Abstract
To better understand how airways produce thick airway mucus, nonvolatile solids were measured in liquid secreted by bronchi from normal pig, cystic fibrosis (CF) human, and non-CF human lungs. Bronchi were exposed to various secretagogues and anion secretion inhibitors to induce a range of liquid volume secretion rates. In all three groups, the relationship of solids concentration (percent nonvolatile solids) to liquid volume secretion rate was curvilinear, with higher solids concentration associated with lower rates of liquid volume secretion. In contrast, the secretion rates of solids mass and water mass as functions of liquid volume secretion rates exhibited positive linear correlations. The y-intercepts of the solids mass-liquid volume secretion relationships for all three groups were positive, thus accounting for the higher solids concentrations in airway liquid at low rates of secretion. Predictive models derived from the solids mass and water mass linear equations fit the experimental percent solids data for the three groups. The ratio of solids mass secretion to liquid volume secretion was 5.2 and 2.4 times higher for CF bronchi than for pig and non-CF bronchi, respectively. These results indicate that normal pig, non-CF human, and CF human bronchi produce a high-percent-solids mucus (>8%) at low rates of liquid volume secretion (≤1.0 μl·cm−2·h−1). However, CF bronchi produce mucus with twice the percent solids (∼8%) of pig or non-CF human bronchi at liquid volume secretion rates ≥4.0 μl·cm−2·h−1.
Keywords: asthma, submucosal glands, mucus, mucin, cystic fibrosis transmembrane conductance regulator
a prominent feature of some airway diseases, notably cystic fibrosis (CF) and status asthmaticus, is the production of an obstructive airway mucus with a high concentration of nonvolatile solids, a complex mixture of biomolecules and inorganic salts. Sputum from CF patients contains >10% solids (8, 23, 26), and sputum produced by asthmatic patients is reported to be 7–8% solids (11). The percent solids in mucus produced by normal, undiseased human airways is difficult to determine, since healthy persons do not produce sputum; however, chronic bronchitis (23) and bronchiectasis (8) patients produce sputum that is ∼6% solids, and mucus collected directly from the ventilation tubes of laryngectomized patients is 5% solids (26). The high solids content of airway liquid in CF and asthmatic patients results in an unusually thick mucus that is poorly cleared and obstructs distal airways (7, 44). The etiology of this thick airway mucus is incompletely understood.
Several theories have been proposed to account for this high-solids airway mucus in these disorders, particularly in CF. Because of the hyperplasia of goblet cells and hypertrophy of submucosal glands in CF lung disease (36) and asthma (1), the presence of thick mucus in the airways has long been attributed to mucus “hypersecretion.” Findings that ciliary clearance of mucus is reduced in many CF patients, profoundly so in patients with advanced disease (31, 45), suggest that the abundance of thick mucus in CF airways could be the consequence of reduced clearance, rather than augmented mucus secretion. Transepithelial Na+ absorption is elevated in CF airways (6), giving rise to the notion that the macromolecular component of mucous liquid is abnormally concentrated by higher-than-normal rates of electrolyte and water absorption by the surface epithelium (12). Observations that secretion of liquid from airway submucosal glands is reduced in CF (19, 34) and that the liquid emerging from CF gland ducts is significantly more viscous than that from non-CF gland ducts (18) support the hypothesis that mucin and liquid components of gland secretion are “uncoupled” in CF (16). That is, loss of the CFTR-dependent anion secretion pathway creates thick mucus by selectively reducing the liquid component of gland secretion from serous cells, which normally express CFTR (14), but not the biomolecular component of gland secretion from the mucous cells, which do not express this anion channel. CF disease is caused by loss-of-function mutations in the gene that codes for the CFTR (33), a Cl− and HCO3− channel, and all pathological consequences of this disease are necessarily linked in some way to the loss of this anion channel. No single genetic cause of asthma has been identified, and the factors that favor formation of the obstructive mucus in this disorder are poorly understood.
In a recent study (25), the efficacies of the glandular secretagogues ACh, substance P, and VIP for induction of mucous liquid secretion from porcine bronchi were compared. ACh and substance P induced relatively high rates of liquid volume secretion, whereas VIP induced significantly lower rates of liquid volume secretion. Surprisingly, the concentration of the nonvolatile solids (i.e., percent solids) in the liquid was significantly greater when induced by VIP than by ACh or substance P. These observations suggest that the percent solids in airway mucus could be negatively related to the rate of liquid volume secretion. Using the same cannulated bronchus technique, where secretion can be assessed quantitatively and normalized to the airway mucosal surface area, we tested this hypothesis in the present study by evaluating the relationships between solids and liquid secretion in normal pig, non-CF human, and CF human bronchi.
MATERIALS AND METHODS
Pig bronchi.
All procedures with swine were approved by the University of South Alabama Institutional Animal Care and Use Committee and complied with the US Public Health Service Policy on Humane Care and Use of Laboratory Animals. Domestic pigs (∼10–20 kg) were obtained from Auburn University Swine Research and Education Center (Auburn, AL) and from a local vendor. Pigs were sedated by intramuscular injection of 1) ketamine (16 mg/kg) and midazolam (2 mg/kg) or 2) ketamine (16 mg/kg) and xylazine (7 mg/kg). Once sedated, the pigs were euthanized with a lethal overdose of pentobarbital sodium administered through an ear vein. The lungs were excised from the chest cavity, and intrapulmonary bronchi (∼30–40 mm long, ∼3–4 mm diameter) were dissected intact from the lung tissues. Side branches of the airways were ligated with suture. The isolated bronchi were immersed in 80 ml of Krebs-Ringer bicarbonate (KRB) solution baths at room temperature and slowly warmed (∼0.25°C/min) to 37°C. Once the solutions reached physiological temperature, the airways were pretreated for 45 min, if necessary, with bumetanide to inhibit transepithelial secretion of Cl−, dimethylamiloride (DMA) to inhibit HCO3− secretion, or the combination of bumetanide and DMA to inhibit secretion of both anions (42). Then the airways were removed from the bath solutions, and all accessible liquid was aspirated from the airway lumina with Intramedic PE-50 tubing (Becton Dickinson, Parsippany, NJ) attached to a syringe. The airways were tied onto polyethylene cannulas with suture and returned to their respective bath solutions, where they were suspended vertically. Cannulation was performed to physically separate the airway lumen, into which liquid was secreted, from the external bath solution. One of three neurotransmitters (ACh, substance P, or VIP) was then added to the bath to induce liquid secretion. At 15 min prior to substance P addition, 1 μM phosphoramidon, a protease inhibitor that prevents breakdown of substance P, was added to the bath. Similarly, 15 min prior to VIP addition, the protease inhibitors phosphoramidon (1 μM) and thiorphan (5 μM) were added to the bath to prevent VIP breakdown.
