Abstract
Disruption of intestinal epithelial homeostasis, including enhanced apoptosis, is a hallmark of inflammatory bowel disease (IBD). We have recently shown that tumor necrosis factor (TNF) increases the kinase activity of ErbB4, a member of the epidermal growth factor receptor family that is elevated in mucosa of IBD patients and that promotes colon epithelial cell survival. In this study, we tested the hypothesis that TNF transactivates ErbB4 through TNF-α converting enzyme (TACE)-mediated ligand release and that this transactivation is necessary to protect colonic epithelial cells from cytokine-induced apoptosis. Using neutralizing antibodies, we show that heparin-binding EGF-like growth factor (HB-EGF) is required for ErbB4 phosphorylation in response to TNF. Pharmacological or genetic inhibition of the metalloprotease TACE, which mediates HB-EGF release from cells, blocked TNF-induced ErbB4 activation. MEK, but not Src or p38, was also required for transactivation. TACE activity and ligand binding were required for ErbB4-mediated antiapoptotic signaling; whereas mouse colon epithelial cells expressing ErbB4 were resistant to TNF-induced apoptosis, TACE inhibition or blockade of ErbB4 ligand binding reversed the survival advantage. We conclude that TNF transactivates ErbB4 through TACE-dependent HB-EGF release, thus protecting colon epithelial cells from cytokine-induced apoptosis. These findings have important implications for understanding how ErbB4 protects the colon from apoptosis-induced tissue injury in inflammatory conditions such as IBD.
Keywords: apoptosis, inflammatory bowel disease, colon
damage to the intestinal epithelium elicits multiple signal transduction pathways necessary for the cell survival, proliferation, and migration responses that repair injury. Coordinating these responses is critical for maintenance of a healthy colonic epithelial barrier, whereas disruption in these pathways can lead to conditions such as inflammatory bowel disease (IBD). We have shown that the tyrosine kinase ErbB4 is upregulated in the damaged epithelium of IBD patients and in mice with chemically induced colitis (8). ErbB4 is the most recently discovered member of the ErbB family of growth factor receptors, which includes epidermal growth factor receptor (EGFR), ErbB2 (HER2), and ErbB3 (HER3). Binding of ErbB4 ligands, which include heparin-binding EGF-like growth factor (HB-EGF), betacellulin (BTC), epiregulin (EPI), and heregulins (HRG) 1–4, induces receptor homodimerization or heterodimerization with ErbBs 1–3. Dimerization triggers activation of intrinsic tyrosine kinase activity and autophosphorylation of the cytoplasmic domain of the receptor, thus providing docking sites for a variety of adaptor proteins involved in the activation of downstream cascades such as the phosphoinositide 3-kinase (PI3K)/Akt pathway (18).
In addition to direct ligand stimulation, ErbBs can be indirectly activated by other stimuli such as G protein-coupled receptor agonists (26), cytokines (37), and bacterial products (38). This cross-communication enables the ErbB family to respond to diverse cellular signals and yield a myriad of biological responses, including survival, proliferation, and migration. Two distinct mechanisms of transactivation have been described: the activation of intracellular kinases and the extracellular release of ligands. Recently we have shown that EGFR and ErbB2 transactivation by the proinflammatory cytokine TNF occurs in an intracellular, Src-dependent manner (37). However, other groups have shown that metalloprotease-driven ligand cleavage mediates EGFR transactivation following stimulation with GPCR-agonists (29). Synthesized as membrane-anchored precursors, ErbB ligands are cleaved by members of the a disintegrin and metalloprotease (ADAM) family (27), releasing soluble growth factors capable of paracrine and autocrine signaling (28). TNF-α converting enzyme (TACE/ADAM17) can cleave several members of the EGF-like ligand family, including HB-EGF, EPI (15), and HRG (23). The mechanism by which cellular stimuli activate TACE is not completely defined; however, data suggest a role for the mitogen-activated protein (MAP) kinases p38 or extracellular signal-regulated (ERK1/2) kinase, which have both been shown to phosphorylate TACE on Thr735 (5, 36).
We have recently demonstrated that ErbB4 expression is upregulated in the colonic epithelium of patients with IBD and in mouse models of colitis (8). Additionally, 24-h TNF exposure stimulates ErbB4 expression and activation in cultured YAMC epithelial cells, through an undetermined mechanism. One of the hallmarks of IBD is disruption of the intestinal epithelial monolayer due to increased apoptosis (6). ErbB4 induction by TNF presumably provides a check on colonocyte apoptosis by promoting cell survival in the presence of inflammatory cytokines (8).
The purpose of this study was to identify the mechanism by which TNF transactivates ErbB4 and to determine the role of this mechanism in antiapoptotic signaling. Herein, we report that HB-EGF release by TACE is necessary for ErbB4 activation following TNF stimulation. Additionally, we show that TNF activates TACE in a MEK-dependent fashion and that TACE activity and ErbB4 ligand binding are required for ErbB4-mediated cell survival in the presence of inflammatory cytokines, as well as activation of the prosurvival PI3K/Akt pathway. By providing the mechanism of the first example of ErbB4 transactivation, this study fills a gap in the knowledge of how ErbB4 functions to protect the gastrointestinal epithelium from inflammation-induced injury, which may lead to novel treatment strategies for protecting the intestinal epithelium from cytokine-induced injury in patients with IBD.
MATERIALS AND METHODS
Cell culture.
Conditionally immortalized young adult mouse colon (YAMC) epithelial cells (34), Madin-Darby canine kidney (MDCK), and Immorto-Min colon epithelial (IMCE) cells were provided by Dr. Robert Whitehead (Vanderbilt University). Generation of TACE−/− lines stably expressing wild-type TACE has previously been published (10). YAMC, IMCE, and TACE−/− lines were all maintained in RPMI 1640 medium containing 5% FBS, 5 U/ml of murine interferon (IFN)-γ (Intergen, Norcross, GA), 5 μg/ml insulin, 5 μg/ml transferrin, 5 μg/ml selenous acid (BD Biosciences, San Jose, CA), and 100 U/ml penicillin and streptomycin and grown under permissive conditions at 33°C with 5% CO2. Prior to all experiments, cells were transferred to 37°C (nonpermissive) conditions and cultured in medium with streptomycin, penicillin, and with either 0.5% FBS or no FBS for 24 h. MDCK cells were maintained in DMEM containing penicillin/ streptomycin and 10% FBS at 37°C. Prior to experiments, MDCK cells were starved of FBS overnight.
