Abstract
Purpose
To evaluate the reproducibility of γ-aminobutyric acid (GABA) and glutamate concentrations derived using three different spectral fitting methods, and to investigate gender-related differences in neurotransmitter levels.
Materials and Methods
Single voxel MEGA-edited PRESS MR spectra were acquired from a 30-mL voxel in the dorsolateral prefrontal cortex in 14 adult volunteers (7 female) at 3 Tesla (3T). For each participant, four consecutive resting spectra were acquired within the same scanning session. Metabolite concentrations were derived using LCModel, jMRUI, and locally written peak fitting software. The within-session reproducibility for each analysis method was calculated as the average coefficient of variation (CV) of the GABA and Glx (glutamate+glutamine) concentrations. Gender differences in GABA and Glx were evaluated using a two-tailed unpaired t-test.
Results
LCModel provided the best reproducibility for both GABA (CV 7%) and Glx (CV 6%). GABA, Glx, and glutamate concentrations were significantly higher in the male participants, (P = 0.02, P = 0.001, and P < 0.001, respectively).
Conclusion
GABA and glutamate can be quantified in vivo with high reproducibility (CV 6–7%) using frequency-domain spectral fitting methods like LCModel. However, the GABA and glutamate concentrations vary significantly between men and women, emphasizing the importance of gender-matching for studies investigating differences in neurotransmitter concentrations between mixed-cohort groups.
Keywords: MR spectroscopy, GABA, glutamate, reproducibility of results, gender differences
As the primary excitatory and inhibitory neurotransmitters in the human brain, glutamate (Glu) and γ-aminobutyric acid (GABA) are important for a variety of neurological and psychiatric disorders including epilepsy, schizophrenia, Parkinson’s disease, and Alzheimer’s dementia (1–3). GABA is also becoming the focus of increasing interest in functional neuroimaging, as resting in vivo GABA levels derived from MR spectroscopy (MRS) have recently been linked to changes in cerebral blood volume, peak gamma oscillation frequency, and blood oxygen level dependent (BOLD) fMRI signal during visual stimulation (4,5), as well as the negative BOLD response in the default mode network (6). Changes in GABA have also been observed with motor learning (7) and changes in glutamate have been detected following acute pain stimulation (8), suggesting that MR spectroscopic measurements of GABA and glutamate are sensitive not only to baseline neurotransmitter levels but also to rapid regional modulations of neurotransmitter activity.
However, despite these encouraging results robust spectroscopic quantitation of glutamate and GABA with MRS remains challenging due to the spectral overlap of these metabolites with each other and with glutamine (Gln). Spectral editing methods like the MEGA-PRESS technique (9,10) provide a promising approach for the discrimination of GABA from glutamate and glutamine, but few studies have examined the reproducibility of the concentrations derived with this method (11,12), and to date the reproducibility of GABA concentrations derived with MEGA-PRESS has not been investigated with a basis set spectral fitting approach like LCModel. In addition, while both GABA and glutamate have been reported to decrease with age (13,14), relatively little is known about the inter-subject variability in neurotransmitter levels arising from gender effects, as one previous study reported higher GABA levels in women than men (14), while another observed the opposite effect (15), and reported gender effects also differ for glutamate (13,15–18). In the present study, we quantify the test–retest reproducibility of the GABA and glutamate concentrations derived with MEGA-PRESS using three different spectral fitting methods, and investigate age-and gender-related differences in neurotransmitter levels in a group of healthy adults.
MATERIALS AND METHODS
Subjects
The subject group consisted of 14 healthy right-handed volunteers (7 female; mean age, 29 years; range, 25–38 years) with no history of neurological or psychiatric illness, illegal substance abuse or use of psychotropic medication. All subjects refrained from caffeine, alcohol, and nicotine for at least 8 h before the experiment. All measurements were performed in the early afternoon.