After 2 h of exposure to the neurotransmitters, the bronchi were removed from their baths and cannulas, the bronchi were sectioned lengthwise, and the luminal mucous liquid was collected with Eppendorf “Reference” 20–100 ml-adjustable pipettes. To facilitate collection of the mucus, which was sometimes thick, the pipette tips were cut 2–3 mm from the ends to create a larger collection orifice. Collection of the mucous liquid from the bronchi required ∼3 min. Independent assessment of the rate of evaporation of water from a mucous liquid sample exposed to room air for 6 h confirmed that this collection time had little effect on the sample volumes. The term “liquid” in this study is considered to represent water and all solutes dissolved or suspended in it, including physiological salts and biomolecules. This collection technique is expected to recover the free liquid and mucous gel in the airway lumen but little, if any, periciliary liquid. The liquid samples were placed in tared aluminum foil weigh boats, immediately weighed to determine their wet weight, and then dried overnight at 80°C. On the following day, the foil boats were weighed again to determine the sample dry weight. Liquid volume was taken from the wet weight of the samples (assuming 1 μl = 1 mg), nonvolatile solids mass was taken from the sample dry weight, and the volatile mass (assumed to be the water mass) was taken from the difference between the wet and dry weights. Mettler H20 (readability = 0.01 mg) and Mettler Toledo XS64 (readability = 0.1 mg) balances were used to weigh the samples. The inner airway surface area of each bronchus was estimated from tissue dimensions, as previously described (42). The rates of total liquid volume secretion were normalized to the surface area (cm2) and time (h). Inner surface areas of the pig bronchi were 2.61 ± 0.06 cm2 (n = 48, maximum = 3.92 cm2, minimum = 2.02 cm2).
Human bronchi.
All human lung tissues were obtained from explanted lungs removed from patients undergoing lung transplantation (Ochsner Clinic Foundation, New Orleans, LA), except for two tissues removed from potential transplant donors whose lungs were deemed unacceptable for transplantation (Life Alliance Organ Recovery Group, University of Miami, Miami, FL). Tissues from the Ochsner Clinic Foundation were collected immediately following surgery and transported by car to the University of South Alabama, where experiments were immediately initiated. The donor tissues were express-mailed overnight to the laboratory, and experiments were initiated immediately upon receipt of the tissue. Non-CF explanted lungs were obtained from two patients with idiopathic pulmonary fibrosis and one patient with emphysema. CF explanted lungs were taken from three ΔF508 (c.1520_1522delTCT) homozygous patients, one ΔF508–3849+10kb C>T (c.3717+12191C>T) compound-heterozygous patient, and one 394delTT (c.395_396delTT)-3905insT (c.3773_3774insT) compound-heterozygous patient. Procedures for procurement of human tissues were approved by the institutional review boards of the participating institutions.
The human bronchi were dissected from the lung tissues, and the secretion experiments were performed as described above for porcine bronchi, except liquid secretion was induced with ACh or forskolin. No anion secretion inhibitors were used with human bronchi. Inner surface areas of the human bronchi (2.01 ± 0.16 cm2, n = 27, maximum = 3.87 cm2, minimum = 0.73 cm2) were significantly smaller (P < 0.05) than those of the pig bronchi. Surface areas of the CF bronchi (1.79 ± 0.26 cm2, n = 13) tended to be smaller than those of the non-CF bronchi (2.21 ± 0.19 cm2, n = 14), but this difference was not statistically significant.
Solutions and drugs.
KRB physiological salt solution (in mM: 112 NaCl, 25 NaHCO3, 11.6 glucose, 4.7 KCl, 2.5 CaCl2, 2.4 MgSO4, and 1.2 KH2PO4) was used for all experiments. Solution pH was maintained at 7.4 by constant bubbling of solutions with 95% O2-5% CO2 gas.
Statistics and data analysis.
All statistical comparisons and linear regression analyses were made using Sigmastat statistical software (version 2.03) and SigmaPlot 2000 graphics software. Modeling calculations were performed using QuatroPro 12 spreadsheet software. Data are expressed as means ± SE. Student's t-test was used to compare parametric data. Nonparametric data were analyzed using the Mann-Whitney rank means test. P > 0.05 was considered the level of statistical significance.
RESULTS
Pig bronchi.
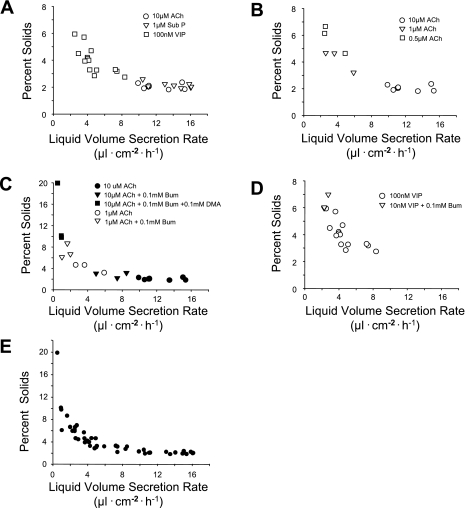
Figure 1A shows that relatively high rates of liquid volume secretion were induced by ACh and substance P (9.9–15.4 and 10.4–16.1 μl·cm−2·h−1, respectively), whereas VIP induced lower liquid volume secretion rates (2.9–8.3 μl·cm−2·h−1). A trend toward increased solids concentration (percent solids) with decreasing rates of liquid volume secretion is evident, particularly with VIP treatment. Figure 1B shows the effect of altering liquid volume secretion rate by treating tissues with variable concentrations of ACh. The liquid volume secretion rates were lower with 0.5–1.0 μM ACh than with 10 μM ACh. Similar to the responses shown in Fig. 1A, the percent solids was substantially higher in the liquid at lower (<6 μl·cm−2·h−1) volume secretion rates. In Fig. 1C, the effect of reducing liquid volume secretion rate with anion secretion inhibitors on percent solids is shown. Liquid volume secretion was induced with 1 or 10 μM ACh. The rate of liquid volume secretion was attenuated in some tissues with the combination of bumetanide, a Cl− secretion inhibitor that targets the basolateral membrane Na+-K+-2Cl− cotransporter in serous cells, and DMA, which blocks HCO3− secretion by inhibiting the Na+/H+ exchanger in the basolateral membrane of serous cells, or bumetanide alone. Again, the percent solids was highest in the liquid volume samples that were secreted at the lowest rates. The highest percent solids, 19.9%, was seen at 0.5 μl·cm−2·h−1. Figure 1D shows that reduction of VIP concentration and pretreatment with bumetanide also reduced liquid volume secretion rate and increased the percent solids. The aggregate data from Fig. 1, A–D, are shown in shown in Fig. 1E, where a consistent trend toward increased solids concentration at lower rates of liquid volume secretion is apparent. This negative relationship between solids concentration and liquid volume secretion rates is clearly curvilinear in this plot.
