Abstract
Aerodigestive reflexes triggered by pharyngeal stimulation can protect the airways by clearing fluid from the pharynx. The objective of this study was to determine the relationship between the maximum capacity of fluid that can safely dwell in the hypopharynx [hypopharyngeal safe volume (HPSV)] before spilling into the larynx and the threshold volumes required to trigger pharyngoglottal closure reflex (PGCR), pharyngo-upper esophageal sphincter contractile reflex (PUCR), and reflexive pharyngeal swallow (RPS). Twenty-five healthy volunteers (mean age 24 yr, 8 males) were studied in the semi-inclined supine position. PGCR, PUCR, and RPS were elicited using techniques of concurrent upper esophageal sphincter manometry and pharyngo-laryngoscopy. The hypopharynx was then anesthetized to abolish RPS. HPSV was determined by infusing water in the pharynx, and perfusion was stopped when the infusate reached the superior margin of the interarytenoid fold. The threshold volumes for triggering PGCR, PUCR, and RPS by slow and rapid injections before pharyngeal anesthesia were 0.18 ± 0.02 and 0.09 ± 0.02 ml; 0.20 ± 0.020 and 0.13 ± 0.04 ml; and 0.61 ± 0.04 and 0.4 ± 0.06 ml, respectively. All of the above volumes were significantly smaller than the HPSV (0.70 ± 0.06 ml, P < 0.01) except for the threshold volume to elicit RPS during slow perfusion, which was not significantly different (P = 0.23). We conclude that pharyngeal aerodigestive reflexes are triggered by both slow and rapid pharyngeal perfusion of water at significantly smaller volumes than the maximum capacity of the hypopharynx to safely hold contents without spilling into the airway. These reflexes thereby aid in prevention of aspiration.
Keywords: aerodigestive reflexes, airway protection, pharyngo-glottal closure reflex, pharyngo-upper esophageal sphincter contractile reflex, reflexive pharyngeal swallow, hypopharyngeal safe volume
despite anatomical contiguity between the gastroesophageal and pharyngolaryngeal lumens, under normal condition, the airway is protected from aspiration of gastric content. The mechanisms of this protection are incompletely understood. While the roles of lower esophageal sphincter, secondary esophageal peristalsis, and the upper esophageal sphincter (UES) are clearly defined, mechanisms that protect the airway when these are overwhelmed and the refluxate has entered the pharynx have not been fully investigated. Aerodigestive reflexes triggered by pharyngeal stimulation such as that which may occur by entry of refluxate into the pharynx or during antegrade transit of substances have been proposed to protect the airways against aspiration. For example, at a threshold volume, fluid in the pharynx can trigger an irrepressible swallow: reflexive pharyngeal swallow (RPS) (9, 11, 2, 6, 8, 4) that not only leads to glottal closure but also clears the pharynx of any residual fluid. At a lower threshold volume, fluid in the pharynx can protect the airways by eliciting glottal closure without a swallow: pharyngoglottal closure reflex (PGCR) (3, 14). During gastroesophageal reflux episodes, fluid entering the pharynx can enhance UES pressure: pharyngo-UES contractile reflex (PUCR) (2, 4, 7, 13) and this in turn may protect against further esophago-pharyngeal reflux. The true relevance of the threshold volume of these pharyngeal reflexes in protecting the airways cannot be determined unless these volumes are compared with the maximum volume that can safely dwell in the hypopharynx beyond which the fluid will spill into the larynx were these reflexes not triggered: hypopharyngeal safe volume (HPSV). The HPSV in health and disease has not been determined to date because of the potential danger of causing aspiration. Using a concurrent manometric, endoscopic, and perfusion/aspiration technique, we have been able to safely measure the HPSV (15, 17). Therefore, we were able to test the hypothesis that the threshold volume to trigger PGCR, PUCR, and RPS will be significantly smaller than the HPSV, thereby postulating that these pharyngeal reflexes protect the airways by not allowing the fluid entering the pharynx to exceed the HPSV.