Transfections and constructs.
Generation of ErbB4-expressing YAMC cells has previously been published (8). Briefly, inserts from pCDNA3.1-ErbB4 (JM-b/CYT-2 isoform) expression vectors (provided by Graham Carpenter, Vanderbilt University) were PCR amplified then cut and ligated into linearized bicistronic LZRS-GFP vector, which was obtained from Albert Reynolds (Vanderbilt University). LZRS-GFP or LZRS-ErbB4-GFP was transiently transfected into Phoenix packaging cells (Steve Hanks, Vanderbilt University) and YAMC or TACE−/− MCE cells were subjected to five rounds of infection with filtered supernatant supplemented with 4 μg/ml polybrene. Infected populations were expanded and GFP-positive cells were sorted at the Vanderbilt Medical Center Flow Cytometry Shared Resource by use of a Becton-Dickinson FACSAria. All ErbB-infected cell lines utilized in this study were infected with the JM-B, CYT-2 isoform, which is not cleavable by TACE in the juxtamembrane domain.
Antibodies, cytokines, and growth factors.
Polyclonal antibodies used for Western blot include phospho-ERK1/2 (Promega) phospho-Y1068 EGFR, phospho-Y1248 ErbB2, active caspase-3, cleaved poly(ADP-ribose) polymerase (PARP), phospho-Y925 FAK, total FAK, phospho-T581 MSK1 (Cell Signaling), TACE, ErbB4 (Santa Cruz), and phospho-T735 TACE (Abcam). Monoclonal antibodies used include phospho-Y1284 ErbB4, phospho-Y1289 ErbB3, phospho-S473 Akt, total Akt (Cell Signaling), and actin (Sigma). Neutralizing antibodies used in cell culture include ErbB4 (Millipore), HRG, HB-EGF, and BTC (R&D Systems). Recombinant murine TNF was purchased from Peprotech (Rocky Hill, NJ). Recombinant HRG, BTC, and HB-EGF were purchased from R&D Systems (Minneapolis, MN). Recombinant EGF was a gift from Stanley Cohen (Vanderbilt University, Nashville, TN). TNF-α protease inhibitor (TAPI)-1 and GM6001 were purchased from EMD Chemicals (Darmstadt, Germany). PMA was purchased from Enzo Life Sciences. U0126, CGP77675, and SB202190 were purchased from Calbiochem.
Cell lysates and Western blot analysis.
Cell lysates were prepared by scraping cells in lysis buffer [10 mM Tris pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 0.1% SDS, 0.2% Na-deoxycholate, and 0.1% phosphatase and protease inhibitor cocktails (Sigma)], incubating on ice for 10 min, clearing by centrifugation, then boiling the supernatants in Laemmli sample buffer. Cell lysates were run on SDS-PAGE gels (7.5–20% as needed) and transferred onto nitrocellulose membranes.
Apoptosis assays.
Apoptosis was measured by cleaved PARP and active caspase-3 Western blot analysis following 3 h of 100 ng/ml TNF and 1 μg/ml cycloheximide treatment. The ApopTag terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling kit (TUNEL; Millipore, Danvers, MA) was also used to label apoptotic cells. YAMC cells expressing either vector or ErbB4 were seeded in 4-well chamber slides (Lab-Tech) at 5 × 104 cells and placed under nonpermissive conditions overnight prior to apoptosis assays. Cells were treated with TNF and cycloheximide for 5 h. The TUNEL assay was then performed following manufacturer's instructions, and cells were visualized by differential interference contrast microscopy on a Leica DM IRB microscope.
Statistics and replicates.
All data are representative of at least three independent experiments. Statistical analyses were performed by using Prism software (GraphPad, La Jolla, CA). Statistical significance of differences was assessed by ANOVA analysis with Bonferroni posttest. Error bars indicate standard error of means.
RESULTS
TNF transactivates ErbB4 in colon epithelial cells.
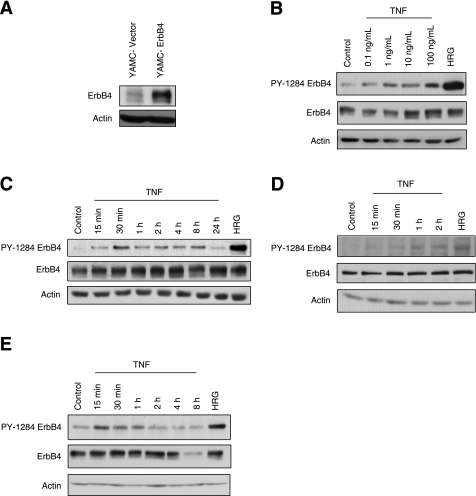
Since ErbB4 expression is upregulated in the colonic epithelium of patients with IBD and in mice with dextran sodium sulfate (DSS)-induced colitis (8), we tested whether TNF could activate ErbB4 in YAMC epithelial cells stably overexpressing ErbB4 (termed YAMC-ErbB4 cells) (Fig. 1A). TNF induced ErbB4 phosphorylation in a dose-dependent manner (Fig. 1B), with the response peaking at 30 min and sustaining for at least 24 h after treatment (Fig. 1C). TNF also activated endogenous ErbB4 in an alternate colon epithelial cell line, IMCE cells (Fig. 1D), as well as in MDCK cells (Fig. 1E), demonstrating that this phenomenon is not restricted to a single cell line or organ.
Fig. 1.
