Abstract
TNF and epidermal growth factor (EGF) are well-known stimuli of cyclooxygenase (COX)-2 expression, and TNF stimulates transactivation of EGF receptor (EGFR) signaling to promote survival in colon epithelial cells. We hypothesized that COX-2 induction and cell survival signaling downstream of TNF are mediated by EGFR transactivation. TNF treatment was more cytotoxic to COX-2−/− mouse colon epithelial (MCE) cells than wild-type (WT) young adult mouse colon (YAMC) epithelial cells or COX-1−/− cells. TNF also induced COX-2 protein and mRNA expression in YAMC cells, but blockade of EGFR kinase activity or expression inhibited COX-2 upregulation. TNF-induced COX-2 expression was reduced and absent in EGFR−/− and TNF receptor-1 (TNFR1) knockout MCE cells, respectively, but was restored upon expression of the WT receptors. Inhibition of mediators of EGFR transactivation, Src family kinases and p38 MAPK, blocked TNF-induced COX-2 protein and mRNA expression. Finally, TNF injection increased COX-2 expression in colon epithelium of WT, but not kinase-defective EGFRwa2 and EGFRwa5, mice. These data indicate that TNFR1-dependent transactivation of EGFR through a p38- and/or an Src-dependent mechanism stimulates COX-2 expression to promote cell survival. This highlights an EGFR-dependent cell signaling pathway and response that may be significant in colitis-associated carcinoma.
Keywords: tumor necrosis factor, cyclooxygenase-2, epidermal growth factor receptor
tumor necrosis factor (TNF) is a cytokine that regulates diverse biological processes, including cell survival, apoptosis, proliferation, and migration, in the gastrointestinal (GI) tract. Dysregulation of TNF signaling can alter the balance between these responses, upset the homeostasis of the epithelial layer, and result in GI diseases (17). TNF has a critical role in the pathogenesis of inflammatory bowel diseases (IBD) and is a therapeutic target for treatment of these disorders (54, 67). Furthermore, mounting evidence suggests that TNF signaling is important in the pathogenesis of GI cancers (47, 50, 78). Specifically, an altered balance between prosurvival and apoptotic signaling stimulated by TNF may be critical for tumorigenesis (5, 57). TNF promotes pro- and antiapoptotic responses in colon epithelial cells through direct signaling by TNF receptors (TNFR) 1 and 2 (TNFR1 and TNFR2) (14, 16, 26, 41, 73). Additionally, there is substantial cross talk between TNFRs and other cell surface receptors, such as epidermal growth factor (EGF) receptor (EGFR), that have critical roles in the maintenance of the GI epithelial layer (76).
EGFR is a member of the ErbB family of receptor tyrosine kinases (77), which regulate proliferation (29, 64), survival (30, 45), and migration of epithelial cells (8, 48, 49, 75). EGFR is also a target of cross talk by other signaling cascades, such as G protein-coupled receptors (9) and receptors for bacterial LPS (36). Furthermore, signaling through TNF/TNFRs stimulates EGFR phosphorylation (33, 63), resulting in cellular proliferation (3), migration (72), and survival (76).
TNF and EGF stimulate the expression of cyclooxygenase (COX)-2, the inducible PG synthase (12, 70). Through generation of PGs, COX-2 regulates diverse biological responses, including cell survival (28, 68, 71), proliferation (58), and migration (10), that promote homeostasis of the colon epithelial monolayer. COX-2 is thought to be important in IBD, because COX-2 protein expression and PG levels are elevated in the GI tract of IBD patients (32, 59, 62). However, whether COX-2 is pathogenic or cytoprotective in IBD is unclear. Chronic elevation of COX-2 protein expression is a risk factor for colorectal adenocarcinomas (37), and chronic nonsteroidal anti-inflammatory drug use is associated with a decreased risk for the development of colorectal cancer (22, 23, 69). Detailed knowledge of COX-2 regulation by TNFRs and EGFR is critical to the development of therapeutic agents for the treatment of a number of GI diseases.
These studies were designed to test the hypothesis that TNF-induced COX-2 expression in GI epithelial cells depends on EGFR transactivation and promotes cell survival. We also tested which TNFR is required for induction of COX-2, since there are conflicting reports about the relative importance of TNFR1 and TNFR2 in this response (39, 50, 52). We report that COX-2 protects colon epithelial cells from TNF-induced cytotoxicity and that TNF-driven COX-2 expression in colon and gastric epithelial cells is via a TNFR1-, EGFR-, Src-, and p38 MAPK-dependent mechanism.
MATERIALS AND METHODS
Cell lines.
The conditionally immortalized young adult mouse colon (YAMC) cell line; TNFR1−/−, TNFR2−/−, and EGFR−/− mouse colon epithelial (MCE) cell lines; COX-1−/− and COX-2−/− MCE cell lines; and TNFR1−/−, TNFR2−/−, COX-1−/−, and COX-2−/− immortalized mouse stomach epithelial (ImSt) cells were isolated from wild-type (WT) or knockout mice crossed with the Immortomouse by Robert Whitehead at the Vanderbilt University Digestive Disease Research Center Novel Cell Line Development Core (74). The cell lines were maintained as previously reported (29). Most of the lines were derived on a C57BL/6 background, but in several cases the mice were of mixed (e.g., EGFR−/− from SV129/C57BL/6) or similar, but not identical (e.g., YAMC from C57BL/10), mice. Thus, to control for the potential variability introduced by any differences in background, all results from experiments using knockout cells were confirmed with inhibitors or add-back experiments as appropriate.
Cell viability/TNF cytotoxicity assay.
YAMC, COX-1−/− MCE, and COX-2−/− MCE cells were plated in growth medium without interferon-γ and incubated for 2 days at 33°C. The cells were then starved in serum-free RPMI at 37°C and treated with 100 ng/ml TNF for 48 or 72 h at 37°C. Cell number was determined using the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) reagent from the CellTiter 96 Aqueous One Solution cell viability assay (Promega, Madison, WI) or the NucleoCounter (New Brunswick, Edison, NJ) automated cell counter according to the manufacturers' instructions. Relative cytotoxicities are expressed as percentage of cell loss stimulated by TNF, calculated as follows: 100% − A490(TNF)/A490(Mock) × 100%, where A490 is absorbance at 490 nm.
Generation of stable cell lines.
The generation of EGFR−/− MCE cells with empty pcDNA3.1/Zeo vector (Vec) or wild-type (WT) EGFR and TNFR1−/− MCE cells with empty bicistronic LZRS-green fluorescent protein retroviral vector (Vec) or hemagglutinin (HA)-tagged WT mouse TNFR1 is described elsewhere (16, 76). An HA-tagged mouse death domain deletion (ΔDD) TNFR1 mutant, in which the TNFR1 protein sequence ends at P348, and HA-tagged mouse WT TNFR2 were cloned from YAMC cells. The resulting amplified sequences were subcloned into the LZRS vector. TNFR1−/− and TNFR2−/− MCE cells were infected with the respective retroviral constructs selected for expression, as previously described (16).
Small interfering RNA transfections.
YAMC cells were transfected with 200 nM siGenome SMARTpool number 2 nontargeting (NT) small interfering RNA (siRNA) or 200 nM mouse EGFR siGenome SMARTpool siRNA (Dharmacon, Lafayette, CO) using the Lipofectamine RNAiMax (Invitrogen, Carlsbad, CA) transfection reagent according to the manufacturer's instructions.
Cell stimulation assays, preparation of cell lysates, and Western blot analysis.
