Abstract
Purpose of the review
Hypoxic pulmonary vasoconstriction (HPV) is driven by the intrinsic response to hypoxia of pulmonary arterial smooth muscle and endothelial cells. These are representatives of a group of specialised O2-sensing cells, defined by their acute sensitivity to relatively small changes in pO2, which have evolved to modulate respiratory and circulatory function in order to maintain O2 supply within physiological limits. The aim of this article is to discuss recent investigations into the mechanism(s) of hypoxia-response coupling and, in light of these, provide a critical assessment of current working hypotheses.
Recent Findings
Upon exposure to hypoxia state-of-the-art technologies have now confirmed that mitochondrial oxidative phosphorylation is inhibited in all O2-sensing cells, including pulmonary arterial smooth muscle cells. Thereafter, evidence has been presented to indicate a role as principal effector for the “gasotransmitters” carbon monoxide and hydrogen sulphide, reactive oxygen species or, in marked contrast, reduced cellular redox couples. Considering recent evidence in favour and against these proposals we suggest that an alternative mechanism may be key, namely the activation of AMP-activated protein kinase (AMPK) consequent to inhibition of mitochondrial oxidative phosphorylation.
Summary
HPV supports ventilation-perfusion matching in the lung by diverting blood flow away from oxygen-deprived areas towards regions rich in O2. However, in diseases such as emphysema and cystic fibrosis, widespread HPV leads to hypoxic pulmonary hypertension and ultimately right heart failure. Determining the precise mechanism(s) that underpins hypoxia-response coupling will therefore advance understanding of the fundamental processes contributing to related pathophysiology and provide for improved therapeutics.
Keywords: hypoxia, pulmonary artery, AMPK, ROS, redox, H2S, CO, HO-2
Introduction
The process of hypoxic pulmonary vasoconstriction (HPV) was first identified in 1894, as a rise in pulmonary arterial pressure upon asphyxia1. Fifty years on it was demonstrated that hypoxia without hypercapnia induced constriction within the pulmonary circulation, and the hypothesis proposed that HPV may assist ventilation-perfusion matching in the lung2. Thus, HPV was recognised as the critical and distinguishing characteristic of pulmonary arteries. In contrast, systemic arteries dilate in response to tissue hypoxemia, in order to match local perfusion to local metabolism3.
Early studies showed that gross excitation of the spinal cord caused vasorelaxation within the systemic circulation, without effect on the pulmonary circulation1. Thereafter it was confirmed that HPV was a local response largely, or entirely, independent of the autonomic nervous system4, being evident after chemical sympathectomy, surgical denervation of the carotid and aortic chemoreceptors or after bilateral cervical vagotomy5, 6. Most significantly, bilateral lung transplants established that HPV remained unaffected following denervation in man7. Therefore, neither central nor local regulation of the autonomic nervous system contributes to HPV.
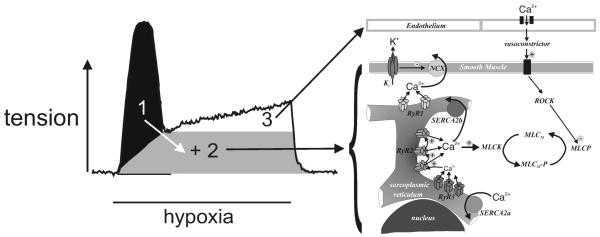
HPV is not induced when the lung is perfused with hypoxic blood at a constant, normoxic alveolar oxygen tension8, but by a fall in airway / alveolar pO29. Moreover, precapillary resistance arteries contribute most to the increase in pulmonary vascular perfusion pressure during alveolar hypoxia, the magnitude of HPV being inversely related to pulmonary artery diameter10. The threshold for HPV is ~60 mmHg and thereafter HPV increases in magnitude in a manner proportional to the degree of hypoxia11, but it fails under near anoxic conditions(~5 mmHg)11. In the perfused and ventilated rat lung a monophasic and sustained increase in perfusion pressure is induced by hypoxia11, 12, while HPV appears biphasic in isolated pulmonary arteries (Fig. 1) comprising a discrete transient constriction (Phase 1; 5-10 min) and a concomitant slow tonic constriction (Phase 2; peak after 30-40 min)13, 14.
Figure 1. Hypoxic pulmonary vasoconstriction.
Experimental record (left) highlights three identified components that underpin the transient and tonic phases of hypoxia-induced constriction of an isolated pulmonary artery ring. In sequence, two discrete mechanisms enhance calcium release from the smooth muscle sarcoplasmic reticulum (1, black; 2, grey) with a third component underpinned by the release of a vasoconstrictor from the endothelium (3, white). The mechanisms involved are described in further detail in the associated schematic (right): Kv, voltage-gated potassium current; NCX, sodium / calcium exchanger; RyR, ryanodine receptor; SERCA, sarco/endoplasmic reticulum Ca2+ ATPase; MLCK, myosin light chain kinase; MLC20, myosin light chain-20; MLCP, myosin phosphatase; ROCK, Rho associated kinase.
Mechanisms intrinsic to the smooth muscle and endothelial cells mediate HPV (Fig. 1), with calcium release from the smooth muscle sarcoplasmic reticulum via ryanodine receptors pivotal11, 12, 14 and subsequent constriction augmented, via myofilament calcium sensitisation, by the release of an as yet unidentified vasoconstrictor from the endothelium14, 15. Hypoxia also modulates the activity of voltage-gated potassium channels (Kv) in the plasma membrane of the smooth muscle cells16-18, although the functional consequence remains contentious19. More contentious still, is the mechanism of hypoxia-response coupling, which is likely common to all O2-sensing cells, including pulmonary arterial smooth muscle and endothelial cells, carotid body type I cells and neonatal adrenomedullary chromaffin cells. These cells have been defined by their acute sensitivity to “activation” by relatively small changes in pO2 and consideration of recent observations on hypoxia-response coupling in all representatives of this group is most revealing.