METHODS
This study was approved by the Institutional Review Board of the Medical College of Wisconsin, and all subjects gave informed written consent before the study. Healthy young volunteers were recruited for the study. All subjects underwent unsedated transnasal endoscopy (12) to rule out any associated silent upper gastrointestinal disorders, since it has been previously shown that, despite no symptoms, ∼20% of subjects have abnormal findings in the upper gastrointestinal tract when screened using unsedated transnasal endoscopy (5). On the day of the study, all subjects filled in a Mayo GERD questionnaire and underwent a brief history and physical examination. All subjects were studied in the semi-inclined supine position (at 45°).
Because fluid can enter the pharynx either slowly or rapidly, we elicited PGCR, PUCR, and RPS by both slow and rapid perfusion of water in the pharynx, and the threshold volumes required to trigger these reflexes were then compared with HPSV.
Slow Pharyngeal Water Perfusion
PGCR, PUCR and RPS.
PGCR, PUCR and RPS were elicited in 25 healthy volunteers (mean age 24 yr, range 17–31; 8 males) using the previously described technique of concurrent unsedated transnasal laryngo-pharyngoscopy, UES manometry (Dentsleeve), and submental electromyogram (EMG) (11, 2, 4, 3, 14, 2, 4) (Fig. 1). UES resting pressure and its response to pharyngeal water stimulation was monitored using a UES sleeve assembly (Dentsleeve, Adelaide, Australia) that incorporated a sleeve device (6 × 0.5 × 0.3 cm) with side-hole recording ports at its proximal and distal ends for manometric positioning. Nasal passages were lubricated by using a nonanesthetic jelly (Surgilube; E. Fougera, Melville, NY) with a cotton-tipped applicator (to prevent the possibility of anesthetizing the pharynx, anesthetic jelly was not used). The manometric assembly was introduced through the nose and positioned such that the manometric port immediately proximal to the sleeve sensor was positioned 2 cm above the UES high-pressure zone and directed posteriorly. This port was used for eliciting the reflexes by perfusing water at 1 ml/min. The posterior orientation of the perfusion port prevented contact of water with the larynx, which was also monitored with an ultrathin laryngo-pharyngoscope passed through the other nostril (Fig. 1). The injection port, the esophageal ports, and the sleeve sensor were connected to pressure transducers in line with a minimally compliant pneumohydraulic pump (Arndorfer Medical Specialties, Greendale, WI). With this arrangement, the onset and offset of water injection and UES pressure were recorded using the MMS Motility System (Medical Measurement Systems, Enschede, Netherlands).
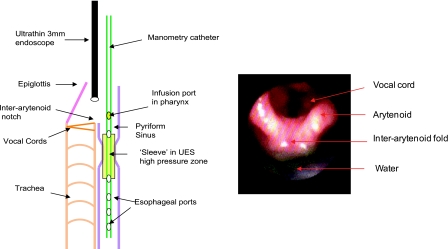
Fig. 1.
A cartoon demonstrating the technique of concurrent unsedated transnasal laryngo-pharyngoscopy and upper esophageal sphincter (UES) manometry (Dentsleeve). The manometry catheter was passed through one nostril, and, to record the glottal response (adduction) to pharyngeal water stimulation (PGCR), the laryngo-pharyngoscope was passed into the other nasal passage and positioned within the pharynx such that the vocal chords were visualized completely. Green-colored water was used for better endoscopic visualization.
To record the glottal response (adduction) to pharyngeal water stimulation (PGCR), a Pentax FNL-10AP (Pentax imaging) laryngo-pharyngoscope was passed in the other nasal passage and positioned within the pharynx such that the vocal chords were visualized completely (3, 14) (Fig. 1). Green-colored water was used for better endoscopic visualization (Fig. 1). The laryngo-pharyngoscope and manometric recording were synchronized using a specially designed timer (Thalner electronics labs, Ann Arbor, MI). Endoscopic images were recorded digitally on a DVD recorder for subsequent analysis in real time and slow motion.