Cells were treated with 100 ng/ml TNF or 10 ng/ml EGF (Peprotech, Rocky Hill, NJ), unless otherwise indicated. For experiments using pharmacological inhibitors, cells were pretreated for 1 h with DMSO vehicle control or 1 μM AG-1478 (Calbiochem, San Diego, CA), 2 μM CGP-77675 (Pharma Research, Basel, Switzerland), 1 μM PP2 (Calbiochem), 10 μM SB-203580 (Calbiochem), or 10 μM SB-202190 (Calbiochem). Primary and secondary antibodies used in Western blot analysis include rabbit anti-EGFR (Millipore, Billerica, MA), mouse anti-actin (Sigma-Aldrich, St. Louis, MO), rabbit anti-HA (Zymed), horseradish peroxidase-conjugated anti-mouse and anti-rabbit polyclonal antibodies (Cell Signaling Technology, Beverly, MA), and goat anti-COX-2 and horseradish peroxidase-conjugated anti-goat polyclonal antibodies (Santa Cruz Biotechnologies, Santa Cruz, CA).
Quantitative load-response Western blotting.
The response in Western blotting in the inhibitor and siRNA studies was quantified as described previously (27). Briefly, varying amounts of uninhibited or NT siRNA-transfected TNF stimulation, corresponding to 100, 75, 50, 25, and 10% of the denatured lysate amount, were loaded onto SDS-polyacrylamide gels. The band volumes from the resulting Western blots were measured with ImageJ, and a standard curve was generated. The band volumes of the controls and treatments were also measured and quantified using the TNF-response standard curve and expressed as a percentage of the uninhibited or NT siRNA-transfected TNF stimulation.
Analysis of cox-2 mRNA levels.
Total RNA from YAMC cells was purified using RNeasy columns (Qiagen, Valencia, CA) following the manufacturer's instructions. iScript reverse transcriptase (Bio-Rad, Hercules, CA) was used to synthesize cDNA, which was subjected to quantitative real-time PCR analysis using SYBR Green reaction mix (Sigma-Aldrich) and an iCycler with IQ5 software (Bio-Rad). Relative input mRNA levels were determined using the cycle threshold (2−ΔΔCT) method, with 18S as the reference, using previously characterized primers (4).
Mice, TNF injections, and tissue preparation.
EGFRwa2 C57BL/6 mice harboring a hypomorphic V743G mutation in EGFR that reduces its kinase activity by 80–95% (38, 76) and EGFRwa5 C57BL/6 mice harboring an antimorphic D833G mutation in EGFR that is kinase-inactive and functions as a dominant-negative EGFR (34) were obtained from David Threadgill (University of North Carolina, Chapel Hill, NC). The mice were intraperitoneally injected with PBS or TNF (104 U) in 2% FBS or PBS. After 24 h, tissues were harvested and fixed as previously described (76). All animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of Vanderbilt University.
COX-2 immunofluorescence.
Paraffin-embedded tissue sections were deparaffinized, rehydrated, and subjected to heat and citrate-antigen retrieval (Vector Laboratories). Antibodies used for immunofluorescence analysis include anti-COX-2 (Cayman Chemical, Ann Arbor, MI), anti-E-cadherin (BD Transduction, San Jose, CA), FITC-conjugated anti-rabbit (Zymed, San Francisco, CA), and Cy3-conjugated anti-mouse (Jackson, Bar Harbor, ME). 4′,6-Diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA) was used to stain nuclei. The number of cells that stained for both COX-2 and E-cadherin in 100 crypts was counted under blinded conditions to quantify epithelial COX-2 induction.
Statistical analysis of experimental data.
Data are representative of at least three experimental trials and were analyzed using GraphPad Prism software (GraphPad Software, La Jolla, CA) by one-way ANOVA with Tukey's multiple comparison test or with Bonferroni's multiple comparison test in which preselected data columns were compared.
RESULTS
COX-2 protects against TNF cytotoxicity in colon epithelial cells.
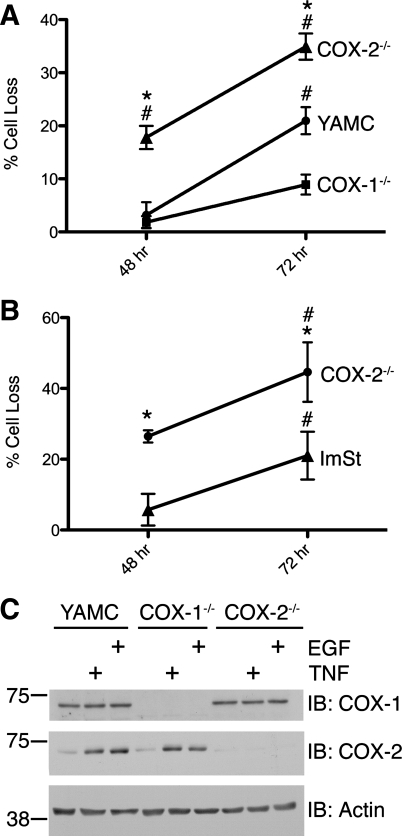
IBD patients have elevated levels of TNF and COX-2 in the epithelial cell layer of the GI tract (40, 46, 62). However, the biological and pathological consequences of COX-2 in the context of elevated TNF levels in normal colon epithelial cells are not well known. Therefore, we tested the effect of TNF on cell viability in a confluent monolayer of WT YAMC cells and COX-1−/− or COX-2−/− MCE cells (Fig. 1A). Treatment of YAMC and COX-1−/− MCE cells with TNF for 48 h resulted in little or no change in the number of viable cells; in contrast, TNF exposure caused a significant decrease in the number of COX-2−/− MCE cells. These differences were sustained for ≥72 h. Similar results were observed in WT and COX-2−/− ImSt cells (Fig. 1B). These data are consistent with our recently published results showing that the COX-2 inhibitor celecoxib enhances cytokine-induced cell death of YAMC cells (20). Additional experiments demonstrated that mock-treated cells were not proliferating over the examined time course (data not shown); therefore, the relative decrease in cell number observed in TNF treatments compared with mock treatment was not the result of a TNF-stimulated blockade of basal cell proliferation. These data suggest that COX-2 protects against TNF-induced cytotoxicity in colon epithelial cells.
Fig. 1.
Cyclooxygenase (COX)-2 protects against TNF cytotoxicity in colon and gastric epithelial cells. A: young adult mouse colon (YAMC), COX-1−/−, and COX-2−/− mouse colon epithelial (MCE) cells were treated with TNF (100 ng/ml) for 48 and 72 h, and cell number was assayed using a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) cell viability assay. Data points represent percentage of cell loss relative to mock treatment. *P < 0.05 vs. YAMC. #P < 0.05 vs. COX-1−/−. B: immortalized mouse stomach epithelial (ImSt) and COX-2−/− ImSt cells were treated with TNF (100 ng/ml) for 48 and 72 h, and cell number was assayed using the NucleoCounter automated cell counter. Data points represent percentage of cell loss relative to mock treatment. *P < 0.05 vs. respective ImSt time point. #P < 0.05 vs. respective 48-h time point. C: COX-1 and COX-2 protein expression in YAMC, COX-1−/−, and COX-2−/− MCE cells. Cells were treated with TNF (100 ng/ml) and epithelial growth factor (EGF, 10 ng/ml) for 24 h, and protein expression in cell lysates was determined by Western blot analysis. IB, immnoblot.
TNF and EGF stimulate COX-2 expression in YAMC and ImSt cells.
To determine the effect of TNF and EGF on COX-2 expression in MCE cells, we treated YAMC and COX-1−/− and COX-2−/− MCE cells with TNF or a receptor-saturating concentration of EGF and assessed COX-2 expression by Western blot analysis (Fig. 1C). TNF and EGF stimulated increased COX-2 protein expression in YAMC and COX-1−/− MCE cells. As expected, no COX-2 expression was detected in COX-2−/− MCE cells under any condition. As expected, COX-1 protein was constitutively expressed and was not induced by TNF or EGF. These data confirm that TNF and EGF stimulate COX-2 protein expression and that this induction correlates with reduced TNF cytotoxicity in YAMC and COX-1−/− cells compared with COX-2−/− cells.
