Abstract
In bile duct-ligated (BDL) rats, cholangiocyte proliferation is regulated by neuroendocrine factors such as α-calcitonin gene-related peptide (α-CGRP). There is no evidence that the sensory neuropeptide substance P (SP) regulates cholangiocyte hyperplasia. Wild-type (WT, +/+) and NK-1 receptor (NK-1R) knockout (NK-1R−/−) mice underwent sham or BDL for 1 wk. Then we evaluated 1) NK-1R expression, transaminases, and bilirubin serum levels; 2) necrosis, hepatocyte apoptosis and steatosis, and the number of cholangiocytes positive by CK-19 and terminal deoxynucleotidyl transferase biotin-dUTP nick-end labeling in liver sections; 3) mRNA expression for collagen 1α and α-smooth muscle (α-SMA) actin in total liver samples; and 4) PCNA expression and PKA phosphorylation in cholangiocytes. In cholangiocyte lines, we determined the effects of SP on cAMP and d-myo-inositol 1,4,5-trisphosphate levels, proliferation, and PKA phosphorylation. Cholangiocytes express NK-1R with expression being upregulated following BDL. In normal NK-1R−/− mice, there was higher hepatocyte apoptosis and scattered hepatocyte steatosis compared with controls. In NK-1R −/− BDL mice, there was a decrease in serum transaminases and bilirubin levels and the number of CK-19-positive cholangiocytes and enhanced biliary apoptosis compared with controls. In total liver samples, the expression of collagen 1α and α-SMA increased in BDL compared with normal mice and decreased in BDL NK-1R−/− compared with BDL mice. In cholangiocytes from BDL NK-1R −/− mice there was decreased PCNA expression and PKA phosphorylation. In vitro, SP increased cAMP levels, proliferation, and PKA phosphorylation of cholangiocytes. Targeting of NK-1R may be important in the inhibition of biliary hyperplasia in cholangiopathies.
Keywords: biliary epithelium, cAMP, innervation, mitosis, sensory innervation
human cholangiocytes are the target cells in a number of cholestatic liver diseases (i.e., cholangiopathies) including primary sclerosing cholangitis and primary biliary cirrhosis (5, 28), which are characterized by an abnormal balance between biliary growth and damage (5, 28). In animal models of cholestasis, changes in biliary growth and damage are achieved by a number of pathophysiological maneuvers including extrahepatic bile duct ligation (BDL) (4, 15, 23), acute administration of carbon tetrachloride (CCl4) (31), cholinergic (30) denervation, and ablation of sensory innervation by knockdown of α-calcitonin gene-related peptide (α-CGRP) (23). In these models, small and large cholangiocytes (lining small and large bile ducts, respectively) (2, 6, 21) show a different biological response, in terms of proliferation, survival, and secretory activity (3, 15, 21, 30, 31). Relevant to this study, in the cholestatic BDL rodent model large but not small cholangiocytes undergo mitosis leading to enhanced large bile duct mass (3, 15, 31). Two second messengers, d-myo-inositol 1,4,5-trisphosphate (IP3) and cyclic adenosine 3′,5′-monophosphate (cAMP), regulate the proliferative, apoptotic, and secretory functions of small and large cholangiocytes (3, 15–17, 23, 30, 31). Although the IP3/Ca2+-dependent signaling modulates the function of small cholangiocytes (16, 31), large cholangiocyte hyperplasia following BDL is regulated by the activation of cAMP-dependent PKA signaling (3, 15, 17, 23, 30).
Two afferent nerve pathways are present in the liver: the vagal and the spinal afferent nerve pathways that run through the dorsal root ganglion (45). Sensory nerves also display an efferent function, which is mediated by the release of sensory neuropeptides such α-CGRP from their peripheral terminals in tissues that they innervate modulating cellular functions (25). Substance P (SP), containing peptidergic nerves, is present in the spinal afferent nerve pathway. SP-positive innervation has been localized in the periportal regions of guinea pig and human liver (43). SP is a member of the tachykinin peptide family that is formed by six members: SP, neurokinin A, neurokinin B, neuropeptide K, neuropeptide γ, and hemokinin (1). SP and neurokinin A are present in the central nervous system and primary sensory afferent neurons innervating peripheral tissues and are released from sensory nerve endings both at the level of the spinal cord and in peripheral tissues (1). The tachykinin receptor family consists of three types of seven transmembrane G protein-coupled receptors: neurokinin-1, -2, and -3 receptors (NK-1R, NK-2R, and NK-3R). SP preferentially binds and signals via the NK-1R (1). Limited data exist regarding the role of sensory innervation in the regulation of biliary functions. Tachykinins are the main nonadrenergic and noncholinergic excitatory neurotransmitters in the common bile duct of guinea pigs (38). Sensory neuropathy has been associated in patients with primary biliary cirrhosis (13). Also, knockout of α-CGRP reduces cholangiocyte hyperplasia in cholestatic BDL mice by downregulation of cAMP signaling (23). No data exist regarding the role of SP in the regulation of biliary hyperplasia during cholestasis induced by BDL.
In many cell types, SP-induced activation of NK-1R (coupled to pertussis toxin-insensitive Gq/GII) activates phospholipase C and subsequent formation of IP3 and diacylglycerol mobilizing intracellular Ca2+ (26, 36). In other cells (27, 37), NK-1R also couple to 1) Gαs resulting in adenylyl cyclase activation (37) and cAMP formation and 2) Gαi that inhibits cAMP formation. On the basis of this background, we aim to demonstrate that SP and its receptor, NK-1R, regulate the proliferation of cAMP-dependent large cholangiocytes in the cholestatic BDL mouse model.
MATERIALS AND METHODS
Materials.
Reagents were purchased from Sigma Chemical (St. Louis, MO) unless otherwise indicated. The nuclear dye 4,6-diamidino-2-phenylindole (DAPI) was obtained from Molecular Probes, Eugene, OR. The NK-1R antibody against the rat COOH-terminal (393–407) peptide was purchased from Enzo Life Sciences International (Plymouth Meeting, PA). SP was purchased from Phoenix Pharmaceuticals (Burlingame, CA). The antibody against proliferating cell nuclear antigen (PCNA) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The mouse anti-cytokeratin-19 (CK-19) antibody (clone RCK105) was purchased from Caltag Laboratories (Burlingame, CA). The cAMP-dependent phospho-PKA catalytic subunit (Thr197) antibody (Cell Signaling, Boston, MA) detects endogenous levels of PKA catalytic subunit (-α, -β, and -γ) only when phosphorylated at Thr197. The cAMP-dependent PKA catalytic subunit-α antibody (Cell Signaling) detects endogenous levels of total PKA catalytic subunit-α. The antibodies for the rabbit anti-ERK1 (which detects p44 and p42) and goat anti-pERK (which detects phosphorylated p44 and p42) were purchased from Santa Cruz Biotechnology RIA kits for the determination of cAMP and IP3 levels were purchased from GE Healthcare (Arlington Heights, IL).
Animal models.