Heme oxygenase-2 as an O2 sensor
Heme oxygenase-2 (HO-2) has been implicated in both pulmonary artery constriction20 and carotid body activation by hypoxia21, 22. This was intriguing given the requirement of this enzyme for NADPH and O2 as co-factors for the generation of carbon monoxide (CO), biliverdin and Fe2+ by catabolism of heme. Thus, it was proposed that under normoxic conditions, HO-2 controlled targets within the signal transduction cascade tonically through the production of CO (Fig. 2D) and that the activity of protein targets would be modulated by hypoxia because the co-factor for CO production, O2, was limiting and / or through modulation by accumulating heme (due to the lack of its degradation by HO-222). However, data from transgenic mice lacking HO-2 are variable. Some report that hypoxia-response coupling in HO-2−/− mice is blunted20, while others suggest that hypoxia-response coupling remains unaltered23-25. Thus, it seems unlikely that HO-2 is physiologically important to hypoxia-response coupling in O2 sensing cells.
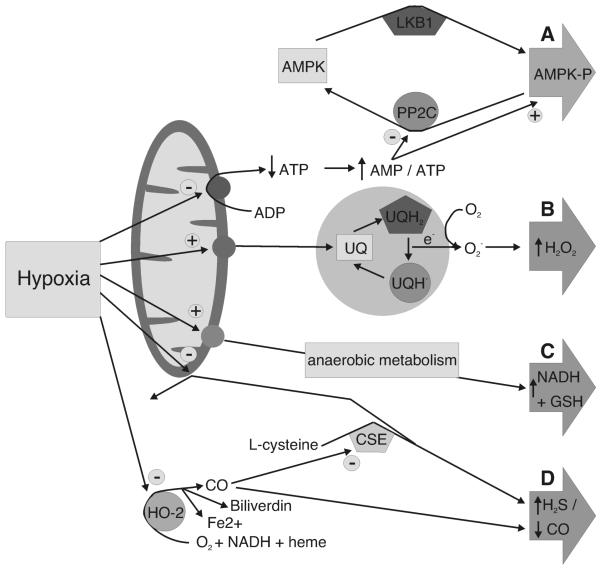
Figure 2. Proposed mechanisms of hypoxia-response coupling.
A, activation of AMP-activated protein kinase (AMPK) initiated by an increase in the cellular AMP / ATP ratio: PP2C, protein phosphatase 2C. B, Electron transfer and ROS production via complex III of the mitochondrial electron transport chain: UQ, ubiquinol; UQH2, reduced ubisemiquinone; UQH., usemibiquinone radical. C, Reduction in the cellular redox status: NADH, reduced β-nicotinamide adenine dinucleotide; GSH, reduced glutathione. D, increased H2S and / or decreased CO: HO-2, heme oxygenase-2; CSE, cystathionine γ-lyase.
Hydrogen sulphide and hypoxia-response coupling
Another gasotransmitter, H2S, has been implicated in HPV26 and carotid body activation by hypoxia27. Thus, hypoxia has been proposed to promote, indirectly, H2S accumulation via cystathionine γ-lyase (which generates H2S in peripheral systems). Two alternative mechanisms have, however, been considered. One suggestion is that under normoxic conditions HO-2 derived CO suppresses H2S production (Fig. 2D), an effect which is depressed by hypoxia because of the requirement of HO-2 for O2 to generate CO27. The alternative proposal is that hypoxia suppresses “normal” mitochondrial H2S metabolism in an O2-dependent manner26. However, recent studies have cast doubt on these hypotheses28, 29, and it is clear that the ability of H2S to mimic HPV may be due to the previously noted inhibition by H2S of mitochondrial oxidative phosphorylation28, 30.
That altered production of any “gasotransmitter” is of central importance to O2 sensing therefore remains open to question, although H2S may contribute to the inhibition of mitochondrial oxidative phosphorylation by hypoxia (see below) and, thereby, CO may influence this process. It should noted, however, that nitric oxide does exert an influence, since inhibitors of nitric oxide synthase potentiate HPV31 and increase carotid body afferent fibre discharge32.
An emerging role for AMP-activated protein kinase in O2 sensing
The ubiquitously expressed AMP-activated protein kinase (AMPK) is a heterotrimer comprising catalytic α and regulatory β and γ subunits, of which there are multiple isoforms33. In response to metabolic stress, AMPK is activated by an increase in the ADP/ATP ratio, which is amplified by adenylate kinase into a greater increase in the AMP/ATP ratio34. Activation of AMPK (>100-fold) is conferred by phosphorylation at Thr-172 within the α subunit by upstream kinases, of which the most important is the tumor suppressor, LKB1. LKB1 appears to phosphorylate Thr-172 constitutively, with binding of AMP to the two exchangeable sites on the γ subunit inhibiting dephosphorylation and yielding the active, phosphorylated form. Binding of AMP to the γ subunit also causes allosteric activation by ~10-fold; the combinatorial effect causing >1000-fold activation. This ensures great sensitivity, with basal AMPK activity kept low in unstressed cells by ATP and ADP competing with AMP for binding at the γ subunit sites. Thus, AMPK can be activated within seconds35 and serves to maintain ATP supply by upregulating catabolic processes, such as β-oxidation of fatty acids, and suppressing non-essential ATP-consuming reactions33. Pertinent to this review, is the new concept that AMPK may contribute to the regulation of O2 and thereby energy (ATP) supply at the whole-body level and in doing so provide for a universal mechanism of hypoxia-response coupling19, 36. That AMPK may regulate aspects of cell function other than metabolism brings us to the mitochondrial hypothesis for O2 sensing.
The first direct indication of a role for mitochondria was provided by studies on the carotid body, in which spectrophotometric analysis of the respiratory chain redox status and fluorometric measurement of the NAD(P)H/NAD(P)+ ratio were related to afferent sinus nerve discharge during hypoxia37. An increase in the NAD(P)H/NAD(P)+ ratio was observed, which correlated with afferent nerve activity over the physiological range of O2 levels. It was suggested that mitochondria of most cells may utilise a high affinity (i.e. normal) cytochrome a3, while the cytochrome a3 incorporated in mitochondria of O2 sensing cells may have a low affinity for O2. However, evidence now points not to the mitochondria themselves, but to their local environment. Firstly, intracellular O2 gradients, and possibly ATP gradients, occur at the cellular level38 and such gradients may vary between tissues. Moreover, there are tissue-specific differences with respect to mitochondrial function that have been attributed to tissue-specific O2 supply, substrate availability and other intracellular variables (including ADP and ATP demand). Such variables can regulate many aspects of mitochondrial function, including the affinity of cytochrome c oxidase for O239. In this respect, it is also notable that recent studies suggest that PKC delta may modulate the rate of O2 consumption and ATP generation by mitochondria40. Therefore, the exquisite sensitivity of O2 sensing cells to a fall in O2 levels could be attributed to a high rate of O2 consumption, as has long been claimed41-43.