MR Data Acquisition
MR imaging and spectroscopy studies were performed with a 3 Tesla (T) GE HDxt MRI scanner (GE Medical Systems, Milwaukee, WI) equipped with TwinSpeed gradients. The scanning protocol included a three-dimensional (3D) fast inversion-recovery prepared gradient echo acquisition (number of slices = 172, slice thickness = 1.2 mm, repetition time (TR) = 9.988 ms, echo time (TE) = 2.916 ms, field of view = 240 mm × 240 mm, flip angle = 20, matrix = 256 × 192, voxel resolution: 0.8 × 0.8 × 1.2 mm), used for localization of the spectroscopy voxels. The volumetric T1-weighted images were also segmented into gray matter, white matter, and cerebrospinal fluid (CSF) maps using statistical parametric mapping (SPM5, Wellcome Dept of Cognitive Neurology) to correct the spectroscopy results for partial volume CSF contamination.
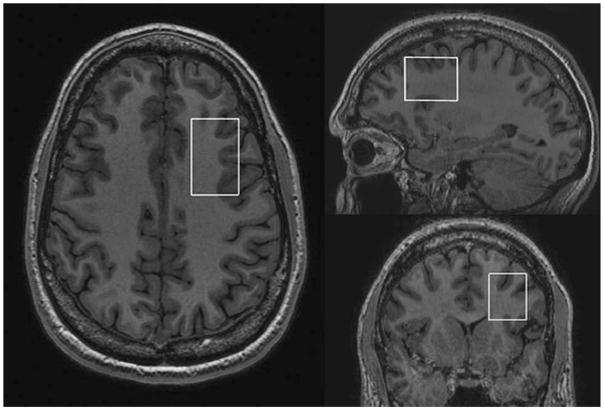
Single voxel edited 1H MR spectra were acquired from a 25 × 40 × 30 mm3 voxel of interest (VOI) positioned in the left dorsolateral prefrontal cortex (DLPFC) using the MEGA-PRESS method (9,10). To achieve a consistent MRS voxel position between subjects, the voxel was positioned on an imaging slice 1.5 mm above the superior margin of the lateral ventricles. The length of the midline was measured on this slice and the center of the voxel was defined at a point one-third of the distance down the midline from the anterior margin of the brain, and in the center of the left hemisphere (half of the distance between the midline and the left lateral border of the brain, on a line perpendicular to the midline, see Fig. 1). A total of 320 spectral averages were acquired for each spectrum with a TR of 1800 ms, an echo time of 68 ms, and an eight-step phase cycle, resulting in an acquisition time of approximately 10 min. MEGA-editing was achieved with 20-ms Gaussian editing pulses applied at 1.9 ppm and 7.5 ppm in alternate spectral lines. For each metabolite spectrum, 16 water reference lines were also acquired as part of the standard PROBE acquisition. For each participant, four consecutive resting GABA spectra were acquired, alternating between eyes open (EO) and eyes closed (EC) rest conditions, with the order (i.e., EC, EO, EC, EO, or EO, EC, EO, EC) counterbalanced between participants.
Figure 1.
Screenshot showing the position of the MEGA-PRESS DLPFC voxel.
MRS/MRI Data Analysis
The spectra were coil combined with weighting factors derived from the first point of the unsuppressed water free induction decay signal from each coil. Water-scaled metabolite concentrations were derived with LCModel version 6.1-4F (19) using a simulated basis set, the Amares algorithm in jMRUI (20), and with locally written peak fitting software in Matlab (5). Spectral fitting in Amares was performed after manually defining the center frequency and linewidth of the GABA and Glx peaks, and modeling the GABA peak as a singlet and the co-edited glutamate + glutamine (Glx) peak as a doublet. In Matlab, the edited GABA signal at 3 ppm and the unsuppressed PRESS water signal were integrated using an automated linear fit of the baseline and a Gaussian fit to the peak, while the co-edited Glx signal at 3.75 ppm was integrated using a linear fit to the baseline and the sum of two Lorentzians to fit the doublet peak. Concentrations were corrected for partial volume CSF contamination and for relaxation effects, using published values for the metabolite relaxation times (21–25).