TNF transactivates ErbB4 in colonic epithelial cells. A: ErbB4 expression in young adult mouse colon (YAMC) cells stably overexpressing ErbB4 (YAMC-ErbB4) or vector (YAMC-vector) was assessed by Western blot with antibodies specific for ErbB4 or actin (loading control). B: YAMC-ErbB4 cells were treated with TNF for 30 min at the indicated concentrations or with 10 ng/ml heregulin (HRG) for 10 min. C: YAMC-ErbB4 cells were treated with TNF (100 ng/ml) for the indicated time points or with HRG (10 ng/ml) for 10 min, as a positive control for activation. D: Immorto-Min colon epithelial (IMCE) cells were treated with 100 ng/ml TNF for the indicated times or with 10 ng/ml HRG for 10 min. E: Madin-Darby canine kidney (MDCK) cells were treated with TNF (100 ng/ml) for the indicated time points or with HRG (10 ng/ml) for 10 min. Cell lysates were analyzed by Western blot with antibodies specific for phosphorylated (PY-1284) ErbB4, total ErbB4, or actin (loading control). All blots are representative of at least 3 experiments.
TNF-induced ErbB4 activation in YAMC cells is mediated by HB-EGF.
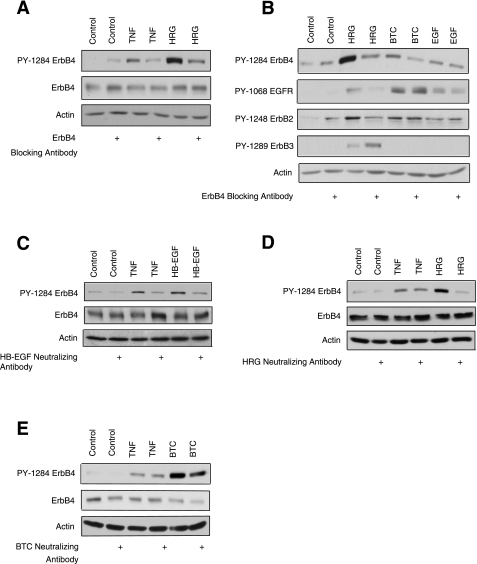
Transactivation of ErbB receptors can occur through either ligand-dependent (38) or ligand-independent (37) mechanisms. To determine which route mediates TNF activation of ErbB4 in intestinal epithelial cells, we utilized a neutralizing antibody that blocks the ligand-binding domain of ErbB4. YAMC-ErbB4 cells were incubated with the blocking antibody for 1 h before treatment with TNF for 30 min or HRG for 10 min. ErbB4 activation was then assessed by Western blot analysis. The ErbB4 blocking antibody inhibited both TNF-induced activation of ErbB4 and direct ligand stimulation (Fig. 2A), indicating that transactivation by TNF requires binding of an extracellular factor.
Fig. 2.
Heparin-binding EGF-like growth factor (HB-EGF) ligand binding mediates ErbB4 transactivation by TNF. A: YAMC-ErbB4 cells were incubated with either control mouse IgG or an ErbB4 blocking antibody for 1 h before the addition of TNF (100 ng/ml) for 30 min or HRG (10 ng/ml) for 10 min. B: YAMC-ErbB4 cells were incubated with an ErbB4 blocking antibody for 1 h before the addition of 10 ng/ml HRG, 1 ng/ml betacellin (BTC), or 1 ng/ml EGF for 10 min. Phosphorylated ErbB4 (PY-1284), EGFR (PY-1068), ErbB2 (PY-1248), ErbB3 (1289), or total actin was assessed by Western blot. YAMC-ErbB4 cells were incubated for 1 h with HB-EGF neutralizing antibody (C), HRG neutralizing antibody (D), or BTC neutralizing antibody (E) before stimulation with 100 ng/ml TNF for 30 min and with 50 ng/ml HB-EGF, 10 ng/ml HRG, or 10 ng/ml BTC for 10 min. Treatment with mouse IgG served as the negative control. Cell lysates were analyzed by Western blot with antibodies specific for phosphorylated ErbB4 (PY-1284), total ErbB4, or actin. All blots are representative of at least 3 experiments.
ErbB4 recognizes a subset of EGF-like ligands, such as HB-EGF and BTC, in addition to members of the heregulin family. To ensure that the ErbB4 blocking antibody did not cross-react with any of the closely related EGFR family members, YAMC-ErbB4 cells were treated with HRG (which binds ErbB3 and ErbB4), BTC (which binds EGFR and ErbB4), and EGF (which only binds EGFR) for 10 min. Figure 2B shows that ErbB4 blocking antibody inhibits ErbB4 phosphorylation by its own ligands, but not by EGF. Importantly, the ErbB4 blocking antibody did not inhibit EGFR activation by BTC and EGF or ErbB3 phosphorylation by HRG.
To determine which of the known ErbB4 ligands is responsible for ErbB4 transactivation by TNF, we treated YAMC-ErbB4 cells with TNF in the presence of HRG, HB-EGF, or BTC neutralizing antibodies. HB-EGF neutralizing antibody blocked both TNF and HB-EGF-stimulated ErbB4 phosphorylation (Fig. 2C). In contrast, neither HRG neutralizing antibody (Fig. 2D) nor BTC neutralizing antibody (Fig. 2E) attenuated TNF activation of ErbB4, although the antibodies were able to inhibit HRG and BTC-induced phosphorylation, respectively. We therefore conclude that HB-EGF is required for ErbB4 transactivation by TNF.
TACE-stimulated release of HB-EGF mediates TNF transactivation of ErbB4.
