Abstract
Background
Previous studies have shown that patients with schizophrenia are impaired on executive tasks where positive and negative feedbacks are used to update task rules or switch attention. However, research to date using saccadic tasks has not revealed clear deficits in task switching in these patients. The present study used an oculomotor “rule switching” task to investigate the use of negative feedback when switching between task rules in people with schizophrenia.
Method
50 patients with first episode schizophrenia and 25 healthy controls performed a task in which the association between a centrally presented visual cue and the direction of a saccade could change from trial to trial. Rule changes were heralded by an unexpected negative feedback indicating that the cue-response mapping had reversed.
Results
Schizophrenia patients were found to make increased errors following a rule switch, but these were almost entirely the result of executing saccades away from the location at which the negative feedback had been presented on the preceding trial. This impairment in negative feedback processing was independent of IQ.
Discussion
The results confirm the existence of a basic deficit in stimulus-response rule switching in schizophrenia, but also suggest that this arises from aberrant processing of response outcomes, resulting in a failure to appropriately update rules. The findings are discussed in the context of neurological and pharmacological abnormalities in the conditions which may disrupt prediction error signalling in schizophrenia.
Keywords: Schizophrenia, Eye movements, Executive function, Reward Processing, Cognitive impairments, Working memory
Introduction
People with schizophrenia have performance deficits on tests of executive function at all stages of the illness (Hutton et al, 1998; Pantelis et al., 1997). An important example of this is impaired cognitive flexibility reflected in increased errors on the Wisconsin Card Sort Test (WCST) (Grant, 1948; Nelson, 1976; Weinberger et al., 1986; Goldberg, 1994; Berman et al., 1995; Prentice et al., 2008) and the CANTAB ID/ED task (Pantelis et al., 1997; Hutton et al.,1998; Murray et al., 2008; Waltz and Gold, 2007; Leeson et al., 2009). Successful performance on these tasks depends on a number of control operations such as maintenance of task goals in working memory, inhibition of pre-potent responses, monitoring of own behaviour and associated feedback. Being able to identify more discrete deficits of this type will allow a greater understanding of the processes contributing to cognitive dysfunction in schizophrenia, their neurobiological basis and how they might impact on clinical outcomes (Carter et al. 2007).
One line of schizophrenia research which has proved fruitful in this regard has focused on more simple paradigms in which the task demands are clearly defined and, by using eye movements, the responses are accurately measured. For example, schizophrenia patients are consistently found to make increased errors in the anti-saccade task (Fukushima et al., 1988; Hutton and Ettinger, 2006), possibly due to weak internal representations of task goals in working memory (Reuter et al., 2007). This has been linked to frontal cortex impairment since patients with lesions in the dorsal and ventrolateral frontal cortex have similar deficits (Guitton, Buchtel, and Douglas, 1985; Walker et al., 1998; Hodgson et al., 2007)
A number of studies have also investigated how patients with schizophrenia switch between two saccadic tasks, which is considered to reflect cognitive flexibility in the sense of being able to adapt behaviour quickly in response to changing environmental contingencies. Manoach et al (2002) required subjects to switch between anti-saccades and “pro-saccades” and surprisingly found that schizophrenia patients were normal, showing similar ‘switch costs’ to healthy controls (see also Greenzang et al., 2007; Franke et al., 2007). However this contrasts to work using non-oculomotor switching tasks in which schizophrenia patients are impaired (Meiran et al., 2000). Given that a number of healthy volunteer studies report no switch cost or even a small benefit when switching between pro- and anti-saccades (Hallett and Adams, 1980; Hodgson et al., 2004; Hunt and Klein, 2002; Parton et al., 2007) it is possible that this finding is an artefact of the testing procedure. Hodgson et al. (2004) suggest that switching to and from a reflexive response - the pro saccade - does not require retrieval and reconfiguration of arbitrary stimulus-response mappings and it is arguably these specific operations that constitute the main challenge of task switching in non-oculomotor paradigms, for example, switching versions of the Stroop task (see Monsell, 2003).
In order to further investigate the processes involved in task switching and how they are affected in schizophrenia we used a novel oculomotor “rule switching” task in which participants learn a rule linking a central symbolic cue with a saccade to either the left or the right (Hodgson et al. 2004). The rule can reverse at different points in the task, as indicated by a change in the feedback presented following the response. This is a closer analogue of non-oculomotor task switching paradigms as it involves coordination of arbitrary stimulus response mappings, requiring suppression of recently correct responses on a subset of trials. Having to suppress particular responses on some trials but not others is of interest because we have found evidence of such an inhibitory impairment in schizophrenia using the stop-signal task (Huddy et al. 2009). We therefore predicted that patients would show increased errors on trials following rule switches.
Two other processes essential for appropriate switching behaviour can be measured with this oculomotor task. One is the ability to monitor responses as reflected in the rate of error correction when a saccade is initially made in the wrong direction (see Husain et al. 2003). Although schizophrenia patients have been shown to correct the majority of errors in the anti-saccade task (Polli et al. 2006), the rule switching task may be more taxing because the demand to inhibit a response varies from trial to trial.
Another process is the requirement to use positive and negative feedback to guide responses. Studies of the WCST and CANTAB ID/ED found that schizophrenia patients do not use negative feedback effectively (Gold et al., 2009; Prentice et al. 2008; Murray et al 2008; Leeson et al 2009). Using the rule switching task in healthy volunteers, Hodgson et al. (2002) found that responses are slower to locations that have just been the site of negative feedback (termed ‘reward-related inhibition of return” in contradistinction to the ‘inhibition of return’ effect seen in studies of covert attention (Posner et al. 1985)). This task therefore allows a further understanding of negative feedback processing in schizophrenia.