For pharyngeal stimulation, water at room temperature was perfused in the pharynx through the dedicated perfusion port at a rate of 1 ml/min using a Harvard infusion pump (model N0975; Harvard Apparatus, Dover, MA) until an irrepressible swallow occurred (RPS). Each injection was started 5–10 s after the UES pressure returned to baseline following a swallow, and subjects withheld swallowing as long as they could. Each injection was performed three times, and subjects were asked to swallow between injections to clear the pharynx of any residual water.
The average end-expiratory UES pressures at baseline (10-s period before the injection) and after the injection (3 of 3 injections) were recorded. The postinjection pressure was defined as the maximum UES pressure following pharyngeal water injection, excluding the 3-s interval before deglutitive relaxation, if a swallow occurred. This 3-s interval was used to avoid counting the commonly seen pressure increase that is registered by the sleeve immediately before its swallow-induced relaxation. We determined, in each subject, the frequency elicitation response and the smallest volume of injected water: threshold volume that in three of three injections triggered glottal adduction (PGCR), rise in UES pressure (PUCR), and an irrepressible pharyngeal swallow (RPS).
Rapid Pharyngeal Water Perfusion
To determine if the threshold volume to elicit PGCR, PUCR, and RPS with rapid pharyngeal water injections is significantly less than the HPSV, 10 consecutive subjects [mean age 23 ± 5 (SD) yr; 5 males] from the above group were also studied with rapid water perfusion in the pharynx.
Using the above setup of concurrent laryngo-pharyngoscopy, UES manometry, and submental EMG, water was rapidly injected in the pharynx through the injection port on the UES sleeve catheter using a hand-held syringe. Water injection was started at 0.05 ml, then 1 ml, and then at increments of 1 ml until the threshold volume to elicit an irrepressible swallow (RPS) was reached. Each injection was repeated three times, and, between injections, the subjects were asked to swallow to clear the pharynx of any residual fluid. Following this command swallow, similar to slow water injections, the UES pressure was allowed to return to baseline before the next injection. The frequency elicitation, UES response, and the minimum volume of water required (3 of 3 injections) to trigger PGCR, PUCR, and RPS were noted.
Pharyngeal Anesthesia and HPSV
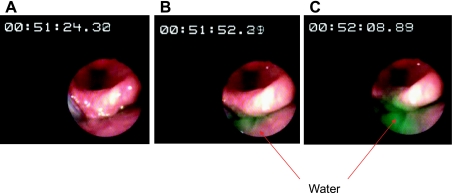
To determine HPSV, reflexive pharyngeal swallow was abolished by anesthetizing the pharynx with 4% topical lidocaine (Roxane, Columbus, OH). The anesthetic agent was applied to the posterior pharyngeal mucosa using an atomizer and spraying additional lidocaine through the biopsy channel of a laryngo-pharyngoscope (FNL-10AP Pentax imaging) positioned just above the hypopharynx. With RPS abolished, colored water was slowly perfused in the pharynx at 1 ml/min as described above. Occurrence of aspiration was documented by endoscopically observing colored water rise up to the superior level of the interarytenoid notch and spill into the larynx (Fig. 2) or when the subjects clearly aspirated and coughed at which point perfusion was stopped and the volunteers were asked to swallow to prevent further spillage into the trachea (monitored endoscopically). The maximum volume of fluid that could safely dwell in the hypopharynx before spilling into the larynx, namely HPSV, was measured. Frequency elicitation of the PGCR, PUCR, and RPS in response to both slow and rapid pharyngeal water injections was also determined after pharyngeal anesthesia.
Fig. 2.
A: green-colored water perfusion was started at 1 ml/min. Note timer is at 00:51:24.30. B: ∼8 s later (timer 00:51:52.29), green-colored water is seen rising up to the interarytenoid folds. C: ∼45 s (00:52:08.89) from the onset of infusion, green-colored water was seen reaching the superior margin of the interarytenoid fold and spilling into the larynx.
