Abstract
Recent studies demonstrate that rapid antidepressant response to ketamine is mediated by activation of the mammalian target of rapamycin (mTOR) signaling pathway, leading to increased synaptic proteins in the prefrontal cortex (PFC) of rats. Our postmortem studies indicate robust deficits in prominent postsynaptic proteins including N-methyl-D-aspartate (NMDA) receptor subunits (NR2A, NR2B), metabotropic glutamate receptor subtype 5 (mGluR5) and postsynaptic density protein 95 kDa (PSD-95) in the PFC in major depressive disorder (MDD). We hypothesize that deficits in the mTOR-dependent translation initiation pathway contribute to the molecular pathology seen in the PFC of MDD subjects, and that a rapid reversal of these abnormalities may underlie antidepressant activity. The majority of known translational regulation occurs at the level of initiation. mTOR regulates translation initiation via its downstream components: p70-kDa ribosomal protein S6 kinase (p70S6K), and eukaryotic initiation factors 4E and 4B (eIF4E, eIF4B). In this study, we examined the expression of mTOR and its core downstream signaling targets: p70S6K, eIF4E, eIF4B in the PFC of 12 depressed subjects and 12 psychiatrically healthy controls using Western blot. Levels of eIF4E phosphorylated at serine 209 (p-eIF4E-Ser209) and eIF4B phosphorylated at serine 504 (p-eIF4B-Ser504) were also examined. Adjacent cortical tissue samples from both cohorts of subjects were used in our previous postmortem analyses. There was a significant reduction in mTOR, p70S6K, eIF4B and p-eIF4B protein expression in MDD subjects relative to controls. No group differences were observed in eIF4E, p-eIF4E or actin levels. Our findings show deficits in mTOR-dependent translation initiation in MDD particularly via the p70S6K/eIF4B pathway, and indicate a potential association between marked deficits in synaptic proteins and dysregulation of mTOR signaling in MDD.
Keywords: prefrontal cortex; translation initiation pathway, major depressive disorder, postmortem
1. Introduction
A major limitation of established antidepressants is the delayed onset of therapeutic response, resulting in non-compliance and dramatically increased risk for suicidal behavior. Of particular relevance is the demonstration that a single dose of ketamine, a glutamate N-methyl-D-aspartate (NMDA) receptor antagonist, induced a rapid (within hours), long lasting (up to 1 week), and robust antidepressant effect in treatment-resistant cases of MDD (Berman et al., 2000; Zarate et al., 2006). Recent animal studies indicate that the fast antidepressant response to NMDA receptor antagonists (ketamine and Ro 25-6981) is mediated by rapid activation of the mammalian target of rapamycin (mTOR) pathway leading to an increase in synaptic signaling proteins and increased number and function of new spine synapses in the prefrontal cortex (PFC) of rats (Li et al., 2010). Moreover, it has been demonstrated that a single dose of these antagonists rapidly reversed the chronic stress induced behavioral and synaptic deficits in an mTOR-dependent manner (Li et al., 2011). Our recent postmortem studies show significant reductions in the expression of prominent postsynaptic proteins involved in glutamate neurotransmission, including NMDA receptor subunits (NR2A, NR2B), metabotropic glutamate receptor subtype 5 (mGluR5) and postsynaptic density 95kDa (PSD-95) in the PFC from depressed subjects (Deschwanden et al., 2011; Feyissa et al., 2009). These studies may indicate an association between marked deficits in synaptic proteins and dysregulation of mTOR signaling in MDD (Karolewicz et al, 2011).
Traditionally, it was thought that the change in the proteome is caused by transcriptional activity. Now it is known that regulation of translation is another way of altering protein production (Nilsson et al., 2004). Protein synthesis is a highly regulated process that can be separated into three general phases: initiation, elongation and termination (Hoeffer and Klann et al., 2010; Klann et al., 2004). The rate-limiting step in the process of protein synthesis is translation initiation (Hoeffer and Klann et al., 2010; Holz et al., 2005). The activity of mTOR, an ubiquitously expressed serine/threonine kinase, is central to the regulation of translation initiation and, consequently, protein synthesis required for long-term potentiation and new synaptic connections (Hashimoto, 2011; Hoeffer and Klann et al., 2010; Klann et al., 2004; Tang et al., 2002; Tang and Schuman, 2002).
