Abstract
Background
Pregnancy is a life stage where excess weight gain may occur and the postpartum period is often characterised by weight retention. The aim of the current study was to evaluate the feasibility of undertaking a randomised controlled trial of a weight loss intervention (WeighWell) in postpartum women living in areas of social disadvantage.
Subjects and methods
The study aimed to recruit 60 women who were not pregnant, 6-18 months postpartum with a BMI >25kg/m2 living in areas of deprivation within Tayside, UK. Recruitment strategies focused on visits to community groups; writing directly to postpartum women living in areas of deprivation and primary care teams who covered the most deprived 15% of the population and advertising in community settings. The 12 week intervention used motivational interviewing techniques to promote an energy deficit diet and increased physical activity, delivered by 3 face to face consultations plus 3 structured telephone calls.
Results
Of 142 women screened, 63 were eligible and 52 (83%) were recruited and randomised to an intervention (n=29) or comparison group (n=23). Over the 12 week intervention, body weight changed significantly by −1.6 ± 2.0kg in the intervention group compared to +0.2 ± 2.2kg in the comparison group indicating the potential efficacy of the intervention. Loss to follow-up was 24% in the intervention group and 39% for the comparison group.
Conclusions
The findings support the development of a definitive trial that embraces personalised recruitment strategies and the development of approaches to improve retention over a clinically relevant intervention period.
Keywords: postpartum, pregnancy, feasibility trial, obesity, weight loss
Introduction
Current projections within the UK suggest that 36% of women will be obese and 32% will be overweight by 2020 (Brown et al., 2010). Pregnancy is a life stage where excess weight gain may occur and the postpartum period is often characterised by weight retention or further increase (Gore et al., 2003). For the overweight mother, weight loss is desirable to meet the physical demands of parenting, to reduce the risk of complications in subsequent pregnancies and reduce the risk of cardiovascular disease and diabetes in later life (Galtier-Dereure et al., 2000).
It is estimated that at 6 weeks postpartum, two thirds of women still weigh more than their pre-pregnancy weight (Walker et al., 2004) and at 6 to 18 months, 15-20% of women have retained at least 5kg (Calfas & Marcus, 2007). In addition to concerns over weight retention, Villamor et al. (2006) have demonstrated that weight gain between pregnancies is associated with maternal and perinatal complications such as gestational diabetes, preeclampsia, macrosomia and stillbirth. Moreover, data collected from antenatal clinics have demonstrated that a significantly greater proportion of women from areas of high deprivation are obese (Heslehurst et al., 2010).
Current guidance on postpartum weight loss recommends that advice for mothers should be tailored to personal circumstances and take account of the demands of caring for a baby including fatigue and any co-existing health problems (NICE, 2010a). A systematic review commissioned by NICE (2010b) included five randomised control trials (RCTs) (Leermakers et al., 1998; Lovelady et al., 2000; O’Toole et al., 2003; Ostbye et al., 2009 and Kuhlmann et al., 2008) that examined postpartum weight loss interventions but all were based in the US and not noted to recruit specifically from disadvantaged groups.
It is likely that adherence to weight loss programmes will be more challenging for low income mothers and studies suggest that the transition from pregnancy to the postpartum phase may be associated with a poorer diet (George et al., 2005). There are many challenges in identifying ways to increase physical activity, restrict intakes of food and drink (when home based) and cope with the demands of child rearing. Multiple physical (and often psychological) morbidities and social isolation may mean that a standard weight loss regime is less acceptable (Peterson et al., 2002). Little is known about the response of low income, postpartum women to weight loss interventions in the UK and evidence from trials is absent. However, undertaking behavioural interventions trials in disadvantaged communities is often characterised by recruitment difficulties and robust design strategies for full trials would benefit from exploratory work as described in the MRC guidance for complex interventions (Craig et al., 2008).
The aim of the current work was to evaluate the feasibility of undertaking a randomised controlled trial of a weight loss programme (WeighWell) in postpartum women living in areas of social disadvantage served by NHS Tayside by conducting a pilot intervention to examine recruitment and retention, feasibility of assessment measures, intervention delivery, and potential efficacy. The study was not designed or analysed to demonstrate a definitive change in body weight. The study used a 1:1 parallel-group randomised controlled trial design.
