Abstract
Mucociliary clearance, vital to lung clearance, is dependent on cilia beat frequency (CBF), coordination of cilia, and the maintenance of periciliary fluid. Adenosine, the metabolic breakdown product of ATP, is an important modulator of ciliary motility. However, the contributions of specific adenosine receptors to key airway ciliary motility processes are unclear. We hypothesized that adenosine modulates ciliary motility via activation of its cell surface receptors (A1, A2A, A2B, or A3). To test this hypothesis, mouse tracheal rings (MTRs) excised from wild-type and adenosine receptor knockout mice (A1, A2A, A2B, or A3, respectively), and bovine ciliated bronchial epithelial cells (BBECs) were stimulated with known cilia activators, isoproterenol (ISO; 10 μM) and/or procaterol (10 μM), in the presence or absence of 5′-(N-ethylcarboxamido) adenosine (NECA), a nonselective adenosine receptor agonist [100 nM (A1, A2A, A3); 10 μM (A2B)], and CBF was measured. Cells and MTRs were also stimulated with NECA (100 nM or 10 μM) in the presence and absence of adenosine deaminase inhibitor, erythro-9- (2-hydroxy-3-nonyl) adenine hydrochloride (10 μM). Both ISO and procaterol stimulated CBF in untreated cells and/or MTRs from both wild-type and adenosine knockout mice by ∼3 Hz. Likewise, CBF significantly increased ∼2–3 Hz in BBECs and wild-type MTRs stimulated with NECA. MTRs from A1, A2A, and A3 knockout mice stimulated with NECA also demonstrated an increase in CBF. However, NECA failed to stimulate CBF in MTRs from A2B knockout mice. To confirm the mechanism by which adenosine modulates CBF, protein kinase activity assays were conducted. The data revealed that NECA-stimulated CBF is mediated by the activation of cAMP-dependent PKA. Collectively, these data indicate that purinergic stimulation of CBF requires A2B adenosine receptor activation, likely via a PKA-dependent pathway.
Keywords: mucociliary clearance, knockout mouse model, bovine bronchial epithelial cells
mucociliary transport is an important host defense mechanism in the lung by which the coordinated beating of cilia continuously clears the lung of aspirated microorganisms and inhaled debris. This transport is dependent on the ciliary motion of airway epithelium, physicochemical properties of periciliary fluid, and mucus secretion (45, 50). Regulation of airway ciliary motility is critical because mucociliary transport is considered the first line of defense between the lung and the environment. Stressful conditions such as exercise or inflammation cause cilia in the airway to beat faster, increase clearance, and move an increased number of inhaled particles (2). As a consequence, mucociliary transport is a regulable host defense that can be activated under numerous conditions. It has been well described that ATP, a signaling molecule released by the airway epithelium after diverse stimuli, including shear forces and increases in intracellular calcium (22, 33), can directly stimulate ciliary activity (19, 23, 35). ATP is rapidly degraded via ecto-nucleotidases and ecto-apyrases to ADP → AMP → adenosine (29). The precise mechanism by which adenosine can stimulate ciliary beating via interaction of its cell-surface receptors is not understood.
Adenosine is known to elicit its cellular responses through its interaction with its cell-surface receptors (A1R, A2AR, A2BR, and A3R) (18). All of these receptors have been implicated in the regulation of pulmonary inflammation either in a cytoprotective and/or anti-inflammatory role (31, 36, 37). Conversely, activation of these receptors, particularly A1R and/or A3R, has been implicated in a proinflammatory role leading to tissue damage (3, 12, 21). In addition, as a G protein-coupled receptor, adenosine receptors have the ability to either activate or inhibit adenylyl cyclase, leading to changes in intracellular cAMP and calcium levels, both critical regulatory messengers for ciliary function (42, 51, 61). In the regulation of airway cilia motility, adenosine is thought to be a natural agonist that is produced in a paracrine fashion via the hydrolysis of ATP. This adenosine release can then act upon the adenosine A2 receptor(s), especially A2B, to increase cAMP, resulting in increased CBF (35).
A growing body of literature demonstrates that many complex factors, including adenosine, regulate ciliary motility and are only partially understood (42). Earlier studies established that adenosine could modulate ciliary activity (57, 59). However, since these early findings, there have been several studies that revealed that the varied response of adenosine in modulating mucociliary velocity depends on its specific interaction with its cell-surface receptors and its ability to activate cAMP (30, 34, 35, 55). Although these studies have established the effects of adenosine on ciliary activities, the results varied depending on the animal model investigated. In addition, most of the studies investigating the role of adenosine in ciliary motility have been gleaned via a combination of indirect pharmacological approaches coupled with molecular analysis of adenosine subtype expression patterns. Given the dearth of subtype-selective antagonists (5), the novelty of our experiments is our ability to use adenosine receptor knockout mice (A1, A2A, A2B, or A3) to delineate their role in modulating ciliary motility. In this study, we evaluated the role of adenosine in modulating ciliary beat frequency (CBF). To gain a better understanding of the contribution of adenosine of specific adenosine receptors to key airway ciliary motility processes, we employed two systems to do this: 1) an ex vivo mouse model that utilizes excised trachea from wild-type and adenosine receptor knockout mice (A1, A2A, A2B, or A3) and 2) a cell model utilizing bovine ciliated bronchial epithelial cells (BBECs). Here we demonstrate that A2B adenosine receptor activation is vital for regulation of ciliary motility through a PKA-dependent pathway.
MATERIALS AND METHODS
Reagents and materials.
M199 medium was purchased from Biofluids (Rockville, MD). Streptomycin, penicillin, protease (type IV), FCS, and fungizone were purchased from Life Technologies (Grand Island, NY). PureCol was purchased from Advanced BioMatrix (San Diego, CA). Phosphocellulose P-81 paper was purchased from Whatman (Clifton, NJ). Heptapeptide substrate for PKA (LRRASLG) was purchased from Peninsula Laboratories (Bachem, San Carlos, CA). [γ-32P] ATP was purchased from GE Healthcare Biosciences (Pittsburgh, PA). Erythro-9- (2-hydroxy-3-nonyl) adenine hydrochloride (EHNA) and 4-(2,3,6,7-tetrahydro-2, 6-dioxo-1-propyl-1H-purin-8-yl)-benzenesulfonic acid potassium salt (PSB1115) were purchased from TOCRIS Bioscience (Ellisville, MO). The nonselective adenosine receptor agonist, 5′-(N-ethylcarboxamido) adenosine (NECA), isoproterenol (ISO), procaterol, and all other reagents not listed were purchased from Sigma (St. Louis, MO).
Ciliated BBEC preparation.
Primary ciliated BBECs were prepared from fresh bronchi by a modification as previously described (53). Bronchi were cut apart from the lung, cleaned, and incubated overnight at 4°C in 0.1% bacterial protease type IV in M199 media. Following overnight incubation, each bronchus lumen was rinsed with M199 containing 10% FCS to collect the epithelial cells lining the lumen. These cells were then filtered through a 40 μm mesh filter, which typically produces a high-viability cell preparation of >95% ciliated cells (48). Cells were plated on 60-mm tissue culture dishes coated with 1% PureCol and maintained in complete M199 media containing 10% FCS, 50 U/ml penicillin and streptomycin, and 2 μg/ml fungizone, in a humidified 95% air-5% CO2 incubator at 37°C. Confluent monolayers of primary BBECs were obtained in 3 days and treated with ISO (10 μM), procaterol (100 nM), and/or NECA (100 nM or 10 μM) for 30 min and were used for CBF studies.
