Abstract
Clinical studies are evaluating the efficacy of synthetic ghrelin agonists in postoperative ileus management. However, the control of ghrelin secretion under conditions of postoperative gastric ileus is largely unknown. Peripheral somatostatin inhibits ghrelin secretion in animals and humans. We investigated the time course of ghrelin changes postsurgery in fasted rats and whether somatostatin receptor subtype 2 (sst2) signaling is involved. Abdominal surgery (laparotomy and 1-min cecal palpation) induced a rapid and long-lasting decrease in plasma acyl ghrelin levels as shown by the 64, 67, and 59% reduction at 0.5, 2, and 5 h postsurgery, respectively, compared with sham (anesthesia alone for 10 min, P < 0.05). Levels were partly recovered at 7 h and fully restored at 24 h. The percentage of acyl ghrelin reduction was significantly higher than that of desacyl ghrelin at 2 h postsurgery and not at any other time point. This was associated with a 48 and 23% decrease in gastric and plasma ghrelin-O-acyltransferase protein concentrations, respectively (P < 0.001). Ghrelin-positive cells in the oxyntic mucosa expressed sst2a receptor and the sst2 agonist S-346-011 inhibited fasting acyl ghrelin levels by 64 and 77% at 0.5 and 2 h, respectively. The sst2 antagonist S-406-028 prevented the abdominal surgery-induced decreased circulating acyl ghrelin but not the delayed gastric emptying assessed 0.5 h postinjection. These data show that activation of sst2 receptor located on gastric X/A-like cells plays a key role in the rapid inhibition of circulating acyl ghrelin induced by abdominal surgery while not being primarily involved in the early phase of postoperative gastric ileus.
Keywords: gastric emptying, somatostatin receptor 2 agonist, somatostatin receptor 2 antagonist, X/A-like cell
postoperative gastric ileus is a condition that develops as a consequence of surgery and is associated with delayed gastric emptying and intestinal transit (41). Since prolonged postoperative ileus (POI) is an iatrogenic condition leading to prolonged hospitalization resulting in increased health care costs, the development of effective management strategies to prevent POI is clinically important (74). Recently, the food intake stimulating and gastroprokinetic effects of the peptide hormone ghrelin is gaining recognition for the treatment of POI (9). The long-acting synthetic agonist Tranzyme Pharma-101 was reported to accelerate the recovery of the upper and lower gastrointestinal tract in patients undergoing major abdominal surgery (47), consistent with preclinical evidence that peripheral administration of ghrelin agonists reversed the abdominal surgery-induced delayed gastric emptying in rats and dogs (69). However, the regulation of ghrelin under conditions of POI is largely unknown. We recently reported that abdominal surgery decreases plasma levels of acyl and desacyl ghrelin, the two major circulating forms of ghrelin (30), at 30 and 90 min postsurgery (58). Only acyl ghrelin binds to the growth hormone secretagogue receptor (GHS-R) 1a (7) [since then renamed ghrelin (GRLN) receptor (17)] to induce a GRLN receptor-dependent stimulation of gastric motility (21) and food intake (70) whereas desacyl ghrelin's actions are exerted independently of the GRLN receptor (60). Recent evidence indicates that coadministration of desacyl ghrelin counteracts the stimulatory effect of intrathecal injection of ghrelin on propulsive contraction of the rat colon (28), the orexigenic action of intraperitoneal injection of acyl ghrelin in rats (32), and the stimulation of pancreatic polypeptide (PP) secretion in isolated mouse islets (36) suggestive of a negative interaction between both peptides, although the physiological role of desacyl ghrelin is still to be established (33, 60).
The present study aims to characterize the pattern of acyl and desacyl ghrelin alterations over a period of 24 h postsurgery using the recently established RAPID method of blood processing that improves the recovery of acyl ghrelin by 80%, allowing the accurate measurement of circulating acyl ghrelin concentrations (64). Since the newly described ghrelin-O-acyltransferase (GOAT) is the only known enzyme to catalyze the acylation of ghrelin (49), we also measured GOAT protein levels in the gastric oxyntic mucosa and circulation, where it has been recently identified in rodents (62), to correlate changes in GOAT expression and the inhibition of circulating acyl ghrelin postsurgery. To investigate whether, in addition to the reduction of the orexigenic and prokinetic gastric hormone acyl ghrelin, intestinal peptides known to inhibit food intake and gastric emptying such as glucagon-like peptide 1 (GLP-1) (51, 68), peptide YY (PYY) (6, 12), and PP (4, 73) were also altered by abdominal surgery, these hormones were measured over the time course of 24 h postsurgery.
Somatostatin is the main inhibitory peptide of gut hormones that is widely expressed in the gastrointestinal tract (56). In the stomach, somatostatin is released from D cells located in the gastric fundus and antrum mucosa (52). Somatostatin's biological actions are mediated through interaction with five somatostatin receptor (sst) subtypes, sst1–5, that belong to the G protein-coupled receptor family (37). Consistent reports established that somatostatin and octreotide, a stable analog with affinity to sst2 > sst5 > sst3, reduce ghrelin levels as demonstrated in vitro (54), ex vivo in isolated stomach preparations (39, 55), and after peripheral administration in vivo in rats (57) as well as in humans (5). Conversely, somatostatin depletion by cysteamine increased circulating acyl and total ghrelin levels in rats (22) and somatostatin knockout mice display elevated plasma levels of ghrelin and gastric ghrelin mRNA (42), indicating that endogenous somatostatin plays a physiological role in the inhibition of ghrelin synthesis and release in rodents. These data coupled with the fact that the sst2 receptor is the major sst subtype expressed in the rodent stomach (53, 57) raised the possibility that sst2 receptor activation may be primarily involved in the observed suppression of ghrelin elicited by abdominal surgery. Therefore, we assessed whether the recently developed selective peptide sst2 agonist S-346-011 (26) mimics the reduction of ghrelin plasma levels as observed after abdominal surgery and whether pretreatment with the selective sst2 antagonist S-406-028 (11) prevents the early drop in ghrelin induced by abdominal surgery and thereby postoperative gastric ileus. In addition, to provide anatomical support for a direct somatostatin action on gastric X/A-like cells producing ghrelin, we investigated whether sst2a is expressed on these cells in the rat gastric oxyntic mucosa.
MATERIALS AND METHODS
Animals
Adult male Sprague-Dawley rats (Harlan, San Diego, CA) weighing 280–350 g were group housed under controlled illumination (0600–1800 h) and temperature (21–23°C) until the start of experiments. Animals had free access to standard rodent chow (Prolab RMH 2500; LabDiet, PMI Nutrition, Brentwood, MO) and tap water. Protocols were approved by the Institutional Animal Care and Use Committee of the Veterans Administration (no. 05-058-02). All experiments were started between 0900–1000 h.
Peptides
The sst2 agonist S-346-011, H2N-CO-DPhe-c[Cys-Aph(CONH2)-DTrp-Lys-Thr-Cys]-Thr-NH2 (S-346-011), molecular weight 1,132.5, compound 2 in Ref. 26 and the sst2 antagonist S-406-028, H2N-pNO2Phe-DCys-Tyr-DAph(Cbm)-Lys-Thr-Cys-2Nal-NH2, molecular weight 1,208.5, compound 4 in Ref. 11 (Clayton Foundation Laboratories, Salk Institute, La Jolla, CA) were synthesized and purity assessed as previously described (11, 26). Peptides were stored in powder form at −80°C and dissolved in saline containing 0.1% bovine serum albumin (BSA) immediately before use.
Procedures
Intravenous catheterization.
Intravenous catheterization of the right external jugular vein was performed as described before (59). Rats were single housed after surgery and allowed to recover for 3 days. During this period, rats were handled to get acquainted to the experiments including light hand restraint for blood withdrawal. Body weight was monitored before and after the catheterization to assess recovery and assure an anabolic state at the start of experiments.
Abdominal surgery.
Rats were housed in single cages and fasted overnight for 17 h with free access to water. Afterward, animals were exposed to isoflurane (4.5% vapor concentration in oxygen; VSS, Rockmart, GA) and abdominal surgery consisting of median laparotomy (2–3 cm), and cecal palpation for 1 min was performed as described before (61). Anesthesia and surgery lasted 10 min and animals recovered the righting reflex within 2–3 min after removal of isoflurane. The sham group was exposed to 10-min anesthesia alone. After anesthesia, animals were singly housed with access to water but not food up to 24 h postsurgery.