The strongest evidence in favour of a requirement for functional mitochondria in O2 sensing comes from recent studies on immortalised neonatal adrenomedullary chromaffin cells that incorporate or lack functional mitochondria. Those with functional mitochondria were found to respond to hypoxia and to inhibitors of mitochondrial oxidative phosphorylation. By contrast those cells lacking functional mitochondria failed to respond to either stimulus44, 45. Moreover, recent studies on the pulmonary vasculature have provided new spectrophotometric evidence that mitochondrial respiration in pulmonary arterial smooth muscle is indeed inhibited by hypoxia over the physiological range, and is particularly sensitive to changes in pO2 when compared with systemic arterial smooth muscle46; that hypoxia increases the NAD(P)H / NAD(P)+ ratio in pulmonary arterial smooth muscle47 supports this view. Significantly, the threshold for inhibition of mitochondrial oxidative phosphorylation was ~60mmHg and inhibition increased in a manner related to the degree of hypoxia, as is the case with HPV11 and carotid body activation by hypoxia48.
Consistent with these findings, comprehensive data indicate that inhibitors of mitochondria (either uncouplers or blockers of specific respiratory chain complexes) mimic hypoxia in their ability to inhibit leak K+ currents and thereby induce voltage-gated Ca2+ entry into carotid body type I cells49. Clear parallels exist with similar studies on the pulmonary vasculature, although there is one twist in the tale. All mitochondrial inhibitors tested thus far mimic the effects of hypoxia at the level of the O2-sensitive Kv current17. Yet only some mitochondrial inhibitors mimic and occlude HPV in the perfused lung50, while others have been shown to block but not mimic HPV in the perfused lung and isolated pulmonary arteries47, 50, 51. This has been a bone of contention in the field and has been cast as inconsistent with the view that HPV, at least, may be triggered by inhibition of mitochondrial oxidative phosphorylation.
However, it has been noted that HPV fails under near anoxic conditions (< 2% O2), i.e. there is a pO2 window within which hypoxia may trigger pulmonary artery constriction52. Moreover, early studies on the carotid body showed that afferent fibre discharge is depressed under anoxic conditions, and so too is the response of the carotid body to mitochondrial inhibition53. It is notable, therefore, that in dorsal root ganglion neurones, which do not serve to monitor O2 supply, no shift in the NAD(P)H/NAD(P)+ ratio is observed until the pO2 falls to ~5 mmHg, at which point HPV and carotid body discharge begin to fail. Why might this be significant? Strictly speaking, it is the “anoxic” and not the “hypoxic” condition that mitochondrial inhibitors would mimic at concentrations that ablate oxidative phosphorylation. Therefore, an explanation for the inconsistency of outcome with respect to the effects of mitochondrial inhibitors on HPV and the pO2 window within which HPV is triggered, may ultimately be provided by a greater understanding of the impact of degrees of metabolic stress. A case in point with respect to mitochondrial inhibitors may be that one such agent, metformin, provides for effective therapy of type II diabetes via AMPK activation, whereas a more potent analogue, phenformin, is no longer prescribed because of related contraindications54.
These considerations bring us back nicely to AMPK, which is activated by all mitochondrial inhibitors in a manner dependent on the degree of inhibition of mitochondrial oxidative phosphorylation. Consider the possibility, therefore, that physiological levels of hypoxia may activate AMPK and thereby precipitate, for example, HPV. It is quite possible that during more extreme metabolic stress, such as anoxia, AMPK may revert to its now classical role and “switch off” non-essential ATP-consuming processes in order to ensure cell survival. In other words, when smooth muscle “energy reserves” fail to maintain a desired level of ATP supply via, for example, β-oxidation of fatty acids55, AMPK may not function itself to drive pulmonary artery constriction. After all, dilating pulmonary arteries in response to anoxia might be a logical “last gasp” in terms of achieving optimal gaseous exchange within the lungs
What then of the evidence in support of a role for AMPK in O2 sensing? Perhaps the most detailed information to date comes from investigations on pulmonary arteries56. Hypoxia precipitates an increase in the AMP/ATP ratio in pulmonary arterial smooth muscle, concomitant activation of AMPK (Fig. 2A) and phosphorylation of acetyl CoA carboxylase (an established marker for AMPK action), despite the fact that cellular ATP levels remain remarkably stable in the presence of hypoxia57. Moreover, AMPK activation is induced in pulmonary arterial smooth muscle both by the mitochondrial inhibitor phenformin and by AICAR, which activates AMPK not by altering the AMP/ATP ratio but by uptake and subsequent metabolism to the AMP mimetic, ZMP56. Each agent also induced an increase in the intracellular Ca2+ concentration in acutely isolated pulmonary arterial smooth muscle cells, by mobilising sarcoplasmic reticulum stores via ryanodine receptors as does hypoxia. As expected, however, only phenformin increased the cellular NAD(P)H autofluorescence. Most significantly, AMPK activation by AICAR evoked a slow, sustained and reversible constriction of pulmonary artery rings that exhibits all the primary characteristics of HPV, namely a requirement for smooth muscle SR Ca2+ release via ryanodine receptors, and Ca2+ influx into and vasoconstrictor release from the endothelium. Moreover, preliminary data also suggest that AMPK modulates plasmalemmal Kv channels in a similar manner to hypoxia58. Consistent with these findings the non-selective AMPK antagonist, compound C, blocks HPV59.
The proposal that AMPK may be of general importance to hypoxia-response coupling in all O2 sensing cells gained notable support from studies on carotid body type I cells56, 60. Firstly, AICAR was shown to elicit a rise in [Ca2+]i in isolated rat type I cells, and increased afferent sensory nerve activity recorded from the carotid sinus nerve - effectively mimicking hypoxia. Importantly, AMPK activation, like hypoxia, triggered these events by selectively inhibiting both BKCa and leak K+ currents in rat type I cells, and thereby causing voltage-gated Ca2+ entry. Furthermore, emerging data from mice lacking the α2 subunit of AMPK indicate an important role for AMPK in the carotid body-mediated ventilatory response to hypoxia19. These observations suggest that hypoxia-response coupling may occur by direct phosphorylation and regulation of O2 sensitive ion channels by AMPK, as AMPK phosphorylates and regulates, for example, recombinant BKCa channels in an AMP-dependent manner60. Thus, this mechanism provides a simple and conceptually satisfying link between the mitochondrial and membrane hypotheses for O2 sensing in both the pulmonary artery and the carotid body.