Using this oculomotor task we investigated rule switching in schizophrenia and how this relates to response inhibition, error monitoring and feedback processing. Findings on this task in patients with focal frontal cortex lesions (Hodgson et al. 2007) also allowed us to make inferences about the possible neurobiological substrates of impaired performance.
Method
Participants
Fifty medicated patients with first-episode psychosis were recruited from the West London longitudinal study (see Huddy 2007 for details). Initial diagnoses were ascertained using The Diagnostic Interview for Psychosis (Jablensky, 1992) and were reviewed one-year later. The final DSM IV diagnoses were schizophrenia (47) and schizoaffective disorder (3). These were compared to 25 healthy volunteers recruited from the same catchment area. Permission to conduct the study was obtained from Merton, Sutton and Wandsworth, Riverside, and Ealing Research Ethics Committees. All participants gave written informed consent and were paid an honorarium for their time.
Procedure
Clinical and Neuropsychological Assessments
Symptoms were assessed at recruitment using Scales for the Assessment of Positive Symptoms (Andreasen, 1984) and Negative Symptoms (Andreasen, 1983) and positive, disorganisation and negative syndrome scores were derived (Huddy et al., 2007). Cognitive assessments were performed a median of 8 days later as follows: premorbid IQ with the Wechsler Test of Adult Reading (Wechsler, 2001); current IQ with four WAIS III subtests (Wechsler, 1999) validated for schizophrenia (Blyler et al., 2000); working memory with CANTAB tests of Spatial Span (Owen et al.,, 1990), which measures the ability to remember the order of sequences of squares presented on the screen in increasing number, and Spatial Working Memory (Owen et al., 1990} where patients are required to ‘open’ sets of boxes to find tokens and errors are recorded when boxes in which tokens have been found are re-opened.
Eye movement recording and analysis
Eye movements were recorded using the Eyelink system (SR Research), a video-based pupil tracker, with head movement compensation system sampling at 250Hz. Subjects sat in front of the display monitor approximately 60 cm from the screen. Pupil position was monitored via two miniature infra-red CCD video cameras mounted on an adjustable headband. Participants were instructed to keep head movements to a minimum and no active restraint of head movements was required to obtain sufficiently accurate gaze position recordings. Eye movements were visualised off-line, saccades were identified and artefacts removed using custom software programs developed within the LabVIEW visual programming environment.
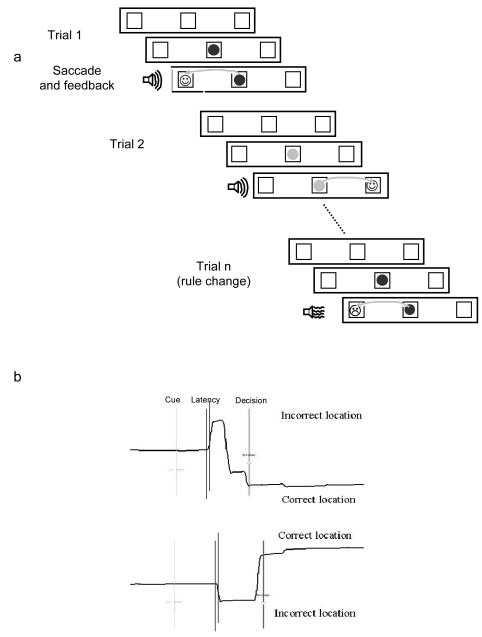
Saccadic rule switching task (Figure 1)
Figure 1.
a) Rule reversal task. Subjects learn a rule linking a coloured shape with a movement to either the left or the right. After a random number of trials the rule can reverse. The task is self-paced with at least 1500ms elapsing between each trial b) Corrective saccades. Feedback is only given following a fixation > 800ms on one of the response boxes. On a proportion of trials participants make saccade errors followed by a corrective movement towards the correct response box. Both examples are corrected errors.
Three boxes, outlined in black on a dark grey coloured background, were presented in the centre and 9 degrees to the left and right of the screen. Each box subtended 3 degrees of visual angle. Trial onset was triggered when the subject had been fixating the central box for 800ms. At this point, a blue or yellow circle was presented in the central box. The subject was instructed to look either to the left or right box whenever a coloured circle appeared. The colour of the cue (yellow / blue) instructed the subject whether to look left or right. The next fixation longer than 800ms on either the left or the right box was taken as the subject’s response on that trial, such that an eye fixation of shorter duration could be made towards the alternate location before the subject made their final decision. Once the viewer had selected one of the boxes by fixating it for >800ms, feedback was given to indicate if the choice was correct or incorrect in the form of a happy/sad face displayed within the selected box accompanied by a high or low pitched tone. Subjects were made aware that the rule linking the colour of the cue and direction of saccade would reverse at several points during the test. Rule changes were indicated by unexpected errors following runs of between 9 and 13 correct response trials. Each subject completed one block of 100 trials, comprising a maximum of 8 possible rule reversals. They were instructed to perform the task as quickly and as accurately as possible and to respond on the basis of the rule they know to be correct at that time, without anticipating the occurrence of a rule change.
Results
See Table 1. Patients and controls were matched for age. Patients scored significantly lower on most neuropsychological tests with a tendency to perform worse on the Spatial Working Memory task.
Table 1.