Methods
The study aimed to recruit 60 women who were not pregnant, were 6-18 months postpartum with a BMI >25kg/m2 and living in areas of moderate to high deprivation (Scottish Index of Multiple Deprivation (SIMD) deciles 1-5) (Scottish Government, 2009) based on postcode. Sample size was chosen to represent 10% of that required to demonstrate a difference in 5% weight loss at 95% power in a definitive trial. This had been estimated to be 303 subjects in each group, based on the body weight variation of 16-50 year old women with a BMI over 25kg/m2 in the 2003 Scottish Health Survey.
Recruitment strategies comprised visits to community groups; writing to GP practices who have at least 30% of their patients classified as living within the most deprived 15% of SIMD (GP, health visitor and practice manager) and support workers (who wrote directly to postpartum women living in appropriate SIMD areas); newspaper advertising and posters in community settings. Recruitment materials offered a Freephone number. Interested individuals were telephone screened for eligibility [by AC], sent a participant information sheet and given a baseline appointment.
The primary outcomes of the study were to assess the feasibility and acceptability of the study procedures (including assessment method and intervention delivery). The following procedures were undertaken at baseline and follow-up.
Height, weight, waist circumference and skinfold measures
Physical activity assessment using a SenseWear™ physical activity monitor (Bodymedia Inc., Pittsburgh, PA, USA) worn for 7 days prior to assessment
Fasting blood sample (for plasma vitamin C analysis)
Questionnaires on socio-demographic background, dietary intake, physical activity, and psychosocial parameters (self efficacy and the theory of planned behaviour constructs on diet and physical activity).
Only the results from the first two of these are reported in this paper. Participants were then randomised to an intervention or comparison group using a 1:1 random sampling procedure in SPSS (Version 17.0, SPSS Inc., Chicago, IL, USA) by the trial manager. Assessments were performed, primarily within a hospital setting but on occasions within the participants’ home, by a research assistant blinded to randomisation allocation.
Intervention and comparator
All participants received a weight loss booklet (British Heart Foundation, 2006). Participants randomised to receive the 12 week intervention were allocated a trained lifestyle counsellor who delivered the intervention by 3 face to face consultations at monthly intervals (at a location most convenient for the participant). Between consultation contact was maintained with the intervention group by the lifestyle counsellor via a minimum of 3 structured telephone calls to identify progress towards goals, achievements and challenges and to provide positive feedback and support.
Motivational interviewing techniques (Rollnick et al., 2010) were employed and a personalised dietary prescription of estimated energy requirements minus 500 kcals was calculated with verbal and written guidance on food groups, frequency of consumption and portion size. Personalised physical activity goals were also set towards achieving 150 minutes of moderate to vigorous activity per week. Participants were provided with 4 week walking plans, a pedometer and a weight logbook for self monitoring.
The comparison group received no further contact beyond the weight loss booklet until follow-up assessment at 12 weeks. The group received a one-off consultation with a lifestyle counsellor after follow-up assessment.
Post-study feedback was sought via semi-structured telephone interviews on a sub-sample of 13 participants from each group. These were undertaken by a research assistant not involved in the study.
Quantitative data analysis was undertaken using SPSS (Version 17.0) utilising ANOVA to assess changes between baseline and follow-up. There was no imputation for missing values; i.e. for the feasibility study’s indicative estimate of effect, we did not do an intention to treat analysis but looked simply at differences between baseline and 12-weeks for those participants for whom weight-loss data were available at both points. Chi-square analysis was undertaken for categorical variables.
Ethical approval was provided by the Fife and Forth Valley NHS Research Ethics Committee 09/S0501/44.
Results
Subjects
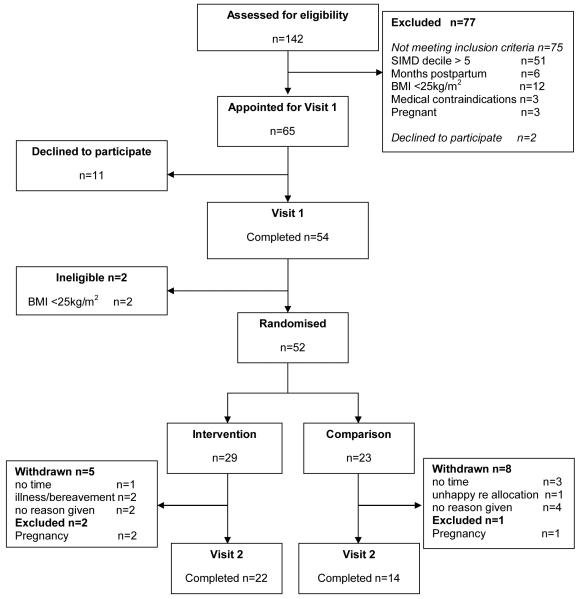
Between August 2009 and February 2010, 142 women responded to the study publicity. Most were ineligible for participation (n=75), largely due to area of residence (SIMD decile). Sixty five women (46%) met the inclusion criteria and were appointed for a baseline visit but 11 women subsequently declined to participate. At baseline assessment two women were excluded due to low BMI. Of the 52 participating women, 29 were randomised to the intervention group and 23 to the control group (Figure 1).
