Abstract
Phosphodiesterase 2A (PDE2A) is stimulated by cGMP to hydrolyze cAMP, a potent endothelial barrier-protective molecule. We previously found that lung PDE2A contributed to a mouse model of ventilator-induced lung injury (VILI). The purpose of the present study was to determine the contribution of PDE2A in a two-hit mouse model of 1-day intratracheal (IT) LPS followed by 4 h of 20 ml/kg tidal volume ventilation. Compared with IT water controls, LPS alone (3.75 μg/g body wt) increased lung PDE2A mRNA and protein expression by 6 h with a persistent increase in protein through day 4 before decreasing to control levels on days 6 and 10. Similar to the PDE2A time course, the peak in bronchoalveolar lavage (BAL) neutrophils, lactate dehydrogenase (LDH), and protein concentration also occurred on day 4 post-LPS. IT LPS (1 day) and VILI caused a threefold increase in lung PDE2A and inducible nitric oxide synthase (iNOS) and a 24-fold increase in BAL neutrophilia. Compared with a control adenovirus, PDE2A knockdown with an adenovirus expressing a short hairpin RNA administered IT 3 days before LPS/VILI effectively decreased lung PDE2A expression and significantly attenuated BAL neutrophilia, LDH, protein, and chemokine levels. PDE2A knockdown also reduced lung iNOS expression by 53%, increased lung cAMP by nearly twofold, and improved survival from 47 to 100%. We conclude that in a mouse model of LPS/VILI, a synergistic increase in lung PDE2A expression increased lung iNOS and alveolar inflammation and contributed significantly to the ensuing acute lung injury.
Keywords: lipopolysaccharide, ventilator-induced, cyclic guanosine monophosphate, inducible nitric oxide synthase, cAMP
severe pneumonia is a frequent cause of acute lung injury (ALI) and the acute respiratory distress syndrome (ARDS) (49). ARDS is characterized by neutrophil infiltration, the generation of toxic cytokines and reactive oxygen species (ROS), increased NO production and the loss of endothelial and epithelial barrier function (4). These features can be reproduced in animal models by the installation of intratracheal (IT) LPS (10, 24, 31), a key component of the gram-negative bacterial cell wall. LPS, through Toll-like receptor-4 signaling, was shown to cause increased NO from phosphorylated endothelial (eNOS) (11) and inducible nitric oxide synthase (iNOS) (24, 31) in alveolar macrophages, neutrophils, epithelium, and endothelium. NO from both eNOS and iNOS was found to play a major pathogenic role in ALI from LPS (24, 31, 42) and in ventilator-induced lung injury (VILI) (33, 39). These injurious effects of endogenous NO have been attributed to a reaction with superoxide to generate peroxynitrite (31, 33). In both LPS-induced ALI (47) and VILI (39), however, NO also activates soluble guanylyl cyclase (sGC), increasing production of cGMP in multiple cell types including endothelium (39), epithelium (9, 17), and macrophages (3).
The effects of cGMP signaling in ALI are complex, depending on cell type, intracellular compartmentalization, and the downstream cGMP targets (39). These targets include cyclic nucleotide-gated cation channels, cGMP-sensitive phosphodiesterases (PDEs), and the cGMP-dependent protein kinase (PKG) (20). Although cGMP-mediated PKG activation prior to injury can protect the endothelial barrier (29) and enhance endothelial antioxidant enzyme concentrations (43), cGMP can also contribute to injury if large cGMP concentrations are generated in the presence of increased phosphodiesterase 2A (PDE2A) expression (39). PDE2A hydrolyzes both cGMP and cAMP but is stimulated by cGMP to preferentially hydrolyze cAMP when cGMP levels are high (8, 44). PDE2A expression was increased by TNF-α in cultured systemic endothelial cells (22, 41, 44) by a p38 MAPK-dependent mechanism (44). Under these conditions, the generation of cGMP by NO donors resulted in the loss of in vitro barrier function through hydrolysis of cAMP (41, 44). We (39) recently identified a role for PDE2A in a mouse model of VILI. More recently, increased lung PDE2A expression was found in a murine model of pneumococcal pneumonia (52). In both studies, pharmacological PDE2A inhibition attenuated the associated ALI (39, 52).
On the basis of these observations, we hypothesized that IT LPS would potently increase lung PDE2A expression. Because of our observation regarding VILI-induced PDE2A upregulation (39), we wondered whether the combination of IT LPS and high tidal volume (HVT) ventilation would produce a more severe injury through an increase in lung PDE2A expression. This is a clinically relevant question because patients with severe pneumonia or sepsis frequently require mechanical ventilation. To test this hypothesis, we developed an intact mouse model of combined IT LPS and VILI and examined lung PDE2A expression and injury. The role of PDE2A was addressed by knocking down lung PDE2A expression with a recombinant, replication-defective adenovirus (Ad) that expresses green fluorescent protein (GFP) and an artificial short hairpin RNA (shRNA) designed to selectively suppress PDE2A expression (Ad.PDE2A-shRNA) in the lung. We found that the combination of HVT ventilation and IT LPS significantly increased lung PDE2A and iNOS expression as well as alveolar inflammation compared with LPS alone. In vivo knockdown of PDE2A expression significantly attenuated bronchoalveolar lavage (BAL) neutrophilia, lactate dehydrogenase (LDH) activity, protein, and chemokine concentrations. Importantly, knockdown of PDE2A also prevented the increase in lung iNOS expression and improved survival. These protective effects were associated with an increased lung cAMP concentration. We conclude that increased lung PDE2A expression significantly contributes to the ALI caused by the combination of HVT ventilation and IT LPS.
MATERIALS AND METHODS
Experimental animals.
Male C57BL/6 mice (20–30 g, 8–10 wk old) were purchased from Jackson Laboratories (Bar Harbor, ME). All animal protocols in this study were approved by The Johns Hopkins Institutions Animal Care and Use Committee. Mice were anesthetized with intraperitoneal ketamine-acetylpromazine (150 and 13.5 mg/kg, respectively) for tracheal intubation and IT instillation procedures as well as during mechanical ventilation. Tracheal intubation was performed by orally inserting a 20-gauge catheter (Jelco-W; Smiths Medical, Southington, CT) under direct tracheal visualization via a midline neck incision (1). After IT administration of adenovirus, LPS, or water, the cannula was removed, the neck incision was closed with glue, and the mice were allowed to recover.
LPS-VILI mouse model.
Following anesthesia and tracheal intubation, Escherichia coli LPS (O55:B5 Sigma L2880, 3.75 μg/g body wt) or an equal volume of water was instilled IT. After 24 h, the mice were reanesthetized, intubated, and ventilated with 20 ml/kg tidal volume at 160 breaths/min for 4 h on room air, by using a volume-controlled ventilator (Harvard Inspira Advanced Safety Ventilator 557058, Harvard Apparatus) with an additional dead space to maintain arterial pH in the normal range as previously described (13, 39).
BAL.
Right lung BAL cell counts and differentials were determined as previously described (1). Total protein concentration (Pierce BCA Protein Assay kit, Thermo Scientific, Rockford, IL) and LDH activity (Promega, Madison, WI) were measured in cell-free supernatants. BAL chemokines LPS-induced CXC chemokine (LIX), macrophage inflammatory protein (MIP-2), and keratinocyte-derived chemokine (KC) were measured by ELISA (R&D Systems, Minneapolis, MN).
Lung PDE2A immunoperoxidase staining.