Immunofluorescent Histochemistry
Immunofluorescent double labeling of paraffin-embedded gastric corpus sections from freely fed naive rats (n = 3) euthanized by decapitation was performed as described previously (63). Briefly, after pretreatment with normal goat serum, sections were incubated overnight at 4°C with mouse monoclonal anti-rat ghrelin antibody (1:2,000, Eli Lilly Research Laboratories, Indianapolis, IN, no. D4–7.1 directed against the carboxy terminus) (27) together with polyclonal rabbit anti-sst2a antibody [1:1,000, CURE no. 9452 in 0.3% Triton X-100 in phosphate-buffered saline (PBS)]. The CURE no. 9452 is directed against the COOH-terminus of mouse sst2a (amino acids 331–340), and its specificity was previously established (65). Fluorescein isothiocyanate (FITC)-labeled goat anti-mouse (1:1,000, Jackson ImmunoResearch Laboratories, West Grove, PA) and tetramethylrhodamine-5-(and 6)-isothiocyanate (TRITC)-conjugated goat anti-rabbit IgG (1:1,000, Jackson ImmunoResearch Laboratories) in 0.3% Triton X-100 in PBS were added for 2 h at room temperature. Each incubation step was followed by 3 × 5 min washing in PBS. Slides were mounted, coverslipped with antifade mounting medium (Vector Laboratory, Burlingame, CA), and visualized and photographed by confocal microscopy (Zeiss, LSM 510, Germany). Five low-power fields (×20 objective) of gastric corpus sections were assessed per rat.
Measurements
Plasma acyl and total ghrelin.
Blood (0.5 ml) was withdrawn through the intravenous (iv) catheter and processed according to the recently developed RAPID method as previously described (64). Briefly, blood was diluted 1:10 in ice-cold buffer (pH 3.6) containing 0.1 M ammonium acetate, 0.5 M NaCl, and enzyme inhibitors (diprotin A, E-64-d, antipain, leupeptin, chymostatin, 1 μg/ml; Peptides International, Louisville, KY) and centrifuged at 3,000 rpm for 10 min at 4°C. The supernatant was subjected to Sep-Pak chromatography (C18 cartridges, 360 mg, 55–105 μm, no. WAT051910, Waters, Milford, MA), and the eluted peptide was dried by vacuum centrifugation and stored at −80°C until further processing. Samples were resuspended in double-distilled H2O immediately before radioimmunoassay according to the original plasma volume and duplicates were used to determine total and acyl ghrelin levels via specific radioimmunoassays (nos. GHRT-89HK and GHRA-88HK, respectively; 100% cross-reactivity with rat total and acyl ghrelin, respectively, Millipore, Billerica, MA). Interassay and intra-assay variability were ≤10 and 2%, respectively. Acyl and total ghrelin plasma levels will also include other forms of ghrelin such as des-Gln14-ghrelin, which were detected in low amounts in the stomach (31) since the antibodies used were raised against the NH2- and COOH-terminus, respectively (according to manufacturer's information). Desacyl ghrelin was calculated as the difference of total minus acyl ghrelin for each individual sample. The acyl-to-total ghrelin ratio was calculated for each rat at each time point.
Plasma gut hormone panel.
Blood was withdrawn through the iv catheter and processed by the RAPID method (64) as described above. The measurement of the intestinal peptide hormones GLP-1, PYY, and PP was performed by using the Luminex xMAP technology for rat gut hormones (sensitivity for GLP-1: 5.2 pg/ml, PYY: 8.4 pg/ml and PP: 2.4 pg/ml; rat gut hormone panel, Millipore). This technology allows the simultaneous determination of multiple hormones without cross-reactivity between the anti-analyte antibodies (manufacturer's information, Millipore). All plasma samples (25 μl in duplicates) were processed in one batch and read via the Luminex 100 (Luminex, Austin, TX). The intra-assay variability was ≤7%.
GOAT protein and mRNA expression.
GOAT corpus and plasma levels were assessed as in our previous studies (59, 62). Crude protein fractions of gastric corpus mucosa and plasma were prepared essentially as described before (62). Briefly, venous blood was transferred to tubes containing aprotinin (0.6 trypsin inhibitory unit; ICN Pharmaceuticals, Costa Mesa, CA), EDTA (7.5%, 10 μl/0.5 ml blood; Sigma, St. Louis, MO) and PMSF (1 mM) and immediately centrifuged at 4°C for 10 min at 3,000 g. The plasma was collected and stored at −80°C. Likewise, the stomachs were immediately opened and rinsed, and the corpus mucosa was scraped off and homogenized in ice-cold PBS containing one tablet of protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN) and PMSF (1 mM). Crude protein was obtained by centrifugation of the homogenates at 12,000 g for 20 min at 4°C to remove cell debris and nuclei, and protein concentrations were determined. Western blots were performed as in our previous studies (59, 62) using the anti-GOAT antibody (GenScript, Piscataway, NJ) raised against the amino acids 273–286 of the rat membrane-bound O-acyl transferase (MBOAT4) protein by using the sequence (SEAGQRPGEERYVP) predicted to be in a large loop structure before transmembrane segment 5 (extracellular epitope) (72). The specificity of the anti-GOAT antibody was previously characterized by Western blot of crude rat and mouse gastric mucosal proteins stained with this anti-GOAT antibody by the alkaline phosphatase detection method showing a 50-kDa band corresponding to the expected molecular weight of the GOAT monomer (62). In the present study, the Western blot was analyzed using Scion Image 4.0.3 (Scion, Frederick, MD). The same blot was stained again for β-actin (molecular weight 45 kDa, 1:1,000, no. 4967, Cell Signaling Technology, Danvers, MA) following the protocol described above. Analysis was performed with Scion Image, and gastric GOAT protein expression was normalized to the housekeeping protein, β-actin. Western blots were repeated twice.
Total RNA from the gastric corpus mucosa was isolated and RNA denatured at 65°C for 5 min and used to synthesize first-strand cDNA by reverse transcription by using the ThermoScript RT-PCR system (Invitrogen, CA). Quantitative RT-PCR for GOAT mRNA expression was performed by using the DNA Engine Opticon 2 Detection System interfaced to the Opticon MONITOR Analysis Software version 2.01 (MJ Research, Waltham, MA) in a 20-μl reaction volume. The optimized reaction contained 10 μl of SYBR1 Premix Ex Taq (Perfect Real Time, Takara Mirus Bio, Madison, WI), 1 μl each of oligonucleotide primers (10 mM), 1 μl of the cDNA synthesis reaction, and 7 μl of H2O. Selected primers for GOAT (GenBank accession number NM 001107317) were GGACGACTCTCTCCTTCACG (forward, f) and TCACAGACCAGCACAGGAAG reverse (r) and for the housekeeping gene, hypoxanthine guanine ribotransferase (HPRT, GenBank accession number NM 012583), CAGTCCCAGCGTCGTGATTA (f) and AGCAAGTCTTTCAGTCCTGTC (r) (10). Each amplification was followed by a melting curve resulting in only one peak for each amplicon, indicative of amplification of only one product. This was confirmed by agarose gel electrophoresis of the RT-PCR products. The cycle of threshold CT was determined as the fluorescent signal (binding of SYBR green to double-stranded cDNA) of 1 SD over background. All reactions were carried out in duplicate, and three separate amplifications for each primer pair were performed. Standard curves were constructed with four serial dilution points of control cDNA (combined cDNA from all samples, 100 ng to 100 pg). Data presented were derived from starting quantity values of each sample normalized to the housekeeping gene HPRT. The relative expression ratio of the target gene compared with the reference gene HPRT was calculated by using the Pfaffl equation (45).
Gastric emptying.
