Abstract
Lung hyperinflation is known to be an important contributing factor in the pathogenesis of ventilator-induced lung injury. Mechanical stretch causes epithelial barrier dysfunction and an increase in alveolar permeability, although the precise mechanisms have not been completely elucidated. p120-catenin is an adherens junction-associated protein that regulates cell-cell adhesion. In this study, we determined the role of p120-catenin in cyclic stretch-induced alveolar epithelial barrier dysfunction. Cultured alveolar epithelial cells (MLE-12) were subjected to uniform cyclic (0.5 Hz) biaxial stretch from 0 to 8 or 20% change in surface area for 0, 1, 2, or 4 h. At the end of the experiments, cells were lysed to determine p120-catenin expression by Western blot analysis. Immunofluorescence staining of p120-catenin and F-actin was performed to assess the integrity of monolayers and interepithelial gap formation. Compared with unstretched control cells, 20% stretch caused a significant loss in p120-catenin expression, which was coupled to interepithelial gap formation. p120-Catenin knockdown with small interfering RNA (siRNA) dose dependently increased stretch-induced gap formation, whereas overexpression of p120-catenin abolished stretch-induced gap formation. Furthermore, pharmacological calpain inhibition or depletion of calpain-1 with a specific siRNA prevented p120-catenin loss and subsequent stretch-induced gap formation. Our findings demonstrate that p120-catenin plays a critical protective role in cyclic stretch-induced alveolar barrier dysfunction, and, thus, maintenance of p120-catenin expression may be a novel therapeutic strategy for the prevention and treatment of ventilator-induced lung injury.
Keywords: mechanical ventilation, adherens junction, ventilator, lung injury, tight junction
ventilator-induced lung injury (VILI) as a result of mechanical stretch is characterized by increased alveolar-capillary permeability that leads to influx of protein-rich edema fluid and inflammatory cells into distal airways and alveoli (1, 38). The alveolar-capillary barrier is composed of epithelial cells of the alveolar wall, endothelial cells of the capillaries, and the basement membrane between adjacent cells. Under physiological conditions, the alveolar epithelium is much less permeable than the lung vascular endothelium. Breakdown of the epithelial barrier has significant consequences for patients with acute lung injury/acute respiratory distress syndrome (43). With each breath and more significantly during mechanical ventilation, pulmonary alveolar epithelial cells undergo biaxial stretch as the surface area of the basement membrane increases. Emerging evidence from both in vivo and in vitro studies has revealed that mechanical stress in the form of cyclic stretch induces structural and cytosolic changes in alveolar epithelial cells that result in alveolar epithelial barrier dysfunction and pulmonary hyperpermeability (5a, 6, 9, 39, 40, 69). Although recent studies suggest that polymerization of the actin cytoskeleton increases paracellular permeability by inducing gap formation between adjacent cells (10), the precise molecular mechanism(s) have not been completely elucidated.
Alveolar epithelial cells form junctional complexes consisting of tight and adherens junctions that restrict the paracellular passage of lipid-insoluble molecules between alveolar and interstitial spaces. The relative impermeability of the alveolar epithelium to paracellular solute diffusion is predominantly regulated by tight junctions (7, 17, 28). Tight junctions consist of integral membrane proteins: occludins, claudins, and junctional adhesion molecules (JAM) (26). In rat alveolar type II cells, cyclic stretch has been demonstrated to decrease peripheral occludin expression, total cellular occludin content, and the degree of cell-cell attachment (7). A recent study also showed that mechanical ventilation increased alveolar epithelial claudin-4 expression during VILI (47). These findings suggest the important role of tight junctions in the mechanism of mechanical ventilation-induced epithelial barrier dysfunction.
The role of adherens junctions in the regulation of mechanical stretch-induced epithelial barrier dysfunction has not been reported. Adherens junctions have been shown to play a role in the adaptation of vascular endothelial cells to long intervals of shear stress (24, 32) and act as transducers, mediating the transduction of shear-stress signals into vascular endothelial cells (34). These findings raise the possibility that epithelial adherens junctions may also contribute to the regulation of mechanical stretch-induced alveolar epithelial barrier dysfunction. Stabilization of adherens junctions is dependent on the association of epithelial (E)-cadherin, β-catenin, p120-catenin (p120), and α-catenin proteins and their linkage to the actin cytoskeleton (44). p120 is the prototypic member of a subfamily of armadillo repeat domain-containing proteins thought to stabilize adherens junctions through interactions with E-cadherins. Thus p120 binds directly to the cytoplasmic domain of E-cadherin and contributes to the regulation of cell-cell junctional integrity (30). p120 is highly expressed in endothelial and epithelial cells, fibroblasts, macrophages, cardiomyocytes, and cells of the nervous system. In mice, four different isoforms of p120 (1A, 1B, 2A, and 2B) have been detected (12, 23).
In this study, we demonstrated that cyclic stretch-induced p120 downregulation and a concomitant increase in actin polymerization result in loss of alveolar epithelial barrier integrity. These findings suggest that maintenance of p120 expression may be a novel therapeutic strategy for the treatment and prevention of VILI.
MATERIALS AND METHODS
Mouse epithelial cell culture.
Mouse lung epithelial cells (MLE-12) were purchased from American Type Culture Collection (Manassas, VA). The cells were plated at a density of 1 × 105 cells/cm2 on fibronectin-coated dishes in DMEM with 10% FBS, and grown to confluence in a humidified incubator at 37°C in 5% CO2-95% air. Confluent monolayers formed on culture dishes or BioFlex plates with elastomer membranes within 24–48 h. MLE-12 cell monolayers were serum-deprived for 2 h prior to experiments. In some experiments, calpain inhibitor I (150 μM) was added into the plate with confluent MLE-12 cell monolayers 15 min prior to stretch.
Cyclic stretching.