The left lung was inflated with 1% low-melting agarose (Invitrogen, Carlsbad, CA) with a constant pressure of 25 cmH2O and then fixed in 4% paraformaldehyde. The lungs were then dehydrated, embedded in paraffin, cut into 5-μm-thick sections, and placed on glass slides. The slides were deparaffinized by rinsing in xylenes and rehydrated by washes in decreasing ethanol concentrations followed by one 5-min wash in double-distilled H2O. Whole mounts were blocked with peroxidase blocking reagent (Dako, Carpinteria, CA) followed by avidin/biotin blocking solutions (Vector Laboratories, Burlingame, CA). Nonspecific protein binding was blocked with goat serum for 1 h and then incubated overnight at 4°C with a polyclonal rabbit anti-PDE2A (1:1,000, FabGennix International, Frisco, TX) or control IgG in PBS containing 0.3% Triton and 1% BSA. Sections were treated with a goat anti-rabbit biotinylated secondary antibody (1:100, Vector Laboratories) for 1 h and staining was detected with avidin-peroxidase reagent (Vectastain Elite ABC Kit, Vector Laboratories) for 1 h followed by 4 min of 3,3′-diaminobenzidine peroxidase substrate (Vector Laboratories) and counterstained with hematoxylin. The sections were visualized with an Olympus-BX51 transmitted light viewing microscope attached to a Q-Color5 digital camera and imported into QCapture Pro6 software.
Lung PDE2A immunofluorescence staining.
The left lung was fixed and processed for immunofluorescence staining using a rabbit polyclonal antibody anti-PDE2A (FabGennix International) as the primary antibody and Alexa Fluor 594 donkey anti-rabbit IgG (Invitrogen-Molecular Probes) as a secondary antibody as previously described (29). All fluorescent images were collected by using an identical exposure time. To quantify the effect of Ad.PDE2A-shRNA on epithelial PDE2A expression, an irregular area of interest was used to outline airway epithelium and determine mean fluorescence per unit area (Image ProPlus, v.5.1) in images from three independent experiments. For each lung, at least 20 individual scores were obtained and averaged to provide a single value.
Quantitative real-time RT-PCR.
RNA was isolated from lung using TRIzol reagent (Invitrogen) and RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. RNA yield was calculated using spectrophotometry (NanoDrop, Wilmington, DE) and purity assessed by A260/A280 ratio. The PDE2A primers were 5′-AGTGTGACGTTGACTCCGT-3′ and 5′-GACTCATCGTACTCCTGCTT-3′. The mouse 18S rRNA primer set used as an internal control was purchased from Qiagen (catalog no. 249900, Qiagen Sciences, Germantown, MD). RNA (0.5 μg) from each sample was converted to cDNA (after genomic DNA wipeout) using QuantiTect Reverse Transcription kit (Qiagen). Quantitative real-time PCR reactions were performed as previously described (43).
Western blot analysis.
Immunoblots for PDE2A, GFP, and iNOS were performed as previously described (34, 39). Briefly, 293A cells were lysed in a Laemmli sample buffer (Bio-Rad) containing 4 mM Na3VO4, 40 mM NaF, 1% mercaptoethanol, and 1:200 dilution of protease inhibitor cocktail (Sigma-Aldrich). Lungs were homogenized in 500 μl of tissue protein extraction reagent (Thermo Scientific, Rockford, IL) with added 100× protease inhibitor cocktail, 1 mM Na3VO4, 1 mM NaF, and 1 mM PMSF (all from Sigma-Aldrich) in a FastPrep tubes containing lysing matrix beads (MP Biomedicals, Solon, OH). The homogenates were centrifuged, and the supernatants were assayed for protein concentration by using a BCA protein assay kit (Pierce, Rockford, IL). Total protein was collected and quantified as described above. Equal amounts of lysates (for lung homogenates 40 μg; for cell homogenates 60 μg) were resolved by a 6–11% SDS-PAGE (Bio-Rad, Hercules, CA) gel and transferred onto nitrocellulose membranes (Bio-Rad). The membranes were blocked with 5% BSA in Tris-buffered saline with 0.1% Tween-20 for 1 h and then incubated with rabbit anti-PDE2A (1:1,000), mouse anti-iNOS (1:5,000; Transduction Laboratories, Rockville, MD), rabbit anti-GFP (1:1,000; Invitrogen, Eugene, OR), followed by horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (1:3,000, Bio-Rad) or anti-mouse IgG (1:3,000 Bio-Rad), respectively. For protein loading, blots were probed with HRP-conjugated anti-GAPDH (1:10,000; Abcam, Cambridge, MA) or HRP-conjugated anti-actin (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA). The blots were developed with enhanced chemiluminescence (ECL; Amersham, Piscataway, NJ) and exposed to X-ray film (Kodak BioMax MR). Bands of interest were quantified by densitometric quantification using UN-SCAN-IT gel automated digitizing system software version 5.1 (Silk Scientific, Orem, UT).
cAMP and cGMP enzyme immunoassay.
Lung cAMP and cGMP tissue concentrations were measured by using a commercially available kit (Assay Design, Ann Arbor, MI) as previously described (39).
Construction of expression plasmid for mouse PDE2A (pcHA-PDE2A).
Mouse PDE2A cDNA was amplified by PCR from pYX-Asc/PDE2A (Open Biosystems, Huntsville, AL). The forward primer used was 5′-CCGGAATTCCATGGCCTATCCATATGACGTCCCAGACTCTGCCGGGCAGGCATGCGCCACTCCATCC-3′, which included the EcoRI restriction site (underlined) and an HA tag 9 peptide YPYDVPDYA (in italics) followed by the second amino acid of PDE2A. The reverse primer was 5′-CCGGAATTCAGCCCTCGAGGCTGCAGCAGCC-3′ which included the EcoRI (underlined). PCR was carried out in a 50-μl reaction volume using 1 μl of 5 ng/μl template pYX-Asc/PDE2A, 1 μl of 10 pM target primers, 1 μl of 10 mM dNTP-Mix, 2 μl of 50 mM MgSO4, 5 μl of 10× High Fidelity PCR buffer, and 0.25 μl of Platinum Taq DNA Polymerase High Fidelity. Cycling conditions were: 94°C for 2 min, followed by 30 cycles at 94°C for 30 s, 56°C for 30 s, and 68°C for 2 min. The resulting 2.8-kb fragment was digested by the EcoRI restriction enzyme (New England Biolabs, Beverly, MA) and cloned into the EcoRI site of the eukaryotic expression vector pcDNA3.1 (Invitrogen). The proper insertion was verified by restriction mapping and nucleotide sequencing.
Adenoviral vectors.
To achieve suppression of PDE2A in the intact mouse lung, we used a recombinant, replication-defective adenovirus that expresses GFP and an artificial microRNA (miRNA) engineered to selectively suppress PDE2A expression (Ad.PDE2A-shRNA). Construction of the artificial miRNA utilized an endogenous murine miRNA (miR-155) (2) in which the shRNA sequences were replaced by shRNA sequences targeting PDE2A. This was then incorporated into a commercially available vector pcDNA6.2-DEST (Gateway recombination cloning system, Invitrogen). In this vector, the shRNA containing the mouse PDE2A antisense sequence (NM_001008548) was placed downstream of a cDNA encoding a modified GFP (EmGFP) so that it was transcribed along with the EmGFP. The target sequence for mouse PDE2A (5′- TGCTGAGAAGTAGCATCCAAGTCAAAGTTTTGGCCACTGACTGACTTTGACTTATGCTACTTCT-3′) used in this study was chosen from among 10 different sequences generated by web-based selection programs in a screen in which individual miRNA-adapted shRNAs (shRNAmiRs) were cotransfected into 293A cells along with the plasmid pcHA-PDE2A that expresses mouse PDE2A. Lysates were prepared 2 days later and probed for PDE2A protein expression. The EmGFP linked to the artificial miRNA region was then transferred into pAd.CMV-DEST by recombinase cloning (Invitrogen). A control adenovirus (Ad.Neg-shRNA) containing EmGFP and a control shRNAmiR that was predicted not to target any known vertebrate gene was reported earlier (54). The initial viral amplification and purification was performed in our laboratory using an AdenopureLS adenovirus purification kit (Puresyn; Malvern, PA) according to the manufacturer's instructions. Large-scale amplification and purification of virus were performed by Puresyn, Malvern, PA.
Statistics.
Multiple treatment groups were compared by one-factor ANOVA with least significant difference post hoc testing. For single comparisons, unpaired Student's t-test was employed. The difference in survival was analyzed by χ2 testing. Nonnormally distributed data were converted to logarithms before analysis. Values presented in text are means ± SE. Differences were considered significant when P ≤ 0.05.