Fig. 1.
Effects of manipulating the rate of liquid volume secretion on percent solids content in pig bronchi. A: effects of 3 neurotransmitters [ACh, substance P (Sub P), and VIP] on liquid secretion rates. B: effects of 0.5, 1, and 10 μM ACh on liquid secretion rates. C: effects of anion transport inhibitors on ACh-induced liquid secretion rates. Bum, bumetanide, an inhibitor of transepithelial Cl− secretion; DMA, dimethylamiloride, an inhibitor of transepithelial HCO3− secretion. D: effect of reduced agonist concentration and anion transport inhibition on VIP-induced liquid secretion. E: summary effects of liquid volume secretion rate on percent solids content. Aggregate data from A–D are shown.
To better define the nature of the relationship between solids and liquid volume secretion, the total solids secretion rates in mass terms (mg·cm−2·h−1) were calculated from the dry weights of the samples and plotted against liquid volume secretion rates (μl·cm−2·h−1) for all samples. Figure 2A shows that the rate of total solids mass secretion fell with reduced liquid volume secretion rates, even though the concentration of solids in the liquid (Fig. 1E) was increased in these same samples. The solids mass secretion rates-liquid volume secretion rates relationship is linear throughout the range of measured values, as shown by the close fit of the regression line (Fig. 2A). This finding was unexpected, given the apparent nonlinear relationship between solids concentration and liquid volume secretion rates in Fig. 1E. Importantly, solids mass secretion rate appears to be directly related to the rate of liquid volume secretion, despite the broad range of manipulations used to alter liquid volume secretion rate. The rate of secretion of the evaporable component of the liquid volume secretion product, assumed to be water mass, was also determined. Water mass (mg) was taken as the difference between the wet weight and the dry weight of the samples. As expected, the rates of water secretion are highly correlated to liquid volume secretion rate (Fig. 2B), since the water mass in the large majority of samples was >90%. The positive linear relationship between solids mass and liquid volume secretion rate also holds when a constant secretagogue concentration (10 μM ACh) was used and the liquid volume secretion rate was altered with anion secretion inhibitors (Fig. 2C). This finding suggests that the liquid volume secretion rate, not the agonist concentration, has the greater influence on the rate of total solids mass secretion in pig bronchi.
Fig. 2.
Effects of liquid volume secretion rates on solids mass and water mass secretion rates in pig bronchi. A: secretion rates of solids mass (mg) plotted against liquid volume (μl) secretion rates. Relationship is linear, with slope = 0.013247, y-intercept = 0.107243, and correlation coefficient (r) = 0.917003. Slope of regression line is significantly different from zero (P < 0.05). B: secretion rates of water mass (mg) plotted against liquid secretion rates. Strongly linear relationship has slope = 0.986874, y-intercept = −0.109216, and r = 0.999980. Slope of regression line is significantly different from zero (P < 0.05). C: solids mass secretion rate is not a function of secretagogue concentration in pig bronchi. All bronchi were exposed to 10 μM ACh. Liquid secretion rate was varied by application of bumetanide and DMA to specifically inhibit Cl− and HCO3− secretion, respectively. Solids mass secretion rate was positively correlated to liquid volume secretion rate, despite a uniform concentration of ACh. Slope = 0.014109 and y-intercept = 0.083465, which closely match values obtained for aggregate data (A). Correlation coefficient (r) = 0.926874, and slope of data is significantly different (P < 0.05) from zero. D: model line prediction of percent solids from rates of liquid volume secretion in pig bronchi. Aggregate data points from Fig. 1E are shown. Model line is drawn from relationship between rates of solids mass and water mass secretion described by Eq. 5.
These data indicate that the concentration of solids (percent solids) in mucous liquid is a function of the two linear relationships shown in Fig. 2, A and B. From Fig. 2A, the linear equation for the relationship of total solids mass secretion rate (Js) to liquid volume secretion rate (Jv) is
| (1) |
From Fig. 2B, the linear equation for the relationship of water mass secretion rate (Jw) to Jv is
| (2) |
Since
| (3) |
the prediction of the percent nonvolatile solids (%solidspred) for any value of Jv will be given by
| (4) |
or
| (5) |
When Eq. 5 is used to construct a line defining the relationship between the percent solids and the Jv values, the model line fits the experimental data very well (Fig. 2D). Thus the negative curvilinear relationship between the percent solids and the rate of liquid volume secretion is caused by the disproportionately high solids mass secretion relative to water mass secretion (signified by the positive y-intercept) at relatively low rates of liquid volume secretion. If the regression lines for the solids mass and water mass secretion rates, as functions of the liquid volume secretion rate, passed through the origin, the percent solids would have been equal at all rates of liquid secretion.
Non-CF human bronchi.
As observed with porcine bronchi, the percent solids were also higher at low rates of liquid volume secretion in non-CF human bronchi (Fig. 3A). This relationship does not appear to be influenced by the disease state. Similar to pig bronchi, the relationships of solids mass and water mass secretion to liquid volume secretion in non-CF bronchi were also linear (Fig. 3, B and C). For non-CF human bronchi, the linear relationship between solids mass secretion and liquid volume secretion is
| (6) |
and the linear relationship between water mass secretion and liquid volume secretion is
| (7) |
Combining these two linear equations for the non-CF human data into a predictive model for percent solids and liquid volume secretion, as performed above for pig bronchi, we obtain
| (8) |
The model prediction line constructed from Eq. 8 is shown in Fig. 3D. This regression line fits the non-CF human data well and, similar to the pig bronchi responses, predicts very high percent solids at low liquid volume secretion rates.
Fig. 3.
Effects of liquid volume secretion rates on percent solids, solids mass secretion rates, and water mass secretion rates in non-cystic fibrosis (non-CF) human bronchi. A: different rates of liquid secretion were induced with 10 μM ACh or 10 μM forskolin. Bronchi were obtained from idiopathic pulmonary fibrosis patients (○, ●), emphysema patients (□, ■), and donors (▵, ▲). A curvilinear relationship similar to that observed in pig bronchi was observed with higher percent solids at lower rates of liquid secretion. B: secretion rates of solids mass (mg) plotted against liquid volume (μl) secretion rates. Relationship is linear, with slope = 0.029110, y-intercept = 0.051814, and r = 0.770612. Slope of regression line is significantly different from zero (P < 0.05). C: secretion rates of water mass (mg) plotted against liquid secretion rate. Strong linear relationship has slope = 0.972820, y-intercept = −0.056100, and r = 0.999763. Slope of regression line is significantly different from zero (P < 0.05). D: model prediction of percent solids at different liquid volume secretion rates for non-CF human bronchi. Aggregate data from A are shown. Model line is drawn from relationship between rates of solids mass and water mass secretion described by Eq. 8.
CF human bronchi.