It has been reported that neuronal mTOR function is influenced by the activity of growth factors, insulin, cytokines, as well as glutamate activity via NMDA receptors and metabotropic glutamate receptors (mGluR) (Antion et al., 2008; Gong et al., 2006; Hay and Sonenberg, 2004; Hoeffer and Klann, 2010) (Fig. 1). Activated mTOR phosphorylates p70-kDa ribosomal protein S6 kinase (p70S6K) followed by p70S6K-induced phosphorylation of eukaryotic initiation factor 4B (eIF4B) which promotes the initiation of protein translation (Raught et al., 2004). mTOR also phosphorylates and inactivates eukaryotic initiation factor 4E-binding protein 1 (4E-BP1), reducing its affinity for eIF4E and releasing eIF4E to facilitate translation initiation. Activated translation initiation factors, particularly eIF4E and eIF4B, are responsible for ribosome recruitment to the 5′ end of mRNA. The 5′ end of all nuclear-transcribed mRNAs possess a cap structure (m7GpppN, in which “m” represents a methyl group and “N”, any nucleotide) that is specifically recognized by eIF4E. Thus, eIF4E guides the ribosome to an mRNA 5′ end and facilitates its binding. On the other hand, eIF4B potentiates ribosome recruitment by stimulating the helicase activity of the eukaryotic initiation factor 4A (eIF4A), to unwind mRNA secondary structure for efficient translation (Gingras et al., 1999; Hay and Sonenberg, 2004; Holz et al., 2005; Rogers et al., 2002). Thus, mTOR controls the efficiency of protein translation within cells via its critical downstream targets (Fig. 1).
Figure 1.
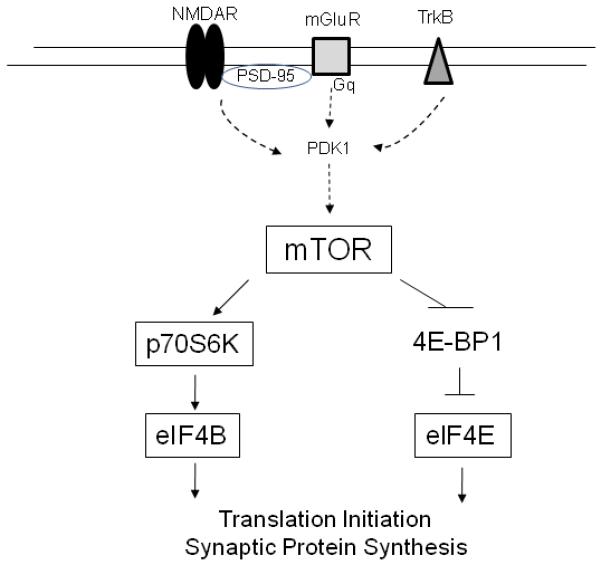
Simplified diagram illustrating the mTOR signaling pathway. Neuronal receptors (NMDAR, mGluR, and TrkB) activate downstream signaling pathways, including PDK1, leading to mTOR activation. Activated mTOR phosphorylates p70S6K followed by p70S6K induced phosphorylation of eIF4B which promotes the initiation of protein translation. mTOR also phosphorylates and inactivates eukaryotic 4E-BP1 reducing its affinity for eIF4E and releasing eIF4E to facilitate translation initiation. Abbreviations: NMDAR, N-methyl-D-aspartate receptor; mGluR, metabotropic glutamate receptor; PSD-95, postsynaptic density-95 kDa; TrkB, tyrosine kinase B receptor; PDK1, phosphoinositide-dependent kinase 1; mTOR, mammalian target of rapamycin; p70S6K, p70 kDa ribosomal protein S6 kinase; eIF4B, eukaryotic initiation factor 4B; 4E-BP1, eukaryotic initiation factor 4E binding protein 1; eIF4E, eukaryotic initiation factor 4E. Diagram drawn using information from Hoeffer and Klann (2010) and Klann et al. (2004).