The majority of the studies were performed in wild-type (WT, +/+) and NK-1R knockout (NK-1R −/−) normal (sham-operated) and 1-wk BDL mice (Table 1); since we did not see any difference in biliary growth between normal and sham-operated mice (not shown), we used normal mice in our studies. Some of the experiments were also performed in normal and 1-wk BDL heterozygous mice (derived from the same breeding) to evaluate hepatocyte apoptosis and steatosis, lobular necrosis, the degree of inflammation and intrahepatic bile duct mass, and biliary apoptosis in vivo. BDL was performed as described (22, 23, 35). The NK-1R−/− mouse model (25–30 g, of the N5 generation) is bred at our Animal Facility; the original breeding pair was a gift from Dr. Norma Gerard (Harvard Medical School, Boston, MA) to Dr. Donald DiPette (coauthor in this article). This is a homozygous−/− model on a C57BL/6 background. The mouse model lacking the NK-1R gene was generated as described (12). Age-matched male C57BL/6 wild-type (WT) mice were purchased from Charles River (Wilmington, MA), whereas heterozygous mice were obtained from our breeding colony. All mice were maintained in a temperature-controlled environment (20–22°C) with 12:12-h light-dark cycles. Before each experimental procedure, animals were injected with pentobarbital sodium (50 mg/kg body wt ip). All animal experiments were performed according to a protocol approved by the Scott and White and Texas A&M Health Science Center Institutional Animal Care and Use Committee.
Table 1.
Evaluation of NK-1R expression, liver and body weight, liver-to-body weight ratio in the selected groups of animals
| Groups | NK-1R Expression | Liver Weight, g | Body Weight, g | Liver/body weight × 100, % |
|---|---|---|---|---|
| WT normal mice (n = 84) | (−/+) | 1.98 ± 0.05 | 26.3 ± 0.5 | 7.58 ± 0.16 |
| NK-1R−/− normal mice (n = 79) | (−) | 1.80 ± 0.05 | 28.3 ± 0.7 | 6.25 ± 0.18* |
| WT BDL mice (n = 41) | (++) | 1.94 ± 0.06 | 21.92 ± 0.3† | 8.84 ± 0.21† |
| NK-1R−/− BDL mice (n = 25) | (−) | 2.07 ± 0.09 | 25.9 ± 0.7‡ | 7.96 ± 0.27‡ |
Data are means ± SE.
ND, not detectable; NK-1R, neurokinin-1 receptor.
P < 0.05 vs. the corresponding value of normal wild-type (WT) mice.
P < 0.05 vs. the corresponding value of WT bile duct-ligated (BDL) mice.
Immortalized and freshly isolated large cholangiocytes.
Since BDL induces the proliferation of large but not small cholangiocytes (21, 23), the signaling studies were performed in freshly isolated large cholangiocytes from WT and NK-1R−/− BDL mice, and our immortalized line of large cholangiocytes, which display phenotypical and functional characteristics similar to those of freshly isolated large cholangiocytes (16, 21, 22). Virtually pure (by CK-19 immunohistochemistry) (16) isolated large cholangiocytes were obtained by centrifugal elutriation followed by immunoaffinity separation (16, 21) by using a monoclonal antibody, mouse IgG2a (provided by Dr. R. Faris, Providence, RI) against an antigen expressed by all intrahepatic cholangiocytes (21).
Evaluation of NK-1R protein expression.
Analysis of NK-1R expression was evaluated by semiquantitative immunohistochemistry in paraffin-embedded liver sections (4–5 μm; 6 slides per treatment group; for each slide 6 nonoverlapping fields were evaluated) (16, 21) from the selected groups of animals. Following immunohistochemistry, photographs of liver sections were taken by Leica Microsystems DM 4500 B Light Microscopy (Weltzlar, Germany) with a Jenoptik Prog Res C10 Plus Videocam (Jena, Germany). The quantitative expression of NK-1R was measured by immunoblots (16) in protein (10 μg) from whole cell lysate from large cholangiocytes from normal and BDL WT mice. The intensity of the bands was determined by scanning video densitometry using the phospho-imager Storm 860 and the ImageQuant TL software version 2003.02 (GE Healthcare, Little Chalfont, Buckinghamshire, UK). The presence of NK-1R was also evaluated by immunofluorescence (14, 16) in immortalized large cholangiocytes. Images were visualized via an Olympus IX-71 confocal microscope. For all immunoreactions, negative controls (with normal serum from the same species substituted for the primary antibody) were included.
Evaluation of serum levels of transaminases and bilirubin, lobular necrosis, inflammation, hepatocyte apoptosis and steatosis, and cholangiocyte proliferation and apoptosis.
In the in vivo studies, we measured 1) liver weight, body weight, and liver-to-body weight ratio (4) and 2) serum levels of transaminases, alanine aminotransferase and aspartate aminotransferase, and total bilirubin using a Dimension RxL Max Integrated Chemistry system (Dade Behring, Deerfield IL) by the Chemistry Department, Scott & White.
We evaluated 1) lobular necrosis and the degree of inflammation by hematoxylin and eosin (H&E) staining; 2) the percentage of apoptotic hepatocytes by terminal deoxynucleotidyl transferase biotin-dUTP nick-end labeling (TUNEL) kit (33) (Apoptag; Chemicon International); and 3) the number of large cholangiocytes (lining large bile ducts, > 15 μm diameter) (2) positive for PCNA (15) and CK-19 (39) or by TUNEL kit (33) in liver sections (4–5 μm; 6 slides per treatment group; for each slide 6 nonoverlapping fields were evaluated) from the selected groups of animals. The degree of hepatocyte steatosis was evaluated in frozen liver sections (4–5 μm thick) by both H&E staining and the oil red O staining kit (32) (IHC World, Woodstock, MD). All sections were examined in a coded fashion by two board certified pathologists by a BX-51 light microscope (Olympus, Tokyo, Japan) equipped with a camera. We also evaluated the damage of a number of tissues/organs in the experimental groups listed in Table 1.
Evaluation of gene expression for collagen 1α and α-smooth muscle actin in total liver samples.
We measured the expression of the messages for collagen 1α and α-smooth muscle actin (α-SMA) in RNA (0.5 μg) in total liver tissue by the RT2 Real-Time assay from SABiosciences (Frederick, MD) (16). A △△CT (delta delta of the threshold cycle) analysis was performed by using RNA from normal WT mice as the control sample. Data were expressed as relative mRNA levels ± SE of the selected gene-to-GAPDH ratio. The primers for collagen 1α and α-SMA (purchased from SABiosciences) were designed according to the NCBI GenBank Accession numbers: NM_007742 (for collagen 1α) (18) and NM_007392 (for α-SMA) (34).
Measurement of PCNA protein expression and phosphorylation of PKA in purified large cholangiocytes.
In protein (10 μg) from whole cell lysate from spleen (positive control) and purified large cholangiocytes from WT and NK-1R−/− BDL mice, we evaluated by immunoblots (16, 21) cholangiocyte proliferation by PCNA protein expression (measured as ratio to β-actin protein expression) (16), and the phosphorylation of cAMP-dependent PKA (expressed as ratio to protein expression of the corresponding total protein), a molecule playing an important role in cAMP-dependent regulation of large cholangiocyte proliferation (8, 15, 17). The intensity of the bands was determined by scanning video densitometry using the phospho-imager Storm 860 and the ImageQuant TL software version 2003.02 (GE Healthcare).