CF human bronchi exhibited greater variability in the percent solids and liquid volume secretion rates than porcine or non-CF human bronchi, and the percent solids were higher in most samples throughout the range of liquid volume secretion (Fig. 4A). Nonetheless, CF bronchi, similar to pig and non-CF bronchi, produced the highest percent solids at low rates of liquid volume secretion. Unexpectedly, forskolin, a direct activator of adenylyl cyclase that is commonly used to identify CFTR-dependent anion secretion, induced appreciable levels of liquid volume secretion in some CF bronchi. The percent solids-liquid volume secretion rate relationship did not appear to be influenced by the CFTR mutation state (Fig. 4A). When the rates of solids mass and water mass secretion are expressed as functions of the liquid volume secretion rate, the relationships are linear (Fig. 4, B and C), as shown above for pig and non-CF human bronchi. The slope of the total solids mass secretion-liquid volume secretion relationship is notably greater than that for pig or non-CF human bronchi (Table 1). For CF human bronchi, the linear relationship between solids mass secretion and liquid volume secretion is
| (9) |
and the linear relationship between water mass secretion and liquid volume secretion is
| (10) |
The predictive model generated from these linear equations for the CF human airway responses is
| (11) |
The model prediction for the CF bronchi responses is shown in Fig. 4D. An increase in the percent solids is predicted at low rates of liquid secretion, as seen with pig and non-CF human bronchi responses.
Fig. 4.
Effects of liquid volume secretion rates on percent solids, solids mass secretion rates, and water mass secretion rates in cystic fibrosis (CF) human bronchi. A: different rates of liquid secretion were induced by treatment with 10 μM ACh or 10 μM forskolin. CFTR mutation status is as follows: ΔF508-ΔF508 (○, ●), ΔF508–3849+10kb C>T (□, ■), and 394delTT-3905insT (▵, ▲). Similar to pig and non-CF human responses, higher percent solids were observed at lower rates of liquid secretion. B: secretion rates of solids mass (mg) plotted against liquid volume (μl) secretion rates. Relationship is linear, with slope = 0.069290, y-intercept = 0.044457, and r = 0.82963. Slope of regression line is significantly different from zero (P < 0.05). C: secretion rates of water mass (mg) plotted against liquid secretion rate. Strong linear relationship has slope = 0.932030, y-intercept = −0.038932, and r = 0.99881. Slope of regression line is significantly different from zero (P < 0.05). D: model prediction of percent solids at different liquid volume secretion rates for CF human bronchi. Data from A are shown. Model line is drawn from relationship between rates of solids mass and water mass secretion described by Eq. 11.
Table 1.
Linear regression parameters from total solids mass secretion vs. liquid volume secretion
| Slope (Js/Jv) | y-Intercept, mg·cm−2·h−1 | |
|---|---|---|
| Pig | 0.013247 | +0.107243 |
| Non-CF human | 0.029110 | +0.051814 |
| CF human | 0.069290 | +0.044457 |
Model prediction plots for all three groups are shown together in Fig. 5. The model lines for pig and non-CF human bronchi are similar, although the maximum rates of liquid volume secretion are higher for the pig. The model for CF human bronchi, however, differs from the pig and non-CF human models, in that the CF human bronchi model predicts higher percent solids at higher liquid volume secretion rates. This effect is emphasized in the model predictions shown in Table 2, where model predictions for percent solids were calculated at specific points throughout the approximate range of observed liquid volume secretion rates for each tissue group. These values indicate that all three groups of tissues produce liquid with very high percent solids (>13%) at very low rates of liquid volume secretion (0.5 μl·cm−2·h−1). However, a divergence in the predicted values occurs at liquid volume secretion rates >2.0 μl·cm−2·h−1. In the CF bronchi, percent solids is predicted to be twice as high as in pig or non-CF bronchi at liquid volume secretion rates ≥4 μl·cm−2·h−1. This finding is consistent with the large slope of the solids mass-liquid volume secretion relationship in CF bronchi compared with pig (5.2-fold greater) or non-CF human (2.4-fold greater) bronchi (Table 1).
Fig. 5.
Comparison of relationship between percent solids and liquid volume secretion for pig, non-CF human, and CF human bronchi. Model lines generated for each group (Figs. 2D, 3D, and 4D) are plotted for comparison.
Table 2.
Model predictions of percent solids in mucous liquid at selected rates of liquid volume secretion
| %Solids |
|||
|---|---|---|---|
| Liquid Volume Secretion Rate, μl·cm−2·h−1 | Pig | Non-CF human | CF human |
| 0.5 | 22.7 | 13.6 | 15.6 |
| 1.0 | 12.1 | 8.1 | 11.3 |
| 2.0 | 6.7 | 5.5 | 9.1 |
| 4.0 | 4.0 | 4.2 | 8.0 |
| 6.0 | 3.1 | 3.8 | 7.7 |
| 10.0 | 2.4 | 3.4 | |
| 16.0 | 2.0 | ||
Liquid volume secretion rates were estimated from regression models described for each airway group. Liquid volume secretion rates represent the approximate ranges of liquid secretion observed for each group.
Contribution of biomolecules to mucous solids.
Mucous solids consist of inorganic physiological salts and organic biomolecules. We assumed that the total inorganic salt concentrations in all the mucous liquid samples were the same as in the KRB physiological salt solution bath liquid, since a prior study documented that the sum of the concentrations of Na+, Cl−, K+, and HCO3− in airway mucous liquid from porcine bronchi approximated that in KRB solution and did not change appreciably at secretion rates of 4–13 μl·cm−2·h−1 (42). The percent dry weight of the KRB physiological salt solution used in the present study was previously reported to be 1.25% (25). For each sample, the salts mass was calculated on the basis of this value and subtracted from the total mucous solids mass to obtain an estimate of the biomolecular mass. The biomolecular mass secretion rate (Jbiomol) was then expressed as a function of liquid volume secretion for all three groups of airways. Figure 6A shows that the rate of biomolecular mass secretion in pig bronchi is relatively constant at all rates of liquid volume secretion. This finding suggests that the fraction of secreted solids that are correlated to liquid volume secretion is entirely composed of the physiological salts, the active and passive secretion of which creates the osmotic gradient to drive water secretion. As shown in Fig. 6, B and C, subtracting the physiological salts from the total solids did not abolish the correlation of the solids mass flux with liquid volume secretion in the non-CF or CF human bronchi. Indeed, the slopes of the Jbiomol-Jv relationship for the non-CF and CF human bronchi (Table 3) were reduced only by the magnitude of the total induced change from the Js-Jv to the Jbiomol-Jv relationship for the pigs (Table 1). The y-intercepts for all three groups were essentially unchanged by the conversion of Js to Jbiomol. When all CF and non-CF human Jbiomol values were compared, no significant difference was observed. However, in samples where Jv was >2 μl·cm−2·h−1, Jbiomol was significantly greater (P < 0.05) in the CF than non-CF bronchi. These findings make several points. 1) The pig bronchi secrete a relatively constant level of biomolecular mass in response to all three secretagogues that is unrelated to the rate of liquid volume secretion and accounts for the high solids at the low rates of liquid volume secretion. 2) A substantial fraction of the rate of biomolecular mass secretion for both human airway groups is indeed correlated to the rate of liquid volume secretion. 3) The ratio of biomolecular solids secretion to liquid volume secretion was substantially higher (3.8 times) in the CF than non-CF bronchi (Table 3).