There is abundant evidence linking mTOR signaling to synaptic plasticity, memory, neurological disorders, and cancer (Gong et al., 2006; Hay and Sonenberg, 2004; Hoeffer and Klann et al., 2010). To date there are no studies that implicate the mTOR signaling pathway in the pathology of MDD. We hypothesize that deficits in the mTOR-dependent translation initiation pathway contribute to the molecular pathology seen in the PFC in MDD. Therefore, the goal of this study is to examine MDD-related changes in the protein level of mTOR and its downstream signaling targets: p70S6K, eIF4E, eIF4B in cortical tissue (PFC BA10) from the same MDD subjects as those used in our previous postmortem studies (Deschwanden et al., 2011; Feyissa et al., 2009). Additionally, levels of eIF4E phosphorylated at serine 209 (p-eIF4E Ser209) and eIF4B phosphorylated at serine 504 (p-eIF4B Ser504) were examined.
2. Methods
2.1. Human Subjects
Postmortem brain samples were collected at autopsy at the Cuyahoga County Coroner’s Office in Cleveland, OH. Informed written consent was obtained from the legal next-of-kin of all subjects. Next-of-kin were interviewed and retrospective psychiatric assessments were conducted in accordance with Institutional Review Board policies at Case Western Reserve University and the University of Mississippi Medical Center. A trained interviewer administered the Schedule for Affective Disorders and Schizophrenia: lifetime version (SADS-L) or the Structured Clinical Interview for DSM-IV Psychiatric Disorders (SCID-IV) to knowledgeable next-of-kin to subjects in the study approximately three months after death to determine current and lifetime Axis I psychopathology (First et al., 1996; Spitzer and Endicott, 1978). Diagnoses for Axis I disorders were assessed independently by a clinical psychologist and a psychiatrist. Consensus diagnosis was reached in conference, using information from knowledgeable informants, The Cuyahoga County Coroner’s Office, and all available inpatient and outpatient medical records. Twelve subjects met criteria for major depressive disorder and twelve subjects did not meet criteria for an Axis I disorder (termed psychiatrically healthy controls) except for nicotine and alcohol dependence based on the Diagnostic and Statistic Manual of Mental Disorders-Revised DSM-IV (Table 1). Among the twelve depressed subjects, 10 were suicide victims. Blood and urine samples from all subjects were examined by the coroner’s office for psychotropic medications and substances of abuse, including ethanol (Table 1 and 2). The average duration of depression was 9.6 (± 3.6) years. Depressed subjects and psychiatrically healthy controls were matched as closely as possible for age, gender, post-mortem interval (PMI), tissue pH, and storage time in freezer (Table 1 and 2).
Table 1.
Demographic data of controls and major depressive subjects
| Control | Age | Sex | PMI | Brain pH | Toxicology | Cause of death |
|---|---|---|---|---|---|---|
| 1 | 37 | F | 13 | 5.93 | Clean | Viral myocarditis |
| 2 | 46 | M | 11 | 6.95 | Clean | Heart disease |
| 3 | 54 | M | 17 | 6.87 | Brompheniramine | Heart disease |
| 4 | 67 | F | 28 | 6.39 | Clean | Heart disease |
| 5 | 69 | M | 26 | 6.7 | Clean | Heart disease |
| 6 | 70 | M | 20 | 6.81 | Clean | Heart disease |
| 7 | 43 | M | 23 | 6.49 | Propoxyphene, norpropoxyphene, oxycodone |
Pulmonary thromboemboli |
| 8 | 59 | M | 6 | 6.79 | Lidocaine | Heart disease |
| 9 | 34 | M | 24 | 6.61 | Ethanol | Thrombophlebitis |
| 10 | 54 | M | 19 | 6.52 | Lidocaine | Heart disease |
| 11 | 52 | M | 17 | 6.28 | Clean | Heart disease |
| 12 | 33 | M | 23 | 6.86 | Clean | Heart Disease |
| MDD | ||||||
|---|---|---|---|---|---|---|
| 1 | 40 | F | 25 | 6.32 | Morphine, codeine, hydrocodone, diphenhydramine |
Heart disease |
| 2 | 46 | M | 17 | 6.26 | Clean | Homicide |
| 3 | 54 | M | 23 | 6.24 | Phenobarbital, phenytoin, CO | Suicide by CO poisoning |
| 4 | 42 | F | 24 | 6.62 | Acetaminophen, propoxyphene | Suicide by overdose of propoxyphene & acetaminophen |
| 5 | 64 | M | 26 | 6.85 | Ethanol | Suicide by gun shot to head |
| 6 | 74 | M | 25 | 6.67 | Diazepam, Acetaminophen | Suicide by gun shot to head |
| 7 | 81 | M | 33 | 6.78 | Clean | Suicide by drowning |
| 8 | 60 | M | 20 | 6.31 | Ethanol | Suicide by gun shot to chest |
| 9 | 42 | M | 20 | 6.8 | Clean | Suicide by gun shot to chest |
| 10 | 52 | M | 17 | 6.48 | CO | Suicide by CO poisoning) |
| 11 | 48 | M | 21 | 6.9 | Flurazepam | Suicide by gun shot to chest |
| 12 | 65 | M | 30 | 6.24 | Codeine | Suicide by gun shot to chest & slashed wrists |
M, male; F, female; PMI, postmortem interval; MDD, major depressive disorder; CO, carbon monoxide.