In vitro effect of SP on cAMP and IP3 levels, proliferation, and phosphorylation of PKA and ERK1/2 of immortalized large cholangiocytes.
For the measurement of cAMP or IP3 levels, large immortalized cholangiocytes were treated at room temperature for 5 (cAMP) (3, 15) or 10 (IP3) (16) min with 0.2% bovine serum albumin (BSA) or SP (10−9 M) before evaluation of the levels of these two molecules by RIA (2, 3, 15, 16). In other experiments, large cholangiocyte lines were treated at 37°C for 24, 48, and 72 h with 0.2% BSA or SP (10−6 to 10−11 M) before evaluation of cell proliferation by CellTiter 96 Cell Proliferation Assay (Promega, Madison, WI) (16). In separate sets of experiments, large cholangiocytes were treated at 37°C for 48 h with 0.2% BSA (basal) or SP (10−9 M) for 48 h in the absence or presence of preincubation with spantide (a specific NK-1R inhibitor, 10−6 M) (24), BAPTA/AM (5 μM) (16), or H89 (a PKA inhibitor, 30 μM) (22) before evaluation of proliferation by CellTiter 96 Cell Proliferation Assay. Absorbance was measured at 490 nm on a microplate spectrophotometer (Molecular Devices, Sunnyvale, CA). Data were expressed as the fold change of treated cells compared with BSA-treated cells. Also, large cholangiocytes were stimulated with 0.2% BSA or SP (10−9 M for 1, 2, and 6 h) before evaluation by immunoblots (19) of the proliferation (by PCNA) and the phosphorylation of PKA. The intensity of the bands was determined by scanning video densitometry (see above). Large cholangiocytes were also stimulated with 0.2% BSA or SP (10−9 M for 1, 2, 3, 5, 7, 10, 20, 30, 60, and 90 min, 1, 2, and 6 h) before evaluation of ERK1/2 phosphorylation by immunoblots (19).
Statistical analysis.
All data are expressed as means ± SE. Differences between groups were analyzed by the Student's unpaired t-test when two groups were analyzed and by ANOVA when more than two groups were analyzed, followed by an appropriate post hoc test.
RESULTS
Expression of NK-1R in liver sections and isolated and immortalized large cholangiocytes.
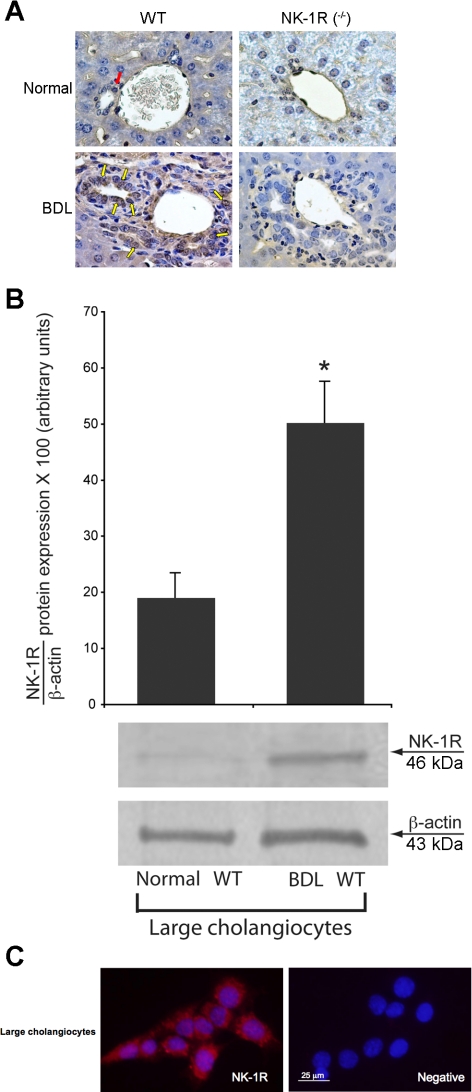
By semiquantitative immunohistochemistry, the expression of NK-1R was low in bile ducts from WT (red arrow) normal mice but increased in bile ducts from WT BDL mice (Fig. 1A; yellow arrows and Table 1). NK-1R was absent in bile ducts from normal and BDL NK-1R−/− mice (Fig. 1A and Table 1). These findings were confirmed by immunoblotting: the expression of NK-1R increased in large cholangiocytes from BDL WT mice compared with large cholangiocytes from normal WT mice (Fig. 1B); NK-1R was also expressed by small bile ducts in liver sections (not shown). By immunofluorescence, specific immunoreactivity for NK-1R in representative fields of large murine cholangiocyte lines (44) is shown in red; cell nuclei were stained with DAPI (blue) (Figs. 1C).
Fig. 1.
A: expression of NK-1 receptor (NK-1R) was low in bile ducts from normal wild-type (WT; red arrow) mice but increased in bile ducts from bile duct-ligated (BDL) WT mice (yellow arrows; see Table 1). NK-1R was absent in intrahepatic bile ducts from normal and BDL NK-1R knockout (NK-1R−/−) mice. Original magnification ×40. B: protein expression of NK-1R increased in large cholangiocytes from BDL WT mice compared with large cholangiocytes from normal WT mice. Data are means ± SE of 6 immunoblots derived from protein obtained from cumulative preparations of cholangiocytes. *P < 0.05 vs. the corresponding value of BDL large cholangiocytes. C: specific immunoreactivity for NK-1R in representative fields of large cholangiocytes is shown in red; cell nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI; blue). Bar size = 25 μm.
Evaluation of serum levels of transaminases and bilirubin, lobular necrosis, inflammation, hepatocyte apoptosis and steatosis, and cholangiocyte proliferation and apoptosis.
Surprisingly, body weight was significantly lower in WT BDL mice compared with NK-1R−/− BDL mice (Table 1). In both normal and BDL NK-1R−/− mice there was a significant decrease in liver-to-body weight ratio (an index of liver growth including cholangiocytes) (4) compared with the corresponding WT mice (Table 1). In agreement with previous studies in rodents (4), the serum levels of transaminases (alanine aminotransferase and aspartate aminotransferase) and total bilirubin were higher in WT BDL mice compared with normal WT mice and decreased in NK-1R−/− BDL mice compared with WT BDL mice (Table 2). No difference in the serum levels of total bilirubin was observed between WT normal mice and NK-1R−/− normal mice (Table 2). Surprisingly, we observed a significant increase in the serum levels of transaminases in normal NK-1R−/− mice compared with WT normal mice (Table 2).
Table 2.