Water-scaled metabolite concentrations for N-acetyl-aspartate (NAA), total creatine (Cr), choline (Cho), and myoinositol (mI) were calculated in LCModel from the unedited spectral lines using a simulated TE = 68 ms basis set. The within-session reproducibility was calculated for each participant and analysis method as the coefficient of variation of the GABA and Glx (Glu+Gln) concentrations. The within-session reproducibility for NAA, Cr, Cho, and mI were also calculated for comparison. After confirming the absence of any difference in GABA or Glx between the EO and EC rest conditions, (either across the participant group as a whole or across the male or female subgroups), the metabolite concentrations from the four resting spectra were averaged for each participant. The average metabolite concentrations for the male and female participants were compared with a two-tailed unpaired t-test, using the concentrations derived with LCModel. The statistical analysis was performed with SPSS version 14.0 (SPSS Inc., Chicago, IL).
RESULTS
A representative MEGA-PRESS spectrum is shown in Figure 2, and the reproducibility of the GABA and Glx concentrations derived using each fitting method are given in Table 1. The GABA peak at 3.0 ppm and the Glx peak at 3.75 ppm were well visualized for all spectra. LCModel provided the best within-session reproducibility for both GABA and Glx (7% and 6%, respectively). The reproducibility was significantly better for LCModel relative to the Matlab peak-fitting method (P < 0.05, two-tailed paired t-test) and showed a trend toward improved reproducibility relative to jMRUI (P = 0.08). LCModel demonstrated a reproducibility of 8% and 48% for glutamate and glutamine, respectively.
Figure 2.
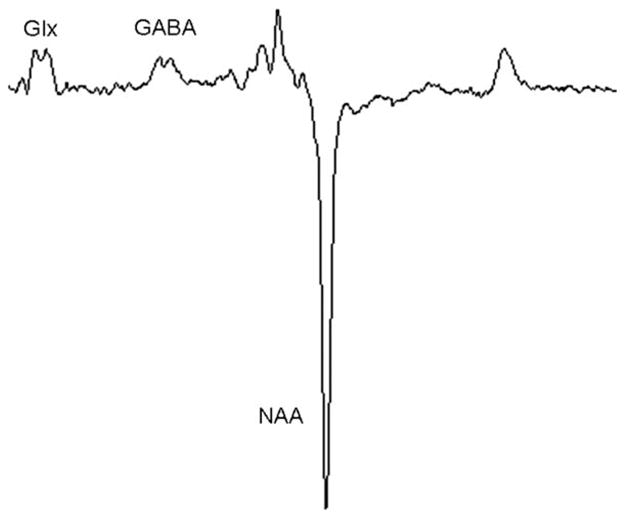
Representative MEGA-PRESS spectrum from one participant. Voxel dimensions: 25 mm (RL) × 40 mm (AP) × 30 mm (SI).
Table 1.
Within-Session Reproducibility for the GABA and Glx Concentrations Derived With LCModel, jMRUI, and Matlab
| Glx (%CV) | GABA (%CV) | |
|---|---|---|
| LCModel | 6% | 7% |
| jMRUI | 18% | 9% |
| Matlab | 9% | 12% |
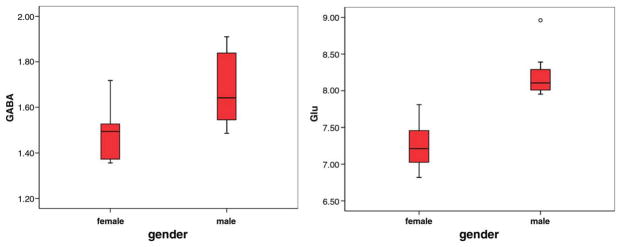
The average metabolite concentrations measured across all subjects and in the male and female participants are given in Table 2. The NAA, Cr, and Cho concentrations were derived from the unedited spectral lines, while the GABA, Glx, and Glu concentrations were derived from the MEGA-PRESS subtraction spectra. GABA, Glx, and Glu concentrations were significantly higher in the male participants, (P = 0.02, P = 0.001, and P < 0.001, respectively, two-tailed), although no significant gender differences were detected for Gln (P = 0.1) or for NAA, Cr, Cho, or mI (P > 0.1). No significant age effects were seen apart from an increase in total creatine with age (R = 0.67, P < 0.01), and no significant gender-related differences in the fraction of gray matter in the MRS voxel were evident (P = 0.9). The distribution of GABA and glutamate concentrations measured for the male and female participants is shown in Figure 3.