Figure 1.
WeighWell CONSORT Flowchart
Loss to follow-up was 31% (24% and 39% for intervention and control group respectively) including three participants who became pregnant during the study and were excluded. Thirty six participants completed follow up assessments by May 2010.
No significant group differences were identified at baseline for age, BMI, ethnicity, education level, household income, SIMD category, employment status or smoking status (Tables 1 and 2).
Table 1.
Baseline characteristics
| Mean ± St. Deviation (range), or n (%)1 |
|||
|---|---|---|---|
| Intervention n=29 |
Comparison n=23 |
||
| Age (years) | 30 ± 5.5 (21 - 39) |
30 ± 6.6 (18 - 44) |
|
| Ethnicity | Caucasian | 27 (93.1) | 22 (9.7) |
| Highest educational Level attained |
Secondary | 3 (10.3) | 6 (26.1) |
| Post school qualification | 18 (62.1) | 9 (39.1) | |
| Degree | 8 (27.6) | 8 (34.8) | |
| Annual household Income | < £15,000 | 7 (24.1) | 3 (13.0) |
| £15,001 - £25,000 | 6 (20.7) | 7 (30.4) | |
| £25,001 - £40,000 | 9 (31.0) | 10 (43.5) | |
| > £40,000 | 7 (24.1) | 3 (13.0) | |
| SIMD Decile21-2 (most deprived) |
15 (51.7) | 13 (56.5) | |
| 3-5 (moderately deprived) | 14 (48.3) | 10 (43.5) | |
| Household Format | Lives alone | 4 (13.8) | 4 (17.4) |
| Lives with partner | 24 (82.8) | 19 (82.6) | |
| Employment Status | Unemployed | 2 (6.9) | 2 (8.7) |
| Full-time parent | 5 (17.2) | 5 (21.7) | |
| Maternity leave | 11 (37.9) | 6 (26.1) | |
| Employed full-time | 4 (13.8) | 5 (21.7) | |
| Employed part-time | 7 (24.1) | 4( 17.4) | |
| Full-time student | - | 1 (4.3) | |
| Smoking status | Current smoker | 4 (13.8) | 3 (13.0) |
| Ex-smoker | 9 (31.0) | 8 (34.8) | |
| Non-smoker | 16 (55.2) | 12 (52.2) | |
No between-group differences were identified for any of the above measures
Individuals living in SIMD deciles 6-10 were excluded at screening
Table 2.
Anthropometric and physical activity measurements
| Intervention Group | Comparison Group | P3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Baeseline n=29 |
Follow-up n=22 |
Difference n=221,5 |
P2 | Baseline n=23 |
Follow-up n=14 |
Difference n=141,5 |
P2 | ||
| Weight (kg) | 83.5± 12.8 (64.4-112.3) |
82.0± 13.5 (63.0- 110.8) |
−1.6± 2.0 | 0.001 | 84.2± 14.8 (59.1-120.8) |
82.1± 14.0 (58.3-100.1) |
0.2± 2.2 | 0.77 | 0.018 |
| Waist Measurement (cm) |
98.2± 11.2 (78.7-117.6) |
94.1± 11.6 (74.4- 116.2) |
−4.4± 3.5 | <0.001 | 102.7± 10.9 (83.0-133.0) |
97.2± 11.8 (83.9-126.3) |
−2.8± 4.5 | 0.04 | 0.23 |
| BMI (kg/m2) | 31.6± 4.7 (25.5-41.8) |
30.7± 4.7 (23.7-39.5) |
−0.7± 0.8 | <0.001 | 31.6± 5.4 (25.3-47.8) |
30.7± 4.3 (25.4-37.2) |
0.1± 0.9 | 0.75 | 0.009 |
| % Body Fat4 | 33.3± 2.6 (28.3-39.1) |
32.0± 2.4 (29.1-38.1) |
−1.5± 0.8 | <0.001 | 34.0± 2.8 (29.7-38.8) |
34.5± 2.6 (30.3-38.9) |
−0.5± 1.4 | 0.32 | 0.029 |
| Minutes moderate- vigorous physical activity / day5 |
83 ± 46 (15 – 209) |
103 ± 51 (44 – 216) |
19 ± 47 | 0.10 | 77 ± 60 (6 – 288) |
79 ± 59 (13 – 228) |
16 ± 48 | 0.25 | 0.86 |
Data are presented as mean ± s.d, with ranges in parentheses.