Evaluation of serum levels of transaminases and bilirubin
| Groups | Alanine Aminotransferase, units/l | Aspartate Aminotransferase, units/l | Total Bilirubin, mg/l |
|---|---|---|---|
| WT normal mice | 20.2 ± 2.4 (n = 8) | 55.2 ± 3.9 (n = 8) | <0.1 (n = 8) |
| NK-1R−/− normal mice | 123.8 ± 20.7 (n = 11) | 229.3 ± 23.2 (n = 12) | <0.1 (n = 16) |
| WT BDL mice | 687.1 ± 119.1* (n = 6) | 1,134.6 ± 212.1* (n = 5) | 13.58 ± 1.74* (n = 7) |
| NK-1R−/− BDL mice | 389 ± 35† (n = 6) | 708.6 ± 50.6† (n = 5) | 9.22 ± 1.1† (n = 7) |
Data are means ± SE of 7 evaluations.
P < 0.05 vs. the corresponding value of normal WT mice.
P < 0.05 vs. the corresponding value of WT mice with BDL for 7 days.
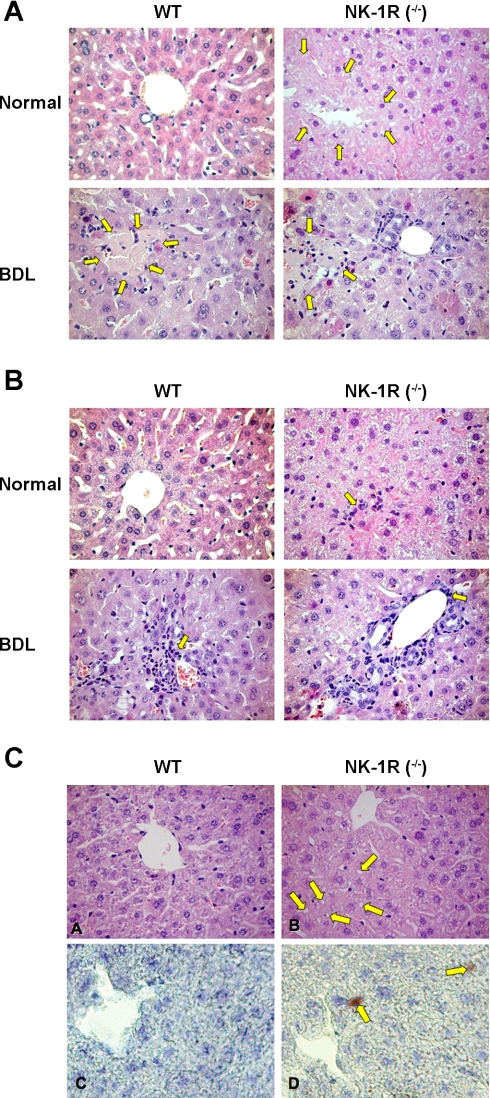
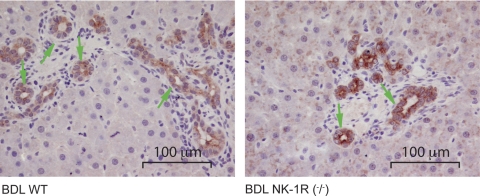
In liver sections from normal NK-1R−/− mice, we observe higher hepatocyte apoptosis (Table 3), some necrotic areas (yellow arrows, Fig. 2A) and inflammatory infiltrates (yellow arrow, Fig. 2B) compared with normal WT mice. There was a decrease in necrotic areas in NK-1R−/− BDL mice compared with BDL WT mice (see yellow arrows) (Fig. 2A). No marked difference in inflammatory infiltrate was observed between WT and NK-1R−/− BDL mice (see yellow arrow) (Fig. 2B). In NK-1R−/− normal mice, centrolobular liver parenchyma shows round-shaped areas (Fig. 2C) and red scattered spots by oil red O staining evocative of steatosis (Fig. 2C), whereas WT (Fig. 2C) and heterozygous (not shown) normal mice samples display normal liver morphology. All BDL liver sections do not present round-shaped areas evocative of steatosis (not shown). No significant differences in these parameters were seen between WT and NK-1R−/− normal mice (not shown). These histomorphological changes (Fig. 2, A–C and Table 3) likely explain the significant increase in the serum levels of transaminases observed in normal NK-1R−/− mice compared with WT normal mice. The number of PCNA-positive cholangiocytes was low and similar in both WT (0.4 ± 0.2) and NK-1R−/− (0.2 ± 0.2) normal mice. In NK-1R−/− BDL mice, there was a decrease in the number of PCNA-positive large cholangiocytes (17.4 ± 1.3) compared with WT BDL mice (12.0 ± 1.3). The number of CK-19-positive large cholangiocytes was similar among WT, heterozygous, and NK-1R−/− normal mice and increased following BDL in both WT and heterozygous BDL mice (Fig. 3 and Table 4). In NK-1R−/− BDL mice, there was a decrease in the number of CK-19-positive large cholangiocytes compared with WT BDL mice (Fig. 3 and Table 4). In heterozygous BDL mice, the number of CK-19-positive cholangiocytes was lower than that of BDL WT mice but higher than NK-1R−/− BDL mice (Table 4). In NK-1R−/− and heterozygous BDL mice, there was a concomitant increase in the number of TUNEL-positive large cholangiocytes compared with the degree of cholangiocyte apoptosis observed in the corresponding WT BDL mice (Table 4). No difference in cholangiocyte apoptosis was observed between WT and heterozygous normal mice and NK-1R−/− normal mice (Table 4). No significant gross postmortem or pathological changes were detected in the body cavities, integumentary, alimentary, respiratory, circulatory, nervous, urogenital, hematopoietic, and musculoskeletal systems of normal WT mice and NK-1R−/− normal mice (not shown).
Table 3.
Evaluation of the percentage of hepatocytes positive by TUNEL
| Groups | Percentage of Hepatocytes Positive by TUNEL |
|---|---|
| WT normal mice | 4.2 ± 0.44 |
| NK-1R−/− normal mice | 4.9 ± 0.48* |
| WT 7 BDL mice | 31.8 ± 2.3* |
| NK-1R−/− BDL mice | 34.0 ± 2.6 |
Data are means ± SE.
TUNEL, terminal deoxynucleotidyltransferase biotin-dUTP nick-end labeling.
P < 0.05 vs. the corresponding value of normal WT mice.
Fig. 2.
Evaluation of necrosis (A), inflammatory infiltrates (by H&E staining) (B), and steatosis [by H&E staining (CA and CB) and oil red O staining (CC and CD)] in liver sections from the experimental groups of Table 1. In sections from normal NK-1R−/− mice, we observed some necrotic areas (yellow arrows; A) and inflammatory infiltrates (yellow arrow B) compared with normal WT mice. There was a decrease in necrotic areas in NK-1R−/− BDL mice compared with BDL WT mice. No marked difference in inflammatory infiltrate was observed between WT and NK-1R−/− BDL mice. By hematoxylin and eosin (H&E; CA and CB) and oil red O (CC and CD) staining, in NK-1R−/− normal mice, centrolobular liver parenchyma shows round-shaped areas (yellow arrows) evocative of steatosis (CB) and scattered red areas highlighted by oil red O stain method, a specific stain for neutral lipids (yellow arrows) (CD). WT normal mice samples show normal liver morphology without oil red O stain (CA and CC). (Light microscopy: CA and CB, H&E, original magnification ×20; CC and CD, oil red O staining in frozen liver sections, original magnification ×40).
Fig. 3.
