Abstract
Idiopathic pulmonary fibrosis is a devastating disease characterized by a progressive, irreversible, and ultimately lethal form of lung fibrosis. Except for lung transplantation, no effective treatment options currently exist. The bleomycin animal model is one of the best studied models of lung injury and fibrosis. A previous study using mouse tumor models observed that liposome-encapsulated bleomycin exhibited reduced lung toxicity. Therefore, we hypothesized that airway delivery of synthetic phosphatidylcholine-containing liposomes alone would protect mice from bleomycin-induced lung toxicity. C57BL/6 mice were administered uncharged multilamellar liposomes (100 μl) or PBS vehicle on day 0 by airway delivery. Bleomycin (3.33 U/kg) or saline vehicle was then given intratracheally on day 1 followed by four additional separate doses of liposomes on days 4, 8, 12, and 16. Fluorescent images of liposomes labeled with 1,1′-dioctadecyl-3,3,3′,3′ tetramethylindocarbocyanine perchlorate confirmed effective and widespread delivery of liposomes to the lower respiratory tract as well as uptake primarily by alveolar macrophages and to a lesser extent by type II alveolar epithelial cells. Results at day 22, 3 wk after bleomycin treatment, showed that airway delivery of liposomes before and after intratracheal administration of bleomycin significantly reduced bleomycin-induced lung toxicity as evidenced by less body weight loss, chronic lung inflammation, and fibrosis as well as improved lung compliance compared with controls. These data indicate that airway-delivered synthetic liposomes represent a novel treatment strategy to reduce the lung toxicity associated with bleomycin in a mouse model.
Keywords: lung injury, pulmonary fibrosis
bleomycin is a chemotherapeutic agent used to treat a variety of cancers including lymphomas, squamous cell carcinomas, and germ cell tumors (21). A major side effect of bleomycin therapy is pulmonary toxicity, which occurs in 5–10% of patients with a progression to pulmonary fibrosis in 1% of cases (21). Pulmonary fibrosis is characterized by the deposition of collagen and extracellular matrix within lung parenchymal tissue. This fibrotic response decreases lung elasticity and impairs gas exchange. Bleomycin-induced lung toxicity is also one of the best studied animal models of lung injury and repair in human subjects including acute respiratory distress syndrome (ARDS) and idiopathic pulmonary fibrosis (IPF) (21, 22). Multiple interventions have proven effective in controlling bleomycin-induced pulmonary fibrosis in animal models (21); however, virtually none have been successfully adapted for use as a safe and effective therapy in either ARDS or IPF (3, 11, 26, 44).
Lung surfactant consists of a mixture of phospholipids, a major component being dipalmitoylphosphatidylcholine (DPPC), and proteins and is critical in the reduction of lung surface tension to prevent alveolar collapse during expiration (5, 28). Surfactant protein (SP)-A and -D are also critical components of innate pulmonary defense (24, 43). Lung surfactant deficiency and/or dysfunction have been shown to exist in a number of pulmonary diseases such as neonatal respiratory distress syndrome (RDS) (1, 8, 14, 37), acute lung injury from drugs or toxins (2, 13, 16, 17, 20, 23, 30, 32, 36, 40), ARDS (3, 10, 11, 26, 27, 38, 44), and IPF (12, 29). Lung surfactant therapy using protein-free natural animal-derived lung surfactant extracts or synthetic surfactants is a mainstay of current therapy for RDS in neonates (1, 8, 14) but has met with minimal success in the treatment of adult acute lung injury/ARDS (3, 11, 26, 44).
Liposomes are synthetic lipid vesicles consisting of concentric phospholipid bilayers, often a major constituent being phosphatidylcholine, and cholesterol that have been used in a number of biological applications including protein and drug delivery (31, 39). For instance, airway delivery of superoxide dismutase covalently bound to phosphatidylcholine-derived molecules reduces bleomycin-induced lung inflammation and fibrosis in mice (34). Intraperitoneal (IP) administration of bleomycin encapsulated with phosphatidylcholine-containing liposomes exhibits increased antitumor activity in mice compared with free bleomycin but interestingly with the additional benefit of decreased lung toxicity as measured by lung hydroxyproline content (4). To test the hypothesis that airway delivery of phosphatidylcholine-containing synthetic liposomes alone would reduce bleomycin-induced lung injury, we airway delivered liposomes to mice with bleomycin-induced lung toxicity. Our findings indicated that airway delivery of liposomes before and after intratracheal (IT) administration of bleomycin significantly reduced bleomycin-induced lung toxicity as evidenced by less weight loss, chronic lung inflammation, and fibrosis as well as improved lung compliance. These data indicate that airway-delivered synthetic liposomes represent a novel treatment and delivery strategy applicable to a mouse model of bleomycin-induced lung toxicity.
MATERIALS AND METHODS
Animals.
Eight- to 10-wk-old female C57BL/6 mice (Charles River Laboratories, Raleigh, NC) were housed under pathogen-free conditions and provided food and water ad libitum. Procedures for the use and care of animals were approved by the National Institute of Environmental Health Sciences (NIEHS) Institutional Animal Care and Use Committee.
Reagents.
Bleomycin sulfate from Streptomyces verticillus was obtained from Calbiochem (Gibbstown, NJ). Uncharged multilamellar liposomes were purchased from Encapsula NanoSciences (Nashville, TN). The concentration of l-α-phosphatidylcholine (lot no. SPC95-162, Avanti Polar Lipids, Alabaster, AL) and cholesterol in the final liposome solution were 24.8 and 10.8 mM, respectively. Light scattering showed that the majority of the liposomes were 1–2 μm in size.
DiI labeling of liposomes.
As previously described by our laboratory for labeling cells prior to airway delivery (25), 2 μl of DiI (1,1′-dioctadecyl-3,3,3′,3′ tetramethylindocarbocyanine perchlorate) (Vybrant DiI cell-labeling solution, Invitrogen, Eugene, OR) was mixed with 0.5 ml of liposome suspension for 30 min at 4°C and then washed twice with 5 ml phosphate-buffered saline (PBS, without Ca and Mg) with centrifugation at 130 g for 8 min. The final pellet was diluted with 0.5 ml PBS and the liposomes were confirmed as DiI labeled by fluorescence microscopy using an Axioplan 2 fluorescence microscope equipped with an AxioCam MRC digital color camera (Carl Zeiss, Oberkochen, Germany).
Airway delivery of liposomes or bleomycin.
Mice were administered liposomes or PBS only on days 0, 4, 8, 12, and 16 by oropharyngeal aspiration. Briefly, mice were anesthetized with 3% isoflurane and then held on an incline plane by the front incisors. While the tongue was gently pulled back with forceps to prevent the swallowing reflex, 100 μl of liposomes or PBS was administered into the oral cavity. The nostrils were then occluded, with the tongue still held back, until the mouse inhaled. Mice were administered bleomycin (3.33 U/kg or 0.06 U/18 g in ∼40 μl volume) or saline vehicle IT on day 1. Briefly, mice were anesthetized with 3% isoflurane, injected IP with the analgesic Buprenex (0.1 mg/kg) and then shaved around the neck area. Iodine and 70% alcohol were applied to the shaved neck region and a small incision was made to expose the trachea. The mice were then held on an incline plane by the front incisors. A ball-tip gavage needle used as a cannula was inserted into the trachea via the oral cavity, which was confirmed by viewing the ball tip within the trachea through the opened incision, and bleomycin or saline was pipetted into the cannula. An empty syringe was then used to flush air into the cannula to complete delivery of the bleomycin solution into the airways and lung tissue. The cannula was removed and the skin incision was resealed using Tissuemend II surgical glue (California Veterinary Supply, Pahrump, NV). There were three treatment groups: 1) liposomes + saline (liposomes/saline), 2) liposomes + bleomycin (liposomes/bleo), and 3) PBS only + bleomycin (PBS/bleo). Experiments were completed 3 wk (day 22) after bleomycin or saline treatment on day 1 with all animals being weighed. Any animal that lost >25% of its initial body weight was euthanized and counted as a death for survival analysis. All mice were euthanized by IP injection with Nembutal (100 mg/kg) followed by terminal surgery [bronchoalveolar lavage (BAL) and harvesting of lung tissue]. In some studies, mice were administered liposomes (or PBS only) on days 0 and 7 and bleomycin (or saline) on day 1, and animals were then euthanized on day 8 followed by collection of BAL and lung tissue.