Demographics and neuropsychological performance of the two patient groups and controls
| Patients with Schizophrenia N = 50 |
Healthy Controls N = 25 |
Statistic | |
|---|---|---|---|
| Age | 24.4 (7.68) | 26.2 (4.3) | t(73) = 1.3 |
| Sex (m / f) | 31 / 19 | 10 / 15 | χ (1) = 3.2 ^ |
| Age finishing education |
16.6 (1.3) | 17.4 (1.1) | t(73) = 2.7 ** |
| Age of illness onset |
23.8 (7.6) | - | - |
| Positive Syndrome |
0.74 (0.23) | - | - |
| Negative Syndrome |
0.33 (0.25) | - | - |
| Disorg. Syndrome |
0.42 (0.31) | - | - |
| WTAR IQ | 91.0 (11.4) | 97.0 (7.7) | t(73) = 2.3 * |
| WAIS IQ | 84.1 (13.0) | 97.7 (10.4) | t(73) = 4.5 ** |
| Spatial Span | 5.2 (1.2) | 6.0 (1.4) | t(73) = 2.8 ** |
| SWM errors | 31.4 (19.7) | 23.8 (15.9) | t(71) = 1.7 ^ |
p < 0.1,
p < 0.05,
p < 0.01
Overall Latencies and errors
Independent sample t-tests revealed significant differences in overall latency (t(73) = 2.1, p < 0.05), overall errors (t(73) = 2.8, p < 0.01) and rules completed (t(73) = 2.8, p < 0.01), patients being slower and more error-prone than controls. Five patients failed to achieve the learning criterion of six consecutive correct responses more than once, and were excluded from subsequent analyses.
Interaction between errors and rule switching
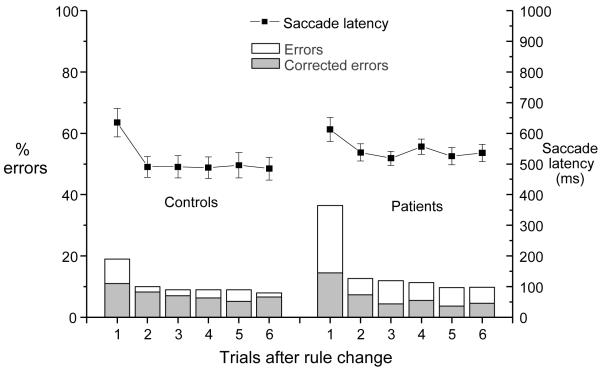
Errors that were subsequently corrected with a secondary saccade prior to the feedback, i.e. corrected errors (Figure 1) were initially separated from errors that were not corrected within the deadline, i.e. uncorrected errors. A mixed three-factor ANOVA was applied using group (controls versus patients), error type (corrected versus uncorrected) and trial (1st to 6th following a rule change).
See Figure 2. There was a main effect of trial (F(5, 340) = 18.7, p < 0.001) and a trend towards a main effect of group (F(1, 68) = 3.1, p < 0.1). This finding was qualified by significant trial × group (F(5, 340) = 3.5, p < 0.01) and error type × group (F(5, 340) = 5.4, p < 0.05) interactions. There was no three way interaction of trial × error type × group (F(5, 340) < 1).
Figure 2.
Latencies and error rates for patients and control groups in the rule switching task, showing proportion of total errors which were corrected, plotted against trial after rule change.
The error type × group interaction was due to patients making proportionally more uncorrected errors than controls overall. Inspection of the data suggested that the patients made more errors of both types on trial 1 only. To investigate this group × trial effect, the ANOVA was repeated for the 2nd to 6th trials inclusive, excluding trial 1,and this revealed that the group × trial interaction was no longer significant (F(4, 272) < 1); there was also no group main effect (F(1, 68) < 1). Thus the significant group × trial interaction found in the first analysis was driven by higher errors in patients on trial 1; this was confirmed by an ANOVA, using group and errors as factors, carried out for trial 1, which showed that patients made more errors than controls (F(1,68) = 10.7, p < 0.01) of both types.
Interaction between response latencies and rule switching
A two-factor ANOVA was applied to mean response latencies for the first saccade following cue onset on correct response trials i.e. when the first saccade was to the correct location; the factors were group and trial (1st to 6th following a rule change). There was a main effect of trial (F(5,335) = 7.9, p < 0.001) but no group difference or trial × group interaction (F(5,335) < 1). Figure 2 indicates that both groups showed a slowing of response times immediately following a rule change. This interpretation was confirmed by an analysis limited to trials 2 - 6 inclusive which demonstrated no main effect of trial (F(4,268) < 1).
In summary, patients made more errors on the first trial following a rule change than the control group. On trials performed entirely correctly, patients showed equivalent slowing of response latencies on trial 1 following a rule change.
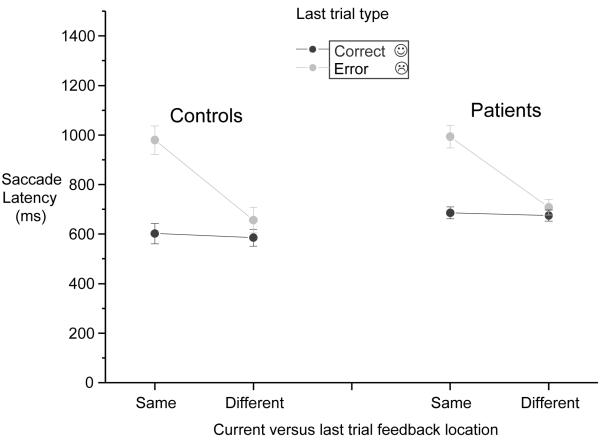
Reward-related inhibition of return effect on latencies
Hodgson et al. (2002, 2004) demonstrated a location specific “inhibition of return” effect of feedback on subsequent response latencies so that responses are slower to locations that have just been the site of negative feedback. A three-factor ANOVA with group, previous trial feedback (error versus correct) and previous feedback location (same versus different) was performed on latency for entirely correct responses (see Figure 3). This allowed us to examine whether there was a bias to make slower responses when the correct saccade was to the side where negative feedback had just been received. There was a significant interaction of location × feedback (F(1, 68) = 48.7 p < 0.001) indicating slower responses to the location of negative feedback on the previous trial compared to the opposite location. The absence of a feedback × direction × group interaction (F(1, 68) = 0.29) indicates that patients and controls showed the same location specific effect of negative feedback in terms of their latencies as the control group.