Table 2.
Mean (±SD) Water-Scaled Metabolite Concentrations Derived With LCModel for All Subjects and for the Male and Female Participants
| Metabolite concentrations (IU)
|
Reproducibility (%CV) | |||
|---|---|---|---|---|
| All subjects | Men | Women | ||
| NAA | 10.1 ± 0.49 | 10.1 ± 0.29 | 10.2 ± 0.65 | 4% |
| Cr | 7.63 ± 0.43 | 7.80 ± 0.42 | 7.46 ± 0.39 | 2% |
| Cho | 1.60 ± 0.21 | 1.57 ± 0.14 | 1.63 ± 0.28 | 3% |
| mI | 4.80 ± 0.58 | 4.76 ± 0.73 | 4.84 ± 0.42 | 10% |
| GABA | 1.61 ± 0.21 | 1.73 ± 0.20 | 1.48 ± 0.13 | 7% |
| Glx | 8.34 ± 0.82 | 8.87 ± 0.77 | 7.73 ± 0.29 | 6% |
| Glu | 7.74 ± 0.60 | 8.23 ± 0.35 | 7.26 ± 0.34 | 8% |
Figure 3.
Distribution of GABA (left) and glutamate (right) concentrations measured for the male and female participants. (Boxplots denote median and interquartile ranges.)
DISCUSSION
The consistently high spectral quality and high sensitivity to GABA seen for all the spectra in this study indicate that the MEGA-PRESS method provides a robust tool for the in vivo detection of GABA and Glx in the DLPFC. However, it is important to note that the GABA values reported in this study also contain contributions from co-edited macromolecule (MM) signals. Results from metabolite nulling studies suggest that the MM resonance can account for nearly half the apparent GABA concentration, although the relative contribution of the MM to the GABA+ peak is thought to remain relatively stable across brain regions and subjects (26). For future studies, it may be possible to explicitly account for the MM contributions by acquiring metabolite nulled spectra for each subject, or by incorporating simulated or experimental MM spectra into the LCModel basis set. However, for the present study the GABA results should be interpreted with caution as GABA+ (i.e., GABA+MM).
The main findings of this study are two-fold: we demonstrate a significant gender-related difference in GABA, Glx, and Glu, and show that frequency-domain spectral fitting methods like LCModel provide the highest precision for quantifying both the GABA and Glx concentrations.
The test–retest reproducibility for the GABA concentrations derived in this study compare favorably with the reproducibility values reported previously for jMRUI and peak fitting approaches (11,12). The glutamate reproducibility values derived here with MEGA-PRESS are also comparable to those derived using STEAM MRS at 4T (27), but slightly worse than those reported for simulation and experimental studies with standard PRESS and JPRESS approaches at 3T (28). The estimated concentrations for NAA, Cr, Cho, mI, Glx are in good agreement with those reported previously for frontal and prefrontal regions (1,16,18,21,29–31). The estimated GABA values (32–35) and the tissue composition of the voxel of interest (VOI) (21,29,35) are also consistent with results from some previous studies, although the relative proportion of white matter seen in the VOI in this study is slightly higher than that reported previously for some studies (33,34) (Table 3).
Table 3.