RESULTS
Effect of IT LPS on lung PDE2A expression and BAL inflammation.
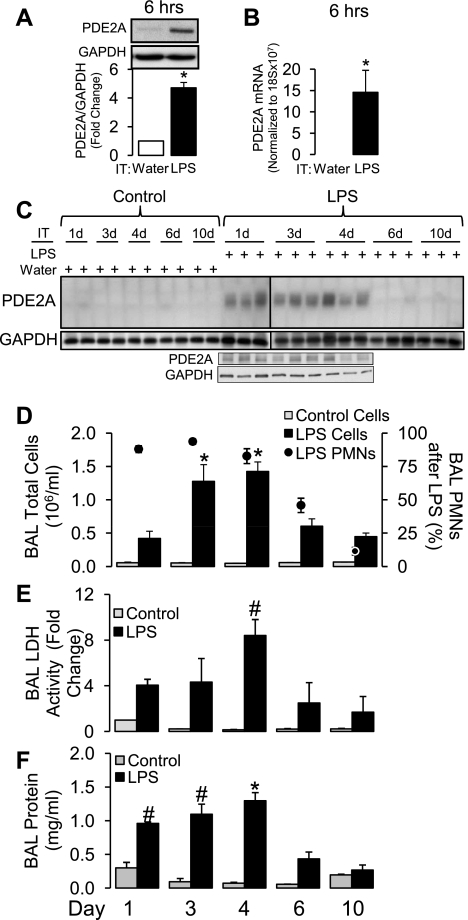
We first determined the time course effect of IT LPS on lung PDE2A protein expression and BAL inflammation from 6 h to 10 days after IT LPS administration (Fig. 1). Figure 1, A and B, shows that PDE2A protein expression and mRNA were increased significantly as early as 6 h post-LPS (P < 0.05). As shown in Fig. 1C, lung PDE2A protein expression remained increased on days 1, 3, and 4 following IT LPS compared with corresponding IT water control mice in which PDE2A expression was nearly undetectable. PDE2A expression on days 6 and 10 post-LPS returned to control levels. Because the immunoblot data in Fig. 1C spanned two separate gels, we elected to show all of the replicates in each group rather than compare by densitometry. To confirm that any differences in expression between gels was not secondary to technical differences in image development, we repeated the day 1-4 LPS lungs on a single gel and found similar results. As shown in Fig. 1, D–F, LPS-induced lung injury in these mice also peaked on day 4 with significant increases in BAL cells (predominantly polymorphonuclear leukocytes), LDH activity, and protein (P < 0.005). Consequently, the resolution of lung PDE2A protein expression to baseline levels on days 6 and 10 was associated with resolution of lung BAL inflammation, cytotoxicity, and protein permeability. This dose of LPS did not cause mortality.
Fig. 1.
Lung phosphodiesterase 2A (PDE2A) protein expression by Western immunoblot (A; n = 5) and lung PDE2A mRNA (B; n = 3) by quantitative real-time PCR 6 h following intratracheal (IT) LPS. The PDE2A protein is normalized to GAPDH loading control and then to time-matched IT water mice (n = 2). The PDE2A mRNA is normalized to 18S ribosomal mRNA. No PDE2A mRNA was detected in IT water control lungs. Values are means ± SE. *P < 0.05. C: time course from 1–10 days (d) of lung PDE2A expression by Western immunoblot following IT water (n = 2 mice for each day) or IT LPS (n = 3 mice for each day) with GAPDH loading control. The vertical line between days 1 and 3 post-LPS separates 2 gels. A separate single immunoblot is shown for lungs from days 1, 3, and 4 following IT LPS to confirm that LPS-induced PDE2A expression was similar from days 1–4 following LPS administration. D: time course of bronchoalveolar lavage (BAL) total cells (bars) following IT water (n = 2-4 mice per day) or IT LPS (n = 3-6 mice per day) and % polymorphonuclear leukocytes (PMN) following LPS (circles). Values are means ± SE. *P < 0.005 vs. IT LPS mice on days 1, 6, and 10 post-LPS. E: time course of BAL lactate dehydrogenase (LDH) activity following IT water (n = 2–3 mice per day) or IT LPS (n = 3–4 mice per day). Values are means ± SE. #P < 0.05 vs. IT LPS mice on days 6 and 10 post-LPS. F: time course of BAL protein concentration following IT water (n = 2–3 mice per day) or IT LPS (n = 3–4 mice per day). Values are means ± SE. *P < 0.0001 vs. IT LPS mice on days 1, 6, and 10 post-LPS. #P < 0.0001 vs. IT LPS mice on days 6 and 10 post-LPS.
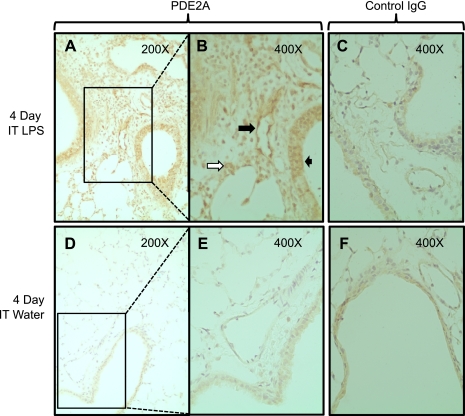
We investigated the cellular localization of the lung PDE2A expression on day 4 post-LPS by immunohistochemistry (Fig. 2). Compared with both the water and IgG controls, PDE2A staining revealed increased expression in both the epithelial (short black arrow in Fig. 2B) and endothelial compartments (long black arrow in Fig. 2B). We also noted strong cytoplasmic PDE2A expression in large mononuclear cells most consistent with macrophages (white arrow in Fig. 2B).
Fig. 2.
Lung immunohistochemistry for PDE2A expression 4 days following IT LPS (A and B) or water (D and E). Rectangles shown in A and D are shown in B and E under higher magnification. Large solid black arrow demonstrates endothelial PDE2A staining in a conduit pulmonary vessel. Small black arrow shows PDE2A staining in airway epithelium. White arrow demonstrates PDE2A staining in a macrophage. C and F: nonspecific IgG staining that was observed predominantly in airway epithelium in an LPS-independent fashion.
Effect of VILI combined with IT LPS on lung PDE2A expression and injury.
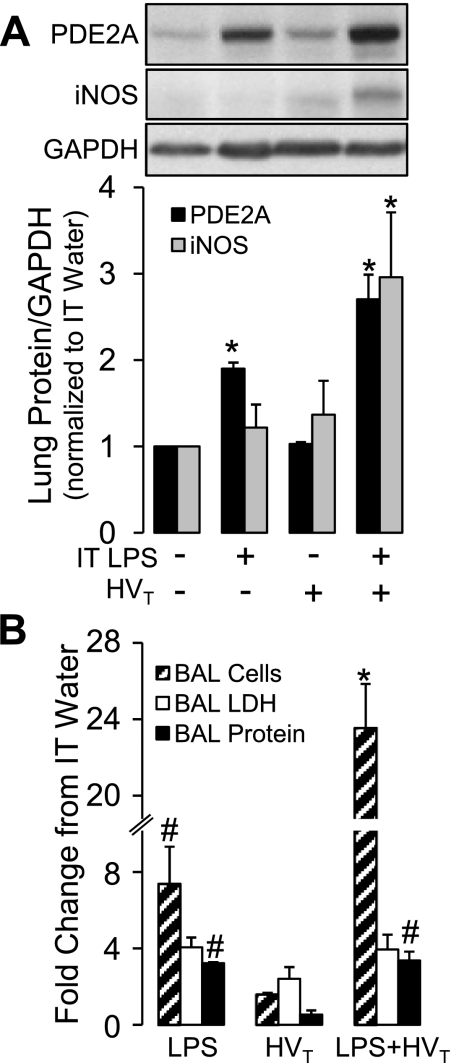
To determine whether the combination of 4 h of HVT ventilation would exacerbate the effects of LPS on PDE2A expression and injury, we compared 24 h of IT LPS or HVT ventilation to the combination of the two. Compared with mice instilled with IT water without HVT ventilation, the combination of LPS and HVT ventilation caused a threefold increase in both PDE2A and iNOS expression (Fig. 3A; P < 0.05) and a 24-fold increase in total BAL cells (96 ± 4% neutrophils; Fig. 3B; P < 0.05). All three effects were significantly greater than either LPS or HVT ventilation alone.
