Abstract
Frequency potentiation of contractile function is a major mechanism of the increase in myocardial performance during exercise. In heart failure (HF), this positive force-frequency relation is impaired, and the abnormal left ventricular (LV)-arterial coupling is exacerbated by tachycardia. A myofilament Ca2+ sensitizer, levosimendan, has been shown to improve exercise tolerance in HF. This may be due to its beneficial actions on the force-frequency relation and LV-arterial coupling (end-systolic elastance/arterial elastance, EES/EA). We assessed the effects of therapeutic doses of levosimendan on the force-frequency relation and EES/EA in nine conscious dogs after pacing-induced HF using pressure-volume analysis. Before HF, pacing tachycardia increased EES, shortened τ, and did not impair EES/EA and mechanical efficiency (stroke work/pressure-volume area, SW/PVA). In contrast, after HF, pacing at 140, 160, 180, and 200 beat/min (bpm) produced smaller a increase of EES or less shortening of τ, whereas EES/EA (from 0.56 at baseline to 0.42 at 200 bpm) and SW/PVA (from 0.52 at baseline to 0.43 at 200 bpm) progressively decreased. With levosimendan, basal EES increased 27% (6.2 mmHg/ml), τ decreased 11% (40.8 ms), EES/EA increased 34% (0.75), and SW/PVA improved by 15% (0.60). During tachycardia, EES further increased by 23%, 37%, 68%, and 89%; τ decreased by 9%, 12%, 15%, and 17%; and EES/EA was augmented by 11%, 16%, 31%, and 33%, incrementally, with pacing rate. SW/PVA was improved (0.61 to 0.64). In conclusion, in HF, treatment with levosimendan restores the normal positive LV systolic and diastolic force-frequency relation and prevents tachycardia-induced adverse effect on LV-arterial coupling and mechanical efficiency.
Keywords: drugs, contractility, diastole, heart rate
under normal conditions, an increase in heart rate (HR) augments myocardial contractility and the rate of relaxation (15, 39, 44). This force-frequency behavior is determined by both calcium handling and myofilament properties (26). It has been shown that the integrated dynamic balance of the intracellular Ca2+ concentration ([Ca2+]i) is the primary cellular mechanism responsible for the force-frequency relation (FFR) and is determined by sarcoplasmic reticulum (SR) Ca2+ load and Ca2+ flux through the sarcolemma via L-type Ca2+ channels and the Na+-Ca2+ exchanger (13). The positive FFR and relaxation-frequency relation (RFR) enhance cardiac performance during stress or exercise (7, 36, 39, 44, 52). In heart failure (HF), the positive FFR and RFR are impaired (16, 24, 28, 29, 44), and the abnormal left ventricular (LV)-arterial coupling is exacerbated by tachycardia. This contributes to exercise intolerance in HF (44, 52). The altered responses of LV contraction and relaxation to HR in HF may at least partially result from a frequency-dependent myofilament Ca2+ desensitization in HF (31). This suggests that the blunted or negative FFR can be opposed by a Ca2+ sensitizer.
Levosimendan, a novel myofilament Ca2+ sensitizer, is an inotropic and vasodilator agent. Unlike most other Ca2+ sensitizers, levosimendan acts through direct binding with troponin C, thereby increasing the affinity of troponin C for Ca2+. A lack of Ca2+ sensitization under low calcium concentrations (i.e., during diastole) has been shown to prevent worsening of diastolic dysfunction in patients with HF and animal models. Levosimendan also leads to peripheral and coronary vasodilation through the opening of ATP-sensitive potassium channels (60). Although the acute hemodynamic benefits with important advances for levosimendan in HF therapy have been documented, there is only limited information about the effect levosimendan on the FFR and RFR. The direct effects of therapeutic doses of levosimendan on the rate-dependent increase in contractility and lusitropy and mechanical efficiency in HF are unclear.
This study was conducted to test the hypothesis that therapeutically relevant concentrations of levosimendan reverse frequency-dependent Ca2+ desensitization in the failing heart (31), lead to the restoration of the positive FFR and RFR, and prevent tachycardia-induced adverse effects on LV arterial-ventricular coupling along with improved mechanical efficiency in HF. To avoid the potential confounding effects of levosimendan-produced changes in loading conditions on conventional measures of LV contractile performance, we evaluated LV contractile performance in the pressure-volume (P-V) plane in conscious dogs with pacing-induced HF that mimics many of the functional and neurohormonal changes of clinical HF (5, 7, 29, 33, 38, 41).
MATERIALS AND METHODS
Instrumentation
This investigation was approved by the Institutional Animal Care and Use Committee and conforms to the Guide for the Care and Use of Laboratory Animals. Nine healthy, male, adult, heartworm-negative, mongrel dogs (25–35 kg body wt) were instrumented to measure three LV internal dimensions, LV pressure, and left atrial (LA) pressure. Myocardial leads (Model 4312; Cardiac Pacemakers, Minneapolis, MN) were implanted within the myocardium of the right ventricle (RV) and right atrium (RA), and the leads were attached to unipolar multiprogrammable pacemakers (Model 8329; Medtronic, Minneapolis, MN) positioned under the skin in the chest. Hydraulic occluders were placed around the venae cavae by a technique described previously (7, 37, 52).
Data Collection
Studies were performed after full recovery from instrumentation (10 days after original surgery) with the dogs standing quietly in a sling. Data were acquired and analyzed using customized software (SPECTRUM, Version 5.0) as described previously (7, 52).
Experimental Protocol
Studies before HF.
NORMAL FFR.
To determine the normal FFR, initial steady-state and transient caval occlusion data were recorded during 15-s periods in the normal animals with sinus rhythm while they were standing quietly and unsedated in a sling. After baseline collections, RA pacing at 140 beat/min (bpm) was performed. After 3–5 min, when the LV pressure had reached a stable level, both steady-state and venae caval occlusion (VCO) data at rest were obtained. RA pacing rate was then increased to 160, 180, and 200 bpm, and the data were acquired in a similar manner.
