Abstract
Postnatal lung development requires proliferation and differentiation of specific cell types at precise times to promote proper alveolar formation. Hyperoxic exposure can disrupt alveolarization by inhibiting cell growth; however, it is not fully understood how this is mediated. The transcription factor CCAAT/enhancer binding protein-α (C/EBPα) is highly expressed in the lung and plays a role in cell proliferation and differentiation in many tissues. After 72 h of hyperoxia, C/EBPα expression was significantly enhanced in the lungs of newborn mice. The increased C/EBPα protein was predominantly located in alveolar type II cells. Silencing of C/EBPα with a transpulmonary injection of C/EBPα small interfering RNA (siRNA) prior to hyperoxic exposure reduced expression of markers of type I cell and differentiation typically observed after hyperoxia but did not rescue the altered lung morphology at 72 h. Nevertheless, when C/EBPα hyperoxia-exposed siRNA-injected mice were allowed to recover for 2 wk in room air, lung epithelial cell proliferation was increased and lung morphology was restored compared with hyperoxia-exposed control siRNA-injected mice. These data suggest that C/EBPα is an important regulator of postnatal alveolar epithelial cell proliferation and differentiation during injury and repair.
Keywords: CCAAT/enhancer binding protein-α, developing lung injury, small interfering RNA, recovery
postnatal lung development involves a series of coordinated events, including active cell proliferation and differentiation, to promote proper alveolar formation. Parenchymal lung cells, fibroblasts, endothelial cells, and epithelial type II cells have distinct growth patterns and specific proliferation rates, beginning in the neonatal period (9). Imbalance of growth factors, inflammatory insults, and oxidative stress (reviewed in Ref. 16) could alter these processes, resulting in impaired lung development. Hyperoxic exposure is well known for disrupting alveolarization in the developing lung, because it inhibits the growth of epithelial, fibroblast, and endothelial cells forming the alveoli (5, 19). Several studies have demonstrated that hyperoxia results in alveolar growth arrest, induction of cell cycle inhibitory proteins (13), altered surfactant protein (SP) production (reviewed in Ref. 4), and disrupted extracellular matrix signaling (1). The effect of hyperoxia on the proliferation of specific lung cell types is not well defined.
The transcription factor CCAAT/enhancer binding protein (C/EBP) family consists of six isoforms. C/EBPα is highly expressed in the lung (6) and plays a role in cell proliferation and differentiation in various tissues (17, 18), as do C/EBPβ and C/EBPδ. Given that the newborn lung proliferates and differentiates rapidly, we hypothesized that C/EBPα regulates neonatal lung cell proliferation in hyperoxia and during recovery.
Here, we demonstrate that C/EBPα expression was enhanced in lungs of newborn mice after 72 h of hyperoxia. The increased C/EBPα protein was predominantly localized to alveolar type II cells, where altered proliferation was observed. Silencing of C/EBPα with a single injection of C/EBPα small interfering RNA (siRNA) prior to exposure did not rescue the altered lung morphology after 72 h of hyperoxia but restored lung morphology after 2 wk of room air recovery by enhancing lung epithelial cell proliferation.
MATERIALS AND METHODS
Animal models and hyperoxic exposures.
Pregnant C57BL/6 female mice at gestational day 18 were purchased from Charles River Laboratories. The mice were kept in a 12:12-h light-dark cycle and allowed access to food and water ad libitum until the time of experimentation. Litters of neonatal (<12-h-old) pups, along with their mothers, were randomly assigned to 21% oxygen or room air or 95% oxygen. Hyperoxic exposure was conducted in an A-chamber (BioSpherix, Redfield, NY), which allows for continuous monitoring and regulation of oxygen and carbon dioxide. Ambient carbon dioxide was maintained at <1,500 ppm by adjustment of the chamber's ventilation. The dams were switched every 24 h between room air and hyperoxia to avoid injury. All procedures were reviewed and approved by the Animal Fair and Care Community of the Children's Hospital of Philadelphia.
Construction of siRNA.
A 19-nucleotide RNA fragment, CGACGAGUUCCUGGCCGAC (15), targeting mouse C/EBPα gene transcription was synthesized in a siSTABLE format to enhance stability of the siRNA (Dharmacon, Chicago, IL). Stock concentration was made at 1 μg/μl in RNase-free water and kept in aliquots at −20°C until use. A control siRNA was prepared using siGENOME Non-Targeting Pool #1 (Dharmacon) and stored as described above. The Non-Targeting siRNA Pool consisted of #1–4 individual RNAs, which were characterized by genome-wide microarray analysis and found to have minimal off-target signatures. For testing efficiency of the transpulmonary delivery and stability of the delivered siRNA, a positive control siRNA, siGLOcyclophilin B (siGLO), conjugated with a fluorophore Cy3 (Thermo Scientific Dharmacon), was purchased and prepared as described above for the C/EBPα siRNA.
Intrapulmonary delivery.
To increase the efficiency of delivery, aliquots of the C/EBPα siRNA, control siRNA, or siGLO were dissolved in saline and mixed with Lipofectamine 2000 at room temperature for 1 h. A 30-μl (3 mg/kg body wt) aliquot of the mixture was injected into the left axilla of the neonatal mouse at the third intercostal space via a 1-ml insulin syringe, as described previously, resulting in intrapulmonary delivery (20). The mice were returned to their mothers and kept in room air for 16 h prior to hyperoxic exposure. The mice received only a single dose of the injected siRNA during the exposure.
Lung morphometric evaluation: radial alveolar counts.
The lungs were inflated to a constant pressure of 25 cmH2O with 4% paraformaldehyde in PBS and immersed in the same fixative for 24 h. Respiratory bronchioles were identified by the presence of epithelial lining in one part of the wall. A perpendicular line was drawn from the center of the respiratory bronchiole to the distal acinus (the pleura or the nearest connective tissue septum). A minimum of 40 lines were drawn on a magnified image of each lung section, and the number of septae intersected by each line was counted (7, 8).
Immunohistochemistry.