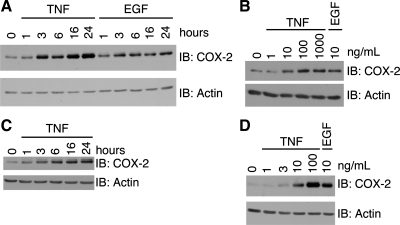
To determine a mechanism by which these two ligands elevate COX-2 protein expression, we tested the kinetics of induction. TNF-stimulated expression of COX-2 began at 3 h of treatment. Expression continued to increase and peaked at 16 h of treatment (Fig. 2A). Stimulation with EGF induced COX-2 protein expression with similar kinetics; however, the amount of COX-2 was not increased appreciably past 6 h. We subsequently performed a dose response with TNF for COX-2 expression (Fig. 2B). There was a noticeable increase in COX-2 expression with 10 ng/ml TNF, and induction was saturated at 100 ng/ml TNF. To determine whether the kinetics and TNF concentration dependence of COX-2 induction were similar in other cell types of the GI tract, we performed time-course and dose-response experiments with TNF in ImSt cells. TNF stimulated increased COX-2 levels by 3 h, and expression peaked at ∼16 h in these cells (Fig. 2C); 100 ng/ml TNF most strongly induced COX-2 expression (Fig. 2D). These data confirm that the concentration of TNF that exhibits a strong cytotoxic effect in COX-2−/− cells is the strongest inducer of COX-2 protein expression and that TNF induction of COX-2 expression is similar in colon and gastric epithelial cells.
Fig. 2.
TNF or EGF stimulates COX-2 expression in YAMC and ImSt cells. YAMC (A) or ImSt (C) cells were treated with TNF (100 ng/ml) or EGF (10 ng/ml) for 0–24 h. YAMC (B) or IMST (D) cells were treated with TNF (0–1,000 ng/ml) or EGF (10 ng/ml) for 24 h. Cell lysates were analyzed for protein expression as described in Fig. 1 legend.
TNF signals through TNFR1 to induce COX-2 expression.
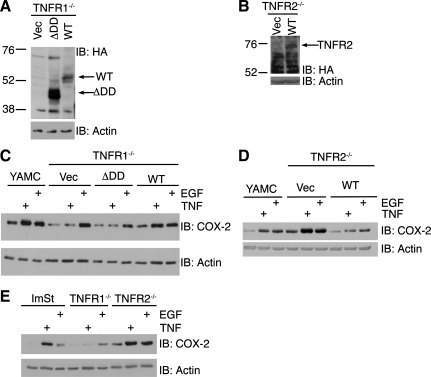
To determine which of the two TNFRs is required for TNF-induced COX-2 expression, we used conditionally immortalized TNFR1−/− or TNFR2−/− MCE to generate TNFR add-back cell lines. TNFR1−/− MCE cells were infected with recombinant retrovirus to introduce a Vec, a ΔDD TNFR1 mutant, or WT TNFR1 (Fig. 3A). TNFR2−/− MCE cells were also infected to introduce Vec or WT TNFR2 (Fig. 3B). TNF did not stimulate COX-2 expression in TNFR1−/− Vec or ΔDD cells (Fig. 3C); however, TNF induction of COX-2 was rescued in TNFR1 WT cells. TNF and EGF induced expression of COX-2 in TNFR2−/− Vec cells (Fig. 3D). Interestingly, the basal and TNF- and EGF-stimulated COX-2 expression levels were lower in TNFR2 WT cells. The same pattern of COX-2 expression occurs in WT, TNFR1−/−, and TNFR2−/− ImSt cells (Fig. 3E). Thus, TNF induces COX-2 expression through TNFR1 in GI epithelial cells.
Fig. 3.
TNF signals through TNF receptor (TNFR) 1 to induce COX-2 expression. Expression of hemagglutinin (HA)-tagged death domain deletion (ΔDD) mutant TNFR1 and wild-type (WT) TNFR1 in TNFR1−/− MCE cells (A) and WT TNFR2 in TNFR2−/− MCE cells (B). YAMC, TNFR1−/− empty vector (Vec), ΔDD TNFR1, and WT TNFR1 MCE cells (C) and TNFR2−/− Vec and WT TNFR2 MCE cells (D) were treated with TNF (100 ng/ml) or EGF (10 ng/ml) for 24 h. E: WT ImSt, TNFR1−/−, and TNFR2−/− cells were treated with TNF or EGF for 24 h. Cell lysates were analyzed for protein expression as described in Fig. 1 legend.
TNF stimulation of COX-2 expression requires EGFR kinase activity.
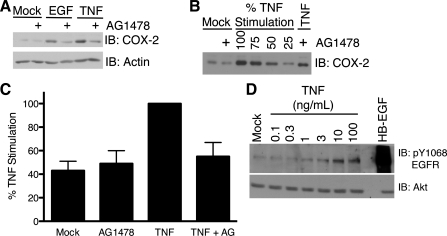
We determined whether EGFR has a role in TNF-induced COX-2 expression by assessing COX-2 expression in the presence of a pharmacological EGFR inhibitor or in the absence of EGFR expression. YAMC cells were treated with TNF in the presence or absence of AG-1478, a small-molecule inhibitor of EGFR kinase activity (Fig. 4A). To quantify the effect of AG-1478 on TNF-stimulated COX-2 expression, we performed a load-response Western blot analysis of the cell lysates (Fig. 4B). AG-1478 reduced TNF-stimulated COX-2 expression to near-basal levels (Fig. 4C). As expected, the inhibitor also blocked EGF-stimulated COX-2 protein expression. In contrast, AG-1478 did not affect basal COX-2 expression. Interestingly, the concentration of TNF that maximally stimulates COX-2 in YAMC and ImSt cells also maximally stimulated transactivation of EGFR in ImSt (Fig. 4D) and YAMC (76) cells. These data indicate that EGFR kinase activity is required for full stimulation of COX-2 protein expression by TNF in YAMC cells.
Fig. 4.
TNF stimulation of COX-2 expression requires EGF receptor (EGFR) kinase activity. A: YAMC cells were treated with EGF (10 ng/ml) or TNF (100 ng/ml) for 24 h in the presence or absence of AG-1478 (1 μM). B: representative load-response Western blot for inhibitor studies. YAMC cells were pretreated with AG-1478 and then treated with TNF for 24 h. Cell lysates and percentages of the uninihibited TNF stimulation cell lysate were analyzed for COX-2 protein expression as described in Fig. 1 legend. C: load-response densitometric analysis of COX-2 protein expression in YAMC cells. Quantified COX-2 protein levels are expressed as percentage of unihibited TNF stimulation. D: ImSt cells were treated with TNF (0.1–100 ng/ml) for 60 min or with heparin-binding EGF-like growth factor (HB-EGF, 30 ng/ml) for 5 min. Protein expression and EGFR phosphorylation in cell lysates were determined by Western blot analysis.
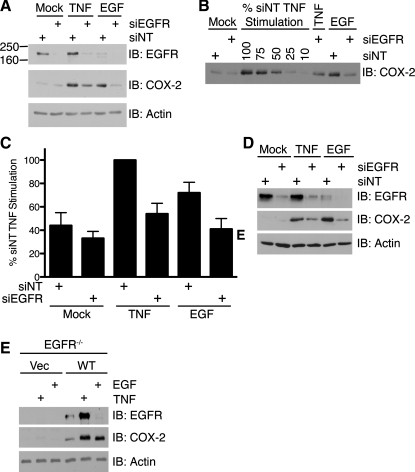
To determine a specific role of EGFR in this pathway, we transfected YAMC cells with siRNA for EGFR and quantified the effect on COX-2 expression using load-response Western blot analysis. Transfection with EGFR siRNA resulted in decreased EGFR expression compared with transfection with NT siRNA. COX-2 induction by TNF or EGF was almost completely blocked in EGFR siRNA-transfected cells (Fig. 5, A–C). Additionally, TNF-stimulated COX-2 expression in ImSt cells was also sensitive to EGFR knockdown (Fig. 5D).
Fig. 5.