Immunohistochemistry for CK-19 in liver sections from WT and NK-1R−/− BDL mice. There was a decrease in the number of large CK-19-positive cholangiocytes in liver sections from NK-1R−/− BDL mice compared with 7-day BDL WT mice (for semiquantitative data, see Table 4). Original magnification ×40.
Table 4.
Evaluation of the number of CK-19 or TUNEL-positive large cholangiocytes
| Groups | Number of CK-19-Positive Cholangiocytes | Number of Cholangiocytes Positive by TUNEL |
|---|---|---|
| WT normal mice | 17.2 ± 1.0 | ND |
| Heterozygous normal mice | 17.3 ± 1.1 | ND |
| NK-1R−/− normal mice | 17.1 ± 1.8 | 0.3 ± 0.1 |
| WT 7 BDL mice | 78.8 ± 4.3* | 1.2 ± 0.2 |
| Heterozygous BDL mice | 65.1 ± 2.0* | 4.2 ± 0.2 |
| NK-1R−/− BDL mice | 53.0 ± 2.6† | 6.6 ± 0.4† |
Data are means ± SE.
P < 0.05 vs. the corresponding value of normal WT mice.
P < 0.05 vs. the corresponding value of WT mice with BDL for 7 days.
mRNA expression for collagen 1α and α-SMA.
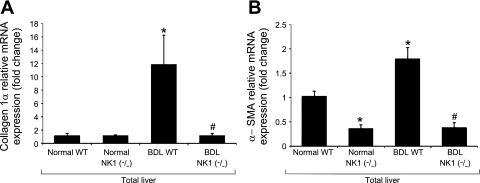
No changes in the mRNA and protein expression of collagen 1α were observed in total liver samples from WT and NK-1R−/− normal mice (Fig. 4A). The expression of α-SMA decreased in total liver samples from normal NK-1R−/− mice compared with normal WT mice (Fig. 4B). The expression of collagen 1α and α-SMA increased in total liver samples from BDL WT mice compared with normal WT mice (Fig. 4, A and B). There was a decrease expression of collagen 1α and α-SMA mRNA expression in total liver samples from BDL NK-1R−/− mice compared with total liver samples from BDL WT mice (Fig. 4, A and B).
Fig. 4.
Evaluation of the mRNA and protein expression of collagen 1α (A) and α-smooth muscle actin (α-SMA; B) in total liver tissue from WT and NK-1R−/− normal and BDL mice. No changes in the expression of collagen 1α were observed in total liver samples from normal WT and normal NK-1R−/− mice. The expression of α-SMA decreased in total liver samples from normal NK-1R−/− mice compared with normal WT mice. The expression of collagen 1α and α-SMA increased in total liver samples from BDL WT mice compared with normal WT mice. There was a decrease expression of collagen 1α and α-SMA mRNA expression in total liver samples from BDL NK-1R−/− mice compared with total liver samples from BDL WT mice. *P < 0.05 vs. the corresponding value of normal WT mice. #P < 0.05 vs. the corresponding value of WT BDL mice.
Measurement of PCNA protein expression, and phosphorylation of cAMP-dependent PKA in isolated large cholangiocytes.
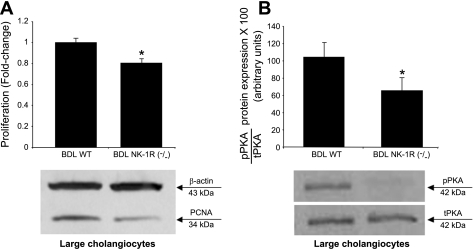
There was a decrease in PCNA expression in large cholangiocytes from NK-1R−/− BDL mice compared with large cholangiocytes from WT BDL mice (Fig. 5A). In large cholangiocytes from NK-1R−/− BDL mice there was a decrease in the phosphorylation of cAMP-dependent PKA compared with large cholangiocytes from WT BDL mice (Fig. 5B).
Fig. 5.
Evaluation of PCNA expression and cAMP-dependent phosphorylation of PKA in large cholangiocytes from WT and NK-1R−/− BDL mice. There was decreased PCNA expression (A) and phosphorylation of PKA (B) in large cholangiocytes from NK-1R−/− BDL mice compared with large cholangiocytes from WT BDL mice. Data are means ± SE of 6 immunoblots derived from protein obtained from cumulative preparations of cholangiocytes. pPKA, phosphorylated PKA; tPKA, total PKA. *P < 0.05 vs. the corresponding value of BDL large cholangiocytes. *P < 0.05 vs. the corresponding value of BDL large cholangiocytes.
In vitro effect of SP on cAMP and IP3 levels, cholangiocyte proliferation, and phosphorylation of PKA and ERK1/2 of large cholangiocytes.
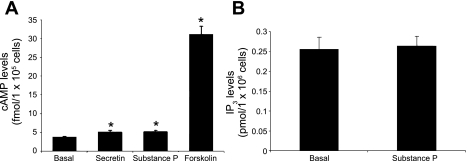
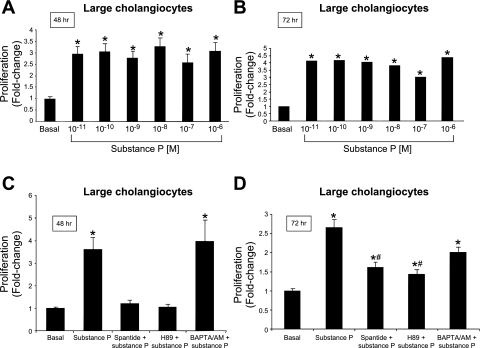
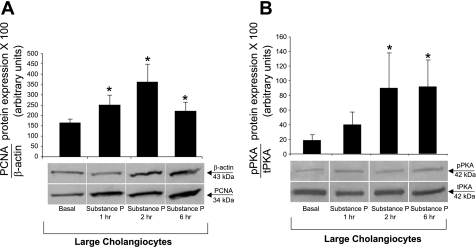
A marked increase in cAMP levels was observed with forskolin (an activator of adenylyl cyclase) (17), whereas secretin and SP induced a modest yet significant increase in cAMP levels in large cholangiocytes (Fig. 6A). The increase in cAMP levels observed with secretin in immortalized large cholangiocytes was similar to that observed in our previous studies (17). SP did not increase IP3 levels of large cholangiocytes (Fig. 6B). By MTS assays, SP induced a similar and sustained (both at 48 and 72 h) increases in the proliferation of large cholangiocytes compared with controls (Fig. 7, A and B); no increase was seen at 24 h of treatment with SP (not shown). SP stimulation of large cholangiocyte growth was blocked by preincubation with spantide and H89 at 48 h and partly at 72 h (Fig. 7 C and D); BAPTA/AM did not block substance stimulation of large cholangiocyte proliferation (Fig. 7 C and D). Short-term treatment with SP increased PCNA protein expression and phosphorylation of PKA (but not ERK1/2, not shown) compared with controls (Fig. 8, A and B).
Fig. 6.