Fig. 3.
A: lung PDE2A and inducible nitric oxide synthase (iNOS) expression by Western blot with densitometric analysis measured after the following conditions in in vivo mice: IT water diluent and spontaneous ventilation, IT LPS and spontaneous ventilation, IT water followed by high tidal volume (HVT) ventilation or IT LPS followed by HVT ventilation (n = 3). All lungs were harvested 24 h after IT treatment; HVT ventilation occurred during the final 4 h of this period. Values are means ± SE. *P < 0.001 vs. all other treatment groups. B: effect of IT LPS, HVT ventilation (following IT water), or the combination on BAL cell count, LDH activity, and protein concentration (n = 3 per group). All values are expressed as fold change from an IT water-alone group (n = 2 per group). All BAL samples obtained 24 h after IT LPS or immediately after HVT ventilation which occurred during the final 4 h of this period. Values are means ± SE. *P < 0.0005 vs. all other treatment groups. #P < 0.0005 vs. HVT ventilation.
Construction of PDE2A-shRNA and viral dose optimization.
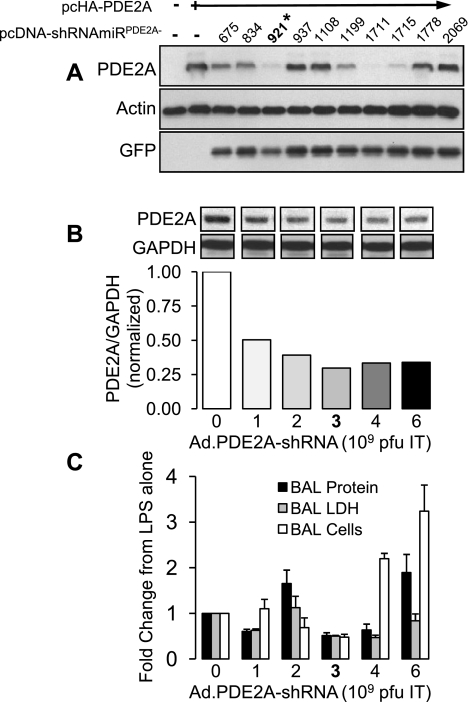
Figure 4A shows the effect of the 10 candidate PDE2A shRNAs cloned into the pcDNA6.2-DEST vector on PDE2A expression in 293A cells transfected with pcHA-PDE2A. Two of 10 candidate shRNA sequences (shRNA-921, and shRNA-1711) reduced PDE2A expression level by 80% or more of control levels. On the basis of this result, the PDE2A shRNA-921 was selected and incorporated into a replication-deficient adenoviral vector to generate Ad.PDE2A-shRNA as described in materials and methods. To determine the optimal dose to be used in vivo, increasing doses [1, 2, 3, 4, and 6 × 109 plaque-forming units (pfu)] of Ad.PDE2A-shRNA were instilled IT in mice 3 days before administration of IT LPS. One day later, Western blot analysis revealed that PDE2A expression was substantially reduced in the lungs of mice that received 35 μl containing 3 × 109 pfu, achieving a 65% knockdown compared with LPS administration alone (Fig. 4B). This same dose of Ad.PDE2A-shRNA maximally attenuated BAL cell count, LDH activity and protein concentration (Fig. 4C) and was chosen for subsequent experiments. Adenoviral dosages greater than 3 × 109 pfu effectively suppressed PDE2A expression but also caused increasing BAL inflammation.
Fig. 4.
A: simultaneous transfection of 293A cells with pcHA-PDE2A to express PDE2A and 10 separate green fluorescent protein (GFP)-tagged pcDNA-shRNAmiRPDE2A constructs designed to suppress PDE2A expression. *pcDNA-shRNAmiRPDE2A-921 was selected for Ad.PDE2A-shRNA [short hairpin RNA (shRNA) designed to selectively suppress PDE2A expression] construction. B: intratracheal dose-response effect of Ad.PDE2A-shRNA (derived from pcDNA-shRNAmiRPDE2A-921) construct on mouse lung PDE2A expression measured 24 h after IT LPS administration. Ad.PDE2A-shRNA was administered 3 days before LPS (n = 2). Representative noncontiguous lanes from the same gel are shown. Values are means normalized to the effect of LPS without adenovirus. C: intratracheal dose-response effect of Ad.PDE2A-shRNA (derived from pcDNA-shRNAmiRPDE2A-921) construct on mouse lung BAL protein, LDH activity, and total cell count 24 h after IT LPS administration. Ad.PDE2A-shRNA was administered 3 days before LPS (n = 2–3). Values are means ± SE normalized to the effect of LPS without adenovirus.
Effect of Ad.PDE2A-shRNA on lung PDE2A expression caused by IT LPS/VILI.
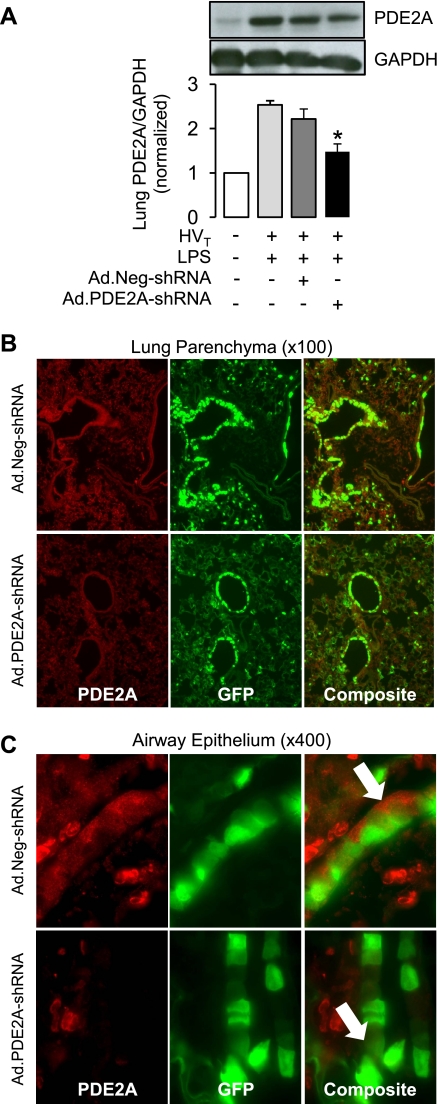
The increase in lung PDE2A caused by the combination of LPS followed by HVT ventilation was not affected by control infection with Ad.Neg-shRNA whereas Ad.PDE2A-shRNA infection significantly decreased PDE2A expression in lung homogenates from mice subjected to LPS/VILI by 33 ± 8% compared with the control virus (Fig. 5A). To determine the distribution of adenoviral infection and PDE2A expression, we performed immunofluorescence for PDE2A in LPS/VILI lungs. PDE2A expression (red) and adenoviral infection (indicated by GFP) were strongest in the airway epithelial compartment although red PDE2A immunofluorescence can also be seen in alveolar cells (Fig. 5B). As shown in Fig. 5C, top and similar to the pattern of expression in Fig. 2, PDE2A expression in Ad.Neg-shRNA infected lungs was present in the airway epithelium (Fig. 5C, white arrow). The composite image in Ad.Neg-shRNA lungs (Fig. 5C, top) shows that PDE2A staining and GFP were observed in most cells. In contrast, Ad.PDE2A-shRNA infection markedly decreased epithelial PDE2A expression in every cell, which demonstrated GFP expression (Fig. 5C, bottom). When we quantified PDE2A immunofluorescence across multiple epithelial cells from three separate experiments, Ad.PDE2A-shRNA infection decreased airway epithelial PDE2A expression by 62 ± 10% compared with lungs from Ad.Neg-shRNA-infected mice (P < 0.005; data not shown).