Mean (±SD) Tissue Composition of the DLPFC Voxel Across the Subject Group
| All subjects | Men | Women | |
|---|---|---|---|
| GM | 0.30 ± 0.03 | 0.30 ± 0.04 | 0.30 ± 0.02 |
| WM | 0.66 ± 0.04 | 0.66 ± 0.03 | 0.66 ± 0.04 |
| CSF | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.03 ± 0.01 |
Age and gender differences have been reported previously for glutamate (17), although a subsequent re-analysis of these results only revealed significant differences in glutamate concentration with age but not gender (13,18). We did not see any significant interactions between glutamate or GABA and age in our sample, possibly due to the relatively limited age range of the participants. However, we did observe strong gender differences in both GABA and glutamate, emphasizing the importance of gender matching for studies aiming to investigate differences in neurotransmitter concentrations between mixed-cohort subject groups. In our sample, GABA concentrations in the DLPFC were significantly higher in the male participants, confirming the results from another recent study examining GABA levels in men and women in the anterior cingulate (15). We also found significantly higher glutamate and Glx concentrations in the male participants, although we did not see any significant differences in glutamine, possibly because of the poorer reproducibility observed for the estimated Gln concentrations. Since the co-edited Glx peak is unaffected by macromolecules (36), the observed gender differences in Glx and glutamate are unlikely to arise from differences in macromolecular concentration between men and women, although gender-based macromolecular differences could affect the estimated GABA+ concentrations. In addition, since no significant gender differences were evident for the water-scaled NAA, Cr, Cho, or mI concentrations, it seems unlikely that the observed gender differences in GABA and Glu are driven by differences in water concentration between men and women. The lack of any significant difference in gray matter fraction in the MRS voxel between the male and female participants also confirms that these gender-related differences in GABA and glutamate are independent of the voxel composition.
The basis for the discrepancy between these results and those of an earlier study reporting higher GABA concentration in women is unclear, but may be due to an interaction between gender and regional variations in GABA concentration, since this study examined GABA levels in the occipital lobe (14). Alternatively, the disparate results may arise from variations in GABA levels with the menstrual cycle in the female participants (32), as we did not control for the phase of the menstrual cycle or collect neurosteroid information at the time of scanning.
While the neurophysiological basis for gender differences in neurotransmitter levels is not yet fully understood, neuroactive steroids such as estrogen, progesterone, testosterone, and dehydroepiandrosterone sulphate (DHEAS) are known to affect both glutamatergic and GABA-ergic neurotransmission, (for review, see Zheng) (37). However, the mechanisms by which neurosteroids affect neurotransmitter release or the responsiveness of postsynaptic receptors are complex, and appear to vary with brain region, functional state, and the type of neurosteroid (37). Therefore, while the observed gender differences in GABA and glutamate observed in this study are likely to reflect differences in the local concentration and action of steroid hormones, further studies will be needed to elucidate the relationship between regional neurotransmitter concentrations and plasma hormone levels.
In conclusion, our results demonstrate that GABA and glutamate can be quantified in vivo with a reliability of 6–7% using frequency-domain spectral fitting methods like LCModel. However, the GABA and glutamate concentrations vary significantly between men and women, emphasizing the importance of gender-matching for studies aiming to investigate differences in neurotransmitter concentrations between mixed-cohort subject groups.
Acknowledgments
Contract grant sponsor: Zurich Center for Integrative Human Physiology (ZIHP).