Mice.
Female C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA) at 8 wk of age and maintained in the animal care facility at the University of Nebraska Medical Center, which is accredited by the American Association of Accreditation of Laboratory Animal Care (AAALAC). Mice received standard rodent chow and water ad libitum. All four homozygous adenosine knockout (AKO) mice (A1AR, A2AAR, A2BAR, and A3AR) were backcrossed onto the C57BL/6 background at the University of Texas-Houston Medical Center, and blinded tracheas that were decoded after data analysis were provided from the laboratory of Dr. Michael Blackburn (University of Texas-Houston Medical Center; Houston, TX). All experiments followed the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the UNMC Institutional Animal Care and Use Committee.
Mouse tracheal extraction and treatment.
Tracheas were removed in a modification as previously described (16) and maintained in serum-free M199 containing penicillin, streptomycin, and fungizone in a closed sterile 15-ml conical tube at room temperature until processing (30–60 min). Tracheal rings were cut from the distal end of the trachea just above the carina (0.5 mm width) and placed in six-well tissue plates containing serum-free M199 for CBF determination. After baseline CBF determination, tracheal rings from wild-type C57BL/6 mice or AKO mice were stimulated with ISO (10 μM) or procaterol (10 μM) and/or NECA (100 nM; 10 μM). Some rings were pretreated with an adenosine deaminase inhibitor, EHNA (10 μM) or with a selective A2B adenosine receptor antagonist, PSB1115 (100 nM), or with a selective and potent cAMP-dependent PKA inhibitor, KT-5720. Rings were incubated for 30 min at 37°C in 5% CO2 and then allowed to equilibrate at 25°C for 10 min, and a final CBF reading was taken.
CBF.
Actively beating ciliated cells from primary BBECs or tracheal rings were observed and their motion quantified using phase-contrast microscopy and computerized frequency spectrum analysis as described by Sisson et al. (52). During CBF measurements, both BBECs and tracheal rings were maintained at a constant temperature (24 ± 0.5°C) by a thermostatically controlled heated stage. Whole-field analysis was performed using software that analyzes the entire captured image of all ciliated cells in a given field. The digital sampling rate was set at 85 frames/s for all experiments. The predominant frequency of cilia was viewed and taken in at least six separate recordings. All frequencies are expressed as means ± SE from six separate fields.
Determination of cAMP-dependent PKA activity.
PKA activity was determined in crude whole cell fractions of ciliated BBECs. The assay used is a modification of procedures previously described (26) with 130 μM PKA substrate heptapeptide (LRRASLG), 10 μM cAMP, 0.2 mM IBMX, 20 mM Mg-acetate, and 0.2 mM [γ-32P] ATP in a 40 mM Tris·Hal buffer (pH 7.5). Samples (20 μl) were added to 50 μl of the above reaction mixture and incubated for 15 min at 30°C. Cell fraction (10 μl) initiated the reactions, and spotting 50 μl of each sample on phosphocellulose papers halted incubation. Papers were then washed five times for 5 min each in 75 mM phosphoric acid, washed once in ethanol, dried, and counted in nonaquaeous scintillant as previously described (38). Negative controls consisted of similar assay samples with or without the substrate peptide or cAMP. Kinase activity is expressed in relation to total cellular protein assayed and was calculated in picomoles of phosphate incorporated per minute per milligram of total protein. All samples were assayed in triplicate, and no fewer than three separate experiments (n = 9) were performed per unique parameter.
Cilia histopathological examination.
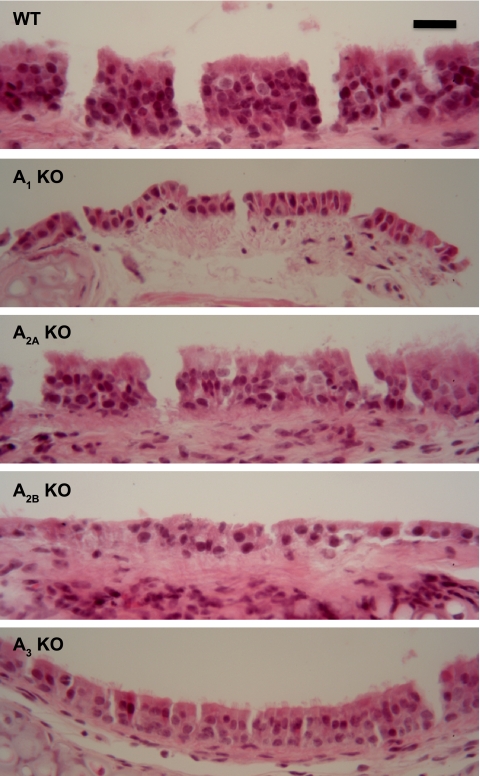
Lungs from AKO and wild-type mice were fixed with 10% neutral buffered formalin (pH 7.2) as previously described by Elliot et al. (15). Following fixation, lungs were paraffin embedded, serial sectioned (4–5 μm), and stained with hematoxylin and eosin, revealing normal appearing cilia and pseudocolumnar epithelium in all four AKO and wild-type mouse tracheal rings (MTRs) (Fig. 1, magnification, ×40).
Fig. 1.
Hematoxylin and eosin staining of mouse tracheal rings (MTRs) from adenosine receptor knockout (AKO) and wild-type (WT) mice. Sections were stained with hematoxylin and eosin, revealing normal-appearing cilia and pseudocolumnar epithelium in all 4 AKO MTRs compared with WT MTRs. Magnification, ×40.
Statistical analysis.
CBF data were statistically analyzed using Student's paired t-test followed by Tukey's multiple-comparison test. Statistical differences between groups were determined using one-way ANOVA followed by Tukey's multiple-comparison test (GraphPad Prism, version 4; GraphPad, San Diego, CA). Significance was assigned at P ≤ 0.05.
RESULTS
ISO stimulation of CBF in MTRs is concentration dependent and comparable to ciliated BBEC.
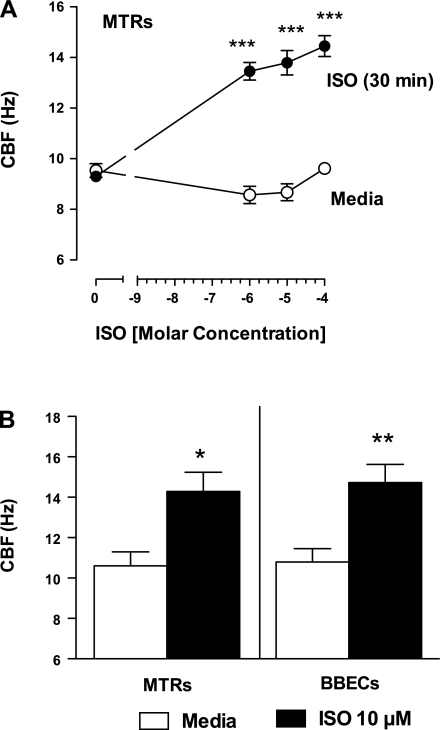
To demonstrate the sensitivity of the tracheal rings with a known activator of cilia motility, wild-type MTRs were stimulated with various concentrations of ISO. Baseline (unstimulated) CBF (∼9 Hz) did not differ among any of the ciliated tracheal rings tested. ISO significantly increased the CBF by 30 min of exposure (∼2–3 Hz; ***P < 0.001) compared with baseline and was concentration dependent (Fig. 2A). On the basis of these findings, we used 10 μM ISO as a positive control for subsequent experiments. Comparable CBF stimulation was achieved by 10 μM ISO in ciliated BBECs (Fig. 2B). These data demonstrate comparable CBF responses in two different models (tracheal rings vs. cultured airway cells) from two different species (murine and bovine).