Statistical Analysis
Frequency of response to pharyngeal stimulation and elicitation of pharyngeal reflexes are expressed as percentage. Threshold volume to elicit these reflexes and the HPSV are presented as means ± SD unless stated otherwise. Interobserver variation was determined by measuring Cohen's kappa value. Comparison of frequency elicitation response of PUCR, PGCR, RPS, and aspiration before and after pharyngeal anesthesia was done using Fischer's exact test. Comparison of UES pressures and threshold volumes to elicit the above reflexes before and after anesthesia was done by paired t-test. For data that did not pass normality, the Mann Whitney Rank Sum test was used. A P value ≤0.05 was considered statistically significant. Analysis was performed using Stats Direct statistical software (StatsDirect).
RESULTS
All subjects completed the study, and there were no adverse events.
Slow Pharyngeal Water Perfusion
Frequency-elicitation.
As shown in Table 1, during slow pharyngeal water perfusion, PGCR, PUCR, and RPS could be elicited in all subjects except for one volunteer in whom PUCR was absent [frequency-elicitation (FrE): PGCR 100%, PUCR 96%, and RPS 100%]. No laryngeal spillage of fluid was noted. After topical pharyngeal anesthesia, RPS and PUCR were abolished in all subjects (FrE 0%), whereas elicitation of PGCR remained unaffected (FrE 100%).
Table 1.
Frequency elicitation of pharyngeal airway protective reflexes with slow and rapid pharyngeal perfusion of water
| PGCR |
PUCR |
RPS* |
Laryngeal Spillage |
|||||
|---|---|---|---|---|---|---|---|---|
| Slow | Rapid | Slow | Rapid | Slow | Rapid | Slow | Rapid | |
| Before anesthesia | 100 | 100 | 96 | 100 | 100 | 100 | 0 | 0 |
| After anesthesia | 100 | 100 | 0 | 30 | 0 | 40 | 100 | 60 |
Units are %. PGCR, pharyngoglottal closure reflex; PUCR, pharyngo-upper esophageal sphincter contractile reflex; RPS, reflexive pharyngeal swallow.
Laryngeal spillage was noted in those in whom RPS was abolished after topical pharyngeal anesthesia.
UES pressure response to pharyngeal water perfusions.
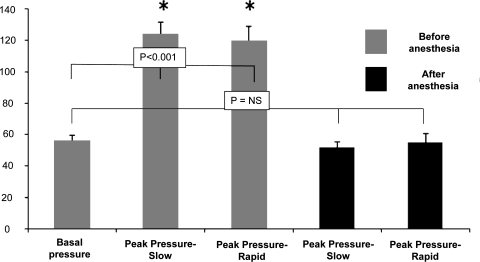
Stimulation of posterior pharynx during slow water perfusion significantly increased the UES pressure. The mean end-expiratory UES pressure at baseline was 56 ± 4 mmHg, and, during pharyngeal stimulation, the UES pressure increased to a mean of 124 ± 8 mmHg, which was a 221% increase from baseline (P < 0.001, Fig. 3). The mean basal UES pressure after topical anesthesia was similar to the basal UES pressure before anesthesia [50 ± 3 and 56 ± 4 mmHg, respectively, P = not significant (NS)]. There was no significant rise in UES pressure in response to slow water stimulation after pharyngeal anesthesia (52 ± 4 mmHg, P = 0.48, Fig. 3).
Fig. 3.
Pharyngeal stimulation with slow and rapid water perfusion resulted in a significant increase in the UES pressure compared with baseline (*P < 0.001). This response was abolished with pharyngeal topical anesthesia.
HPSV and threshold volumes to elicit PGCR, PUCR, and RPS.
