Dear Sirs,
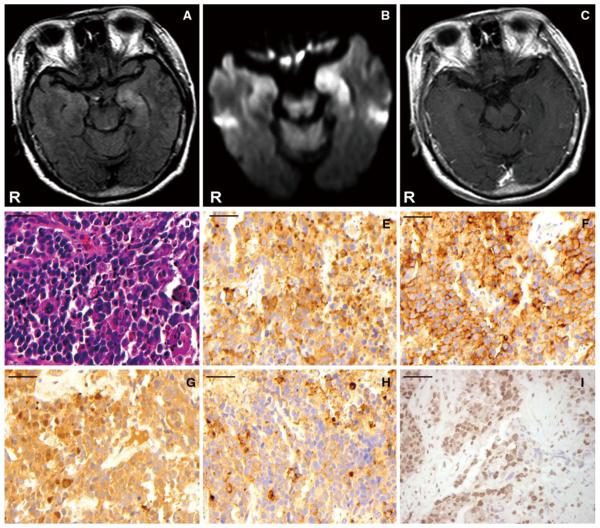
A 65-year-old woman was admitted to the neurology ward of our hospital with a suspected diagnosis of acute meningo-encephalitis. No antecedent flu-like episode was reported. Past medical history included a pelvic tumor which was initially diagnosed as a leiomyoma of the uterus. It had enlarged to a diameter of 10 cm in her pelvic space. On admission, her temperature was 37.6°C; her eyes were open but she was disoriented. The Glasgow Coma Scale score was 14 (E4 V4 M5) points. Signs of meningeal irritation were present. Other neurologic findings were normal. No involuntary movements were evident at admission. CSF analysis on admission showed leukocytes 51/mm3 with 134 monocular cells and 18 polymorphonuclear leukocytes, total protein concentration 23 mg/100 ml, and glucose 67 mg/100 ml (plasma glucose 119 mg/100 ml). HSV and HHV-6 DNA were not detected in CSF using the polymerase chain reaction method. CSF culture and cytology were negative. She was initially treated with acyclovir, panipemem–betamipron (a carbapenem-like antibiotic), vancomycin, and corticosteroids. However, on the 2nd hospital day, she became comatose, and her electroencephalogram demonstrated marked generalized slowing consisting of δ and θ waves. On the 8th hospital day, she exhibited tonic–clonic seizures and oral dyskinesia, which were treated with intravenous anticonvulsants. Her respiration was controlled with an endotracheal tube without the assistance of mechanical ventilation. Cranial MRI revealed abnormalities of high intensity in both, predominantly in the left, limbic area, mainly hippocampus and amygdala, on FLAIR and diffusion images, whereas gadolinium-enhanced T1-weighted image revealed no abnormality in these areas (Fig. 1). Her neurological condition did not improve despite the above treatments, and her comatose state persisted. The anti-NMDA-R antibody was detected in both serum and cerebrospinal fluid. On the 40th hospital day, she underwent surgical removal of the uterus and bilateral accessory organs. The pathological diagnosis was carcinosarcoma with neuroendocrine differentiation of the uterus. Microscopic findings of the tumor were as follows: on HE staining, solid nests of viable atypical cells were observed in the uterine tumor, though this tumor exhibited extensive necrosis. The majority of the atypical cells had scant cytoplasm and spheroid nuclei with granular chromatin, exhibiting frequent mitoses and marked venous/lymphatic infiltration. On immunohistochemical examination, most of this tumor was frequently positive for synaptophysin, neuron-specific enolase (NSE), and CD56. Chromogranin immunoreactivity was also noted. Immunoreactivity for keratin, myogenin, and desmin was detected in only a few neoplastic cells. We also examined whether the tumor exhibited NR1/NR2 subunits ectopically by immunohistochemical staining using a monoclonal antibody to NR1. The tumor was found to exhibit membranous NR1 staining (Fig. 1). She died from complications of multiple organ failure. No autopsy was performed.
Fig. 1.