For those participants who completed both baseline and follow-up measurements
Repeated Measures ANOVA
One Way ANOVA
% Body Fat measurements successful in n=21 (baseline) and n=17 (follow-up) for the intervention group and n=15 (baseline) and n=9 (follow-up) for the comparison group
Participants wearing the SenseWear monitor for a minimum of 4 days: n=28 at baseline and n=19 at follow-up for the intervention group, and n=20 at baseline and n=14 at follow-up for the comparison group.
Feasibility of assessment measures
Height, weight and waist circumference were obtained from all participants who took part in baseline and follow up assessments. Skinfold measurements were attempted in all participants and obtained from 37 (71%) at baseline and 26 (72%) at follow up. The remainder were not collected due to measurement difficulties. Fasting blood samples were obtained from 35 (67%) participants at baseline and 20 (56%) at follow up. The activity monitors were worn for a minimum of 4 days by 49 (94%) at baseline with a similar proportion at follow up (97%).
Feasibility of intervention delivery
The intervention face to face consultations (3) and telephone contact calls (3) were successfully delivered to 100% of the Intervention Group who completed the study. Most (76%) of the participants were seen at home.
Potential efficacy
Body weight loss was achieved by 73% of the intervention group compared to 36% of the comparison group (Table 2). Weight loss of clinical significance (reduction of 5% body weight SIGN, 2010) was achieved by 9% of the intervention group, compared to 0% in the comparison group. Whilst the study was not powered to show change between intervention and comparator there was a significantly greater change in the intervention compared to the comparison group for body weight, BMI and % body fat. No differences were detected between groups for change in waist circumference, nor objectively measured physical activity.
Post-study interviews
Post-study interviews revealed that all participants reported their experience positively overall, regardless of group. However, many comparison group participants noted a feeling of disappointment about their allocation despite acknowledging their awareness of the randomisation procedure prior to their enrolment on the study.
Baseline and follow-up assessments were largely acceptable, with most difficulties relating to either the discomfort experienced whilst wearing the SenseWear™ physical activity arm band and/or a small number of questionnaire items. However, a number of positive comments were received overall about the usefulness of the feedback received from the physical activity monitors.
Whilst the comparison group received only a weight loss booklet, many did report having made some improvements to their lifestyle, either as a result of having an increased awareness of aspects of their lifestyle requiring improvement after having carried out the assessment procedures and/or after reading the British Heart Foundation booklet provided.
The intervention was particularly well received with respect to the format (one-to-one rather than group sessions, duration and frequency), the supportive approach of the counsellors, and the literature provided, with all recipients describing the intervention as useful. Intervention group participants all reported having made sustained improvements to aspects of their diet (e.g. increases in fruit and vegetable intake and cooking of fresh food; and reductions to portion sizes, frequency of eating out, and intakes of snacks and sugary foods and drinks). In most cases these were reported to have extended to the wider family. Although not as consistent as the reported dietary changes, most also reported having improved their levels of physical activity.
Discussion
The findings of this feasibility study suggest the potential efficacy of the programme for initiating postpartum weight loss. Though not designed or powered to provide evidence of effect, the study showed a promising weight loss over the 12 week period by the intervention group was modest and in contrast to the weight gain found in the comparison group. The comparison group had all received written information on weight loss, a condition of ethical approval, so cannot be regarded as a true control group receiving usual care only. However, the results suggest that without health professional support the norm may well be to gain weight, despite a perception of having made positive lifestyle changes. These results are similar to a recent intervention trial on prevention of weight gain in young mothers (with primary school aged children) which reported a significant weight loss of −0.20kg after a 12 month intervention compared to a gain of 0.83kg in their control group (Lombard et al., 2010). Such findings highlight the challenges of avoiding weight gain in societies where social contacts focus on encouraging eating, drinking and sedentary activities.