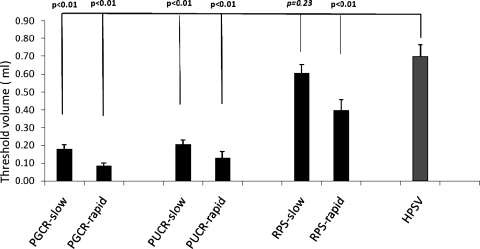
During slow pharyngeal water perfusion, HPSV, namely, the maximum volume of water that can safely dwell in the pharynx beyond which laryngeal spillage was noted, was 0.7 ± 0.06 ml. The threshold volume to elicit PGCR was 0.18 ± 0.02 ml, which was significantly smaller than HPSV (P < 0.001) and also significantly smaller than the threshold volume required to elicit RPS (RPS 0.61 ± 0.04 ml, P < 0.001) (Fig. 4). The threshold volume to elicit PUCR was 0.2 ± 0.02 ml, which was again significantly smaller than HPSV and the threshold volume required to trigger RPS (P < 0.001). There was no significant difference between threshold volume required to elicit RPS and the HPSV (0.61 ± 0.04 and 0.7 ± 0.06 ml, respectively; P = 0.46) (Fig. 4).
Fig. 4.
The threshold volume for triggering PGCR, pharyngo-UES contractile reflex (PUCR), and reflexive pharyngeal swallow (RPS) by slow and rapid injections before pharyngeal anesthesia was 0.18 ± 0.02 and 0.09 ± 0.02 ml; 0.20 ± 0.020 and 0.13 ± 0.04 ml; 0.61 ± 0.04 and 0.4 ± 0.06 ml, respectively. All of the above volumes were significantly smaller than the maximum volume that can safely dwell in the hypopharynx hypopharyngeal safe volume (HPSV) of 0.70 ± 0.06 ml (P < 0.01) except for the threshold volume to elicit RPS during slow perfusion, which was not significantly different form HPSV (P = 0.23).
Laryngeal spillage.
Topical pharyngeal anesthesia abolished RPS in all subjects. Concurrent endoscopic monitoring showed laryngeal spillage of the colored fluid in all subjects (100%, Table 1) (Fig. 2).
Rapid Pharyngeal Water Perfusion
Frequency-elicitation.
During rapid pharyngeal water injections, PGCR, PUCR, and RPS could be elicited in all subjects (FrE 100%, Table 1), and no laryngeal spillage of fluid was seen. After topical pharyngeal anesthesia, similar to slow pharyngeal water perfusion, PGCR was preserved in all subjects during rapid water injections. After anesthesia, during rapid pharyngeal water infusion, frequency elicitations of PUCR and RPS were 30 and 40%, respectively, compared with slow water injection where both of these reflexes were abolished in all subjects after pharyngeal anesthesia. Absent RPS resulted in laryngeal spillage of water.
UES pressure response to pharyngeal water perfusions.
Similar to slow water perfusion, stimulation of the posterior pharynx with rapid water injection significantly increased the UES pressure compared with baseline (basal 56 ± 4 mmHg; rapid injection 120 ± 9 mmHg; 214% increase over baseline; P < 0.001, Fig. 3). There was no significant difference in the peak rise in UES pressure between slow and rapid pharyngeal water perfusions (slow 124 ± 8 mmHg, rapid 120 ± 9 mmHg; P = ns). After pharyngeal anesthesia, similar to slow water perfusion, there was no significant rise in UES pressure in response to rapid water stimulation (basal 56 ± 4 mmHg; rapid injection 55 ± 6 mmHg, P = ns, Fig. 3).
HPSV and threshold volumes to elicit PGCR, PUCR, and RPS.
During rapid pharyngeal water injection, the threshold volumes to elicit PGCR, PUCR, and RPS were 0.09 ± 0.02, 0.13 ± 0.04, and 0.4 ± 0.06 ml, respectively. All of these threshold volumes were significantly smaller than the HPSV (0.7 ± 0.06 ml; P < 0.01) (Fig. 4).