Alveolar epithelial cell monolayers on flexible membranes were exposed to cyclic stretch using FX-4000T Flexercell Tension Plus system (Flexcell International, McKeesport, PA) equipped with a 25-mm BioFlex loading station as previously described (33). Briefly, MLE-12 epithelial cells were seeded at standard densities (8 × 105 cells/well) onto collagen I-coated flexible bottom BioFlex plates. After 48 h of culture, the medium was changed in each plate, and experimental plates with cell monolayers were mounted onto the Flexercell system. Based on our preliminary data, we used a pattern of cyclic stretch at a frequency of 30 cycles/min (0.5 Hz), with a stretch-to-relaxation relation of 1:1. Cyclic stretch was conducted at 8 and 20% change in basement membrane surface area applied in a cyclic manner. These surface area changes correspond to 50 and 80% of total lung capacity, respectively (11, 18, 39). Cells were stretched for 1, 2, and 4 h at 37°C in a humidified incubator containing 5% CO2. The flexible cell-covered elastomer membranes were stretched by applying an oscillating vacuum to the underside of the membranes. A computer controlled the duration, amplitude, and frequency of the applied stretch. Nonstretched cells (static cells) were used as controls. Comparisons were made between stretched cells and control cells cultured on the same plates in the absence of cyclic strain.
Transient transfection of siRNA.
p120 and calpain-1 small interfering RNAs (siRNAs), which are composed of a pool of three target-specific 20- to 25-nt siRNAs (concentration range of 15–80 nM), were added to 50–70% confluent MLE-12 cells in DMEM + 10% FBS to deplete p120 and calapin-1 according to the manufacturer's protocol. Successful depletion of p120 and calpain-1 was confirmed by Western blot analysis. All experiments were performed 48 h after siRNA transfection (14, 35).
Virus packaging and infection.
p120 1A cDNA and empty vector (control) were transfected into Phoenix 293 packaging cells with Lipofectamine 2000 to produce retrovirus (16). MLE-12 cells were then infected with LZRS-MS-IRES-neo virus containing p120 1A cDNA or vector as described previously (41). The transfection efficiency and expression levels were determined by Western blot analysis and immunofluorescence of green fluorescent protein retroviral vector.
Western blot analysis.
At the end of the experiment, cell lysates were collected and protein levels in stretched and control (nonstretched) cells determined by Western blot analysis (14, 35). Briefly, cells were lysed following cyclic stretch in lysis buffer (50 mM Tris·HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.25% sodium deoxycholate, 1.0% Nonidet P-40, 0.1% SDS, 1 mM Na3VO4, 1 mM NaF, 1 mM PMSF, and protease inhibitor mixture). Protein concentrations were measured by using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL) according to the manufacturer's instructions. Equal amounts of protein were loaded on 10% acrylamide gels, separated by SDS-PAGE, and transferred to nitrocellulose membranes. Nonspecific binding was blocked with 5% nonfat dry milk in Tris-buffered saline (TBS) for 1 h at room temperature. The membranes were washed three times with TBS-T solution (0.05% Tween 20 in TBS) and incubated with tubulin (1:3,000), p120 (1:6,000), calpain-1 (1:800), or β-actin (1:3,000) antibodies in 5% BSA solution (in TBS-T). Incubation was carried out overnight; the membranes were washed three times for 5 min each and then incubated for 60 min with goat anti-rabbit (polyclonal) or anti-mouse (monoclonal) Ig G conjugated to horseradish peroxidase (∼1:5,000–8,000). Membranes were washed three times for 5 min each, and the protein bands were detected by use of the ECL SuperSignal reagent (Pierce, Rockford, IL). Relative band densities of the various proteins were measured from scanned films using ImageJ Software [http://rsb.info.nih.gov/ij; National Institutes of Health (NIH), Bethesda, MD].
Immunofluorescence staining and quantitative analysis of gap formation.
At the end of the stretch period, epithelial monolayers were fixed with formaldehyde and immunostained to determine intercellular gap formation. Briefly, cells were washed with PBS, fixed with 4% paraformaldehyde in HBSS, and then permeabilized for 30 min with 0.2% Triton X-100 in HBSS. Cells were blocked with 3% BSA in PBS and then incubated with anti-p120 antibody (1:600) followed by incubation with Alexa-labeled secondary antibody for another 2 h. Actin filaments were stained with Texas red-conjugated phalloidin diluted in the blocking solution. Control immunostaining was performed by using the respective nonspecific IgG. Cell nuclei were labeled with 4′,6-diamidino-2-phenyl indole dihydrochloride (DAPI). DAPI (1 μg/ml) was added immediately after cell fixation for 20 min and the cells were rinsed three times. The Flex membranes were excised with a razor blade and then mounted on glass slides with ProLong antifade mounting medium (Molecular Probes). Confocal images were acquired with a laser-scanning confocal microscope (Zeiss LSM 510 META) using Hg lamp and UV filters to detect DAPI [band pass (BP) 385–470 nm emission], 488 nm laser excitation to detect Alexa 488 (BP 505–530 nm emission), and 543 nm ex laser to detect Texas Red (BP 550 nm emission). Optical sections of <1 μm (pinhole set to achieve 1 Airy) were acquired with four frames averaging of 1,020 × 1,020 pixels. The 16-bit images were analyzed by using MetaVue 4.6 software (Universal Imaging, Downington, PA). Paracellular gaps were manually marked out, and images were differentially segmented between gaps and cells based on image grayscale levels. Quantitative analysis of gap formation was performed using ImageJ software (NIH). For each experimental condition, at least five ×60 fields for each independent experiment were analyzed from different areas (both central and peripheral) of the total field (4, 14, 35). Confocal images of epithelial monolayers which represent an average value across the surface area of the Silastic membrane were taken to illustrate effects of cyclic stretch on interepithelial gap formation. Gap formation was expressed as a ratio of the gap area to the area of the whole image.
Drugs and reagents.
p120 and calpain-1 siRNAs were purchased from Dharmacon. β-Actin and α-tubulin horseradish peroxidase-conjugated goat anti-rabbit antibodies were from Santa Cruz Biotechnology; DAPI, goat anti-mouse, and anti-rabbit IgG labeled with Alexa 488 and Alexa 546 phalloidin were from Molecular Probes.
All other chemicals and reagents used were obtained from Sigma Chemical (St. Louis, MO) unless otherwise stated. cDNA transfection reagent Lipofectamine 2000 was purchased from Invitrogen. HBSS containing NaHCO3 (4.2 mM) and HEPES (10 mM) was adjusted to pH 7.4. Calpain-1 inhibitor I was dissolved in DMSO. DMEM and FBS were obtained from Vec Technologies (Rensselaer, NY) and Hyclone (Logan, UT), respectively.