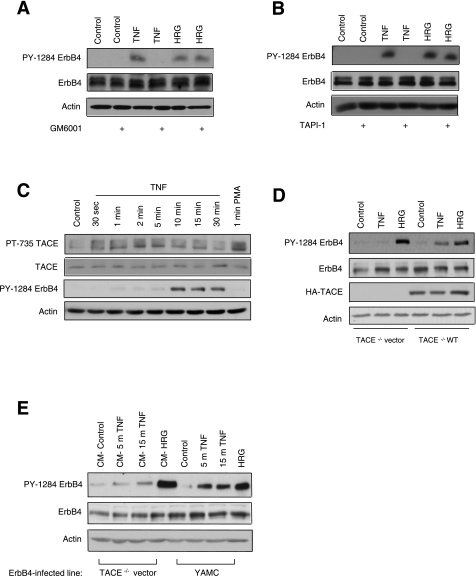
TNF signaling through TNFRs is known to activate metalloproteinases, which are proteases that can cleave membrane-anchored ligands (16). To investigate whether ligand cleavage is necessary for ErbB4 transactivation, we treated YAMC-ErbB4 cells with the broad-spectrum metalloproteinase inhibitor GM6001 (50 μM) for 30 min, followed by TNF for 30 min or HRG for 10 min. GM6001 blocked ErbB4 activation in response to TNF, but not HRG (Fig. 3A). Since the metalloproteinase TACE has been specifically implicated in the cleavage of ErbB4 ligands (15), we also treated YAMC-ErbB4 cells with the selective TACE inhibitor TAPI-1 (10 μM, 30 min) before TNF or HRG exposure. TNF-mediated ErbB4 phosphorylation was completely reversed by TACE inhibition, whereas HRG-induced activation was not altered (Fig. 3B), suggesting that TACE-mediated cleavage of an ErbB4 ligand mediates ErbB4 transactivation.
Fig. 3.
TNF-α converting enzyme (TACE) mediates TNF transactivation of ErbB4 in colonic epithelial cells. YAMC-ErbB4 cells were incubated for 30 min with 50 μM of the broad spectrum metalloproteinase inhibitor GM6001 (A) or 10 μM of the TACE selective inhibitor TNF-α protease inhibitor (TAPI)-1 (B), then stimulated with TNF (100 ng/ml, 30 min) or HRG (1 ng/ml, 10 min). C: YAMC-ErbB4 cells were treated with 100 ng/ml TNF for various time points or with 20 ng/ml PMA for 1 min. Cell lysates were analyzed by Western blot with antibodies specific for phosphorylated TACE (PT-735), ERK, or total actin. D: TACE−/− colonic epithelial cells infected with ErbB4 and with either wild-type TACE or vector added back were treated with 100 ng/ml TNF for 30 min or 10 ng/ml HRG for 10 min. E: YAMC-ErbB4 cells were treated directly with 100 ng/ml TNF for 5 or 15 min or 10 ng/ml HRG for 10 min. TACE−/− colon epithelial cells expressing ErbB4 were treated with conditioned media (CM) from the corresponding YAMC cells for 10 min. Cell lysates were then analyzed by Western blot with antibodies specific for phosphorylated ErbB4 (PY-1284), total ErbB4, or actin. All blots are representative of at least 3 experiments.
To confirm that TACE is activated by TNF in YAMC-ErbB4 cells, cultures were treated with TNF for up to 30 min or with 20 ng/ml PMA for 1 min, then lysed and analyzed for phosphorylation at a known TACE activation site (5). In response to TNF, TACE was phosphorylated at T735, with activation peaking between 2 and 5 min. PMA, a known TACE stimulus (13), also induced TACE phosphorylation (Fig. 3C). To further verify that TACE is in fact the metalloproteinase responsible for TNF transactivation of ErbB4, we stably expressed ErbB4 in TACE−/− mouse colon epithelial (MCE) cells transfected with either wild-type TACE or vector. ErbB4 was not phosphorylated in response to TNF in TACE−/− MCE cells expressing vector; however, reexpression of wild-type TACE restored TNF-induced ErbB4 activation (Fig. 3D). We then took advantage of the observation that TNF cannot promote ErbB4 phosphorylation in the TACE−/− MCE line, by transferring conditioned media from YAMC-ErbB4 cells treated with TNF to the TACE-null line and assessing ErbB4 phosphorylation by Western blot. In TACE−/− MCE cells, ErbB4 was phosphorylated by conditioned media transferred from YAMC cells treated with 100 ng/ml TNF for 15 min (Fig. 3E), further supporting the hypothesis that ErbB4 transactivation by TNF is mediated by TACE cleavage of an ErbB4 ligand.
ErbB4 activation by TNF requires MEK kinase activity.
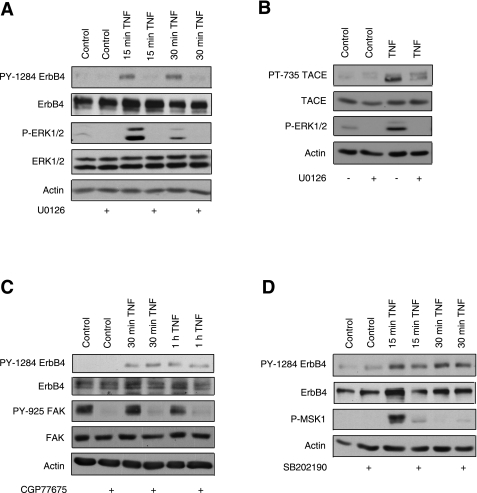
There are multiple routes by which TNF could stimulate TACE. One common mechanism of TNF-induced TACE activation is through intracellular kinases. Specifically, ERK1/2 stimulates TACE phosphorylation at T735 in response to TNF treatment of HeLa cells (5), p38 mediates TACE activation in lymphocytes and monocytes treated with TNF (19), and Src is required for gastrin-releasing peptide-induced TACE activity in squamous cell carcinoma of the head and neck cell lines (39). Since we demonstrated TNF-induced TACE phosphorylation at the ERK site T735 (Fig. 3C), we tested the possibility that the MEK/ERK cascade is required for TNF transactivation of ErbB4. YAMC-ErbB4 cells were treated with the MEK inhibitor U0126 for 30 min before addition of TNF or HRG. MEK inhibition completely blocked ErbB4 phosphorylation in response to TNF, as assessed by Western blot analysis (Fig. 4A). To further test the hypothesis that ERK-induced TACE activation promotes ErbB4 transactivation by TNF, we pretreated YAMC-ErbB4 cells with U0126 before TNF stimulation. Blocking MEK activity reversed the ability of TNF to activate TACE on a known ERK consensus site (Fig. 4B). In contrast to MEK inhibition, Src inhibition by CGP77675 (Fig. 4C) or p38 inhibition by SB202190 (Fig. 4D) or did not affect transactivation.