References
- 1.Helms G, Ciumas C, Kyaga S, Savic I. Increased thalamus levels of glutamate and glutamine (Glx) in patients with idiopathic generalised epilepsy. J Neurol Neurosurg Psychiatry. 2006;77:489–494. doi: 10.1136/jnnp.2005.074682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin WRW. MR Spectroscopy in neurodegenerative disease. Mol Imaging Biol. 2007;9:196–203. doi: 10.1007/s11307-007-0087-2. [DOI] [PubMed] [Google Scholar]
- 3.Stone JM, Morrison PD, Pilowsky LS. Glutamate and dopamine dysregulation in schizophrenia - A synthesis and selective review. J Psychopharmacol (Oxf) 2007;21:440–452. doi: 10.1177/0269881106073126. [DOI] [PubMed] [Google Scholar]
- 4.Donahue MJ, Near J, Blicher JU, Jezzard P. Baseline GABA concentration and fMRI response. Neuroimage. 2010;53:392–398. doi: 10.1016/j.neuroimage.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Muthukumaraswamy SD, Edden RAE, Jones DK, Swettenham JB, Singh KD. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci U S A. 2009;106:8356–8361. doi: 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Northoff G, Walter M, Schulte RF, et al. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat Neurosci. 2007;10:1515–1517. doi: 10.1038/nn2001. [DOI] [PubMed] [Google Scholar]
- 7.Floyer-Lea A, Wylezinska M, Kincses T, Matthews PM. Rapid modulation of GABA concentration in human sensorimotor cortex during motor learning. J Neurophysiol. 2006;95:1639–1644. doi: 10.1152/jn.00346.2005. [DOI] [PubMed] [Google Scholar]
- 8.Gussew A, Rzanny R, Erdtel M, et al. Time-resolved functional H-1 MR spectroscopic detection of glutamate concentration changes in the brain during acute heat pain stimulation. Neuroimage. 2010;49:1895–1902. doi: 10.1016/j.neuroimage.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Edden RAE, Barker PB. Spatial effects in the detection of gamma-aminobutyric acid: improved sensitivity at high fields using inner volume saturation. Magn Reson Med. 2007;58:1276–1282. doi: 10.1002/mrm.21383. [DOI] [PubMed] [Google Scholar]
- 10.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11:266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 11.Bogner W, Gruber S, Doelken M, et al. In vivo quantification of intracerebral GABA by single-voxel H-1-MRS-How reproducible are the results? Eur J Radiol. 2010;73:526–531. doi: 10.1016/j.ejrad.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Evans CJ, McGonigle DJ, Edden RAE. Diurnal stability of gamma-aminobutyric acid concentration in visual and sensorimotor cortex. J Magn Reson Imaging. 2010;31:204–209. doi: 10.1002/jmri.21996. [DOI] [PubMed] [Google Scholar]
- 13.Chang L, Jiang CS, Ernst T. Effects of age and sex on brain glutamate and other metabolites. Magn Reson Imaging. 2009;27:142–145. doi: 10.1016/j.mri.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanacora G, Mason GF, Rothman DL, et al. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56:1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- 15.Sheffield P, Noseworthy MD. Simultaneously assessed GABA/Glutamate/Glutamine concentration gender differences at 3.0T. Proceedings of the 18th Annual Meeting of ISMRM; Stockholm, Sweden. 2010. p. abstract 940. [Google Scholar]
- 16.Pouwels PJW, Frahm J. Regional metabolite concentrations in human brain as determined by quantitative localized proton MRS. Magn Reson Med. 1998;39:53–60. doi: 10.1002/mrm.1910390110. [DOI] [PubMed] [Google Scholar]
- 17.Sailasuta N, Ernst T, Chang L. Regional variations and the effects of age and gender on glutamate concentrations in the human brain. Magn Reson Imaging. 2008;26:667–675. doi: 10.1016/j.mri.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaiser LG, Schuff N, Cashdollar N, Weiner MW. Age-related glutamate and glutamine concentration changes in normal human brain: H-1 MR spectroscopy study at 4T. Neurobiol Aging. 2005;26:665–672. doi: 10.1016/j.neurobiolaging.2004.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Provencher SW. Estimation of metabolite concentrations from localized in-vivo proton NMR-spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 20.Naressi A, Couturier C, Devos JM, et al. Java-based graphical user interface for the MRUI quantitation package. Magn Reson Mater Phys Biol Med. 2001;12:141–152. doi: 10.1007/BF02668096. [DOI] [PubMed] [Google Scholar]