In vitro effect of forskolin (10−4 M), secretin (100 nM) and substance P (10 μM) on cAMP (A) and substance P (10 μM) on d-myo-inositol 1,4,5-trisphosphate (IP3) levels (B) of large cholangiocytes. A: a massive increase in cAMP levels was observed with forskolin, whereas secretin and substance P induced a modest albeit significant increase in cAMP levels in large cholangiocytes. B: substance P did not increase IP3 levels of large cholangiocytes. Substance P increased cAMP (A) but not IP3 (B) levels of large cholangiocytes. Data are means ± SE of 6 values obtained from cumulative preparations of cholangiocytes. *P < 0.05 vs. the corresponding basal value of large cholangiocytes treated with 0.2% BSA (basal).
Fig. 7.
A and B: by MTS assays, substance P induced a similar (at all the doses used) and sustained (24 and 72 h) increase in the proliferation of large cholangiocytes compared with BSA-treated cells. Data are means ± SE of 6 experiments. *P < 0.05 vs. the corresponding basal value of large cholangiocytes. C and D: effect of 0.2% BSA (basal) or substance P (10−9 M) for 48 h in the absence or presence of preincubation with spantide (specific NK-1R inhibitor), H89 (PKA inhibitor), or BAPTA/AM (intracellular Ca2+ chelator). Substance P stimulation of large cholangiocyte growth was blocked by preincubation with spantide and H89 but not BAPTA/AM. Data are means ± SE of 6 experiments. *P < 0.05 vs. the corresponding basal value of large cholangiocytes.
Fig. 8.
Effect of 0.2% BSA (basal) or substance P (10−9 M for 1, 2, and 6 h) on the proliferation (by PCNA) and phosphorylation of PKA of large cholangiocytes. Substance P increased PCNA protein expression (A) and the phosphorylation of PKA (B) compared with their corresponding basal values. Data are means ± SE of 10 immunoblots for PCNA (A) and 7 immunoblots for PKA derived from protein (10 μg) (B) obtained from cumulative preparations of cholangiocytes. *P < 0.05 vs. the corresponding value of large cholangiocytes treated with BSA.
DISCUSSION
Previous studies have demonstrated that 1) circulating levels of the sensory neuropeptides CGRP, SP, and adrenomedullin are elevated in humans, and rodent models of cirrhosis and biliary hyperplasia (11, 23, 29, 40); and 2) SP serum levels are elevated in cholestatic patients and BDL rats (42). Our study provides the first evidence regarding the role of the SP→NK-1R axis in sustaining the proliferation of large cholangiocytes by activation of cAMP signaling. We found an increase in the serum levels of transaminases in normal NK-1R−/− mice compared with WT normal mice. The serum levels of transaminases and total bilirubin were decreased in NK-1R−/− BDL mice compared with WT BDL mice. We demonstrated the presence of NK-1R in large cholangiocytes that was higher in BDL compared with normal rats. Knockdown of the NK-1R gene in BDL mice induces a decrease (∼40%) in the number of large cholangiocytes (associated with enhanced biliary apoptosis) compared with BDL WT mice. There was decreased PCNA protein expression and phosphorylation of PKA in large cholangiocytes from NK-1R−/− BDL mice compared with controls. The expression of collagen 1α and α-SMA increased in total liver samples from BDL WT mice compared with normal WT mice and decreased in BDL NK-1R−/− mice compared with total liver samples from BDL WT mice. In vitro, SP increased cAMP levels, enhanced the phosphorylation of PKA but not ERK1/2, and induced a sustained increase in the proliferation of large cholangiocytes. Pharmacological targeting of NK-1R may be important in the inhibition of biliary proliferation in cholestatic liver disorders.
In support for the presence of NK-1R in liver, previous studies have demonstrated the presence of NK-1R in hepatocytes (9, 10). Although both small (not shown) and large cholangiocytes express NK-1R, we evaluated the role of the SP→NK-1R axis on the regulation of large cholangiocyte growth since large, but not small, cholangiocytes proliferate in response to BDL (3, 15, 31). Previous studies have emphasized the importance of cAMP/PKA/ERK1/2 signaling in the regulation of large biliary functions (8). For example, the stimulation of adenylyl cyclase by forskolin stimulates large cholangiocyte proliferation (17). Maintenance of cholangiocyte cAMP levels by administration of forskolin prevents the functional damage of bile ducts induced by vagotomy (30). Since 1) small cholangiocytes (whose function is regulated by IP3/Ca2+) (16, 20) express NK-1R and 2) SP exerts its cellular function by the activation of both cAMP and IP3/Ca2+ signaling (26, 36), studies aimed to evaluate the role of SR in small cholangiocyte functions are necessary. Also, further experiments aimed to evaluate the effects of SP on the phosphorylation of other MAPK isoforms such as JNK and p38 in large cholangiocytes are underway and part of another project.
In our NK-1R−/− BDL model, the extent of the reduction (∼40%) of biliary mass is consistent with the concept that cholangiocyte proliferation is coordinately modulated by a number of stimulatory/inhibitory neuroendocrine factors (7). A similar reduction in biliary was observed in α-CGRP BDL−/− mice (23) since other sensory neuropeptides such as β-CGRP stimulate cholangiocyte proliferation during cholestasis. Also, knockout of the secretin receptor gene induces a similar decrease in biliary mass in mice with BDL (22). The reduction of the serum levels of transaminases and bilirubin observed in NK-1R−/− BDL mice further supports the concept that blockage of the NK-1R induced signaling is important in the reduction of liver damage and biliary hyperplasia. The increase in hepatocyte apoptosis and steatosis likely explains the significant increase in the serum levels of transaminases observed in normal NK-1R−/− mice compared with WT normal mice. This finding also suggests that SP signaling may play a role in hepatic metabolism and that lack of the NK-1R may trigger hepatocyte steatosis (41) that was we speculate was resolved during cholestasis induced by BDL. Compared with WT animals, in NK-1R−/− mice a significant reduction in the mRNA expression of collagen 1α and α-SMA after BDL. These data reflect the reduction of the expansion of the biliary tree in NK-1R−/− mice, with a consequent reduction of biliary fibrogenesis, which is mostly likely due to reduced hepatic stellate cell activity (46). In addition to alterations in hepatic stellate cell activation, we cannot rule out that an ancillary part of those differences may be accounted to reduced collagen deposition by hepatocytes, by cholangiocytes, and, in particular, by inflammatory cells, since SP is a one of the mediators of neurogenic inflammation and NK1-R antagonist have been shown to protect mice from cytokine, CD95 and TNF-α mediated liver injury (9, 10).
The biological and pathophysiological significance of our findings is supported by a number of studies. For example, the neurokinin-1 receptor antagonists CP-96,345 and L-733,060 protect mice from cytokine-mediated liver injury, most likely by inhibiting SP effects (10). NK-1R antagonists have been shown to protect mice from CD95- and TNF-α-mediated liver damage (9). As a direct outgrowth of the present study, since a number of neuroendocrine factors regulate biliary functions by autocrine mechanisms, we propose to evaluate the possible autocrine role of SP in the growth and damage of the biliary epithelium.