Mitochondria, hypoxia and reactive oxygen species (ROS)
Schumacker, Chandel and colleagues were the first to propose that hypoxia may trigger a paradoxical increase in ROS production at complex III of the electron transport chain61, 62 (Fig. 2B). That this may mediate HPV has gained significant prominence and support over the last 10 years47, 63. However, all measures of ROS production have been carried out under relatively severe hypoxia (≤ 5mm Hg). Moreover, it is notable that measured ROS generation occurred over the same range of pO2 in (cultured) pulmonary arterial smooth muscle62 and a wide range of other cultured cell types64-67 that do not serve to monitor / sense O2 supply. At face value this would not allow for selective regulation by hypoxia of O2 sensing cells. Moreover, both HPV and carotid body discharge are depressed by the degree of hypoxia utilised.
Most recently, it was suggested that mitochondrial ROS can lead to activation of AMPK without a shift in the AMP/ATP ratio68, 69. However, these studies were carried out on embryonic fibroblasts and osteosarcoma cells, which again are not recognised O2 sensors. Therefore, outcomes most likely reflect a generalised cellular response to severe hypoxia. Nonetheless, it was reported that AMPK was activated via LKB1 only when functional mitochondria were present, with no change in the AMP/ATP ratio, and that AMPK activation was blocked by an antioxidant. Moreover, in mitochondria-deficient cells AMPK was activated by exogenous application of H2O2 and in cells lacking mitochondrial cytochrome b (which would not be expected to consume O2, while allowing ROS generation at complex III) AMPK was activated by hypoxia. Importantly, however, in LKB1 deficient cells the response to H2O2 was markedly attenuated, suggesting that H2O2 must necessarily, like AMP, inhibit dephosphorylation of AMPK at Thr-172. Collectively, therefore, it may be reasonable to conclude that hypoxia could activate O2 sensing cells through ROS-dependent activation of AMPK, but available evidence suggests this scenario is unlikely. Thus, Hawley et al. (2010) have demonstrated conclusively, using HEK293 cells expressing either wild-type AMPK or an AMP-insensitive mutant, that H2O2 activates AMPK indirectly and in an LKB1-dependent manner, by inhibiting mitochondrial function and increasing the AMP/ATP ratio70. Therefore, if mitochondrial ROS were to contribute to AMPK activation by hypoxia this would likely be by way of facilitating mitochondrial inhibition.
There are, however, other issues that remain to be accounted for with respect to the ROS hypothesis:
Antimycin A increases mitochondrial ROS production71 and ablates but does not mimic HPV50, whilst mitochondrial inhibitors that do not increase ROS also mimic and / or occlude HPV47, 50. By contrast all mitochondrial inhibitors examined, antimycin A included, modulate in a manner consistent with the effects of hypoxia all O2 sensitive K channels in pulmonary arterial smooth muscle17, carotid body type I cells49 and neonatal adrenomedullary chromaffin cells45.
In carotid body type I cells H2O2 does not increase [Ca2+]i, nor does it interfere with the hypoxic response of type I cells. Furthermore, in neonatal adrenomeduallary chromaffin cells H2O2 opposes hypoxia-response coupling72.
Hyperoxia, which precipitates ROS formation in all cell types73, is without significant effect on the pulmonary vasculature74 and attenuates carotid body output75.
Application of oxidising and reducing agents provides inconsistent outcomes in terms of the functional response to hypoxia of pulmonary arteries63, 76-79 and carotid body type I cells80, 81.
In studies on isolated mitochondria an increase in mitochondrial ROS has been observed under hyperoxia, but not in response to hypoxia82, 83. So, can mitochondria per se provide for both increased ROS generation and release in response to hypoxia in O2 sensing cells46, 84?
Finally, it remains a concern that some laboratories consistently measure an increase in mitochondrial ROS in response to hypoxia irrespective of the cell type46, 62, 64, 65, while others observe either no change or the exact opposite in all O2 sensing cells16, 72, 80, 85. Either way, experimental observations are less than robust and likely provide as much evidence against as for the proposal that changes in the cellular redox status (Fig. 2C) or ROS per se underpin hypoxia-response coupling. Nonetheless, it is clear that physiological levels of hypoxia increase the NAD(P)H / NAD(P)+ ratio, and this may contribute to hypoxia-response coupling(see for example86) in addition to the regulation of AMPK by the AMP/ATP ratio.
Key Points.
It is unlikely that CO / H2S and ROS contribute to hypoxia-response coupling other than by inhibiting mitochondrial oxidative phosphorylation.
Accumulating evidence supports a central role for AMPK, which is likely necessary and sufficient for hypoxia-response coupling in O2 sensing cells.
The precise nature of the functional outcome triggered by AMPK activation will depend not only on the AMPK heterotrimers present, but on the types of AMPK-sensitive and/or AMPK-insensitive ion channels (or other protein targets) expressed by a given cell type. Thereby AMPK may also differentially regulate a variety of cells that respond to metabolic signals other than hypoxia, such as glucose.
Identification of the ion channels and transporters regulated by AMPK will account for cell- and system-specific responses vital to whole body energy homeostasis.
It is our view, therefore, that AMPK regulates O2 and energy (ATP) supply at the whole body level.
Acknowledgements
These studies were supported by two Programme Grants from the Wellcome Trust (81195, to AME, CP and DGH).
Footnotes
There are no commercial interests or conflicts of interest.