Gastric emptying of a nonnutrient viscous solution was determined by the phenol red methylcellulose method as described previously (58). Methylcellulose (Sigma) was dispersed in warm water at a final concentration of 1.5% under continuous stirring. The solution was allowed to cool at 37°C, then phenol red (50 mg/100 ml, Sigma) was added. The phenol red in aqueous methylcellulose was freshly made before use and kept under constant intensity agitation and temperature throughout the experiment. An orogastric gavage of the phenol red methylcellulose solution (1.5 ml) was given to overnight fasted rats that were euthanized 20 min later by CO2 inhalation followed by thoracotomy. Standards were obtained from stomachs collected immediately after intragastric delivery of the solution. The abdominal cavity was opened, pylorus and cardia were clamped, and the stomach was removed, rinsed in saline, and processed for measurement of gastric emptying as detailed in our previous studies (15). Briefly, stomachs were placed in 100 ml of 0.1 N NaOH and homogenized (Polytron, Brinkmann Instruments) for 30 s. The suspension was allowed to settle for 60 min at room temperature and 5 ml of the supernatant added to 0.5 ml of trichloroacetic acid (20% wt/vol). After centrifugation for 20 min, the supernatant was added to 4 ml of 0.5 N NaOH and absorbance of the sample was read at a wavelength of 560 nm with a Shimazu-UV260 spectrophotometer. Gastric emptying was calculated according to the formula: 1 − [amount of phenol red recovered from test stomach/average amount of phenol red recovered from standard stomachs] × 100.
Experimental Protocols
Except otherwise stated, all experiments were performed in rats chronically equipped with an iv catheter and deprived of food with free access to water overnight and throughout the duration of the experiment in keeping with the general clinical practice of fasting before surgical interventions.
Time course of changes in plasma acyl and total ghrelin, GLP-1, PYY, and PP levels induced by abdominal surgery.
Rats were subjected to abdominal surgery or sham (anesthesia alone), and blood (0.5 ml) was withdrawn before and at 2, 5, 7, and 24 h after the procedure and processed for acyl and total ghrelin RIA measurements as well as the plasma intestinal hormones with the Luminex panel.
Changes in plasma GOAT levels and gastric GOAT expression at 2 h after abdominal surgery.
Naive rats underwent abdominal surgery or sham procedure and were euthanized by decapitation 2 h later. Trunk blood and stomach were collected to assess plasma and gastric corpus GOAT protein concentration and gastric GOAT mRNA expression.
Effect of intravenous injection of sst2 agonist on plasma acyl and total ghrelin levels.
Conscious rats were injected iv twice at a 30-min interval with the selective sst2 agonist S-346-011 (100 μg/rat in 200 μl) or vehicle. Blood was withdrawn before the second injection at 0.5 and at 2 h, and then processed for acyl and total ghrelin measurement.
Effect of sst2 antagonist on abdominal surgery-induced decreased plasma acyl and total ghrelin levels.
Conscious rats were injected iv (200 μl) with the sst2 antagonist S-406-028 (100 μg/rat) or vehicle followed by intraperitoneal injection of 25% urethane (1 ml/300 g body wt) known to increase gastric somatostatin mRNA expression and peptide release (71). The control group did not receive any treatment. Rats were euthanized by decapitation 30 min after urethane and trunk blood was processed for acyl and total ghrelin measurement. In other groups of conscious rats, the sst2 antagonist S-406-028 (100 μg/rat) or vehicle was injected iv (200 μl) followed by abdominal surgery and sham procedure. Blood was withdrawn at 0.5 and 1 h after the end of the procedure and processed for acyl and total ghrelin measurement. The dose of the sst2 antagonist was based on our previous functional studies in rats injected with the sst2 antagonist PRL-2903 (35) and adjusted for the 10-times higher binding affinity of S-406-028 than PRL-2903 to the sst2 receptor (11, 50).
Effect of sst2 antagonist on abdominal surgery-induced delayed gastric emptying.
Conscious rats were injected iv (200 μl) with the sst2 antagonist S-406-028 (100 μg/rat) or vehicle followed by abdominal surgery or sham procedure. Rats regained the righting reflex in their cages and 10 min after the end of anesthesia received an orogastric gavage of a nonnutrient phenol red methylcellulose viscous solution. Gastric emptying was assessed 20 min later.
Statistical Analysis
Data are expressed as means ± SE and analyzed by one-way ANOVA followed by Tukey post hoc test or two-way ANOVA followed by Holm-Sidak method. P < 0.05 was considered significant.
RESULTS
Abdominal Surgery Decreases Plasma Acyl and Desacyl Ghrelin Levels
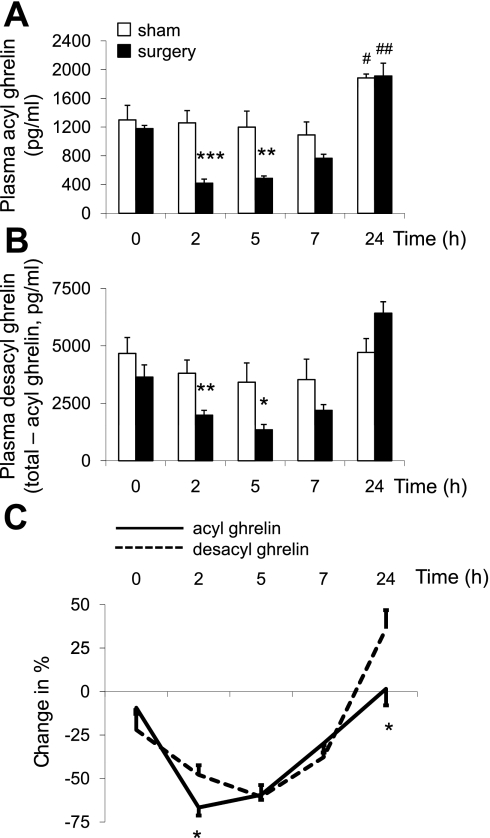
In overnight fasted rats, before procedure, plasma levels of acyl and desacyl ghrelin were not significantly different (P > 0.05; Fig. 1, A and B). Abdominal surgery reduced acyl ghrelin levels by 67 and 59% at 2 and 5 h, respectively, compared with rats exposed to anesthesia alone (P < 0.01). Thereafter, levels were partially recovered at 7 h (−30%) and were fully restored at 24 h with values that were significantly higher at 24 h (41 h fasting) vs. 0 h (17 h fasting) in both abdominal surgery and sham groups (P < 0.05; Fig. 1A). Two-way ANOVA showed a significant impact of treatment (F(1,40) = 21.8, P < 0.001), time (F(4,40) = 22.3, P < 0.001), and treatment × time (F(4,40) = 4.1, P < 0.01). Similarly, desacyl ghrelin plasma levels significantly decreased following abdominal surgery at 2 and 5 h by 48 and 61%, respectively (P < 0.01), partly recovered at 7 h (−38%), and values were fully restored at 24 h compared with sham and similar to those observed at time point 0 h (Fig. 1B). Two-way ANOVA indicated a significant influence of treatment (F(1,40) = 7.3, P < 0.05), time (F(4,40) = 11.7, P < 0.001), and treatment × time (F(4,40) = 4.0, P < 0.01). Comparison of the percentage of suppression showed a significantly higher decrease of acyl ghrelin than desacyl ghrelin at 2 h postsurgery and a higher increase in desacyl ghrelin than acyl ghrelin at 24 h compared with sham (P < 0.05; Fig. 1C). The acyl-to-desacyl ghrelin ratio was 1:5 at 2 h postsurgery compared with 1:3 in sham and both groups at all other time points (P < 0.05).
Fig. 1.
Time course of changes in plasma acyl and desacyl ghrelin levels induced by abdominal surgery in overnight-fasted rats implanted with an intrajugular catheter. After abdominal surgery or sham procedure (anesthesia alone) under 10-min isoflurane exposure, repeated blood samplings were performed through the catheter in conscious rats before and at 2, 5, 7, and 24 h postprocedure and processed for acyl (A) and total ghrelin measurement. Desacyl ghrelin (B) was calculated as the difference of total minus acyl ghrelin for each individual sample. The percentages of changes from basal values in plasma acyl and desacyl ghrelin levels induced by abdominal surgery are shown in C. Each bar represents the means ± SE of 5 rats/group. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. sham at respective time point; #P < 0.05 and ##P < 0.01 vs. respective group at time point 0 h.
In contrast to the pronounced and long-lasting decrease in ghrelin, plasma levels of GLP-1 were not altered, those of PYY were decreased significantly by 36% at 2 h, and PP levels increased by 52% at 5 h postsurgery compared with the sham group and not changed at other time points (P < 0.05; Table 1).
Table 1.