Fig. 6.
Effects of liquid volume secretion rates on biomolecular solids mass secretion rates in pig (A), non-CF human (B), and CF human (C) bronchi. For each sample, extracellular salts mass was subtracted from total solids mass to estimate biomolecular solids mass. A: slope of biomolecular solids mass secretion rate-liquid secretion rate relationship was not significantly different from zero, with r = 0.128326. B: slope of biomolecular solids mass secretion rate-liquid secretion rate relationship was significantly different from zero (P < 0.05), with r = 0.569590. C: slope of biomolecular solids mass secretion rate-liquid secretion rate relationship was significantly different from zero (P < 0.05), with r = 0.772658. Slopes and y-intercepts for the 3 groups of tissues are shown in Table 3.
Table 3.
Linear regression parameters from biomolecular solids mass secretion vs. liquid secretion
| Slope (Jbiomol/Jv) | y-Intercept, mg·cm−2·h−1 | |
|---|---|---|
| Pig | 0.000746 | +0.107260 |
| Non-CF human | 0.014680 | +0.056101 |
| CF human | 0.055568 | +0.038517 |
Biomolecular solids mass secretion (Jbiomol) values were determined by subtraction of estimated extracellular salts mass from total mucous solids mass. Regression parameters are from data plotted in Fig. 6.
DISCUSSION
Several important findings are reported in this study. 1) We observed that the percent solids in mucous liquid is negatively related to the liquid volume secretion rate in a curvilinear manner for all three groups of airways: normal pig, non-CF human, and CF human. That is, airways from all three groups secreted a very high-percent-solids mucus at low rates of liquid volume secretion. 2) We determined that the secretion of total solids mass by bronchial airways is directly related to the rate of liquid volume secretion, not to the concentration of the secretagogue. Coincident salts mass secretion accounts fully for this correlated mass secretion observed from pig bronchi, but not from non-CF human or CF human bronchi. 3) We found that CF bronchi secrete a high-percent-solids liquid, not only at low rates of liquid volume secretion, but also, on average, at higher rates of liquid volume secretion (>2 μl·cm−2·h−1) compared with pig or non-CF human airways. This behavior of CF airways appears to be due to a higher rate of biomolecule secretion in these tissues than in tissues from pig or non-CF human airways.
The curvilinear relationship between the percent solids and the rate of liquid volume secretion was determined to be the consequence of two distinct linear processes, solids mass secretion and water mass secretion, as functions of the rate of liquid volume secretion. For all three groups of tissues, the y-intercept of the slope of the solids mass secretion-liquid volume secretion relationship is positive relative to the water mass secretion-liquid volume secretion relationship. Thus, as the rate of liquid volume secretion decreases, the concentration of solids (i.e., percent solids) in the mucous liquid increases, because the fractional solids mass increases in proportion to the fractional water mass. This effect becomes pronounced at very low rates of liquid volume secretion. Indeed, the models predict that the percent solids in the secreted liquid approaches 100% as the rate of liquid volume secretion approaches zero. This limit is unlikely to be reached in a physiological system. There is certainly some very low level of liquid volume secretion at which secretion of solids mass ceases, but this end point is not definable from these data.
Our data indicate that pig and human bronchi, even CF human bronchi, exhibit this property of producing a very high-percent-solids mucus at low rates of liquid volume secretion. Importantly, these findings indicate that even healthy airways have the potential to produce mucous liquid that is as thick as that measured in the airways of CF patients. Why then do normal airways not become obstructed by this high-solids mucous liquid when the rate of liquid secretion is low, the condition that likely dominates in the undiseased, unstimulated lung of healthy persons? We speculate that submucosal glands of the airways secrete liquid in response to an appropriate transient stimulus, such as local irritation and/or inflammation. The ability of submucosal glands in normal airways to cease liquid volume secretion entirely or to periodically secrete a large-volume, low-percent-solids liquid when needed probably protects against accumulation of the thick mucus within the airway lumen. Normal regulation of the airway surface liquid volume, particularly the depth of the periciliary liquid, by anion secretion and Na+ absorption across the airway surface epithelium may be capable of maintaining mucociliary transport and clearance of even very high-percent-solids mucous liquid as long as the volumes are relatively small. Because of the loss of CFTR activity in CF airways, the capacity for anion secretion by the surface epithelium of these tissues is likely to be fractionally reduced (40), and mucociliary transport capacity is probably compromised to some degree by this reduced capability to augment periciliary liquid depth (4, 39). Thus CF airways are expected to have greater difficulty clearing a high-percent-solids mucus load than non-CF airways. A persistent liquid volume secretion stimulus in a CF airway, such as a local infection, would probably exacerbate the clearance defect, as much larger volumes of high-percent-solids liquid would be produced from the glands. This notion is consistent with initial observations from CF pig airways, where densely staining mucous plaques are localized in early disease to focal areas, rather than all airway regions (38). In non-CF human airways or normal pig airways, the same inflammatory stimulus would likely result in a larger volume of much thinner, lower-solids liquid that would facilitate, rather than impede, mucociliary clearance.
Our findings indicate that pig bronchi produce the biomolecular mass component of mucous solids at a relatively consistent level over a wide range of liquid secretion rates and secretagogue concentrations (Fig. 6A). This observation is seemingly at odds with the well-known actions of muscarinic agonists as efficacious inducers of mucous liquid secretion from glands. However, Dwyer and co-workers (13), using freshly isolated submucosal gland cells from swine tracheas, determined that maximum glycoconjugate secretion was induced with only 30 nM ACh. The lowest concentration of ACh used in the present study was 500 nM; thus glycoconjugate secretion would be expected to be at its peak rate in all tissues treated with ACh. On the basis of previous studies with nonpulmonary tissues (27, 43), mucous glycoprotein secretion should also be near maximal rates with the concentrations of substance P and VIP used in the present study. Consequently, the rate of biomolecular solids secretion should be relatively constant at all rates of liquid volume secretion, as seen in Fig. 6A. Indeed, the mucous liquid volume secretion rates in pig bronchi vary only by the quantities of physiological salts and water that are added to the biomolecular component, which is secreted at a constant rate. On the basis of findings from previous studies (3), we expect that all three of these agonists induce macromolecular secretion principally from submucosal glands. The goblet cells in the surface epithelium are poorly innervated and express few neurotransmitter receptors, suggesting that they would minimally contribute to macromolecular secretion induced by these agents (10). However, secondary release of ATP and/or UTP from surrounding tissues could induce mucin release from goblet cells.