TNF stimulation of COX-2 expression requires EGFR expression. A: YAMC cells were transfected with nontargeting small interfering RNA (siNT) or EGFR small interfering RNA (siEGFR) and then treated with TNF or EGF for 24 h. B: representative load-response Western blot for siRNA experiments. Cell lysates and percentages of siNT-transfected TNF stimulation cell lysate were analyzed for COX-2 protein expression as described in Fig. 1 legend. C: load-response densitometric analysis of COX-2 protein expression. Quantified COX-2 protein levels are expressed as percentage of siNT-transfected TNF stimulation. D: ImSt cells were transfected with siNT or siEGFR and then treated with TNF (100 ng/ml) or EGF (10 ng/ml) for 24 h. Cell lysates were analyzed for protein expression as described in Fig. 1 legend. E: EGFR−/− Vec and EGFR−/− WT MCE cells were treated with TNF or EGF for 24 h. Cell lysates were analyzed for protein expression as described in Fig. 1 legend.
To further confirm these results, we assessed TNF stimulation of COX-2 in EGFR−/− MCE cells transfected with Vec or WT EGFR. TNF stimulated COX-2 expression in EGFR−/− WT EGFR, but not EGFR−/− Vec, cells (Fig. 5E). EGF-stimulated COX-2 expression was also ablated in Vec cells and restored in WT EGFR cells. Interestingly, whereas EGF stimulated downregulation of EGFR, a well-known mechanism for modulating receptor signaling (11, 35), TNF did not. Taken together, these data demonstrate that EGFR is a critical signaling molecule in TNF-stimulated COX-2 expression and that TNF-stimulated EGFR activation is distinct from EGF-stimulated EGFR activation.
TNF- and EGF-stimulated COX-2 protein expression requires Src and p38 MAPK activity.
A potential mechanism of EGFR transactivation is through activation of matrix metalloproteinases (MMP), such as ADAM metallopeptidase domain 17 (ADAM-17) (9). To test for their involvement in TNF-induced COX-2 expression, we treated YAMC cells with TNF in the presence or absence of the broad-spectrum MMP inhibitor GM-6001 or the ADAM-17 inhibitor TAPI-1 and assessed COX-2 expression. Neither of these inhibitors blocked TNF-stimulated COX-2 expression in colon and stomach epithelial cells, and COX-2 expression was not affected in ImSt ADAM17−/− cells (data not shown).
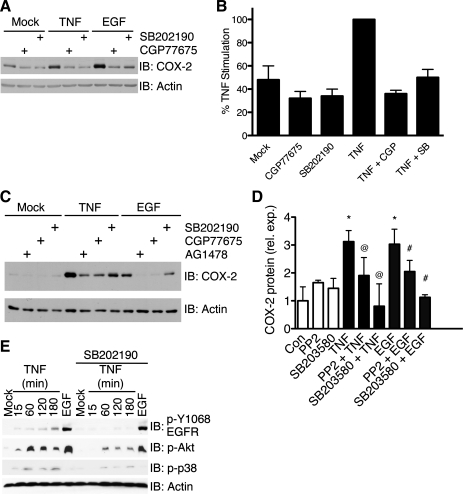
An additional mode of ErbB receptor transactivation is through the activation of nonreceptor tyrosine kinases, such as Src, which results in phosphorylation of EGFR (2, 13, 43). Another component of TNF signaling, p38 MAPK, has also been shown to modulate EGFR phosphorylation and activity (19, 63). To test whether TNF-stimulated COX-2 expression requires Src or p38 kinase activity, we treated YAMC cells with TNF in the presence or absence of the Src family kinase inhibitor CGP-77675 or the p38 inhibitor SB-202190. The Src and p38 inhibitors blocked TNF- and EGF-stimulated COX-2 expression in YAMC cells (Fig. 6, A and B). Furthermore, the Src inhibitor also blocked TNF stimulation of COX-2 expression in ImSt cells (Fig. 6C). To confirm the involvement of Src and p38 pathways in COX-2 induction using additional pharmacological inhibitors, YAMC cells were pretreated with the Src inhibitor PP2 or the p38 inhibitor SB-203580 before stimulation with TNF or EGF. PP2 and SB-203580 blocked COX-2 induction (Fig. 6D), showing that the results with CGP-77675 and SB-202190 are not off-target effects of individual inhibitors. These data indicate that Src family kinases and p38 are required for TNF- and EGF-stimulated COX-2 expression in GI cells.
Fig. 6.
TNF- and EGF-stimulated COX-2 expression requires Src and p38 MAPK activity, and TNF-stimulated transaction of EGFR requires p38 activity. A: YAMC cells were pretreated with CGP-77675 (2 μM) or SB-202190 (10 μM) for 1 h and then treated with TNF (100 ng/ml) or EGF (10 ng/ml) for 24 h. B: load-response densitometric analysis of COX-2 protein expression. Quantified COX-2 protein levels are expressed as percentage of unihibited TNF stimulation. C: ImSt cells were pretreated with AG-1478, CGP-77675, or SB-202190 and then treated with TNF or EGF for 24 h. D: YAMC cells were treated with TNF or EGF in the presence or absence of PP2 (1 μM) or SB-203580 (10 μM). COX-2 expression was determined by quantitative Western blot. *P < 0.05 vs. control. @P < 0.05 vs. TNF. #P < 0.05 vs. EGF. E: YAMC cells were treated with TNF for 15–180 min in the absence or presence of SB-201290. Cell lysates were analyzed for protein expression and protein phosphorylation using the respective antibodies as described in Fig. 1 legend.
To determine whether p38 regulates TNF induction of COX-2 by transactivating EGFR, we stimulated YAMC cells with TNF in the presence or absence of SB-20190 (Fig. 6E). TNF stimulated noticeable levels of EGFR phosphorylation after 15 min of treatment. EGFR phosphorylation continued to increase to a maximum after 180 min of treatment. The p38 inhibitor reduced TNF stimulation of EGFR phosphorylation at the observed time points and also reduced phosphorylation of Akt, a downstream signaling target of EGFR. Thus, TNF signaling requires p38 activation to transactivate EGFR and stimulate COX-2 expression.
TNF-stimulated cox-2 mRNA expression requires EGFR, Src, and p38 activity.
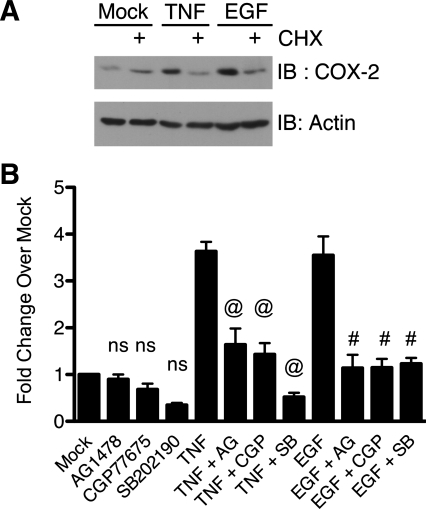
The data from the TNF and EGF time-course experiment suggest de novo synthesis of COX-2 protein from mRNA. To test whether the induced expression was the result of protein synthesis, we stimulated YAMC cells with TNF or EGF in the presence or absence of cycloheximide, an inhibitor of protein synthesis (Fig. 7A). Cycloheximide blocked TNF- and EGF-stimulated COX-2 protein expression, indicating that the increase in COX-2 protein expression stimulated by TNF and EGF requires de novo protein synthesis. This suggests that the change in COX-2 protein levels is a result of a change in COX-2 translation or cox-2 mRNA levels. Therefore, we next sought to determine whether EGFR, Src kinases, and p38 regulate TNF- and EGF-stimulated cox-2 mRNA levels by assessing the effect of the respective kinase inhibitors (Fig. 7B). TNF and EGF stimulated an increase in cox-2 mRNA levels to a similar extent. The EGFR, Src, and p38 inhibitors blocked TNF- and EGF-stimulated cox-2. These data indicate that EGFR, Src kinases, and p38 are required for TNF- and EGF-stimulated cox-2 mRNA expression.
Fig. 7.