Table 2.
Summary of demographic characteristics of subjects
| Parameter | Controls (n=12) | Major Depression (n=12) |
|---|---|---|
| Age* | 51 (3.8) years | 55 (3.8) years |
| Postmortem interval* | 19 (1.9) hours | 23 (1.4) hours |
| pH* | 6.62 (0.09) | 6.54 (0.07) |
| Gender (female/male) |
2/10 | 2/10 |
| Medication history a | none | Sertraline (n=2) Fluoxetine (n=1) Paroxetine, fluoxetine & amitriptyline (n=1) |
| Comorbid diagnosis | History of alcohol abuse (n=1) History of alcohol dependence (n=2) |
Alcohol abuse (n=1) History of alcohol abuse (n=1) Dysthymia (n=1) Polysubstance dependence & bulimia nervosa (n=1) |
| Smoking | Smokers (n=2) History of smoking (n=2) |
Smokers (n=3) |
| Suicide | none | n=10 |
Mean (SEM)
prescriptions for antidepressants within 4 weeks prior to death; none of the 12 depressed subjects had antidepressants present in their postmortem toxicology screening.
2.2. Immunoblotting
Tissue samples were dissected from the anterior region of the prefrontal cortex (PFC) containing Brodmann’s area 10 (BA10). Frozen blocks were cut into 50 um-thick sections and tissue punches containing all six cortical layers of the gray matter were collected and used. Western blot experiments were performed as described previously (Feyissa et al., 2009; Feyissa et al., 2010; Deschwanden et al., 2011). Immunoreactivities of mTOR, p70S6K, eIF4E, p-eIF4E (Ser209), eIF4B, and p-eIF4B (Ser504) were investigated in twelve depressed subjects and twelve psychiatrically healthy controls. Immunoblots of six pairs of subjects were on the same gel with duplicates on separate gels. All mTOR signaling pathway components were detected using rabbit monoclonal antibodies (1:1000, Epitomics Burlingame, CA, USA) and secondary anti-rabbit antibody (1:3000, Amersham Biosciences, Piscataway, NJ, USA). As a control for transfer and loading, actin was detected on each blot using mouse anti-actin primary antibody (1:10,000; Millipore, Temecula, CA, USA) and anti-mouse secondary antibody (1:5,000; Amersham Biosciences). In order to ensure that actin is not affected by depression, amounts of actin immunoreactivity from depressed subjects were compared to amounts of actin immunoreactivity of matched controls. Actin immunoreactivity detected in depressed subjects was not changed compared to controls (t=1.09; df=11, p=0.299, not shown). The unchanged level of actin between study groups supports the suitability of this protein as internal control.
2.3. Data Analysis
Immunoreactive bands were analyzed using MCID Elite 7.0 (Imaging Research, St. Catherines, ON, Canada). To control for accuracy of tissue loading and efficiency of transfer, data were normalized to actin detected on the same blots. The final data are expressed as a ratio of the relative optical density (ROD) of protein of interest to ROD of actin. For each protein, statistical analyses compared expression values in MDD vs. matched controls. A maximum-likelihood mixed-models test was used to estimate parameters of the models, assuming pairs and subjects within pairs were random components (SAS; Version 9.1, SAS Institute Inc., Cary, NC, USA). As a first step, unadjusted models were fit to compare depressives versus controls without adjusting for potential confounders. Adjusted models included the main effect for comparing depressives versus controls and covariates for age, PMI, and tissue pH. Interactions between the main effect, depressives versus controls, and each of the potentially confounding covariates were investigated and dropped from the model. The covariate adjusted analyses produced similar results; the results for the unadjusted model are reported for simplicity. Results for the depressed and control groups are reported as mean ± SEM based on the mixed model. In order to adjust for multiple comparisons (six signaling proteins analyzed) the p value of 0.05 was adjusted to <0.0083 (threshold for significance).