Fig. 2.
A: isoproterenol (ISO)-stimulated ciliary beat frequency (CBF) in MTRs is concentration dependent. WT MTRs were stimulated with various concentrations of ISO. Unstimulated CBF among each group was ∼9 Hz (○). ISO increased CBF by 30 min of exposure (14–16 Hz; ●; ***P < 0.001 vs. media control). B: ISO stimulates ciliary motility in MTRs and bovine bronchial epithelial cells (BBECs). Baseline CBF from both WT MTRs and ciliated BBECs were similar. ISO (10 μM) significantly stimulated CBF in both WT MTRs and ciliated BBECs over media control (4.85 ± 0.435 Hz and 4.975 ± 0.7763 Hz, respectively). All frequencies are expressed as means ± SE from 6 separate fields. These data are representative of 3 independent experiments (MTRs *P < 0.05 vs. media control; BBECs **P < 0.01 vs. media control).
NECA, activation of adenosine A2B receptor stimulates CBF in both MTRs and ciliated BBEC.
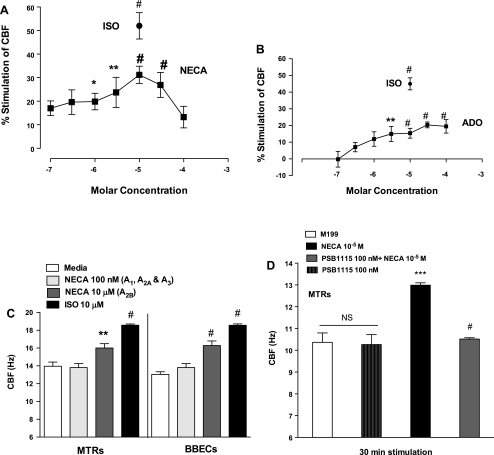
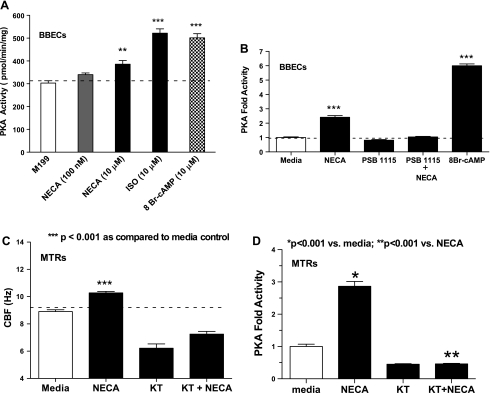
NECA is a potent nonselective adenosine agonist that is resistant to hydrolysis by adenosine deaminase, an enzyme that degrades adenosine. Baseline CBF of ∼12 Hz was maintained up to 30 min. NECA increased CBF in a concentration-dependent fashion at a threshold of ∼1 μM (EC50 = 2.25 μM) with maximal stimulation of CBF occurring at 10 μM (#P < 0.001 compared with media control), returning almost to baseline at 100 μM (Fig. 3A). ISO (10 μM) was used as positive control. Similarly, MTRs stimulated with adenosine increased CBF in a concentration-dependent fashion (EC50 = 1.5 μM; Fig. 3B) with maximal stimulation of CBF occurring at 30 μM (#P < 0.001 compared with media control), demonstrating that NECA had a greater potency than adenosine. To determine the subtype of adenosine cell surface receptor(s) responsible for stimulated ciliary motility, wild-type MTRs and ciliated BBECs were treated with NECA (100 nM, activates A1, A2A, and A3; and 10 μM, activates A2B) (47). Stimulation with 10 μM NECA significantly increased CBF in both wild-type MTRs and ciliated BBECs, comparable to 10 μM ISO. Stimulation with 100 nM did not elicit a significant increase in CBF (Fig. 3C). Wild-type MTRs were pretreated with a highly selective A2BR antagonist, PSB1115, to confirm the A2BR activation affect on ciliary beating. PSB1115 completely abrogated NECA-mediated CBF and had no effect on baseline CBF (Fig. 3D). These data suggest that activation of the A2B receptor(s) stimulates CBF in both the ex vivo MTRs and primary ciliated BBECs.
Fig. 3.
A: 5′-(N-ethylcarboxamido) adenosine (NECA) (a nonselective adenosine agonist) and adenosine stimulates CBF in MTRs in a concentration-dependent manner. MTRs were stimulated with various concentrations of NECA (■) for 30 min, and CBF was measured. NECA at high concentrations (1–30 μM) significantly increased CBF. Lower concentrations of NECA (10–100 nM) did not significantly stimulate CBF. ISO (10 μM; ●; #P < 0.001 compared with media control) served as a positive control. B: similarly, MTRs were stimulated with various concentrations of adenosine (■) for 30 min, and CBF was measured. Adenosine at high concentration (30–100 μM) significantly increased CBF (#P < 0.001 compared with media control). C: NECA stimulates CBF in both WT MTRs and primary ciliated BBECs. MTRs and BBECs were treated with NECA at concentrations designed to specifically activate specific adenosine (ADO) receptors (100 nM, A1, A2A, and A3; white bar) and (10 μM, A2B; gray bar). NECA at 10 μM significantly increased CBF in both WT MTRs and ciliated BBECs over baseline (2.33 ± 0.50 Hz and 4.87 ± 0.94 Hz, respectively). Exposure to a low concentration of NECA at 100 nM (A1, A2A, and A3) did not significantly increase CBF in either WT MTRs or ciliated BBECs compared with media control. ISO-treated MTRs and BBECs were used as positive control [ISO-treated **P < 0.001 vs. media control; NECA (10 μM)-treated *P < 0.05 vs. media control]. D: PSB1115 (highly selective A2B AR antagonist) blocks NECA-mediated CBF. MTRS were pretreated with or without PSB1115 (100 nM) for 30 min and then stimulated with NECA (10 μM). PSB1115 had no effect on baseline CBF. However, PSB1115 significantly inhibited NECA-stimulated CBF (***P < 0.001 vs. media control; #P < 0.001 vs. NECA-treated). These data are representative of 3 independent experiments performed in triplicate ± SE.
NECA pretreatment potentiates ISO-stimulation of CBF in MTRs.