Liposome deposition in the lung.
To determine liposome deposition within lung tissue, select mice were airway delivered 100 μl of DiI-labeled liposomes by oropharyngeal aspiration on day 0 followed by IT saline on day 1 with euthanasia on day 2. Whole lungs were harvested without lavage or perfusion. The freshly isolated lungs were instilled with 50% Tissue-Tek OCT compound (Sakura Finetek USA, Torrance, CA) in saline to inflate the lungs to total lung capacity and subsequently frozen in liquid nitrogen. To obtain the maximal cross-sectional area of each side of the whole lung, and thereby reveal each lobe, frozen lungs were cut through the cranial-caudal plane at the first bifurcation of bronchi. The frozen lung tissue was cryosectioned into 7-μm sections using a cryo-microtome (CM1850, Leica Microsystems, Nussolch, Germany) and imaged by fluorescence microscopy.
Immunohistochemistry.
Frozen whole lung sections were fixed in methanol and 3% H2O2 for 10 min at 4°C to reduce nonspecific staining of lung tissue and then were permeabilized with 0.8% Triton X-100 for 10 min at room temperature. Slides were rinsed with PBS before and after fixation and permeabilization. After blocking with PBS containing 0.25% normal goat serum and 0.5% bovine serum albumin, lung sections were immunostained with rabbit anti-pro-surfactant protein C (SPC) (Research Diagnostics, Flanders, NJ) at a 1:100 dilution for 1 h at 4°C followed by goat anti-rabbit secondary antibody (IgG) conjugated to Alexa Fluor 488 (Molecular Probes, Eugene, OR) at a 1:300 dilution for 1 h at room temperature to label type II alveolar epithelial (AT II) cells. Cell nuclei were stained with the nuclear counterstain diamidino-2-phenylindole dihydrochloride (DAPI) (Roche Diagnostics, Indianapolis, IN) according to the manufacturer's instructions.
NBT assay.
Intracellular superoxide anion (O2−) generation by stimulated macrophages was measured by a quantitative nitroblue tetrazolium (NBT) assay as previously described (7). Mouse macrophages (RAW 264.7 cells) were plated for 1 h in serum-free DMEM at 37°C in 5% CO2 in 24-well culture plates (5 × 105 cells/well). The cells were treated for 1 h with PMA (0.5 μg/ml) as a positive control or liposomes in 100 μl PBS + 100 μl NBT reagent (0.5 mg/ml) (Sigma-Aldrich, St. Louis, MO). After treatment, cells were washed two times with warm PBS, fixed in methanol, and air-dried. Then 120 μl of 2 M KOH and 140 μl DMSO were added to the cells to solubilize the cell membranes and to dissolve the blue formazan, respectively, while the plate was gently shaken for 10 min at room temperature. Dissolved formazan was then transferred to a 96-well plate and absorbance was measured at 630 nm via a spectrophotometer.
BAL and lung tissue preparation.
After IP injection with Nembutal (100 mg/kg), the trachea was surgically exposed and a cannula was inserted into the trachea and tied off to collect BAL as previously described (25). Briefly, a 1-ml syringe was attached to the cannula and the airways and lungs were flushed 10 times with 0.9 ml of cold PBS (without Ca and Mg) with a volume recovery rate of 80–90%. The first two lavage fractions were pooled and centrifuged to pellet cells, and the supernatant was collected, frozen, and stored at −80°C for measurement of protein content later by use of a standard BCA protein assay kit (Thermo Scientific, Waltham, MA). The cells from the first two lavage fractions were combined with the cells from the remaining eight lavage fractions and centrifuged to pellet cells, and red blood cells were lysed, washed with PBS, and counted by Trypan blue exclusion to determine the number of viable cells. Lungs were perfused with 20 ml cold PBS. For Masson's trichrome histology, lungs were inflated with formalin to total lung capacity (and the trachea tied off), removed, placed in formalin overnight at room temperature, and then stored in 70% ethanol at 4°C. For hydroxyproline measurements, lungs were removed, separated into left and right lungs, cut into small pieces, transferred to 1.5-ml Eppendorf tubes, and quick frozen in liquid nitrogen followed by storage at −80°C.
Measurements of lung mechanics.
Mice were anesthetized by IP injection with Nembutal (50–75 mg/kg). A small incision was made to expose the trachea, and a cannula was inserted into the trachea and tied off. A differential pressure transducer was connected to the tracheal cannula to allow for measurements of airway resistance, lung compliance, and other lung function parameters by use of a FlexiVent mechanical ventilator and data-acquisition system (SCIREQ, Montreal, Canada). Mice were ventilated with a tidal volume of 8–10 ml/kg (e.g., ∼0.5–0.7 ml) at a rate of 130–200 breaths per minute, with an end-expiratory pressure of 2–3 cmH2O. Lung function was measured two to four times and the data averaged for each mouse.
Hydroxyproline assay.
To quantify collagen content, lung hydroxyproline was measured by a standard assay (6). Briefly, frozen lung tissue was thawed in 1 ml of distilled water (dH2O), homogenized by use of a hand-held tissue homogenizer, and transferred to heat-resistant glass tubes. Next 1 ml of 6 N HCl was added to the homogenized lung tissue, which was then baked for 20 h at 105°C. After baking, the dried pellets were reconstituted with 2 ml of dH2O and hydroxyproline content in samples was determined by colorimetric assay. Sample values calculated based on a standard curve were multiplied by a factor of 10 to account for the total initial sample volume (2 ml). Data are reported as the total amount (μg) of hydroxyproline measured separately in left or right lung tissue as differences in fibrosis commonly exist between left and right lungs after IT delivery of bleomycin.
Histology and scoring.
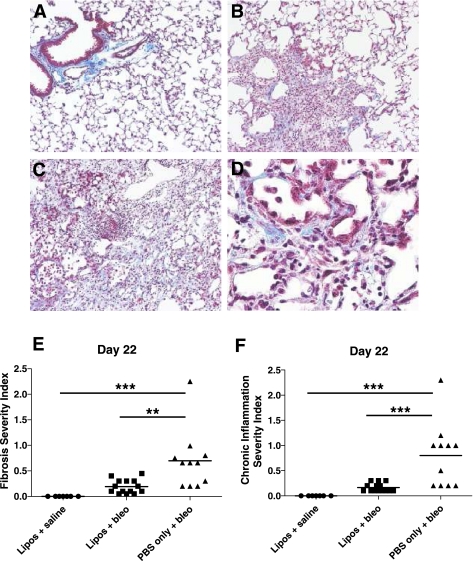
Formalin-fixed lungs were embedded in paraffin, sectioned at 5 μm and stained with Masson's trichrome to identify collagen (NIEHS pathology/histology support group). The day 22 stained lung sections were then graded by a pathologist independent from our group (W. G. Lieuallen, Charles River Laboratories, Pathology Associates, Raleigh, NC) to determine 1) a severity score for fibrosis or chronic inflammation and 2) the distribution (% area affected) of fibrosis or chronic inflammation throughout the lung tissue. For fibrosis, severity scoring was subjectively determined by the intensity of Masson's trichrome collagen staining of affected areas within the lung section. The fibrosis scoring was as follows: 0 = within normal limits (no treatment-related lesions), 1 = minimal (thin, wispy fibrils), 2 = mild (small areas of fibril coalescence), 3 = moderate (larger areas of more solid collagen deposition), and 4 = marked. For chronic inflammation, severity scoring was subjectively based on 1) the number of inflammatory cells present within the lung section and 2) the degree and extent of alveolar septal wall thickening that was not due to fibrosis. The chronic inflammation scoring was also as follows: 0 = within normal limits (no treatment-related lesions), 1 = minimal, 2 = mild, 3 = moderate, and 4 = marked. The distribution (% area affected) of fibrosis or chronic inflammation was a semiquantitative assessment of the percentage of the whole lung section in which fibrosis or chronic inflammation were present (0–100%). Severity score (0–4) × % area affected (0–100%/100) = severity index. Representative photomicrographs showing severity scoring of day 22 stained lung sections from the different treatment groups are shown in Fig. 6, A–D. Day 8 lung sections were similarly scored blinded by a pathologist (M. Cesta, Cellular and Molecular Pathology Branch, NIEHS) according to the severity of inflammation or fibrosis.