TNF-stimulated COX-2 induction requires de novo protein synthesis, and induction of cox-2 mRNA expression requires EGFR, Src, and p38 activity. A: YAMC cells were pretreated with cycloheximide (CHX, 5 μg/ml) and then treated with TNF (100 ng/ml) or EGF (10 ng/ml) for 6 h. Cell lysates were analyzed for protein expression as described in Fig. 1 legend. B: YAMC cells were pretreated with AG-1478, CGP-77675, or SB-202190 and then treated with TNF or EGF for 24 h. cox-2 mRNA levels were determined by quantitative RT-PCR analysis. ns, Nonsignificant vs. Mock. @P < 0.05 vs. TNF. #P < 0.05 vs. EGF.
TNF-stimulated COX-2 protein expression requires EGFR kinase activity in vivo.
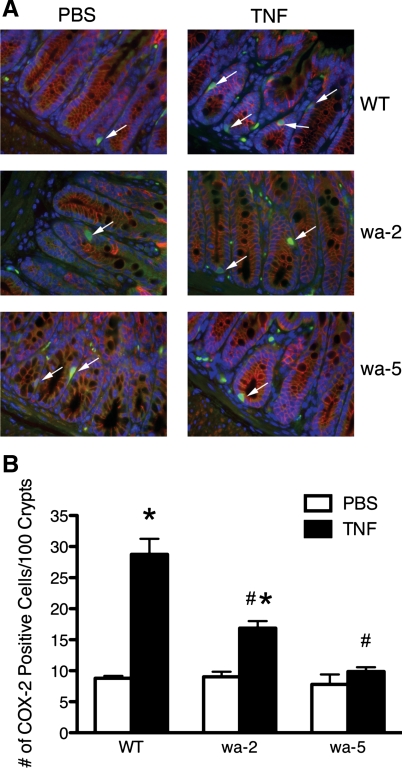
The experiments with cultured GI epithelial cells indicate that EGFR kinase activity is required for stimulation of COX-2 expression by TNF. To determine the in vivo relevance of this TNFR-EGFR-COX-2 pathway, we assessed induction of COX-2 protein expression in colon epithelial cells following intraperitoneal injection of TNF in WT mice, EGFRwa2 hypomorphic EGFR mice (38), and EGFRwa5 antimorphic EGFR mice expressing a dominant-negative mutation (34). We quantified TNF induction of COX-2 expression among the WT and mutant mice in colon epithelial cells by counting the number of cells per 100 colon crypts that stained for both COX-2 and E-cadherin, an epithelial cell marker (Fig. 8). TNF induced increased numbers of COX-2-expressing colon epithelial cells in WT mice, consistent with our findings in vitro. TNF induced a lower number of COX-2-expressing colon epithelial cells in EGFRwa2 mice and no increase in COX-2-expressing colon epithelial cells in EGFRwa5 mice. Thus, EGFR kinase activity is also critical to TNF induction of COX-2 expression in vivo.
Fig. 8.
TNF induction of COX-2 in vivo requires EGFR kinase activity. A: representative immunofluorescence imaging of colon sections from WT, EGFRwa2 (wa-2), and EGFRwa5 (wa-5) mice injected with PBS or TNF (104 U) for 24 h. Blue represents 4′,6-diamidino-2-phenylindole-positive nuclei, green represents COX-2-positive cells, and red represents E-cadherin-positive cells. White arrows indicate cells that stained for both COX-2 and E-cadherin. B: number of epithelial cells that stained for both COX-2 and E-cadherin. *P < 0.05 vs. respective PBS treatment. #P < 0.05 vs. WT TNF.
DISCUSSION
In this study, we investigated whether TNF transactivation of EGFR regulates the induction of COX-2 and whether induced COX-2 expression promotes GI epithelial cell survival. We have demonstrated that TNF induction of COX-2 protein expression in colon and gastric epithelial cells occurs through a TNFR1/EGFR-dependent pathway and that the induced COX-2 protects cells from the cytotoxic effect of high concentrations of TNF. Blocking EGFR kinase activity or expression attenuated COX-2 induction by TNF (Figs. 4A and 5A), while TNF-induced COX-2 protein expression was rescued in EGFR−/− MCE cells expressing WT EGFR (Fig. 5E). Furthermore, we confirmed that the requirement of EGFR kinase activity for TNF induction of COX-2 exists in vivo. The increase in COX-2 expression observed in response to TNF in colon sections from WT (high COX-2 induction), EGFRwa2 (moderate COX-2 induction), and EGFRwa5 (no COX-2 induction) mice correlated with their respective levels of EGFR kinase activity: WT >> EGFRwa2 > EGFRwa5 (Fig. 8) (34, 38). Despite this evidence demonstrating a role for EGFR, there was a residual stimulation of COX-2 protein expression by TNF, even if EGFR kinase activity or expression was inhibited in the cultured colon epithelial cells (Figs. 4A and 5A). Additionally, steady-state EGFR knockdown with siRNA did not affect basal COX-2 levels (Fig. 5, A and D). This suggests that while there are EGFR-dependent mechanisms promoting COX-2 expression and the greatest increase in TNF-stimulated COX-2 expression is EGFR-dependent, there is also EGFR-independent regulation.
Our findings also show that TNF and EGF stimulate COX-2 protein expression by increasing steady-state cox-2 mRNA levels, and blocking EGFR kinase activity attenuates this response (Fig. 7B). It is not clear how EGFR activity is increasing cox-2 expression, but future experiments will determine whether TNF-transactivated EGFR increases cox-2 transcription or regulates the stability of the mRNA through stabilizing factors such as the embryonic lethal abnormal vision (ELAV)-like HuR (15, 61, 65) or RNA-binding motif (RBM3) (66). Interestingly, TNF consistently stimulated more COX-2 protein induction than did EGF, but these ligands stimulated comparable levels of cox-2 mRNA. Thus differences between TNF and EGF stimulation exist at cox-2 posttranscription or translation.
A recent study from our laboratory detailing TNF transactivation of EGFR concluded that the mechanism for transactivation in YAMC cells involved the activity of Src family kinases (76); TNF-induced phosphorylation of the receptor also occurs by p38 MAPK (63). We have demonstrated that Src family kinase and p38 activities are required for TNF and EGF stimulation of COX-2 protein (Fig. 6) and mRNA expression (Fig. 7B). We also demonstrated that p38 activity is required for full TNF stimulation of EGFR phosphorylation and subsequent activation of the downstream signaling molecule Akt (Fig. 6E). These data suggest that p38 and Src family kinases regulate TNF stimulation of COX-2 expression through EGFR transactivation. It is not clear whether p38 activation is upstream or downstream of Src kinase activation. Determining the relative position of p38 and Src may be complicated by additional stimulation of these signaling molecules by transactivated EGFR. Nonetheless, this mechanism of EGFR transactivation may account for a difference between TNF and EGF downregulation of activated EGFR. In our experiments, EGF promoted EGFR activation, followed by downregulation (Fig. 5, A, D, and E), a well-known phenomenon (11, 35). In contrast, even though TNF stimulates EGFR phosphorylation (Fig. 6E) (76), there was no noticeable downregulation of EGFR in response to the cytokine. It is possible that TNF may not stimulate phosphorylation on specific EGFR tyrosines that drive receptor internalization (24). It is also possible that, because of differences in localization or kinetics of phosphorylation, TNF-transactivated EGFR does not result in the activation of proteins that are involved in the downregulation of EGFR. This may have a significant impact on how EGFR couples to downstream signaling molecules and may stimulate cellular responses that distinguish TNF-stimulated EGFR signaling from EGF-stimulated signaling.