References
- 1.Bradford JR, Dean HP. The Pulmonary Circulation. The Journal of physiology. 1894;16(1-2):34–158. 125. doi: 10.1113/jphysiol.1894.sp000493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Euler US, Liljestrand G. Observations on the pulmonary arterial blood pressure in the cat. Acta physiologica Scandinavica. 1946;12:301–320. [Google Scholar]
- 3.Roy CS, Sherrington CS. On the Regulation of the Blood-supply of the Brain. The Journal of physiology. 1890;11(1-2):85–158. 117. doi: 10.1113/jphysiol.1890.sp000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nisell O. The influence of blood gases on the pulmonary vessels of the cat. Acta physiologica Scandinavica. 1951;23(1):85–90. doi: 10.1111/j.1748-1716.1951.tb00821.x. [DOI] [PubMed] [Google Scholar]
- 5.Naeije R, Lejeune P, Leeman M, Melot C, Closset J. Pulmonary vascular responses to surgical chemodenervation and chemical sympathectomy in dogs. J Appl Physiol. 1989;66(1):42–50. doi: 10.1152/jappl.1989.66.1.42. [DOI] [PubMed] [Google Scholar]
- 6.Lejeune P, Vachiery JL, Leeman M, Brimioulle S, Hallemans R, Melot C, Naeije R. Absence of parasympathetic control of pulmonary vascular pressure-flow plots in hyperoxic and hypoxic dogs. Respiration physiology. 1989;78(2):123–133. doi: 10.1016/0034-5687(89)90046-7. [DOI] [PubMed] [Google Scholar]
- 7.Robin ED, Theodore J, Burke CM, Oesterle SN, Fowler MB, Jamieson SW, Baldwin JC, Morris AJ, Hunt SA, Vankessel A, et al. Hypoxic pulmonary vasoconstriction persists in the human transplanted lung. Clin Sci (Lond) 1987;72(3):283–287. doi: 10.1042/cs0720283. [DOI] [PubMed] [Google Scholar]
- 8.Duke HN, Killick EM. Pulmonary vasomotor responses of isolated perfused cat lungs to anoxia. The Journal of physiology. 1952;117(3):303–316. doi: 10.1113/jphysiol.1952.sp004750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergofsky EH, Haas F, Porcelli R. Determination of the sensitive vascular sites from which hypoxia and hypercapnia elicit rises in pulmonary arterial pressure. Federation proceedings. 1968;27(6):1420–1425. [PubMed] [Google Scholar]
- 10.Kato M, Staub NC. Response of small pulmonary arteries to unilobar hypoxia and hypercapnia. Circulation research. 1966;19(2):426–440. doi: 10.1161/01.res.19.2.426. [DOI] [PubMed] [Google Scholar]
- 11.Dipp M, Evans AM. Cyclic ADP-ribose is the primary trigger for hypoxic pulmonary vasoconstriction in the rat lung in situ. Circulation research. 2001;89(1):77–83. doi: 10.1161/hh1301.093616. [DOI] [PubMed] [Google Scholar]
- 12.Morio Y, McMurtry IF. Ca(2+) release from ryanodine-sensitive store contributes to mechanism of hypoxic vasoconstriction in rat lungs. J Appl Physiol. 2002;92(2):527–534. doi: 10.1152/jappl.2002.92.2.527. [DOI] [PubMed] [Google Scholar]
- 13.Leach RM, Robertson TP, Twort CH, Ward JP. Hypoxic vasoconstriction in rat pulmonary and mesenteric arteries. The American journal of physiology. 1994;266(3 Pt 1):L223–231. doi: 10.1152/ajplung.1994.266.3.L223. [DOI] [PubMed] [Google Scholar]
- 14.Dipp M, Nye PC, Evans AM. Hypoxic release of calcium from the sarcoplasmic reticulum of pulmonary artery smooth muscle. American journal of physiology. 2001;281(2):L318–325. doi: 10.1152/ajplung.2001.281.2.L318. [DOI] [PubMed] [Google Scholar]
- 15.Robertson TP, Ward JP, Aaronson PI. Hypoxia induces the release of a pulmonary-selective, Ca(2+)-sensitising, vasoconstrictor from the perfused rat lung. Cardiovascular research. 2001;50(1):145–150. doi: 10.1016/s0008-6363(01)00192-4. [DOI] [PubMed] [Google Scholar]
- 16.Archer SL, Huang J, Henry T, Peterson D, Weir EK. A redox-based O2 sensor in rat pulmonary vasculature. Circulation research. 1993;73(6):1100–1112. doi: 10.1161/01.res.73.6.1100. [DOI] [PubMed] [Google Scholar]
- 17.Firth AL, Yuill KH, Smirnov SV. Mitochondria-dependent regulation of Kv currents in rat pulmonary artery smooth muscle cells. American journal of physiology. 2008;295(1):L61–70. doi: 10.1152/ajplung.90243.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Platoshyn O, Yu Y, Ko EA, Remillard CV, Yuan JX. Heterogeneity of hypoxia-mediated decrease in I(K(V)) and increase in [Ca2+](cyt) in pulmonary artery smooth muscle cells. American journal of physiology. 2007;293(2):L402–416. doi: 10.1152/ajplung.00391.2006. [DOI] [PubMed] [Google Scholar]
- 19.Evans AM, Hardie DG, Peers C, Wyatt CN, Viollet B, Kumar P, Dallas ML, Ross F, Ikematsu N, Jordan HL, Barr BL, Rafferty JN, Ogunbayo O. Ion channel regulation by AMPK: the route of hypoxia-response coupling in thecarotid body and pulmonary artery. Annals of the New York Academy of Sciences. 2009;1177:89–100. doi: 10.1111/j.1749-6632.2009.05041.x. [DOI] [PubMed] [Google Scholar]
- 20.Adachi T, Ishikawa K, Hida W, Matsumoto H, Masuda T, Date F, Ogawa K, Takeda K, Furuyama K, Zhang Y, Kitamuro T, Ogawa H, Maruyama Y, Shibahara S. Hypoxemia and blunted hypoxic ventilatory responses in mice lacking heme oxygenase-2. Biochemical and biophysical research communications. 2004;320(2):514–522. doi: 10.1016/j.bbrc.2004.05.195. [DOI] [PubMed] [Google Scholar]
- 21.Prabhakar NR. Endogenous carbon monoxide in control of respiration. Respiration physiology. 1998;114(1):57–64. doi: 10.1016/s0034-5687(98)00072-3. [DOI] [PubMed] [Google Scholar]
- 22.Williams SE, Wootton P, Mason HS, Bould J, Iles DE, Riccardi D, Peers C, Kemp PJ. Hemoxygenase-2 is an oxygen sensor for a calcium-sensitive potassium channel. Science (New York, N.Y. 2004;306(5704):2093–2097. doi: 10.1126/science.1105010. [DOI] [PubMed] [Google Scholar]
- 23.Ortega-Saenz P, Pascual A, Gomez-Diaz R, Lopez-Barneo J. Acute oxygen sensing in heme oxygenase-2 null mice. The Journal of general physiology. 2006;128(4):405–411. doi: 10.1085/jgp.200609591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ortega-Saenz P, Pascual A, Piruat JI, Lopez-Barneo J. Mechanisms of acute oxygen sensing by the carotid body: lessons from genetically modified animals. Respiratory physiology & neurobiology. 2007;157(1):140–147. doi: 10.1016/j.resp.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Roth M, Rupp M, Hofmann S, Mittal M, Fuchs B, Sommer N, Parajuli N, Quanz K, Schubert D, Dony E, Schermuly RT, Ghofrani HA, Sausbier U, Rutschmann K, Wilhelm S, Seeger W, Ruth P, Grimminger F, Sausbier M, Weissmann N. Heme oxygenase-2 and large-conductance Ca2+-activated K+ channels: lung vascular effects of hypoxia. American journal of respiratory and critical care medicine. 2009;180(4):353–364. doi: 10.1164/rccm.200806-848OC. *HO-2 KO is without effect on the vascular response to alveolar hypoxia
- 26.Olson KR, Whitfield NL, Bearden SE, St Leger J, Nilson E, Gao Y, Madden JA. Hypoxic pulmonary vasodilation: a paradigm shift with a hydrogen sulfide mechanism. Am J Physiol Regul Integr Comp Physiol. 2010;298(1):R51–60. doi: 10.1152/ajpregu.00576.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng YJ, Nanduri J, Raghuraman G, Souvannakitti D, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR. H2S mediates O2 sensing in the carotid body. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(23):10719–10724. doi: 10.1073/pnas.1005866107. *Cystathionine γ-lyase KO reduces basal afferent fibre discharge from the carotid body and attenuates afferent fibre discharge in response to hypoxia. However, it is notable that minute ventilation of the KO mice is reduced relative to control, and the ventilatory response is retained albeit blunted.