Statistical analysis.
One-way ANOVA and Student-Newman-Keuls test for post hoc comparisons were used to determine differences between control and experimental groups. Student's t-test was performed for paired samples. Parameter changes between different groups over time were evaluated by a two-way ANOVA with repeated measures. Data are expressed as means ± SE, and differences in group means were considered significant at P < 0.05.
RESULTS
Cyclic stretch induced intercellular gap formation and downregulation of p120 protein expression in murine alveolar epithelial cells.
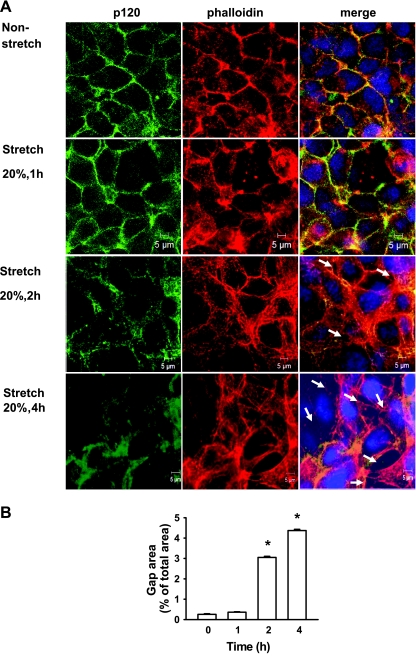
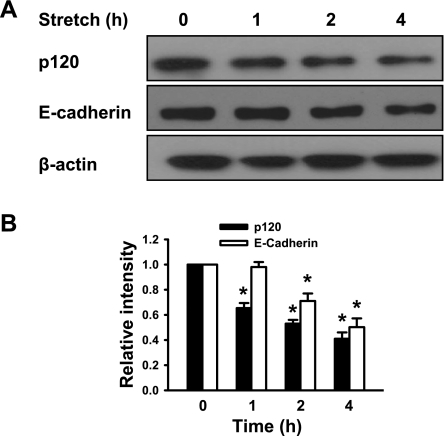
We initially examined the effect of cyclic stretch on epithelial monolayer integrity. A cyclic stretch of 20% of the surface area caused a time-dependent increase in intercellular gaps (Fig. 1A), consistent with previous findings (9). Cells exposed to 20% cyclic stretch for 1 h revealed relatively normal patterns of cytoskeletal arrangement characterized by circumferential (cortical) F-actin staining. Two hour-cyclic stretch dramatically induced stress fiber formation and paracellular gap formation. These responses were even greater in epithelial monolayers at 4 h exposed to 20% cyclic stretch. Quantitative analysis of stretch-induced gap formation (Fig. 1B) confirmed these observations and showed that total gap area after exposure of cells for 4 h increased 16-fold compared with static control monolayers. In static controls, p120 staining was observed primarily at sites of cell-cell contact. However, after 2- or 4-h cyclic stretch, total p120 expression (fluorescence intensity) was substantially reduced. To confirm a change in p120 protein expression, we analyzed the expression of p120 in the mouse epithelial cells by Western blot analysis following cyclic stretch. p120 protein level in the epithelial cells was reduced in a time-dependent manner following cyclic stretch. After exposure of epithelial cells to 20% cyclic stretch for 4 h, p120 protein level decreased by 60% (Fig. 2). Consistently, E-cadherin expression was also decreased after exposure of epithelial monolayers to cyclic stretch.
Fig. 1.
Effects of cyclic stretch on epithelial monolayer integrity. A: MLE-12 epithelial cell monolayers were exposed to 20% cyclic stretch for 0, 1, 2, and 4 h. At the end of experiments, cells were fixed, permeabilized, and then incubated with anti-p120 primary antibody followed by Alexa-conjugated secondary antibody (green). Actin cytoskeletal remodeling was examined by immunofluorescent staining with Texas red-conjugated phalloidin. Intercellular gaps induced by cyclic stretch are marked by arrows. The nucleus (blue) was stained with 4′,6-diamidino-2-phenyl indole dihydrochloride (DAPI). Scale bars = 5 μm. B: quantitative analysis of cyclic stretch-induced gap formation in alveolar epithelial cells exposed to cyclic stretch. Shown are representative results of 5 independent experiments. *P < 0.05 vs. static control.
Fig. 2.
Time course of cyclic stretch-induced degradation of p120 protein in alveolar epithelial cells. MLE-12 epithelial cells were exposed to 20% cyclic stretch for 0, 1, 2, and 4 h. p120 and E-cadherin protein expressions were determined by Western blot analysis. A: representative Western blots of p120 and E-cadherin protein expressions. B: the density of proteins in control (static) group was used as a standard (1 arbitrary unit) to compare relative densities in the other groups. *P < 0.05, compared with control groups (static). Data are representative of 3 independent experiments.
Depletion of p120 enhanced cyclic stretch-induced intercellular gap formation in murine epithelial cells.