There has been conflicting evidence regarding whether TNFR1 (52) or TNFR2 (39) is responsible for TNF-induced COX-2 expression. Our studies performed in TNFR1−/− and TNFR2−/− MCE cells in which the respective WT TNFRs have been reexpressed controlled for unrelated differences that may exist between TNFR−/− MCE and WT YAMC cells. Expression of WT TNFR1 in TNFR1−/− MCE cells restored TNF-stimulated COX-2 expression lost in the vector control, whereas stimulated COX-2 expression was not enhanced in TNFR2−/− cells, relative to the vector control, by addition of WT TNFR2 (Fig. 3, A and B). Furthermore, TNF stimulation of COX-2 expression was lost in TNFR1−/− ImSt, but not TNFR2−/− ImSt, cells (Fig. 3E). Thus these studies demonstrate that TNF signals for COX-2 expression through TNFR1. Expression of a ΔDD TNFR1 mutant in the TNFR1−/− cells did not restore TNF stimulation of COX-2 expression (Fig. 3A). Therefore, TNF potentially stimulates COX-2 expression through death domain signaling; however, the ΔDD mutant used in this study contained a stop codon that resulted in a TNFR1 that lacked not only the death domain but also the protein sequence COOH terminus to the death domain. As a result, it is possible that elements within this sequence contribute to signaling that promotes COX-2 expression. Interestingly, basal COX-2 expression in TNFR2−/− cells was nearly as high as induced COX-2 expression in WT cells, and induced COX-2 expression was also very high, but upon expression of TNFR2, COX-2 expression was lowered (Fig. 3, D and E). This suggests that TNFR2 plays a role in negatively regulating COX-2 expression.
We have shown that COX-2 expression is cytoprotective in an environment of high TNF concentration (Fig. 1). Notably, this same concentration of TNF (100 ng/ml) stimulates transactivation of EGFR in YAMC cells, and this transactivation is required for colon epithelial cell survival in vitro and in vivo, as described in our previous study (76). Our previous finding (76) that Src activity is required for EGFR transactivation and cell survival is consistent with our finding in the present study that Src activity is also necessary for COX-2 accumulation in response to TNF. The specific role of p38 in cell survival following TNF exposure is likely more complicated, as this MAPK promotes pro- and antiapoptotic signals. Our results clearly show that p38 is required for full TNF transactivation of EGFR and COX-2 induction; these pathways presumably represent a balancing survival signal to cell death-promoting events downstream of p38.
The lower level of COX-2 expression induced by TNF in the EGFRwa2 than WT mice (Fig. 8) correlates with an increase in apoptosis in the EGFRwa2 mice in our previous work (76). The lack of strong COX-2 induction in the EGFRwa2 mice (Fig. 8) may contribute to the increased apoptosis. Hence, it is apparent that COX-2 is at least one of the cell survival effectors downstream of EGFR transactivation by TNF; however, further experiments are needed to confirm such a role for COX-2 in vivo. The survival role of COX-2 in an environment of high TNF concentration may explain why nonsteroidal anti-inflammatory drugs, including selective COX-2 inhibitors, can exacerbate IBD (7, 18, 31, 42, 51). For instance, administration of selective COX-2 inhibitors to IL-10−/− mice that develop spontaneous colitis, in which TNF levels are elevated (6) and demonstrably critical to the progression of the colitis (56), augmented the severity of colitis (25). More specifically, a recent study investigating the respective roles of COX-1 and COX-2 in the course of disease in a dextran sulfate sodium model of colitis demonstrated that COX-2 was critical for protection against ulceration in a later stage of disease (60).
The finding that COX-2 is a survival effector of TNF-transactivated EGFR also has relevance to cancers of the GI tract, since chronic inflammatory conditions provide an environment that permits the development and progression of cancers. For example, prolonged ulcerative colitis is a known risk factor for the development of epithelial dysplasia and adenocarcinoma (44, 55). Also, studies of colitis-associated neoplasia in humans show COX-2 overexpression in neoplastic lesions (1). Furthermore, in an animal model of colitis-associated cancer, increased mucosal EGFR phosphorylation and COX-2 expression have been reported (21). This correlation between EGFR phosphorylation and increased COX-2 expression in an inflammatory environment is certainly consistent with our results, and it may define a mechanism that explains the different outcomes of tissue toward ulceration or neoplasia. It is possible that sites of ulceration in IBD represent compartments of tissue where COX-2, which is protective, is not adequately expressed in response to TNF as a result of a lack/dysregulation of components of a TNFR-EGFR-COX-2 axis. In contrast, sites at which neoplasias develop may represent compartments where COX-2 is expressed in large quantities as a result of adequate or overactive components of this axis. For instance, our data suggest that tissues that lack TNFR2 expression would be expected to have high basal and TNF-inducible levels of COX-2 expression and, thus, more cell survival. Survival responses in an inflammatory environment may permit the perpetuation of cell populations that harbor mutations resulting from oxidative DNA damage that occurs in IBD (53) and, thus, facilitate the accumulation of additional mutations that could affect proliferative, migratory, or survival responses in tumorigenesis.
In summary, the data presented in the present study provide evidence for a previously unknown mechanism for the induction of COX-2 by TNF through TNFR1 in colon and stomach epithelial cells by a p38- and/or Src-dependent TNF transactivation of EGFR that also upregulates COX-2 expression in vivo. Subsequently, this induction of COX-2 protein expression protects a confluent monolayer of colon epithelial cells from TNF cytotoxicity. Thus our studies specify signaling events linking inflammation to cell survival that may be critical to the progression of carcinomas in IBD through a TNFR-EGFR-COX-2 axis.
GRANTS
This work was supported by National Institutes of Health Awards T32 CA-106183 (to S. S. Hobbs), DK-56008 and DK-54993 (to D. B. Polk), DK-077956 (to M. R. Frey), and DK-058404 (through the Vanderbilt University Digestive Disease Research Center).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Agoff SN, Brentnall TA, Crispin DA, Taylor SL, Raaka S, Haggitt RC, Reed MW, Afonina IA, Rabinovitch PS, Stevens AC, Feng Z, Bronner MP. The role of cyclooxygenase 2 in ulcerative colitis-associated neoplasia. Am J Pathol 157: 737–745, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andreev J, Galisteo ML, Kranenburg O, Logan SK, Chiu ES, Okigaki M, Cary LA, Moolenaar WH, Schlessinger J. Src and Pyk2 mediate G-protein-coupled receptor activation of epidermal growth factor receptor (EGFR) but are not required for coupling to the mitogen-activated protein (MAP) kinase signaling cascade. J Biol Chem 276: 20130–20135, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Argast GM, Campbell JS, Brooling JT, Fausto N. Epidermal growth factor receptor transactivation mediates tumor necrosis factor-induced hepatocyte replication. J Biol Chem 279: 34530–34536, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Bachar O, Rose AC, Adner M, Wang X, Prendergast CE, Kempf A, Shankley NP, Cardell LO. TNF-α reduces tachykinin, PGE2-dependent, relaxation of the cultured mouse trachea by increasing the activity of COX-2. Br J Pharmacol 144: 220–230, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balkwill F. TNF-α in promotion and progression of cancer. Cancer Metastasis Rev 25: 409–416, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Berg DJ, Davidson N, Kühn R, Müller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4+ TH1-like responses. J Clin Invest 98: 1010–1020, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berg DJ, Zhang J, Weinstock JV, Ismail HF, Earle KA, Alila H, Pamukcu R, Moore S, Lynch RG. Rapid development of colitis in NSAID-treated IL-10-deficient mice. Gastroenterology 123: 1527–1542, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Blay J, Brown KD. Epidermal growth factor promotes the chemotactic migration of cultured rat intestinal epithelial cells. J Cell Physiol 124: 107–112, 1985 [DOI] [PubMed] [Google Scholar]