Fig. 6.
Liposomes decrease lung fibrosis and chronic inflammation in bleomycin-treated mice. Bleomycin-treated mice ± liposomes and liposomes/saline-treated control mice were euthanized and lung tissue was collected on day 22. Formalin-fixed lungs were embedded in paraffin, sectioned, stained for collagen with Masson's trichrome, and then numerically scored for 1) the severity and 2) the distribution (% area affected) of fibrosis or chronic inflammation. A--D: representative photomicrographs showing severity scoring of day 22 Masson's trichrome-stained lung sections. A: lung sections from the liposomes/saline group are within normal limits (severity score = 0) (×100 magnification). B: lung sections from the liposomes/bleo group reveal mild fibrosis and chronic inflammation (severity score = 2) (×100 magnification). C: lung sections from the PBS/bleo group reveal moderate fibrosis and chronic inflammation (severity score = 3) (×100 magnification). D: lung sections from the PBS/bleo group showing evidence of lymphocyte and macrophage infiltration, AT II cell proliferation, and fibrosis (×400 magnification). E: fibrosis was significantly reduced in lungs of liposomes/bleo-treated mice compared with PBS/bleo-treated mice (P < 0.01) and was not significantly different from the liposomes/saline control mice. F: chronic inflammation was significantly reduced in lungs of liposomes/bleo-treated mice compared with PBS/bleo-treated mice (P < 0.001) and was not significantly different from the liposomes/saline control mice. Results are from 2 combined experiments (liposomes/saline group, n = 7; liposomes/bleo group, n = 14; PBS/bleo group, n = 11). Bars represent mean values. Statistical analysis was performed by 1-way ANOVA and Tukey's multiple-comparison test; **P < 0.01, ***P < 0.001.
Statistics.
Statistical analysis was performed by one-way ANOVA and Tukey's multiple-comparison tests, log-rank (Mantel-Cox) test for survival analysis, or unpaired Student's t-test with GraphPad PRISM 5 software (La Jolla, CA). Data are reported as means ± SD with a P value < 0.05 considered statistically significant.
RESULTS
Lung deposition of DiI-labeled liposomes.
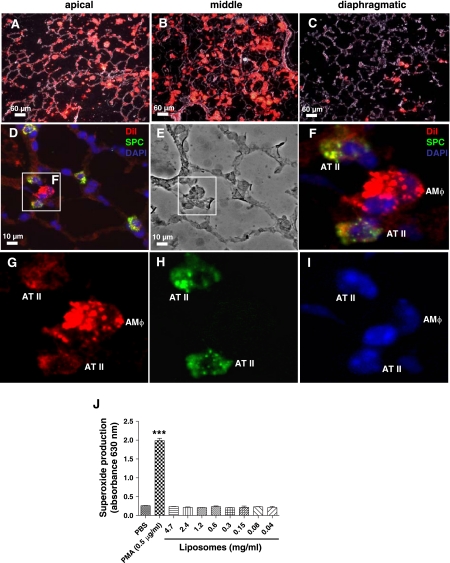
Airway delivery of a single dose of DiI-liposomes resulted in diffuse alveolar deposition of the left lung on day 2 with most of the liposomes in the apical and middle regions and fewer present in the diaphragmatic region (Fig. 1, A–C). Immunostaining revealed that the DiI-liposomes were internalized by both alveolar macrophages and pro-SPC (+) AT II cells, although uptake was predominantly by alveolar macrophages and to a lesser extent by AT II cells (Fig. 1, D, F, G, and H). Treatment of mouse macrophages (RAW 264.7 cells) with liposomes did not induce superoxide anion production in an in vitro NBT assay (Fig. 1J), suggesting that liposomes do not induce macrophage activation.
Fig. 1.
Evidence of alveolar deposition of airway-delivered 1,1′-dioctadecyl-3,3,3′,3′ tetramethylindocarbocyanine perchlorate (DiI)-labeled liposomes. C57BL/6 mice received 100 μl of DiI-labeled or unlabeled liposomes on day 0 by oropharyngeal aspiration followed by saline intratracheally (IT) on day 1. Mice were euthanized on day 2 and whole lungs were prepared for fluorescence microscopy as previously described. A–C: merged fluorescent and phase images. Lung deposition following a single delivery of DiI-labeled liposomes (red) reveals diffuse alveolar deposition mostly in the apical and middle regions of the left lung and fewer liposomes evident in the diaphragmatic region. B: there were increased numbers of DiI-labeled liposomes evident more proximally near larger bronchi. D–I: DiI fluorescent liposomes were mostly present in alveolar macrophages (AMΦ) and to a lesser extent in type II alveolar epithelial (AT II) cells. AT II cells were identified by immunofluorescence of the antibody to prosurfactant protein C (SPC) (green). Cell nuclei were stained with diamidino-2-phenylindole dihydrochloride (DAPI; blue). The phase image of D is shown (E) and the enlarged image from D (F) reveals an example of 2 AT II cells and 1 alveolar macrophage with internalized DiI-labeled liposomes. J: liposomes do not induce macrophage superoxide anion production in vitro. Mouse macrophages (RAW 264.7 cells) were treated in duplicate wells for 1 h with liposomes or the positive control PMA (0.5 μg/ml) and intracellular superoxide anion generation was measured quantitatively via a nitroblue tetrazolium assay. Results are from a single representative experiment. Data are expressed as means ± SD. Statistical analysis was performed by 1-way ANOVA and Tukey's multiple-comparison test; ***P < 0.001 (compared with all other groups).
Airway delivery of liposomes protects from bleomycin-induced weight loss.