Figure 3.
Effect of relative location of previous feedback on correct trial latencies for low and average premorbid IQ patients and control groups.
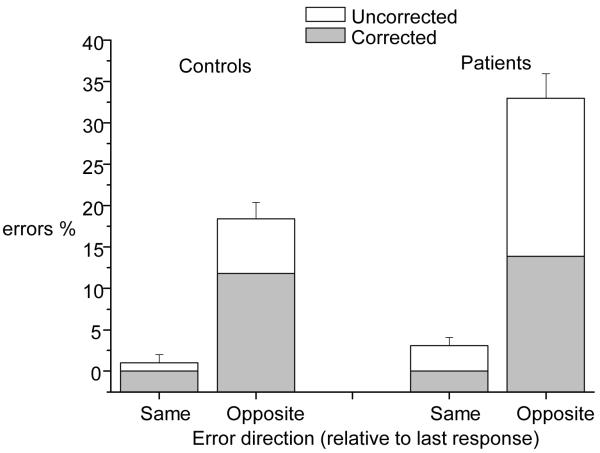
Reward-related inhibition of return effect on errors
An analysis was also conducted to determine if the bias to avoid making saccades to the location of previous error feedback impacted on the nature of the errors. A three-factor ANOVA was conducted only on the 1st trial following rule switches to examine the effect of the previous location of feedback (same versus different), error type (corrected versus uncorrected) and group. There were significant main effects of location (F(1, 68) = 30.1 p < 0.001) and group (F(1, 68) = 10.2 p < 0.01) but no three-way interaction of feedback, location and group (F(1, 68) < 1). The location × group interaction was significant (F(1, 68) = 8.2 p < 0.01) while the group × error type interaction was not (F(1, 68) = 2.6 p = 0.11). Figure 4 indicates that both groups made more errors of both types by directing their gaze away from the previous location of error feedback but patients showed a significant exacerbation of this effect.
Figure 4.
Effect of relative direction on error rates on rule switch trials for patients and control groups
In summary, both patients and controls were equally slow to make saccades in the direction of previous negative feedback. Both groups also tended to make errors in this condition by incorrectly looking away from the location of previous negative feedback but patients made significantly more. This latter effect explains the finding of increased trial 1 errors in the patients that is reported above.
Correlation between rule switching and neuropsychology
To reduce the number of comparisons, corrected and uncorrected errors were collapsed into a total errors score and this was compared only on trial 1 where group differences emerged (see Table 2). There were no strong relationships between errors, spatial working memory or spatial span in patients with schizophrenia. However, the control group showed a strong correlation between spatial span and switching errors. Premorbid and current IQ were moderately associated with rule switch errors in patients.
Table 2.
Correlations between switch task errors [Sw ers] (trial 1), working memory (spatial span and spatial working memory errors and IQ (WTAR and WAIS)). Correlations above the diagonal are patients with schizophrenia (N = 45) and below are controls (N= 25).
| Sw ers | span | SWM | WTAR | WAIS | |
|---|---|---|---|---|---|
| Sw. Ers. | - | −.05 | .06 | −.30* | −.26* |
| SPAN | −.65** | - | −.22 | .18 | .34* |
| SWM(68) | .34 | −.15 | - | −.1 | −.16 |
| WTAR | .14 | −.23 | −.18 | - | .67** |
| WAIS | −.01 | −.07 | −.61** | .40* | - |
The influence of IQ on switching
As patients had a significantly lower IQ than controls we examined the effect of IQ by extracting a subgroup of patients with average IQ using a WAIS cut-off score of 90 (N = 17); these were matched to the controls on current IQ (patients: mean = 100.2, SD = 4.8; controls: mean = 97.7, SD = 10.4; t(39) = −0.9). A three-factor ANOVA (group, error type and trial) revealed a significant group × trial interaction (F(5, 200) = 3.0 p < 0.05) indicating that patients with schizophrenia who have IQ in the average range and equivalent to controls make more errors immediately following rule shifts.
In contrast, there was no error type × group interaction (F(5, 200) < 1). This indicates that the group × error type interaction reported in the full group of patients above may be due to IQ differences between the groups. To determine which factors predicted error correction a hierarchical multiple regression was conducted with proportion of errors (proportion of all errors that were followed by a corrective saccade within the deadline) as the dependent variable and group and current IQ as predictors. IQ was entered first and group second. IQ explained a significant amount of the variance (R2 = .20, p < 0.01) in the first step but group failed to predict further variance in the second step (R2 change = .00, ns). This finding suggested that error correction is related to IQ rather than group membership.
In summary, the ability to correct erroneous responses within the time limit was a function of IQ. Patients with normal IQ, equivalent to that of the control group, made more errors of any type than controls immediately following the rule change.
Correlation between error rates and symptoms
Correlations were conducted between trial 1 errors and the three symptom syndromes. There was a significant moderate correlation between the severity of the negative syndrome and trial 1 errors (r = 0.36, p < 0.05) but no correlations with positive or disorganisation syndromes.
Discussion
In this study we examined rule switching performance in patients with first episode schizophrenia using an oculomotor task. Patients made more errors and had longer response latencies overall relative to control participants. The increase in errors was particularly marked immediately following a change in the rule. Further examination revealed that this effect was almost completely the result of saccades executed away from the location of a previous negative feedback, i.e. rather than responding on the basis of the new rule, patients with schizophrenia made a saccade to the location opposite to the last response. Further, when patients responded correctly, they showed the same slower response latencies as controls to the location of a recently presented negative feedback (i.e the so called “reward-dependent” inhibition of return effect described by Hodgson et al., 2002). Thus patients with schizophrenia demonstrated abnormalities on a task that required oculomotor switching between rules triggered by symbolic cues. This is in contrast to findings of unimpaired oculomotor switching between reflexive pro-saccades and anti-saccades. We suggest that this difference occurs because the current task involves repeated reconfiguration and retrieval of stimulus response mappings that do not involve a reflexive response.