Fig. 5.
A: effect of Ad.PDE2A-shRNA on lung PDE2A expression following LPS and HVT compared with control adenovirus (Ad.Neg-shRNA) infection. PDE2A expression is also shown for lungs subjected to LPS plus HVT ventilation or IT water without HVT ventilation, all in the absence of viral infection. Total lung PDE2A knockdown was quantified by Western immunoblot (with GAPDH loading control) shown normalized to PDE2A expression in the IT water control group. Values are means ± SE; n = 4 for all groups. *P ≤ 0.05. Representative images of lung parenchyma (×400; B) and airway epithelial (×100; C) anti-PDE2A immunofluorescence (red) in a lung from a mouse treated with Ad.Neg-shRNA (top) or Ad.PDE2A-shRNA (bottom) prior to LPS and 4 h of HVT ventilation. Arrow highlights cytoplasmic PDE2A staining. Middle images show GFP indicating adenoviral infection.
Role of PDE2A in LPS/VILI.
Figure 6 shows the effect of PDE2A knockdown with Ad.PDE2A-shRNA on the injury caused by the combination of IT LPS and HVT ventilation. When we compared Ad.Neg-shRNA to Ad.PDE2A-shRNA infection in the surviving double-hit mice, we found that PDE2A knockdown significantly decreased BAL cells, LDH activity, and protein concentration by 60, 57, and 37%, respectively (P < 0.05; Fig. 6, A–C). The difference in BAL cell count was in neutrophils because the cell differentials did not differ consisting of >93% neutrophils in all groups (data not shown). Because the adenoviral infection itself potentially introduced an additional injury, we also included the comparison group of LPS/VILI mice without viral infection. Compared with these mice, Ad.Neg-shRNA infection resulted in a similar BAL cell count and protein concentration, suggesting that this concentration of adenovirus did not contribute to neutrophil influx and protein leak. The BAL LDH activity was increased by control adenoviral infection, suggesting a component of adenoviral cytotoxicity.
Fig. 6.
Effect of PDE2A knockdown with Ad.PDE2A-shRNA infection (n = 6) vs. control infection with Ad.Neg-shRNA (n = 7) in mice subjected to 1 day of LPS followed by HVT ventilation on BAL total cell count (A), BAL LDH concentration (B), and BAL protein concentration (C). The effects of HVT ventilation 1 day after IT LPS in the absence of adenoviral infection are also shown (n = 3) along with the effects of IT water without HVT ventilation (n = 2). LDH is shown normalized to the IT water control group. Values are means ± SE. *P < 0.05 vs. Ad.Neg-shRNA group.
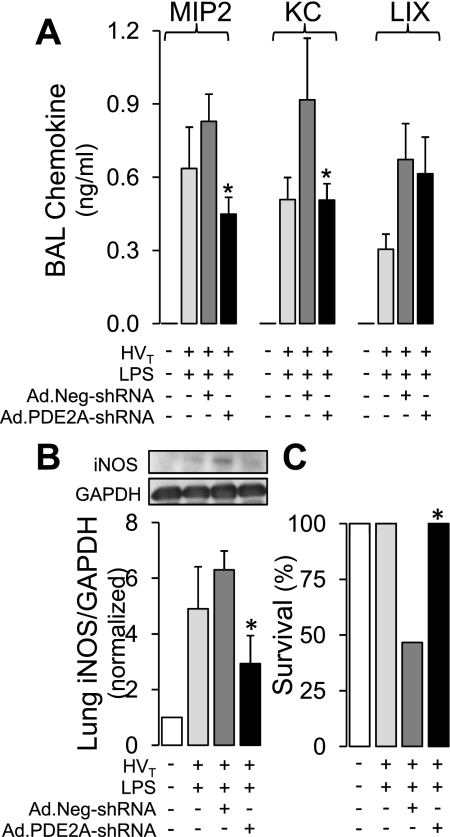
As shown in Fig. 7A, the reduction in BAL neutrophils caused by PDE2A knockdown was associated with a 44 ± 10% decrease in BAL MIP2 concentration (P < 0.001) and a 51 ± 7% reduction in KC (P < 0.001) compared with LPS/VILI mice infected with control adenovirus. PDE2A knockdown did not affect the chemokine LIX. There were no detectable BAL chemokine concentrations in the IT water controls. Similar to the BAL LDH result, the addition of control adenoviral infection to mice subjected to the combination of LPS and HVT ventilation tended to further increase BAL chemokine levels compared with LPS/HVT without virus although none reached statistical significance. Importantly, PDE2A knockdown also resulted in a significant 53 ± 16% reduction in iNOS expression (Fig. 7B).
Fig. 7.
A: effect of PDE2A knockdown with Ad.PDE2A-shRNA infection (n = 6) vs. control infection with Ad.Neg-shRNA (n = 4) in mice subjected to 1 day of LPS followed by HVT ventilation on BAL chemokines macrophage inflammatory protein (MIP2), keratinocyte-derived chemokine (KC), and LPS-induced CXC chemokine (LIX). The effects of IT water without HVT ventilation (n = 4) and IT LPS plus HVT (n = 3), all in the absence of viral infection are also shown. B: effect of PDE2A knockdown with Ad.PDE2A-shRNA infection (n = 4) vs. control infection with Ad.Neg-shRNA (n = 4) in mice subjected to 1 day of LPS followed by HVT ventilation on lung iNOS expression. The effects of IT water without HVT ventilation (n = 4) or IT LPS plus HVT (n = 4), all in the absence of viral infection are also shown. (n = 4). C: effect of PDE2A knockdown with Ad.PDE2A-shRNA infection (n = 6) vs. control infection with Ad.Neg-shRNA (n = 15) on survival during the 4 h of HVT ventilation. The survival of mice subjected to IT water without HVT ventilation (n = 15) or IT LPS plus HVT (n = 4), all in the absence of viral infection, is also shown. Values are means ± SE. *P < 0.05 vs. Ad.Neg-shRNA group.
The presence of control adenoviral infection with Ad.Neg-shRNA prior to LPS/VILI resulted in a survival of only 47% (7 of 15 mice) during the 4 h of HVT ventilation. All deaths occurred during the 4 h HVT period. PDE2A knockdown with Ad.PDE2A-shRNA, however, was associated with 100% survival (6 of 6 mice) through the protocol (P < 0.05; Fig. 7C), identical to the 100% survival we found in uninfected mice subjected to LPS/VILI (4 of 4) or IT water without HVT ventilation (15 of 15).
Effect of in vivo PDE2A knockdown on lung cAMP and cGMP concentration.
As shown in Fig. 8A, knockdown of PDE2A before LPS/VILI resulted in a significant near twofold increase in lung cAMP concentration. There was no comparable effect on lung cGMP; in fact, the mean cGMP value tended to be less in the Ad.PDE2A-shRNA lungs (Fig. 8B). There was no statistically significant effect of control adenoviral infection on either cyclic nucleotide although there was a trend toward an increase in cGMP (P = 0.07) that might have reached statistical significance with additional replicates.
Fig. 8.
Effect of PDE2A knockdown with Ad.PDE2A-shRNA infection (n = 4) vs. control infection with Ad.Neg-shRNA (n = 4) in mice subjected to 1 day of LPS followed by HVT ventilation on lung cAMP concentration (A) and lung cGMP concentration (B). To examine the contribution of adenoviral infection, the effect of HVT ventilation 1 day after IT LPS in the absence of adenoviral infection on cyclic nucleotide concentrations is shown (n = 3) along with a group of IT water, no HVT controls (n = 3). Values are means ± SE. *P < 0.002 vs. Ad.Neg-shRNA group.