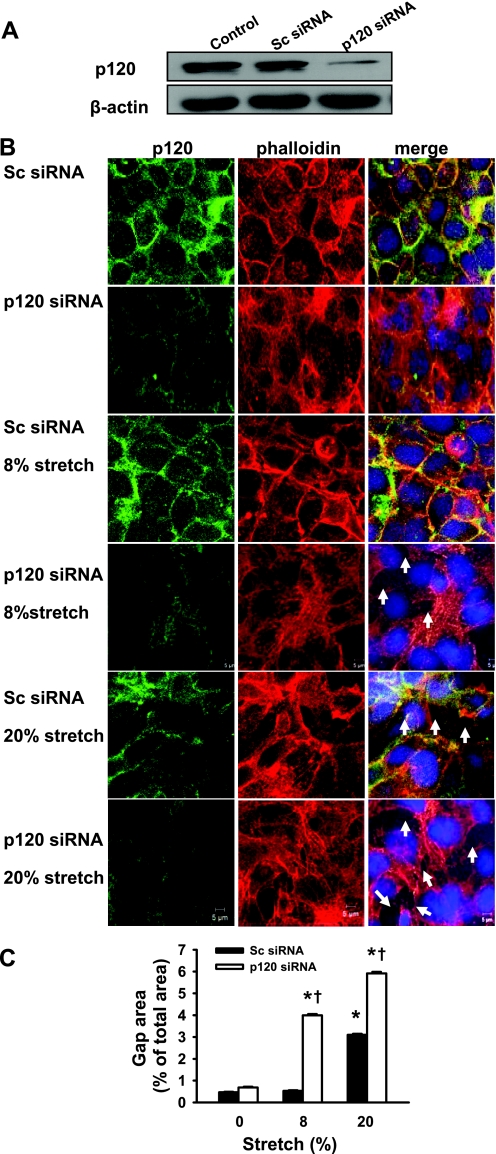
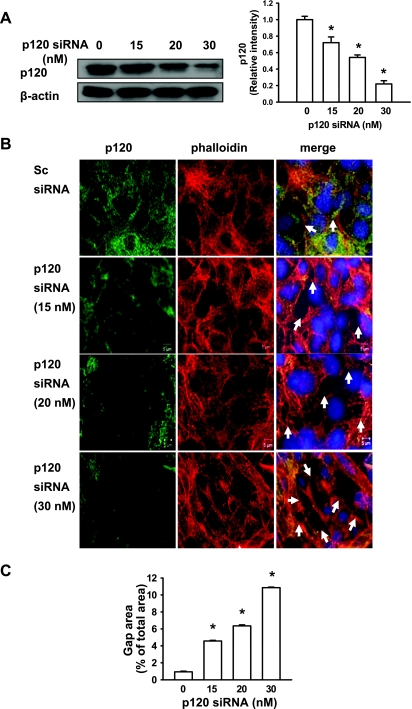
To determine whether downregulation of p120 contributes to stretch-induced epithelial barrier dysfunction, we treated epithelial cells with a specific p120 siRNA that reduced p120 protein expression by 80% (Fig. 3A). Reduced p120 expression per se did not result in gap formation between adjacent cells in the absence of cyclic stretch (Fig. 3B). Cyclic stretch of 8% of the surface area did not induce stress fiber formation or epithelial barrier disruption in scrambled siRNA-treated monolayers. However, depletion of p120 in epithelial monolayers with a specific siRNA significantly increased 8% cyclic stretch-induced stress fiber formation and intercellular gap area (Fig. 3B). Consistently, 20% cyclic stretch increased gap formation in scrambled siRNA-treated monolayers, whereas these effects were exaggerated by p120 knockdown in epithelial cell monolayers. A small number of detached cells were also found in p120 siRNA-treated epithelial cells in response to stretch. We next manipulated p120 expression with different concentrations of siRNA in epithelial cells, separated these data into three levels (∼70, 50, and 20%) of p120 expression (Fig. 4A) and determined the effect of 20% cyclic stretch on gap formation relative to the level of p120 expression. We observed an inverse correlation between stretch-induced gap formation and the level of p120 expression (Fig. 4, B and C), suggesting that cyclic stretch-induced alveolar epithelial gap formation may be dependent on the level of the p120 knockdown.
Fig. 3.
Effects of different magnitudes of cyclic stretch on epithelial barrier integrity in epithelial cells transfected with scrambled (Sc) and p120 small interfering RNAs (siRNAs). MLE-12 epithelial cells were transfected with scrambled and p120 siRNAs. At 48 h posttransfection, cells were exposed to cyclic stretch (8 and 20%) for 2 h. A: p120 was downregulated with specific p120 siRNA as determined by Western blot. Actin was used as a loading control. B: after fixation and permeabilization, cells were incubated with anti-p120-catenin primary antibody followed by Alexa-conjugated secondary antibody (green). The F-actin and nucleus were stained with Alexa 546 phalloidin (red) and DAPI (blue), respectively. Scale bars = 5 μm. C: quantitative analysis of cyclic stretch-induced gap formation in alveolar epithelial cells exposed to low- and high-magnitude of cyclic stretch was performed as described in materials and methods. Shown are representative results of 5 independent experiments. *P < 0.05 vs. control group (static). †P < 0.05 vs. respective Sc siRNA groups.
Fig. 4.
p120 siRNA increased cyclic stretch-induced gap formation in a dose-dependent manner. MLE-12 epithelial cells were transfected with scrambled and p120 siRNAs. At 48 h posttransfection, cells were then exposed to cyclic stretch (20%) for 2 h. A: the p120 siRNA specifically knocked down p120 expression as demonstrated at the protein levels in a dose-dependent manner. Left: representative Western blots of p120 protein expression. Right: the density of proteins in the control group was used as a standard (1 arbitrary unit) to compare relative densities in the other groups. *P < 0.05, compared with control groups. Data are representative of 3 independent experiments. B: after fixation and permeabilization, cells were incubated with anti-p120-catenin primary antibody followed by Alexa-conjugated secondary antibody (green). The F-actin and nucleus were stained with Alexa 546 phalloidin (red) and DAPI (blue), respectively. Scale bars = 5 μm. C: quantitative analysis of cyclic stretch-induced gap formation in alveolar epithelial cells transfected with p120 siRNA was performed as described in materials and methods. Shown are representative results of 5 independent experiments. *P < 0.05 vs. control group (static).
Overexpression of p120 prevented stretch-induced gap formation in epithelial cells.
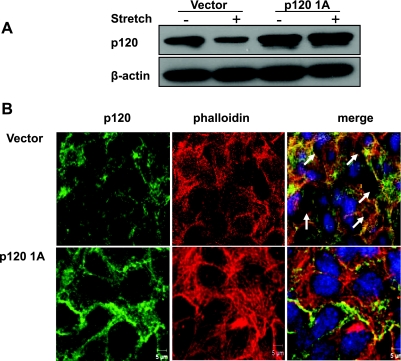
To confirm the observation that p120 plays a role in the mechanism of epithelial barrier dysfunction caused by cyclic stretch, we transfected epithelial cells with p120 1A cDNA or the empty vector. At 48 h posttransfection, Western blot analysis showed that p120 expression doubled in cells transfected with p120 1A cDNA compared with vector-transfected cells (Fig. 5A). Interestingly, after 2 h epithelial cell exposure to 20% cyclic stretch, p120 protein expression was reduced in control cells transfected with empty vector, whereas the level of p120 protein expression in p120 1A-overexpressing cells was not affected by mechanical stretch. p120 overexpression alone induced no change in cell-cell junctions (data not shown). Cyclic stretch of 20% of the surface area caused a significant increase in gap formation in epithelial cell monolayers transfected with the empty vector, whereas this effect was abolished by overexpression of p120 (Fig. 5B).