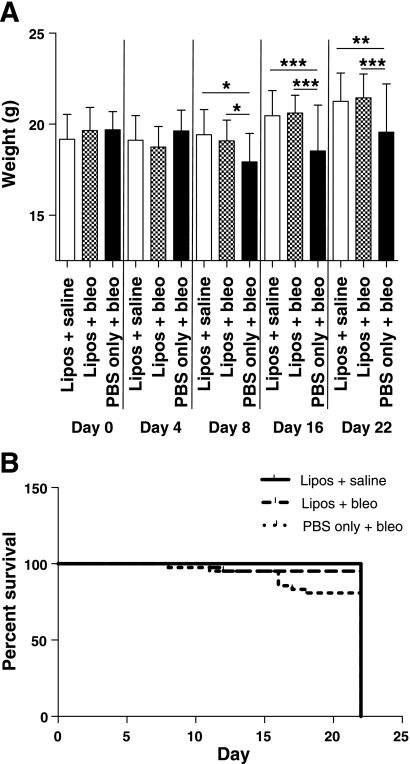
The weight of mice treated with bleomycin alone was significantly lower than that of mice that received bleomycin with liposomes (Fig. 2A). On days 0 and 4, there were no significant differences in weights between the three treatment groups. However, on day 8, the mean weight of mice that received PBS/bleo (17.9 ± 1.56 g) was significantly lower than the mean weight of those that received liposomes/saline (19.4 ± 1.39 g) (P < 0.05) and liposomes/bleo (19.1 ± 1.13 g) (P < 0.05). By day 16, the mean weight of the mice in the PBS/bleo group (18.5 ± 2.52 g) was significantly lower than that of mice in both the liposomes/bleo group (20.6 ± 0.97 g) (P < 0.001) and the liposomes/saline group (20.5 ± 1.38 g) (P < 0.001). By day 22, the mean weight of the mice in the PBS/bleo group (19.6 ± 2.64 g) remained significantly lower compared with the mean weights in both the liposomes/bleo group (21.5 ± 1.32 g) (P < 0.001) and the liposomes/saline group (21.3 ± 1.56 g) (P < 0.01). Weights were not significantly different for liposomes/bleo-treated mice on days 0, 4, 8, 16, and 22 compared with liposomes/saline-treated control mice. Therefore, over the course of these studies, bleomycin-treated mice that received liposomes had significantly less weight loss than bleomycin-treated mice that received a PBS control and were indistinguishable from the weights of control mice (no bleomycin) at each time point. Mortality in the PBS/bleo treatment group indicated that one mouse died on day 17 and a total of seven others were euthanized for excessive weight loss (> 25%) on days 8 (n = 1), 11 (n = 1), 16 (n = 4), and 18 (n = 1). In contrast, only two mice in the liposomes/bleo treatment group did not survive to day 22, with one mouse dying on day 12 and another euthanized on day 11 because of excessive weight loss (> 25%). Therefore, survival to day 22 was increased in bleomycin-treated mice that were airway delivered liposomes compared with those that did not receive liposomes (Fig. 2B); however, differences were not statistically significant.
Fig. 2.
Airway-delivered liposomes (lipos) protect against bleomycin (bleo)-induced weight loss. C57BL/6 mice received 100 μl of liposomes or PBS only on days 0, 4, 8, 12, and 16 by oropharyngeal aspiration and bleomycin (3.33 U/kg) or saline IT on day 1. A: weights were significantly decreased on days 8, 16, and 22 in PBS/bleo-treated mice compared with liposomes/saline-treated control mice (P < 0.05 for day 8; P < 0.001 for day 16; P < 0.01 for day 22). In contrast, in the liposomes/bleo mice, weight loss was significantly less compared with PBS/bleo mice on day 8 (P < 0.05) as well as on days 16 and 22 (P < 0.001 for both time points). Weights were not significantly different between liposomes/bleo-treated mice on days 0, 4, 8, 16, and 22 compared with liposomes/saline-treated control mice. There were no significant differences in weights between the three treatment groups on days 0 and 4. Results are from 3 combined experiments (liposomes/saline group, n = 21; liposomes/bleo group, n = 43; PBS/bleo group, n = 42). Statistical analysis was performed by 1-way ANOVA and Tukey's multiple-comparison test; *P < 0.05, **P < 0.01, ***P < 0.001. B: survival curves are shown; % survival to day 22 was increased in liposomes/bleo-treated mice compared with PBS/bleo mice; however, differences were not statistically significant. Statistical analysis was performed by a log-rank (Mantel-Cox) test.
Liposomes decrease bleomycin-induced lung fibrosis and chronic inflammation.
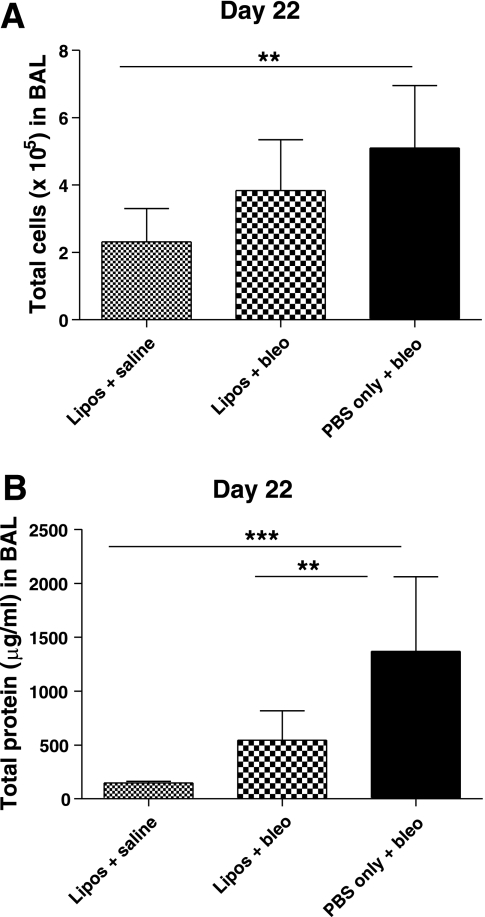
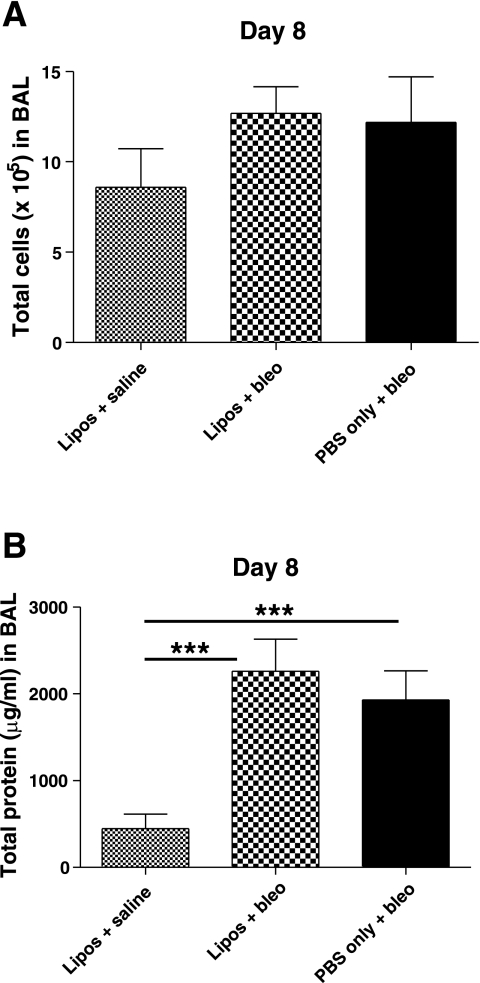
The mean total number of cells in BAL on day 22 was significantly increased in mice treated with PBS/bleo (5.10 ± 1.86 × 105) compared with the liposomes/saline control group (2.31 ± 0.99 × 105) (P < 0.01) (Fig. 3A). The mean total number of cells for the liposomes/bleo group (3.84 ± 1.51 × 105) was less than that for the PBS/bleo group but not statistically significant. However, the liposomes/bleo group was also not significantly different from the control liposomes/saline group. In some studies, BAL was collected earlier on day 8 (1 wk after IT treatment with bleomycin), after administration of liposomes on days 0 and 7. Mean total cell numbers were also elevated in bleomycin-treated compared with liposomes/saline-treated mice on day 8; however, there were no differences between mice treated with bleomycin ± liposomes on day 8 (Fig. 4A).
Fig. 3.
Airway-delivered liposomes decrease bleomycin-induced bronchoalveolar lavage (BAL) protein at day 22. BAL was collected 21 days after IT bleomycin on day 1 and cell counts and protein levels were determined. A: total cells were significantly increased in BAL of PBS/bleo-treated mice compared with the liposomes/saline-treated control group (P < 0.01). In liposomes/bleo mice, there was a reduction in the BAL cell counts compared with PBS/bleo mice, but the difference did not reach significance. Results are from a single experiment (liposomes/saline group, n = 8; liposomes/bleo group, n = 14; PBS/bleo group, n = 12). Data are expressed as means ± SD. B: total protein content (μg/ml) in cell-free BAL supernatants was elevated in PBS/bleo-treated mice compared with liposomes/saline-treated control mice (P < 0.001). However, the protein levels in the liposomes/bleo mice were significantly reduced compared with the PBS/bleo mice (P < 0.01) and were not significantly different from the liposomes/saline control mice. Results are from 2 combined experiments (liposomes/saline group, n = 10; liposomes/bleo group, n = 20; PBS/bleo group, n = 19). Bars represent mean values. Statistical analysis was performed by 1-way ANOVA and Tukey's multiple-comparison test; **P < 0.01, ***P < 0.001.