Laryngeal spillage.
During rapid pharyngeal water injection, topical pharyngeal anesthesia abolished RPS in 60% of subjects (compared with in 100% of subjects during slow water perfusion). As the volume of water that was being injected rapidly in the pharynx was increased by 0.1-ml increments, laryngeal splashing of the colored water was seen in these subjects with absent RPS (60%, Table 1).
Interobserver Variation
Observed agreement was 96.67%, expected agreement was 66.06%, and kappa was equal to 0.9018.
DISCUSSION
Aspiration of pharyngeal contents is influenced by 1) the volume of material that can safely dwell in the pharynx without spilling in to the airway (HPSV), and 2) activation of pharyngeal airway protective reflexes. Because material can enter the pharynx slowly or rapidly, previous studies have shown that these reflexes can be triggered by slow and rapid pharyngeal water injections. The relationship between the threshold volume of water required to elicit these reflexes with slow and rapid pharyngeal infusion and the HPSV has not been previously studied. In this study, we evaluated this relationship.
Aerodigestive reflexes that are triggered by stimulation of the pharynx have been proposed to protect the airways against aspiration during antegrade and retrograde transit of food. This study was designed to evaluate the threshold volume of fluid required to elicit pharyngeal reflexes in relation to HPSV irrespective of the mode of entry of fluid in the hypopharynx (spill from oral cavity, saliva, nasal drip, or esophago-pharyngeal reflux). To stimulate pharyngeal reflexes, water was directly injected in the hypopharynx and not in the esophagus (retrograde transit to the hypopharynx) nor in the oral cavity (antegrade transit to the hypopharynx). Hence the results of this study translate more to what happens when the fluid enters the pharynx, and, although not directly demonstrated in this study, these results may have implications during antegrade or retrograde transit of food material.
One of the pharyngeal aerodigestive reflexes proposed to protect the airways include reflexive vocal cord adduction triggered by rapid or slow injection of fluid into the pharynx, PGCR (3, 14). At a larger threshold volume, fluid in the pharynx can trigger an irrepressible swallow that not only lifts and closes the glottis but also clears the pharynx of any residual fluid: RPS (2, 4, 11). Injection of water into the hypopharynx can augment the UES pressure, PUCR (2, 4, 7, 13). Although this may appear as counterproductive in pharyngeal clearance during antegrade transit of material, it has been proposed that, during retrograde transit of food, refluxate entering the pharynx can trigger PUCR, thereby preventing further entry of fluid in the pharynx. Based on these mechanisms, it has been proposed that these aerodigestive reflexes triggered by pharyngeal stimulation protect the airways against aspiration. However, so far, there was no direct evidence to support this hypothesis. This was because there were no techniques to determine the maximum volume of water that can safely dwell in the hypopharynx (HPSV) beyond which water will spill in the larynx and to correlate this volume with the threshold volumes required to trigger the above reflexes.
In this study, for the first time, we have shown that the threshold volumes of water required to trigger PGCR, PUCR, and RPS were significantly smaller than the HPSV. Direct evidence on the airway protective function of these reflexes was further demonstrated by abolishing RPS with topical pharyngeal anesthesia and perfusing water into the pharynx. By doing so, HPSV was exceeded and water was seen spilling into the larynx. All subjects in this study were evaluated at a 45-degree inclined head-up supine position. These results may not represent what happens in other body positions. In a pilot study, we have shown that HPSV can alter with body position (16), and further studies will be needed to determine the influence of body position on HPSV and its interaction with aerodigestive reflexes. Similarly, body position can also alter spatial distribution of infused fluid in the pharynx that can influence triggering of reflexive swallow (10). An altered anatomy of the hypopharynx may also influence HPSV. This study was primarily focused on determining the relationship between HPSV and the threshold volumes to elicit aerodigestive reflexes in normal subjects. Our further studies will focus on this relationship in patients with neurologic disorders resulting in aspiration and in subjects with deteriorated aerodigestive reflexes, for example, in chronic cigarette smokers (2, 3, 14).