Fig. 5.
Effects of p120 overexpression on cyclic stretch-induced gap formation. MLE-12 cells were transfected with p120 1A cDNA or empty vector. At 48 h posttransfection, cells were exposed to cyclic stretch (20%) for 2 h. A: p120 protein expression was determined by Western blot analysis. B: after fixation and permeabilization, cells were incubated with anti-p120-catenin primary antibody followed by Alexa-conjugated secondary antibody (green). The F-actin and nucleus were stained with Alexa 546 phalloidin (red) and DAPI (blue), respectively. Scale bars = 5 μm.
Calpain-1 mediates stretch-induced p120 loss and subsequent gap formation.
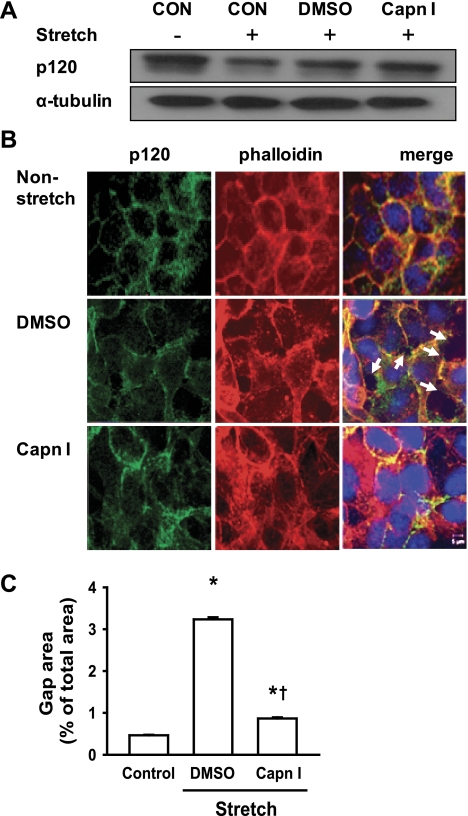
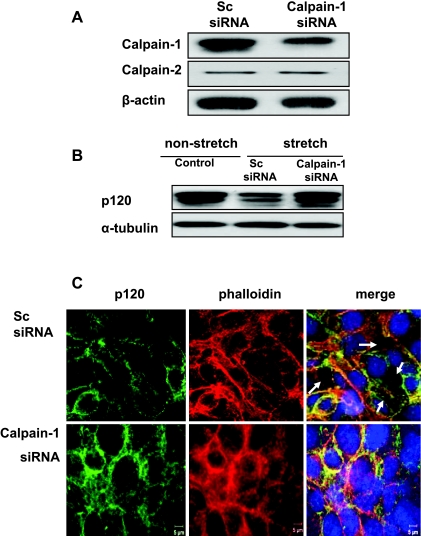
Calpain, a Ca2+-dependent protease, has been shown to mediate the degradation of p120 in ischemic SH-SY5Y cells (25) and vascular endothelial growth factor-treated endothelial cells (19). We therefore sought to address whether calpain-1 activation plays an important role in cyclic stretch-induced p120 degradation and subsequent epithelial barrier dysfunction. We found that pharmacological calpain inhibitor prevented cyclic stretch-induced p120 degradation compared with vehicle (DMSO) treatment (control) (Fig. 6A). Confocal imaging showed that in DMSO-treated monolayers (control), 20% cyclic stretch induced gap formation which was prevented by calpain inhibitor (Fig. 6, B and C). To clarify the contribution of calpain-1 and calpain-2 to the increase of calpain activity, we used siRNA to specifically knock down calpain-1 expression. Epithelial cells were transfected with calpain-1 siRNA or a scrambled siRNA as control. The efficacy of siRNA inhibition of calpain-1 and calpain-2 protein expression was analyzed by Western blot. As shown in Fig. 7A, calpain-1 protein expression was reduced by ∼80% with calpain-1 siRNA compared with scrambled siRNA, whereas calpain-2 expression was not affected. Forty-eight hours after transfection, cell monolayers were exposed to 20% cyclic stretch for 2 h. Compared with control cells, 20% stretch induced p120 loss in scrambled siRNA-treated cells, whereas this effect was completed blocked in calpain-1-depleted cells (Fig. 7B). Consistent with experiments using the pharmacological calpain inhibitor, confocal imaging demonstrated that depletion of calpain-1 prevented cyclic stretch-induced gap formation that occurred in scrambled siRNA-treated epithelial cells (Fig. 7C).
Fig. 6.
Effects of calpain inhibitor I on cyclic stretch-induced p120 loss and gap formation. MLE-12 cells were pretreated with calpain inhibitor I (Capn I, 150 μM) for 15 min and then exposed to cyclic stretch (20%) for 2h. A: effect of calpain inhibitor I on stretch-induced p120 protein expression. p120 protein expression was determined by Western blot analysis. CON, control. B: after fixation and permeabilization, cells were incubated with anti-p120-catenin primary antibody followed by Alexa-conjugated secondary antibody (green). The F-actin and nucleus were stained with Alexa 546 phalloidin (red) and DAPI (blue), respectively. Scale bars = 5 μm. C: quantitative analysis of cyclic stretch-induced gap formation in alveolar epithelial cells was performed as described in materials and methods. Shown are representative results of 5 independent experiments. *P < 0.05 vs. control group (static). †P < 0.05 vs. DMSO group.
Fig. 7.