Fig. 4.
Airway-delivered liposomes had no effect on day 8 bleomycin-induced BAL cell counts and protein levels. Liposomes (or PBS only) were administered on days 0 and 7. BAL was collected 7 days after IT bleomycin (or saline) on day 1 and cell counts and protein levels were determined. A: mean total cell numbers were increased in BAL of both PBS/bleo-treated and liposomes/bleo-treated mice compared with the liposomes/saline-treated control group; however, the differences were not statistically significant. Results are from a single experiment (liposomes/saline group, n = 3; liposomes/bleo group, n = 5; PBS/bleo group, n = 6). Data are expressed as means ± SD. B: total protein content (μg/ml) in cell-free BAL supernatants was significantly elevated in both PBS/bleo-treated and liposomes/bleo-treated mice compared with liposomes/saline-treated control mice (P < 0.001). However, the protein levels in the liposomes/bleo mice were not significantly different compared with the PBS/bleo mice. Results are from a single experiment (liposomes/saline group, n = 4; liposomes/bleo group, n = 6; PBS/bleo group, n = 6). Data are expressed as means ± SD. Statistical analysis was performed by 1-way ANOVA and Tukey's multiple-comparison test; ***P < 0.001.
The mean total protein levels on day 22 in BAL from mice treated with PBS/bleo (1,368 ± 694 μg/ml) were significantly increased compared with mice in both the liposomes/bleo group (544 ± 273 μg/ml) (P < 0.01) and the liposomes/saline group (150 ± 13.0 μg/ml) (P < 0.001) (Fig. 3B). Protein levels in the liposomes/bleo mice were not significantly different from the liposomes/saline mice. However, in studies in which mice were treated with liposomes on days 0 and 7, total protein levels were significantly elevated in BAL of bleomycin-treated compared with liposomes/saline-treated mice on day 8, but there were no significant differences between mice treated with bleomycin ± liposomes on day 8 (Fig. 4B). These data indicate that the airway delivery of liposomes partially decreased or ameliorated the development of a protein-rich edema, cell damage, and/or other causes of increased protein within the airways and lung tissue of bleomycin-treated mice later on, at day 22.
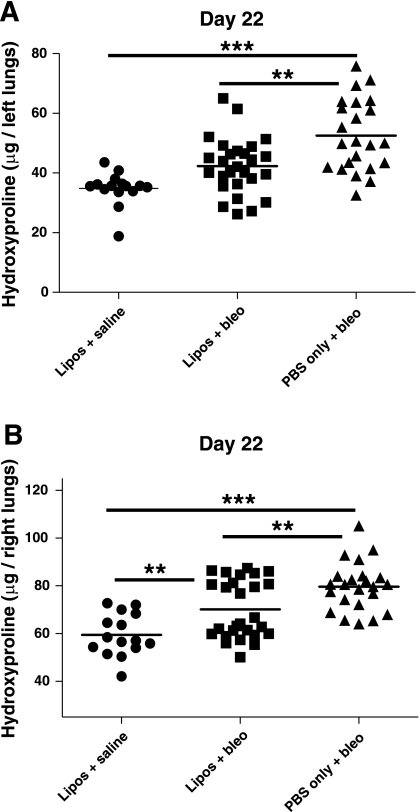
Lung tissue was harvested to assess inflammation and fibrosis both quantitatively and histologically. Hydroxyproline levels were measured in lung tissue from treated mice on day 22 (Fig. 5). The left and right lungs from each mouse were assayed separately. Mean hydroxyproline levels in the left lungs (Fig. 5A) were significantly increased in mice that received PBS/bleo (52.6 ± 12 μg) compared with mice treated with liposomes/bleo (42.3 ± 9.4 μg) (P < 0.01) and liposomes/saline (34.8 ± 5.5 μg) (P < 0.001). Mean hydroxyproline levels in the left lungs were not significantly different for the liposomes/bleo and liposomes/saline groups. Mean hydroxyproline levels in the right lungs (Fig. 5B) were also significantly increased in mice that received PBS/bleo (79.6 ± 10.1 μg) compared with mice treated with liposomes/bleo (70.1 ± 12.2 μg) (P < 0.01) or liposomes/saline (59.4 ± 8.9 μg) (P < 0.001). Mean hydroxyproline levels in the right lungs were significantly increased in mice that received liposomes/bleo compared with mice treated with liposomes/saline (P < 0.01). These data indicate that the airway delivery of liposomes partially decreased the total collagen content of bleomycin-treated lungs on day 22, suggesting that there was less fibrosis with liposome treatment.
Fig. 5.
Airway-delivered liposomes protect from bleomycin-induced increase in lung hydroxyproline. Hydroxyproline levels (μg/lung) were measured on day 22 in lung tissue by a standard hydroxyproline assay with A representing the left lungs and B representing the right lungs. Hydroxyproline was significantly increased in both the left and right lungs of PBS/bleo-treated mice compared with the respective lungs of mice that received liposomes/bleo (P < 0.01) or liposomes/saline (P < 0.001). The reduction in the left lungs by liposome treatment was sufficient to bring hydroxyproline levels statistically down to control levels, whereas a more modest reduction in the right lungs was still significantly above control levels (P < 0.01). Results are from 3 combined experiments (liposomes/saline group, n = 15; liposomes/bleo group, n = 27; PBS/bleo group, n = 23). Bars represent mean values. Statistical analysis was performed by 1-way ANOVA and Tukey's multiple-comparison test; **P < 0.01, ***P < 0.001.