- 28.Connolly M, Prieto-Lloret J, Shaifta Y, Ward JP, Aaronson PI. Hydrogen sulphide mimics rather than mediates hypoxic pulmonary vasoconstriction in rat inrapulmonary arteries. Proc Physiol Soc. 2010;19:C93. [Google Scholar]
- 29.Fitzgerald RS, Shirahata M, Kostuk E. Hypoxia vs hydrogen sulfide (H2S) acting at teh carotid body (CB) and elsewhere systemically. FASEB J. 2010;24 1026.1022. [Google Scholar]
- 30.Cooper CE, Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. Journal of bioenergetics and biomembranes. 2008;40(5):533–539. doi: 10.1007/s10863-008-9166-6. [DOI] [PubMed] [Google Scholar]
- 31.Robertson BE, Warren JB, Nye PC. Inhibition of nitric oxide synthesis potentiates hypoxic vasoconstriction in isolated rat lungs. Experimental physiology. 1990;75(2):255–257. doi: 10.1113/expphysiol.1990.sp003399. [DOI] [PubMed] [Google Scholar]
- 32.Prabhakar NR. NO and CO as second messengers in oxygen sensing in the carotid body. Respiration physiology. 1999;115(2):161–168. doi: 10.1016/s0034-5687(99)00019-5. [DOI] [PubMed] [Google Scholar]
- 33.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nature reviews. 2007;8(10):774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 34.Hardie DG, Hawley SA. AMP-activated protein kinase: the energy charge hypothesis revisited. Bioessays. 2001;23(12):1112–1119. doi: 10.1002/bies.10009. [DOI] [PubMed] [Google Scholar]
- 35.Tamas P, Hawley SA, Clarke RG, Mustard KJ, Green K, Hardie DG, Cantrell DA. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. The Journal of experimental medicine. 2006;203(7):1665–1670. doi: 10.1084/jem.20052469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans AM. AMP-activated protein kinase and the regulation of Ca2+ signalling in O2-sensing cells. The Journal of physiology. 2006;574(Pt 1):113–123. doi: 10.1113/jphysiol.2006.108381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mills E, Jobsis FF. Mitochondrial respiratory chain of carotid body and chemoreceptor response to changes in oxygen tension. Journal of neurophysiology. 1972;35(4):405–428. doi: 10.1152/jn.1972.35.4.405. [DOI] [PubMed] [Google Scholar]
- 38.Jones DP. Intracellular diffusion gradients of O2 and ATP. The American journal of physiology. 1986;250(5 Pt 1):C663–675. doi: 10.1152/ajpcell.1986.250.5.C663. [DOI] [PubMed] [Google Scholar]
- 39.Gnaiger E, Lassnig B, Kuznetsov A, Rieger G, Margreiter R. Mitochondrial oxygen affinity, respiratory flux control and excess capacity of cytochrome c oxidase. The Journal of experimental biology. 1998;201(Pt 8):1129–1139. doi: 10.1242/jeb.201.8.1129. [DOI] [PubMed] [Google Scholar]
- 40.Acin-Perez R, Hoyos B, Gong J, Vinogradov V, Fischman DA, Leitges M, Borhan B, Starkov A, Manfredi G, Hammerling U. Regulation of intermediary metabolism by the PKC{delta} signalosome in mitochondria. Faseb J. 2010 doi: 10.1096/fj.10-166934. *Identification of a molecular signalling cascade that allows for cell-specific modulation of intermediary metabolism.
- 41.Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiological reviews. 1994;74(4):829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- 42.Duchen MR, Biscoe TJ. Relative mitochondrial membrane potential and [Ca2+]i in type I cells isolated from the rabbit carotid body. The Journal of physiology. 1992;450:33–61. doi: 10.1113/jphysiol.1992.sp019115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duchen MR, Biscoe TJ. Mitochondrial function in type I cells isolated from rabbit arterial chemoreceptors. The Journal of physiology. 1992;450:13–31. doi: 10.1113/jphysiol.1992.sp019114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson RJ, Farragher SM, Cutz E, Nurse CA. Developmental regulation of O(2) sensing in neonatal adrenal chromaffin cells from wild-type and NADPH-oxidase-deficient mice. Pflugers Arch. 2002;444(4):539–548. doi: 10.1007/s00424-002-0853-6. [DOI] [PubMed] [Google Scholar]
- 45.Buttigieg J, Brown ST, Lowe M, Zhang M, Nurse CA. Functional mitochondria are required for O2 but not CO2 sensing in immortalized adrenomedullary chromaffin cells. Am J Physiol Cell Physiol. 2008;294(4):C945–956. doi: 10.1152/ajpcell.00495.2007. [DOI] [PubMed] [Google Scholar]
- 46.Sommer N, Pak O, Schorner S, Derfuss T, Krug A, Gnaiger E, Ghofrani HA, Schermuly RT, Huckstorf C, Seeger W, Grimminger F, Weissmann N. Mitochondrial cytochrome redox states and respiration in acute pulmonary oxygen sensing. Eur Respir J. 2010 doi: 10.1183/09031936.00013809. *Direct measurements of mitochondrial respiratory function over the physiological range of pO2.