Cranial MRI (a–c). Abnormalities of high intensity in both, predominantly in the left, limbic area, mainly hippocampus and amygdala, were revealed on FLAIR (a) and diffusion images (b), whereas gadolinium-enhanced T1-weighted image (c) shown no abnormality in these areas. Microscopic findings for the removed tumor (d–i). On hematoxylin–eosin staining, solid nests of viable atypical cells with frequent mitoses and marked venous/lymphatic infiltration were observed in the uterine tumor, though it exhibited extensive necrosis (d). On immunohistochemical examination, most of this tumor was frequently positive for synaptophysin (e), CD56 (f), and neuron-specific enolase (g). Chromogranin immunoreactivity was also noted in the tumor (h). Based on these findings, the tumor was diagnosed as a carcinosarcoma with neuroendocrine differentiation of the uterus. The tumor expressed ectopic NR1 subunits of NMDAR, as determined using a monoclonal antibody to mouse IgG. The tumor also exhibited membranous NR1 staining (i). All scale bars are 50 μm, with original magnification ×400
Anti-NMDA-R encephalitis is characterized by prominent psychiatric symptoms, dyskinesias, seizures, autonomic instability, and central hypoventilation [1, 2, 4]. The clinical manifestations in our patient were typical of this disorder. Dalmau and colleagues reported that about 55% of women with anti-NMDA-R encephalitis older than 18 years had an underlying tumor, usually mature or immature ovarian teratomas [2]. They confirmed that components of ovarian teratomas that contained nervous tissue exhibited NR1/NR2 subunits of NMDAR ectopically [1, 3]. Other tumors previously found in association with anti-NMDAR encephalitis did not exhibit subunits of NMDAR. The tumor in our patient was composed mostly of poorly differentiated neuroendocrine carcinoma, a histopathologically rare tumor of the uterus. There are no previous cases of carcinosarcoma with neuroendocrine differentiation reported in association with any paraneoplastic neurological syndromes. This is the first report of tumor cells with neuroendocrine differentiation associated with a paraneoplastic neurological syndrome and exhibiting NR1/NR2 subunits of NMDAR.
Contributor Information
Makoto Hara, Division of Neurology, Department of Medicine, Nihon University School of Medicine, 30-1 Oyaguchi-kamicho, Itabashi-ku, Tokyo 173-8610, Japan.
Akihiko Morita, Division of Neurology, Department of Medicine, Nihon University School of Medicine, 30-1 Oyaguchi-kamicho, Itabashi-ku, Tokyo 173-8610, Japan.
Satoshi Kamei, Division of Neurology, Department of Medicine, Nihon University School of Medicine, 30-1 Oyaguchi-kamicho, Itabashi-ku, Tokyo 173-8610, Japan.
Mai Yamaguchi, Division of Neurology, Department of Medicine, Nihon University School of Medicine, 30-1 Oyaguchi-kamicho, Itabashi-ku, Tokyo 173-8610, Japan.
Taku Homma, Department of Pathology, Nihon University School of Medicine, Tokyo, Japan.
Norimichi Nemoto, Department of Pathology, Nihon University School of Medicine, Tokyo, Japan.
Kenji Sugita, Department of Obstetrics and Gynecology, Nihon University School of medicine, Tokyo, Japan.
Tatsuo Yamamoto, Department of Obstetrics and Gynecology, Nihon University School of medicine, Tokyo, Japan.
Josep Dalmau, Division of Neuro-oncology, Department of Neurology, University of Pennsylvania, Philadelphia, PA, USA.
References
- 1.Dalmau J, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-d-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seki M, Suzuki S, Iizuka T, et al. Neurological response to early removal of ovarian teratoma in anti-NMDAR encephalitis. J Neurol Neurosurg Psychiatry. 2008;79:324–326. doi: 10.1136/jnnp.2007.136473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Florance NR, Davis RL, Lam C, et al. Anti-N-methyl-d-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol. 2009;66:11–18. doi: 10.1002/ana.21756. [DOI] [PMC free article] [PubMed] [Google Scholar]