For visualization of C/EBPα protein expression in the lung, 5-μm paraffin-embedded tissue sections were incubated with a 1:100 dilution of polyclonal anti-C/EBPα (14AA, sc-61, Santa Cruz Biotechnology, Santa Cruz, CA) specific to the C/EBPα isoform overnight and then with a 1:500 dilution of anti-rabbit IgG (Alexa Fluor 488, A11008, Invitrogen, Carlsbad, CA) for 1 h. Subsequently, sections were costained with a monoclonal antibody for the type II cell marker ATP-binding cassette subfamily A member 3 (ABCA3; WMAB-ABCA3-13, Seven Hills Bioreagents, Cincinnati, OH) at a 1:100 dilution overnight and a 1:500 dilution of anti-mouse IgG (Alexa Fluor 594, A11005, Invitrogen) for 1 h. Additionally, sections for evaluation of proliferating cell nuclear antigen (PCNA) by costaining of the type II cell marker pro-SP-C were prepared similarly. Specific antibodies were a 1:100 dilution of a monoclonal anti-PCNA (PC10, sc-56, Santa Cruz) and a 1:100 dilution of a polyclonal anti-pro-SP-C serum (AB3786, Millipore, Temecula, CA). After tissues were immunostained, sections were mounted with a drop of mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA) and visualized with a fluorescence microscope (model IX70, Olympus America, Center Valley, PA). Images were captured with a digital camera (model C4742-95, Hamamatsu).
Quantitative immunohistochemistry for type II cell proliferation.
For quantification of proliferating type II cells, images of random fields of terminal alveolar regions were acquired with the fluorescence microscope. Ten fields per lung were obtained from three separate mice in each group. For counting PCNA or pro-SP-C-positive cells, regions for DAPI fluorescence-positive cells were randomly selected to obviate bias toward signals generated with secondary antibodies. Images were digitally merged to identify dual-positive cells. Quantification was achieved with Metamorph software (MetaMorph Imaging System, Universal Imaging, West Chester, PA). Briefly, the ratios of pro-SP-C to DAPI, PCNA to DAPI, and PCNA to pro-SP-C were obtained from each field, and the average of 10 fields per animal was graphed.
Evaluation of protein levels in lung homogenates.
Lungs were flushed with PBS to exclude red blood cells and frozen in liquid nitrogen. To obviate the difference in protein or mRNA levels due to injection variability, the entire injected lung was quickly pulverized in liquid nitrogen and mixed with 50 μl of PBS. An aliquot was taken for Western analysis, and the remaining lung homogenates were dissolved in TRIzol reagent for mRNA extraction. Western analysis was performed to evaluate protein levels for markers of cell proliferation, type II and type I cells, and vascular endothelial cells. The procedure is described elsewhere (22). The antibodies and dilutions were as follows: a 1:1,000 dilution of polyclonal anti-C/EBPα (14AA, sc-61) for C/EBPα, a 1:1,000 dilution of hamster anti-T1α (clone 8.1.1, Iowa Hybridoma Bank, Iowa City, IA) for the marker of type I cells (T1α), a 1:1,000 dilution of polyclonal anti-pro-SP-B or anti-pro-SP-C serum (Ab 3780 or Ab 3786, Millipore) for pro-SP-B or pro-SP-C, a 1:1,000 dilution of monoclonal anti-PCNA (PC10, sc-56) for PCNA, a 1:800 dilution of polyclonal anti-p21 (C-19, sc-397) for p21, a 1:200 dilution of goat anti-PECAM-1 (M-20, sc-1,506) for platelet/endothelial cell adhesion molecule-1 (PECAM-1), a 1:10,000 dilution of polyclonal anti-caveolin-1 (N20, sc-894) for caveolin-1, a 1:20,000 dilution of polyclonal anti-calnexin (SPA-860, Stressgen, Victoria, BC, Canada) for calnexin, and a 1:10,000 dilution of monoclonal anti-GAPDH (MAb 374, Millipore) for GAPDH.
Quantitative real-time PCR.
Steady-state mRNA levels were evaluated by quantitative real-time PCR using the TaqMan gene expression system (Applied Biosystems). Briefly, total lung RNA was extracted with TRIzol reagent (Invitrogen). First-strand cDNA was synthesized with SuperScript II reverse transcriptase (Invitrogen) and random primers. Gene-specific mRNA levels were determined using TaqMan gene expression assays (each primer at 900 nM and probe at 250 nM; Applied Biosystems) designed over exon-to-exon boundaries. Specific primers for each gene are listed in Table 1. All reactions were performed in 384-well plates with a final volume of 10 μl. Real-time PCR plates were analyzed using the Prism 7900HT sequence detection system with Prism SDS2.1 software (Applied Biosystems). Relative quantitation was achieved by normalization to the value of 18S mRNA using the cycle threshold (ΔΔCT) method.
Table 1.
ABI primers used for quantitative real-time PCR
| Gene Symbol | ABI Assay ID | Gene Name |
|---|---|---|
| C/EBPα | Mm01265914_s1 | CCAAT/enhancer binding protein-α |
| Nkx2-1 | Mm00447558_m1 | NK2 homeobox 1 or thyroid transcription factor1 (TTF1) |
| FoxA2 | Mm00839704_mH | Forkhead box A2 or hepatocyte nuclear factor 3β (HNF3β) |
| Myc | Mm00487803_m1 | Myelocytomatosis oncogene |
| TGFb1 | Mm01178819_m1 | Transforming growth factor-β1 |
| Sftpb | Mm00455681_m1 | Surfactant protein B |
| Sftpc | Mm00488144_m1 | Surfactant protein C |
| Aqp5 | Mm00437578_m1 | Aquaporin 5 |
| T1α | Mm00494716_m1 | TypeI (T1)-α |
Statistical analysis.
For comparison between treatment groups, the null hypothesis that there is no difference between treatment means was tested by a single-factor ANOVA for multiple groups or unpaired t-test for two groups (InStat 3, GraphPad Software). Statistical significance (P < 0.05, P < 0.01, or P < 0.001) between and within groups was determined by Tukey's method of multiple comparisons.
RESULTS
Hyperoxia induces C/EBPα expression in neonatal mouse lung.