The current work raises several considerations for the design of a future RCT. The response of women to recruitment strategies suggest that current approaches might be enhanced by identifying eligible women at postnatal discharge and sending personal contact letters through GPs. Loss to follow up was similar to that reported by Leermakers et al. (1998) of 27% and less than the 40% reported by O’Toole et al. (2003) but highlights a major challenge. The exclusion of 5% due to pregnancy suggests that the intervention might be more appropriately offered within the first 12 months postpartum. A further challenge is retention of the comparison group. Those who completed the study expressed dissatisfaction at their allocation even though women were offered a consultation with a counsellor after completion of follow up measures. One potential solution to this in a future trial would be the use of an active comparison group, which improves loyalty by providing a similar level of support to that provided in the intervention group but in an area that is not expected to affect weight loss, such as a focus on positive aspects of baby development or other aspects of maternal wellbeing such as stress reduction.
The measurements utilised were intended to provide subjective and objective measures of factors influencing weight loss. A future trial would have weight loss as its primary outcome and measuring this was not a problem in this feasibility study. However, difficulties in obtaining skinfold thickness measurements (as also reported by Gray et al., 1990) and fasting blood analysis suggests these measures may be of limited use in a future trial. In addition, these may have added to subject burden as they necessitated a visit to the research centre and may partially account for interested participants withdrawing. There was a clear preference expressed for intervention visits to be at home and future work should reconsider essential measurements in this light. Given the importance of body fat as an outcome measure, this should include reconsideration as to the methods of body fat measurement. Bioelectrical impedance analysis may offer a quick and portable alternative less prone to observer bias than skinfold thickness measurements, which would be important in a larger RCT (Deurenberg, 2009).
Whilst it is recognised that home visits may be costly, women recruited to studies of postpartum weight loss have been reported as having little time available, competing responsibilities above their own health, and find rigid interventions (such as group based classes) difficult to attend (Carter-Edwards et al., 2009). A recent trial utilising diet and activity classes and telephone counselling found no difference in weight change between intervention and control groups noting that engaging women during this busy period is challenging and concluding that home based interventions may be more successful (O’Toole et al., 2003).
This feasibility study was aimed at delivering a 3-month intervention to explore intervention delivery, recruitment and retention with post-partum women and, additionally, to give an indication of the intervention’s ability to support weight loss. The results are encouraging although a future RCT should embrace wider, personalised recruitment strategies and the development of specific approaches to improve study retention over a clinically relevant intervention period.
Supplementary Material
Acknowledgements
The authors acknowledge the financial support of the Medical Research Council (Ref GO701771), and NHS Research Scotland (NRS) through NHS Tayside, to carry out this work. The authors would like to thank Amy Gregor who assisted with recruitment and screening of participants and in the development of study materials and Margaret Robertson, the project lifestyle counsellor. They would also like to thank Professor Peter Howat, Director of the Centre for Behavioural Research in Cancer Control at Curtin University, Australia for guidance in project design and assessment.
Funded by: Medical Research Council (Ref GO701771)
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- British Heart Foundation So you want to lose weight… for good. [Accessed 21 September 2010]. 2006. http://www.bhf.org.uk/publications/view_publication.aspx?ps=1000807.
- Brown M, Byatt T, Marsh T, Mcpherson K. A prediction of obesity trends for adults and their associated diseases. [Accessed 21 September 2010]. 2010. http://nhfshare.heartforum.org.uk/RMAssets/NHFreports/NHF_adultobese_short_170210.pdf.
- Calfas KJ, Marcus BH. Postpartum weight retention: a mothers weight to bear. Am J Prev Med. 2007;32(4):356–357. doi: 10.1016/j.amepre.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Carter-Edwards L, Ostbye T, Bastian LA, Yarnall KSH, Krause KM, Simmons TJ. Barriers to adopting a healthy lifestyle: insight from postpartum women. BMC Res Notes. 2009;2:161. doi: 10.1186/1756-0500-2-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. [Accessed 21 September 2010]. 2009. http://www.bmj.com/content/337/bmj.a1655.full.