Effects of calpain knockdown on cyclic stretch-induced p120 loss and gap formation. MLE-12 epithelial cells were transfected with scrambled and calpain-1 siRNAs. At 48 h posttransfection, cells were then exposed to cyclic stretch (20%) for 2 h. Calpain-1, calpain-2, and p120 protein expression and epithelial barrier function were determined by Western blot analysis (A) and immunofluorescent staining, respectively (B). A: effect of calpain-1 siRNA on calpain-1 protein expression. B: effects of calpain-1 knockdown on cyclic stretch-induced p120 protein expression. C: after fixation and permeabilization, cells were incubated with anti-p120-catenin primary antibody followed by Alexa-conjugated secondary antibody (green). The F-actin and nucleus were stained with Alexa 546 phalloidin (red) and DAPI (blue), respectively. Scale bars = 5 μm.
DISCUSSION
Using genetic and confocal imaging approaches, we have demonstrated that adherens junction protein p120 is an important determinant of cyclic stretch-induced epithelial barrier disruption. Cyclic stretch caused calpain-1-mediated p120 degradation, which predisposes cell-cell junctions to a high susceptibility to mechanical stress-induced epithelial barrier dysfunction. Our results suggest that mechanical ventilation may increase alveolar epithelial permeability by inducing the cleavage of adherens junction protein p120.
Several lines of evidence in this study suggest that cyclic stretch-induced downregulation of adherens junction protein p120 contributes to alveolar epithelial barrier dysfunction in response to mechanical stress. We initially found that cyclic stretch caused interepithelial gap formation in a time-dependent manner, which was coupled to reduced adherens junction protein p120 expression. Furthermore, we observed a dose-response effect of p120 siRNA on epithelial barrier dysfunction caused by cyclic stretch. The magnitude of cyclic stretch (8%) that did not induce gap formation in scrambled siRNA-treated cells had a disruptive effect on alveolar barrier in epithelial cells transfected with p120 siRNA. Cyclic stretch at 20% exaggerated gap formation in p120 knockdown cells compared with scrambled siRNA-treated cells. Finally, overexpression of p120 in epithelial cells abolished cyclic stretch-induced gap formation. Given all the evidence obtained so far, our results raise the possibility that the expression level of p120 may represent a threshold factor for alveolar epithelial barrier dysfunction during mechanical ventilation. These findings clearly suggest an important role of adherens junction protein p120 in stabilizing the alveolar epithelial barrier against mechanical stress.
Pulmonary epithelial adherens junctions are formed mainly by E-cadherin which is a transmembrane molecule that links neighboring cells through homophilic interactions. E-cadherin contains an extracellular domain (five homologous repeats) and a conserved cytoplasmic domain. p120 binds to the cytoplasmic domain in juxtamembrane regions via its central armadillo domain (20) whereas α-catenin interacts through β-catenin with the distal portion of the E-cadherin cytoplasmic domain (44). Accumulating evidence indicates that p120 acts as a critical regulator of cell-cell adhesion by stabilizing E-cadherin hemophilic interactions and by maintaining the total level of E-cadherin expression in epithelial cells (29). p120 binding to E-cadherin prevents the endocytosis and degradation of E-cadherin. p120-uncoupled E-cadherin not only increases E-cadherin internalization from the cell surface but also decreases its recycling back to the cell surface (21). As expected, in the present study, E-cadherin expression decreased in epithelial cells as p120 expression decreased. Notably, p120 knockdown per se did not cause interepithelial gap formation, consistent with our previous findings in pulmonary endothelial cells (41). In agreement with these results, p120-null epidermis displayed a marked reduction in E-cadherin at intercellular borders. Under normal conditions, depletion of p120 did not alter tight junction formation and cell-cell contacts within the epidermis (27). However, reduction in adherens junction complexes imposed by loss of p120 resulted in adhesive defects under conditions of mechanical stress. Therefore, stretch-induced p120 loss may weaken the adhesive forces between cells and contribute to epithelial barrier dysfunction induced by mechanical stimulation. Taken together, our results indicate an important role of disruption of adherens junctions in the breakdown of alveolar epithelial barrier integrity during mechanical ventilation.
Studies have suggested that adherens junctions and tight junctions are functionally and structurally interconnected and that the assembly of tight junctions is generally dependent on the formation and maintenance of adherens junctions (22, 36). Vascular endothelial-cadherin at adherens junctions upregulates expression of the tight junction protein claudin-5, and disruption of adherens junctions leads to the destabilization of tight junctions (36). In addition, tight junction components zonula occludens (ZO)-1 and ZO-2 have been implicated in the crosstalk between these two junctional structures (15). Thus it is likely that cyclic stretch-induced degradation of adherens junction protein p120 weakens tight junctions by disrupting tight junction protein expressions and complex integrity.
Calpains are a family of Ca2+-dependent intracellular cysteine proteases, encompassing the ubiquitously expressed calpain-1 (μM Ca2+-requiring, also known as μ-calpain) and calpain-2 (mM Ca2+-requiring, also known as m-calpain). By cleaving their protein substrates, both calpain-1 and calpain-2 are implicated in a wide variety of biological functions (13). Calpain-1 has been shown to mediate the degradation of p120 in ischemic SH-SY5Y cells (25) and endothelial cells (19). To address whether calpain also mediates p120 loss caused by mechanical stretch in alveolar epithelial cells, we utilized a genetic approach as well as pharmacological inhibitor to elucidate the role of calpain-1 vs. calpain-2 in stretch-induced p120 loss and subsequent epithelial gap formation. We found that pretreatment of epithelial cells with calpain inhibitor prevented cyclic stretch-induced loss of p120 and interepithelial gap formation. Consistently, specific knockdown of calpain-1 also abolished stretch-induced p120 degradation and gap formation. These results suggest that calpain-1, but not calpain-2, mediates mechanical stretch-induced p120 degradation and subsequent epithelial barrier dysfunction. Rat alveolar type II epithelial cells exposed to mechanical stretch in culture exhibited elevated Ca2+ mobilization (46). Therefore, in our study, it is possible that cyclic stretch activates calpain-1 via an increase in intracellular Ca2+ concentration.