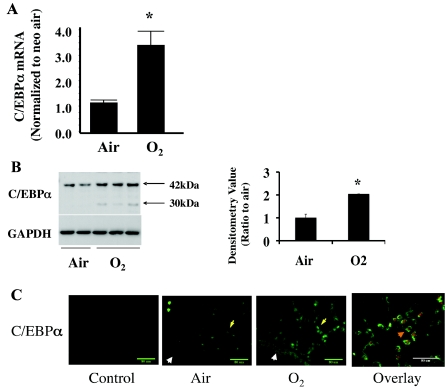
Levels of C/EBPα mRNA were increased threefold in neonatal mouse lung exposed to 72 h of hyperoxia as measured by quantitative real-time PCR (Fig. 1A). Protein levels of C/EBPα were increased twofold in lung homogenates as determined by Western analysis (Fig. 1B). The induced C/EBPα appeared to be selective only in the lung, as liver and thymus homogenates did not show increased C/EBPα protein in the hyperoxia-exposed animal (data not shown). This induction was not observed in similarly exposed adult animals (data not shown).
Fig. 1.
Hyperoxic induction of lung CCAAT/enhancer binding protein-α (C/EBPα) mRNA and its epithelial localization in neonatal (<12-h-old) mouse lung after 72 h of room air (Air) and 95% O2 (O2) exposure. A: C/EBPα mRNA increases in hyperoxia after normalization to room air. B: Western blots of C/EBPα immunoreactive protein levels (left) and densitometric evaluation of 42-kDa C/EBPα (right) in hyperoxia. Blots represent results from 4 experiments. Arrows indicate the 2 isoforms for C/EBPα protein. Values are means ± SE of 3 mice in each group. *P < 0.05 vs. Air. C: immunohistofluorescent staining of hyperoxia-induced C/EBPα in lung epithelial cells. Bronchiolar epithelial cells are indicated by white arrowheads and alveolar epithelial cells by yellow arrows. Overlay shows colocalization (orange arrowhead) of hyperoxia-induced C/EBPα (red staining) and ATP-binding cassette subfamily A member 3 (ABCA3, green staining), a marker of alveolar type II cells.
Hyperoxia-induced C/EBPα protein is preferentially localized in lung alveolar type II cells.
To understand which lung cells expressed C/EBPα, immunofluorescent staining of paraffin-embedded lung tissue slides was performed. Enhanced staining of alveolar epithelial cells was observed with antibodies against C/EBPα in room air and further increased in hyperoxia (Fig. 1C). When the lung slides were costained with ABCA3, a type II cell-specific marker, C/EBPα expression was predominantly observed in type II cells (Fig. 1C, overlay). These data suggest that hyperoxia-induced C/EBPα may play a specific role in lung alveolar type II cells.
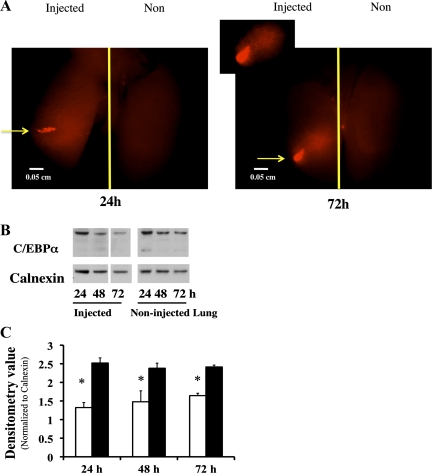
Intrapulmonary delivery of C/EBPα siRNA decreases C/EBPα protein for up to 72 h postinjection.
Because of the small size of the newborn mouse pups, we encountered significant difficulties when administering small volumes of siRNA via intranasal inhalation, and in preliminary experiments, we could not demonstrate inhibition of C/EBPα in the neonatal mouse lung with this method (data not shown). We therefore chose an intrapulmonary injection method to deliver the C/EBPα siRNA (20). Stability of the injected siRNA was evaluated by injection of a Cy3-labeled control siRNA (siGLO), and fluorescent signals were monitored at 24 and 72 h after injections. The siRNA signal was visible in the injected lung and was above the level in the noninjected lung (background signal) at 24 h postinjection. This elevated level persisted for 72 h (Fig. 2A), indicating that the delivered siRNA was stable for at least that period. The inhibitory effect of the delivered siRNA was assessed quantitatively by Western analysis with densitometry. Injection of C/EBPα siRNA into the neonatal mouse lung resulted in a twofold decrease in C/EBPα protein levels 24 h postinjection that was sustained for up to 72 h (Fig. 2, B and C).
Fig. 2.
Evaluation of small interfering RNA (siRNA) stability and specific target gene inhibition via intrapulmonary injection. A: a Cy3-conjugated siRNA to cyclophilin B (siGLO) was injected into left lung of neonatal mouse, and a fluorescent micrograph was obtained at 24 and 72 h after injections. Arrows indicate injection sites. Inset: focused view of injected area. B: Western blots of C/EBPα protein levels after intrapulmonary injection. Blots represent results from 3 experiments. Only 1 of the 2 samples originally loaded on the gel is shown. Inadequately loaded lanes were omitted (white spaces). C: densitometric evaluation of 42-kDa C/EBPα protein. Values are means ± SE of 3 mice in each group. Open bars, injected left lungs; solid bars, corresponding noninjected right lungs. *P < 0.05 vs. noninjected lungs.
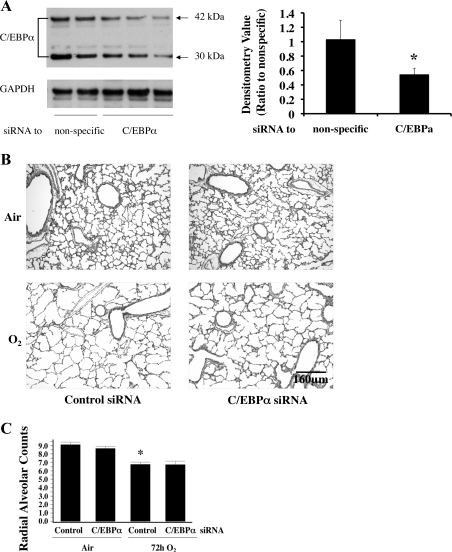
Intrapulmonary delivery of C/EBPα siRNA does not rescue disrupted lung architecture.
