clostridium botulinum C3 exozyme in the Rho-guanine nucleotide dissociation inhibitor-1 complex. J Biol Chem 278: 28523–28527, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Harnett KM, Cao W, Biancani P. Signal-transduction pathways that regulate smooth muscle function I. Signal transduction in phasic (esophageal) and tonic (gastroesophageal sphincter) smooth muscles. Am J Physiol Gastrointest Liver Physiol 288: G407–G416, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Ishizaki T, Uehata M, Tamechika I, Keel J, Nonomura K, Maekawa M. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol Pharmacol 57: 976–983, 2000 [PubMed] [Google Scholar]
- 12. Kandabashi T, Shimokawa H, Miyata K, Kunihiro I, Eto Y, Morishige K, Matsumoto Y, Obara K, Nakayama K, Takahashi S, Takeshita A. Evidence for protein kinase C-mediated activation of Rho-kinase in a porcine model of coronary artery spasm. Arterioscler Thromb Vasc Biol 23: 2209–2214, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Kobayashi E, Nakano H, Morimoto M, Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun 15: 548–553, 1989 [DOI] [PubMed] [Google Scholar]
- 14. Matsumoto T, Nishiyama M, Kobayashi T, Kasuya Y, Kamata K. Effect of phorbol 12,13-dibutyrate on smooth muscle tone in rat stomach fundus. J Smooth Muscle Res 41: 107–116, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Mitsui M, Karaki H. Contractile and relaxant effects of phorbol ester in the intestinal smooth muscle of guinea-pig taenia caeci. Br J Pharmacol 109: 229–233, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol 68: 345–374, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Murthy KS, Grider JR, Kuemmerle JF, Makhlouf GM. Sustained muscle contraction induced by agonists, growth factors, and Ca2+ mediated by distinct PKC isozymes. Am J Physiol Gastrointest Liver Physiol 279: G201–G210, 2000 [DOI] [PubMed] [Google Scholar]
- 18. O'Kelly T, Brading A, Mortensen N. Nerve mediated relaxation of the human internal anal sphincter: the role of nitric oxide. Gut 34: 689–693, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park SY, Song HJ, Sohn UD. Participation of Rho-associated kinase in electrical stimulated and acetylcholine-induced contraction of feline esophageal smooth muscle. Eur J Pharmacol 607: 220–225, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Patel CA, Rattan S. Cellular regulation of basal tone in internal anal sphincter smooth muscle by RhoA/ROCK. Am J Physiol Gastrointest Liver Physiol 292: G1747–G1756, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Patil SB, Bitar KN. RhoA- and PKC-α-mediated phosphorylation of MYPT and its association with HSP27 in colonic smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 290: G83–G95, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Puetz S, Lubomirov LT, Pfitzer G. Regulation of smooth muscle contraction by small GTPases. Physiology 24: 342–356, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Rattan S, Benjamin P, Maxwell PJ IV. RhoA/ROCK-kinase: pathophysiologic and therapeutic implications in gastrointestinal smooth muscle tone and relaxation. Gastroenterology 138: 13–18, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rattan S, Chakder S. Role of nitric oxide as a mediator of internal anal sphincter relaxation. Am J Physiol Gastrointest Liver Physiol 262: G107–G112, 1992 [DOI] [PubMed] [Google Scholar]
- 25. Rattan S, De Godoy MAF, Patel CA. Rho kinase as a novel molecular therapeutic target for hypertensive internal anal sphincter. Gastroenterology 131: 108–116, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Sakai H, Kurihara Y, Hashimoto H, Chiba Y, Misawa M. Involvement of multiple PKC isoforms in phorbol 12,13-dibutyrate-induced contraction during high K+ depolarization in bronchial smooth muscle of mice. J Smooth Muscle Res 46: 225–233, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Sakai H, Yamamoto M, Chiba Y, Misawa M. Some different effect of PKC inhibitors on the acetylcholine, and endothelin-1-induced contractions of rat bronchial smooth muscle. Eur J Pharmacol 618: 58–62, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Sasaguri T, Watson SP. Phorbol esters inhibit smooth muscle contractions through activation of Na+-K+-ATPase. Br J Pharmacol 99: 237–242, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sims SM, Chrones T, Preiksaitis HG. Calcium sensitization in human esophageal muscle: role for RhoA kinase in maintenance of lower esophageal sphincter tone. J Pharmacol Exp Ther 327: 178–186, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Somara S, Bitar KN. Direct association of calponin with specific domains of PKC-α. Am J Physiol Gastrointest Liver Physiol 295: G1246–G1254, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Somara S, Gilmont RR, Dennis RG, Bitar KN. Bioengineered internal anal sphincter derived from isolated human internal anal sphincter smooth muscle cells. Gastroenterology 137: 53–61, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Xiao L, Eto M, Kazanietz MG. ROCK mediates phorbol ester-induced apoptosis in prostate cancer cells via p21Cip1 up-regulation and JNK. J Biol Chem 284: 29365–29375, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]