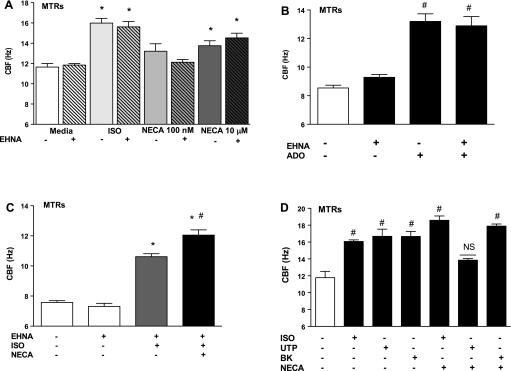
β-Adrenergic stimulation of CBF is limited by the desensitization of the β-adrenergic receptors. Because adenosine is a natural ligand for regulating ciliary motility, we investigated whether A2B-mediated activation would potentiate β-adrenergic stimulation of CBF. Wild-type MTRs were pretreated with ±10 μM EHNA followed by a subsequent pretreatment with 10 μM NECA for 30 min then exposed with 10 μM ISO for 30 min. CBF was then measured for 30 min. Control experiments indicated that EHNA had no effect on baseline CBF (Fig. 4A). Similarly, when MTRs were treated with adenosine, EHNA had no effect on baseline or adenosine-stimulated CBF (Fig. 4B). ISO-stimulated MTRs pretreated with EHNA demonstrated a significant increase in CBF (∼3–4 Hz; **P < 0.001) compared with media controls. MTRs pretreated with NECA further enhanced ISO stimulation of CBF (∼2–3 Hz, #P < 0.001) compared with ISO-stimulated MTRs and significantly increased CBF compared with media control (∼4–6 Hz, P < 0.001; Fig. 4C). MTRs pretreated with NECA did not enhance bradykinin (BK)-mediated CBF, a nonpeptide (100 nM; 17.90 ± 0.243 Hz compared with BK-treated group, 16.67 ± 0.597 Hz; P > 0.05) (56). Furthermore, pretreatment with NECA blunted uridine 5′-triphosphate-mediated CBF, a P2Y2 purinoceptor (P2Y2-R; UTP; 100 μM; 13.85 ± 0.193 Hz compared with UTP-treated group, 16.68 ± 0.851 Hz; **P < 0.01) (35) (Fig. 4D). Consistently, we observed that pretreatment with NECA significantly potentiated ISO-mediated CBF (18.59 ± 0.505 Hz compared with ISO-treated group 16.07 ± 0.190 Hz; *P < 0.05; Fig. 4D). These data indicate that NECA potentiates ISO-stimulated CBF in MTRs and suggest a novel interaction between purinergic and β-adrenergic stimulation of ciliary motility in our ex vivo model.
Fig. 4.
A: pretreatment with erythro-9- (2-hydroxy-3-nonyl) adenine hydrochloride (EHNA), an adenosine deaminase inhibitor, does not affect NECA-mediated CBF. Control experiments were conducted and revealed that pretreatment with EHNA did not affect baseline CBF stimulated with either NECA (100 nM, A1, A2A, A3; or 10 μM, A2B) or ISO (10 μM) and (B) also did not affect baseline CBF stimulated with adenosine (C). Pretreatment of NECA potentiates ISO-stimulated CBF in MTRs. ISO significantly stimulated CBF in MTRs pretreated with EHNA (∼3–4 Hz, gray bar; *P < 0.001) compared with media controls ± EHNA. MTR pretreatment with NECA further enhanced ISO stimulation of CBF (2–3 Hz; P < 0.001) compared with ISO-stimulated MTRs and revealed significantly increased CBF compared with both media control ± EHNA (∼ 4–6 Hz; *P < 0.001). D: pretreatment of NECA does not potentiate bradykinin (BK)- or UTP-stimulated CBF in MTRs. MTRs stimulated with either BK or UTP induced significant increases in CBF (3–4 Hz; #P < 0.001). Pretreatment with NECA did not further enhance BK or UTP stimulation of CBF. These data are representative of 3 independent experiments performed in triplicate ± SE.
NECA-stimulation of CBF requires activation of cAMP-dependent PKA activity in ciliated BBECs and MTRs.
The cAMP-dependent PKA is established as an important regulator of the cilia stimulation pathway (2, 42, 51, 62). To determine the mechanism of NECA-stimulation of CBF, we measured PKA activity in our primary ciliated BBEC model. Ciliated BBECs were stimulated for 30 min with NECA [100 nM (A1, A2A, A3), 10 μM (A2B), ISO (10 μM), and 8-bromoadenosine-3′,5′-cyclic monophosphate (8-Br-cAMP), an analog of cAMP], and PKA activity was measured. ISO-stimulated PKA activity increased approximately twofold over the media control group (***P < 0.001). Cells stimulated with 10 μM NECA also significantly increased PKA activity (**P < 0.01; Fig. 5A). However, cells stimulated with 100 nM NECA for 30 min did not increase PKA activity (P > 0.05). The positive control group (cells stimulated with 8-Br-cAMP) displayed a significant increase in PKA activity (*P < 0.001). To confirm a role for A2BR-mediated activation of PKA involvement in ciliary motility, cells pretreated with 100 nM PBS1115 significantly blocked A2B-mediated activation of PKA (P < 0.001; Fig. 5B). To demonstrate the temporal effect of PKA activation and A2B-stimulated CBF, some MTRs were pretreated with a potent PKA inhibitor, KT-5720 (1 μM) and stimulated with 10 μM NECA for 30 min. NECA significantly stimulated CBF compared with media control group (***P < 0.001), whereas cells pretreated with KT-5720 markedly blocked A2B-stimulated CBF below baseline as well as significantly reduced baseline CBF compared with media-stimulated cells (***P < 0.001; Fig. 5C). Similarly, MTRs stimulated with 10 μM NECA for 30 min significantly increased PKA compared with media-stimulated group (*P < 0.001; Fig. 5D). KT-5720 pretreatment significantly blocked NECA-stimulated PKA activation in MTRs (**P < 0.001; Fig. 5D). Collectively, these data indicate that A2B receptor-mediated stimulation of CBF requires activation of PKA in both the ex vivo MTRs and primary ciliated BBECs, suggesting PKA activation as a likely regulator of A2B-mediated cilia stimulation.
Fig. 5.
A: NECA activates cAMP-dependent PKA activity in ciliated BBECs and MTRs. PKA activity was measured in ciliated BBECs exposed to NECA (100 nM for A1, A2A, A3; 10 μM for A2B), and ISO (10 μM). ISO stimulated PKA activity 2-fold over baseline (***P < 0.001). Cells stimulated with 10 μM NECA displayed a significant increase in PKA activity (**P < 0.01). However, cells treated with 100 nM NECA for as little as 30 min did not demonstrate an increase in PKA activity (P > 0.05). B: PSB1115 blocks A2BAR-mediated PKA activation. Confluent ciliated BBECs were pretreated with or without PSB1115 (100 nM) for 30 min and stimulated with NECA (10 μM for A2B). NECA stimulated ∼2.5-fold increase activity of PKA (***P < 0.001 vs. media control). Pretreatment with PSB1115 significantly reduced A2BAR-mediated activation of PKA. 8-Bromoadenosine-3′,5′-cyclic monophosphate (8-Br-cAMP) (10 μM) was used as positive control (***P < 0.001 vs. media control). C: KT-5720, a potent PKA inhibitor, reduced NECA-stimulated CBF in MTRS. MTRs were pretreated with or without KT-5720 (1 μM) for 30 min and stimulated with 10 μM NECA, and CBF was measured. NECA stimulated CBF ∼2.5 Hz (***P < 0.001 vs. media control). Pretreatment with KT-5720 significantly reduced NECA-stimulated CBF. Representative of 3 independent experiments performed in triplicate ± SE, n = 9. D: KT-5720 blocks A2BAR-mediated activation of PKA in MTRs. MTRs were treated as mentioned above, and PKA activity was accessed. NECA stimulated an ∼3-fold increase in PKA activity (*P < 0.001 vs. media control). Pretreatment with KT-5720 significantly reduced A2BAR-mediated activation of PKA (**P < 0.001 vs. NECA). These data are representative of 3 independent experiments performed in triplicate ± SE.