After 72 h of hyperoxia, C/EBPα protein levels were reduced by 50% in the C/EBPα siRNA-injected lungs compared with similarly exposed control siRNA-injected lungs (Fig. 3A). The increased C/EBPα protein level in the right lung (without injection) of the same animal remained high (data not shown), indicating that the injected siRNA only had a localized effect and was able to reduce C/EBPα protein to the basal level throughout the 72-h exposure.
Fig. 3.
Evaluation of lung architecture and function after intrapulmonary injection of C/EBPα siRNA into neonatal lung exposed to 72 h of hyperoxia (O2). A: Western blots of C/EBPα protein levels (left) and densitometric evaluation of 42-kDa C/EBPα protein (right) after hyperoxic exposure preceded by injection of nonspecific (Control) or C/EBPα siRNA. Blots represent results from 3 experiments. Values are means ± SE of 4 mice in each group. *P < 0.05 vs. nonspecific. B: hematoxylin-eosin-stained lung sections obtained from A. C: radial alveolar counts (RACs) in lungs of pups obtained from A. Values are means ± SE of 3–5 mice in each group. *P < 0.01 vs. Control in Air.
Despite evidence of simplified alveolar septae in both injected groups exposed to hyperoxia, there were no obvious changes in lung morphology between the C/EBPα siRNA- and the control siRNA-injected lungs exposed to room air or hyperoxia for 72 h (Fig. 3B). To corroborate this finding, radial alveolar counts (RACs) as a measure of disrupted alveolarization were significantly reduced in C/EBPα siRNA- and control siRNA-injected groups exposed to hyperoxia for 72 h (Fig. 3C); however, injection of C/EBPα siRNA did not correct the RAC (Fig. 3C).
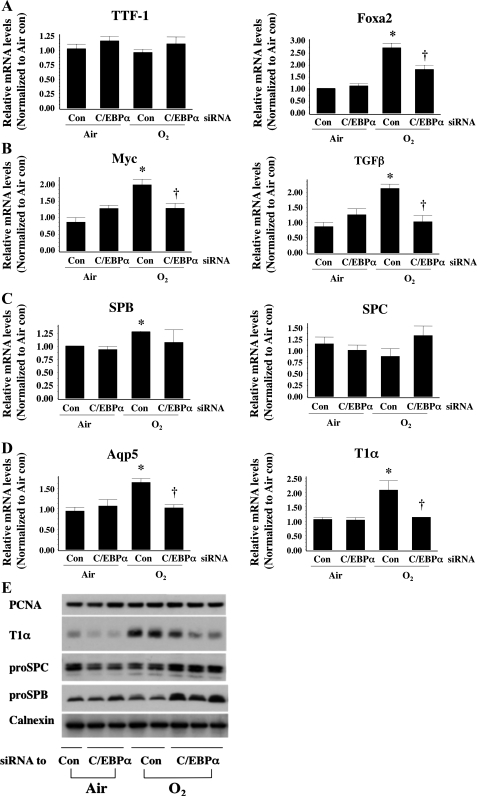
Silencing C/EBPα reduces hyperoxia-induced markers of differentiation and of type I cells.
To understand whether hyperoxia induced C/EBPα-modified type II cell development and affected differentiation of type II to type I cells, we evaluated mRNA levels for markers that were specific for development and differentiation of type II cells, as well as others that are specific for type II and type I cells. Thyroid transcription factor 1 (TTF1), a key transcription factor in type II cell development, did not increase in hyperoxia (Fig. 4A), consistent with a previous report (14). Furthermore, C/EBPα siRNA injection did not change TTF1 mRNA levels in hyperoxia. However, another important regulator of type II cell maturation, hepatocyte nuclear factor-3β (FoxA2), was significantly increased in hyperoxia and was reversed by C/EBPα siRNA (Fig. 4A). Myelocytomatosis oncogene (myc) and transforming growth factor β1 (TGFβ), general markers of cell differentiation, were increased in hyperoxia and reversed by injection of C/EBPα siRNA (Fig. 4D). In addition, SP-B, a known type II cell marker, increased in hyperoxia but was not altered by C/EBPα siRNA (Fig. 4B). However, type I cell markers, aquaporin 5 and T1α, were increased in hyperoxia and reversed by C/EBPα siRNA (Fig. 4C). Overall, these data indicate that hyperoxia-induced C/EBPα regulates alveolar cell differentiation, as well as expression of type I, but not type II, cell markers.
Fig. 4.
mRNA levels of thyroid transcription factor 1 (TTF1) and FoxA2 as markers of lung development (A), myelocytomatosis oncogene (myc) and transforming growth factor-β (TGFβ) as markers of cell differentiation (B), surfactant proteins B and C (SP-B and SP-C) as markers of type II cells (C), and aquaporin 5 (Aqp5) and T1α as markers of type I cells (D) after intrapulmonary injection of C/EBPα siRNA into neonatal lung exposed to 72 h of hyperoxia (O2). Values are means ± SE of 3 mice. *P < 0.05 vs. control siRNA-injected group (Con) in 72-h air exposure (Air). †P < 0.05 vs. Con in O2. E: representative Western blots of protein levels of alveolar type II and type I cell markers, as well as proliferating cell nuclear antigen (PCNA), a general cell proliferation marker.
Despite differences in mRNA levels for the various markers, it was important to determine whether protein levels of type II and type I cell markers were equally modulated after injection with C/EBPα siRNA in hyperoxia. Therefore, SP-B (pro-SP-B), SP-C (pro-SP-C), and T1α levels were evaluated in lung homogenates by Western analysis. After 72 h of hyperoxia, there were no changes in pro-SP-B or pro-SP-C proteins (Fig. 4E). However, T1α protein levels were significantly increased (Fig. 4E). Injection of C/EBPα siRNA prior to hyperoxia did not reduce pro-SP-B and pro-SP-C protein levels but attenuated T1α protein levels, in a reciprocal manner to the hyperoxia-induced increase (Fig. 4E).
C/EBPα does not regulate lung epithelial cell proliferation after 72 h of hyperoxia.