GRANTS
This work was supported by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology to Dr. Alpini from Scott & White Hospital, a Veterans Affairs (VA) Research Scholar Award, a VA Merit Award, and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant DK062975 to Dr. Alpini. Portions of this study were supported by 1) by a Scott & White grant award (no. 060483) from Scott & White and the NIDDK RO1 Grant (DK081442) to Dr. Glaser and 2) a grant from Health and Labor Sciences Research Grants for the Research on Measures for Intractable Diseases (from the Ministry of Health, Labor and Welfare of Japan) and from Grant-in Aid for-Scientific Research C (21590822) from Japan Society for the Promotion of Science to Y. Ueno.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We acknowledge Bryan Moss, Scott & White, Medical Illustration Department for assistance with figures, and the Scott & White Hospital animal facility staff assistance with animal surgical models. We thank Anna Webb and the Texas A&M Health Science Center Microscopy Imaging Center for assistance with confocal microscopy.
REFERENCES
- 1. Almeida TA, Rojo J, Nieto PM, Pinto FM, Hernandez M, Martin JD, Candenas ML. Tachykinins and tachykinin receptors: structure and activity relationships. Curr Med Chem 11: 2045–2081, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Alpini G, Glaser S, Robertson W, Rodgers RE, Phinizy JL, Lasater J, LeSage G. Large but not small intrahepatic bile ducts are involved in secretin-regulated ductal bile secretion. Am J Physiol Gastrointest Liver Physiol 272: G1064–G1074, 1997 [DOI] [PubMed] [Google Scholar]
- 3. Alpini G, Glaser S, Ueno Y, Pham L, Podila PV, Caligiuri A, LeSage G, LaRusso NF. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am J Physiol Gastrointest Liver Physiol 274: G767–G775, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Alpini G, Lenzi R, Sarkozi L, Tavoloni N. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest 81: 569–578, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alpini G, Prall RT, LaRusso NF. The pathobiology of biliary epithelia. In: The Liver: Biology & Pathobiology ( 4th ed.), Arias IM, Boyer JL, Chisari FV, Fausto N, Jakoby W, Schachter D, Shafritz DA. Philadelphia, PA: Lippincott Williams & Wilkins, 2001, p.421–435 [Google Scholar]
- 6. Alpini G, Roberts S, Kuntz SM, Ueno Y, Gubba S, Podila PV, LeSage G, LaRusso NF. Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology 110: 1636–1643, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Alvaro D, Mancino MG, Glaser S, Gaudio E, Marzioni M, Francis H, Alpini G. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology 132: 415–431, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Alvaro D, Onori P, Metalli VD, Svegliati-Baroni G, Folli F, Franchitto A, Alpini G, Mancino MG, Attili AF, Gaudio E. Intracellular pathways mediating estrogen-induced cholangiocyte proliferation in the rat. Hepatology 36: 297–304, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Bang R, Biburger M, Neuhuber WL, Tiegs G. Neurokinin-1 receptor antagonists protect mice from CD95- and tumor necrosis factor-alpha-mediated apoptotic liver damage. J Pharmacol Exp Ther 308: 1174–1180, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Bang R, Sass G, Kiemer AK, Vollmar AM, Neuhuber WL, Tiegs G. Neurokinin-1 receptor antagonists CP-96,345 and L-733,060 protect mice from cytokine-mediated liver injury. J Pharmacol Exp Ther 305: 31–39, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Bendtsen F, Schifter S, Henriksen JH. Increased circulating calcitonin gene-related peptide (CGRP) in cirrhosis. J Hepatol 12: 118–123, 1991 [DOI] [PubMed] [Google Scholar]
- 12. Bozic CR, Lu B, Hopken UE, Gerard C, Gerard NP. Neurogenic amplification of immune complex inflammation. Science 273: 1722–1725, 1996 [DOI] [PubMed] [Google Scholar]
- 13. Charron L, Peyronnard JM, Marchand L. Sensory neuropathy associated with primary biliary cirrhosis. Histologic and morphometric studies. Arch Neurol 37: 84–87, 1980 [DOI] [PubMed] [Google Scholar]
- 14. DeMorrow S, Glaser S, Francis H, Venter J, Vaculin B, Vaculin S, Alpini G. Opposing actions of endocannabinoids on cholangiocarcinoma growth: recruitment of Fas and Fas ligand to lipid rafts. J Biol Chem 282: 13098–13113, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Francis H, Franchitto A, Ueno Y, Glaser S, DeMorrow S, Venter J, Gaudio E, Alvaro D, Fava G, Marzioni M, Vaculin B, Alpini G. H3 histamine receptor agonist inhibits biliary growth of BDL rats by downregulation of the cAMP-dependent PKA/ERK1/2/ELK-1 pathway. Lab Invest 87: 473–487, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Francis H, Glaser S, DeMorrow S, Gaudio E, Ueno Y, Venter J, Dostal D, Onori P, Franchitto A, Marzioni M, Vaculin S, Vaculin B, Katki K, Stutes M, Savage J, Alpini G. Small mouse cholangiocytes proliferate in response to H1 histamine receptor stimulation by activation of the IP3/CaMK I/CREB pathway. Am J Physiol Cell Physiol 295: C499–C513, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Francis H, Glaser S, Ueno Y, LeSage G, Marucci L, Benedetti A, Taffetani S, Marzioni M, Alvaro D, Venter J, Reichenbach R, Fava G, Phinizy JL, Alpini G. cAMP stimulates the secretory and proliferative capacity of the rat intrahepatic biliary epithelium through changes in the PKA/Src/MEK/ERK1/2 pathway. J Hepatol 41: 528–537, 2004 [DOI] [PubMed] [Google Scholar]
- 18. French BT, Lee WH, Maul GG. Nucleotide sequence of a cDNA clone for mouse pro alpha 1(I) collagen protein. Gene 39: 311–312, 1985 [DOI] [PubMed] [Google Scholar]
- 19. Glaser S, Benedetti A, Marucci L, Alvaro D, Baiocchi L, Kanno N, Caligiuri A, Phinizy JL, Chowdhury U, Papa E, LeSage G, Alpini G. Gastrin inhibits cholangiocyte growth in bile duct-ligated rats by interaction with cholecystokinin-B/gastrin receptors via d-myo-inositol 1,4,5-triphosphate-, Ca(2+)-, and protein kinase C alpha-dependent mechanisms. Hepatology 32: 17–25, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Glaser S, DeMorrow S, Wise C, Francis H, Venter J, Gaudio E, Kopriva S, Franchitto A, Ueno Y, Onori P, Carpino G, Alpini G. The α1-adrenergic receptor agonist, phenylephrine, stimulates the proliferation of small mouse cholangiocytes by activation of the Ca2+-dependent transcription factors, NFATc1 and NFATc4. Hepatology 50: 2009 [Google Scholar]