Time course of changes in plasma levels of intestinal hormones after abdominal surgery in rats
| Hormone Plasma Level, pg/ml |
||||||
|---|---|---|---|---|---|---|
| GLP-1 |
PYY |
PP |
||||
| Time point, h | Sham | Surgery | Sham | Surgery | Sham | Surgery |
| 0 | 39.6 ± 3.4 | 42.3 ± 3.5 | 75.0 ± 7.4 | 78.1 ± 13.5 | 66.0 ± 9.7 | 80.0 ± 10.5 |
| 2 | 37.6 ± 2.6 | 36.8 ± 3.7 | 65.0 ± 7.0 | 41.9 ± 3.6* | 55.5 ± 8.4 | 50.4 ± 7.6 |
| 5 | 37.4 ± 4.9 | 39.9 ± 2.5 | 73.4 ± 13.8 | 51.8 ± 4.6 | 64.2 ± 11.4 | 97.3 ± 8.1* |
| 24 | 37.2 ± 5.3 | 27.8 ± 1.8 | 110.4 ± 45.2 | 113.2 ± 26.1 | 105.1 ± 10.2 | 100.9 ± 18.2 |
Rats were fasted overnight and underwent abdominal surgery consisting of laparotomy and cecal palpation (1 min) or anesthesia alone (sham) and levels of glucagon-like peptide 1 (GLP-1), peptide YY (PYY), and pancreatic polypeptide (PP) were assessed before, and at 2, 5, 7, and 24 h postsurgery. Data are presented as means ± SE of 5 rats/group;
P < 0.05 vs. sham.
Abdominal Surgery Decreases Plasma and Stomach GOAT Protein Concentrations
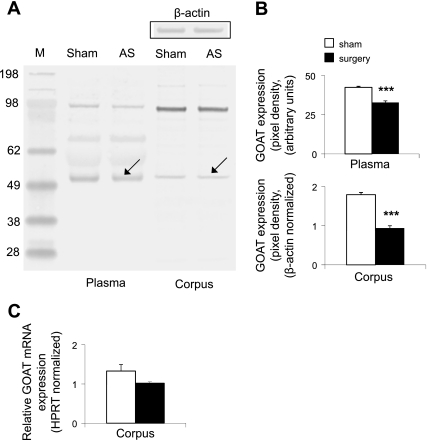
Considering the more pronounced decrease of acyl ghrelin than desacyl ghrelin at 2 h postsurgery, we investigated whether it was associated with changes in GOAT protein expression. Gastric corpus and plasma GOAT protein levels were decreased by 48 and 23%, respectively, compared with sham-treated rats at 2 h postsurgery in overnight fasted rats (P < 0.001, Fig. 2, A and B). At the same time, gastric corpus GOAT mRNA expression assessed by quantitative RT-PCR was not significantly modified in the surgery vs. sham group (1.02 ± 0.04, vs. 1.33 ± 0.17, P = 0.1; Fig. 2C).
Fig. 2.
Abdominal surgery decreases plasma and gastric corpus ghrelin-O-acyltransferase (GOAT) protein concentrations but does not alter gastric GOAT mRNA expression at 2 h postsurgery. Abdominal surgery or sham (n = 5/group) was performed under 10 min isoflurane exposure and 2 h later, animals were euthanized by decapitation, and trunk blood and stomach were collected to assess circulating and gastric GOAT protein concentrations and gastric GOAT mRNA expression. Lane 1, molecular weight standard marker (M); lane 2, plasma proteins after sham; lane 3, plasma proteins after abdominal surgery (AS); lane 4, gastric corpus mucosa proteins after sham; lane 5, gastric corpus mucosa proteins after abdominal surgery (A). Western blot shows 2 dominant bands at ∼50 and ∼100 kDa. The 50-kDa band most likely represents monomeric GOAT, whereas the 100-kDa band most likely represents an SDS-stable dimer. Abdominal surgery reduced the 50-kDa band (arrow) compared with sham, indicating reduced plasma and gastric GOAT protein concentrations quantified in B. Restaining of the Western blot for β-actin demonstrated equal gastric corpus mucosal protein concentration (A, inset). GOAT mRNA levels were assessed by real-time quantitative RT-PCR. Data are derived from starting quantity values of each sample normalized to the housekeeping gene HPRT. The relative expression ratio of the target gene compared with the reference gene HPRT was calculated via the Pfaffl equation (C). ***P < 0.001 vs. sham.
The sst2 Agonist Decreases Fasting Plasma Acyl and Desacyl Ghrelin Levels
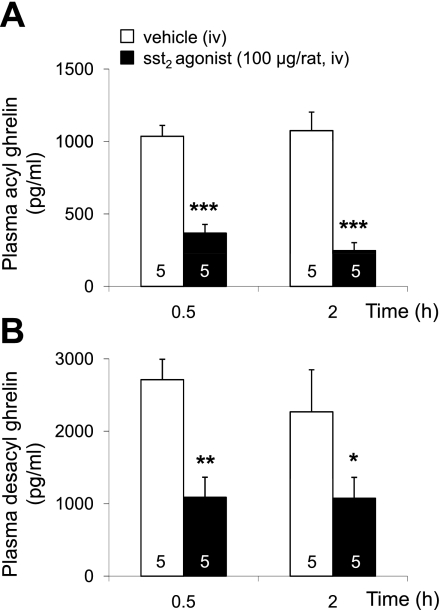
The sst2 agonist S-346-011 (100 μg/rat) injected iv twice at a 30-min interval significantly reduced fasting acyl ghrelin levels compared with iv vehicle at 0.5 and 2 h by 64 and 77%, respectively (P < 0.001, Fig. 3A). Likewise, desacyl ghrelin levels were reduced by 60 and 53%, respectively, at 0.5 and 2 h compared with vehicle (P < 0.05, Fig. 3B). Because of the more pronounced decrease of acyl ghrelin than desacyl ghrelin at 2 h, the acyl-to-desacyl ghrelin ratio was reduced to 1:5 compared with 1:3 in vehicle-treated rats (P < 0.05). Two-way ANOVA showed a significant impact of treatment (F(1,15) = 89.3, P < 0.001), whereas time had no effect (F(1,15) = 0.3, P > 0.05).
Fig. 3.
The somatostatin receptor subtype 2 (sst2) agonist decreases circulating acyl and desacyl ghrelin levels in overnight-fasted rats implanted with an intrajugular catheter. The sst2 agonist (100 μg/rat in 200 μl saline containing 0.1% BSA) or vehicle (saline containing 0.1% BSA) was injected intravenously (iv) twice at 0 and 0.5 h. Blood was withdrawn before the second injection at 0.5 and at 2 h and processed for acyl (A) and total ghrelin measurement. Desacyl ghrelin (B) was calculated as the difference of total minus acyl ghrelin for each individual sample. Each bar represents the means ± SE of number of rats indicated at the bottom of the column. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. vehicle.
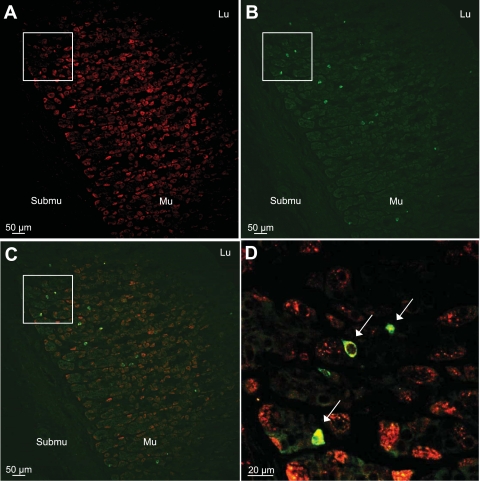
Ghrelin-Positive Cells Express the sst2a Receptor in Naive Rats
Fluorescent immunostaining in naive rats detected sst2a-positive cells distributed throughout the gastric oxyntic mucosa (Fig. 4A). Ghrelin-positive cells were localized mainly in the mid portion as well as the lower third of the rat gastric corpus glands (Fig. 4B). All ghrelin-positive cells were also immunoreactive for sst2a (Fig. 4, C and D). Omission of the primary antibody resulted in complete absence of the immunosignals.
Fig. 4.
Ghrelin-positive X/A-like cells in the gastric oxyntic mucosa express the sst2a receptor in naive rats. Paraffin-embedded gastric corpus sections of ad libitum-fed rats were processed for immunofluorescent double labeling. Confocal microscopy shows the localization of sst2a-immunopositive cells throughout the gastric oxyntic mucosa (A, red), whereas ghrelin-positive cells are localized mostly in the middle and the lower part of the glands (B, green). The picture in C and the higher magnification (D) show that ghrelin-positive cells express the sst2a receptor (arrow). Lu, lumen; Mu, mucosa; Submu, submucosa.