There were no changes in levels of PCNA protein, a general marker for cell proliferation, with hyperoxia or C/EBPα siRNA delivery (Fig. 4E), suggesting that, despite its effect on epithelial differentiation and type II and type I cell protein levels, C/EBPα siRNA does not alter lung overall cell proliferation.
Injection of C/EBPα siRNA prior to hyperoxia followed by 2 wk of room air recovery partially rescued disrupted lung architecture and reduced RAC.
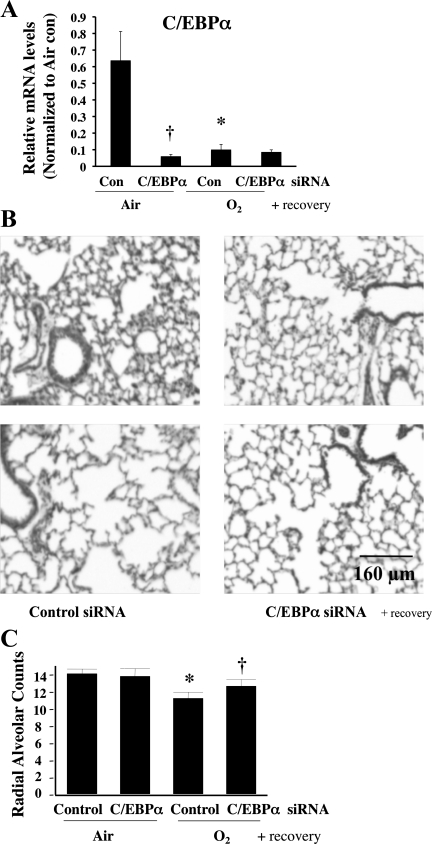
Although C/EBPα siRNA did not improve hyperoxia-induced disruption of lung architecture at 72 h, its effects on markers of cell differentiation and lung epithelial cells suggest that C/EBPα siRNA might influence lung epithelial cell growth and alveolarization during repair. To this extent, we assigned neonatal pups to four groups: 1) injection with one dose of control siRNA 16 h prior to a 72-h air exposure followed by room air recovery for up to 2 wk (control siRNA air/air), 2) room air recovery as in group 1 and injection with C/EBPα siRNA 16 h prior to a 72-h air exposure (C/EBPα siRNA air/air), 3) room air recovery as in group 1 and injection with control siRNA 16 h prior to 72 h of hyperoxia (control siRNA O2/air), and 4) room air recovery as in group 1 and injection with C/EBPα siRNA 16 h prior to 72 h of hyperoxia (C/EBPα siRNA O2/air). After recovery, C/EBPα mRNA levels were lower in the C/EBPα siRNA air/air and C/EBPα siRNA O2/air groups; it is unclear why C/EBPα mRNA was also decreased in the control siRNA O2/air group (Fig. 5A). Perhaps there was an inhibitory response to the initial C/EBPα induction during room air recovery. This has not been explored in detail. Interestingly, the disrupted lung architecture and loss of RAC after 72 h of hyperoxia worsened after 2 wk of room air recovery (Fig. 5, B and C), while C/EBPα siRNA improved lung architecture and partially restored RAC loss (Fig. 5, B and C).
Fig. 5.
Effect of C/EBPα siRNA on lung architecture and RAC in lungs of neonatal mice exposed to hyperoxia and allowed to recover in room air. A: mRNA levels in C/EBPα siRNA-injected lung allowed to recover in room air. Values are means ± SE of 6 animals in each group. *P < 0.05 vs. control siRNA-injected lung (Con) in 72-h air exposure (Air). †P < 0.05 vs. C/EBPα siRNA-injected lung (C/EBPα siRNA) in Air. B: representative hematoxylin-eosin-stained lung sections. C: RAC in control (nonspecific) siRNA- and C/EBPα siRNA-injected lungs after room air recovery. Values are means ± SE of 5 animals in each group. *P < 0.05 vs. Control in Air. †P < 0.05 vs. Control in 72-h O2 exposure (O2).
Markers for overall cell differentiation and for specific type II and type I cells remain low in C/EBPα siRNA-injected lungs following recovery.
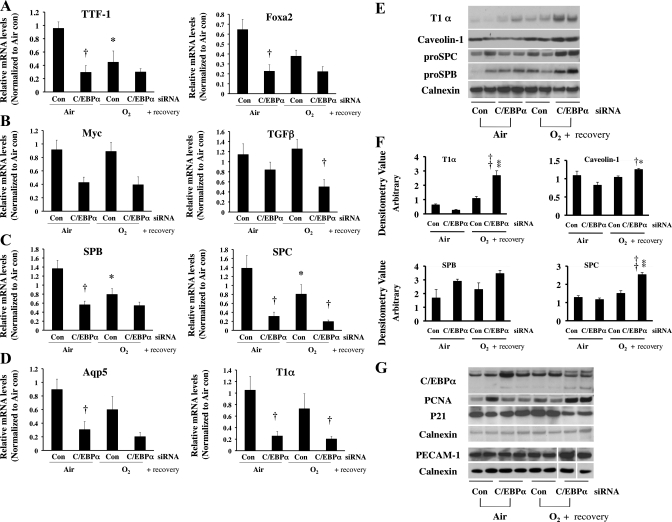
To understand whether the effects of C/EBPα siRNA on alveolar cell development and differentiation were sustained after room air recovery, we evaluated differentiation markers and type I and II markers as described above. After room air recovery, the hyperoxia-induced decreases in mRNA levels of the cell differentiation markers myc and TGFβ and the type I cell markers aquaporin 5 and T1α were further diminished (Fig. 6, B and D). Interestingly, markers for type II cell development, TTF1 and FoxA2, and markers specific for type II cells, pro-SP-B and pro-SP-C, were decreased in the control siRNA O2/air group compared with the control siRNA air/air group (Fig. 6, A and C), suggesting that hyperoxia disrupts type II cell development and differentiation during recovery. Paradoxically, C/EBPα siRNA further decreased mRNA levels of TGFβ, pro-SP-C, and T1α (Fig. 6, B–D) with room air recovery.