Studies during the development of HF.
Following completion of the normal studies, as previously described, rapid RV pacing (at 220–240 bpm) was initiated. After pacing for 4–5 wk, when the LV end-diastolic pressure (PED) during the nonpaced period had increased by more than 15 mmHg over the prepacing control level, HF data were obtained.
Studies after the onset of HF.
FFR AFTER HF.
During the stable HF period, the RA pacing protocol was repeated. Briefly, the pacemaker was turned off, and the animal was allowed to stabilize for at least 1 h. Steady-state and VCO data were collected in the animal with sinus rhythm. Data were then acquired again during RA pacing at 140, 160, 180, and 200 bpm, respectively.
EFFECTS OF LEVOSIMENDAN ON FFR IN HF.
After HF control FFR data collections, the pacemaker was turned off and the dog was allowed to equilibrate. When the LV pressure had reached a stable level, levosimendan was started with loading doses of 24 μg/kg administered for 10 min followed by infusion of levosimendan at rates of 0.2 μg/kg per min for 40 min (52). The dosing protocol of levosimendan used in this study was identified from our initial dosage-response study, and its efficiency was verified in our past study (52). A similar dosing paradigm was used by many investigators including clinical trials (9) and experimental studies (37, 46). Of note, in humans, levosimendan is completely metabolized before excretion. Approximately 5% of a dose is converted to OR-1855 and then to a highly active metabolite, OR-1896, which has hemodynamic effects similar to those of levosimendan (9). In contrast, in dogs, neither levosimendan nor OR-1855 is metabolized to OR-1896 (3). The blood samples for levosimendan concentration were collected as previously described (37). The pacing protocol was then repeated, and data were again collected as described above.
Data Processing and Analysis
LV volume, time constant of relaxation (τ), LV end-systolic pressure (PES)-end-systolic volume (VES) relation, and its slope (end-systolic elastance, EES) as well as the slope (dE/dtmax) of the relation between LV dP/dtmax and end-diastolic volume (VED), a load-independent index of LV contractile performance (35), were analyzed as previously described (7, 37). V90,ES, the volume associated with a PES of 90 mmHg, was calculated (45). LV-arterial coupling was quantitated as the ratio of EES to arterial elastance (EA). EA was calculated as PES/stroke volume (SV). The LV P-V area (PVA), which represents the total mechanical energy, was determined as the area under the end-systolic P-V relation and systolic P-V trajectory above the PED-V curve. The efficiency of conversion of mechanical energy to external work of the heart was calculated as mechanical efficiency (stoke work/PVA, SW/PVA) (36, 42).
Determination of plasma LS concentrations.
A 1-ml blood sample was drawn from the peripheral venous catheter (different side without intravenous drug infusion), placed into precooled, sterile Vacutainer tubes, and centrifuged at 3,000 revolution/min for 10 min. The resulting plasma from each sample was stored at −80°C for subsequent analysis. The concentrations of levosimendan were determined by liquid chromatography tandem mass spectrometry at the Department of Pharmacokinetics and Bioanalytics, Orion Pharma (Orion, Espoo, Finland) (37).
Statistical Analysis
Data are expressed as means ± SD or means ± SE where otherwise stated. Comparisons between normal and HF were made using two-tailed Student's t-test. Pairwise comparisons were made using Student's paired t-test. LV function parameters before and during pacing were compared using repeat-measure ANOVA. P < 0.05 was considered significant.
RESULTS
Development of Pacing-Induced HF
As summarized in Table 1, consistent with our past reports (7, 38, 41, 52), chronic ventricular rapid pacing in a canine model produced progressive LV systolic and diastolic dysfunction. After 4–5 wk of rapid pacing, the LV PED was elevated (158%; HF: 29.9 ± 5.1, normal: 11.6 ± 3.3 mmHg). Both LV VES and VED were significantly increased, whereas SV was decreased. The time constant (τ) increased (47%; 45.7 ± 6.9 vs. 31.1 ± 1.7 ms) (Table 1). The details of the LV P-V analysis are shown in Table 2. LV contractility significantly decreased as indicated by marked rightward shifts of the PES-VES relation with reductions in the slopes of EES (26%; 4.9 ± 1.0 vs. 6.6 ± 1.5 mmHg/ml) (Table 2, Figs. 1 and 2). EA was increased (34%; 9.8 ± 4.4 vs. 7.3 ± 1.2 mmHg/ml). Thus the EES/EA ratio significantly decreased (39%; 0.56 ± 0.14 vs. 0.92 ± 0.25) accompanied by reduced LV mechanical efficiency of SW/PVA (15%; 0.52 ± 0.06 vs. 0.61 ± 0.07) (P < 0.05).
Table 1.