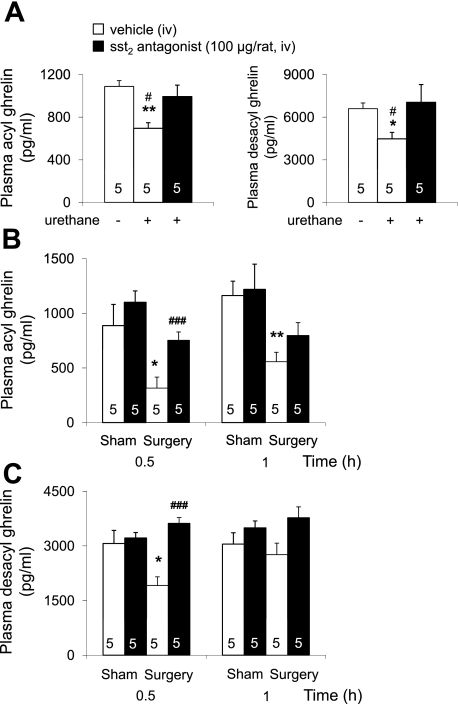
The sst2 Antagonist Prevents the Abdominal Surgery-Induced Decrease in Acyl and Desacyl Ghrelin Levels Whereas the Delayed Gastric Emptying Is Not Altered
We first assessed the acyl ghrelin response and sst2 antagonist effect to an intraperitoneal injection of 25% urethane known to induce a somatostatin-dependent inhibition of circulating gastrin and gastric acid secretion (71). Urethane decreased fasting acyl and desacyl ghrelin plasma levels within 30 min by 36 and 32%, respectively, in iv vehicle-injected rats compared with overnight-fasted conscious nontreated rats (P < 0.05; Fig. 5A). The sst2 antagonist S-406-028 (100 μg/rat iv) restored acyl and desacyl ghrelin levels in urethane-anesthetized rats to those of fasted naive animals (Fig. 5A).
Fig. 5.
The sst2 antagonist blocks urethane-induced reduction of ghrelin and prevents abdominal surgery-induced decrease of circulating acyl and desacyl ghrelin levels in overnight fasted rats. A: the sst2 antagonist (100 μg/rat in 200 μl saline containing 0.1% BSA) or vehicle (saline containing 0.1% BSA) was injected iv followed by intraperitoneal injection of 25% urethane compared with rats that received no treatment. Animals were euthanized by decapitation 30 min later, and trunk blood was processed for acyl and total ghrelin measurement. *P < 0.05 and **P < 0.01 vs. vehicle/no urethane; #P < 0.05 vs. sst2 antagonist/urethane. B and C: the sst2 antagonist (100 μg/rat in 200 μl saline containing 0.1% BSA) or vehicle (saline containing 0.1% BSA) was injected iv followed by abdominal surgery or sham procedure. Blood was withdrawn at 0.5 and 1 h after the end of the procedure and processed for acyl ghrelin measurement (B), and desacyl ghrelin (C) was calculated as the difference of total minus acyl ghrelin for each individual sample. *P < 0.05 and **P < 0.01 vs. vehicle/sham; ###P < 0.001 vs. sst2 antagonist/surgery. Each bar represents the means ± SE of number of rats indicated at the bottom of the column.
In iv vehicle-pretreated rats, abdominal surgery significantly reduced acyl ghrelin plasma levels by 64 and 52% at 0.5 and 1 h, respectively, postsurgery compared with sham (P < 0.05; Fig. 5B). By contrast, in rats injected with the sst2 antagonist S-406-028 (100 μg/rat iv) at a dose preventing endogenous somatostatin action on ghrelin secretion, the suppression of acyl ghrelin was completely prevented at 0.5 h and partially at 1 h postsurgery. Likewise, the sst2 antagonist completely prevented the abdominal surgery-induced decrease in desacyl ghrelin plasma levels at 0.5 h postsurgery (Fig. 5C). In the sham group, the sst2 antagonist S-406-028 had no significant effect on fasting acyl and desacyl ghrelin levels (Figs. 5, B and C).
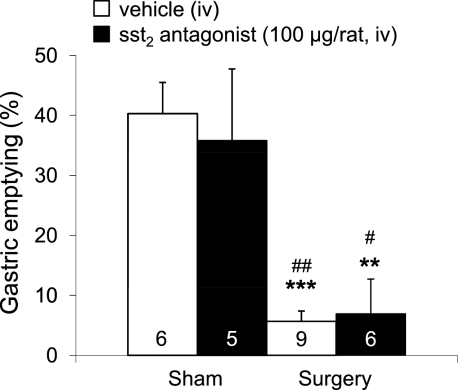
Abdominal surgery delayed gastric emptying by 86% compared with sham (P < 0.001), as assessed at 0.5 h postsurgery. However, gastric ileus was not altered by pretreatment with the sst2 antagonist S-406-028 (Fig. 6). The sst2 antagonist had no effect on gastric emptying under sham treatment conditions (Fig. 6).
Fig. 6.
The sst2 antagonist does not prevent the abdominal surgery-induced decrease in gastric emptying. Overnight-fasted rats were injected iv with sst2 antagonist (100 μg/rat in 200 μl saline containing 0.1% BSA) or vehicle (saline containing 0.1% BSA) followed by abdominal surgery or sham procedure. Rats received an orogastric gavage of a nonnutrient solution (1.5 ml) and gastric emptying was assessed 20 min later. Each bar represents the means ± SE of number of rats indicated at the bottom of the column. **P < 0.01 and ***P < 0.001 vs. vehicle/sham; #P < 0.05 and ##P < 0.01 vs. sst2 antagonist/sham.
DISCUSSION
In the present study we show that abdominal surgery consisting of laparotomy and short cecal palpation decreases circulating acyl and desacyl ghrelin levels with a rapid onset (within 30 min) that was maintained reduced by 67–59% for 5 h and then levels were fully restored at 24 h postsurgery. The suppression of ghrelin levels was induced by the abdominal surgery per se and did not result from the short anesthesia since the sham group exposed to the same duration (10 min) of anesthesia without surgery had stable acyl and desacyl ghrelin plasma levels throughout the 7-h observation period. These data expand our previous report showing that abdominal surgery performed under similar conditions reduced acyl ghrelin plasma levels at 30 and 90 min postsurgery (58). Plasma acyl ghrelin in fasted rats during the 30 min to 5 h period postsurgery were in the 315.3–486.6 pg/ml range, which were similar to levels of ad libitum-fed rats (391.5 pg/ml) previously detected with the RAPID method of blood processing (64). These data are indicative that abdominal surgery induces a fed state level of acyl ghrelin in fasted rats. In contrast, circulating concentrations of other gut hormones inhibiting food intake and gastric emptying, namely GLP-1, PYY, and PP (4, 6, 12, 51, 68, 73), were not or were only temporarily altered with a 36% decrease of PYY at 2 h and a 52% increase of PP at 5 h postsurgery and no changes in GLP-1 compared with sham. These results point toward the specificity of abdominal surgery to impact primarily on circulating levels of acyl ghrelin, inducing a rapid-onset and long-lasting suppression of this hormone. Whether the suppression of acyl ghrelin is a hallmark response to any type of surgery or specific to abdominal surgery remains to be assessed. However, the definition of postoperative ileus does not require abdominal surgery (40) and equally applies to other surgical interventions. Previously, we showed in rats that trepanation, similarly to abdominal surgery, induced a rapid-onset delayed gastric emptying that involves activation of brain corticotropin-releasing factor signaling pathways (66). Therefore, it is likely that also other types of surgery can affect circulating ghrelin levels.
During the 30 min to 2 h period postsurgery, circulating acyl ghrelin levels dropped more sharply than those of desacyl ghrelin, resulting in a decreased acyl-to-desacyl ghrelin ratio of 1:5 compared with 1:3 in the sham group at these same time points or the surgery group at 5, 7 or 24 h. The drop in acyl-to-desacyl ghrelin at 2 h postsurgery was investigated in the context of recent demonstration that GOAT is the unique enzyme highly conserved across vertebrates able to catalyze the octanoylation of ghrelin, leading to the active form of the peptide (27, 72). In the present study, abdominal surgery induced a 48% reduction of GOAT protein expression in the gastric corpus mucosa and there was also a 23% reduction in plasma GOAT at 2 h postsurgery compared with sham. The decrease of GOAT protein expression in the gastric corpus mucosa was not associated with a significant reduction of GOAT mRNA levels. The observed reduction in gastric and plasmatic GOAT protein may have a bearing with the parallel 67% decrease in circulating acyl ghrelin observed 2 h after abdominal surgery. However, this will need to be ascertained also at the level of GOAT enzyme activity by use of enzyme-linked click-chemistry (cat-ELCCA), which was recently developed (25). Furthermore, in addition to reduced GOAT protein expression, it cannot be ruled out that other mechanisms participating in the secretion rate of acyl ghrelin or changes in the activity of other enzymes, namely hepatic esterases involved in deacylation (19) may also contribute to the observed altered ratio of acyl to desacyl ghrelin.