Fig. 4.
ErbB4 transactivation by TNF requires MEK, but not p38 or Src kinases. A: YAMC-ErbB4 cells were incubated with 10 μM U0126 (MEK inhibitor) for 1 h before the addition of TNF (100 ng/ml) for either 15 or 30 min. B: YAMC-ErbB cells were pretreated with U0126 before 5-min TNF treatment. C: YAMC-ErbB4 cells were incubated with 2 μM of the Src inhibitor CGP77675 for 1 h before the addition of TNF for the indicated time points. D: YAMC-ErbB4 cells were incubated with 10 μM SB20190 (p38 inhibitor) for 1 h before TNF treatment for 15 or 30 min. Phosphorylation of ErbB4 (PY-1284), TACE (PT-735), ERK1/2, MSK1 (positive control for p38 inhibition), or FAK (PY-925, positive control for Src inhibition) or total ErbB4, TACE, ERK1/2, FAK, or actin was determined by Western blot analysis. All blots are representative of at least 3 experiments.
ErbB4 transactivation by TNF promotes colonic epithelial cell survival.
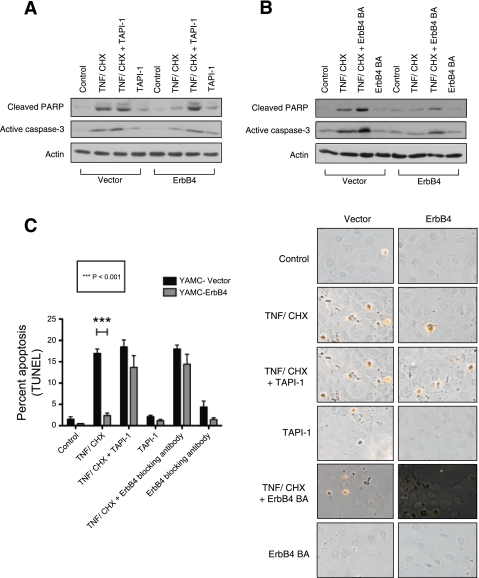
We have previously shown that ErbB4 overexpression protects colon epithelial cells from inflammatory cytokine-induced apoptosis (8). To test the hypothesis that TACE-mediated transactivation of the receptor is necessary for this survival response, we compared apoptosis levels in YAMC-ErbB4 cells and YAMC-vector cells following 3 h treatment with 100 ng/ml TNF and 1 μg/ml cycloheximide (to stimulate apoptosis) with or without 30 min pretreatment with 10 μM TAPI-1 (to inhibit transactivation). After TNF and cycloheximide exposure, YAMC cells overexpressing ErbB4 exhibited lower levels of the apoptotic markers cleaved PARP and active caspase-3 than cells expressing vector alone. TAPI-1 treatment blocked the ability of ErbB4 to protect against apoptosis, indicating a requirement for TACE activity (Fig. 5A). To corroborate these results, YAMC cells expressing either ErbB4 or vector were subjected to the same apoptosis assay after incubation with ErbB4 blocking antibody. Similar to TACE inhibition, the blocking antibody reversed the ability of ErbB4 to protect against cytokine-induced apoptosis (Fig. 5B). Finally, TUNEL staining was used as an independent method to verify apoptosis. After 5 h TNF and cycloheximide exposure, substantially fewer YAMC-ErbB4 cells were TUNEL positive compared with vector controls. However, blocking ligand binding (by 1 h pretreatment with ErbB4 blocking antibody) or TACE activity (by 30-min pretreatment with TAPI-1) abrogated the ability of ErbB4 to protect from apoptosis (Fig. 5C). Thus ErbB4 transactivation via TACE protects colonic epithelial cells from cytokine-induced apoptosis.
Fig. 5.
ErbB4 transactivation by TNF protects colonic epithelial cells from cytokine-induced apoptosis. YAMC cells expressing either vector (YAMC-vector) or ErbB4 (YAMC-ErbB4) were incubated with 10 μM TAPI-1 for 30 min (A) or ErbB4 blocking antibody for 1 h (B) before treatment with 1 μg/ml cycloheximide (CHX) and 100 ng/ml TNF for 3 h. Caspase-3 activation and poly(ADP-ribose) polymerase (PARP) cleavage were assessed by Western blot analysis of whole cell lysates. Actin was used as a loading control. C: vector and ErbB4-expressing YAMC cells were incubated with 10 μM TAPI-1 for 30 min or with an ErbB4 blocking antibody for 1 h before treatment with 1 μg/ml cycloheximide and 100 ng/ml TNF for 5 h. Subsequently, cells were subjected to terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling kit (TUNEL) apoptosis assay. Labeled nuclei from at least 5 random fields per condition were counted in each of 3 experiments.
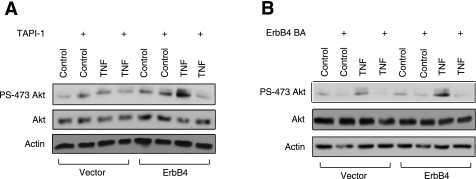
Members of the ErbB family can promote survival through activation of the PI3K/Akt signaling pathway (4, 8). To determine whether this is a result of TACE-mediated transactivation, YAMC-vector and YAMC-ErbB4 cells were incubated with TAPI-1 for 30 min before treatment with TNF for 15 min. Western blot analysis with an antibody specific for Akt phosphorylation (S473) showed that ErbB4 expression stimulates increased levels of TNF-induced Akt activation and that inhibiting transactivation with TAPI-1 abrogates this response (Fig. 6A). Pretreatment of YAMC-ErbB4 cells with an ErbB4 blocking antibody also inhibits ErbB4's ability to promote Akt activation following TNF stimulation (Fig. 6B). In conjunction with our previous results showing that PI3K blockade reverses ErbB4-induced cell survival (8), these data indicate Akt signaling is involved in the prosurvival pathway elicited by TNF-induced ErbB4 transactivation in colon epithelial cells.
Fig. 6.