Fig. 6.
mRNA levels of TTF1 and FoxA2 as markers of lung development (A), myc and TGFβ as markers of cell differentiation (B), SP-B and SP-C as markers of type II cells (C), and aquaporin 5 and T1α as markers of type I cells (D) after intrapulmonary injection of C/EBPα siRNA into neonatal lung exposed to 72 h of hyperoxia (O2) and allowed to recover in room air. Values are means ± SE of 6 mice. *P < 0.05 vs. control siRNA-injected group (Con) in 72-h air exposure (Air). †P < 0.05 vs. C/EBPα siRNA-injected group (C/EBPα siRNA) in Air or O2. E: representative Western blots of type I (T1α and caveolin-1) and type II (pro-SP-C and pro-SP-B) cell marker proteins. F: densitometric evaluations of T1α, caveolin-1, pro-SP-B, and pro-SP-C protein levels from 6 lungs in each group. Values are means ± SE of 6 mice. *P < 0.05 vs. C/EBPα siRNA in Air. †P < 0.05 vs. Con in O2. **P < 0.001 vs. C/EBPα siRNA in Air. ††P < 0.001 vs. Con in O2. G: representative Western blots of protein levels of C/EBPα, PCNA (a general cell proliferation marker), p21 (a cell cycle inhibitor protein), and platelet/endothelial cell adhesion marker (PECAM-1, a vascular endothelial cell marker). In PECAM-1 immunoreactive protein evaluations, 3 lanes were omitted because of collapsed wells on acrylamide gel (white spaces). Each blot was normalized to corresponding calnexin protein levels to obviate loading variability.
Surprisingly, changes in pro-SP-C and T1α mRNA did not correspond to changes in protein levels. In fact, pro-SP-C and T1α protein levels were increased in the C/EBPα siRNA O2/air group compared with the control siRNA O2/air group (Fig. 6, E and F) in a reverse of the mRNA levels (Fig. 6, C and D). To confirm these results, additional markers for type II cells, pro-SP-B, and for type I cells, caveolin-1, were evaluated. Both markers were similarly increased, as with pro-SP-C and T1α, respectively (Fig. 6E).
C/EBPα siRNA increases PCNA, a marker of cell proliferation, and decreases cyclin-dependent kinase inhibitor p21 in recovery.
Protein levels of the cell proliferation marker PCNA were enhanced and the cell cycle inhibitor protein p21 was decreased in the C/EBPα siRNA O2/air group (Fig. 6G), suggesting that C/EBPα siRNA improves overall cell proliferation after recovery from hyperoxia, in contrast to 72 h of hyperoxia alone.
C/EBPα siRNA increases PECAM-1, a marker of vascularization.
To understand whether C/EBPα siRNA altered lung vasculature, protein levels of PECAM-1 were evaluated. PECAM-1 protein levels were increased in the C/EBPα siRNA O2/air group compared with the siRNA O2/air controls (Fig. 6G). Additionally, protein levels of caveolin-1, another endothelial cell and type I cell marker (Fig. 6E), were increased in this group, suggesting improved vasculature with C/EBPα siRNA in recovery.
C/EBPα siRNA reverses loss of type II cells and gain of proliferating type II cells in alveolar epithelia after room air recovery.
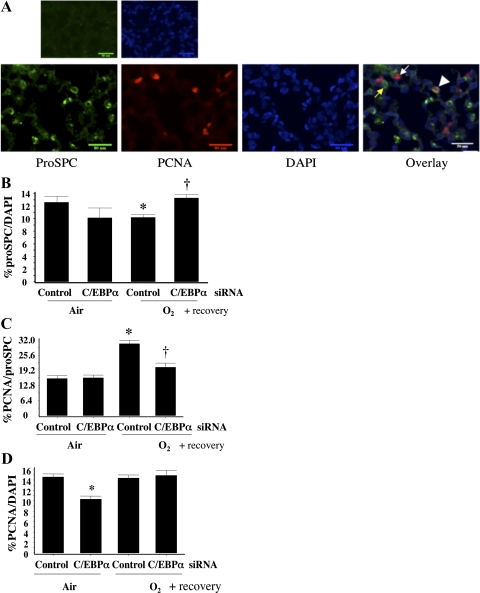
A previous study showed that hyperoxia reduced the number of total type II cells yet increased proliferating type II cells in neonatal mice allowed to recover in room air for 2 wk (24). To understand whether hyperoxia-induced C/EBPα was responsible for these effects, pro-SP-C-positive cells and cells that were positive for both pro-SP-C and PCNA were counted in lung slices (Fig. 7A). There was a significant decrease in the number of type II cells (Fig. 7B) but an increase in PCNA-positive type II cells (Fig. 7C) in the control siRNA O2/air group, in agreement with the literature (24). Both effects were reversed in the C/EBPα siRNA O2/air group (Fig. 7, B and C). We therefore wanted to know whether the decreased proliferating type II cells merely reflected an overall decrease in alveolar cell proliferation. PCNA-positive cells were counted and normalized to DAPI-positive cells. Despite decreased PCNA-positive cells in the C/EBPα siRNA air/air group, there was no difference in PCNA-positive cells in the control siRNA-injected groups with or without hyperoxia (Fig. 7D), suggesting that the decreased proliferation observed with C/EBPα siRNA injection was specific to type II cells.
Fig. 7.
Evaluation of type II cell numbers and proliferating type II cells in control siRNA- and C/EBPα siRNA-injected lung after room air recovery. A: immunofluorescent staining of lung slides for pro-SP-C (indicator of type II cells), PCNA (indicator of overall proliferating cells), and 4′,6-diamidino-2-phenylindole (DAPI, indicator of total cells). Overlay shows cells positive for both pro-SP-C- and PCNA. Yellow arrow indicates pro-SP-C-positive cell, white arrow indicates PCNA-positive cell, and arrowhead indicates cell positive for both pro-SP-C and PCNA. B: pro-SP-C-positive cells as percentage of DAPI-positive cells. *P < 0.05 vs. control siRNA-injected lung (Control) in 72-h air exposure (Air). †P < 0.05 vs. Control in 72-h O2 exposure (O2). C: cells positive for both pro-SP-C and PCNA as percentage of DAPI-positive cells. *P < 0.05 vs. Control in Air. †P < 0.05 vs. Control in O2. D: PCNA-positive cells as percentage of DAPI-positive cells. Ten high-power fields were counted on each lung section at the distal alveolar epithelial level. *P < 0.05 vs. Control in Air. Values were obtained from an average of 3 lungs and an average of 10 high-power fields per lung.