In addition to p120, other cell-cell junction proteins have been reported to be substrates of calpain. Calpain activation has been shown to induce cleavage of adherens junction proteins E-cadherin and β-catenin in airway, prostate, and mammary tumor epithelial cells (8, 31). Furthermore, calpain also degraded tight junction protein occludin but not claudin-1 or JAM-1, indicating that calpains target specific junctional proteins (8). Thus we cannot exclude the possibility that cyclic stretch-induced calpain activation may have also caused degradation of these other junction proteins, resulting in gap formation. However, since the specific depletion of p120 with siRNA enhanced stretch-induced epithelial barrier dysfunction and overexpression of p120 abolished epithelial gap formation caused by cyclic stretch, our results clearly demonstrate the important role of p120 in cyclic stretch-induced epithelial barrier disruption.
The signaling mechanisms by which calpain-1-mediated, stretch-induced downregulation of p120 leads to gap formation are not clear. Activation of Rho kinases has been shown to play a role in cyclic stretch-induced gap formation in human pulmonary artery endothelial cells (5). Recent evidence indicates that p120 inhibits RhoA and activates Rac and Cdc42 (2). Thus the possible mechanism may be that calpain-1-mediated p120 loss in response to cyclic stretch induces alveolar epithelial barrier dysfunction via activation of RhoA and inhibition of Rac and Cdc 42.
In the present study, MLE-12 cells were utilized to determine the role of p120 in the regulation of alveolar epithelial barrier during mechanical stimulation. The MLE-12 cell line has been used as a model for alveolar type II epithelial cells to study mechanisms of mechanical stretch-mediated lung edema clearance (42, 46). MLE-12 cells have a higher proliferative index compared with primary alveolar epithelial cells and may represent a subtype of respiratory epithelial cells. This characteristic of MLE-12 cells may be associated with a higher tolerance for mechanical stretch than primary alveolar epithelial type II cells (3, 45). The relevance of our present study to in vivo animal and human clinical studies requires further investigation. However, we believe these studies provide valuable insight into the important role of adherens junction protein p120 in alveolar epithelial barrier dysfunction during mechanical ventilation.
In summary, this study provides evidence for the novel concept that cyclic stretch downregulates adherens junction protein p120 expression and weakens the epithelial barrier, thereby increasing the sensitivity of cell-cell contacts to mechanical stress. Calpain-1 activation mediates cyclic stretch-induced p120 degradation and subsequent epithelial barrier dysfunction. Our results suggest that p120 may be an important mechanosensitive molecule, and, thus, inhibition of p120 degradation may be a novel and effective target for the prevention and treatment of VILI.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute grant 5R01HL104092 (G. Hu) and American Heart Association Scientist Development Grant 0730331N (G. Hu) and National Heart, Lung, and Blood Institute grants 5R01 HL071626 and P01 HL060678 (R. D. Minshall).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Brenda Russell, Ph.D. (Department of Physiology and Biophysics, University of Illinois at Chicago) for access and training in the use of FX-4000T Flexercell Tension Plus system and Erik Swanson (Department of Physiology and Biophysics, University of Illinois at Chicago) for technical assistance.
REFERENCES
- 1. Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342: 1301–1308, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Anastasiadis PZ. p120-ctn: a nexus for contextual signaling via Rho GTPases. Biochim Biophys Acta Mol Cell Res 1773: 34–46, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Arold SP, Wong JY, Suki B. Design of a new stretching apparatus and the effects of cyclic strain and substratum on mouse lung epithelial-12 cells. Ann Biomed Eng 35: 1156–1164, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Birukova AA, Chatchavalvanich S, Rios A, Kawkitinarong K, Garcia JG, Birukov KG. Differential regulation of pulmonary endothelial monolayer integrity by varying degrees of cyclic stretch. Am J Pathol 168: 1749–1761, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Birukova AA, Moldobaeva N, Xing J, Birukov KG. Magnitude-dependent effects of cyclic stretch on HGF- and VEGF-induced pulmonary endothelial remodeling and barrier regulation. Am J Physiol Lung Cell Mol Physiol 295: L612–L623, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a. Cavanaugh KJ, Cohen TS, Margulies SS. Stretch increases alveolar epithelial permeability to uncharged micromolecules. Am J Physiol Cell Physiol 290: C1179–C1188, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cavanaugh KJ, Margulies SS. Measurement of stretch-induced loss of alveolar epithelial barrier integrity with a novel in vitro method. Am J Physiol Cell Physiol 283: C1801–C1808, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Cavanaugh KJ, Jr, Oswari J, Margulies SS. Role of stretch on tight junction structure in alveolar epithelial cells. Am J Respir Cell Mol Biol 25: 584–591, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Chun J, Prince A. TLR2-induced calpain cleavage of epithelial junctional proteins facilitates leukocyte transmigration. Cell Host Microbe 22: 47–58, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cohen TS, Cavanaugh KJ, Margulies SS. Frequency and peak stretch magnitude affect alveolar epithelial permeability. Eur Respir J 32: 854–861, 2008 [DOI] [PubMed] [Google Scholar]
- 10. DiPaolo BC, Lenormand G, Fredberg JJ, Margulies SS. Stretch magnitude and frequency-dependent actin cytoskeleton remodeling in alveolar epithelia. Am J Physiol Cell Physiol 299: C345–C353, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Geiger RC, Kaufman CD, Lam AP, Budinger GR, Dean DA. Tubulin acetylation and histone deacetylase 6 activity in the lung under cyclic load. Am J Respir Cell Mol Biol 40: 76–82, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Golenhofen N, Drenckhahn D. The catenin, p120ctn, is a common membrane-associated protein in various epithelial and non-epithelial cells and tissues. Histochem Cell Biol 114: 147–155, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev 83: 731–801, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Hu G, Vogel SM, Schwartz DE, Malik AB, Minshall RD. Intercellular adhesion molecule-1-dependent neutrophil adhesion to endothelial cells induces caveolae-mediated pulmonary vascular hyperpermeability. Circ Res 102: e120–e131, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ikenouchi J, Umeda K, Tsukita S, Furuse M, Tsukita S. Requirement of ZO-1 for the formation of belt-like adherens junctions during