Effect of levosimendan on hemodynamic and LV diastolic functional responses to heart rate in HF
| Pacing Rate, bpm |
|||||
|---|---|---|---|---|---|
| Baseline | 140 | 160 | 180 | 200 | |
| Normal | |||||
| Heart rate, bpm | 101 ± 9.8 | 140 ± 0.2* | 157 ± 1.2* | 179 ± 1.2* | 199 ± 0.7* |
| Peak +dP/dt, mmHg/s | 2163 ± 249 | 2086 ± 504 | 2120 ± 528 | 2122 ± 489 | 2090 ± 524 |
| Peak −dP/dt, mmHg/s | −1835 ± 195 | −1836 ± 334 | −1862 ± 309 | −1865 ± 319 | −1810 ± 354 |
| LV end-systolic pressure, mmHg | 97.2 ± 11.1 | 93.7 ± 17.3 | 93.4 ± 14.7 | 92.4 ± 16.1 | 88.7 ± 17.7* |
| LV end-diastolic pressure, mmHg | 11.6 ± 3.3 | 7.5 ± 2.4* | 6.4 ± 1.2* | 6.7 ± 1.0* | 5.9 ± 1.9* |
| LV end-systolic volume, ml | 26.8 ± 10.7 | 25.5 ± 10.4* | 25.4 ± 10.5* | 24.6 ± 10.6* | 23.9 ± 10.1* |
| LV end-diastolic volume, ml | 40.3 ± 12.0 | 35.4 ± 12.5* | 34.5 ± 12.5* | 32.7 ± 12.4* | 31.6 ± 11.9* |
| Stroke volume, ml | 13.4 ± 1.6 | 9.8 ± 2.3* | 9.1 ± 2.4* | 8.2 ± 1.8* | 7.6 ± 1.9* |
| Cardiac output, ml/min | 1363 ± 209 | 1373 ± 326 | 1428 ± 378 | 1465 ± 328 | 1517 ± 384 |
| Time constant τ, ms | 31.1 ± 1.7 | 29.0 ± 2.0 | 26.6 ± 2.1* | 25.6 ± 1.8* | 24.9 ± 2.1* |
| HF Control | |||||
| Heart rate, bpm | 120 ± 12.5 | 140 ± 0.1* | 157 ± 0.4* | 179 ± 1.2* | 198 ± 1.6* |
| Peak +dP/dt, mmHg/s | 1401 ± 370† | 1363 ± 413† | 1424 ± 349† | 1448 ± 358† | 1437 ± 356† |
| Peak −dP/dt, mmHg/s | −1425 ± 257† | −1410 ± 280† | −1464 ± 240† | −1487 ± 237† | −1482 ± 210† |
| LV end-systolic pressure, mmHg | 94.2 ± 8.2 | 93.4 ± 8.5 | 95.9 ± 7.3 | 92.9 ± 8.0 | 91.8 ± 8.4* |
| LV end-diastolic pressure, mmHg | 29.9 ± 5.1† | 26.2 ± 5.4*† | 24.7 ± 5.0*† | 23.1 ± 4.2*† | 24.0 ± 5.4*† |
| LV end-systolic volume, ml | 41.0 ± 14.5† | 41.5 ± 14.5† | 40.9 ± 14.4† | 40.3 ± 14.2† | 39.3 ± 14.0† |
| LV end-diastolic volume, ml | 52.2 ± 17.6† | 50.7 ± 16.4† | 49.9 ± 16.8*† | 47.9 ± 16.0*† | 46.2 ± 15.7*† |
| Stroke volume, ml | 11.3 ± 3.8 | 9.2 ± 2.1* | 8.9 ± 2.6* | 7.6 ± 2.2* | 6.9 ± 2.0* |
| Cardiac output, ml/min | 1331 ± 365 | 1286 ± 295 | 1393 ± 415 | 1373 ± 403 | 1362 ± 395 |
| Time constant τ, ms | 45.7 ± 6.9† | 45.9 ± 7.8† | 44.1 ± 7.2† | 42.0 ± 6.5*† | 41.7 ± 4.9*† |
| HF & LS | |||||
| Heart rate, bpm | 119 ± 19.1 | 140 ± 1.8* | 157 ± 0.5* | 179 ± 2.1* | 198 ± 1.7* |
| Peak +dP/dt, mmHg/s | 1717 ± 471‡ | 1654 ± 451‡ | 1709 ± 459‡ | 1741 ± 435‡ | 1805 ± 391‡ |
| Peak −dP/dt, mmHg/s | −1629 ± 266‡ | −1597 ± 210‡ | −1665 ± 224‡ | −1668 ± 215‡ | −1732 ± 197‡ |
| LV end-systolic pressure, mmHg | 96.5 ± 4.7 | 93.0 ± 5.8 | 94.7 ± 7.1 | 93.6 ± 7.1 | 94.6 ± 7.1 |
| LV end-diastolic pressure, mmHg | 26.1 ± 6.3 | 20.6 ± 4.8‡ | 19.6 ± 5.9*‡ | 18.7 ± 4.2*‡ | 19.5 ± 4.5*‡ |
| LV end-systolic volume, ml | 37.4 ± 12.5‡ | 36.7 ± 12.2*‡ | 36.3 ± 11.1‡ | 35.5 ± 10.8‡ | 35.0 ± 11.1*‡ |
| LV end-diastolic volume, ml | 50.4 ± 15.6 | 47.3 ± 14.6*‡ | 46.4 ± 13.6*‡ | 44.8 ± 13.5*‡ | 44.1 ± 13.9* |
| Stroke volume, ml | 12.9 ± 3.8‡ | 10.6 ± 3.1* | 10.1 ± 3.0*‡ | 9.3 ± 3.1*‡ | 9.1 ± 3.2*‡ |
| Cardiac output, ml/min | 1562 ± 468‡ | 1496 ± 433 | 1590 ± 479‡ | 1678 ± 566‡ | 1800 ± 640*‡ |
| Time constant τ, ms | 40.8 ± 6.3‡ | 37.2 ± 6.6*‡ | 35.8 ± 6.8*‡ | 34.7 ± 6.2*‡ | 34.1 ± 4.8*‡ |
Values are mean ± SD.
P < 0.05 vs. corresponding baseline values;
P < 0.05, differences between normal and heart failure (HF) control;
P < 0.05, differences between HF control and HF with levosimendan (HF & LS) at each pacing rate. LV, left ventricle; bpm, beats/min.
Table 2.