DISCUSSION
We demonstrated that hyperoxia increases C/EBPα mRNA and protein expression in the newborn lung and thereby impacts the development of epithelial type II cells, a major target for hyperoxic injury in the developing lung (24). We showed that increased C/EBPα expression in hyperoxia parallels the disrupted lung architecture and decreased radial alveolar counts and that these changes persist and even worsen after 2 wk of recovery in room air. Inhibition of the induced C/EBPα in hyperoxia improved lung architecture and partially rescued RAC loss after recovery, indicating that C/EBPα plays a crucial role in the regulation of alveolar formation in the neonate. Additionally, C/EBPα siRNA enhanced type II cell numbers and dampened type II cell hyperproliferation in recovery, which suggests that C/EBPα controls type II cell differentiation in hyperoxia and during recovery.
Postnatal lung development involves a series of coordinated events, including active cell proliferation and differentiation, to promote proper alveolar formation. A primary effect of hyperoxia is inhibition of cell proliferation. In the rat, numbers of the four predominant lung cell types (fibroblasts, endothelial cells, type I cells, and type II cells) are highest on postnatal day 10. Specific type II cell proliferation peaks at 7 days of life and decreases to adult levels after 14 days (9). This is when septal crest outgrowth occurs (9), leading to a mature lung architecture. In preliminary studies, it was observed that the expression pattern of C/EBPα mRNA is reciprocal to proliferation of type II cells during normal postnatal lung development (G. Yang, personal communication), which corroborates its antiproliferative role in this cell type. In hyperoxia, C/EBPα protein levels were predominantly induced in the type II cells. We suspect that overexpression of C/EBPα results in disruption of normal type II cell proliferation, leading to the simplified lung architecture observed in hyperoxia.
In a conditional knockout mouse model where C/EBPα was deleted specifically in the lung, animals had hyperproliferating type II cells without lamellar body synthesis and no type I cell formation (3, 10). This established an essential role for C/EBPα as a master regulator of type II cell proliferation and differentiation during normal lung development. Adult mice with disruption of C/EBPα were more susceptible to hyperoxic exposure (21). The effects of C/EBPα in the neonatal lung after hyperoxia had not been studied previously. Our observation that hyperoxia-induced C/EBPα resulted in decreased abundance of type II cells with air recovery in neonates supports the role for C/EBPα in regulating type II cell proliferation during injury and repair in the neonates. The difference in responses between adults and newborns exposed to hyperoxia, when C/EBPα is silenced or disrupted, suggests that specific tissue levels of C/EBPα mediate beneficial or detrimental effects. Whereas neonates, with hyperoxia-induced C/EBPα, benefit from reduction of this level to baseline, adults, without hyperoxia-induced C/EBPα, may be harmed by complete disruption of this protein.
It seems counterintuitive that the total number of type II cells was reduced after hyperoxia followed by air recovery but that these cells were actively proliferating. Perhaps hyperproliferation was a compensatory response for the overall loss of type II cells. C/EBPα siRNA injection appeared to reduce this hyperproliferation by increasing the number of type II cells but not by changing overall proliferating lung cells in the alveoli. Thus the increased PCNA protein levels in whole lung homogenate reflect enhancement of PCNA expression by C/EBPα siRNA during recovery. Although we did not measure cell death in our experiments, minimal type II cell apoptosis has been demonstrated when neonatal mice are exposed to hyperoxia followed by air recovery. Therefore, it is unlikely that the type II cells die in large numbers with hyperoxic exposure. Thus C/EBPα siRNA promotes type II cell proliferation in recovery.
An additional explanation for the hyperproliferating type II cells would be that type II cells are differentiating to type I cells. Because of the known challenges of counting type I cells on lung slides, the actual number of type I cells was not assessed in our study. However, T1α and caveolin-1 protein levels did not increase significantly in lung homogenates after recovery, negating an overall increase in type I cells in the lungs. C/EBPα siRNA injection significantly increased type I cell marker protein levels, suggesting that C/EBPα promotes overall lung epithelial cell proliferation in recovery. The preservation of markers of cell differentiation, myc and TGFβ, suggests that type II cells may have differentiated to other intermediate cell types, although this needs to be further investigated. In fact, C/EBPα siRNA injection significantly decreased myc and TGFβ mRNA levels in the acute phase of hyperoxic exposure, but the decrease was only seen with TGFβ in recovery, indicating that C/EBPα may also affect TGFβ signaling, which is known to be beneficial to alveolarization (12). Whether altered type II cell proliferation and differentiation caused the impaired alveolarization seen after hyperoxia needs clarification.
We did not observe a difference in PCNA protein levels, an index of cell proliferation, in whole lung homogenates at 72 h after hyperoxia. This may be due to the fact that proliferating type II cells are in low abundance on postnatal day 3 (9) and that the many other cell types in the lung may have variable proliferation, thereby masking the specific changes in type II cell proliferation. However, this does not preclude subtle changes in levels within individual cell types or a shift in subcellular distribution without changes in absolute levels (2, 11).
Hyperoxia alters many signaling pathways in the neonatal lung (22, 23). Although increased C/EBPα protein may function as a transcription factor modulating specific lung genes, it could also directly bind to cyclin-dependent kinases to inhibit cell cycle progression via protein-protein interactions, as shown by others (18). In fact, p21, a cell cycle inhibitor gene downstream of C/EBPα, is upregulated in the hyperoxia-exposed neonatal lung (17). We did not observe a significant difference in p21 protein levels in lungs at 72 h of hyperoxia (data not shown), but this protein was decreased twofold in hyperoxia-exposed C/EBPα siRNA-injected mice allowed to recover in room air compared with the control siRNA-injected lung after room air recovery. This could suggest that C/EBPα-mediated p21 expression contributes to the inhibition of type II cell proliferation in hyperoxia and recovery.