- 9. Blobel C. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol 6: 32–43, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Buchanan FG, Wang D, Bargiacchi F, DuBois RN. Prostaglandin E2 regulates cell migration via the intracellular activation of the epidermal growth factor receptor. J Biol Chem 278: 35451–35457, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Carpenter G, Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol 71: 159–171, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chun KS, Surh YJ. Signal transduction pathways regulating cyclooxygenase-2 expression: potential molecular targets for chemoprevention. Biochem Pharmacol 68: 1089–1100, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Daub H, Wallasch C, Lankenau A, Herrlich A, Ullrich A. Signal characteristics of G protein-transactivated EGF receptor. EMBO J 16: 7032–7044, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dempsey PW, Doyle SE, He JQ, Cheng G. The signaling adaptors and pathways activated by TNF superfamily. Cytokine Growth Factor Rev 14: 193–209, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Denkert C, Koch I, von Keyserlingk N, Noske A, Niesporek S, Dietel M, Weichert W. Expression of the ELAV-like protein HuR in human colon cancer: association with tumor stage and cyclooxygenase-2. Mod Pathol 19: 1261–1269, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Edelblum KL, Goettel JA, Koyama T, McElroy SJ, Yan F, Polk DB. TNFR1 promotes TNF-mediated mouse colon epithelial cell survival through Raf activation of NF-κB. J Biol Chem 483: 29485–29494, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Edelblum KL, Yan F, Yamaoka T, Polk DB. Regulation of apoptosis during homeostasis and disease in the intestinal epithelium. Inflamm Bowel Dis 12: 413–424, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Felder JB, Korelitz BI, Rajapakse R, Schwarz S, Horatagis AP, Gleim G. Effects of nonsteroidal anti-inflammatory drugs on inflammatory bowel disease: a case-control study. Am J Gastroenterol 95: 1949–1954, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Frey MR, Dise RS, Edelblum KL, Polk DB. p38 kinase regulates epidermal growth factor receptor downregulation and cellular migration. EMBO J 25: 5683–5692, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Frey MR, Hilliard VC, Mullane MT, Polk DB. ErbB4 promotes cyclooxygenase-2 expression and cell survival in colon epithelial cells. Lab Invest 90: 1415–1424, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fukata M, Chen A, Vamadevan AS, Cohen J, Breglio K, Krishnareddy S, Hsu D, Xu R, Harpaz N, Dannenberg AJ, Subbaramaiah K, Cooper HS, Itzkowitz SH, Abreu MT. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology 133: 1869–1881, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Aspirin use and the risk for colorectal cancer and adenoma in male health professionals. Ann Intern Med 121: 241–246, 1994 [DOI] [PubMed] [Google Scholar]
- 23. Greenberg ER, Baron JA, Freeman DH, Mandel JS, Haile R. Reduced risk of large-bowel adenomas among aspirin users. The Polyp Prevention Study Group. J Natl Cancer Inst 85: 912–916, 1993 [DOI] [PubMed] [Google Scholar]
- 24. Grøvdal LM, Stang E, Sorkin A, Madshus IH. Direct interaction of Cbl with pTyr 1045 of the EGF receptor (EGFR) is required to sort the EGFR to lysosomes for degradation. Exp Cell Res 300: 388–395, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Hegazi RA, Mady HH, Melhem MF, Sepulveda AR, Mohi M, Kandil HM. Celecoxib and rofecoxib potentiate chronic colitis and premalignant changes in interleukin 10 knockout mice. Inflamm Bowel Dis 9: 230–236, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Hehlgans T, Männel DN. The TNF-TNF receptor system. Biol Chem 383: 1581–1585, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Hobbs SS, Cameron EM, Hammer RP, Le AT, Gallo RM, Blommel EN, Coffing SL, Chang H, Riese DJ. Five carboxyl-terminal residues of neuregulin2 are critical for stimulation of signaling by the ErbB4 receptor tyrosine kinase. Oncogene 23: 883–893, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Joseph RR, Yazer E, Hanakawa Y, Stadnyk AW. Prostaglandins and activation of AC/cAMP prevents anoikis in IEC-18. Apoptosis 10: 1221–1233, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Kaiser GC, Polk DB. Tumor necrosis factor-α regulates proliferation in a mouse intestinal cell line. Gastroenterology 112: 1231–1240, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Karnes WE, Weller SG, Adjei PN, Kottke TJ, Glenn KS, Gores GJ, Kaufmann SH. Inhibition of epidermal growth factor receptor kinase induces protease-dependent apoptosis in human colon cancer cells. Gastroenterology 114: 930–939, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Laudanno OM, Cesolari JA, Esnarriaga J, Rista L, Piombo G, Maglione C, Aramberry L, Sambrano J, Godoy A, Rocaspana A. Gastrointestinal damage induced by celecoxib and rofecoxib in rats. Dig Dis Sci 46: 779–784, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Lauritsen K, Laursen LS, Bukhave K, Rask-Madsen J. Effects of topical 5-aminosalicylic acid and prednisolone on prostaglandin E2 and leukotriene B4 levels determined by equilibrium in vivo dialysis of rectum in relapsing ulcerative colitis. Gastroenterology 91: 837–844, 1986 [DOI] [PubMed] [Google Scholar]
- 33. Lee CW, Lin CC, Lin WN, Liang KC, Luo SF, Wu CB, Wang SW, Yang CM. TNF-α induces MMP-9 expression via activation of Src/EGFR, PDGFR/PI3K/Akt cascade and promotion of NF-κB/p300 binding in human tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 292: L799–L812, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Lee D, Cross SH, Strunk KE, Morgan JE, Bailey CL, Jackson IJ, Threadgill DW. Wa5 is a novel ENU-induced antimorphic allele of the epidermal growth factor receptor. Mamm Genome 15: 525–536, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Levkowitz G, Waterman H, Zamir E, Kam Z, Oved S, Langdon WY, Beguinot L, Geiger B, Yarden Y. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev 12: 3663–3674, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lin WN, Luo SF, Wu CB, Lin CC, Yang CM. Lipopolysaccharide induces VCAM-1 expression and neutrophil adhesion to human tracheal smooth muscle cells: involvement of Src/EGFR/PI3-K/Akt pathway. Toxicol Appl Pharmacol 228: 256–268, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Liu CH, Chang SH, Narko K, Trifan OC, Wu MT, Smith E, Haudenschild C, Lane TF, Hla T. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem 276: 18563–18569, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Luetteke N, Phillips H, Qiu T, Copeland N, Earp H, Jenkins N, Lee D. The mouse waved-2 phenotype results from a point mutation in the EGF receptor tyrosine kinase. Genes Dev 8: 399–413, 1994 [DOI] [PubMed] [Google Scholar]
- 39. Luo JC, Shin VY, Yang YH, Wu WK, Ye YN, So WH, Chang FY, Cho CH. Tumor necrosis factor-α stimulates gastric epithelial cell proliferation. Am J Physiol Gastrointest Liver Physiol 288: G32–G38, 2005 [DOI] [PubMed] [Google Scholar]
- 40. MacDonald TT, Hutchings P, Choy MY, Murch S, Cooke A. Tumour necrosis factor-α and interferon-γ production measured at the single cell level in normal and inflamed human intestine. Clin Exp Immunol 81: 301–305, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. MacEwan DJ. TNF receptor subtype signalling: differences and cellular consequences. Cell Signal 14: 477–492, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Matuk R, Crawford J, Abreu MT, Targan SR, Vasiliauskas EA, Papadakis KA. The spectrum of gastrointestinal toxicity and effect on disease activity of selective cyclooxygenase-2 inhibitors in patients with inflammatory bowel disease. Inflamm Bowel Dis 10: 352–356, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Maudsley S, Pierce KL, Zamah AM, Miller WE, Ahn S, Daaka Y, Lefkowitz RJ, Luttrell LM. The β2-adrenergic receptor mediates extracellular signal-regulated kinase activation via assembly of a multi-receptor complex with the epidermal growth factor receptor. J Biol Chem 275: 9572–9580, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Morson BC. Precancer and cancer in inflammatory bowel disease. Pathology 17: 173–180, 1985 [DOI] [PubMed] [Google Scholar]