Effect of levosimendan on the PES-VES relations, LV-arterial coupling, and mechanical efficiency responses to heart rate in HF
| Pacing Rate, bpm |
|||||
|---|---|---|---|---|---|
| Baseline | 140 | 160 | 180 | 200 | |
| Normal | |||||
| EES, mmHg/ml | 6.6 ± 1.5 | 8.6 ± 2.9 | 10.2 ± 3.0* | 10.8 ± 2.5* | 14.4 ± 5.1* |
| EA, mmHg/ml | 7.3 ± 1.2 | 9.8 ± 2.3* | 10.8 ± 3.2* | 11.5 ± 2.1* | 12.1 ± 3.4* |
| EES/EA | 0.92 ± 0.25 | 0.91 ± 0.39 | 1.0 ± 0.4 | 0.96 ± 0.31 | 1.3 ± 0.67 |
| SW/PVA | 0.61 ± 0.07 | 0.59 ± 0.10 | 0.61 ± 0.08 | 0.59 ± 0.07 | 0.63 ± 0.11 |
| V90, ES | 23.8 ± 9.0 | 23.8 ± 8.3 | 23.8 ± 8.7 | 23.4 ± 8.8 | 23.3 ± 9.0 |
| HF Control | |||||
| EES, mmHg/ml | 4.9 ± 1.0† | 5.3 ± 1.0† | 5.5 ± 0.7*† | 5.6 ± 0.8*† | 5.9 ± 1.2*† |
| EA, mmHg/ml | 9.8 ± 4.4 | 10.8 ± 3.1 | 11.7 ± 3.6* | 13.7 ± 3.7* | 14.7 ± 4.9* |
| EES/EA | 0.56 ± 0.14† | 0.51 ± 0.13† | 0.47 ± 0.12*† | 0.42 ± 0.12*† | 0.42 ± 0.11*† |
| SW/PVA | 0.52 ± 0.06† | 0.49 ± 0.05† | 0.47 ± 0.05*† | 0.43 ± 0.05*† | 0.43 ± 0.05*† |
| V90, ES | 35.7 ± 12.8 | 36.6 ± 13.4 | 37.1 ± 13.3 | 36.6 ± 13.4 | 36.1 ± 12.3 |
| HF & LS | |||||
| EES, mmHg/ml | 6.2 ± 1.6‡ | 7.6 ± 0.8‡ | 8.5 ± 1.3*‡ | 10.4 ± 2.3*‡ | 11.7 ± 2.6*‡ |
| EA, mmHg/ml | 8.4 ± 2.1 | 9.8 ± 2.7 | 10.5 ± 3.3*‡ | 11.3 ± 3.7*‡ | 12.2 ± 4.4*‡ |
| EES/EA | 0.75 ± 0.15‡ | 0.83 ± 0.23‡ | 0.87 ± 0.28‡ | 0.98 ± 0.33‡ | 1.0 ± 0.37‡ |
| SW/PVA | 0.60 ± 0.06‡ | 0.61 ± 0.05‡ | 0.61 ± 0.07‡ | 0.63 ± 0.07‡ | 0.64 ± 0.08‡ |
| V90, ES | 32.6 ± 11.9‡ | 33.3 ± 12.1‡ | 33.4 ± 12.0‡ | 33.2 ± 11.1‡ | 33.1 ± 11.1‡ |
Values are means ± SD.
P < 0.05, vs. baseline;
P < 0.05, differences between normal and HF control;
P < 0.05, differences between HF control and HF & LS at each pacing rate. PES, end-systolic pressure; VES, end-systolic volume; EES, end-systolic elastance; EA, effective arterial elastance; SW, stroke work; PVA, pressure-volume area; V90, ES, end-systolic volume at end-systolic pressure equal to 90 mmHg.
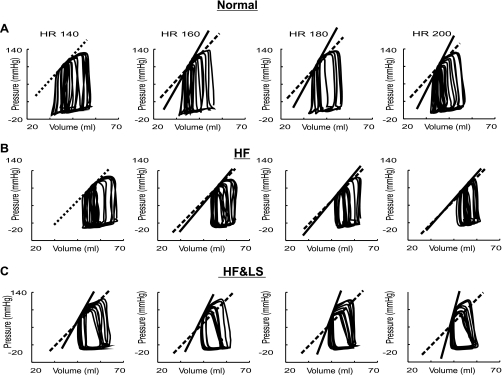
Fig. 1.
Examples of the effect of levosimendan (LS) on left ventricular (LV) contractile function at matched heart rate (HR) during pacing in increments of 20 to 200 beat/min (bpm) after heart failure (HF). Variably loaded pressure-volume (P-V) loops during pacing obtained in the same animal before heart failure (HF) (A), after HF (B), and HF following acute LS administration (C). The end-systolic P-V (PES-VES) relation is indicated by the solid line (ESPVR). The dashed line indicates ESPVR with pacing rate of 140 bpm before drug administration. A: before HF, during pacing, there were serially progressive leftward shifts of the PES-VES relation with increased slopes, which indicate progressively increased LV contractility. B: after HF, during pacing, there were no marked shifts in the positions of PES-VES relationships, and the increases in end-systolic elastance (EES) were significantly smaller at each pacing rate compared with that before HF, demonstrating a blunted frequency potentiation of LV contractility. In contrast, in HF treated with LS (C), tachycardia caused similar incremental increases in the slopes of PES-VES relations as seen before HF, consistent with restoration of normal HR response on ESPVR in HF.
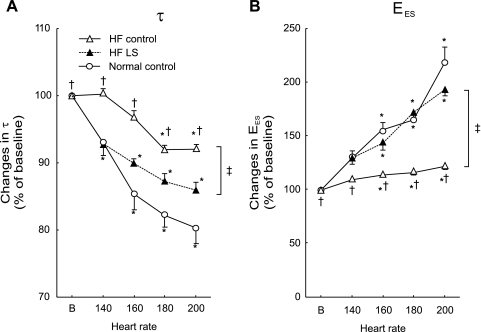
Fig. 2.