In summary, we have demonstrated that hyperoxia increases C/EBPα mRNA and protein expression in neonatal lung type II cells. This was associated with disrupted lung architecture and reduction of RAC immediately after hyperoxia and following room air recovery. Altered type II and type I cell protein makers were evident at 72 h of hyperoxia, and reduced type II cell numbers were observed, despite compensatory increases in proliferation. Inhibition of C/EBPα reversed these effects and improved lung morphology. We speculate that C/EBPα is an important regulator of postnatal type II cell proliferation in hyperoxia and, thus, modulates alveolar injury and repair in the neonate.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grant HL-58752 (P. A. Dennery).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We are grateful to Peggy McDonald and Cheryn Jarvis for administrative assistance and Li Zhi and Nantale Nsibiwa for technical assistance.
The monoclonal antibody T1α, developed by Andrew Farr, was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by The University of Iowa, Department of Biological Sciences (Iowa City, IA).
REFERENCES
- 1. Alejandre-Alcazar MA, Kwapiszewska G, Reiss I, Amarie OV, Marsh LM, Sevilla-Perez J, Wygrecka M, Eul B, Kobrich S, Hesse M, Schermuly RT, Seeger W, Eickelberg O, Morty RE. Hyperoxia modulates TGF-β/BMP signaling in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 292: L537–L549, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Andreyev AY, Shen Z, Guan Z, Ryan A, Fahy E, Subramaniam S, Raetz CR, Briggs S, Dennis EA. Application of proteomic marker ensembles to subcellular organelle identification. Mol Cell Proteomics 9: 388–402, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Basseres DS, Levantini E, Ji H, Monti S, Elf S, Dayaram T, Fenyus M, Kocher O, Golub T, Wong KK, Halmos B, Tenen DG. Respiratory failure due to differentiation arrest and expansion of alveolar cells following lung-specific loss of the transcription factor C/EBPα in mice. Mol Cell Biol 26: 1109–1123, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boggaram V. Regulation of surfactant protein gene expression by hyperoxia in the lung. Antioxid Redox Signal 6: 185–190, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Bonikos DS, Bensch KG, Ludwin SK, Northway WH., Jr Oxygen toxicity in the newborn. The effect of prolonged 100% O2 exposure on the lungs of newborn mice. Lab Invest 32: 619–635, 1975 [PubMed] [Google Scholar]
- 6. Cassel TN, Nord M. C/EBP transcription factors in the lung epithelium. Am J Physiol Lung Cell Mol Physiol 285: L773–L781, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Cooney TP, Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal. 1. Postnatal lung growth. Thorax 37: 572–579, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cooney TP, Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal. 2. Intrauterine and early postnatal lung growth. Thorax 37: 580–583, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kauffman SL, Burri PH, Weibel ER. The postnatal growth of the rat lung. II. Autoradiography. Anat Rec 180: 63–76, 1974 [DOI] [PubMed] [Google Scholar]
- 10. Martis PC, Whitsett JA, Xu Y, Perl AK, Wan H, Ikegami M. C/EBPα is required for lung maturation at birth. Development 133: 1155–1164, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Naryzhny SN, Lee H. The post-translational modifications of proliferating cell nuclear antigen: acetylation, not phosphorylation, plays an important role in the regulation of its function. J Biol Chem 279: 20194–20199, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-β activation contributes to pathogenesis in Marfan syndrome. Nat Genet 33: 407–411, 2003 [DOI] [PubMed] [Google Scholar]
- 13. O'Reilly MA, Staversky RJ, Watkins RH, Reed CK, de Mesy Jensen KL, Finkelstein JN, Keng PC. The cyclin-dependent kinase inhibitor p21 protects the lung from oxidative stress. Am J Respir Cell Mol Biol 24: 703–710, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Pogach MS, Cao Y, Millien G, Ramirez MI, Williams MC. Key developmental regulators change during hyperoxia-induced injury and recovery in adult mouse lung. J Cell Biochem 100: 1415–1429, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Qiao L, MacLean PS, You H, Schaack J, Shao J. Knocking down liver CCAAT/enhancer-binding protein-α by adenovirus-transduced silent interfering ribonucleic acid improves hepatic gluconeogenesis and lipid homeostasis in db/db mice. Endocrinology 147: 3060–3069, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Speer CP. Chorioamnionitis, postnatal factors and proinflammatory response in the pathogenetic sequence of bronchopulmonary dysplasia. Neonatology 95: 353–361, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Timchenko NA, Harris TE, Wilde M, Bilyeu TA, Burgess-Beusse BL, Finegold MJ, Darlington GJ. CCAAT/enhancer binding protein-α regulates p21 protein and hepatocyte proliferation in newborn mice. Mol Cell Biol 17: 7353–7361, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang H, Iakova P, Wilde M, Welm A, Goode T, Roesler WJ, Timchenko NA. C/EBPα arrests cell proliferation through direct inhibition of Cdk2 and Cdk4. Mol Cell 8: 817–828, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Warner BB, Stuart LA, Papes RA, Wispe JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol Lung Cell Mol Physiol 275: L110–L117, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Weng YH, Tatarov A, Bartos BP, Contag CH, Dennery PA. HO-1 expression in type II pneumocytes after transpulmonary gene delivery. Am J Physiol Lung Cell Mol Physiol 278: L1273–L1279, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Xu Y, Saegusa C, Schehr A, Grant S, Whitsett JA, Ikegami M. C/EBPα is required for pulmonary cytoprotection during hyperoxia. Am J Physiol Lung Cell Mol Physiol 297: L286–L298, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang G, Abate A, George AG, Weng YH, Dennery PA. Maturational differences in lung NF-κB activation and their role in tolerance to hyperoxia. J Clin Invest 114: 669–678, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang G, Madan A, Dennery PA. Maturational differences in hyperoxic AP-1 activation in rat lung. Am J Physiol Lung Cell Mol Physiol 278: L393–L398, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Yee M, Vitiello PF, Roper JM, Staversky RJ, Wright TW, McGrath-Morrow SA, Maniscalco WM, Finkelstein JN, O'Reilly MA. Type II epithelial cells are critical target for hyperoxia-mediated impairment of postnatal lung development. Am J Physiol Lung Cell Mol Physiol 291: L1101–L1111, 2006 [DOI] [PubMed] [Google Scholar]