ErbB4 transactivation by TNF promotes increased Akt activation. YAMC-vector and YAMC-ErbB4 cells were incubated with 10 μM TAPI-1 for 30 min (A) or with ErbB4 blocking antibody for 1 h (B) before treatment with 100 ng/ml TNF for 15 min. Cell lysates were then analyzed by Western blot with antibodies against phosphorylated Akt (PS-473), total Akt, ErbB4, or actin. All blots are representative of at least 3 experiments.
DISCUSSION
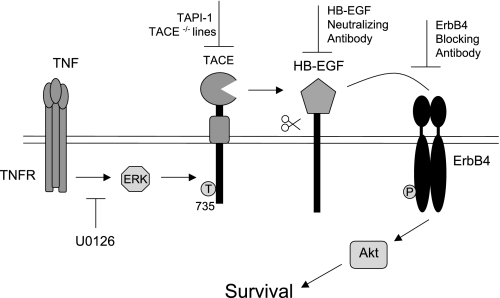
This study describes a novel signaling cascade that promotes colonic epithelial cell survival following TNF exposure. We determined the pathway through which TNF induces ErbB4 activation, providing the first mechanism of ErbB4 transactivation by an inflammatory cytokine. The data show that TNF stimulates ErbB4 phosphorylation through TACE-dependent HB-EGF cleavage. Additionally, MEK, but not p38 or Src, kinase activity was required for TNF-induced ErbB4 activation. Furthermore, TACE-dependent ErbB4 transactivation is necessary for resistance from cytokine-induced apoptosis through increased Akt signaling (Fig. 7). These results suggest that in the inflamed colon, TNF-induced ErbB4 activation may protect the epithelial monolayer from the high levels of epithelial apoptosis and ulcerations that are a hallmark of IBD (12, 17).
Fig. 7.
Model of ErbB4 transactivation by TNF in colonic epithelial cells TNF stimulation of colonic epithelial cells leads to activation of ERK1/2, which stimulates TACE activity. TACE cleaves membrane-bound HB-EGF, which then binds to ErbB4 and stimulates phosphorylation and activation. ErbB4 transactivation by TNF then mediates colonic epithelial cell survival through increased Akt signaling.
Interestingly, although EGFR and ErbB4 are highly related members of the same receptor tyrosine kinase family, in colon epithelial cells ErbB4 transactivation by TNF occurs through a different mechanism than EGFR transactivation by TNF. TNF activates EGFR through an intracellular, Src-dependent mechanism (37), whereas TNF activates ErbB4 through extracellular, TACE-mediated ligand release (Figs. 2 and 3). Given the wealth of data implicating EGFR in the pathogenesis of colon cancer (25) and the emerging role of ErbB4 in protecting colon cells from apoptosis, delineating the differences in activation and signaling pathways between these two receptors is important for potential therapies. Understanding the mechanisms by which inflammatory cytokines cross talk with ErbB family members may also provide more targets for treatment of colitis or for interfering with the prosurvival signaling that leads to carcinogenesis.
In IBD, high levels of inflammatory cytokines, such as TNF and interferon-γ, contribute to colon epithelial cell apoptosis, resulting in loss of mucosal integrity and barrier dysfunction (6). ErbB4 is likely important for protecting colon epithelial cells and limiting tissue damage during active inflammation. However, there is also a growing literature that suggests ErbB4 may contribute to colorectal cancer. Somatic mutation of the ErbB4 kinase domain occurs in colorectal cancers (31), increased ErbB4 coexpression with ErbB2 has been found in late stage carcinomas (21), and high ErbB4 expression is seen in colorectal tumors (22). We have recently demonstrated that ErbB4-overexpressing colon epithelial cells acquire the ability to form colonies in soft agar in a cyclooxygenase-2-dependent manner (9). Furthermore, ErbB4 deletion in human colon cancer lines increases apoptosis (20).
It is interesting that we observed ErbB4 transactivation leading to increased Akt phosphorylation (Fig. 6); aberrant PI3K/AKT activity is implicated in apoptosis resistance in a variety of cancers (14). An endocrine-resistant human breast carcinoma cell line that displays both ErbB4 upregulation and increased levels of ErbB ligands (including HB-EGF, the ligand which activates ErbB4 in the present study) shows constitutive activation of the PI3K/Akt pathway (11). HB-EGF is expressed in a variety of cancers, including gastric and colon cancer (35). In breast cancer cells, sequestering HB-EGF with a mutant form of the diphtheria toxin CRM197 attenuated Akt phosphorylation and led to significant apoptotic cell death. Our demonstration that HB-EGF-mediated ErbB4 transactivation by TNF leads to increased Akt activation may suggest a mechanism for chronic inflammation-induced carcinogenesis. Whereas ErbB4 transactivation by TNF may provide the short-term benefit of protecting the colon epithelium from apoptosis-induced ulceration during inflammatory conditions, the long-term consequences could potentially include colitis-associated carcinogenesis.
Although TACE levels and activity are upregulated in patients with IBD and TACE-mediated TNF cleavage could possibly lead to the increased levels of soluble TNF seen in inflammatory diseases (1), it has not yet been resolved whether inhibiting TACE would be a viable therapy for IBD. For instance, in mice with TNBS-induced colitis, TACE activity was elevated, resulting in concomitant increases in soluble TNF. Treatment with a pharmacological TACE inhibitor ameliorated TNBS-induced colonic damage and inflammation (3). On the other hand, when TACE expression and activity are reduced to <5% normal levels via genetic strategies, mice are far more susceptible to DSS-induced colitis (2, 32), supporting a role for TACE in protecting the GI tract from acute injury-induced inflammation. Likewise, when HT29 colon cells are grown to confluence, inhibiting TACE pharmacologically (with TAPI-2) or physiologically [with recombinant tissue inhibitor of metalloproteinase 3 (TIMP3)] sensitizes the intestinal epithelial monolayer to TNF-induced barrier disruption, as measured by FITC-dextran flux (7). Our studies underscore the potentially complex outcomes of TACE blockade; whereas the metalloprotease may enhance TNF release it is also required for the survival advantage conferred by TNF-induced ErbB4 activation. When TACE is inhibited, TNF is no longer able to activate ErbB4 in colonic epithelial cells and, instead, the proapoptotic effects of TNF are increased (Fig. 5).