Unlike pig airways, human bronchi secrete a fraction of biomolecular solids that positively correlates to liquid volume secretion (Fig. 6, B and C). Species differences in the sensitivity of biomolecule secretion processes to the secretagogues could account for the variation in responses between pigs and humans. Alternatively, the diseased human airways, particularly the CF tissues, are expected to exhibit goblet cell metaplasia in the surface epithelium and hyperplasia of mucous and serous cells of the submucosal glands (36), and such remodeling could increase the capacity of these airways for secreting macromolecular mass. Additionally, chronic inflammation in the CF airways likely induces a hyperstimulated state that could result in a downregulation of neurotransmitter receptor expression and a shift in the EC50 for the secretagogues to higher levels. We do not know which biomolecules account for the higher solids mass in the CF mucous liquid. Mucous plugs that form in airway lumina of CF airways stain avidly with antibodies for MUC5AC and MUC5B (7), suggesting that goblet and glandular mucous cells contribute to this mass; however, because degradation products of MUC5B and MUC5AC are present in CF mucus, it is difficult to conclude which mucin is the dominant secreted macromolecular species (9). The observation that the viscosity of pilocarpine-induced mucous liquid at the duct openings of CF airway submucosal glands is elevated approximately threefold compared with mucous liquid from normal human glands (34) suggests that an important fraction of the high-solids mucus in CF mucus is secreted from the submucosal glands, which should be dominated by MUC5B. It should be considered that the biomolecular solids in CF mucus are not simply due to increased secretion of gel-forming mucins. Sputum induced from healthy persons contains >100 different biomolecules, with the gel-forming mucins only accounting for 20–30% of the total mass (20). Thus non-gel-forming proteins, perhaps secreted by gland serous cells, could contribute substantially to the high-biomolecular-solids mucus in CF. While the ratio of biomolecular mass to liquid volume secretion was lower in non-CF human than CF human bronchi, it was nonetheless higher than that in normal pig bronchi (Table 3). We expect that this was also due to an elevated level of biomolecule secretion from these diseased tissues. Indeed, increased sputum production and goblet cell hyperplasia are characteristic of chronic obstructive pulmonary disease (32), and cystic air spaces in the lungs of pulmonary fibrosis patients are frequently filled with mucin (21).
The relationship we observed between percent solids and the rate of liquid volume secretion in the present study may have significant relevance to another important airway disease, status asthmaticus, a severe intractable asthma, where the central and peripheral airways become obstructed with mucus (15, 44). The etiology of this complication of asthma is poorly understood. Typically, asthmatic patients are treated with inhaled β2-adrenergic agonists to dilate airway smooth muscle and reduce airflow obstruction. Severe exacerbations in some status asthmaticus patients have been linked to the overuse of β2-adrenergic agonists and, particularly, the use of long-acting β2-agonists (41). Feline airway submucosal glands, when treated with the β-agonist isoprenaline (isoproterenol), have been shown to induce liquid secretion at rates that are only ∼10% of that induced by the muscarinic agonist bethanechol (30). In the present study, one-tenth of the ACh-induced rate of liquid secretion by human bronchi corresponds to ∼8–10% solids, which is comparable to that previously reported for CF sputum (8) and severe asthma (11). Therefore, it is plausible that sustained, low rates of glandular liquid secretion induced by β2-agonists are sufficient to produce thick airway mucus that, over time, could occlude the lumen of the airways. Indeed, MUC5B, which is secreted principally from mucous cells of the submucosal glands, was found to account for >95% of the total mucin fraction from a mucous plug obstructing the airways of a status asthmaticus patient who died from this disorder (35). This pathogenic process could be further exacerbated by the coadministration of cholinergic receptor antagonists, which are commonly prescribed along with β2-agonists to maximally dilate airway smooth muscle in asthmatic patients (44). These anticholinergic agents likely promote the production of thick mucus by blocking the normal muscarinic secretion pathway that is capable of inducing the high-volume, low-solids liquid secretion from submucosal glands that would augment clearance. In this way, the glandular airways in these patients could become locked into a state of high-solids mucus secretion, a condition that outwardly resembles the mucous obstruction that occurs in CF airways.
The surface epithelium of the bronchial and tracheal airways of most mammals studied actively absorbs Na+ (5), a process that provides the driving force for liquid absorption and regulates airway surface liquid volume (39). The rate of active Na+ absorption is greater across CF nasal epithelium than non-CF tissues (6). This greater absorptive force across the surface epithelium of CF airways is thought to create the high-percent-solids mucus that typifies this disease (12). Because the mucous liquid collected in the present study represents a “net” secretion product, it is plausible that the higher ratio of solids mass secretion to liquid volume secretion in the CF airways could have been due to reduced liquid secretion or increased liquid absorption. That is, the higher-percent-solids mucus from CF airways could have been generated by reduced liquid secretion or increased liquid absorption in the face of uniform solids secretion. Indeed, if 73.5% of the liquid volume that was produced by the non-CF human bronchi was reabsorbed across the surface epithelium without changing the solids secretion rate, the slope of the Jbiomol-Jv relationship would approximate that observed for the CF human bronchi. On the other hand, a reduction in liquid volume secretion rate of approximately the same magnitude would also produce a comparable concentration increase in the slope of the Jbiomol-Jv relationship. Two observations argue against a significant role of Na+ hyperabsorption in these results. 1) We found that the biomolecular solids secretion rates were significantly higher in the CF than the non-CF airways at liquid volume secretion rates >2 μl·cm−2·h−1, which favors the hypersecretion of biomolecular solids hypothesis. 2) Uniform hyperabsorption of salt and water from all samples from the CF bronchi should have caused a uniform leftward shift in the Js-Jv curves, which would have raised the y-intercept relative to that of the non-CF bronchi. However, the y-intercepts of these relationships from the CF and non-CF bronchi are very similar (Table 1). On the other hand, it is possible that higher rates of salt and water absorption occur in CF airways but that the time required to reabsorb sufficient salt and water from the surface liquid to generate a high-percent-solids mucus is well beyond the 2-h time frame of the present study. Resolution of this issue requires additional study.