3. Results
Amounts of mTOR, p70S6K, eIF4E, eIF4B, p-eIF4E (Ser209), and p-eIF4B (Ser504) were analyzed in the PFC BA10 from 12 pairs of subjects with MDD and matched healthy controls. Fig. 2 shows representative immunoblots from 3 pairs of subjects used in the analysis. The amount of mTOR immunoreactivity from depressed subjects (0.42±0.06) was significantly lower compared to controls (0.616±0.055; t=4.13 df=11, p=0.0017, Fig.3). Similarly, there was a robust reduction in the level of p70S6K in depressed subjects (0.37±0.04) compared to control subjects (0.54±0.046; t=4.42 df=11, p=0.001, Fig.3). The amount of eIF4B immunoreactivity from depressed subjects (0.26±0.10) was also significantly lower compared to control subjects (0.78±0.14; t=3.64 df=11, p=0.0039, Fig.3). The amount of p-eIF4B (Ser504) from depressed subjects (1.18±0.32) was significantly lower compared to control subjects (2.18±0.36; t=5.09 df=11, p=0.0004, Fig.4). However, there were no changes in the expression of eIF4E or p-eIF4E (Ser209) between the two groups (t=2.66 df=11, p=0.022 and t=0.161 df=10, p=0.87, respectively, Fig. 3 and Fig.4).
Figure 2.
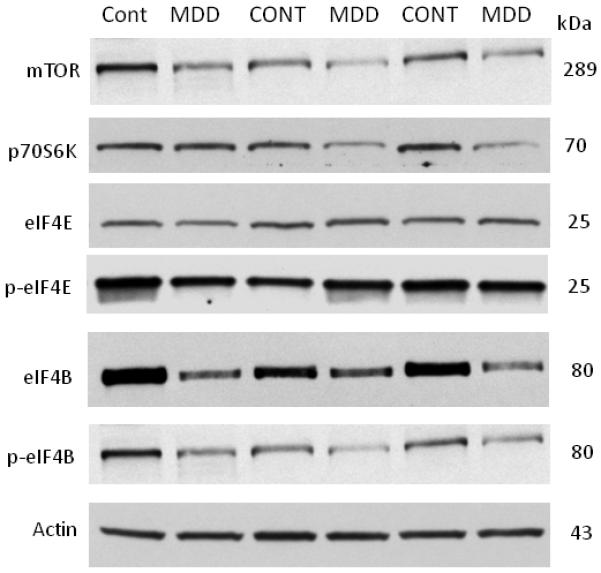
Immunoblots of mTOR, p70S6K, eIF4E, p-eIF4E, eIF4B, p-eIF4B and actin from six representative subjects used in the analysis. Each well was loaded with 20ug of total protein. Cont, control; MDD, major depressive disorder.
Figure 3.
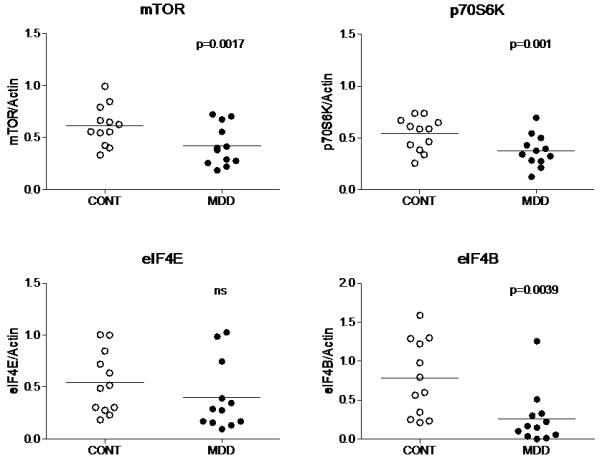
Scatter plots of mTOR, p70S6K, eIF4E, and eIF4B protein levels normalized to actin. Significant reductions in mTOR, p70S6K, and eIF4B immunoreactivities were observed in depressed subjects (filled circles; n=12) as compared to controls (open circle; n=12). Normalized optical density values for the individual subjects and mean values (horizontal lines) are presented. A p-value <0.0083 was considered as a threshold for significance.
Figure 4.