Because aspiration can occur during abrupt or slow entry of fluid into the pharynx, we elicited PGCR, PUCR, and RPS by rapid injection and slow perfusion of water in the pharynx. As seen in Table 1, in the absence of reflexive pharyngeal swallow, rapid injection of water in the pharynx simulating abrupt entry of material in the pharyngeal resulted in penetration of the airway because of splashing of the injected water even when the injected volume is smaller than the safe pharyngeal volume, indicating the pivotal role of reflexive pharyngeal swallow in protection of airway during both rapid and slow entry of material into the pharynx.
Except for RPS during slow perfusion, the threshold volume of water required to trigger these reflexes during rapid and slow injections was significantly smaller than HPSV. The reason why the threshold volume to elicit RPS during slow water perfusion was not significantly different from HPSV is not known. In a previous study, we had shown that the threshold volume to elicit RPS can be dependent on the pharyngeal perfusion rate with the volume being larger with perfusion rate of 1 ml/min compared with 5.5 ml/min (1). We speculate that, during slow perfusion of water into the hypopharynx, afferent receptors at the superior margin of the interarytenoid fold rather than hypopharyngeal afferents may be involved in triggering RPS, and, since we used this level to also measure HPSV after abolishing RPS, the threshold volume for RPS at a slow perfusion rate was similar to HPSV. This concept will need further evaluation.
In this study, it was also observed that topical pharyngeal anesthesia had different effects on the frequency elicitation of PGCR, PUCR, and RPS. For example, despite pharyngeal anesthesia, PGCR could still be elicited in all subjects during rapid and slow water injections while pharyngeal anesthesia abolished RPS in all subjects during slow water perfusion and in 60% during rapid water injections. Similarly, pharyngeal anesthesia abolished PUCR in all subjects during slow perfusion and in 70% during rapid injections. To elicit these three reflexes, since water was always injected at the same site through a dedicate injection port positioned 2 cm above the UES high-pressure zone and endoscopic monitoring confirmed no splashing of water into the larynx, different afferent receptors could explain these differential responses. Incomplete pharyngeal anesthesia with the technique used could be another possibility. However, this is unlikely since, after topical pharyngeal anesthesia, subjects were not aware of fluid rising in the hypopharynx during perfusion that resulted in laryngeal spillage of water. Even if one were to assume that the pharynx may not have been completely anesthetized, what is clear from this study is that preserved PGCR at the level of pharyngeal anesthesia that abolishes PUCR and RPS suggests different afferent receptors.
In summary, this study has for the first time shown that, in healthy young individuals, the threshold volume to elicit aerodigestive reflexes by pharyngeal water stimulation is significantly smaller than the maximum capacity of the hypopharynx to safely hold contents without spilling into the airway. These reflexes thereby aid in the prevention of aspiration. By abolishing these reflexes with pharyngeal anesthesia, this study has also shown that the safe volume can be exceeded, resulting in laryngeal spillage of water. These observations directly demonstrate the airway protective role of these reflexes. Further studies are planned to evaluate the effect of body positions on the interaction between these reflexes and the safe volume and to study patients with deteriorated aerodigestive reflexes.
GRANTS
This work is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant 1P01DK-068051-01A1.
DISCLOSURES
None of the authors of this study have any potential conflict of interest relevant to this manuscript and have nothing to disclose.
ACKNOWLEDGMENTS
Part of this work was presented at the Neurogastroenterology and Motility (oral presentation) meeting, 2008 and at the Digestive Disease Week (oral presentation), 2010.