We observed that several CF airways secreted relatively high rates of liquid volume (5–7 μl·cm−2·h−1) when treated with forskolin, an activator of adenylyl cyclase. Since CFTR is activated by cAMP-elevating agonists (2), this secretagogue is often used to distinguish between CFTR-dependent and -independent anion secretion. However, at least two prior studies indicate that a substantial transepithelial anion secretion response can be generated in CF respiratory epithelia by application of forskolin. Sustained transepithelial Cl− secretion requires activation of 1) apical membrane anion channels, which provide the anion efflux pathway from the cells' interior, and 2) basolateral membrane K+ channels, which raise the electrical driving force for anion efflux by hyperpolarizing the cells. MacVinish et al. (24) showed that forskolin stimulated anion secretion in nasal epithelia of CF and wild-type mice. They attributed this response to forskolin-induced elevation of intracellular Ca2+, which activated basolateral membrane K+ channels, and the relatively high resting activity of murine apical membrane Ca2+-activated Cl− channels, which provides an apical membrane efflux pathway for anions, despite loss of functional CFTR. Lee and Foskett (22) also demonstrated that forskolin elevates intracellular Ca2+ concentrations in gland serous cells from humans and pigs. They showed that forskolin alone induced increases in intracellular Ca2+ concentrations sufficient to activate basolateral K+, but not apical Ca2+-activated Cl−, channels. However, forskolin importantly potentiated the anion secretion responses of other Ca2+-elevating secretagogues sufficiently to induce secretion responses, even in CF pig and CF human serous cells. In the present study, we used freshly dissected, intact airways obtained from explanted lungs of patients with end-stage CF lung disease. Numerous autacoids and inflammatory substances, including substance P (17), bradykinin (29), neurokinin A (28), and ATP (37), would be expected to be present at some level in the interstitium of these inflamed tissues. All these agents are glandular secretagogues that elevate intracellular Ca2+. We speculate that forskolin could have potentiated the background secretion responses induced by these agonists.
In summary, we observed that the percent solids present in airway liquid was negatively related to the liquid volume secretion rate and that high percent solids, comparable to that seen in asthma and CF, was induced in normal airways when the secretion rates were low. Additionally, we found that bronchi from CF explanted lungs, on average, produced mucous liquid with higher percent solids than pig or non-CF human bronchi at higher rates of liquid volume secretion. Our observations have important relevance not just to CF airway disease, but perhaps to intractable asthma as well.
GRANTS
This work was funded by the National Institutes of Health Grant HL-063302.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Drs. James M. Downey and Mary I. Townsley for helpful comments and discussions and the Ochsner Clinic Foundation (New Orleans, LA) and the Life Alliance Organ Recovery group at the University of Miami (Miami, FL) for procurement of human lung tissues.
Present address of C. J. Martens: University of Washington School of Medicine, 1959 NE Pacific St., Seattle, WA 98195.
REFERENCES
- 1. Aikawa T, Shimura S, Sasaki H, Ebina M, Takishima T. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest 101: 916–921, 1992 [DOI] [PubMed] [Google Scholar]
- 2. Anderson MP, Gregory RJ, Thompson S, Sousa DW, Paul S, Mulligan RC, Smith AE, Welsh MJ. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science 253: 202–205, 1991 [DOI] [PubMed] [Google Scholar]
- 3. Ballard ST, Inglis SK. Liquid secretion properties of airway submucosal glands. J Physiol 556: 1–10, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ballard ST, Trout L, Mehta A, Inglis SK. Liquid secretion inhibitors reduce mucociliary transport in glandular airways. Am J Physiol Lung Cell Mol Physiol 283: L329–L335, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Boucher RC, Narvarte J, Cotton C, Stutts MJ, Knowles MR, Finn AL, Gatzy JT. Sodium absorption in mammalian airways. In: Fluid and Electrolyte Abnormalities in Exocrine Glands in Cystic Fibrosis, edited by Quinton PM, Martinez JR, Hopfer U. San Francisco, CA: San Francisco Press, 1982, p. 271–287 [Google Scholar]
- 6. Boucher RC, Stutts MJ, Knowles MR, Cantley L, Gatzy JT. Na+ transport in cystic fibrosis respiratory epithelia. Abnormal basal rate and response to adenylate cyclase activation. J Clin Invest 78: 1245–1252, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burgel PR, Montanni D, Danel C, Dusser DJ, Nadel JA. A morphometric study of mucins and small airway plugging in cystic fibrosis. Thorax 62: 153–161, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chernick WS, Barbero GJ. Composition of tracheobronchial secretions in cystic fibrosis of the pancreas and bronchiectasis. Pediatrics 24: 739–745, 1959 [PubMed] [Google Scholar]
- 9. Davies JR, Svitacheva N, Lannefors L, Kornfalt R, Carlstedt I. Identification of MUC5B, MUC5AC and small amounts of MUC2 mucins in cystic fibrosis airway secretions. Biochem J 344: 321–330, 1999 [PMC free article] [PubMed] [Google Scholar]
- 10. Davis CW, Dickey BF. Regulated airway goblet cell mucin secretion. Annu Rev Physiol 70: 487–512, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Daviskas E, Anderson SD, Young IH. Inhaled mannitol changes the sputum properties in asthmatics with mucus hypersecretion. Respirology 12: 683–691, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Donaldson SH, Boucher RC. Update on pathogenesis of cystic fibrosis lung disease. Curr Opin Pulmon Med 9: 486–491, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Dwyer TM, Szebeni M, Diveki K, Farley JM. Transient cholinergic glycoconjugate secretion from swine trachea submucosal gland cells. Am J Physiol Lung Cell Mol Physiol 262: L418–L426, 1992 [DOI] [PubMed] [Google Scholar]
- 14. Engelhardt JF, Yankaskas JR, Ernst ST, Yang Y, Marino CR, Boucher RC, Cohn JA, Wilson JM. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat Genet 2: 240–248, 1992 [DOI] [PubMed] [Google Scholar]
- 15. Groneberg DA, Eynott PR, Lim S, Oates T, Wu R, Carlstedt I, Roberts P, McCann B, Nicholson AG, Harrison BD, Chung KF. Expression of respiratory mucins in fatal status asthmaticus and mild asthma. Histopathology 40: 367–373, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Inglis SK, Corboz MR, Taylor AE, Ballard ST. Effect of anion transport inhibition on mucus secretion by airway submucosal glands. Am J Physiol Lung Cell Mol Physiol 272: L372–L377, 1997 [DOI] [PubMed] [Google Scholar]