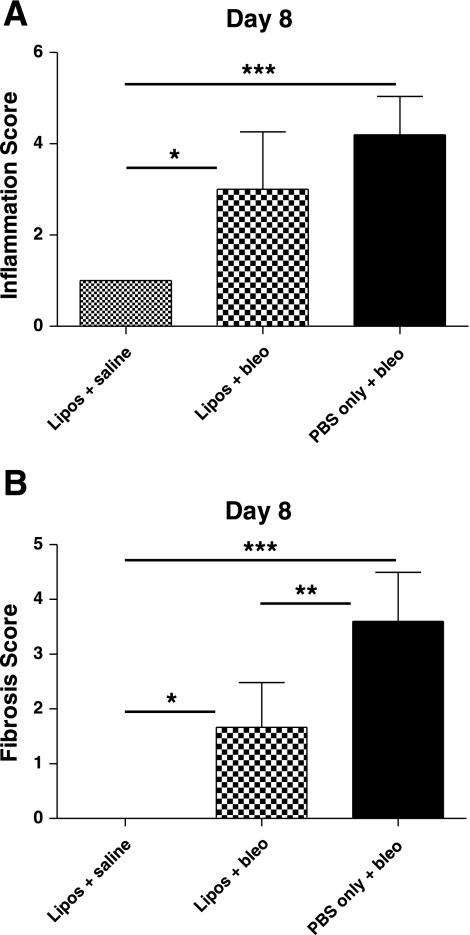
Lung sections from treated mice on day 22 were stained for collagen with Masson's trichrome and then numerically scored to determine 1) the severity and 2) the distribution (% area affected) of fibrosis or chronic inflammation within the lung tissue (Fig. 6). The fibrosis severity index (Fig. 6E) was significantly increased in day 22 lung sections from mice treated with PBS/bleo (0.70 ± 0.58) compared with mice in the liposomes/bleo group (0.19 ± 0.14) (P < 0.01) or the liposomes/saline group (0.00 ± 0.00) (P < 0.001). Fibrosis was not significantly different in day 22 lungs from the liposomes/bleo-treated mice compared with the liposomes/saline-treated control mice. The chronic inflammation severity index (Fig. 6F) was also significantly increased in day 22 lung sections from mice treated with PBS/bleo (0.80 ± 0.64) compared with mice in the liposomes/bleo group (0.16 ± 0.08) (P < 0.001) or the liposomes/saline group (0.00 ± 0.00) (P < 0.001) with increased macrophage and lymphocyte infiltration as well as AT II cell proliferation (Fig. 6D). Chronic inflammation was not significantly different in day 22 lungs from the liposomes/bleo-treated mice compared with the liposomes/saline-treated control mice. Although the severity of fibrosis (severity score) revealed in the day 22 lung sections did not significantly differ between the PBS/bleo (mean of 2.4 with range of 1–3) and the liposomes/bleo groups (mean of 2.1 with range of 1–4), the distribution (% area affected) of fibrosis throughout the lung tissue was significantly increased in animals from the PBS/bleo group (mean of 29% with range of 10–75%) compared with the liposomes/bleo group (mean of 9% with range of 5–15%). However, unlike with the fibrosis severity index, both the severity of chronic inflammation (severity score) and distribution of inflammation throughout the lung tissue (% area affected) were significantly increased in mice treated with PBS/bleo (mean of 2.6 with range of 2–3 and mean of 29% with range of 10–75%, respectively) compared with the liposomes/bleo group (mean of 1.4 with range 1–2 and mean of 9% with range 5–15%, respectively) (data not shown). Lung histology indicated that there was more inflammation on day 8, which included increased neutrophils and lymphocytes (data not shown), in bleomycin-treated compared with liposomes/saline-treated mice on day 8. There was also a trend for increased inflammation in PBS/bleo-treated mice compared with liposomes/bleo-treated mice on day 8, but the difference was not significant (Fig. 7A). Fibrosis score was significantly increased in day 8 lung sections from bleomycin-treated mice compared with both liposomes/bleo-treated mice as well as control liposomes/saline-treated mice (Fig. 7B). Taken together, these histological findings indicate that airway delivery of liposomes significantly protected mice from bleomycin-induced lung inflammation and fibrosis.
Fig. 7.
Effect of airway-delivered liposomes on day 8 lung inflammation and fibrosis scores in bleomycin-treated mice. Liposomes (or PBS only) were administered on days 0 and 7. Lung tissue was collected 7 days after IT bleomycin (or saline) on day 1. Formalin-fixed lungs from bleomycin-treated mice ± liposomes and liposomes/saline-treated control mice were embedded in paraffin, sectioned, stained for collagen with Masson's trichrome, and then numerically scored for the severity of inflammation or fibrosis. A: inflammation was significantly increased in lungs of liposomes/bleo-treated and PBS/bleo-treated mice (P < 0.05 and P < 0.001, respectively) compared with the liposomes/saline control mice. However, liposomes/bleo-treated and PBS/bleo-treated mice were not significantly different. B: fibrosis was significantly increased in lungs of liposomes/bleo-treated and PBS/bleo-treated mice (P < 0.01 and P < 0.001, respectively) compared with the liposomes/saline control mice. However, unlike with inflammation, fibrosis scores were significantly reduced in lungs of liposomes/bleo-treated compared with PBS/bleo-treated mice (P < 0.01). Results are from a single experiment (liposomes/saline group, n = 4; liposomes/bleo group, n = 6; PBS/bleo group, n = 5). Data are expressed as means ± SD. Statistical analysis was performed by 1-way ANOVA and Tukey's multiple-comparison test; *P < 0.05, **P < 0.01, ***P < 0.001.
Liposomes improve lung function in bleomycin-treated mice.
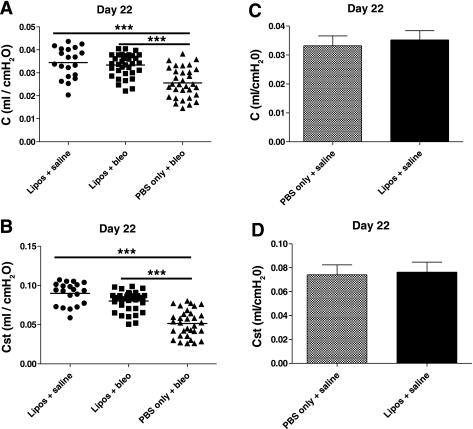
Airway delivery of liposomes significantly protected mice from bleomycin-induced loss of lung function on day 22 (Fig. 8). Both mean dynamic compliance (C, Fig. 8A) and static compliance (Cst, Fig. 8B) were significantly decreased in mice that received PBS/bleo (0.026 ± 0.007 and 0.052 ± 0.017 ml/cmH2O, respectively) compared with those that were treated either with liposomes/bleo (0.033 ± 0.005 and 0.080 ± 0.013 ml/cmH2O) (P < 0.001 for both C and Cst) or liposomes/saline (0.034 ± 0.007 and 0.090 ± 0.014 ml/cmH2O) (P < 0.001 for both C and Cst). C and Cst values were not significantly different in liposomes/bleo mice compared with liposomes/saline control mice. Airway-delivered liposomes alone also had no effect on lung compliance on day 22 since mean C and Cst values were not significantly different between PBS/saline (0.033 ± 0.003 and 0.074 ± 0.008 ml/cmH2O) and liposomes/saline (0.035 ± 0.003 and 0.076 ± 0.008 ml/cmH2O) control mice (Fig. 8, C and D). These data indicate that the administration of liposomes significantly protected bleomycin-treated mice from a loss of lung compliance, a physiological correlate to the fibrotic process.
Fig. 8.
Airway-delivered liposomes improve lung compliance in bleomycin-treated mice. Lung compliance was measured on day 22. A: dynamic lung compliance (C, ml/cmH2O) was significantly decreased in lungs of PBS/bleo-treated mice compared with the liposomes/saline-treated control mice (P < 0.001). In contrast, the liposomes/bleo mice had dynamic compliance values that were significantly improved compared with PBS/bleo mice (P < 0.001) and indistinguishable from those values for liposomes/saline control mice. B: static lung compliance (Cst, ml/cmH2O) was also significantly decreased in lungs of PBS/bleo-treated mice compared with the liposomes/saline-treated control mice (P < 0.001). Similar to A, static lung compliance was markedly improved in the liposomes/bleo-treated mice compared with the PBS/bleo-treated mice (P < 0.001) and indistinguishable from those values for the liposomes/saline control mice. Results are shown for 3 combined experiments (liposomes/saline group, n = 20; liposomes/bleo group, n = 35; PBS/bleo group, n = 32). Bars represent mean values. Statistical analysis was performed by 1-way ANOVA and Tukey's multiple-comparison test; ***P < 0.001. C and D: airway-delivered liposomes alone have no effect on day 22 lung compliance. Dynamic lung compliance (ml/cmH2O) (C) and static lung compliance (ml/cmH2O) (D) were not significantly different on day 22 in lungs of PBS/saline-treated mice compared with liposomes/saline-treated control mice. Results are from a single experiment (PBS/saline group, n = 5; liposomes/saline group, n = 6). Data are expressed as means ± SD. Statistical analysis was performed by an unpaired Student's t-test.
DISCUSSION
This study indicates that airway delivery of uncharged synthetic liposomes before and after IT treatment with bleomycin protected mice against bleomycin-induced lung toxicity. Specifically, liposome-treated mice that received bleomycin showed significantly less weight loss, chronic lung inflammation, and fibrosis and improved lung compliance compared with PBS/bleo-treated mice.