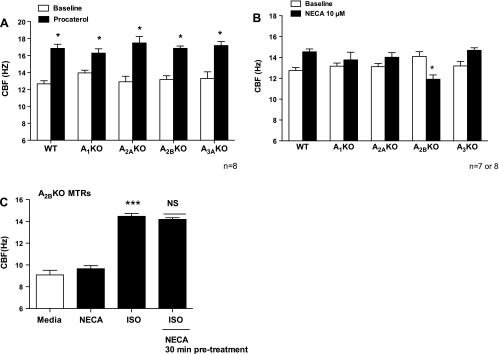
Procaterol stimulates CBF in tracheal rings from both wild-type and AKO mice.
To determine functional evidence of CBF, both MTRs from AKO and wild-type mice were stimulated with procaterol, a long acting β2-adrenergic agonist used in treatment of asthma. MTRs from wild-type and AKO mice demonstrated baseline CBF of ∼12 Hz, which was maintained up to 30 min. Cilia exposed with 10 μM procaterol significantly induced maximal beating (∼3–4 Hz; *P < 0.01 to each matched baseline; Fig. 6A). These data indicate that the β2-adrenergic receptor signaling system from AKO mice MTRs is intact.
Fig. 6.
A: procaterol stimulates CBF in MTRs from both WT and AKO mice. MTRs from both WT and AKO were exposed with procaterol (10 μM) for 30 min, and CBF was measured. Baseline CBF from WT and AKO mice MTRs were similar. Procaterol significantly increased CBF in WT MTRs and all 4 AKO mice MTRs over their respective baseline. B: NECA failed to stimulate CBF in tracheal rings from A2BKO mice. Both WT and AKO MTRs were stimulated with NECA (10 μM) for 30 min, and CBF was measured. NECA significantly increased CBF in WT MTRs over baseline. In contrast, NECA did not enhance CBF in A2BKO mice but instead caused a significant decrease in CBF back to baseline levels after 30-min exposure. Representative of 2 independent experiments performed in quadruplicate ± SE, n = 7 or 8. C: NECA-stimulated MTRs from A2BKO mice failed to potentiate ISO-stimulated CBF. A2BKO MTRs were pretreated with 10 μM NECA and stimulated ± ISO. ISO significantly increased CBF (***P < 0.001 vs. media control). NECA failed to potentiate ISO-stimulated CBF (P > 0.05 vs. ISO-treated group). These data are representative of 3 independent experiments performed in triplicate ± SE.
NECA failed to stimulate CBF in tracheal rings from A2B knockout mice.
To confirm implication that activation of A2B receptor in adenosine-mediated cilia stimulation, both wild-type and AKO MTRs were exposed with NECA (10 μM) for 30 min, and CBF was measured. NECA significantly stimulated CBF in wild-type mice MTRs (14.54 ± 0.275 Hz; **P = 0.01 compared with WT baseline CBF 12.17 ± 0.316 Hz; Fig. 6B). NECA did not stimulate CBF in A2B KO mice. In fact, we observed depressed CBF following NECA exposure in A2BKO (*P = 0.037 compared with A2BKO baseline CBF 14.09 ± 0.456 Hz). NECA also induced a marked increase of CBF in MTRs from A3 knockout (A3KO) compared with A3KO baseline CBF (13.18 ± 0.436 Hz). However, this increased CBF was not significant compared with wild-type-stimulated CBF (∼0.5 Hz; P > 0.05). MTRs from both A1KO and A2AKO stimulated with NECA did not significantly increase CBF compared with their respective baselines (A1KO baseline CBF 13.16 ± 0.300; P = 0.531 and A2AKO baseline CBF 13.11 ± 0.301; P = 0.07). These data corroborate our findings that purinergic stimulation of CBF requires the one A2B adenosine receptor.
NECA-stimulated MTRs from A2BKO mice failed to potentiate ISO-stimulated CBF.
To confirm the novel interaction between purinergic and β-adrenergic stimulation of ciliary motility, MTRs from A2BKO mice were stimulated with media, 10 μM NECA or ISO for 30 min. Some A2BKO MTRs were pretreated with NECA and then exposed to ISO. NECA did not stimulate CBF in A2BKO mice; however, ISO significantly stimulated A2BKO MTRs compared with the media-treated group (***P < 0.001; Fig. 6C). Moreover, pretreatment with NECA failed to potentiate ISO-stimulated CBF compared with ISO-treated group (P > 0.05; Fig. 6C). These data indicate that stimulation either by β1- or β2-adrenergic agonists necessitates the activation of the A2B receptor.
DISCUSSION
Adenosine is well established as a natural ligand that can stimulate CBF (42). Our study provides evidence of the contribution of the specific role adenosine receptors play in regulating ciliary motility. We demonstrate using pharmacological and genetic approaches in two distinct models of ciliary motility, ex vivo MTRs and ciliated BBECs, and adenosine activation of the A2B receptor(s) is likely responsible for the role of adenosine in cilia control. In addition, our data indicate that stimulation of the A2B receptor(s) activates cAMP-dependent PKA, a known kinase regulator of CBF. Furthermore, we demonstrate a novel observation that purinergic stimulation potentiates β-adrenergic stimulation of CBF, suggesting a previously undescribed interaction between the β-adrenergic and adenosine receptor(s). Most importantly, dose-dependent responses to NECA, receptor blocking studies, and KO mice analysis confirm a complex role for adenosine in cilia regulation.
β-Agonists are known stimulators of ciliary motility (2, 58, 62). To demonstrate the sensitivity of our ex vivo model, tracheal rings from wild-type MTRs were stimulated with various concentrations of ISO. We found that our ex vivo MTR model functions similarly to our in vitro bovine ciliated model. Furthermore, the responsiveness of MTRs support other findings that ex vivo lung tissue models are useful in determinations of kinetic descriptors for a variety of lung functions (40) and provide a useful tool to predict in vivo responses in ciliary function.