- Deurenberg P. Body Composition. In: Gibney MJ, Vorster HH, Kok FJ, editors. Introduction to Human Nutrition. Blackwell Publishing; Chichester: 2009. pp. 23–26. [Google Scholar]
- Galtier-Dereure F, Boegner C, Bringer J. Obesity and pregnancy: complications and cost. Am J Clin Nutr. 2000;71(5):S1242–S1248. doi: 10.1093/ajcn/71.5.1242s. [DOI] [PubMed] [Google Scholar]
- George GC, et al. Food choices of low-income women during pregnancy and postpartum. J Am Diet Assoc. 2005;105(6):899–907. doi: 10.1016/j.jada.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Gore SA, Brown DM, West DS. The role of postpartum weight retention in obesity among women: a review of the evidence. Ann Behav Med. 2003;26(2):149–159. doi: 10.1207/S15324796ABM2602_07. [DOI] [PubMed] [Google Scholar]
- Gray DS, Bray GA, Bauer M, Kaplan K, Gemayel N, Wood R, et al. Skinfold thickness measurements in obese subjects. Am J Clin Nutr. 1990;51:571–577. doi: 10.1093/ajcn/51.4.571. [DOI] [PubMed] [Google Scholar]
- Heslehurst N, Rankin J, Wilkinson JR, Summerbell CD. A nationally representative study of maternal obesity in England, UK: trends in incidence and demographic inequalities in 619 323 births, 1989–2007. Int J Obes. 2010;34(3):420–428. doi: 10.1038/ijo.2009.250. [DOI] [PubMed] [Google Scholar]
- Kuhlmann AK, Dietz PM, Galavotti C, England LJ. Weight management interventions for pregnant or postpartum women. Am J Prev Med. 2008;34(6):523–528. doi: 10.1016/j.amepre.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Leermakers EA, Anglin K, Wing RR. Reducing postpartum weight retention through a correspondence intervention. Int J Obes. 1998;22(11):1103–1109. doi: 10.1038/sj.ijo.0800734. [DOI] [PubMed] [Google Scholar]
- Lombard C, Deeks A, Jolley D, ball K, Teede H. A low intensity, community based lifestyle programme to prevent weight gain in women with young children: cluster ransomised trail. [Accessed 21 September 2010]. 2010. http://www.bmj.com/content/341/bmj.c3215.full. [DOI] [PMC free article] [PubMed]
- Lovelady CA, Garner KE, Moreno KL, Williams JP. The effect of weight loss in overweight, lactating women on the growth of their infants. N Engl J Med. 2000;342(7):449–453. doi: 10.1056/NEJM200002173420701. [DOI] [PubMed] [Google Scholar]
- National Institute for Heath and Clinical Excellence Dietary interventions and physical activity interventions for weight management before, during and after pregnancy. [Accessed 21 September 2010]. 2010a. http://www.nice.org.uk/nicemedia/live/13056/49926/49926.pdf.
- National Institute for Heath and Clinical Excellence Systematic review of weight management interventions after childbirth. [Accessed 21 September 2010]. 2010b. http://www.nice.org.uk/nicemedia/live/13056/49952/49952.pdf.
- Ostbye T, Krause KM, Lovelady CA, Morey MC, Bastian LA, Peterson BL, et al. Active mothers postpartum: a randomized controlled weight-loss intervention trial. Am J Prev Med. 2009;37(3):173–180. doi: 10.1016/j.amepre.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole ML, Sawicki MA, Artal R. Structured diet and physical activity prevent postpartum weight retention. J Womens Health (Larchmt) 2003;12(10):991–998. doi: 10.1089/154099903322643910. [DOI] [PubMed] [Google Scholar]
- Peterson KE, Sorenson G, Pearson M, Hebert JR, Gottlieb BR, McCormick MC. Design of an intervention addressing multiple levels of influence on dietary and activity patterns of low-income, postpartum women. Health Educ Res. 2002;17(5):531–40. doi: 10.1093/her/17.5.531. [DOI] [PubMed] [Google Scholar]
- Rollnick S, Butler CC, Kinnersley P, Gregory J, Mash B. Motivational interviewing. BMJ. 2010;340:1242–1245. doi: 10.1136/bmj.c1900. [DOI] [PubMed] [Google Scholar]
- Scottish Government Scottish Index of Multiple Deprivation. [Accessed 21 September 2010]. 2009. http://www.scotland.gov.uk/Topics/Statistics/SIMD/
- Scottish Intercollegiate Guidelines Network Guideline 115 Management of Obesity NHS Scotland. [Accessed 21 September 2010]. 2010. http://www.sign.ac.uk/pdf/sign115.pdf.
- Villamor E, Cnattingius S. Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. Lancet. 2006;368(9542):1164–1170. doi: 10.1016/S0140-6736(06)69473-7. [DOI] [PubMed] [Google Scholar]
- Walker LO, Timmerman GM, Sterling BS, Kim M, Dickson P. Do low-income women attain their pre-pregnant weight by the 6th week of postpartum? Ethn Dis. 2004;14(1):119–126. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.