In a previous study, Arndt et al. (4) observed that liposome-encapsulated bleomycin administered IP exhibited increased antitumor activity in mouse tumor models compared with free bleomycin, perhaps due to a decreased local clearance of bleomycin from the tumor site. In addition, they found that lung hydroxyproline levels were decreased in animals after treatment with liposome-encapsulated bleomycin compared with free bleomycin. This suggests that the use of liposomes decreased the lung toxicity of bleomycin while enhancing the antitumor properties and thus did not interfere with the functional activity of bleomycin. Thus this study suggested to us the hypothesis that direct airway delivery of liposomes alone might be protective from bleomycin-induced lung injury and perhaps other forms of lung injury as well.
DiI labeling of liposomes confirmed that the liposomes were delivered diffusely throughout the lung parenchyma and were incorporated primarily by alveolar macrophages and to a lesser extent by AT II cells (Fig. 1). Liposomes present within these cells may potentially modulate alveolar macrophage and/or alveolar epithelial cell activation and function, for example, by the induction of anti-inflammatory cytokines (15, 35), which in turn may dampen the destructive impact of free bleomycin within the lung microenvironment, or by suppression of profibrotic cytokines such as TGF-β1. However, TGF-β1 protein was measured by ELISA in BAL, and although it was found to be significantly elevated in bleomycin-treated compared with liposomes/saline-treated mice on day 8, there were no significant differences in TGF-β1 between mice treated with bleomycin ± liposomes on day 8 (data not shown). We demonstrated in vitro that liposomes did not affect superoxide generation by macrophages, consistent with other studies showing that both liposomes (9) as well as exogenous surfactant (19) do not activate murine alveolar macrophages. Alternatively, liposomes may act as an extracellular “sink” to free bleomycin and/or bleomycin-generated oxygen radicals preventing bleomycin from directly interacting with alveolar epithelial cells as well as reducing the interaction of free radicals with the vulnerable cell surface. No protective effect was observed when liposomes were given only after bleomycin (data not shown); however, further optimization of the number and timing of liposome doses postbleomycin might enhance the protective role.
Airway delivery of these liposomes, which consisted largely of phosphatidylcholine, may have had a “surfactant-like” effect. Since a major component of lung surfactant is phosphatidylcholine, it is possible that liposomes may have been partly protective by reducing lung surface tension and/or by enhancing fluid clearance in response to bleomycin-induced alveolar edema (42). Because surfactant replacement therapy has been successful in the treatment of neonatal respiratory distress syndrome (1, 8, 14), it has been considered for the treatment of respiratory diseases in which lung surfactant deficiency or dysfunction also exist, such as acute lung injury or ARDS (2, 3, 11, 13, 20, 26, 27, 32, 38, 44) and pulmonary fibrosis or IPF (17, 40), but these applications have had minimal success.
In a study by Volchkov et al. (40), inhalation of a cattle-derived natural surfactant reduced bleomycin-induced lung alveolitis and atelectasis. In another study, IT treatment with artificial surfactant in rats decreased bleomycin-induced lung toxicity as evidenced by decreased body weight loss, lung wet weight, and collagen content (17). Interestingly, in a study by Suwabe et al. (33), data from in vitro studies indicated that exogenous artificial surfactant promoted neutrophil apoptosis, suggesting this as one possible mechanism that could reduce neutrophil-mediated inflammation in the lung. Furthermore, phosphatidylcholine-containing liposomes in turn have been used to successfully treat lung surgical wounds (18) as well as lung damage induced by X-ray irradiation (41) in animal models. Thus there are multiple examples to suggest that exogenous and endogenous phospholipids may ameliorate lung injury; and yet, determining the specific mechanisms of how this actually occurs in vivo remains unclear.
In our study, bleomycin was administered IT to mice. Intravenous (IV) or IP systemic delivery of bleomycin would be more similar to how bleomycin is administered therapeutically in human subjects; however, these routes of delivery in animal models require more time for the induction of lung fibrosis, are less effective, and typically produce more animal-to-animal variability compared with IT delivery (22). Future work is therefore needed to assess whether lung toxicity is still reduced when liposomes are airway delivered before and after systemic (IP or IV) administration of bleomycin, and to determine whether the antineoplastic activity of bleomycin is preserved. In summary, our study demonstrates that airway-delivered synthetic liposomes can significantly reduce bleomycin-induced acute injury and fibrosis in a mouse model of pulmonary toxicity.
GRANTS
This research was supported by the Intramural Research Program of the NIEHS, National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We are grateful to the members of the NIEHS pathology/histology support group for processing and histochemical staining of lung sections. We also thank Charles River Laboratories, Pathology Associates, specifically Warren G. Lieuallen, D.V.M, Ph.D., for the pathological evaluation and scoring of day 22 lung sections.
REFERENCES
- 1. Ainsworth SB, Milligan DW. Surfactant therapy for respiratory distress syndrome in premature neonates: a comparative review. Am J Respir Med 1: 417–433, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Anderson BM, Jackson F, Jr, Moxley MA, Longmore WJ. Effects on experimental acute lung injury 24 hours after exogenous surfactant instillation. Exp Lung Res 18: 191–204, 1992 [DOI] [PubMed] [Google Scholar]
- 3. Anzueto A, Baughman RP, Guntupalli KK, Weg JG, Wiedemann HP, Raventos AA, Lemaire F, Long W, Zaccardelli DS, Pattishall EN. Aerosolized surfactant in adults with sepsis-induced acute respiratory distress syndrome. Exosurf Acute Respiratory Distress Syndrome Sepsis Study Group. N Engl J Med 334: 1417–1421, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Arndt D, Zeisig R, Bechtel D, Fichtner I. Liposomal bleomycin: increased therapeutic activity and decreased pulmonary toxicity in mice. Drug Deliv 8: 1–7, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Batenburg JJ. Surfactant phospholipids: synthesis and storage. Am J Physiol Lung Cell Mol Physiol 262: L367–L385, 1992 [DOI] [PubMed] [Google Scholar]
- 6. Bergman I, Loxley R. Lung tissue hydrolysates: studies of the optimum conditions for the spectrophotometric determination of hydroxyproline. Analyst 94: 575–584, 1969 [DOI] [PubMed] [Google Scholar]
- 7. Choi HS, Kim JW, Cha YN, Kim C. A quantitative nitroblue tetrazolium assay for determining intracellular superoxide anion production in phagocytic cells. J Immunoassay Immunochem 27: 31–44, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Donn SM, Dalton J. Surfactant replacement therapy in the neonate: beyond respiratory distress syndrome. Respir Care 54: 1203–1208, 2009 [PubMed] [Google Scholar]
- 9. Gonzalez-Rothi RJ, Straub L, Cacace JL, Schreier H. Liposomes and pulmonary alveolar macrophages: functional and morphologic interactions. Exp Lung Res 17: 687–705, 1991 [DOI] [PubMed] [Google Scholar]