The differential effects of adenosine to stimulate and/or inhibit ciliary motility and clearance have been investigated in numerous models (34, 35, 55, 57), yet the net effects of adenosine receptor activation on ciliary function remain unclear. We chose an ex vivo model using both pharmacological and genetic manipulations to provide further clarity into the complex roles of adenosine in the regulation of mammalian ciliary beating. Our pharmacological studies revealed that wild-type MTRs exposed to NECA (10 μM; A2B; EC50 of 2.2 μM) had a sustained CBF response similar to that observed with β-adrenergic stimulation of ISO. We also observed a similar profile when wild-type MTRs were exposed to adenosine (10 μM; A2B; EC50 of 1.5 μM) although NECA had a greater potency than adenosine. NECA is a potent nonselective agonist, and it can bind to A1, A2A, and A3 receptors (lower nanomolar concentration). Considering that A2BR have low affinity for adenosine (∼5 μM), the responses elicited by NECA at concentrations in the low micromolar range (1–10 μM) in this study are characteristic of A2B receptor (9, 17). As confirmation, wild-type MTRs pretreated with the highly selective A2B antagonist, PSB1115 (100 nM), revealed a significant attenuation of the A2BAR-mediated stimulation of CBF. Conversely, wild-type MTRs exposed to NECA (100 nM; A1, A2A, A3) did not demonstrate an increase in CBF, suggesting that activation of the adenosine A2B receptor(s) is required. Our data are consistent with the findings observed in canine tracheal in vivo adenosine delivery of 10 μM aerosol-stimulated ciliary activity (60) and in explants of human nasal epithelium exposed to 1 μM NECA (EC50 of 0.09 μM) (35) showing that NECA is stimulatory in its action on ciliary activity via its activation of the A2B receptor. In addition, our study reiterates that there are differences emerging between various mammalian models in understanding the purinergic signal transduction pathways involved in regulation of ciliary beat function.
To gain a better understanding of the adenosine-mediated ciliary beating, we investigated the effects of EHNA, an adenosine deaminase inhibitor that has been characterized also as a dynein ATPase inhibitor. EHNA has been implicated in blocking flagella and cilia-like movement (1, 7); the evidence is clear that dynein ATPases in the axoneme regulate motility (8). Although we did not measure ATPase activity, our data suggest that pretreatment with EHNA did not alter NECA-mediated or adenosine-mediated stimulation of CBF, making cilia dynein inhibition unlikely. Furthermore, the data imply that baseline CBF is not affected by endogenous adenosine in our ex vivo model.
It is clear that β-adrenergic agonists increase CBF although there is still little information regarding the relationship between adenosine and β-adrenergic receptor(s). Both of these receptors are members of the G protein-coupled receptor class and require activation of adenylyl cyclase that couples to the G-α subunit (2, 3, 42). Engagement of these receptors leads to changes in intracellular cAMP and calcium levels, both essential for increased CBF activity (9, 24, 39, 44). Our laboratory previously demonstrated that cAMP-dependent phosphorylation via activation of PKA mediates increased CBF in ciliated BBECs (2, 51, 61, 62). Likewise, it has been demonstrated that adenosine activation of A2 receptors, particularly A2B, increases cAMP, thereby increasing CBF (35, 42). Understanding that both of these receptors share similar signal transduction pathways in regulating CBF, we investigated their potential kinetic interaction. We found that pretreatment with NECA potentiates ISO-stimulated CBF, and this potentiation was not detected when MTRs were stimulated with BK. One of the surprising observations in this study was that pretreatment with NECA significantly decreased UTP-stimulated CBF, suggesting that activation of A2B receptor may in fact act as a autocrine/paracrine mediator (42). Furthermore, these findings support the existence of a novel interaction between adenosine and β-adrenergic receptors. In addition, stimulation of either NECA or ISO in ciliated epithelial cells leads to the activation of PKA (41, 42, 62). As proof of concept, pretreatment with PSB1115 revealed significant inhibition of NECA-mediated activation of PKA. Likewise, MTRs pretreated with KT-5720 significantly attenuated NECA stimulation of CBF below media baseline CBF, demonstrating a nonfunctional CBF, implying that NECA-stimulation of CBF beyond baseline beating is mediated via activation of PKA. Collectively, these studies support that both types of G protein-coupled receptors share PKA as a common signal transduction element in regulating CBF.
Pharmacological manipulations are useful tools; however, they are subject to nonspecific and potential cross-talk interactions among the receptor(s) investigated. To further support our findings, we utilized a genetic approach to delineate an adenosine role in mucociliary transport and clearance. AKO mouse models have been quite useful in understanding the complex properties of adenosine (10, 43, 63). In our studies, we demonstrated the equipotent characteristics between β1 (ISO) and/or β2 (procaterol) adrenergic receptors (49, 64), as cilia from AKO MTRs (A1, A2A, A2B, and A3) and wild-type all responded to procaterol stimulation. However, with AKO MTRs stimulated with NECA, the A2BKO MTRs paradoxically revealed a significant decrease in CBF. Furthermore, A2BKO MTRs pretreated with NECA failed to potentiate ISO-stimulated CBF, suggesting that activation of A2B receptor is essential for this novel interaction. In addition, our findings were consistent with pharmacological studies of mouse lateral ventricle ependymal cells demonstrating that enhanced CBF was attributable to A2B receptor activation, which was subsequently confirmed by using A2BKO/β-galactosidase reporter gene knock-in mice (20). Both A1KO and A2AKO MTRs failed to be stimulated with NECA, which indicates that A3 receptor may act as a negative regulator of A2B activation. The slight but nonsignificant increase in CBF in A3KO MTRs further supports that activation of A3R might play a role in slowing CBF and requires further investigation. These findings confirm our pharmacological studies demonstrating that purinergic stimulation of CBF is dependent on A2B adenosine receptor activation.
The A2B receptor(s) is the least characterized of the adenosine purinergic receptors (P1), has been defined as the “low-affinity” adenosine receptor(s), and pharmacologically lacks potent and selective agonists. Despite these limitations, growing evidence demonstrates that the activation of A2B receptors regulates a wide range of physiological and pathophysiological events (4, 11, 13, 14, 17, 27, 29, 46). Genetic-linked diseases such as cystic fibrosis and primary ciliary dyskinesia, both characterized with impaired mucociliary clearance, demonstrated evidence involving the A2B receptor subtype in determining secretion stimulation of epithelial transports, i.e., chloride secretion via direct activation of the cAMP-activated chloride channel cystic fibrosis transmembrane conductance regulator (25, 28, 32, 54). Overall these studies indicate that purinergic regulation of mucociliary function either via the epithelial transport or direct stimulation of A2B receptor(s) may act as a natural defense system working to “sweep away” injuries caused by cellular damage or inflammation (6, 32, 35).
In summary, we observed that activation of the adenosine A2B receptor stimulates CBF in MTRs excised from wild-type and AKO mice and BBECs. A2BR-stimulated CBF is likely mediated by the activation of PKA. Moreover, we demonstrated a novel interaction of purinergic stimulation that potentiates β-adrenergic stimulation of CBF. Collectively, the data revealed that adenosine response is tissue and species specific, which can account for the varied response in modulating mucociliary function, and that future experiments should focus on understanding whether purinergic dysfunction contributes to pathological conditions such as chronic obstructive pulmonary disease and asthma.