DISCUSSION
The major new findings of this study are that 1) the combination of IT LPS and HVT ventilation potently upregulates lung PDE2A, iNOS, and alveolar inflammation and 2) knockdown of lung PDE2A expression with an adenoviral-generated shRNA significantly attenuates all three. These results serve to confirm the emerging idea that PDE2A is a key proinflammatory enzyme that enhances the cellular and humoral inflammation associated with common forms of tissue injury (14, 41, 44, 52).
In mammalian cells, cyclic nucleotides are hydrolyzed by 11 known families of PDEs. In pulmonary endothelium (30, 44, 46, 55, 56) and epithelium (36), PDE2A, 3, 4, and 5 appear to represent the most significant forms. PDE2A is a dual-function PDE capable of hydrolyzing both cAMP and cGMP. PDE2A contains a regulatory GAF domain (cGMP-activated PDEs, adenylyl cyclase, and Fh1A) that alters catalytic function when bound by cGMP. Specifically, elevated cGMP stimulates PDE2A to preferentially hydrolyze cAMP over cGMP even when cAMP is present at lower substrate concentration (12, 44). Thus PDE2A can serve to lower intracellular cAMP concentrations when conditions result in the expression of PDE2A and the simultaneous generation of increased cGMP from soluble or particulate guanylyl cyclase (44).
Consistent with this concept, we (39) recently showed that high tidal volume (HVT) ventilation alone simultaneously increased NO, lung cGMP, and PDE2A expression in a buffer-perfused isolated mouse lung model of VILI. Inhibition of eNOS, sGC, or PDE2A prevented endothelial barrier dysfunction whereas further enhancement of cGMP production with a direct sGC activator exacerbated the injury (39). More recently, an injurious role for increased lung PDE2A expression was found in a mouse model of pneumococcal pneumonia (52). Specifically, Streptococcus pneumoniae serotype-3 infection in intact mice was shown to increase PDE2A mRNA and protein at 24 h postinfection with no changes noted in mRNA for PDEs 3A, 3B, 4A, 4B, 4C, 4D, 5A, or 7A. A continuous subcutaneous infusion of the PDE2A inhibitor hydroxyl-PDP inhibited a pneumonia-induced increase in BAL albumin but had no effect on the increased BAL neutrophils caused by pneumococcal infection (52).
In the present study, we examined the role of PDE2A in an intact mouse model of ALI that combined IT LPS and VILI. We chose this combination of insults because gram-negative bacterial pneumonia is a frequent cause of severe healthcare-associated pneumonia (35) and gram-negative bacilli are also a common cause of ventilator-associated pneumonia in critically ill patients requiring mechanical ventilation (48). Moreover, TNF-α, a proinflammatory cytokine generated by LPS stimulation, was shown to increase PDE2A expression in aortic (22) and human umbilical vein (41, 44) endothelium. On the basis of these data, and our previous observation that HVT ventilation alone caused a PDE2A-mediated injury (39), we reasoned that the combination of LPS and HVT could represent a particularly potent stimulus of PDE2A expression in the lung.
Because the effect of LPS alone on lung PDE2A expression has not previously been reported, we first examined the time course of PDE2A expression following a nonlethal dose of IT LPS alone in intact mice. We found a rapid increase in lung PDE2A mRNA and a prolonged increase in protein expression that corresponded with the peak changes in BAL neutrophils, LDH, and protein following LPS (Fig. 1, C–F). We examined day 4 LPS-treated lungs by immunohistochemistry (Fig. 2) and found that PDE2A expression was increased in epithelium, endothelium, and cells that appeared to be alveolar macrophages. This cellular pattern of PDE2A expression is consistent with previous reports indicating that PDE2A expression can be detected in pulmonary endothelial (37) and epithelial (36) cells. PDE2A expression has not been reported in alveolar macrophages but the presence of PDE2A in peritoneal (51), neuronal (6), and peripheral monocyte-derived macrophages (7) is consistent with the presence of PDE2A in the pulmonary macrophage population.
When we combined the effects of IT LPS with HVT we found that HVT significantly boosted the LPS-induced increase in both lung PDE2A and iNOS expression (Fig. 3A). Of note, we did not see a significant increase in lung PDE2A or iNOS with HVT alone at this time point. Although we previously showed that 4 h of 20 ml/kg HVT ventilation increased protein extravasation and lung water in this preparation by a sGC-dependent mechanism, HVT-induced increased lung PDE2A expression was examined only at 40 and 80 min in buffer-perfused mouse lungs (39). Thus it is possible that an increase in PDE2A from HVT alone was not sustained for 4 h. A similar situation may exist for iNOS, which was previously shown to increase at 2 h in this intact mouse VILI model (33).
If PDE2A and iNOS are simultaneously increased in the same or even neighboring cells, the increased NO from iNOS could serve to generate additional cGMP from sGC, which in turn would stimulate the newly formed PDE2A to degrade cAMP. Thus this combination of effects could represent a potent mechanism for cAMP regulation in the lung. The synergistic effect of LPS and HVT ventilation was also observed in the BAL neutrophil count with a 24-fold increase after the combined injury compared with <8-fold increases after either LPS or HVT ventilation alone (Fig. 3B). The combined effect of LPS and HVT in the present study is also consistent with previous studies showing that lung ventilatory stretch combined with bacteria or bacterial products synergistically exacerbated ALI with increased BAL neutrophilia and lung chemokine production including MIP-2, MIP-1α, and IL-1β (25, 27). A similar phenomenon was recently observed with the combination of lung viral infection and HVT ventilation (5). Neither PDE2A nor iNOS were evaluated in these studies. The inability of HVT ventilation alone to increase BAL inflammation or protein is consistent with previous work showing that isolated VILI decreases endothelial barrier function with only small changes in BAL leukocytes and protein (33).
To determine the role of the increased PDE2A observed in our LPS/VILI model, we chose to knock down PDE2A protein expression with an shRNA generated by an adenovirus. Although specific pharmacological inhibitors for PDE family members exist, the uncertainty of drug tissue concentration and off-target inhibition of other PDEs following systemic administration in intact animals remains problematic. The use of an shRNA directed against PDE2A avoided this problem. To our knowledge, this study is the first to utilize this method to examine the contribution of PDE2A in any intact animal model of acute tissue injury.
As shown in Fig. 5, we were able to significantly knock down PDE2A in the lung by IT administration of Ad.PDE2A-shRNA. Although we achieved adenoviral infection throughout the parenchyma, the airway epithelial compartment was primarily infected, successfully knocking down the marked upregulation of PDE2A that we observed in this cellular population after the combined injury (Fig. 5). Both IT LPS (32, 53) and VILI (45) have been shown to cause airway injury with increased airway edema associated with increased airway epithelial permeability and apoptosis. Thus it is possible that PDE2A knockdown in this cellular compartment could significantly contribute to the beneficial effects we observed in the overall LPS/VILI insult in mice infected with Ad.PDE2A-shRNA.
Compared with control adenoviral infection, infection with Ad.PDE2A-shRNA significantly attenuated the alveolar inflammation and protein leak resulting from the combination of IT LPS and HVT ventilation (Fig. 6). The protection conferred by interfering with PDE2A expression was accompanied by a significant reduction in MIP2 and KC, key neutrophil chemoattractants in mouse models of LPS lung injury (1) and VILI (18, 50). Moreover, MIP2 is known to be synergistically increased when these two injuries are combined (25, 27). PDE2A knockdown also significantly decreased iNOS expression (Fig. 7B). Experiments utilizing NO synthase inhibitors or iNOS knockout mice in IT LPS or VILI models underscore the critical importance of iNOS in these injuries (24, 31, 33, 42). For example, in IT LPS, NO production from iNOS was upstream of MIP-2 and TNF-α (31, 42) and contributed significantly to alveolar neutrophil influx (31, 42), and alveolar-capillary barrier dysfunction (24, 31, 42). Indeed, NO synthase inhibition was more effective in blocking LPS-induced epithelial barrier dysfunction than either TNF-α antibody or neutrophil depletion (24). To our knowledge, our data are the first to show that iNOS upregulation requires increased PDE2A expression in any form of acute tissue injury.