- 21.Choi CH, Coupland NJ, Bhardwaj PP, et al. T-2 measurement and quantification of glutamate in human brain in vivo. Magn Reson Med. 2006;56:971–977. doi: 10.1002/mrm.21055. [DOI] [PubMed] [Google Scholar]
- 22.Ethofer T, Mader I, Seeger U, et al. Comparison of longitudinal metabolite relaxation times in different regions of the human brain at 1. 5 and 3 Tesla. Magn Reson Med. 2003;50:1296–1301. doi: 10.1002/mrm.10640. [DOI] [PubMed] [Google Scholar]
- 23.Mlynarik V, Gruber S, Moser E. Proton T-1 and T-2 relaxation times of human brain metabolites at 3 Tesla. NMR Biomed. 2001;14:325–331. doi: 10.1002/nbm.713. [DOI] [PubMed] [Google Scholar]
- 24.Traber F, Block W, Lamerichs R, Gieseke J, Schild HH. H-1 metabolite relaxation times at 3. 0 tesla: measurements of T1 and T2 values in normal brain and determination of regional differences in transverse relaxation. J Magn Reson Imaging. 2004;19:537–545. doi: 10.1002/jmri.20053. [DOI] [PubMed] [Google Scholar]
- 25.Zaaraoui W, Fleysher L, Fleysher R, Liu ST, Soher BJ, Gonen O. Human brain-structure resolved T-2 relaxation times of proton metabolites at 3 Tesla. Magn Reson Med. 2007;57:983–989. doi: 10.1002/mrm.21250. [DOI] [PubMed] [Google Scholar]
- 26.Kegeles L, Mao X, Gonsalez R, Shungu DC. Evaluation of anatomic variation in macromolecule contribution to the GABA signal using metabolite nulling and the J-editing technique at 3T. Proceedings of the 15th Annual Meeting of ISMRM; Berlin, Germany. 2007. p. abstract 391. [Google Scholar]
- 27.Bartha R, Drost DJ, Menon RS, Williamson PC. Comparison of the quantification precision of human short echo time H-1 spectroscopy at 1.5 and 4. 0 Tesla. Magn Reson Med. 2000;44:185–192. doi: 10.1002/1522-2594(200008)44:2<185::aid-mrm4>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 28.Hancu I. Optimized glutamate detection at 3T. J Magn Reson Imaging. 2009;30:1155–1162. doi: 10.1002/jmri.21936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brambilla P, Stanley JA, Nicoletti MA, et al. H-1 magnetic resonance spectroscopy investigation of the dorsolateral prefrontal cortex in bipolar disorder patients. J Affect Disord. 2005;86:61–67. doi: 10.1016/j.jad.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Geurts JJG, Barkhof F, Castelijns JA, Uitdehaag BMJ, Polman CH, Pouwels PJW. Quantitative H-1-MRS of healthy human cortex, hippocampus, and thalamus: metabolite concentrations, quantification precision, and reproducibility. J Magn Reson Imaging. 2004;20:366–371. doi: 10.1002/jmri.20138. [DOI] [PubMed] [Google Scholar]
- 31.Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- 32.Epperson CN, Haga K, Mason GF, et al. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2002;59:851–858. doi: 10.1001/archpsyc.59.9.851. [DOI] [PubMed] [Google Scholar]
- 33.McLean MA, Busza AL, Wald LL, Simister RJ, Barker GJ, Williams SR. In vivo GABA+ measurement at 1. 5T using a PRESS-localized double quantum filter. Magn Reson Med. 2002;48:233–241. doi: 10.1002/mrm.10208. [DOI] [PubMed] [Google Scholar]
- 34.Simister RJ, McLean MA, Barker TJ, Duncan JS. A proton magnetic resonance spectroscopy study of metabolites in the occipital lobes in epilepsy. Epilepsia. 2003;44:550–558. doi: 10.1046/j.1528-1157.2003.19102.x. [DOI] [PubMed] [Google Scholar]
- 35.Tayoshi S, Nakataki M, Sumitani S, et al. GABA concentration in schizophrenia patients and the effects of antipsychotic medication: a proton magnetic resonance spectroscopy study. Schizophr Res. 2010;117:83–91. doi: 10.1016/j.schres.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Kaiser LG, Marjanska M, Matson GB, et al. H-1 MRS detection of glycine residue of reduced glutathione in vivo. J Magn Reson. 2010;202:259–266. doi: 10.1016/j.jmr.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng P. Neuroactive steroid regulation of neurotransmitter release in the CNS: action, mechanism and possible significance. Prog Neurobiol. 2009;89:134–152. doi: 10.1016/j.pneurobio.2009.07.001. [DOI] [PubMed] [Google Scholar]