- 21. Glaser S, Gaudio E, Rao A, Pierce LM, Onori P, Franchitto A, Francis HL, Dostal DE, Venter JK, DeMorrow S, Mancinelli R, Carpino G, Alvaro D, Kopriva SE, Savage JM, Alpini G. Morphological and functional heterogeneity of the mouse intrahepatic biliary epithelium. Lab Invest 89: 456–469, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Glaser S, Lam IP, Franchitto A, Gaudio E, Onori P, Chow BK, Wise C, Kopriva S, Venter J, White M, Ueno Y, Dostal D, Carpino G, Mancinelli R, Butler W, Chiasson V, DeMorrow S, Francis H, Alpini G. Knockout of secretin receptor reduces large cholangiocyte hyperplasia in mice with extrahepatic cholestasis induced by bile duct ligation. Hepatology 52: 204–214, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Glaser S, Ueno Y, DeMorrow S, Chiasson VL, Katki KA, Venter J, Francis HL, Dickerson IM, DiPette DJ, Supowit SC, Alpini G. Knockout of alpha-calcitonin gene-related peptide reduces cholangiocyte proliferation in bile duct ligated mice. Lab Invest 87: 914–926, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Hakanson R, Wang ZY, Folkers K. Comparison of spantide II and CP-96,345 for blockade of tachykinin-evoked contractions of smooth muscle. Biochem Biophys Res Commun 178: 297–301, 1991 [DOI] [PubMed] [Google Scholar]
- 25. Holzer P. Efferent-like roles of afferent neurons in the gut: blood flow regulation and tissue protection. Auton Neurosci 125: 70–75, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khawaja AM, Rogers DF. Tachykinins: receptor to effector. Int J Biochem Cell Biol 28: 721–738, 1996 [DOI] [PubMed] [Google Scholar]
- 27. Laniyonu A, Sliwinski-Lis E, Fleming N. Different tachykinin receptor subtypes are coupled to the phosphoinositide or cyclic AMP signal transduction pathways in rat submandibular cells. FEBS Lett 240: 186–190, 1988 [DOI] [PubMed] [Google Scholar]
- 28. Lazaridis KN, Strazzabosco M, LaRusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology 127: 1565–1577, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Lee FY, Lin HC, Tsai YT, Chang FY, Lu RH, Hou MC, Li CP, Chu CJ, Wang SS, Lee SD. Plasma substance P levels in patients with liver cirrhosis: relationship to systemic and portal hemodynamics. Am J Gastroenterol 92: 2080–2084, 1997 [PubMed] [Google Scholar]
- 30. LeSage G, Alvaro D, Benedetti A, Glaser S, Marucci L, Baiocchi L, Eisel W, Caligiuri A, Phinizy JL, Rodgers R, Francis H, Alpini G. Cholinergic system modulates growth, apoptosis, and secretion of cholangiocytes from bile duct-ligated rats. Gastroenterology 117: 191–199, 1999 [DOI] [PubMed] [Google Scholar]
- 31. LeSage G, Glaser S, Marucci L, Benedetti A, Phinizy JL, Rodgers R, Caligiuri A, Papa E, Tretjak Z, Jezequel AM, Holcomb LA, Alpini G. Acute carbon tetrachloride feeding induces damage of large but not small cholangiocytes from BDL rat liver. Am J Physiol Gastrointest Liver Physiol 276: G1289–G1301, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Lillie RD, Ashburn LL. Supersaturated solutions of fat stains in dilute isopropanol for demonstration of acute fatty degeneration not shown by Herxheimer's technique. Arch Pathol 36: 432–440, 1943 [Google Scholar]
- 33. Mancinelli R, Onori P, Gaudio E, DeMorrow S, Franchitto A, Francis H, Glaser S, Carpino G, Venter J, Alvaro D, Kopriva S, White M, Kossie A, Savage J, Alpini G. Follicle-stimulating hormone increases cholangiocyte proliferation by an autocrine mechanism via cAMP-dependent phosphorylation of ERK1/2 and Elk-1. Am J Physiol Gastrointest Liver Physiol 297: G11–G26, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34. Min BH, Foster DN, Strauch AR. The 5′-flanking region of the mouse vascular smooth muscle alpha-actin gene contains evolutionarily conserved sequence motifs within a functional promoter. J Biol Chem 265: 16667–16675, 1990 [PubMed] [Google Scholar]
- 35. Miyoshi H, Rust C, Roberts PJ, Burgart LJ, Gores GJ. Hepatocyte apoptosis after bile duct ligation in the mouse involves Fas. Gastroenterology 117: 669–677, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Mizuta K, Gallos G, Zhu D, Mizuta F, Goubaeva F, Xu D, Panettieri RA, Jr, Yang J, Emala CW., Sr Expression and coupling of neurokinin receptor subtypes to inositol phosphate and calcium signaling pathways in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 294: L523–L534, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Narumi S, Maki Y. Stimulatory effects of substance P on neurite extension and cyclic AMP levels in cultured neuroblastoma cells. J Neurochem 30: 1321–1326, 1978 [DOI] [PubMed] [Google Scholar]
- 38. Patacchini R, De Giorgio R, Bartho L, Barbara G, Corinaldesi R, Maggi CA. Evidence that tachykinins are the main NANC excitatory neurotransmitters in the guinea-pig common bile duct. Br J Pharmacol 124: 1703–1711, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Taffetani S, Glaser S, Francis H, DeMorrow S, Ueno Y, Alvaro D, Marucci L, Marzioni M, Fava G, Venter J, Vaculin S, Vaculin B, Lam IP, Lee VH, Gaudio E, Carpino G, Benedetti A, Alpini G. Prolactin stimulates the proliferation of normal female cholangiocytes by differential regulation of Ca2+-dependent PKC isoforms. BMC Physiol 7: 6, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tahan V, Avsar E, Karaca C, Uslu E, Eren F, Aydin S, Uzun H, Hamzaoglu HO, Besisik F, Kalayci C, Okten A, Tozun N. Adrenomedullin in cirrhotic and non-cirrhotic portal hypertension. World J Gastroenterol 9: 2325–2327, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Trevenzoli IH, Rodrigues AL, Oliveira E, Thole AA, Carvalho L, Figueiredo MS, Toste FP, Neto JF, Passos MC, Lisboa PC, Moura EG. Leptin treatment during lactation programs leptin synthesis, intermediate metabolism, and liver microsteatosis in adult rats. Horm Metab Res 42: 483–490, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Trivedi M, Bergasa NV. Serum concentrations of substance P in cholestasis. Ann Hepatol 9: 177–180, 2010 [PubMed] [Google Scholar]
- 43. Ueno T, Tanikawa K. Intralobular innervation and lipocyte contractility in the liver. Nutrition 13: 141–148, 1997 [DOI] [PubMed] [Google Scholar]
- 44. Ueno Y, Alpini G, Yahagi K, Kanno N, Moritoki Y, Fukushima K, Glaser S, LeSage G, Shimosegawa T. Evaluation of differential gene expression by microarray analysis in small and large cholangiocytes isolated from normal mice. Liver Int 23: 449–459, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Uyama N, Geerts A, Reynaert H. Neural connections between the hypothalamus and the liver. Anat Rec A Discov Mol Cell Evol Biol 280: 808–820, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Xia X, Demorrow S, Francis H, Glaser S, Alpini G, Marzioni M, Fava G, LeSage G. Cholangiocyte injury and ductopenic syndromes. Semin Liver Dis 27: 401–412, 2007 [DOI] [PubMed] [Google Scholar]