- 17. Ishihara H, Shimura S, Sato M, Masuda T, Ishide N, Miura M, Sasaki T, Sasaki H, Takishima T. Intracellular calcium concentration of acinar cells in feline tracheal submucosal glands. Am J Physiol Lung Cell Mol Physiol 259: L345–L350, 1990 [DOI] [PubMed] [Google Scholar]
- 18. Jayaraman S, Joo NS, Reitz B, Wine JJ, Verkman AS. Submucosal gland secretions in airways from cystic fibrosis patients have normal [Na+] and pH but elevated viscosity. Proc Natl Acad Sci USA 98: 8119–8123, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Joo NS, Irokawa T, Wu JV, Robbins RC, Whyte RI, Wine JJ. Absent secretion to vasoactive intestinal peptide in cystic fibrosis airway glands. J Biol Chem 277: 50710–50715, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Kesimer M, Kirkham S, Pickles RJ, Henderson AG, Alexis NE, DeMaria G, Knight D, Thornton DJ, Sheehan JK. Tracheobronchial air-liquid interface cell culture: a model for innate mucosal defense of the upper airways? Am J Physiol Lung Cell Mol Physiol 296: L92–L100, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. King TE. Interstitial lung diseases. In: Harrison's Principles of Internal Medicine (17th ed.), edited by Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL. New York: McGraw Hill Medical, 2008, vol. 2, p. 1643–1651 [Google Scholar]
- 22. Lee RJ, Foskett JK. Requirement for cAMP-activated Ca2+ signaling for CFTR-mediated porcine and human airway submucosal gland serous cell fluid secretion. J Clin Invest 120: 3137–3148, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lethem MI, James SL, Marriott C. The role of mucous glycoproteins in the rheologic properties of cystic fibrosis sputum. Am Rev Respir Dis 142: 1053–1058, 1990 [DOI] [PubMed] [Google Scholar]
- 24. MacVinish LJ, Hickman ME, Mufti DAH, Durrington HJ, Cuthbert AW. Importance of basolateral K+ conductance in maintaining Cl− secretion in murine nasal and colonic epithelia. J Physiol 510: 237–247, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martens CJ, Ballard ST. Effects of secretagogues on net and unidirectional liquid fluxes across porcine bronchial airways. Am J Physiol Lung Cell Mol Physiol 298: L270–L276, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matthews LW, Spector S, Lemm J, Potter JL. Studies on pulmonary secretions. I. The over-all chemical composition of pulmonary secretions from patients with cystic fibrosis, bronchiectasis, and laryngectomy. Am Rev Respir Dis 88: 199–204, 1963 [DOI] [PubMed] [Google Scholar]
- 27. Mullol J, Rieves RD, Baranhiuk JN, Lundgren JD, Merida M, Hausfeld JH, Shelhamer JH, Kaliner MA. The effects of neuropeptides on mucous glycoprotein secretion from human nasal mucosa in vitro. Neuropeptides 21: 231–238, 1992 [DOI] [PubMed] [Google Scholar]
- 28. Nagaki M, Ishihara H, Shimura S, Sasaki T, Takishima T, Shirato K. Tachykinins induce a [Ca2+]i rise in the acinar cells of feline tracheal submucosal gland. Respir Physiol 98: 111–120, 1994 [DOI] [PubMed] [Google Scholar]
- 29. Nagaki M, Shimura S, Irokawa T, Sasaki T, Oshiro T, Nara M, Kakuta Y, Shirato K. Bradykinin regulation of airway submucosal gland secretion: role of bradykinin receptor subtype. Am J Physiol Lung Cell Mol Physiol 270: L907–L913, 1996 [DOI] [PubMed] [Google Scholar]
- 30. Quinton PM. Composition and control of secretions from tracheal bronchial submucosal glands. Nature 279: 551–552, 1979 [DOI] [PubMed] [Google Scholar]
- 31. Regnis JA, Robinson M, Bailey DL, Cook P, Hooper P, Chan HK, Gonda I, Bautovich G, Bye PT. Mucociliary clearance in patients with cystic fibrosis and in normal subjects. Am J Respir Crit Care Med 150: 66–71, 1995 [DOI] [PubMed] [Google Scholar]
- 32. Reilly JJ, Silverman EK, Shapiro SD. Chronic obstructive pulmonary disease. In: Harrison's Principles of Internal Medicine (17th ed.), edited by Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL. New York: McGraw Hill Medical, 2008, vol. 2, p. 1635–1643 [Google Scholar]
- 33. Riordan JR, Rommens JM, Kerem BS, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, Drumm ML, Iannuzzi MC, Collins FS, Tsui LC. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245: 1066–1073, 1989 [DOI] [PubMed] [Google Scholar]
- 34. Salinas D, Haggie PM, Thiagarajah JR, Song Y, Rosbe K, Finkbeiner WE, Nielson DW, Verkman AS. Submucosal gland dysfunction as a primary defect in cystic fibrosis. FASEB J 19: 431–433, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Sheehan JK, Howard M, Richardson PS, Longwill T, Thornton DJ. Physical characterization of a low-charge glycoform of the MUC5B mucin comprising the gel-phase of an asthmatic respiratory mucous plug. Biochem J 338: 507–513, 1999 [PMC free article] [PubMed] [Google Scholar]
- 36. Sheppard MN. The pathology of cystic fibrosis. In: Cystic Fibrosis, edited by Hodson ME, Geddes DM. London, UK: Chapman & Hall, 1995, p. 131–149 [Google Scholar]
- 37. Shimura S, Sasaki T, Nagaki M, Takishima T, Shirato K. Extracellular ATP regulation of feline tracheal submucosal gland secretion. Am J Physiol Lung Cell Mol Physiol 267: L159–L164, 1994 [DOI] [PubMed] [Google Scholar]
- 38. Stoltz DA, Meyerholz DK, Pezzulo AA, Ramachandran S, Rogan MP, Davis GJ, Hanfland RA, Wohlford-Lenane C, Dohrn CL, Bartlett JA, Nelson GA, Chang EH, Taft PJ, Ludwig PS, Estin M, Hornick EE, Launspach JL, Samuel M, Rokhlina T, Karp PH, Ostedgaard LS, Uc A, Starner TD, Horswill AR, Brogden KA, Prather RS, Richter SS, Shilyansky J, McCray PB, Zabner J, Welsh MJ. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med 2: ra31, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tarran R. Regulation of airway surface liquid volume and mucus transport by active ion transport. Proc Am Thorac Soc 1: 42–46, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Tarran R, Trout L, Donaldson SH, Boucher RC. Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis superficial airway epithelia. J Gen Physiol 127: 591–560, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Taylor RD. The β-agonist saga and its clinical relevance: on and on it goes. Am J Respir Crit Care Med 179: 976–978, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Trout L, Gatzy JT, Ballard ST. Contribution of chloride and bicarbonate transport to acetylcholine-induced liquid secretion in porcine bronchial epithelium. Am J Physiol Lung Cell Mol Physiol 275: L1095–L1099, 1998 [DOI] [PubMed] [Google Scholar]
- 43. Turner JT, Camden JM. The influence of vasoactive intestinal peptide receptors in dispersed acini from rat submandibular gland on cyclic AMP production and mucin release. Arch Oral Biol 35: 103–108, 1990 [DOI] [PubMed] [Google Scholar]
- 44. Werner HA. Status asthmaticus in children. Chest 119: 1913–1929, 2001 [DOI] [PubMed] [Google Scholar]
- 45. Yeates DB, Sturgess JM, Kahn SR, Levison H, Aspin N. Mucociliary transport in trachea of patients with cystic fibrosis. Arch Dis Child 51: 28–33, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]