The adenoviral infection contributed to some aspects of the LPS/VILI injury including an exacerbation of BAL LDH release, a trend toward increased BAL chemokines, and an increased mortality during the 4-h HVT period. We do not know the cellular source of the increased BAL LDH nor the cause of death associated with control adenoviral infection when combined with LPS/VILI. The fact that PDE2A knockdown successfully protected against these adverse outcomes suggests that adenoviral infection may have contributed to increased PDE2A activity since control adenoviral infection did not further increase PDE2A expression (Fig. 5A). The near-significant increase in lung cGMP induced by control adenovirus in LPS/VILI lungs (Fig. 8B) could be responsible for this PDE2A stimulation. We speculate that adenoviral infection may have increased NO from iNOS, which in turn stimulated sGC in a critical cell population.
The mechanism behind the ability of PDE2A to regulate iNOS and chemokine production and enhance injury likely involves the preferential loss of cAMP when PDE2A is stimulated by increased cGMP levels. Consistent with this notion, we were able to demonstrate a significant increase in lung cAMP concentration when PDE2A was knocked down by Ad.PDE2A-shRNA (Fig. 8A). There was no significant effect on cGMP, again consistent with the enhanced activity of stimulated PDE2A toward cAMP. cAMP is a key defensive signaling molecule in the injured lung, opposing LPS- and ROS-mediated cell death, preserving endothelial and epithelial intercellular junctions, and enhancing edema clearance (16, 21, 23, 26, 28, 38). For example, increased PDE2A expression in human umbilical vein endothelium was recently reported to increase thrombin-induced NADPH oxidase activity and ROS production by a loss of cAMP inhibition of rac1 activity (14). cAMP also serves as a critical regulator of lung macrophage function suppressing cytokine and chemokine production as well as iNOS expression (40). Given the critical importance of lung macrophages in mediating both LPS-induced lung injury (15) and VILI (19), our results raise the possibility that increased macrophage PDE2A expression may play an important role in our combined injury model. Additional experiments are planned to elucidate the specific cellular populations responsible for the PDE2A-mediated effects when LPS and VILI are combined.
In summary, we found that IT LPS and HVT ventilation synergistically increased lung PDE2A and iNOS expression and caused ALI. Successful knockdown of PDE2A expression with Ad.PDE2A-shRNA proved to be markedly protective with decreased BAL inflammation and lung iNOS expression and increased lung cAMP, suggesting that PDE2A played a critical role in this double-hit ALI model.
GRANTS
This work was supported by National Heart, Lung and Blood Institute Grant HL067189 (D. B. Pearse).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Aggarwal NR, D'Alessio FR, Tsushima K, Files DC, Damarla M, Sidhaye VK, Fraig MM, Polotsky VY, King LS. Moderate oxygen augments lipopolysaccharide-induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol 298: L371–L381, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Angelini DJ, Su Q, Yamaji-Kegan K, Fan C, Skinner JT, Champion HC, Crow MT, Johns RA. Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELMα) induces the vascular and hemodynamic changes of pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 296: L582–L593, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arias-Diaz J, Garcia-Verdugo I, Casals C, Sanchez-Rico N, Vara E, Balibrea JL. Effect of surfactant protein A (SP-A) on the production of cytokines by human pulmonary macrophages. Shock 14: 300–306, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Bauer TT, Ewig S, Rodloff AC, Muller EE. Clinical practice: acute respiratory distress syndrome and pneumonia: a comprehensive review of clinical data. Clin Infect Dis 43: 748–756, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bem RA, van Woensel JBM, Bos AP, Koski A, Farnand AW, Domachowske JB, Rosenberg HF, Martin TR, Matute-Bello G. Mechanical ventilation enhances lung inflammation and caspase activity in a model of mouse pneumovirus infection. Am J Physiol Lung Cell Mol Physiol 296: L46–L56, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bender AT, Beavo JA. Specific localized expression of cGMP PDEs in Purkinje neurons and macrophages. Neurochem Int 45: 853–857, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Bender AT, Ostenson CL, Giordano D, Beavo JA. Differentiation of human monocytes in vitro with granulocyte-macrophage colony-stimulating factor and macrophage colony-stimulating factor produces distinct changes in cGMP phosphodiesterase expression. Cell Signal 16: 365–374, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 58: 488–520, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Bove PF, Wesley UV, Greul AK, Hristova M, Dostmann WR, van der Vliet A. Nitric oxide promotes airway epithelial wound repair through enhanced activation of MMP-9. Am J Respir Cell Mol Biol 36: 138–146, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chignard M, Balloy V. Neutrophil recruitment and increased permeability during acute lung injury induced by lipopolysaccharide. Am J Physiol Lung Cell Mol Physiol 279: L1083–L1090, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Connelly L, Jacobs AT, Palacios-Callender M, Moncada S, Hobbs AJ. Macrophage endothelial nitric-oxide synthase autoregulates cellular activation and pro-inflammatory protein expression. J Biol Chem 278: 26480–26487, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem 76: 481–511, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Damarla M, Hasan E, Boueiz A, Le A, Pae HH, Montouchet C, Kolb T, Simms T, Myers A, Kayyali US, Gaestel M, Peng X, Reddy SP, Damico R, Hassoun PM. Mitogen activated protein kinase activated protein kinase 2 regulates actin polymerization and vascular leak in ventilator associated lung injury. PLoS ONE 4: e4600, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Diebold I, Djordjevic T, Petry A, Hatzelmann A, Tenor H, Hess J, Gorlach A. Phosphodiesterase 2 mediates redox-sensitive endothelial cell proliferation and angiogenesis by thrombin via Rac1 and NADPH oxidase 2. Circ Res 104: 1169–1177, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Farley KS, Wang L, Mehta S. Septic pulmonary microvascular endothelial cell injury: role of alveolar macrophage NADPH oxidase. Am J Physiol Lung Cell Mol Physiol 296: L480–L488, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Friedman GB, Taylor CT, Parkos CA, Colgan SP. Epithelial permeability induced by neutrophil transmigration is potentiated by hypoxia: role of intracellular cAMP. J Cell Physiol 176: 76–84, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Geary CA, Goy MF, Boucher RC. Synthesis and vectorial export of cGMP in airway epithelium: expression of soluble and CNP-specific guanylate cyclases. Am J Physiol Lung Cell Mol Physiol 265: L598–L605, 1993 [DOI] [PubMed] [Google Scholar]
- 18. Halbertsma FJ, Vaneker M, Scheffer GJ, van der Hoeven JG. Cytokines and biotrauma in ventilator-induced lung injury: a critical review of the literature. Neth J Med 63: 382–392, 2005 [PubMed] [Google Scholar]
- 19. Hamanaka K, Jian MY, Townsley MI, King JA, Liedtke W, Weber DS, Eyal FG, Clapp MM, Parker JC. TRPV4 channels augment macrophage activation and ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 299: L353–L362, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hofmann F, Ammendola A, Schlossmann J. Rising behind NO: cGMP-dependent protein kinases. J Cell Sci 113: 1671–1676, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Kim MJ, Lee JH, Park SY, Hong KW, Kim CD, Kim KY, Lee WS. Protection from apoptotic cell death by cilostazol, phosphodiesterase type III inhibitor, via cAMP-dependent protein kinase activation. Pharmacol Res 54: 261–267, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Koga S, Morris S, Ogawa S, Liao H, Bilezikian JP, Chen G, Thompson WJ, Ashikaga T, Brett J, Stern DM, Pinsky DJ. TNF modulates endothelial properties by decreasing cAMP. Am J Physiol Cell Physiol 268: C1104–C1113, 1995 [DOI] [PubMed] [Google Scholar]