Several sets of pharmacological and anatomical evidence support that mechanisms underlying the reduction of plasma ghrelin levels induced by abdominal surgery involve the activation of peripheral gastric sst2 receptors. First, it is well recognized that ghrelin-positive X/A-like cells distributed throughout the gastric oxyntic mucosa (16, 43) (present study) are the main source of circulating acyl ghrelin (3) as demonstrated by the decline in ghrelin levels following gastrectomy in rats (38). Second, we provided the first evidence that sst2a receptor protein is localized on ghrelin-positive X/A-like cells of the rat oxyntic mucosa. Third, iv injection of the selective sst2 agonist S-346-011 (IC50 7.5–20 nM) (26) induced a rapid decrease in fasting acyl ghrelin levels as well as reduced the acyl-to-desacyl ghrelin ratio to 1:5, mimicking the rapid suppression and 1:5 ratio induced by abdominal surgery. Lastly, the selective sst2 antagonist S-406-028 (IC50 2.6 ± 0.7 nM) (11) completely prevented the abdominal surgery-induced rapid decrease in circulating acyl ghrelin levels at 0.5 h postsurgery. The partial reduction at 1 h could be due to the relatively short half-life of the compound as previously reported for another peptide sst2 antagonist (35). We also used urethane anesthesia, known to increase rat gastric somatostatin mRNA levels and activate endogenous gastric somatostatin signaling within 30 min postinjection (71). In this urethane model, fasting plasma levels of acyl ghrelin were inhibited and the sst2 antagonist injected iv restored ghrelin levels within 0.5 h to those observed in fasted conscious nontreated rats. We previously demonstrated that urethane under similar conditions induces a somatostatin-dependent inhibition of plasma levels of gastrin (71). The present findings expand the inhibitory effect of urethane mediated by the activation of somatostatin signaling to another gastric hormone, acyl ghrelin. The sst2-dependent inhibition of ghrelin release observed after abdominal surgery suggests the release of somatostatin under these conditions. Potential mechanisms may involve capsaicin-sensitive afferents containing calcitonin gene-related peptide (CGRP) signaling known to be activated by abdominal surgery (29, 46, 75). There is also an established linkage between capsaicin-sensitive afferent stimulation containing CGRP and the increase in gastric somatostatin release (8, 34, 48). Collectively, these data support the contention that the reduction of circulating ghrelin levels in rats induced by peripheral administration of somatostatin (18, 55, 57) or sst2 agonist (present study) and by endogenous somatostatin released by urethane or likely by abdominal surgery involves the activation of sst2 receptors expressed on gastric X/A-like cells. Of interest, recent in vitro studies in mouse primary pituitary cell cultures showed that somatostatin inhibits GOAT mRNA expression monitored after 24-h incubation and, conversely, somatostatin knockout mice displayed high GOAT mRNA expression (23). Therefore, it can be speculated that the observed reduced gastric GOAT protein expression induced by abdominal surgery may also be related to the increased somatostatin signaling under these conditions.
Although the iv injection of sst2 antagonist completely prevents the decrease of acyl and desacyl ghrelin plasma levels, the abdominal surgery-induced delayed gastric emptying is not altered by pretreatment with the sst2 antagonist. These data are indicative that normalizing ghrelin levels to physiological fasting concentrations immediately postsurgery is not sufficient to restore basal gastric emptying. In addition, this shows that peripheral sst2 receptors are not playing a primary role in the delayed gastric emptying immediately postsurgery. However, the functional significance of preventing the sustained drop in circulating ghrelin in the overall recovery of postoperative ileus and food intake will need to be further ascertained with treatment resulting in sustained blockade of the somatostatin-sst2 signaling. These findings are, however, congruent with clinical studies reporting that only pharmacological doses of ghrelin exert gastroprokinetic effects under conditions of gastroparesis (44, 67), whereas lower doses that are able to stimulate growth hormone release do not enhance gastric emptying (13). Similarly, in rats gastric electrical stimulation increased gastric ghrelin production and release associated with increased central hunger signaling, whereas gastric emptying was not altered (24).
In conclusion, we provided the first evidence that abdominal surgery induces a rapid and long-lasting reduction of circulating levels of acyl ghrelin. This response is selective to ghrelin since abdominal surgery did not similarly alter other gut hormones, namely GLP-1, PP, and PYY. Pharmacological interventions with sst2 agonist and antagonist along with the colocalization of sst2 receptor immunoreactivity on ghrelin cells of the gastric mucosa support that the reduction of fasting ghrelin levels by abdominal surgery is mediated through activation of gastric sst2 receptor signaling. In addition, the rapid drop in plasma acyl ghrelin was associated with a prominent decrease of GOAT protein concentrations in the gastric corpus mucosa and, to a lesser extent, in the plasma supporting a link between the drop in GOAT protein and acyl ghrelin levels induced by abdominal surgery. Clinical relevance of these pathways is supported by the increase of somatostatin serum levels following surgery involving the peritoneal cavity (1), the recent localization of sst2a immunoreactivity on ghrelin cells in the human gastric mucosa (20), and the demonstration that the preferential sst2 agonist octreotide also inhibits ghrelin release in humans (5). Whether sustained prevention of the long-lasting drop in ghrelin levels by reduction of somatostatin-sst2 signaling after abdominal surgery will restore feeding behavior and digestive functions linked with increased circulating levels of ghrelin (2, 14) will need to be further investigated by sustained blockade of this pathway. However, based on the present and previous studies (69), the restoration of surgically inhibited gastrointestinal motor function seems to require supraphysiological doses of ghrelin to exert a prokinetic effect within the immediate period postsurgery.
GRANTS
This research was supported by National Institutes of Health (NIH) Grant R01 DK-33061 (Y. Taché), NIH Center Grant DK-41301 (Animal Core, Y. Taché), and Veterans Affairs (VA) Merit Awards (Y. Taché and N. W. G. Lambrecht) and VA Research Career Scientist Award (Y. Taché). J. Rivier is the Dr. Frederik Paulsen Chair in Neuroscience Professor.
DISCLOSURES
A. Stengel, M. Gobel-Stengel, L. Wang, A. Shaikh, N. W. G. Lambrecht, and Y. Taché have nothing to disclose. J. Rivier is Founder of Sentia Medical Sciences; no conflicts of interest exist.
ACKNOWLEDGMENTS
We thank Dr. Tamer Coskun (Eli Lilly) for providing the anti-ghrelin antibody, Honghui Liang for excellent technical support, and Eugenia Hu for reviewing the manuscript.