The finding that errors on this task were particularly related to impaired negative feedback processing have broader implications for understanding the nature of rule and attention switching deficits in other contexts. It is important to emphasise differences in the structure of superficially similar paradigms when comparing studies which measure rule and attention switching in schizophrenia patients. The rule switching test described, WCST and CANTAB attentional set-shifting tasks all require participants to update behavioural rules on the basis of response contingent feedback. However, the cognitive operations resulting from feedback may lead to variations from task to task. As with the present task, in the WCST, biasing responses away from the card which had just been associated with a negative feedback on the last trial would be a maladaptive strategy. Indeed, a meta analysis of WCST errors profile in schizophrenia has shown that non-perseverative errors of this type constitute a large proportion of the total errors on the WCST (Li and Park, 2004), so a similar ‘avoid negative feedback’ response strategy may contribute to the deficit on this task. However, aversive responses away from specific stimuli that have been subject to negative feedback would be adaptive for mediating straightforward response switches between trials or ID (intra-dimentional) attentional switches, explaining why patients show a clearer deficit at the ED (extra-dimensional) shift stage of the ID/ED task (Hutton et al., 1998). Also consistent with this common explanation of deficit in schizophrenia, Waltz and Gold (2007) report marked impairments in patients using a probabilistic version of a reversal learning task (Swainson et al., 2000). In their study, the standard parameters of reversal learning were modified by the introducing of a probabilistic component, so that erroneous feedback was given on a minority (20%) of “correct” trials. Under these conditions, participants must avoid being influenced by the location of recent negative feedbacks and instead attend to the feedback likelihood over a series of trials to determine the current rule. The marked impairment that patients with schizophrenia demonstrate is again indicative of negative feedback processing deficits in this group.
Our findings therefore suggest that schizophrenia patients have rule switching deficits and that this is mainly due to impaired negative feedback processing. Previous studies of more complex rule switching tasks such as the WCST schizophrenia have been unable to clarify whether the impairments are secondary to an abnormally blunted impact of negative feedback or occur because patients fail to use negative feedback correctly to guide behaviour despite appreciating the affective valence (Gold et al., 2008; Prentice et al, 2008; Leeson 2009; Murray et al., 2008). Our results are more unequivocal in this matter as patients were ultra-sensitive to negative feedbacks, as evidenced by their pattern of errors following a rule change, but failed to update conditional stimulus-response rules as a result of the error. This conclusion agrees with that of Heerey et al (2007) who showed that patients with schizophrenia have a general difficulty in ‘translating experience into action’.
In this regard it is important to understand whether our findings can be explained by the IQ difference between our patients and controls. Generalised cognitive impairment is probably an intrinsic feature of schizophrenia (Woodberry et al, 2008) and can explain many of the deficits found on neuropsychological tests (Dickinson et al., 2009). We examined this possibility in a subset of patients matched for current IQ and found they too showed increased errors immediately after negative feedback explained by the reward-related inhibition of return effect. The main difference was that the average IQ patient corrected errors to the same degree as controls and regression analysis showed that error correction was related to IQ and not to diagnostic group. Thus in the full group of schizophrenia patients, it can be concluded that lower IQ is related to the inability to self-monitor and correct errors whereas impaired negative feedback processing Is independent of IQ effects.
Rather than reflecting failures to update rules, an alternative explanation for the present results would be that patients’ have correct knowledge of task rules but have a weak representation of motoric goals (Hunt et al., 2004; Reuter, 2004) such that prepotent saccadic responses dominate. In the current context, the aversion to negative feedback is the analogous prepotent response which dominates saccadic responding. A previous study of the oculomotor rule switching task in patients with frontal lobe lesions (Hodgson et al., 2007) reports that increased errors after a rule switch is associated with lesions of the right ventrolateral prefrontal cortex, an area thought to be part of a network involved in inhibitory control of responding, including task switching (Aron et al., 2004) and response inhibition, particularly stopping (Aron et al., 2003). We have also previously demonstrated impaired stopping in the same group of patients with schizophrenia (Huddy et al., 2009) and other researchers have shown an attenuation of inferior frontal cortex activity in patients (Kaladjian et al., 2007). However, if inhibitory failure was solely responsible for patients impairment on the task, it would be expected that patients would correct a substantial proportion of their errors (Polli et al., 2006) - and the fact they did not may support the previous suggestion that the abnormality lies in the use of negative feedback in rule abstraction. Another possibility is that the deficit on this task represents both rule updating and inhibitory impairment in schizophrenia.
Another brain region which may be important in attention and rule switching is the orbitofrontal cortex. This region is activated in healthy people and non-human primates during reversal learning tasks and it has been suggested that it serves to maintain a representation of the negative value of stimuli for action selection and in detecting breaches in expected positive outcomes and learning from them (Nobre et al.,1999; Tremblay and Schultz 2000; Takahashi et al., 2009). A single case study of a patient with a circumscribed bilateral orbitofrontal cortex excision reported their performance on the same oculomotor rule switching task described here (Hodgson et al 2002). It was found that this patient showed a reduction in the magnitude of the reward based inhibition of return effect, i.e. the opposite effect to that found in patients with schizophrenia in the present study, who made increased errors and whose behaviour was dominated by an overt inhibition of return bias. This would seem to indicate that the orbitofrontal cortex is over active in patients with schizophrenia, leading to an apparent over sensitivity to negative feedback. However, heightened Orbitofrontal (OFC) activity conflicts with other research indicating under active OFC function in schizophrenia (Leeson et al., 2008; Waltz and Gold, 2007; Murray et al., 2008). Given that rule representation is likely to be mediated by the dorsal frontal cortex and outcome value is an aspect of OFC function, it may be that dysfunction in schizophrenia is best explained by abnormal interactions between dorsal and orbital frontal cortex (see also Gold et al., 2008), rather than abnormality of the OFC per se.