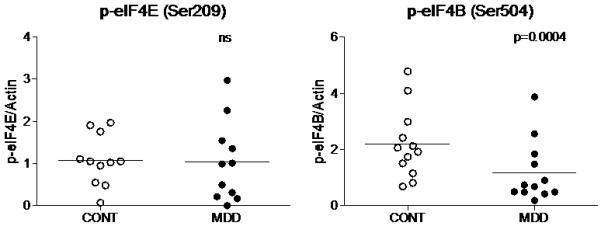
Scatter plots of p-eIF4E, and p-eIF4B protein levels normalized to actin. Significant reduction in p-eIF4B immunoreactivity was observed in depressed subjects (filled circles; n=12) as compared to controls (open circle; n=12). Normalized optical density values for the individual subjects and mean values (horizontal lines) are presented. A p-value <0.0083 was considered as a threshold for significance.
4. Discussion
The present study is the first to analyze levels of the mTOR-dependent translation initiation factors in the PFC from subjects diagnosed with MDD. Significant reductions in the expression of mTOR, p70S6K, eIF4B, and p-eIF4B were observed in MDD subjects as compared to psychiatrically healthy controls. In contrast, levels of eIF4E and p-eIF4E were unchanged in depressed subjects. Previously, we reported marked deficits in prominent postsynaptic proteins involved in glutamate neurotransmission such as NMDA receptor subunits (NR2A, NR2B), mGluR5, and PSD-95 (Deschwanden et al., 2011; Feyissa et al., 2009) in the PFC from the same depressed subjects used in this study. Taken together, these findings support the hypothesis that deficits in the mTOR-dependent translation initiation pathway contribute to the molecular pathology seen in the PFC in MDD, and a rapid reversal of these abnormalities may underlie antidepressant activity. Given that mTOR function is influenced by the activity of neuronal receptors including NMDA receptors, mGluR5 or tyrosine kinase (TrkB) a receptor for neurotrophic factors, additional studies will be required to elucidate whether deficits in these receptors, reported previously in MDD (Deschwanden et al., 2011; Feyissa et al., 2009, Thompson et al., 2011), are the reason for, or consequence of, mTOR signaling pathology.
It is generally accepted that mTOR acts as a node of convergence downstream of the aforementioned receptors and several signaling pathways, including phosphoinositide dependent kinase-1 (PDK1), phosphoinositide-3-kinase (PI3K), and Akt/protein kinase-B (Akt) (Hoeffer and Klann et al., 2010; Klann et al., 2004). A significant decrease in Akt1 activity has been previously reported in the PFC of suicide victims (Karege et al., 2007; confirmed by Dwivedi et al., 2010) and schizophrenics (Zhao et al., 2006), indicating an association between dysregulation of Akt/mTOR signaling and psychiatric disorders. Several core components of the mTOR signaling pathway are present in dendrites and enriched at postsynaptic sites (Tang et al., 2002), and the involvement of mTOR signaling in dendritic protein synthesis has been recently characterized (Gong et al., 2006).
In this study we have investigated eIF4B and eIF4E which are involved in translation initiation, the rate-limiting step of ribosome recruitment to the 5′ end of an mRNA (Gingras et al., 1999). In contrast to eIF4E, we have shown specific reductions in the levels of eIF4B (unphosphorylated and phosphorylated forms). Reduced level of phosphorylated eIF4B may indicate decreased eIF4B function. Given that eIF4B is phosphorylated/activated by p70S6K, reduced p-eIF4B would suggest reduced activity of p70S6K, indicating a dysfunction in mTOR/p70S6K/eIF4B pathway. Thus, based on our observation we hypothesize that the eIF4B-dependent steps in translation initiation are most likely impaired in depressed individuals.