REFERENCES
- 1. Antal A, Nemeth J, Szolcsanyi J, Pozsgai G, Pinter E. Abdominal surgery performed under general anesthesia increases somatostatin-like immunoreactivity in human serum. Neuroimmunomodulation 15: 153–156, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Ariga H, Tsukamoto K, Chen C, Mantyh C, Pappas TN, Takahashi T. Endogenous acyl ghrelin is involved in mediating spontaneous phase III-like contractions of the rat stomach. Neurogastroenterol Motil 19: 675–680, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, Shirakami G, Usui T, Shimatsu A, Doi K, Hosoda H, Kojima M, Kangawa K, Nakao K. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab 86: 4753–4758, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Asakawa A, Inui A, Ueno N, Fujimiya M, Fujino MA, Kasuga M. Mouse pancreatic polypeptide modulates food intake, while not influencing anxiety in mice. Peptides 20: 1445–1448, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Barkan AL, Dimaraki EV, Jessup SK, Symons KV, Ermolenko M, Jaffe CA. Ghrelin secretion in humans is sexually dimorphic, suppressed by somatostatin, and not affected by the ambient growth hormone levels. J Clin Endocrinol Metab 88: 2180–2184, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature 418: 650–654, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Bednarek MA, Feighner SD, Pong SS, McKee KK, Hreniuk DL, Silva MV, Warren VA, Howard AD, Van Der Ploeg LH, Heck JV. Structure-function studies on the new growth hormone-releasing peptide, ghrelin: minimal sequence of ghrelin necessary for activation of growth hormone secretagogue receptor 1a. J Med Chem 43: 4370–4376, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Bunnett NW, Helton WS, Debas HT, Ensinck JW. CGRP stimulates the release of pro-somatostatin-derived peptides from the gastric fundus. Am J Physiol Gastrointest Liver Physiol 258: G316–G319, 1990 [DOI] [PubMed] [Google Scholar]
- 9. Camilleri M, Papathanasopoulos A, Odunsi ST. Actions and therapeutic pathways of ghrelin for gastrointestinal disorders. Nat Rev Gastroenterol Hepatol 6: 343–352, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Caminos JE, Bravo SB, Gonzalez CR, Garces MF, Cepeda LA, Gonzalez AC, Cordido F, Lopez M, Dieguez C. Food-intake-regulating-neuropeptides are expressed and regulated through pregnancy and following food restriction in rat placenta. Reprod Biol Endocrinol 6: 14, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cescato R, Erchegyi J, Waser B, Piccand V, Maecke HR, Rivier JE, Reubi JC. Design and in vitro characterization of highly sst2-selective somatostatin antagonists suitable for radiotargeting. J Med Chem 51: 4030–4037, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chelikani PK, Haver AC, Reidelberger RD. Comparison of the inhibitory effects of PYY(3–36) and PYY(1–36) on gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol 287: R1064–R1070, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Cremonini F, Camilleri M, Vazquez Roque M, McKinzie S, Burton D, Baxter K, Zinsmeister AR. Obesity does not increase effects of synthetic ghrelin on human gastric motor functions. Gastroenterology 131: 1431–1439, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50: 1714–1719, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Czimmer J, Million M, Taché Y. Urocortin 2 acts centrally to delay gastric emptying through sympathetic pathways while CRF and urocortin 1 inhibitory actions are vagal dependent in rats. Am J Physiol Gastrointest Liver Physiol 290: G511–G518, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 141: 4255–4261, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Davenport AP, Bonner TI, Foord SM, Harmar AJ, Neubig RR, Pin JP, Spedding M, Kojima M, Kangawa K. International Union of Pharmacology. LVI. Ghrelin receptor nomenclature, distribution, and function. Pharmacol Rev 57: 541–546, 2005 [DOI] [PubMed] [Google Scholar]
- 18. de la Cour CD, Norlen P, Hakanson R. Secretion of ghrelin from rat stomach ghrelin cells in response to local microinfusion of candidate messenger compounds: a microdialysis study. Regul Pept 143: 118–126, 2007 [DOI] [PubMed] [Google Scholar]
- 19. De Vriese C, Gregoire F, Lema-Kisoka R, Waelbroeck M, Robberecht P, Delporte C. Ghrelin degradation by serum and tissue homogenates: identification of the cleavage sites. Endocrinology 145: 4997–5005, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Fischer T, Doll C, Jacobs S, Kolodziej A, Stumm R, Schulz S. Reassessment of sst2 somatostatin receptor expression in human normal and neoplastic tissues using the novel rabbit monoclonal antibody UMB-1. J Clin Endocrinol Metab 93: 4519–4524, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Fujino K, Inui A, Asakawa A, Kihara N, Fujimura M, Fujimiya M. Ghrelin induces fasted motor activity of the gastrointestinal tract in conscious fed rats. J Physiol 550: 227–240, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fukuhara S, Suzuki H, Masaoka T, Arakawa M, Hosoda H, Minegishi Y, Kangawa K, Ishii H, Kitajima M, Hibi T. Enhanced ghrelin secretion in rats with cysteamine-induced duodenal ulcers. Am J Physiol Gastrointest Liver Physiol 289: G138–G145, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Gahete MD, Cordoba-Chacon J, Salvatori R, Castano JP, Kineman RD, Luque RM. Metabolic regulation of ghrelin O-acyl transferase (GOAT) expression in the mouse hypothalamus, pituitary, and stomach. Mol Cell Endocrinol 317: 154–160, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gallas S, Sinno MH, Boukhettala N, Coeffier M, Dourmap N, Gourcerol G, Ducrotte P, Dechelotte P, Leroi AM, Fetissov SO. Gastric electrical stimulation increases ghrelin production and inhibits catecholaminergic brainstem neurons in rats. Eur J Neurosci 33: 276–284, 2011 [DOI] [PubMed] [Google Scholar]
- 25. Garner AL, Janda KD. cat-ELCCA: a robust method to monitor the fatty acid acyltransferase activity of ghrelin O-acyltransferase (GOAT). Angew Chem Int Ed Engl 49: 9630–9634, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grace CR, Erchegyi J, Koerber SC, Reubi JC, Rivier J, Riek R. Novel sst2-selective somatostatin agonists. Three-dimensional consensus structure by NMR. J Med Chem 49: 4487–4496, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, Witcher DR, Luo S, Onyia JE, Hale JE. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA 105: 6320–6325, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hirayama H, Shiina T, Shima T, Kuramoto H, Takewaki T, BF J, Shimizu Y. Contrasting effects of ghrelin and des-acyl ghrelin on the lumbo-sacral defecation center and regulation of colorectal motility in rats. Neurogastroenterol Motil 22: 1124–1131, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Holzer P, Lippe IT, Holzer-Petsche U. Inhibition of gastrointestinal transit due to surgical trauma or peritoneal irritation is reduced in capsaicin-treated rats. Gastroenterology 91: 360–363, 1986 [DOI] [PubMed] [Google Scholar]
- 30. Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun 279: 909–913, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Hosoda H, Kojima M, Matsuo H, Kangawa K. Purification and characterization of rat des-Gln14-Ghrelin, a second endogenous ligand for the growth hormone secretagogue receptor. J Biol Chem 275: 21995–22000, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Inhoff T, Mönnikes H, Noetzel S, Stengel A, Goebel M, Dinh QT, Riedl A, Bannert N, Wisser AS, Wiedenmann B, Klapp BF, Taché Y, Kobelt P. Desacyl ghrelin inhibits the orexigenic effect of peripherally injected ghrelin in rats. Peptides 29: 2159–2168, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Inhoff T, Wiedenmann B, Klapp BF, Mönnikes H, Kobelt P. Is desacyl ghrelin a modulator of food intake? Peptides 30: 991–994, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Inui T, Kinoshita Y, Yamaguchi A, Yamatani T, Chiba T. Linkage between capsaicin-stimulated calcitonin gene-related peptide and somatostatin release in rat stomach. Am J Physiol Gastrointest Liver Physiol 261: G770–G774, 1991 [DOI] [PubMed] [Google Scholar]