- 23. Lawrence DW, Comerford KM, Colgan SP. Role of VASP in reestablishment of epithelial tight junction assembly after Ca2+ switch. Am J Physiol Cell Physiol 282: C1235–C1245, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Li X, Donaldson K, MacNee W. Lipopolysaccharide-induced alveolar epithelial permeability. The role of nitric oxide. Am J Respir Crit Care Med 157: 1027–1033, 1998 [DOI] [PubMed] [Google Scholar]
- 25. Lin SM, Lin HC, Lee KY, Huang CD, Liu CY, Wang CH, Kuo HP. Ventilator-induced injury augments interleukin-1beta production and neutrophil sequestration in lipopolysaccharide-treated lungs. Shock 28: 453–460, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Lum H, Jaffe HA, Schulz IT, Masood A, RayChaudhury A, Green RD. Expression of PKA inhibitor (PKI) gene abolishes cAMP-mediated protection to endothelial barrier dysfunction. Am J Physiol Cell Physiol 277: C580–C588, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Martin TR. Interactions between mechanical and biological processes in acute lung injury. Proc Am Thorac Soc 5: 291–296, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matthay MA, Robriquet L, Fang X. Alveolar epithelium: role in lung fluid balance and acute lung injury. Proc Am Thorac Soc 2: 206–213, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Moldobaeva A, Welsh-Servinsky LE, Shimoda LA, Stephens RS, Verin AD, Tuder RM, Pearse DB. Role of protein kinase G in barrier-protective effects of cGMP in human pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 290: L919–L930, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Moore TM, Chetham PM, Kelly JJ, Stevens T. Signal transduction and regulation of lung endothelial cell permeability. Interaction between calcium and cAMP. Am J Physiol Lung Cell Mol Physiol 275: L203–L222, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Okamoto T, Gohil K, Finkelstein EI, Bove P, Akaike T, van der Vliet Multiple contributing roles for NOS2 in LPS-induced acute airway inflammation in mice. Am J Physiol Lung Cell Mol Physiol 286: L198–L209, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Ooi H, Arakawa M, Ozawa H. A morphological study of acute respiratory tract lesions in a lipopolysaccharide instilled rat model. Arch Histol Cytol 57: 87–105, 1994 [DOI] [PubMed] [Google Scholar]
- 33. Peng X, Abdulnour RE, Sammani S, Ma SF, Han EJ, Hasan EJ, Tuder R, Garcia JG, Hassoun PM. Inducible nitric oxide synthase contributes to ventilator-induced lung injury. Am J Respir Crit Care Med 172: 470–479, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rentsendorj O, Mirzapoiazova T, Adyshev D, Servinsky LE, Renne T, Verin AD, Pearse DB. Role of vasodilator-stimulated phosphoprotein in cGMP-mediated protection of human pulmonary artery endothelial barrier function. Am J Physiol Lung Cell Mol Physiol 294: L686–L697, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Restrepo MI, Anzueto A. The role of gram-negative bacteria in healthcare-associated pneumonia. Semin Respir Crit Care Med 30: 61–66, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Rousseau E, Gagnon J, Lugnier C. Biochemical and pharmacological characterization of cyclic nucleotide phosphodiesterase in airway epithelium. Mol Cell Biochem 140: 171–175, 1994 [DOI] [PubMed] [Google Scholar]
- 37. Sadhu K, Hensley K, Florio VA, Wolda SL. Differential expression of the cyclic GMP-stimulated phosphodiesterase PDE2A in human venous and capillary endothelial cells. J Histochem Cytochem 47: 895–906, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Sato Y. Modulation of PMN-endothelial cells interactions by cyclic nucleotides. Curr Pharm Des 10: 163–170, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Schmidt EP, Damarla M, Rentsendorj O, Servinsky LE, Zhu B, Moldobaeva A, Gonzalez A, Hassoun PM, Pearse DB. Soluble guanylyl cyclase contributes to ventilator-induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol 295: L1056–L1065, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Serezani CH, Ballinger MN, Aronoff DM, Peters-Golden M. Cyclic AMP: master regulator of innate immune cell function. Am J Respir Cell Mol Biol 39: 127–132, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Seybold J, Thomas D, Witzenrath M, Boral S, Hocke AC, Burger A, Hatzelmann A, Tenor H, Schudt C, Krull M, Schutte H, Hippenstiel S, Suttorp N. Tumor necrosis factor-α-dependent expression of phosphodiesterase 2: role in endothelial hyperpermeability. Blood 105: 3569–3576, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Shanley TP, Zhao B, Macariola DR, Denenberg A, Salzman AL, Ward PA. Role of nitric oxide in acute lung inflammation: lessons learned from the inducible nitric oxide synthase knockout mouse. Crit Care Med 30: 1960–1968, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Stephens RS, Rentsendorj O, Servinsky LE, Moldobaeva A, Damico R, Pearse DB. cGMP increases antioxidant function and attenuates oxidant cell death in mouse lung microvascular endothelial cells by a protein kinase G-dependent mechanism. Am J Physiol Lung Cell Mol Physiol 299: L323–L333, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Surapisitchat J, Jeon KI, Yan C, Beavo JA. Differential regulation of endothelial cell permeability by cGMP via phosphodiesterases 2 and 3. Circ Res 101: 811–818, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Syrkina O, Jafari B, Hales CA, Quinn DA. Oxidant stress mediates inflammation and apoptosis in ventilator-induced lung injury. Respirology 13: 333–340, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Thompson WJ, Ashikaga T, Kelly JJ, Liu L, Zhu B, Vemavarapu L, Strada SJ. Regulation of cyclic AMP in rat pulmonary microvascular endothelial cells by rolipram-sensitive cyclic AMP phosphodiesterase (PDE4). Biochem Pharmacol 63: 797–807, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Tsubochi H, Suzuki S, Kubo H, Ueno T, Yoshimura T, Suzuki T, Sasano H, Kondo T. Early changes in alveolar fluid clearance by nitric oxide after endotoxin instillation in rats. Am J Respir Crit Care Med 167: 205–210, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Valencia M, Torres A. Ventilator-associated pneumonia. Curr Opin Crit Care 15: 30–35, 2009 [DOI] [PubMed] [Google Scholar]
- 49. Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000 [DOI] [PubMed] [Google Scholar]
- 50. Wilson MR, Choudhury S, Goddard ME, O'Dea KP, Nicholson AG, Takata M. High tidal volume upregulates intrapulmonary cytokines in an in vivo mouse model of ventilator-induced lung injury. J Appl Physiol 95: 1385–1393, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Witwicka H, Kobialka M, Siednienko J, Mitkiewicz M, Gorczyca WA. Expression and activity of cGMP-dependent phosphodiesterases is up-regulated by lipopolysaccharide (LPS) in rat peritoneal macrophages. Biochim Biophys Acta 1773: 209–218, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Witzenrath M, Gutbier B, Schmeck B, Tenor H, Seybold J, Kuelzer R, Grentzmann G, Hatzelmann A, van Laak V, Tschernig T, Mitchell TJ, Schudt C, Rosseau S, Suttorp N, Schutte H. Phosphodiesterase 2 inhibition diminished acute lung injury in murine pneumococcal pneumonia. Crit Care Med 37: 584–590, 2009 [DOI] [PubMed] [Google Scholar]
- 53. Z'graggen BR, Tornic J, Muller-Edenborn B, Reyes L, Booy C, Beck-Schimmer B. Acute lung injury: apoptosis in effector and target cells of the upper and lower airway compartment. Clin Exp Immunol 161: 324–331, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang M, Koitabashi N, Nagayama T, Rambaran R, Feng N, Takimoto E, Koenke T, O'Rourke B, Champion HC, Crow MT, Kass DA. Expression, activity, and pro-hypertrophic effects of PDE5A in cardiac myocytes. Cell Signal 20: 2231–2236, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhu B, Kelly J, Vemavarapu L, Thompson WJ, Strada SJ. Activation and induction of cyclic AMP phosphodiesterase (PDE4) in rat pulmonary microvascular endothelial cells. Biochem Pharmacol 68: 479–491, 2004 [DOI] [PubMed] [Google Scholar]
- 56. Zhu B, Strada S, Stevens T. Cyclic GMP-specific phosphodiesterase 5 regulates growth and apoptosis in pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol 289: L196–L206, 2005 [DOI] [PubMed] [Google Scholar]