Recent animal studies indicate that the fast antidepressant response to NMDA receptor antagonists (ketamine and Ro25-6981) is mediated by rapid activation of the mTOR pathway leading to an increase in synaptic signaling proteins and increased number and function of new spine synapses in the PFC of rats (Li et al., 2010). Moreover, blockade of mTOR signaling, using its specific inhibitor rapamycin, completely blocked ketamine-induced synaptogenesis and antidepressive effects in animal screening procedures (Li et al., 2010). Furthermore, it has been shown that a single dose of these antagonists rapidly reversed the chronic unpredictable stress-induced behavioral and synaptic deficits in an mTOR dependent manner (Li et al., 2011). The activation of mTOR and related proteins was also observed in rat cortical tissue after chronic treatment with NMDA receptor antagonist MK-801 (Yoon et al., 2008). Interestingly, chronic but not acute treatment with fluoxetine was shown to induce hyperphosphorylation of eIF4E, a key regulator of protein translation, suggesting that regulation of the translational machinery was involved in the mechanism of action of chronic fluoxetine administration (Dagestad et al., 2006). Therefore, the activation of the mTOR pathway may be related to the common effect of NMDA receptor antagonists and antidepressants, however, mechanisms underlying the induction of mTOR signaling are currently unclear. Therefore, further characterization of the mTOR signaling pathway in MDD and its involvement in antidepressant activity has the potential to identify novel therapeutic targets for antidepressant drug development.
In summary, the data reported herein, in conjunction with recent animal studies, implicate the involvement of mTOR signaling particularly via p70S6K/eIF4B pathway in the pathophysiology of depression and antidepressive activity. Reduced activity of critical core components of mTOR signaling may underlie the synaptic deficits previously reported in the PFC in MDD. These findings further confirm the potential of targeting the mTOR signaling cascade as an innovative and valuable strategy for the discovery of novel, fast-acting antidepressant medications.
*Highlights.
Depression is associated with deficits in synaptic proteins
We investigated levels of mTOR-dependent translation initiation factors in depression
Reductions in mTOR, p70S6K, eIF4B and p-eIF4B were indentified
No differences seen in eIF4E, p-eIF4E or actin levels.
An association between deficits in synaptic proteins in depression and dysregulation of mTOR/p70S6K/eIF4B signaling is evident
Acknowledgements
We thank Dr. Feyza Aricioglu for comments on the manuscript. The authors thank Drs. Dorota Maciag and Warren May for assistance in statistical analysis. We gratefully acknowledge the assistance of Drs James C Overholser, George Jurjus, Herbert Y Meltzer, and Ginny Dilley and Lisa Konick, MA, in the establishment of retrospective psychiatric diagnoses. The excellent assistance of the Cuyahoga County Coroner’s Office, Cleveland, OH, is greatly appreciated. We thank the next-of-kin for their participation and support. This study was supported by grant from the National Center for Research Resources (NCRR, RR17701), a component of NIH.
Abbreviations
- MDD
major depressive disorder
- PFC
prefrontal cortex
- NMDA
N-methyl-D-aspartate
- NR2A
NMDA receptor subunit 2A
- NR2B
NMDA receptor subunit 2B
- mGluR5
metabotropic glutamate receptor subtype 5
- PSD-95
postsynaptic density-95 kDa
- mTOR
mammalian target of rapamycin
- p70S6K
70-kDa ribosomal protein S6 kinase
- eIF4B
eukaryotic initiation factor 4B
- eIF4E
eukaryotic initiation factor 4E
- p-eIF4E (Ser209)
eukaryotic initiation factor 4E phosphorylated at serine 209
- p-eIF4B (Ser504)
eukaryotic initiation factor 4B phosphorylated at serine 504
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antion MD, Hou L, Wong H, Hoeffer CA, Klann E. mGluR-dependent long-term depression is associated with increased phosphorylation of S6 and synthesis of elongation factor 1A but remains expressed in S6K-deficient mice. Mol Cell Biol. 2008;28:2996–3007. doi: 10.1128/MCB.00201-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;15:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Dagestad G, Kuipers SD, Messaoudi E, Bramham CR. Chronic fluoxetine induces region-specific changes in translation factor eIF4E and eEF2 activity in the rat brain. Eur J Neurosci. 2006;23:2814–2818. doi: 10.1111/j.1460-9568.2006.04817.x. [DOI] [PubMed] [Google Scholar]