- 35. Kawakubo K, Coy DH, Walsh JH, Taché Y. Urethane-induced somatostatin mediated inhibition of gastric acid: reversal by the somatostatin 2 receptor antagonist PRL-2903. Life Sci 65: PL115–PL120, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Kumar R, Salehi A, Rehfeld JF, Hoglund P, Lindstrom E, Hakanson R. Proghrelin peptides: desacyl ghrelin is a powerful inhibitor of acylated ghrelin, likely to impair physiological effects of acyl ghrelin but not of obestatin A study of pancreatic polypeptide secretion from mouse islets. Regul Pept 164: 65–70, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Kumar U, Grant M. Somatostatin and somatostatin receptors. Results Probl Cell Differ 50: 137–184, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Lehto-Axtelius D, Chen D, Surve VV, Hakanson R. Post-gastrectomy osteopenia in the rat: bone structure is preserved by retaining 10%-30% of the oxyntic gland area. Scand J Gastroenterol 37: 437–443, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Lippl F, Kircher F, Erdmann J, Allescher HD, Schusdziarra V. Effect of GIP, GLP-1, insulin and gastrin on ghrelin release in the isolated rat stomach. Regul Pept 119: 93–98, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Livingston EH, Passaro EP., Jr Postoperative ileus. Dig Dis Sci 35: 121–132, 1990 [DOI] [PubMed] [Google Scholar]
- 41. Luckey A, Livingston E, Taché Y. Mechanisms and treatment of postoperative ileus. Arch Surg 138: 206–214, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Luque RM, Gahete MD, Hochgeschwender U, Kineman RD. Evidence that endogenous SST inhibits ACTH and ghrelin expression by independent pathways. Am J Physiol Endocrinol Metab 291: E395–E403, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Mizutani M, Atsuchi K, Asakawa A, Matsuda N, Fujimura M, Inui A, Kato I, Fujimiya M. Localization of acyl ghrelin- and des-acyl ghrelin-immunoreactive cells in the rat stomach and their responses to intragastric pH. Am J Physiol Gastrointest Liver Physiol 297: G974–G980, 2009 [DOI] [PubMed] [Google Scholar]
- 44. Murray CD, Martin NM, Patterson M, Taylor SA, Ghatei MA, Kamm MA, Johnston C, Bloom SR, Emmanuel AV. Ghrelin enhances gastric emptying in diabetic gastroparesis: a double blind, placebo controlled, crossover study. Gut 54: 1693–1698, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Plourde V, Wong HC, Walsh JH, Raybould HE, Taché Y. CGRP antagonists and capsaicin on celiac ganglia partly prevent postoperative gastric ileus. Peptides 14: 1225–1229, 1993 [DOI] [PubMed] [Google Scholar]
- 47. Popescu I, Fleshner PR, Pezzullo JC, Charlton PA, Kosutic G, Senagore AJ. The Ghrelin agonist TZP-101 for management of postoperative ileus after partial colectomy: a randomized, dose-ranging, placebo-controlled clinical trial. Dis Colon Rectum 53: 126–134, 2010 [DOI] [PubMed] [Google Scholar]
- 48. Ren J, Young RL, Lassiter DC, Harty RF. Calcitonin gene-related peptide mediates capsaicin-induced neuroendocrine responses in rat antrum. Gastroenterology 104: 485–491, 1993 [DOI] [PubMed] [Google Scholar]
- 49. Romero A, Kirchner H, Heppner K, Pfluger P, Tschoep M, Nogueiras R. GOAT-the master switch for the ghrelin system? Eur J Endocrinol 163: 1–8, 2010 [DOI] [PubMed] [Google Scholar]
- 50. Rossowski WJ, Cheng BL, Taylor JE, Datta R, Coy DH. Human urotensin II-induced aorta ring contractions are mediated by protein kinase C, tyrosine kinases and Rho-kinase: inhibition by somatostatin receptor antagonists. Eur J Pharmacol 438: 159–170, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Ruttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology 150: 1174–1181, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sachs G, Zeng N, Prinz C. Physiology of isolated gastric endocrine cells. Annu Rev Physiol 59: 243–256, 1997 [DOI] [PubMed] [Google Scholar]
- 53. Schindler M, Humphrey PP. Differential distribution of somatostatin sst2 receptor splice variants in rat gastric mucosa. Cell Tissue Res 297: 163–168, 1999 [DOI] [PubMed] [Google Scholar]
- 54. Seoane LM, Al-Massadi O, Barreiro F, Dieguez C, Casanueva FF. Growth hormone and somatostatin directly inhibit gastric ghrelin secretion. An in vitro organ culture system. J Endocrinol Invest 30: RC22–RC25, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Shimada M, Date Y, Mondal MS, Toshinai K, Shimbara T, Fukunaga K, Murakami N, Miyazato M, Kangawa K, Yoshimatsu H, Matsuo H, Nakazato M. Somatostatin suppresses ghrelin secretion from the rat stomach. Biochem Biophys Res Commun 302: 520–525, 2003 [DOI] [PubMed] [Google Scholar]
- 56. Shulkes A. Somatostatin: physiology and clinical applications. Baillieres Clin Endocrinol Metab 8: 215–236, 1994 [DOI] [PubMed] [Google Scholar]
- 57. Silva AP, Bethmann K, Raulf F, Schmid HA. Regulation of ghrelin secretion by somatostatin analogs in rats. Eur J Endocrinol 152: 887–894, 2005 [DOI] [PubMed] [Google Scholar]
- 58. Stengel A, Goebel M, Luckey A, Yuan PQ, Wang L, Taché Y. Cold ambient temperature reverses abdominal surgery-induced delayed gastric emptying and decreased plasma ghrelin levels in rats. Peptides 31: 2229–2235, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Stengel A, Goebel M, Wang L, Reeve JR, Jr, Taché Y, Lambrecht NW. Lipopolysaccharide differentially decreases plasma acyl and desacyl ghrelin levels in rats: potential role of the circulating ghrelin-acylating enzyme GOAT. Peptides 31: 1689–1696, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Stengel A, Goebel M, Wang L, Taché Y. Ghrelin, des-acyl ghrelin and nesfatin-1 in gastric X/A-like cells: role as regulators of food intake and body weight. Peptides 31: 357–369, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Stengel A, Goebel M, Wang L, Taché Y. Abdominal surgery activates nesfatin-1 immunoreactive brain nuclei in rats. Peptides 31: 263–270, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stengel A, Goebel M, Wang L, Taché Y, Sachs G, Lambrecht NW. Differential distribution of ghrelin-O-acyltransferase (GOAT) immunoreactive cells in the mouse and rat gastric oxyntic mucosa. Biochem Biophys Res Commun 392: 67–71, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stengel A, Goebel M, Yakubov I, Wang L, Witcher D, Coskun T, Taché Y, Sachs G, Lambrecht NW. Identification and characterization of nesfatin-1 immunoreactivity in endocrine cell types of the rat gastric oxyntic mucosa. Endocrinology 150: 232–238, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Stengel A, Keire D, Goebel M, Evilevitch L, Wiggins B, Taché Y, Reeve JR., Jr The RAPID method for blood processing yields new insight in plasma concentrations and molecular forms of circulating gut peptides. Endocrinology 150: 5113–5118, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sternini C, Wong H, Wu SV, de Giorgio R, Yang M, Reeve J, Jr, Brecha NC, Walsh JH. Somatostatin 2A receptor is expressed by enteric neurons, and by interstitial cells of Cajal and enterochromaffin-like cells of the gastrointestinal tract. J Comp Neurol 386: 396–408, 1997 [PubMed] [Google Scholar]
- 66. Taché Y, Barquist E, Stephens RL, Rivier J. Abdominal surgery- and trephination-induced delay in gastric emptying is prevented by intracisternal injection of CRF antagonist in the rat. J Gastrointest Motil 3: 19–25, 1991 [Google Scholar]
- 67. Tack J, Depoortere I, Bisschops R, Verbeke K, Janssens J, Peeters T. Influence of ghrelin on gastric emptying and meal-related symptoms in idiopathic gastroparesis. Aliment Pharmacol Ther 22: 847–853, 2005 [DOI] [PubMed] [Google Scholar]
- 68. Tolessa T, Gutniak M, Holst JJ, Efendic S, Hellstrom PM. Glucagon-like peptide-1 retards gastric emptying and small bowel transit in the rat: effect mediated through central or enteric nervous mechanisms. Dig Dis Sci 43: 2284–2290, 1998 [DOI] [PubMed] [Google Scholar]
- 69. Venkova K, Greenwood-Van Meerveld B. Application of ghrelin to gastrointestinal diseases. Curr Opin Investig Drugs 9: 1103–1107, 2008 [PubMed] [Google Scholar]
- 70. Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, Batterham RL, Taheri S, Stanley SA, Ghatei MA, Bloom SR. Ghrelin causes hyperphagia and obesity in rats. Diabetes 50: 2540–2547, 2001 [DOI] [PubMed] [Google Scholar]
- 71. Yang H, Wong H, Wu V, Walsh JH, Taché Y. Somatostatin monoclonal antibody immunoneutralization increases gastrin and gastric acid secretion in urethane-anesthetized rats. Gastroenterology 99: 659–665, 1990 [DOI] [PubMed] [Google Scholar]
- 72. Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 132: 387–396, 2008 [DOI] [PubMed] [Google Scholar]
- 73. Young AA, Gedulin BR, Rink TJ. Dose-responses for the slowing of gastric emptying in a rodent model by glucagon-like peptide (7–36) NH2, amylin, cholecystokinin, and other possible regulators of nutrient uptake. Metabolism 45: 1–3, 1996 [DOI] [PubMed] [Google Scholar]
- 74. Zeinali F, Stulberg JJ, Delaney CP. Pharmacological management of postoperative ileus. Can J Surg 52: 153–157, 2009 [PMC free article] [PubMed] [Google Scholar]
- 75. Zittel TT, Reddy SN, Plourde V, Raybould HE. Role of spinal afferents and calcitonin gene-related peptide in the postoperative gastric ileus in anesthetized rats. Ann Surg 219: 79–87, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]