Although it is generally accepted that TACE plays a pivotal role in a range of inflammatory diseases such as IBD, rheumatoid arthritis (24), and heart disease (33), it is not fully understood how TACE activity is induced and regulated. Given the variety of stimuli that activate TACE and the wide range of substrates that TACE cleaves, there must be tight control over enzymatic activity. There have been multiple reports that phosphorylation at T735 in the cytoplasmic tail of TACE by either p38 or ERK is important for trafficking and regulation of the metalloprotease (5, 30, 36). In our study, we show that TACE is phosphorylated at T735 following TNF stimulation and that inhibiting MEK attenuates both TNF-induced TACE phosphorylation as well as ErbB4 transactivation by TNF (Figs. 3 and 4). These data support a model in which TACE is activated by ERK at T735 following TNF stimulation in colonic epithelial cells, and that this activation is essential for ErbB4 transactivation by TNF. It is important to note that p38 activity is not required for transactivation, since in some cell lines p38 is responsible for TACE activation.
In summary, our data demonstrate that ErbB4 transactivation by the proinflammatory cytokine TNF protects colon epithelial cells from apoptosis through a TACE-dependent mechanism leading to downstream Akt signaling. This study links two known mediators of inflammatory signaling, TNF and TACE, to activation of the ErbB4 growth factor receptor. We have determined the mechanism by which TNF transactivates ErbB4: TNF-stimulated ERK activates TACE, which cleaves and releases membrane-bound HB-EGF. Upon HB-EGF-induced ErbB4 phosphorylation, Akt is activated and promotes downstream survival signaling (Fig. 7). Since TNF upregulates and activates ErbB4, and ErbB4 has recently been implicated in colorectal carcinogenesis, delineating the steps of this mechanism could provide additional therapeutic targets for treatment of IBD and prevention of colitis-associated carcinogenesis.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01DK56008 (D. B. Polk), K01DK077956 and K01DK077956-S1 (M. R. Frey), and P01CA116087 (D. B. Polk, R. M. Peek) and by the Vanderbilt Digestive Diseases Research Center NIH Grant P30DK058404.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Jeremy Goettel, Anna Means, and Michael Rosen for thoughtful discussions and manuscript critiques.
REFERENCES
- 1. Brynskov J, Foegh P, Pedersen G, Ellervik C, Kirkegaard T, Bingham A, Saermark T. Tumour necrosis factor alpha converting enzyme (TACE) activity in the colonic mucosa of patients with inflammatory bowel disease. Gut 51: 37–43, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chalaris A, Adam N, Sina C, Rosenstiel P, Lehmann-Koch J, Schirmacher P, Hartmann D, Cichy J, Gavrilova O, Schreiber S, Jostock T, Matthews V, Hasler R, Becker C, Neurath MF, Reiss K, Saftig P, Scheller J, Rose-John S. Critical role of the disintegrin metalloprotease ADAM17 for intestinal inflammation and regeneration in mice. J Exp Med 207: 1617–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Colon AL, Menchen LA, Hurtado O, De Cristobal J, Lizasoain I, Leza JC, Lorenzo P, Moro MA. Implication of TNF-alpha convertase (TACE/ADAM17) in inducible nitric oxide synthase expression and inflammation in an experimental model of colitis. Cytokine 16: 220–226, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Danielsen AJ, Maihle NJ. The EGF/ErbB receptor family and apoptosis. Growth Factors 20: 1–15, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Diaz-Rodriguez E, Montero JC, Esparis-Ogando A, Yuste L, Pandiella A. Extracellular signal-regulated kinase phosphorylates tumor necrosis factor alpha-converting enzyme at threonine 735: a potential role in regulated shedding. Mol Biol Cell 13: 2031–2044, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Edelblum KL, Yan F, Yamaoka T, Polk DB. Regulation of apoptosis during homeostasis and disease in the intestinal epithelium. Inflamm Bowel Dis 12: 413–424, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Freour T, Jarry A, Bach-Ngohou K, Dejoie T, Bou-Hanna C, Denis MG, Mosnier JF, Laboisse CL, Masson D. TACE inhibition amplifies TNF-alpha-mediated colonic epithelial barrier disruption. Int J Mol Med 23: 41–48, 2009 [PubMed] [Google Scholar]
- 8. Frey MR, Edelblum KL, Mullane MT, Liang D, Polk DB. The ErbB4 growth factor receptor is required for colon epithelial cell survival in the presence of TNF. Gastroenterology 136: 217–226, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frey MR, Hilliard VC, Mullane MT, Polk DB. ErbB4 promotes cyclooxygenase-2 expression and cell survival in colon epithelial cells. Lab Invest 90: 1415–1424, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garton KJ, Gough PJ, Philalay J, Wille PT, Blobel CP, Whitehead RH, Dempsey PJ, Raines EW. Stimulated shedding of vascular cell adhesion molecule 1 (VCAM-1) is mediated by tumor necrosis factor-alpha-converting enzyme (ADAM 17). J Biol Chem 278: 37459–37464, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Ghayad SE, Vendrell JA, Larbi SB, Dumontet C, Bieche I, Cohen PA. Endocrine resistance associated with activated ErbB system in breast cancer cells is reversed by inhibiting MAPK or PI3K/Akt signaling pathways. Int J Cancer 126: 545–562 [DOI] [PubMed] [Google Scholar]