GRANTS
This study is the result of work supported with resources and the use of facilities at the Omaha VA Medical Center, Omaha, NE [Dept. of Veterans Affairs (VA Merit Review) to T. Wyatt]. This work was supported by NIH-NHLBI (K01HL084684) to D. Allen-Gipson, NIH-NIAAA (R37AA008669) to J. Sisson, and NIH-NIAAA (R01AA017663) to T. Wyatt.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors recognize Jane DeVasure for expert technical support and Lisa Chudomelka for editorial expertise.
REFERENCES
- 1. Alexander J, Burns RG. Differential inhibition by erythro-9-[3-(2-hydroxynonyl)]adenine of flagella-like and cilia-like movement of Leishmania promastigotes. Nature 305: 313–315, 1983 [DOI] [PubMed] [Google Scholar]
- 2. Allen-Gipson DS, Romberger DJ, Forget MA, May KL, Sisson JH, Wyatt TA. IL-8 inhibits isoproterenol-stimulated ciliary beat frequency in bovine bronchial epithelial cells. J Aerosol Med 17: 107–115, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Allen-Gipson DS, Wong J, Spurzem JR, Sisson JH, Wyatt TA. Adenosine A2A receptors promote adenosine-stimulated wound healing in bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 290: L849–L855, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Allersma MW, Wang L, Axelrod D, Holz RW. Visualization of regulated exocytosis with a granule-membrane probe using total internal reflection microscopy. Mol Biol Cell 15: 4658–4668, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Auchampach JA, Kreckler LM, Wan TC, Maas JE, van der Hoeven D, Gizewski E, Narayanan J, Maas GE. Characterization of the A2B adenosine receptor from mouse, rabbit, and dog. J Pharmacol Exp Ther 329: 2–13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baraldi PG, Tabrizi MA, Fruttarolo F, Romagnoli R, Preti D. Recent improvements in the development of A(2B) adenosine receptor agonists. Purinergic Signal 4: 287–303, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bouchard P, Cosson MP. [Molecular basis of sperm movement (author's transl)]. Ann Endocrinol (Paris) 42: 398–406, 1981 [PubMed] [Google Scholar]
- 8. Bouchard P, Penningroth SM, Cheung A, Gagnon C, Bardin CW. erythro-9-[3-(2-Hydroxynonyl)]adenine is an inhibitor of sperm motility that blocks dynein ATPase and protein carboxylmethylase activities. Proc Natl Acad Sci USA 78: 1033–1036, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bruns RF, Lu GH, Pugsley TA. Characterization of the A2 adenosine receptor labeled by [3H]NECA in rat striatal membranes. Mol Pharmacol 29: 331–346, 1986 [PubMed] [Google Scholar]
- 10. Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, Moskowitz MA, Fink JS, Schwarzschild MA. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci 19: 9192–9200, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clancy JP, Ruiz FE, Sorscher EJ. Adenosine and its nucleotides activate wild-type and R117H CFTR through an A2B receptor-coupled pathway. Am J Physiol Cell Physiol 276: C361–C369, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Dickenson JM, Hill SJ. Coupling of histamine H1 and adenosine A1 receptors to phospholipase C in DDT1MF-2 cells: synergistic interactions and regulation by cyclic AMP. Biochem Soc Trans 21: 1124–1129, 1993 [DOI] [PubMed] [Google Scholar]
- 13. Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood 111: 2024–2035, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eckle T, Koeppen M, Eltzschig HK. Role of extracellular adenosine in acute lung injury. Physiology (Bethesda) 24: 298–306, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Elliott MK, Sisson JH, West WW, Wyatt TA. Differential in vivo effects of whole cigarette smoke exposure versus cigarette smoke extract on mouse ciliated tracheal epithelium. Exp Lung Res 32: 99–118, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Elliott MK, Sisson JH, Wyatt TA. Effects of cigarette smoke and alcohol on ciliated tracheal epithelium and inflammatory cell recruitment. Am J Respir Cell Mol Biol 36: 452–459, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Feoktistov I, Biaggioni I. Adenosine A2B receptors. Pharmacol Rev 49: 381–402, 1997 [PubMed] [Google Scholar]
- 18. Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J. International union of pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 53: 527–552, 2001 [PMC free article] [PubMed] [Google Scholar]
- 19. Geary CA, Davis CW, Paradiso AM, Boucher RC. Role of CNP in human airways: cGMP-mediated stimulation of ciliary beat frequency. Am J Physiol Lung Cell Mol Physiol 268: L1021–L1028, 1995 [DOI] [PubMed] [Google Scholar]
- 20. Genzen JR, Yang D, Ravid K, Bordey A. Activation of adenosine A2B receptors enhances ciliary beat frequency in mouse lateral ventricle ependymal cells (Abstract). Cerebrospinal Fluid Res 6: 15, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gessi S, Merighi S, Varani K, Leung E, MacLennan S, Borea PA. The A3 adenosine receptor: an enigmatic player in cell biology. Pharmacol Ther 117: 123–140, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Guyot A, Hanrahan JW. ATP release from human airway epithelial cells studied using a capillary cell culture system. J Physiol 545: 199–206, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hameister WM, Wong LB, Yeates DB. Tracheal ciliary beat frequency in baboons: effects of peripheral histamine and capsaicin. Agents Actions 35: 200–207, 1992 [DOI] [PubMed] [Google Scholar]
- 24. Hochstrasser M, Carlson GL, Walczak CE, Nelson DL. Paramecium has two regulatory subunits of cyclic AMP-dependent protein kinase, one unique to cilia. J Eukaryot Microbiol 43: 356–362, 1996 [DOI] [PubMed] [Google Scholar]
- 25. Huang P, Lazarowski ER, Tarran R, Milgram SL, Boucher RC, Stutts MJ. Compartmentalized autocrine signaling to cystic fibrosis transmembrane conductance regulator at the apical membrane of airway epithelial cells. Proc Natl Acad Sci USA 98: 14120–14125, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jiang H, Colbran JL, Francis SH, Corbin JD. Direct evidence for cross-activation of cGMP-dependent protein kinase by cAMP in pig coronary arteries. J Biol Chem 267: 1015–1019, 1992 [PubMed] [Google Scholar]
- 27. Kode A, Yang SR, Rahman I. Differential effects of cigarette smoke on oxidative stress and proinflammatory cytokine release in primary human airway epithelial cells and in a variety of transformed alveolar epithelial cells (Abstract). Respir Res 7: 132, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lazarowski ER, Mason SJ, Clarke L, Harden TK, Boucher RC. Adenosine receptors on human airway epithelia and their relationship to chloride secretion. Br J Pharmacol 106: 774–782, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lazarowski ER, Tarran R, Grubb BR, van Heusden CA, Okada S, Boucher RC. Nucleotide release provides a mechanism for airway surface liquid homeostasis. J Biol Chem 279: 36855–36864, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lieb T, Frei CW, Frohock JI, Bookman RJ, Salathe M. Prolonged increase in ciliary beat frequency after short-term purinergic stimulation in human airway epithelial cells. J Physiol 538: 633–646, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol 41: 775–787, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Livraghi A, Randell SH. Cystic fibrosis and other respiratory diseases of impaired mucus clearance. Toxicol Pathol 35: 116–129, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, Boucher RC. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell 95: 1005–1015, 1998 [DOI] [PubMed] [Google Scholar]