epithelial cell polarization. J Cell Biol 176: 779–786, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ireton RC, Davis MA, van Hengel J, Mariner DJ, Barnes K, Thoreson MA, Anastasiadis PZ, Matrisian L, Bundy LM, Sealy L, Gilbert B, van Roy F, Reynolds AB. A novel role for p120 catenin in E-cadherin function. J Cell Biol 159: 465–476, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jain M, Sznajder JI. Effects of hypoxia on the alveolar epithelium. Proc Am Thorac Soc 2: 202–205, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Kaufman CD, Geiger RC, Dean DA. Electroporation- and mechanical ventilation-mediated gene transfer to the lung. Gene Ther 17: 1098–1104, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kusaba T, Okigaki M, Matui A, Murakami M, Ishikawa K, Kimura T, Sonomura K, Adachi Y, Shibuya M, Shirayama T, Tanda S, Hatta T, Sasaki S, Mori Y, Matsubara H. Klotho is associated with VEGF receptor-2 and the transient receptor potential canonical-1 Ca2+ channel to maintain endothelial integrity. Proc Natl Acad Sci USA 107: 19308–19313, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu Q, Paredes M, Medina M, Zhou J, Cavallo R, Peifer M, Orecchio L, Kosik KS. δ-catenin, an adhesive junction-associated protein which promotes cell scattering. J Cell Biol 144: 519–532, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miyashita Y, Ozawa M. Increased internalization of p120-uncoupled E-cadherin and a requirement for a dileucine motif in the cytoplasmic domain for endocytosis of the protein. J Biol Chem 282: 11540–11548, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Miyoshi J, Takai Y. Molecular perspective on tight-junction assembly and epithelial polarity. Adv Drug Deliv Rev 57: 815–855, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Mo YY, Reynolds AB. Identification of murine p120cas isoforms and heterogeneous expression of p120cas isoforms in human tumor cell lines. Cancer Res 56: 2633–2640, 1996 [PubMed] [Google Scholar]
- 24. Noria S, Cowan DB, Gotlieb AI, Langille BL. Transient and steady-state effects of shear stress on endothelial cell adherens junctions. Circ Res 85: 504–514, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Ohno H, Uemura K, Shintani-Ishida K, Nakamura M, Inomata M, Yoshida K. Ischemia promotes calpain-mediated degradation of p120-catenin in SH-SY5Y cells. Biochem Biophys Res Commun 353: 547–552, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Paris L, Tonutti L, Vannini C, Bazzoni G. Structural organization of the tight junctions. Biochim Biophys Acta 1778: 646–659, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Perez-Moreno M, Davis MA, Wong E, Pasolli HA, Reynolds AB, Fuchs E. p120-catenin mediates inflammatory responses in the skin. Cell 124: 631–644, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rajasekaran SA, Palmer LG, Moon SY, Peralta Soler A, Apodaca GL, Harper JF, Zheng Y, Rajasekaran AK. Na,K-ATPase activity is required for formation of tight junctions, desmosomes, and induction of polarity in epithelial cells. Mol Biol Cell 12: 3717–3732, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reynolds AB. p120-catenin: Past and present. Biochim Biophys Acta 1773: 2–7, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reynolds AB, Roesel DJ, Kanner SB, Parsons JT. Transformation-specific tyrosine phosphorylation of a novel cellular protein in chicken cells expressing oncogenic variants of the avian cellular src gene. Mol Cell Biol 9: 629–638, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rios-Doria J, Kuefer R, Ethier SP, Day ML. Cleavage of beta-catenin by calpain in prostate and mammary tumor cells. Cancer Res 64: 7237–7240, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Schnittler HJ, Püschel B, Drenckhahn D. Role of cadherins and plakoglobin in interendothelial adhesion under resting conditions and shear stress. Am J Physiol Heart Circ Physiol 273: H2396–H2405, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Senyo SE, Koshman YE, Russell B. Stimulus interval, rate and direction differentially regulate phosphorylation for mechanotransduction in neonatal cardiac myocytes. FEBS Lett 581: 4241–4247, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shay-Salit A, Shushy M, Wolfovitz E, Yahav H, Breviario F, Dejana E, Resnick N. VEGF receptor 2 and the adherens junction as a mechanical transducer in vascular endothelial cells. Proc Natl Acad Sci USA 99: 9462–9467, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sun Y, Hu G, Zhang X, Minshall RD. Phosphorylation of caveolin-1 regulates oxidant-induced pulmonary vascular permeability via paracellular and transcellular pathways. Circ Res 105: 676–685, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, Potente M, Daly C, Dimmeler S, Dejana E. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol 10: 923–934, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Tremblay LN, Slutsky AS. Ventilator-induced lung injury: from the bench to the bedside. Intensive Care Med 32: 24–33, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Tschumperlin DJ, Margulies SS. Alveolar epithelial surface area-volume relationship in isolated rat lungs. J Appl Physiol 86: 2026–2033, 1999 [DOI] [PubMed] [Google Scholar]
- 40. Tschumperlin DJ, Margulies SS. Equibiaxial deformation-induced injury of alveolar epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol 275: L1173–L1183, 1998 [DOI] [PubMed] [Google Scholar]
- 41. Wang YL, Malik AB, Sun Y, Hu S, Reynolds AB, Minshall RD, Hu G. Innate immune function of the adherens junction protein p120-catenin in endothelial response to endotoxin. J Immunol 186: 3180–3187, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Waters CM, Ridge KM, Sunio G, Venetsanou K, Sznajder JI. Mechanical stretching of alveolar epithelial cells increases Na+-K+-ATPase activity. J Appl Physiol 87: 715–721, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Weinacker AB, Vaszar LT. Acute respiratory distress syndrome: physiology and new management strategies. Annu Rev Med 52: 221–237, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci 121: 727–735, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Wikenheiser KA, Vorbroker DK, Rice WR, Clark JC, Bachurski CJ, Oie HK, Whitsett JA. Production of immortalized distal respiratory epithelial cell lines from surfactant protein C/simian virus 40 large tumor antigen transgenic mice. Proc Natl Acad Sci USA 90: 11029–11033, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wirtz HR, Dobbs LG. Calcium mobilization and exocytosis after one mechanical stretch of lung epithelial cells. Science 250: 1266–1269, 1990 [DOI] [PubMed] [Google Scholar]
- 47. Wray C, Mao Y, Pan J, Chandrasena A, Piasta F, Frank JA. Claudin-4 augments alveolar epithelial barrier function and is induced in acute lung injury. Am J Physiol Lung Cell Mol Physiol 297: L219–L227, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.