These findings can also be viewed as being consistent with putative neurotransmitter dysfunction in schizophrenia. A wealth of evidence points towards abnormal dopamine function in psychosis (Anden et al., 1970; Creese et al., 1976; Seeman et al.,, 1976; Abi-Dargham et al., 2000). The mesolimbic dopamine system may be recruited to signal breaches in behaviour-outcome predictions which demand updating of cognitive representations. This dopaminergic “prediction error” signal (Schultz and Dickinson, 2000; Waelti et al., 2001) may be disrupted in schizophrenia compared to healthy individuals, contributing to the elaboration of delusional beliefs (Corlett et al., 2007; Kapur, 2003). This would fit with the present findings in which patients show an enhanced behavioural response to negative feedbacks (i.e. outcome prediciton breaches).
In summary, the current study demonstrated that patients in the early course of schizophrenia were more sensitive than healthy controls to negative feedbacks in the context of a simple rule switching task. The response profile suggested that the while patients had intact appreciation of the negative valence of punishing events they more often reacted instinctively rather than using the information to update rules that adaptively guide responses. This finding builds on previous reports of impaired negative feedback processing in schizophrenia by further specifying this deficit at the level of integration of negative feedback into a rule set, with intact basic responsiveness to feedback in the immediate context. As well as being consistent with hypothesised neuroanatomical and pharmacological abnormalities in the condition, the findings also support the use of cognitive rehabilitation packages that particularly focus on strategies such as an emphasis on errorless learning and positive feedback (e.g. Wykes et al., 2007).
Acknowledgements
This study was supported by Wellcome Trust programme grant 064607 to EMJ, MR and TREB and a Wellcome Trust Sabbatical Award to TLH. We are grateful to the consultants and nurses of West London and South West London and St George’s Mental Health National Health Service (NHS) Trusts for greatly facilitating the study and to Isobel Harrison and Stan Mutsatsa for patient assessments.
Footnotes
Declaration of Interest: TREB has acted as a consultant for Servier, Johnson & Johnson and Bristol-Myers Squibb. VH, TLH, MAR and EMJ have no biomedical financial interests or potential conflicts of interest
This manuscript describes work conducted at the Division of Neuroscience and Mental Health, Imperial College London
Contributor Information
Vyv C Huddy, Institute of Psychiatry, Kings College London..
Timothy L Hodgson, School of Psychology, University of Exeter..
Maria A Ron, Institute of Neurology, University College London..
Thomas RE Barnes, Division of Neuroscience and Mental Health, Imperial College London.
Eileen M Joyce, Institute of Neurology, University College London..
References
- Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, et al. Increased baseline occupancy of D-2 receptors by dopamine in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) The University of Iowa; Iowa City, Iowa: 1983. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) The University of Iowa; Iowa City, IA: 1984. [Google Scholar]
- Anden NE, Butcher SG, Corrodi H, Fuxe K, Ungerstedt U. Receptor activity and turnover of dopamine and noradrenaline after neuroleptics. Eur J Pharmacol. 1970;11:303–14. doi: 10.1016/0014-2999(70)90006-3. [DOI] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8(4):170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Berman KF, Ostrem JL, Randolph C, Gold J, Goldberg TE, Coppola R, et al. Physiological Activation of A Cortical Network During Performance of the Wisconsin Card Sorting Test - A Positron Emission Tomography Study. Neuropsychologia. 1995;33:1027–1046. doi: 10.1016/0028-3932(95)00035-2. [DOI] [PubMed] [Google Scholar]
- Blyler CR, Gold JM, Iannone VN, Buchanan RW. Short form of the ‘WAIS-III for use with patients with schizophrenia. Schizophrenia Research. 2000;46:209–215. doi: 10.1016/s0920-9964(00)00017-7. [DOI] [PubMed] [Google Scholar]
- Carter CS, Barch DM, Buchanan RW, Bullmore E, Krystal JH, Cohen J, et al. Identifying cognitive mechanisms targeted for treatment development in schizophrenia : an overview of the first meeting of the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia Initiative. Biological Psychiatry. 2008;64:4–10. doi: 10.1016/j.biopsych.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlett PR, Murray GK, Honey GD, Aitken MRF, Shanks DR, Robbins TW, et al. Disrupted prediction-error signal in psychosis: evidence for an associative account of delusions. Brain. 2007;130:2387–2400. doi: 10.1093/brain/awm173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese I, Burt DR, Snyder SH. Dopamine receptor-binding predicts clinical and pharmacological potencies of anti-schizophrenic drugs. Science. 1976;192(4238):481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Harvey PD. Systemic hypotheses for generalized cognitive deficits in schizophrenia: A new take on an old problem. Schizophrenia Bulletin. 2009;35:403–414. doi: 10.1093/schbul/sbn097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke C, Reuter B, Schulz L, Kathmann N. Schizophrenia patients show impaired response switching in saccade tasks. Biological Psychology. 2007;76:91–99. doi: 10.1016/j.biopsycho.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Fukushima J, Fukushima K, Chiba T, Tanaka S, Yamashita I, Kato M. Disturbances of voluntary control of saccadic eye movements in schiophrenic patients. Biological Psychiatry. 1988;23:670–677. doi: 10.1016/0006-3223(88)90050-9. [DOI] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: A deficit in the representation of value. Schizophrenia Bulletin. 2008;34:835–847. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Weinberger DR. Schizophrenia, Training Paradigms, and the Wisconsin Card Sorting Test Redux. Schizophrenia Research. 1994;11:291–296. doi: 10.1016/0920-9964(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Grant DA, Berg EA. A Behavioral Analysis of Degree of Reinforcement and Ease of Shifting to New Responses in A Weigl-Type Card-Sorting Problem. Journal of Experimental Psychology. 1948;38:404–411. doi: 10.1037/h0059831. [DOI] [PubMed] [Google Scholar]