- 45. Moyer JD, Barbacci EG, Iwata KK, Arnold L, Boman B, Cunningham A, DiOrio C, Doty J, Morin MJ, Moyer MP, Neveu M, Pollack VA, Pustilnik LR, Reynolds MM, Sloan D, Theleman A, Miller P. Induction of apoptosis and cell cycle arrest by CP-358774, an inhibitor of epidermal growth factor receptor tyrosine kinase. Cancer Res 57: 4838–4848, 1997 [PubMed] [Google Scholar]
- 46. Murch SH, Braegger CP, Walker-Smith JA, MacDonald TT. Location of tumour necrosis factor-α by immunohistochemistry in chronic inflammatory bowel disease. Gut 34: 1705–1709, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oguma K, Oshima H, Aoki M, Uchio R, Naka K, Nakamura S, Hirao A, Saya H, Taketo M, Oshima M. Activated macrophages promote Wnt signalling through tumour necrosis factor-α in gastric tumour cells. EMBO J 27: 1671–1681, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Polk DB. Ontogenic regulation of phospholipase C-γ1 activity and expression in the rat small intestine. Gastroenterology 107: 109–116, 1994 [DOI] [PubMed] [Google Scholar]
- 49. Polk DB, Tong W. Epidermal and hepatocyte growth factors stimulate chemotaxis in an intestinal epithelial cell line. Am J Physiol Cell Physiol 277: C1149–C1159, 1999 [DOI] [PubMed] [Google Scholar]
- 50. Popivanova B, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, Mukaida N. Blocking TNF-α in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest 118: 560–570, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Reuter BK, Asfaha S, Buret A, Sharkey KA, Wallace JL. Exacerbation of inflammation-associated colonic injury in rat through inhibition of cyclooxygenase-2. J Clin Invest 98: 2076–2085, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Riehl T, Newberry RD, Lorenz RG, Stenson W. TNFR1 mediates the radioprotective effects of lipopolysaccharide in the mouse intestine. Am J Physiol Gastrointest Liver Physiol 286: G166–G173, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Roessner A, Kuester D, Malfertheiner P, Schneider-Stock R. Oxidative stress in ulcerative colitis-associated carcinogenesis. Pathol Res Pract 204: 511–524, 2008 [DOI] [PubMed] [Google Scholar]
- 54. Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR, de Villiers WJ, Present D, Sands BE, Colombel JF. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 353: 2462–2476, 2005 [DOI] [PubMed] [Google Scholar]
- 55. Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M, Williams C, Price A, Talbot I, Forbes A. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology 126: 451–459, 2004 [DOI] [PubMed] [Google Scholar]
- 56. Scheinin T, Butler DM, Salway F, Scallon B, Feldmann M. Validation of the interleukin-10 knockout mouse model of colitis: antitumour necrosis factor-antibodies suppress the progression of colitis. Clin Exp Immunol 133: 38–43, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sethi G, Sung B, Aggarwal BB. TNF: a master switch for inflammation to cancer. Front Biosci 13: 5094–5107, 2008 [DOI] [PubMed] [Google Scholar]
- 58. Shao J, Sheng GG, Mifflin RC, Powell DW, Sheng H. Roles of myofibroblasts in prostaglandin E2-stimulated intestinal epithelial proliferation and angiogenesis. Cancer Res 66: 846–855, 2006 [DOI] [PubMed] [Google Scholar]
- 59. Sharon P, Stenson WF. Enhanced synthesis of leukotriene B4 by colonic mucosa in inflammatory bowel disease. Gastroenterology 86: 453–460, 1984 [PubMed] [Google Scholar]
- 60. Sigthorsson G, Simpson RJ, Walley M, Anthony A, Foster R, Hotz-Behoftsitz C, Palizban A, Pombo J, Watts J, Morham SG, Bjarnason I. COX-1 and 2, intestinal integrity, and pathogenesis of nonsteroidal anti-inflammatory drug enteropathy in mice. Gastroenterology 122: 1913–1923, 2002 [DOI] [PubMed] [Google Scholar]
- 61. Singer CA, Baker KJ, McCaffrey A, AuCoin DP, Dechert MA, Gerthoffer WT. p38 MAPK and NF-κB mediate COX-2 expression in human airway myocytes. Am J Physiol Lung Cell Mol Physiol 285: L1087–L1098, 2003 [DOI] [PubMed] [Google Scholar]
- 62. Singer II, Kawka DW, Schloemann S, Tessner T, Riehl T, Stenson WF. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology 115: 297–306, 1998 [DOI] [PubMed] [Google Scholar]
- 63. Singhirunnusorn P, Ueno Y, Matsuo M, Suzuki S, Saiki I, Sakurai H. Transient suppression of ligand-mediated activation of epidermal growth factor receptor by tumor necrosis factor-α through the TAK1-p38 signaling pathway. J Biol Chem 282: 12698–12706, 2007 [DOI] [PubMed] [Google Scholar]
- 64. Sizemore N, Cox AD, Barnard JA, Oldham SM, Reynolds ER, Der CJ, Coffey RJ. Pharmacological inhibition of Ras-transformed epithelial cell growth is linked to down-regulation of epidermal growth factor-related peptides. Gastroenterology 117: 567–576, 1999 [DOI] [PubMed] [Google Scholar]
- 65. Subbaramaiah K, Marmo TP, Dixon DA, Dannenberg AJ. Regulation of cyclooxgenase-2 mRNA stability by taxanes: evidence for involvement of p38, MAPKAPK-2, and HuR. J Biol Chem 278: 37637–37647, 2003 [DOI] [PubMed] [Google Scholar]
- 66. Sureban SM, Ramalingam S, Natarajan G, May R, Subramaniam D, Bishnupuri KS, Morrison AR, Dieckgraefe BK, Brackett DJ, Postier RG, Houchen CW, Anant S. Translation regulatory factor RBM3 is a proto-oncogene that prevents mitotic catastrophe. Oncogene 27: 4544–4556, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Targan SR, Hanauer SB, van Deventer SJ, Mayer L, Present DH, Braakman T, DeWoody KL, Schaible TF, Rutgeerts PJ. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor-α for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med 337: 1029–1035, 1997 [DOI] [PubMed] [Google Scholar]
- 68. Tessner TG, Muhale F, Riehl T, Anant S, Stenson W. Prostaglandin E2 reduces radiation-induced epithelial apoptosis through a mechanism involving Akt activation and bax translocation. J Clin Invest 114: 1676–1685, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Thun MJ, Namboodiri MM, Calle EE, Flanders WD, Heath CW. Aspirin use and risk of fatal cancer. Cancer Res 53: 1322–1327, 1993 [PubMed] [Google Scholar]
- 70. Tsatsanis C, Androulidaki A, Venihaki M, Margioris AN. Signalling networks regulating cyclooxygenase-2. Int J Biochem Cell Biol 38: 1654–1661, 2006 [DOI] [PubMed] [Google Scholar]
- 71. Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell 83: 493–501, 1995 [DOI] [PubMed] [Google Scholar]
- 72. Ueno Y, Sakurai H, Matsuo M, Choo M, Koizumi K, Saiki I. Selective inhibition of TNF-α-induced activation of mitogen-activated protein kinases and metastatic activities by gefitinib. Br J Cancer 92: 1690–1695, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ 10: 45–65, 2003 [DOI] [PubMed] [Google Scholar]
- 74. Whitehead RH, Robinson PS. Establishment of conditionally immortalized epithelial cell lines from the intestinal tissue of adult normal and transgenic mice. Am J Physiol Gastrointest Liver Physiol 296: G455–G460, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wilson AJ, Gibson PR. Role of epidermal growth factor receptor in basal and stimulated colonic epithelial cell migration in vitro. Exp Cell Res 250: 187–196, 1999 [DOI] [PubMed] [Google Scholar]
- 76. Yamaoka T, Yan F, Cao H, Hobbs SS, Dise RS, Tong W, Polk DB. Transactivation of EGF receptor and ErbB2 protects intestinal epithelial cells from TNF-induced apoptosis. Proc Natl Acad Sci USA 105: 11772–11777, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2: 127–137, 2001 [DOI] [PubMed] [Google Scholar]
- 78. Zins K, Abraham D, Sioud M, Aharinejad S. Colon cancer cell-derived tumor necrosis factor-α mediates the tumor growth-promoting response in macrophages by up-regulating the colony-stimulating factor-1 pathway. Cancer Res 67: 1038–1045, 2007 [DOI] [PubMed] [Google Scholar]