Group means of increasing HR-caused percent changes on τ (A) and EES (B). In normal (○), compared with baseline, the percent decreases in τ and increases in EES were significantly increased incrementally with pacing rate, indicating frequency-dependent positive inotropic and lusitropic effects. After HF (▵), increasing HR produced blunted responses with significantly smaller percent changes in τ and EES at each pacing rate. In contrast, concomitant LS (▴) administration with pacing caused percent decreases in τ and increases in EES similar to that observed before HF. Error bar indicates SE. *P < 0.05 vs. baseline; †P < 0.05 vs. before HF; ‡P < 0.05 on LS effects on HR change.
Responses to Increasing HR Before and After HF
Before HF.
As shown in Table 1, in the normal heart, compared with baseline, increased HR resulted in progressively significant decreases in LV PED with relatively unchanged LV PES. LV VES and LV VED were decreased. Cardiac output still increased (from 1,363 ± 209 ml at baseline to 1,517 ± 384 ml at 200 bpm) despite reduced SV. EA was significantly increased. Increasing HR failed to cause significant changes of LV peak +dP/dt and peak −dP/dt. With incremental changes in HR with pacing, τ was progressively shortened from 31.1 ± 1.7 ms at baseline to 29.0 ± 2.0, 26.6 ± 2.1, 25.6 ± 1.8, and 24.9 ± 2.1 ms (Table 1).
As presented in Table 2 and exhibited in Fig. 1, the PES-VES relationships were progressively shifted to the left, and the slopes of EES increased incrementally with pacing rate from 6.6 ± 1.3 mmHg/ml at baseline to 8.6.0 ± 2.9, 10.2 ± 3.0, 10.8 ± 2.5, and 14.4 ± 5.1 mmHg/ml. Consistently, the slopes of LV dP/dtmax and VED relationship (dE/dtmax) were also progressively increased during pacing tachycardia (96 ± 33, 112 ± 33, 125 ± 40, and 136 ± 31 mmHg/ml per s with 140, 160, 180, and 200 bpm, respectively). The τ decreased by 20% and EES increased by 118% at 200 bpm, respectively (Fig. 2). These alterations are consistent with positive FFR and RFR. Of note, increased HR not only affected the PES-VES relation but also altered the arterial system. Pacing caused similar incremental increases in EA. Thus EES/EA ratios were not significantly altered with increased HR. SW/PVA ratios also remained unchanged (from 0.61 ± 0.07 at baseline to 0.63 ± 0.11 at 200 bpm) (P = NS) during pacing-induced tachycardia (Table 2 and Fig. 3).
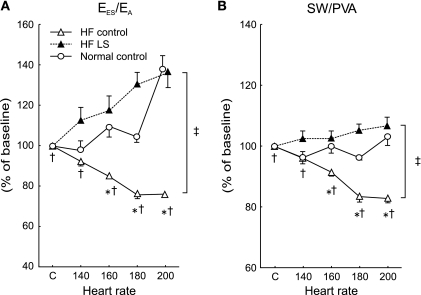
Fig. 3.
Group means of increasing HR-caused percent changes on the EES/arterial elastance (EA) (A) and stroke work/pressure-volume area (SW/PVA). In normal (○), compared with baseline, EES/EA and SW/PVA remained relatively unchanged. In contrast, after HF (▵), EES/EA and SW/PVA were progressively decreased. Following LS administration in HF (▴), this altered response to HR in EES/EA and SW/PVA was prevented. Error bar indicates SE. *P < 0.05 vs. corresponding baseline; †P < 0.05 vs. before HF; ‡ P < 0.05 on LS effects on HR change.
After HF.
In the failing heart, compared with baseline, increased HR also caused significant decreases in LV PED, VES, VED, and SV, but no significant alterations in PES, peak +dP/dt and peak −dP/dt. In contrast to before HF, increased HR failed to increase cardiac output. As shown in Fig. 2, pacing at 140, 160, 180, and 200 bpm only produced modest changes in τ (from 45.7 ± 6.9 ms at baseline to 45.9 ± 7.8, 44.1 ± 7.2, 42.0 ± 6.5, and 41.7 ± 4.9 ms), amounting to 0–8% reductions. Similar to before HF, EA was increased incrementally at each pacing rate. There were no marked shifts in the positions of PES-VES relationships, and the increases in the slopes of EES and dE/dtmax were significantly smaller. As exhibited in Fig. 2, pacing at 140, 160, 180 and 200 bpm only produced modest increases in EES (from 4.9 ± 1.0 mmHg/ml at baseline to 5.3 ± 1.0, 5.5 ± 0.7, 5.6 ± 0.8, and 5.9 ± 1.2 mmHg/ml), amounting to 8–20% increases. Consistently, dE/dtmax was modestly increased during pacing tachycardia (74 ± 3, 87 ± 8, 84 ± 3, and 89 ± 7 mmHg/ml per s with 140, 160, 180, and 200 bpm, respectively). Moreover, in contrast to the effects before HF, EES/EA progressively decreased, which led to significantly incremental reductions in SW/PVA at each increased pacing rate (Table 2 and Fig. 3).
Effect of Levosimendan on the Responses to Increasing HR in HF
In HF, after levosimendan intravenous administration with loading doses of 24 μg/kg administered for 10 min followed by infusion of levosimendan at rates of 0.2 μg/kg per min for 40 min, the steady-state plasma levosimendan concentrations were 68.3 ± 12.1 ng/ml, within reported working range (37). The plasma levels of levosimendan were similar to previous human (9, 30, 48) and animal experimental investigations (3, 37, 46, 52).