- 10. Gregory TJ, Longmore WJ, Moxley MA, Whitsett JA, Reed CR, Fowler AA, 3rd, Hudson LD, Maunder RJ, Crim C, Hyers TM. Surfactant chemical composition and biophysical activity in acute respiratory distress syndrome. J Clin Invest 88: 1976–1981, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gunther A, Ruppert C, Schmidt R, Markart P, Grimminger F, Walmrath D, Seeger W. Surfactant alteration and replacement in acute respiratory distress syndrome. Respir Res 2: 353–364, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gunther A, Schmidt R, Nix F, Yabut-Perez M, Guth C, Rosseau S, Siebert C, Grimminger F, Morr H, Velcovsky HG, Seeger W. Surfactant abnormalities in idiopathic pulmonary fibrosis, hypersensitivity pneumonitis and sarcoidosis. Eur Respir J 14: 565–573, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Harris JD, Jackson F, Jr, Moxley MA, Longmore WJ. Effect of exogenous surfactant instillation on experimental acute lung injury. J Appl Physiol 66: 1846–1851, 1989 [DOI] [PubMed] [Google Scholar]
- 14. Holm BA. Surfactant replacement therapy. New levels of understanding. Am Rev Respir Dis 148: 834–836, 1993 [DOI] [PubMed] [Google Scholar]
- 15. Holt PG. Down-regulation of immune responses in the lower respiratory tract: the role of alveolar macrophages. Clin Exp Immunol 63: 261–270, 1986 [PMC free article] [PubMed] [Google Scholar]
- 16. Horiuchi T, Ikegami M, Cherniack RM, Mason RJ. Increased surface tension of the lung and surfactant in bleomycin-induced pulmonary fibrosis in rats. Am J Respir Crit Care Med 154: 1002–1005, 1996 [DOI] [PubMed] [Google Scholar]
- 17. Ito M, Suwabe A, Suzuki T, Tominaga M, Takahashi K. [The effects of surfactant-TA on bleomycin-induced lung injury and lung fibroblast proliferation]. Nihon Kyobu Shikkan Gakkai Zasshi 35: 1163–1172, 1997 [PubMed] [Google Scholar]
- 18. Kornilova ZK, Selishcheva AA, Perel'man MI. Effect of phosphatidylcholine liposome on regeneration of surgical wound in guinea pig lung. Bull Exp Biol Med 131: 191–194, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Kramer BW, Jobe AH, Ikegami M. Exogenous surfactant changes the phenotype of alveolar macrophages in mice. Am J Physiol Lung Cell Mol Physiol 280: L689–L694, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Lewis J, Ikegami M, Higuchi R, Jobe A, Absolom D. Nebulized vs. instilled exogenous surfactant in an adult lung injury model. J Appl Physiol 71: 1270–1276, 1991 [DOI] [PubMed] [Google Scholar]
- 21. Moeller A, Ask K, Warburton D, Gauldie J, Kolb M. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int J Biochem Cell Biol 40: 362–382, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moore BB, Hogaboam CM. Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 294: L152–L160, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Osanai K, Takahashi K, Sato S, Iwabuchi K, Ohtake K, Sata M, Yasui S. Changes of lung surfactant and pressure-volume curve in bleomycin-induced pulmonary fibrosis. J Appl Physiol 70: 1300–1308, 1991 [DOI] [PubMed] [Google Scholar]
- 24. Pastva AM, Wright JR, Williams KL. Immunomodulatory roles of surfactant proteins A and D: implications in lung disease. Proc Am Thorac Soc 4: 252–257, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pasula R, Weaver T, Martinez MA, Martin WJ., 2nd Morphologic detection and functional assessment of reconstituted normal alveolar macrophages in the lungs of SCID mice. J Immunol 169: 4504–4510, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Playfor SD, Nootigattu VK. Exogenous surfactant in paediatric acute lung injury and acute respiratory distress syndrome. Curr Drug Saf 1: 159–168, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Rozenberg OA, Danilov LN, Volchkov VA, Lebedeva ES, Dubrovskaia VF, Val'kovich AA, Klestova OV, Kirillov IuA, Seiliev AA, Shaldzhian AA, Loshakova LV, Shul'ga AE, Zhuikov AG. [Pharmacological properties and therapeutic efficacy of the domestic preparations of lung surfactants]. Biull Eksp Biol Med 126: 455–458, 1998 [PubMed] [Google Scholar]
- 28. Rugonyi S, Biswas SC, Hall SB. The biophysical function of pulmonary surfactant. Respir Physiol Neurobiol 163: 244–255, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schmidt R, Meier U, Markart P, Grimminger F, Velcovsky HG, Morr H, Seeger W, Gunther A. Altered fatty acid composition of lung surfactant phospholipids in interstitial lung disease. Am J Physiol Lung Cell Mol Physiol 283: L1079–L1085, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Schmidt R, Ruppert C, Markart P, Lubke N, Ermert L, Weissmann N, Breithecker A, Ermert M, Seeger W, Gunther A. Changes in pulmonary surfactant function and composition in bleomycin-induced pneumonitis and fibrosis. Toxicol Appl Pharmacol 195: 218–231, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Schwendener RA. Liposomes in biology and medicine. Adv Exp Med Biol 620: 117–128, 2007 [DOI] [PubMed] [Google Scholar]
- 32. So KL, de Buijzer E, Gommers D, Kaisers U, van Genderen PJ, Lachmann B. Surfactant therapy restores gas exchange in lung injury due to paraquat intoxication in rats. Eur Respir J 12: 284–287, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Suwabe A, Otake K, Yakuwa N, Suzuki H, Ito M, Tomoike H, Saito Y, Takahashi K. Artificial surfactant (Surfactant TA) modulates adherence and superoxide production of neutrophils. Am J Respir Crit Care Med 158: 1890–1899, 1998 [DOI] [PubMed] [Google Scholar]
- 34. Tanaka K, Ishihara T, Azuma A, Kudoh S, Ebina M, Nukiwa T, Sugiyama Y, Tasaka Y, Namba T, Sato K, Mizushima Y, Mizushima T. Therapeutic effect of lecithinized superoxide dismutase on bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 298: L348–L360, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Thepen T, Hoeben K, Breve J, Kraal G. Alveolar macrophages down-regulate local pulmonary immune responses against intratracheally administered T-cell-dependent, but not T-cell-independent antigens. Immunology 76: 60–64, 1992 [PMC free article] [PubMed] [Google Scholar]
- 36. Thrall RS, Swendsen CL, Shannon TH, Kennedy CA, Frederick DS, Grunze MF, Sulavik SB. Correlation of changes in pulmonary surfactant phospholipids with compliance in bleomycin-induced pulmonary fibrosis in the rat. Am Rev Respir Dis 136: 113–118, 1987 [DOI] [PubMed] [Google Scholar]
- 37. Tooley WH, Clements JA, Muramatsu K, Brown CL, Schlueter MA. Lung function in prematurely delivered rabbits treated with a synthetic surfactant. Am Rev Respir Dis 136: 651–656, 1987 [DOI] [PubMed] [Google Scholar]
- 38. van Helden HP, Kuijpers WC, Langerwerf PE, Langen RC, Haagsman HP, Bruijnzeel PL. Efficacy of Curosurf in a rat model of acute respiratory distress syndrome. Eur Respir J 12: 533–539, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Voinea M, Simionescu M. Designing of ‘intelligent’ liposomes for efficient delivery of drugs. J Cell Mol Med 6: 465–474, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Volchkov VA, Dubrovskaya VF, Klestova OV, Val'kovich AA, Serzhanina VA, Seiliev AA, Rosenberg OA. Therapeutic efficiency of early and late administration of surfactant-BL during bleomycin-induced damage to rat lungs. Bull Exp Biol Med 141: 682–684, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Volchkov VA, Kirillov JA, Dubrovskaja VF, Rosenberg OA. Modification of X-ray induced pathology of lung by intratracheal administration of PC-chol liposomes (Abstract). J Liposome Res 8: 120–121, 1998 [Google Scholar]
- 42. Wang PM, Ashino Y, Ichimura H, Bhattacharya J. Rapid alveolar liquid removal by a novel convective mechanism. Am J Physiol Lung Cell Mol Physiol 281: L1327–L1334, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Waters P, Vaid M, Kishore U, Madan T. Lung surfactant proteins A and D as pattern recognition proteins. Adv Exp Med Biol 653: 74–97, 2009 [DOI] [PubMed] [Google Scholar]
- 44. Weg JG, Balk RA, Tharratt RS, Jenkinson SG, Shah JB, Zaccardelli D, Horton J, Pattishall EN. Safety and potential efficacy of an aerosolized surfactant in human sepsis-induced adult respiratory distress syndrome. JAMA 272: 1433–1438, 1994 [PubMed] [Google Scholar]