- Greenzang C, Manoach DS, Goff DC, Barton JJS. Task-switching in schizophrenia: active switching costs and passive carry-over effects in an antisaccade paradigm. Experimental Brain Research. 2007;181:493–502. doi: 10.1007/s00221-007-0946-8. [DOI] [PubMed] [Google Scholar]
- Guitton D, Buchtel HA, Douglas RM. Frontal-Lobe Lesions in Man Cause Difficulties in Suppressing Reflexive Glances and in Generating Goal-Directed Saccades. Experimental Brain Research. 1985;58:455–472. doi: 10.1007/BF00235863. [DOI] [PubMed] [Google Scholar]
- Hallett PE, Adams BD. The Predictability of Saccadic Latency in A Novel Voluntary Oculomotor Task. Vision Research. 1980;20:329–339. doi: 10.1016/0042-6989(80)90019-x. [DOI] [PubMed] [Google Scholar]
- Heerey EA, Robinson BM, McMahon RP, Gold JM. Delay discounting in schizophrenia. Cognitive Neuropsychiatry. 2007;12:213–21. doi: 10.1080/13546800601005900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson T, Chamberlain M, Parris B, James M, Gutowski N, Husain M, et al. The role of the ventrolateral frontal cortex in inhibitory oculomotor control. Brain. 2007;130:1525–1537. doi: 10.1093/brain/awm064. [DOI] [PubMed] [Google Scholar]
- Hodgson TL, Golding C, Molyva D, Rosenthal CR, Kennard C. Eye movements during task switching: Reflexive, symbolic, and affective contributions to response selection. Journal of Cognitive Neuroscience. 2004;16:318–330. doi: 10.1162/089892904322984599. [DOI] [PubMed] [Google Scholar]
- Hodgson TL, Mort D, Chamberlain MM, Hutton SB, O’Neill KS, Kennard C. Orbitofrontal cortex mediates inhibition of return. Neuropsychologia. 2002;40(12):1891–1901. doi: 10.1016/s0028-3932(02)00064-7. [DOI] [PubMed] [Google Scholar]
- Huddy VC, Aron AR, Harrison M, Barnes TRE, Robbins TW, Joyce EM. Impaired conscious and preserved unconscious inhibitory processing in recent onset schizophrenia. Psychological Medicine. 2009;39:907–916. doi: 10.1017/S0033291708004340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huddy VC, Hodgson TL, Kapasi M, Mutsatsa SH, Harrison I, Bames TRE, et al. Gaze strategies during planning in first-episode psychosis. Journal of Abnormal Psychology. 2007;116:589–598. doi: 10.1037/0021-843X.116.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt AR, Klein RM. Eliminating the cost of task set reconfiguration. Memory & Cognition. 2002;30:529–539. doi: 10.3758/bf03194954. [DOI] [PubMed] [Google Scholar]
- Hunt AR, Olk B, von Muhlenen A, Kingstone A. Integration of competing saccade programs. Cognitive Brain Research. 2004;19:206–208. doi: 10.1016/j.cogbrainres.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Husain M, Parton A, Hodgson TL, Mort D, Rees G. Self-control during response conflict by human supplementary eye field. Nature Neuroscience. 2003;6:117–118. doi: 10.1038/nn1005. [DOI] [PubMed] [Google Scholar]
- Hutton SB, Ettinger U. The antisaccade task as a research tool in psychopathology: A critical review. Psychophysiology. 2006;43:302–313. doi: 10.1111/j.1469-8986.2006.00403.x. [DOI] [PubMed] [Google Scholar]
- Hutton SB, Puri BK, Duncan L-J, Robbins TW, Barnes TRE, Joyce EM. Executive function in first-episode Schizophrenia. Psychological Medicine. 1998;28:463–473. doi: 10.1017/s0033291797006041. [DOI] [PubMed] [Google Scholar]
- Jablensky A, Sartorius N, Ernberg G, Anker M, Korten A, Cooper J, et al. Schizophrenia: manifestations, incidence and course in different cultures. A World Health Organization ten country study. Psychological Medicine: Mongraph Supplement. 1992;20:1–97. doi: 10.1017/s0264180100000904. [DOI] [PubMed] [Google Scholar]
- Kaladjian A, Jeanningros R, Azorin JM, Grimault S, Anton JL, Mazzola-Pomietto P. Blunted activation in right ventrolateral prefrontal cortex during motor response inhibition in schizophrenia. Schizophrenia Research. 2007;97:184–193. doi: 10.1016/j.schres.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: A framework linking biology, phenomenology, and pharmacology in schizophrenia. American Journal of Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Leeson V, Robbins TW, Matheson E, Hutton SB, Ron M, Barnes TRE, et al. Discrimination learning, reversal and set-shifting in first episode schizophrenia: stability over six years and specific associations with medication type and disorganisation syndrome. Biological Psychiatry. 2009;15:586–93. doi: 10.1016/j.biopsych.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Park S. Do schizophrenia patients make more perseverative than non-perseverative errors on the Wisconsin Card Sorting Test? A meta-analytic study. Psychiatry Research. 2004;129:179–90. doi: 10.1016/j.psychres.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Lindgren KA, Cherkasova MV, Goff DC, Halpern EF, Intriligator J, et al. Schizophrenic subjects show deficient inhibition but intact task switching on saccadic tasks. Biological Psychiatry. 2002;51:II. doi: 10.1016/s0006-3223(01)01356-7. [DOI] [PubMed] [Google Scholar]