As shown in Tables 1 and 2, consistent with past reports (37, 46, 52), this dosing protocol was well tolerated by all animals and caused no side effects such as tachycardia, arrhythmias, or excessive preload reduction with hypotension in HF. The maintenance of HR observed during levosimendan administration to HF dogs may be related to the attenuation of baroreceptor reflex activity that is known to occur in HF (22, 46). Levosimendan caused significant enhancement of LV contractility and relaxation that took about 40 min to fully develop and was then sustained for more than 3 h. Levosimendan significantly reduced EA, PED, and τ along with an increased SV (12.9 ± 3.8 vs. 11.3 ± 3.8 ml) and cardiac output. Basal EES increased 27% (6.2 mmHg/ml), τ decreased 11% (40.8 ms), and EES/EA improved 34% (0.75). SW/PVA augmented 15% (from 0.52 ± 0.06 to 0.60 ± 0.06) (P < 0.05).
Importantly, these beneficial actions of levosimendan on basal LV function in sinus rhythm were accentuated at increasing HR during pacing. Levosimendan restored the normal frequency-dependent upregulation of LV contractility and relaxation rate in HF. Compared with baseline, significant increases in SV and cardiac output were observed over a wide range of HR, resulting from significantly greater reductions in LV VES. PES was unchanged, and EA decreased over a wide range of HR. Typical examples of the effect of pacing tachycardia on variably loaded PES-VES relations from one conscious chronically instrumented animal are presented in Fig. 1. As shown in Table 2 and Figs. 1 and 2, with levosimendan, during pacing at 140, 160, 180, and 200 bpm, τ decreased by 9%, 12%, 15%, and 17%, and EES further increased by 23%, 37%, 68%, and 89%, respectively. The LV PES-VES relationship was shifted leftward, manifested by significant reductions in V90,ES at each HR after levosimendan administration (Table 2). Consistently, with levosimendan, significantly greater incremental increases in dE/dtmax were achieved during pacing at 140, 160, 180, and 200 bpm (from 98 ± 7, 130 ± 14, 181 ± 86, to 182 ± 57 mmHg/ml per s, respectively). EES/EA was progressively augmented by 11%, 16%, 31%, and 33%. Thus, with the treatment of levosimendan, SW/PVA further improved from 0.60 ± 0.06 to 0.64 ± 0.08, which is close to the values of normal controls before HF (Table 2 and Fig. 3).
DISCUSSION
We found that a clinically relevant dosage of levosimendan restores the positive LV FFR and RFR in HF and prevents adverse effects of tachycardia on LV-arterial coupling and mechanical efficiency. These data demonstrate an important feature of levosimendan, which not only enhances basal cardiac function but also improves LV contractile and relaxation responses to chronotropic reserve for the failing heart (5, 8).
Reversal of FFR and RFR Defects in HF by Levosimendan
Consistent with past reports, in the present study, normal conscious dogs showed a positive FFR and RFR in response to pacing tachycardia. The increased HR caused progressively enhanced contractile function (EES increased by 118% at 200 bpm). The increased HR also permits the LV to relax more rapidly (τ decreased by 20% at 200 bpm) to augment the SV and to decrease the VES, thereby producing increased cardiac output. In contrast, dogs with pacing-induced HF exhibited an altered FFR with blunted frequency potentiation of LV contractility and relaxation. It appears that the failing heart was unable to adequately increase cardiac output at higher HR, which is consistent with observations in patients with HF (57). As highlighted in Figs. 1–3, pacing tachycardia only produced modest reductions in τ and caused significantly smaller increases in EES and dE/dtmax at each pacing rate. EES only increased by 20% at 200 bpm, amounting to an 83% smaller than normal increase in EES with an increase in HR from baseline to 200 bpm, constituting a significant loss of contractile reserve. Importantly, this abnormal force-frequency abnormality in HF was reversed with the treatment of levosimendan. After levosimendan in HF, during pacing tachycardia, τ was progressively shortened (τ decreased by 17% at 200 bpm), and EES incrementally increased by 89% at 200 bpm. Thus levosimendan resulted in a greater contractile force (positive FFR) and faster relaxation (positive RFR) at higher pacing frequencies.
Our present observation of the restoration of a positive FFR in HF with levosimendan concurs with several earlier clinical studies that have shown that levosimendan improves the frequency-induced force generation in human failing myocardium (20) and converts a negative FFR into a positive relationship (6, 25). However, our observations are somewhat different from those of Givertz et al. (16), who studied intracoronary infusion with levosimendan of patients with LV systolic dysfunction and concluded that levosimendan exerts positive inotropic and lusitropic effects over a range of heart rates but does not alter the slope of the FFR or RFR. This discrepancy may result from several factors, including the use of a load-sensitive index such as dP/dtmax as the measurement of contractility, lower dosages of levosimendan administered intracoronary, and the effect of pharmacotherapy on the failing human heart. For example, they used LV dP/dtmax as a measure of contractility of the intact human heart, but dP/dtmax is preload dependent. In contrast, in the present study, by using the load-insensitive index of contractility, EES and dE/dtmax, we clearly demonstrated that impaired FFR of contractility seen in HF was significantly restored with levosimendan up to 200 bpm (Table 2 and Figs. 2 and 3). In addition, in that study, the human group received multiple cardiovascular specific medications for HF. Chronic treatment with cardiovascular-specific medications (such as β-blockers and angiotensin-converting enzyme inhibitors) may alter the primary defect in the contractile properties of the heart, thus further modifying LV functional response to increased HR in HF. Our study demonstrated a more pronounced relaxation response to levosimendan at baseline and during tachycardia compared with the study by Givertz et al. (16).
It is evident that levosimendan can act through at least three signaling pathways to elicit beneficial effects in HF: 1) increase the Ca2+ sensitivity of contractile proteins by binding to troponin C, 2) inhibit phosphodiesterase (PDE)-III, and 3) activate ATP-dependent potassium channel in vascular smooth muscle cells and mitochondrial membranes (27, 59, 60). Although the mechanisms of the beneficial effect of levosimendan on FFR in HF are not entirely clear, enhancing the calcium sensitivity of the contractile proteins and PDE III inhibition are believed to play a key role in the levosimendan-caused positive FFR in HF.