- 47.Leach RM, Hill HM, Snetkov VA, Robertson TP, Ward JP. Divergent roles of glycolysis and the mitochondrial electron transport chain in hypoxic pulmonary vasoconstriction of the rat: identity of the hypoxic sensor. The Journal of physiology. 2001;536(Pt 1):211–224. doi: 10.1111/j.1469-7793.2001.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar P. Sensing hypoxia in the carotid body: from stimulus to response. Essays in biochemistry. 2007;43:43–60. doi: 10.1042/BSE0430043. [DOI] [PubMed] [Google Scholar]
- 49.Wyatt CN, Buckler KJ. The effect of mitochondrial inhibitors on membrane currents in isolated neonatal rat carotid body type I cells. The Journal of physiology. 2004;556(Pt 1):175–191. doi: 10.1113/jphysiol.2003.058131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weissmann N, Ebert N, Ahrens M, Ghofrani HA, Schermuly RT, Hanze J, Fink L, Rose F, Conzen J, Seeger W, Grimminger F. Effects of mitochondrial inhibitors and uncouplers on hypoxic vasoconstriction in rabbit lungs. American journal of respiratory cell and molecular biology. 2003;29(6):721–732. doi: 10.1165/rcmb.2002-0217OC. [DOI] [PubMed] [Google Scholar]
- 51.Waypa GB, Chandel NS, Schumacker PT. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circulation research. 2001;88(12):1259–1266. doi: 10.1161/hh1201.091960. [DOI] [PubMed] [Google Scholar]
- 52.Dipp M, Thomas JM, Galione A, Evans AM. A PO2 window for smooth muscle cADPR accumulation and constriction by hypoxia in rabbit pulmonary artery smooth muscle. Proc. Phys. Soc. 2003;547P:C72. [Google Scholar]
- 53.Eyzaguirre C, Koyano H. Effects of hypoxia, hypercapnia, and pH on the chemoreceptor activity of the carotid body in vitro. The Journal of physiology. 1965;178(3):385–409. doi: 10.1113/jphysiol.1965.sp007634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holstein A, Stumvoll M. Contraindications can damage your health--is metformin a case in point? Diabetologia. 2005;48(12):2454–2459. doi: 10.1007/s00125-005-0026-1. [DOI] [PubMed] [Google Scholar]
- 55.Sutendra G, Bonnet S, Rochefort G, Haromy A, Folmes KD, Lopaschuk GD, Dyck JR, Michelakis ED. Fatty acid oxidation and malonyl-CoA decarboxylase in the vascular remodeling of pulmonary hypertension. Sci Transl Med. 2010;2(44):44ra58. doi: 10.1126/scitranslmed.3001327. [DOI] [PubMed] [Google Scholar]
- 56.Evans AM, Mustard KJ, Wyatt CN, Peers C, Dipp M, Kumar P, Kinnear NP, Hardie DG. Does AMP-activated protein kinase couple inhibition of mitochondrial oxidative phosphorylation by hypoxia to calcium signaling in O2-sensing cells? The Journal of biological chemistry. 2005;280(50):41504–41511. doi: 10.1074/jbc.M510040200. [DOI] [PubMed] [Google Scholar]
- 57.Leach RM, Sheehan DW, Chacko VP, Sylvester JT. Energy state, pH, and vasomotor tone during hypoxia in precontracted pulmonary and femoral arteries. American journal of physiology. 2000;278(2):L294–304. doi: 10.1152/ajplung.2000.278.2.L294. [DOI] [PubMed] [Google Scholar]
- 58.Dallas ML, Rafferty JN, Ikematsu N, Ross FA, Fedida D, Hardie DG, Peers C, Evans AM. Modulation of Kv2.1 and Kv1.5 channels by AMP-activated protein kinase (AMPK) Proc Physiol Soc. 2010;20 C02 and PC02. [Google Scholar]
- 59.Robertson TP, Mustard KJ, Lewis TH, Clark JH, Wyatt CN, Blanco EA, Peers C, Hardie DG, Evans AM. AMP-activated protein kinase and hypoxic pulmonary vasoconstriction. European journal of pharmacology. 2008;595(1-3):39–43. doi: 10.1016/j.ejphar.2008.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wyatt CN, Mustard KJ, Pearson SA, Dallas ML, Atkinson L, Kumar P, Peers C, Hardie DG, Evans AM. AMP-activated protein kinase mediates carotid body excitation by hypoxia. The Journal of biological chemistry. 2007;282(11):8092–8098. doi: 10.1074/jbc.M608742200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(20):11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Waypa GB, Marks JD, Guzy R, Mungai PT, Schriewer J, Dokic D, Schumacker PT. Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circulation research. 2010;106(3):526–535. doi: 10.1161/CIRCRESAHA.109.206334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Snetkov VA, Smirnov SV, Kua J, Aaronson PI, Ward JP, Knock GA. Superoxide Differentially Controls Pulmonary and Systemic Vascular Tone through Multiple Signalling Pathways. Cardiovascular research. 2010 doi: 10.1093/cvr/cvq275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kulisz A, Chen N, Chandel NS, Shao Z, Schumacker PT. Mitochondrial ROS initiate phosphorylation of p38 MAP kinase during hypoxia in cardiomyocytes. American journal of physiology. 2002;282(6):L1324–1329. doi: 10.1152/ajplung.00326.2001. [DOI] [PubMed] [Google Scholar]
- 65.Pearlstein DP, Ali MH, Mungai PT, Hynes KL, Gewertz BL, Schumacker PT. Role of mitochondrial oxidant generation in endothelial cell responses to hypoxia. Arteriosclerosis, thrombosis, and vascular biology. 2002;22(4):566–573. doi: 10.1161/01.atv.0000012262.76205.6a. [DOI] [PubMed] [Google Scholar]
- 66.Bell EL, Klimova TA, Eisenbart J, Schumacker PT, Chandel NS. Mitochondrial reactive oxygen species trigger hypoxia-inducible factor-dependent extension of the replicative life span during hypoxia. Molecular and cellular biology. 2007;27(16):5737–5745. doi: 10.1128/MCB.02265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guzy RD, Sharma B, Bell E, Chandel NS, Schumacker PT. Loss of the SdhB, but Not the SdhA, subunit of complex II triggers reactive oxygen species-dependent hypoxia-inducible factor activation and tumorigenesis. Molecular and cellular biology. 2008;28(2):718–731. doi: 10.1128/MCB.01338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Emerling BM, Weinberg F, Snyder C, Burgess Z, Mutlu GM, Viollet B, Budinger GR, Chandel NS. Hypoxic activation of AMPK is dependent on mitochondrial ROS but independent of an increase in AMP/ATP ratio. Free radical biology & medicine. 2009;46(10):1386–1391. doi: 10.1016/j.freeradbiomed.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zmijewski JW, Banerjee S, Bae H, Friggeri A, Lazarowski ER, Abraham E. Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. The Journal of biological chemistry. 2010 doi: 10.1074/jbc.M110.143685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hawley SA, Ross FA, Chevtzoff C, Green KA, Evans A, Fogarty S, Towler MC, Brown LJ, Ogunbayo OA, Evans AM, Hardie DG. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell metabolism. 2010;11(6):554–565. doi: 10.1016/j.cmet.2010.04.001. ** HEK293 cells expressing an AMP-insensitive γ subunit variant demonstrate that H2O2 activates AMPK by inhibiting mitochondrial oxidative phosphorylation and identifies the mechanism of AMPK activation by a diverse group of compounds.