- Deschwanden A, Karolewicz B, Feyissa AM, Treyer V, Ametamey SM, Johayem A, et al. Reduced Metabotropic Glutamate Receptor 5 Density in Major Depression Determined by [11C]ABP688 PET and Postmortem Study. Am J Psychiatry. 2011 April 15; doi: 10.1176/appi.ajp.2011.09111607. published online. doi: 10.1176/appi.ajp.2011.09111607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Zhang H, Roberts RC, Conley RR, Pandey GN. Modulation in activation and expression of phosphatase and tensin homolog on chromosome ten, Akt1, and 3-phosphoinositide-dependent kinase 1: further evidence demonstrating altered phosphoinositide 3-kinase signaling in postmortem brain of suicide subjects. Biol Psychiatry. 2010;67:1017–1025. doi: 10.1016/j.biopsych.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:70–75. doi: 10.1016/j.pnpbp.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyissa AM, Woolverton WL, Miguel-Hidalgo JJ, Wang Z, Kyle PB, Hasler G, et al. Elevated level of metabotropic glutamate receptor 2/3 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:279–283. doi: 10.1016/j.pnpbp.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Donovan S, Frances A. Nosology of chronic mood disorders. Psychiatr Clin North Am. 1996;19:29–39. doi: 10.1016/s0193-953x(05)70271-9. [DOI] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem. 1999;68:913–63. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- Gong R, Park CS, Abbassi NR, Tang SJ. Roles of glutamate receptors and the mammalian target of rapamycin (mTOR) signaling pathway in activity-dependent dendritic protein synthesis in hippocampal neurons. J Biol Chem. 2006;281:18802–18815. doi: 10.1074/jbc.M512524200. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. Role of the mTOR signaling pathway in the rapid antidepressant action of ketamine. Expert Rev Neurother. 2011;11:33–36. doi: 10.1586/ern.10.176. [DOI] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–45. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell. 2005;123:569–580. doi: 10.1016/j.cell.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Karege F, Perroud N, Burkhardt S, Schwald M, Ballmann E, La Harpe R, et al. Alteration in kinase activity but not in protein levels of protein kinase B and glycogen synthase kinase-3beta in ventral prefrontal cortex of depressed suicide victims. Biol Psychiatry. 2007;61:240–245. doi: 10.1016/j.biopsych.2006.04.036. [DOI] [PubMed] [Google Scholar]
- Karolewicz B, Cetin M, Aricioglu F. Beyond the glutamate N-methyl D-aspartate receptor in major depressive disorder: the mTOR signaling pathway. Bulletin of Clinical Psychopharmacology. 2011;21:1–6. [Google Scholar]
- Klann E, Antion MD, Banko JL, Hou L. Synaptic plasticity and translation initiation. Learn Mem. 2004;11:365–372. doi: 10.1101/lm.79004. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J, Sengupta J, Frank J, Nissen P. Regulation of eukaryotic translation by the RACK1 protein: a platform for signalling molecules on the ribosome. EMBO Rep. 2004;5:1137–41. doi: 10.1038/sj.embor.7400291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raught B, Peiretti F, Gingras AC, Livingstone M, Shahbazian D, Mayeur GL, et al. Phosphorylation of eukaryotic translation initiation factor 4B Ser422 is modulated by S6 kinases. EMBO J. 2004;23:1761–1769. doi: 10.1038/sj.emboj.7600193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers GW, Jr, Komar AA, Merrick WC. eIF4A: the godfather of the DEAD box helicases. Prog Nucleic Acid Res Mol Biol. 2002;72:307–331. doi: 10.1016/s0079-6603(02)72073-4. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Endicott J. Schedule for Affective Disorders and Schizophrenia (SADS) 3rd ed. New York State Psychiatric Institute; New York: 1978. [Google Scholar]
- Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci U S A. 2002;99:467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SJ, Schuman EM. Protein synthesis in the dendrite. Philos Trans R Soc Lond B Biol Sci. 2002;357:521–529. doi: 10.1098/rstb.2001.0887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson Ray M, Weickert CS, Wyatt E, Webster MJ. Decreased BDNF, trkB-TK+ and GAD(67) mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. J Psychiatry Neurosci. 2011;36(1):100048. doi: 10.1503/jpn.100048. doi: 10.1503/jpn.100048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SC, Seo MS, Kim SH, Jeon WJ, Ahn YM, Kang UG, et al. The effect of MK-801 on mTOR/p70S6K and translation-related proteins in rat frontal cortex. Neurosci Lett. 2008;434:23–28. doi: 10.1016/j.neulet.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Ksiezak-Reding H, Riggio S, Haroutunian V, Pasinetti GM. Insulin receptor deficits in schizophrenia and in cellular and animal models of insulin receptor dysfunction. Schizophr Res. 2006;84:1–14. doi: 10.1016/j.schres.2006.02.009. [DOI] [PubMed] [Google Scholar]