Earlier observations indicated that the dependence of cardiac contractility on HR is attributable in principle to an increased Ca2+ transient associated with enhanced SR Ca2+ loading from a calmodulin II-dependent pathway (34, 47). The integrated dynamic balance of the [Ca2+]i is determined by SR Ca2+ load and Ca2+ flux through the sarcolemma via L-type Ca2+ channels and the Na+-Ca2+ exchanger (4, 13). The Ca2+-ATPase of the SR (50) also plays an important role in the regulation of FFR.
Both the alteration of Ca2+ handling and myofilament Ca2+ sensitivity could contribute to the deterioration of positive FFR in HF. Recent evidence further demonstrated that, in normal hearts, the positive FFR is primarily attributable to an increase in Ca2+ influx per unit of time. In failing hearts, frequency-dependent Ca2+ desensitization of the myofilaments contributes to the blunted, flat, or negative FFR (31). Increasing inotropic activity through an elevation in intracellular calcium has been the common denominator of inotropic drugs such as β-adrenergic agonists. It has been shown that some inotropic agents are able to improve the FFR in HF acting through the elevation of [Ca2+]i coupled with a cAMP-mediated activation (13). The effect of the cAMP-accumulating agents is complex, which depends on the combination of the extent of cAMP accumulation and the frequency-dependent Ca2+ regulation (13). The increase in cAMP leads to phosphorylation of phospholamban, calcium channels, and contractile element proteins via protein kinase A. However, contractility with these agents is increased at the expense of increased myocardial energy consumption, risk of ischemia, and arrhythmias (58). Distinct from traditional inotropic agents, Ca2+ sensitizers are pharmacological agents that improve cardiac contractility without increasing [Ca2+]i. Indeed, although levosimendan increases cellular responsiveness to calcium, it does not increase the intracellular concentration of Ca2+ within the cardiac myocyte (6, 11). The inotropic effect of levosimendan is mediated by calcium concentration-dependent conformational changes in troponin C during systole leading to sensitization of the contractile apparatus to calcium ions (17, 18). This mode of action of levosimendan may lead to frequency-dependent Ca2+ sensitization of the myofilaments, thereby reversing the abnormalities of the FFR in HF. It is well recognized that, in HF, the positive inotropic effects of cAMP-dependent inotropes are attenuated, but Ca2+ sensitizer-produced myofilament Ca2+ sensitization is preserved. Increased experimental studies and clinical data have shown that levosimendan has some advantages over other positive inotropic agents in the treatment of HF (2, 6, 14, 25, 40, 46, 55).
cAMP may be at least partially involved in the positive inotropic action of levosimendan (31, 33). It has been shown that levosimendan acts, preferentially, as a calcium sensitizer at low concentrations, whereas, at high concentrations, PDE III inhibition may play some role in its action on positive inotropic effect (11). In contrast, levosimendan was reported as a cardiotonic agent with calcium-sensitizing and calcium-mobilizing actions over identical concentration ranges (12, 49). Thus this frequency-dependent gain in contractility that we observed with levosimendan treatment in HF may also be caused in part by PDE III inhibition that leads to activation of protein kinase A and phosphorylation of phospholamban, which may result in an increased [Ca2+]i (11, 19, 29, 31, 33).
Importantly, our present study also reveals a clear, positive, rate-dependent lusitropic effect after LS administration in HF as opposed to impaired relaxation by other calcium sensitizers in vitro (such as EMD 50733 and 53998, ORG 30029, and CGP 48506). We found that increasing stimulation frequencies caused significant rate-dependent reductions of τ, despite that LV PES was relatively unchanged during pacing. Thus the progressively decreased τ during pacing in our study may well represent a frequency-dependent acceleration of relaxation. Our findings are consistent with several prior experimental studies (21, 52) and supported by evidence in vitro indicating that levosimendan accelerates and does not impair relaxation in isolated cardiac muscle from end-stage failing human hearts (10, 20) and by a recent clinical combined hemodynamic and Doppler echocardiographic study in patients with severe HF.
The mechanisms governing cardiac RFR are not as thoroughly investigated. The pathways responsible for mediating levosimendan-caused positive RFR in HF are less clear.
Recently, Varian and Janssen (56) reported that, in skinned fiber preparations of normal rabbit heart, myofilament calcium sensitivity is decreased at higher frequencies, playing a prominent role in frequency-dependent acceleration of relaxation. Diastolic dysfunction in HF exacerbated during tachycardia may be attributed to the loss of the ability to sufficiently desensitize the myofilaments at higher HR. Calcium-sensitizing effect of levosimendan is unlikely to explain the rate-dependent improvement of LV relaxation that we observed after levosimendan in HF; rather it may have been mainly attributable to a lack of Ca2+ sensitization of levosimendan under low-prevailing calcium concentrations during diastole. It has been shown that levosimendan exerts, not only a Ca2+-dependent influence, but also a use-dependent influence on Ca2+-triggered, cross-bridge interaction (i.e., increased contractility when intracellular Ca2+ is high and may favor relaxation under low-Ca2+ conditions) (6). This use dependency allows also a rapid relaxation.
It is well recognized that β-adrenergic receptor stimulation and some inotropic agents accelerate relaxation through the actions of cAMP to 1) accelerate reuptake of calcium by the SR, 2) reduce calcium sensitivity of the contractile apparatus, and 3) accelerate the rate of myofilament cross-bridge detachment. Thus it appears that these findings that we observed may also be indicative of a cAMP-dependent facilitation of relaxation by levosimendan (e.g., by a PDE inhibitory pharmacodynamic action) (12, 17).
Of note, recent demonstrated actions of levosimendan, such as increased activity of the Na+-Ca2+ exchange (20) and anti-ischemia (2, 40), may also contribute to the rate-related enhancements of LV contractile performance and relaxation. However, the relative contribution of each of the factors is unclear and awaits further study.