REFERENCES
- 1. Bajaj JS, Dua K, Bajaj S, Rittmann T, Shaker R. Effect of infusion rate on elicitation of pharyngeal reflexes and development of aspiration among smokers (Abstract). Neurogastroenterol Motil 16: 672, 2004 [Google Scholar]
- 2. Dua K, Bardan E, Ren J, Sui Z, Shaker R. Effect of chronic and acute cigarette smoking on the pharyngo-upper oesophageal sphincter contractile reflex and reflexive pharyngeal swallow. Gut 43: 537–541, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dua K, Bardan E, Ren J, Sui Z, Shaker R. Effect of chronic and acute cigarette smoking on the pharyngoglottal closure reflex. Gut 51: 771–775, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dua KS, Surapaneni SN, Santharam R, Knuff D, Hofmann C, Shaker R. Effect of systemic alcohol and nicotine on airway protective reflexes. Am J Gastroenterol 104: 2431–2438, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dua KHM, Surapaneni S, Tatro L, Shaker R. Prevalence of abnormal upper GI findings in apparently healthy volunteers enrolled for research studies. Gastrointest Endosc 69: AB350–AB351, 2009 [Google Scholar]
- 6. Lang IM, Dana N, Medda BK, Shaker R. Mechanisms of airway protection during retching, vomiting, and swallowing. Am J Physiol Gastrointest Liver Physiol 283: G529–G536, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Medda BK, Lang IM, Layman R, Hogan WJ, Dodds WJ, Shaker R. Characterization and quantification of a pharyngo-UES contractile reflex in cats. Am J Physiol Gastrointest Liver Physiol 267: G972–G983, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Medda BK, Kern M, Ren J, Xie P, Ulualp SO, Lang IM, Shaker R. Relative contribution of various airway protective mechanisms to prevention of aspiration during swallowing. Am J Physiol Gastrointest Liver Physiol 284: G933–G939, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Nishino T. Swallowing as a protective reflex for the upper respiratory tract. Anesthesiology 79: 588–560, 1993 [DOI] [PubMed] [Google Scholar]
- 10. Pouderoux P, Logemann J, Kahrilas P. Pharyngeal swallowing elicited by fluid infusion: role of volition and vallecular containment. Am J Physiol Gastrointest Liver Physiol 270: G347–G354, 1996 [DOI] [PubMed] [Google Scholar]
- 11. Shaker R, Ren J, Zamir Z, Sarna A, Liu J, Sui Z. Effect of aging, position, and temperature on the threshold volume triggering pharyngeal swallows. Gastroenterology 107: 396–402, 1994 [DOI] [PubMed] [Google Scholar]
- 12. Shaker R. Unsedated trans-nasal pharyngoesophagogastroduodenoscopy (T-EGD): technique. Gastrointest Endosc 40: 346–348, 1994 [DOI] [PubMed] [Google Scholar]
- 13. Shaker R, Ren J, Xie P, Lang IM, Bardan E, Sui Z. Characterization of the pharyngo-UES contractile reflex in humans. Am J Physiol Gastrointest Liver Physiol 273: G854–G858, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Shaker R, Ren J, Bardan E, Easterling C, Dua K, Xie P, Kern M. Pharyngoglottal closure reflex: characterization in healthy young, elderly and dysphagic patients with predeglutitive aspiration. Gerontology 49: 12–20, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Surapaneni S, Dua K, Kuribayashi S. Mechanism of airway protection against aspiration of refluxate: relationship between critical volume and threshold volume (Abstract). Neurogastroenterol Motil 20: 21, 2008 [Google Scholar]
- 16. Surapaneni SN, Dua KS, Kuribayashi S, Hafeezullah M, Tatro L, Shaker R. Influence of position on the maximum volume of fluid that can safely dwell in the hypo-pharynx; “Hypopharyngeal Safe Volume” (HPSV) (Abstract). Gastroenterology 136: A734, 2008 [Google Scholar]
- 17. Surapaneni SN, Dua KS, Kuribayashi S, Hafeezullah M, Amaris MA, Shaker R. Aerodigestive protective reflexes are triggered before the safe capacity of pharynx is exceeded (Abstract). Gastroenterology 138: S13, 2010 [Google Scholar]