- Meiran N, Levine J, Meiran N, Henik A. Task set switching in schizophrenia. Neuropsychology. 2000;14:471–482. doi: 10.1037//0894-4105.14.3.471. [DOI] [PubMed] [Google Scholar]
- Monsell S. Task switching. Trends in Cognitive Sciences. 2003;7:134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Murray GK, Cheng F, Clark L, Barnett JH, Blackwell AD, Fletcher PC, et al. Reinforcement and reversal learning in first-episode psychosis. Schizophrenia Bulletin. 2008;34:848–855. doi: 10.1093/schbul/sbn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson HE. Modified Card Sorting Test Sensitive to Frontal Lobe Defects. Cortex. 1976;12:313–324. doi: 10.1016/s0010-9452(76)80035-4. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Coull JT, Frith CD, Mesulam MM. Orbitofrontal cortex is activated during breaches of expectation in tasks of visual attention. Nature Neuroscience. 1999;2(1):11–12. doi: 10.1038/4513. [DOI] [PubMed] [Google Scholar]
- Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and Spatial Working Memory Following Frontal-Lobe Lesions in Man. Neuropsychologia. 1990;28:1021–1034. doi: 10.1016/0028-3932(90)90137-d. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Barnes TRE, Nelson HE, Tanner S, Weatherley L, Owen AM, Robbins TW. Fronto-striatal cognitive deficits in patients with chronic schizophrenia. Brain. 1997;120:1823–1843. doi: 10.1093/brain/120.10.1823. [DOI] [PubMed] [Google Scholar]
- Parton A, Nachev P, Hodgson TL, Mort D, Thomas D, Ordidge R, et al. Role of the human supplementary eye field in the control of saccadic eye movements. Neuropsychologia. 2007;45:997–1008. doi: 10.1016/j.neuropsychologia.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polli FE, Barton JJS, Vangel M, Goff DC, Iguchi L, Manoach DS. Schizophrenia patients show intact immediate error-related performance adjustments on an antisaccade task. Schizophrenia Research. 2006;82:191–201. doi: 10.1016/j.schres.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rafal RD, Choate LS, Vaughan J. Inhibition of return: neural basis and function. Cognitive Neuropsychology. 1985;2:211–38. [Google Scholar]
- Prentice KJ, Gold JM, Buchanan RW. The Wisconsin Card Sorting impairment in schizophrenia is evident in the first four trials. Schizophrenia Research. 2008;106:81–87. doi: 10.1016/j.schres.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter B, Jager M, Bottlender R, Kathmann N. Impaired action control in schizophrenia: The role of volitional saccade initiation. Neuropsychologia. 2007;45:1840–1848. doi: 10.1016/j.neuropsychologia.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Reuter B, Kathmann N. Using saccade tasks as a tool to analyze executive dysfunctions in schizophrenia. Acta Psychologica. 2004;115:255–269. doi: 10.1016/j.actpsy.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annual Review of Neuroscience. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Seeman P, Chau-Wong M, Tedesco J, Wong K. Dopamine receptors in human and calf brains, using [3H]apomorphine and an antipsychotic drug. Proc Natl Acad Sci. 1976;73:4354–8. doi: 10.1073/pnas.73.12.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swainson R, Rogers RD, Sahakian BJ, Summers BA, Polkey CE, Robbins TW. Probabilistic learning and reversal deficits in patients with Parkinson’s disease or frontal or temporal lobe lesions: possible adverse effects of dopaminergic medication. Neuropsychologia. 2000;38:596–612. doi: 10.1016/s0028-3932(99)00103-7. [DOI] [PubMed] [Google Scholar]
- Takahashi YK, Roesch MR, Stainaker TA, et al. The Orbitofrontal Cortex and Ventral Tegmental Area Are Necessary for Learning from Unexpected Outcomes. Neuron. 2009;62(2):269–280. doi: 10.1016/j.neuron.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Reward-related neuronal activity during gonogo task performance in primate orbitofrontal cortex. JOURNAL OF NEUROPHYSIOLOGY. 2000;83(4):1864–1876. doi: 10.1152/jn.2000.83.4.1864. [DOI] [PubMed] [Google Scholar]
- Waelti P, Dickinson A, Schultz W. Dopamine responses comply with basic assumptions of formal learning theory. Nature. 2001;412:43–48. doi: 10.1038/35083500. [DOI] [PubMed] [Google Scholar]
- Walker R, Husain M, Hodgson TL, Harrison J, Kennard C. Saccadic eye movement and working memory deficits following damage to human prefrontal cortex. Neuropsychologia. 1998;36:1141–1159. doi: 10.1016/s0028-3932(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. Third Edition Psychological Corporation; London: 1999. [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading. Third Edition Psychological Corporation; London: 2001. [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia .1. Regional cerebral blood-flow evidence. Archives Of General Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Waltz JA, Gold JM. Probabilistic reversal learning impairments in schizophrenia: Further evidence of orbitofrontal dysfunction. Schizophrenia Research. 2007;93:296–303. doi: 10.1016/j.schres.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: a meta-analytic review. American Journal of Psychiatry. 2008;165:579–587. doi: 10.1176/appi.ajp.2008.07081242. [DOI] [PubMed] [Google Scholar]
- Wykes T, Reeder C, Landau S, Everitt B, Knapp M, Patel A, Romeo R. Cognitive remediation therapy in schizophrenia: randomised controlled trial. British Journal of Psychiatry. 2007;190:421–27. doi: 10.1192/bjp.bp.106.026575. [DOI] [PubMed] [Google Scholar]