- 71.Meany DL, Poe BG, Navratil M, Moraes CT, Arriaga EA. Superoxide released into the mitochondrial matrix. Free radical biology & medicine. 2006;41(6):950–959. doi: 10.1016/j.freeradbiomed.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 72.Thompson RJ, Buttigieg J, Zhang M, Nurse CA. A rotenone-sensitive site and H2O2 are key components of hypoxia-sensing in neonatal rat adrenomedullary chromaffin cells. Neuroscience. 2007;145(1):130–141. doi: 10.1016/j.neuroscience.2006.11.040. [DOI] [PubMed] [Google Scholar]
- 73.Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. The Biochemical journal. 1973;134(3):707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hambraeus-Jonzon K, Bindslev L, Mellgard AJ, Hedenstierna G. Hypoxic pulmonary vasoconstriction in human lungs. A stimulus-response study. Anesthesiology. 1997;86:308–315. doi: 10.1097/00000542-199702000-00006. [DOI] [PubMed] [Google Scholar]
- 75.Biscoe TJ, Pallot DJ. The carotid body chemoreceptor: an investigation in the mouse. Quarterly journal of experimental physiology (Cambridge, England) 1982;67(4):557–576. doi: 10.1113/expphysiol.1982.sp002676. [DOI] [PubMed] [Google Scholar]
- 76.Thuringer D, Findlay I. Contrasting effects of intracellular redox couples on the regulation of maxi-K channels in isolated myocytes from rabbit pulmonary artery. The Journal of physiology. 1997;500(Pt 3):583–592. doi: 10.1113/jphysiol.1997.sp022044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu MF, Gorenne I, Su X, Moreland RS, Kotlikoff MI. Sodium hydrosulfite contractions of smooth muscle are calcium and myosin phosphorylation independent. The American journal of physiology. 1998;275(5 Pt 1):L976–982. doi: 10.1152/ajplung.1998.275.5.L976. [DOI] [PubMed] [Google Scholar]
- 78.Olschewski A, Hong Z, Peterson DA, Nelson DP, Porter VA, Weir EK. Opposite effects of redox status on membrane potential, cytosolic calcium, and tone in pulmonary arteries and ductus arteriosus. American journal of physiology. 2004;286(1):L15–22. doi: 10.1152/ajplung.00372.2002. [DOI] [PubMed] [Google Scholar]
- 79.Park MK, Lee SH, Ho WK, Earm YE. Redox agents as a link between hypoxia and the responses of ionic channels in rabbit pulmonary vascular smooth muscle. Experimental physiology. 1995;80(5):835–842. doi: 10.1113/expphysiol.1995.sp003891. [DOI] [PubMed] [Google Scholar]
- 80.Agapito MT, Sanz-Alfayate G, Gomez-Nino A, Gonzalez C, Obeso A. General redox environment and carotid body chemoreceptor function. Am J Physiol Cell Physiol. 2009;296(3):C620–631. doi: 10.1152/ajpcell.00542.2008. [DOI] [PubMed] [Google Scholar]
- 81.Gomez-Nino A, Agapito MT, Obeso A, Gonzalez C. Effects of mitochondrial poisons on glutathione redox potential and carotid body chemoreceptor activity. Respiratory physiology & neurobiology. 2009;165(1):104–111. doi: 10.1016/j.resp.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 82.Hoffman DL, Salter JD, Brookes PS. Response of mitochondrial reactive oxygen species generation to steady-state oxygen tension: implications for hypoxic cell signaling. Am J Physiol Heart Circ Physiol. 2007;292(1):H101–108. doi: 10.1152/ajpheart.00699.2006. [DOI] [PubMed] [Google Scholar]
- 83.Skulachev VP. Role of uncoupled and non-coupled oxidations in maintenance of safely low levels of oxygen and its one-electron reductants. Quarterly reviews of biophysics. 1996;29(2):169–202. doi: 10.1017/s0033583500005795. [DOI] [PubMed] [Google Scholar]
- 84.Starkov AA. The role of mitochondria in reactive oxygen species metabolism and signaling. Annals of the New York Academy of Sciences. 2008;1147:37–52. doi: 10.1196/annals.1427.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weir EK, Archer SL. The role of redox changes in oxygen sensing. Respiratory physiology & neurobiology. 2010 doi: 10.1016/j.resp.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilson HL, Dipp M, Thomas JM, Lad C, Galione A, Evans AM. Adp-ribosyl cyclase and cyclic ADP-ribose hydrolase act as a redox sensor. a primary role for cyclic ADP-ribose in hypoxic pulmonary vasoconstriction. The Journal of biological chemistry. 2001;276(14):11180–11188. doi: 10.1074/jbc.M004849200. [DOI] [PubMed] [Google Scholar]