- 34. Morales B, Barrera N, Uribe P, Mora C, Villalon M. Functional cross talk after activation of P2 and P1 receptors in oviductal ciliated cells. Am J Physiol Cell Physiol 279: C658–C669, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Morse DM, Smullen JL, Davis CW. Differential effects of UTP, ATP, and adenosine on ciliary activity of human nasal epithelial cells. Am J Physiol Cell Physiol 280: C1485–C1497, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Perretti M. Endogenous mediators that inhibit the leukocyte-endothelium interaction. Trends Pharmacol Sci 18: 418–425, 1997 [DOI] [PubMed] [Google Scholar]
- 37. Polosa R. Adenosine-receptor subtypes: their relevance to adenosine-mediated responses in asthma and chronic obstructive pulmonary disease. Eur Respir J 20: 488–496, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Roskoski R., Jr Assays of protein kinase. Methods Enzymol 99: 3–6, 1983 [DOI] [PubMed] [Google Scholar]
- 39. Ryzhov S, Goldstein AE, Biaggioni I, Feoktistov I. Cross-talk between G(s)- and G(q)-coupled pathways in regulation of interleukin-4 by A(2B) adenosine receptors in human mast cells. Mol Pharmacol 70: 727–735, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Sakagami M. In vivo, in vitro and ex vivo models to assess pulmonary absorption and disposition of inhaled therapeutics for systemic delivery. Adv Drug Deliv Rev 58: 1030–1060, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Salathe M. Effects of beta-agonists on airway epithelial cells. J Allergy Clin Immunol 110: S275–281, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Salathe M. Regulation of mammalian ciliary beating. Annu Rev Physiol 69: 401–422, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Salvatore CA, Tilley SL, Latour AM, Fletcher DS, Koller BH, Jacobson MA. Disruption of the A(3) adenosine receptor gene in mice and its effect on stimulated inflammatory cells. J Biol Chem 275: 4429–4434, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Sanderson MJ, Charles AC, Boitano S, Dirksen ER. Mechanisms and function of intercellular calcium signaling. Mol Cell Endocrinol 98: 173–187, 1994 [DOI] [PubMed] [Google Scholar]
- 45. Satir P, Sleigh MA. The physiology of cilia and mucociliary interactions. Annu Rev Physiol 52: 137–155, 1990 [DOI] [PubMed] [Google Scholar]
- 46. Schingnitz U, Hartmann K, Macmanus CF, Eckle T, Zug S, Colgan SP, Eltzschig HK. Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury. J Immunol 184: 5271–5279, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schulte G, Fredholm BB. Human adenosine A(1), A(2A), A(2B), and A(3) receptors expressed in Chinese hamster ovary cells all mediate the phosphorylation of extracellular-regulated kinase 1/2. Mol Pharmacol 58: 477–482, 2000 [PubMed] [Google Scholar]
- 48. Shoji S, Rickard KA, Ertl RF, Linder J, Rennard SI. Lung fibroblasts produce chemotactic factors for bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 257: L71–L79, 1989 [DOI] [PubMed] [Google Scholar]
- 49. Siegel SC, Katz RM, Rachelefsky GS, Brandon ML, Borgen LA. A placebo-controlled trial of procaterol: a new long-acting oral beta 2-agonist in bronchial asthma. J Allergy Clin Immunol 75: 698–705, 1985 [DOI] [PubMed] [Google Scholar]
- 50. Silberberg A. Biorheological matching: mucociliary interaction and epithelial clearance. Biorheology 20: 215–222, 1983 [DOI] [PubMed] [Google Scholar]
- 51. Sisson JH, May K, Wyatt TA. Nitric oxide-dependent ethanol stimulation of ciliary motility is linked to cAMP-dependent protein kinase (PKA) activation in bovine bronchial epithelium. Alcohol Clin Exp Res 23: 1528–1533, 1999 [PubMed] [Google Scholar]
- 52. Sisson JH, Stoner JA, Ammons BA, Wyatt TA. All-digital image capture and whole-field analysis of ciliary beat frequency. J Microsc 211: 103–111, 2003 [DOI] [PubMed] [Google Scholar]
- 53. Sisson JH, Tuma DJ, Rennard SI. Acetaldehyde-mediated cilia dysfunction in bovine bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 260: L29–L36, 1991. [Errata. Am J Physiol Lung Cell Mol Physiol 262(2) and 262(6): L, 1992.] [DOI] [PubMed] [Google Scholar]
- 54. Strohmeier GR, Reppert SM, Lencer WI, Madara JL. The A2b adenosine receptor mediates cAMP responses to adenosine receptor agonists in human intestinal epithelia. J Biol Chem 270: 2387–2394, 1995 [DOI] [PubMed] [Google Scholar]
- 55. Taira M, Tamaoki J, Nishimura K, Nakata J, Kondo M, Takemura H, Nagai A. Adenosine A(3) receptor-mediated potentiation of mucociliary transport and epithelial ciliary motility. Am J Physiol Lung Cell Mol Physiol 282: L556–L562, 2002 [DOI] [PubMed] [Google Scholar]
- 56. Tamaoki J, Kobayashi K, Sakai N, Chiyotani A, Kanemura T, Takizawa T. Effect of bradykinin on airway ciliary motility and its modulation by neutral endopeptidase. Am Rev Respir Dis 140: 430–435, 1989 [DOI] [PubMed] [Google Scholar]
- 57. Tamaoki J, Kondo M, Takizawa T. Adenosine-mediated cyclic AMP-dependent inhibition of ciliary activity in rabbit tracheal epithelium. Am Rev Respir Dis 139: 441–445, 1989 [DOI] [PubMed] [Google Scholar]
- 58. Verdugo P, Johnson NT, Tam PY. beta-Adrenergic stimulation of respiratory ciliary activity. J Appl Physiol 48: 868–871, 1980 [DOI] [PubMed] [Google Scholar]
- 59. Weiss T, Gheber L, Shoshan-Barmatz V, Priel Z. Possible mechanism of ciliary stimulation by extracellular ATP: involvement of calcium-dependent potassium channels and exogenous Ca2+. J Membr Biol 127: 185–193, 1992 [DOI] [PubMed] [Google Scholar]
- 60. Wong LB, Yeates DB. Luminal purinergic regulatory mechanisms of tracheal ciliary beat frequency. Am J Respir Cell Mol Biol 7: 447–454, 1992 [DOI] [PubMed] [Google Scholar]
- 61. Wyatt TA, Sisson JH. Chronic ethanol downregulates PKA activation and ciliary beating in bovine bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 281: L575–L581, 2001 [DOI] [PubMed] [Google Scholar]
- 62. Wyatt TA, Spurzem JR, May K, Sisson JH. Regulation of ciliary beat frequency by both PKA and PKG in bovine airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 275: L827–L835, 1998 [DOI] [PubMed] [Google Scholar]
- 63. Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, Jones MR, St Hilaire C, Seldin DC, Toselli P, Lamperti E, Schreiber BM, Gavras H, Wagner DD, Ravid K. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest 116: 1913–1923, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zapol WM, Snider MT. Pulmonary hypertension in severe acute respiratory failure. N Engl J Med 296: 476–480, 1977 [DOI] [PubMed] [Google Scholar]