- 12. Hagiwara C, Tanaka M, Kudo H. Increase in colorectal epithelial apoptotic cells in patients with ulcerative colitis ultimately requiring surgery. J Gastroenterol Hepatol 17: 758–764, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Hahn D, Pischitzis A, Roesmann S, Hansen MK, Leuenberger B, Luginbuehl U, Sterchi EE. Phorbol 12-myristate 13-acetate-induced ectodomain shedding and phosphorylation of the human meprinbeta metalloprotease. J Biol Chem 278: 42829–42839, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov 4: 988–1004, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Hinkle CL, Sunnarborg SW, Loiselle D, Parker CE, Stevenson M, Russell WE, Lee DC. Selective roles for tumor necrosis factor alpha-converting enzyme/ADAM17 in the shedding of the epidermal growth factor receptor ligand family: the juxtamembrane stalk determines cleavage efficiency. J Biol Chem 279: 24179–24188, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Huovila AP, Turner AJ, Pelto-Huikko M, Karkkainen I, Ortiz RM. Shedding light on ADAM metalloproteinases. Trends Biochem Sci 30: 413–422, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Iwamoto M, Koji T, Makiyama K, Kobayashi N, Nakane PK. Apoptosis of crypt epithelial cells in ulcerative colitis. J Pathol 180: 152–159, 1996 [DOI] [PubMed] [Google Scholar]
- 18. Kaushansky A, Gordus A, Budnik BA, Lane WS, Rush J, MacBeath G. System-wide investigation of ErbB4 reveals 19 sites of Tyr phosphorylation that are unusually selective in their recruitment properties. Chem Biol 15: 808–817, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Killock DJ, Ivetic A. The cytoplasmic domains of TNFalpha-converting enzyme (TACE/ADAM17) and L-selectin are regulated differently by p38 MAPK and PKC to promote ectodomain shedding. Biochem J 428: 293–304 [DOI] [PubMed] [Google Scholar]
- 20. Lee D, Yu M, Lee E, Kim H, Yang Y, Kim K, Pannicia C, Kurie JM, Threadgill DW. Tumor-specific apoptosis caused by deletion of the ERBB3 pseudo-kinase in mouse intestinal epithelium. J Clin Invest 119: 2702–2713, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee JC, Wang ST, Chow NH, Yang HB. Investigation of the prognostic value of coexpressed erbB family members for the survival of colorectal cancer patients after curative surgery. Eur J Cancer 38: 1065–1071, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Leung SP, Griffith OL, Masoudi H, Gown A, Jones S, Phang T, Wiseman SM. Clinical utility of type 1 growth factor receptor expression in colon cancer. Am J Surg 195: 604–610, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Montero JC, Yuste L, Diaz-Rodriguez E, Esparis-Ogando A, Pandiella A. Differential shedding of transmembrane neuregulin isoforms by the tumor necrosis factor-alpha-converting enzyme. Mol Cell Neurosci 16: 631–648, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Newton RC, Solomon KA, Covington MB, Decicco CP, Haley PJ, Friedman SM, Vaddi K. Biology of TACE inhibition. Ann Rheum Dis 60, Suppl 3: iii25–iii32, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ponz-Sarvise M, Rodriguez J, Viudez A, Chopitea A, Calvo A, Garcia-Foncillas J, Gil-Bazo I. Epidermal growth factor receptor inhibitors in colorectal cancer treatment: what's new? World J Gastroenterol 13: 5877–5887, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature 402: 884–888, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Rose-John SCA, Adam N. Intestinal inflammation is coordinated by the metalloprotease ADAM17. Cytokine 48: 51–52, 2009 [Google Scholar]
- 28. Sanderson MP, Dempsey PJ, Dunbar AJ. Control of ErbB signaling through metalloprotease mediated ectodomain shedding of EGF-like factors. Growth Factors 24: 121–136, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Schafer B, Marg B, Gschwind A, Ullrich A. Distinct ADAM metalloproteinases regulate G protein-coupled receptor-induced cell proliferation and survival. J Biol Chem 279: 47929–47938, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Soond SM, Everson B, Riches DW, Murphy G. ERK-mediated phosphorylation of Thr735 in TNFalpha-converting enzyme and its potential role in TACE protein trafficking. J Cell Sci 118: 2371–2380, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Soung YH, Lee JW, Kim SY, Wang YP, Jo KH, Moon SW, Park WS, Nam SW, Lee JY, Yoo NJ, Lee SH. Somatic mutations of the ERBB4 kinase domain in human cancers. Int J Cancer 118: 1426–1429, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology 138: 2101–2114.e5 [DOI] [PubMed] [Google Scholar]
- 33. Wang X, Oka T, Chow FL, Cooper SB, Odenbach J, Lopaschuk GD, Kassiri Z, Fernandez-Patron C. Tumor necrosis factor-alpha-converting enzyme is a key regulator of agonist-induced cardiac hypertrophy and fibrosis. Hypertension 54: 575–582, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Whitehead RH, VanEeden PE, Noble MD, Ataliotis P, Jat PS. Establishment of conditionally immortalized epithelial cell lines from both colon and small intestine of adult H-2Kb-tsA58 transgenic mice. Proc Natl Acad Sci USA 90: 587–591, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu WK, Tse TT, Sung JJ, Li ZJ, Yu L, Cho CH. Expression of ErbB receptors and their cognate ligands in gastric and colon cancer cell lines. Anticancer Res 29: 229–234, 2009 [PubMed] [Google Scholar]
- 36. Xu P, Derynck R. Direct activation of TACE-mediated ectodomain shedding by p38 MAP kinase regulates EGF receptor-dependent cell proliferation. Mol Cell 37: 551–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yamaoka T, Yan F, Cao H, Hobbs SS, Dise RS, Tong W, Polk DB. Transactivation of EGF receptor and ErbB2 protects intestinal epithelial cells from TNF-induced apoptosis. Proc Natl Acad Sci USA 105: 11772–11777, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yan F, Cao H, Chaturvedi R, Krishna U, Hobbs SS, Dempsey PJ, Peek RM, Jr, Cover TL, Washington MK, Wilson KT, Polk DB. Epidermal growth factor receptor activation protects gastric epithelial cells from Helicobacter pylori-induced apoptosis. Gastroenterology 136: 1297–1307, e1–e3, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang Q, Thomas SM, Lui VW, Xi S, Siegfried JM, Fan H, Smithgall TE, Mills GB, Grandis JR. Phosphorylation of TNF-alpha converting enzyme by gastrin-releasing peptide induces amphiregulin release and EGF receptor activation. Proc Natl Acad Sci USA 103: 6901–6906, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]