Reversal of the Adverse Effects of Tachycardia on LV-Arterial Coupling and Mechanical Efficiency in HF by Levosimendan
LV-arterial coupling, which regulates SV at a given preload (51) and closely relates to LV mechanical efficiency (43), is a key determinant of optimal cardiovascular function. Previously, we demonstrated that normal-functioning LV and arterial system are nearly optimally coupled to produce SW both at rest and during exercise. In the present study, we found that, before HF, the LV and arterial system are optimally coupled with EES/EA ratio close to 1.0, resulting in near maximal production of SW. During increases in HR, both EES and EA increase simultaneously (Table 2). The increases in the magnitude of EES and EA were appropriately matched, with EES/EA thus remaining close to 1.0 (0.92–1.30) (Table 2). In contrast, after HF, EES/EA was lower than normal (0.56) at baseline and progressively decreased with increasing HR (0.51 to 0.43) as EA increased, resulting in rapid declines of SW. Therefore, as a consequence, after HF, the LV and arterial system were not optimally coupled at baseline in sinus rhythm, and such impaired coupling was exacerbated during tachycardia. As predicted, we found that mechanical efficiency (i.e., the transfer of myocardial potential energy to external work) was reduced after HF and was further impaired with increases in HR. The adverse effect of increased HR on LV-arterial coupling in HF with resulting impairment of SW and mechanical efficiency may contribute to impaired exercise tolerance in HF (52).
Importantly, we found that treatment with levosimendan, not only caused an increase in the basal EES/EA ratio with resulting near-maximum SW, but also progressively increased EES/EA ratio incrementally with pacing rate attributable to greater increases in EES that more than offset the tachycardia-induced increase in EA. This finding is in agreement with clinical observations that levosimendan exhibits enhanced contractility without increasing myocardial oxygen demand (53, 55). The mechanisms of normalization of LV-arterial coupling and mechanical efficiency responses to tachycardia in HF may involve all of the known pharmacological actions of levosimendan, including the enhancement of calcium sensitivity to contractile proteins and PDE III inhibition with the opening of ATP-dependent potassium channels on vascular smooth muscle (60).
Study Limitations
Several methodological issues should be considered in the interpretation of our data. First, we studied an animal model of RV pacing-induced HF that reproduces many of the functional and neurohormonal features (5, 7, 8, 29, 33, 54) of clinical HF. Although this experimental model demonstrated biventricular chamber dilatation with increased LV and RV filling pressures and striking abnormalities in systolic and diastolic function similar to those found in patients with dilated cardiomyopathy, we cannot be certain that our results apply to HF of other causes such as hypertrophic cardiomyopathy. Second, we studied the acute effects of levosimendan treatment. We do not know the effects of prolonged treatment with levosimendan. Moreover, in the present study, levosimendan was started with loading doses and followed by infusion. Although, during the study period, therapeutically relevant plasma concentrations of levosimendan were achieved without side effects, this dosing protocol may not be necessarily suitable in the clinical setting. In humans, unlike in dogs, levosimendan has metabolites with long elimination half-life. One of those metabolites, OR-1896, is active and has similar hemodynamic properties as levosimendan. This may play an important role in both the acute and long-term actions of the drug and may induce some side effects such as HR disturbance and hypotensive effects (1, 32). Third, we used atrial pacing to study the effect of increased HR. Although the FFR and RFR are well documented in the literature, pacing tachycardia remains artificial, as tachycardia in vivo is mediated by sympathetic activation. Fourth, we used τ for the assessment of LV relaxation. Although, in the present study, levosimendan accelerates the isovolumic phase of LV diastolic performance in HF during pacing, we did not examine the effects of levosimendan on LV filling or the diastolic pressure-volume relation during pacing in this investigation. Further studies are needed to focus on detailed frequency-dependent LV diastolic performance in HF.
In conclusion, after HF, treatment with intravenous therapeutic doses of levosimendan restores the positive LV FFR and RFR and prevents tachycardia-induced adverse effects on LV-arterial coupling and mechanical efficiency. The present investigation reveals an important feature of levosimendan, which, not only enhances basal cardiac function, but also improves LV contractile and relaxation responses to chronotropic reserve for the failing heart. Recently, the importance of increased HR as a predictor of cardiovascular mortality has been emphasized (23). Patients with HF tolerate increases in HR poorly. The present data provide the first demonstration of a pharmacological intervention of levosimendan amplifying the myocardial FFR and RFR at a substantial energetic saving in HF.
GRANTS
This study was supported, in part, by grants from the National Institutes of Health HL074318 and AA12335 and American Heart Association (AHA) Mid-Atlantic Grant-in-Aid 0855079E (C.-P. Cheng), The National American Heart Association Scientist Development Grant 0530079N (H.-J. Cheng), and the Alcoholic Beverage Medical Research Foundation (H.-J. Cheng).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
This abstract was presented at an American Heart Association Meeting. The authors acknowledge the computer programming of Dr. Ping Tan, the technical assistance of Michael Cross and Chun Xian Zhang, and the administrative assistance of Amanda Burnette.
REFERENCES
- 1.Antila S, Pesonen U, Lehtonen L, Tapanainen P, Nikkanen H, Vaahtera K, Scheinin H. Pharmacokinetics of levosimendan and its active metabolite OR-1896 in rapid and slow acetylators. Eur J Pharm Sci 23: 213–222, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Avgeropoulou C, Andreadou I, Markantonis-Kyroudis S, Demopoulou M, Missovoulos P, Androulakis A, Kallikazaros I. The Ca2+-sensitizer levosimendan improves oxidative damage, BNP and pro-inflammatory cytokine levels with advanced decompensated heart failure in comparison to dobutamine. Eur J Heart Fail 7: 882–887, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Banfor PN, Preusser LC, Campbell TJ, Marsh KC, Polakowski JS, Reinhart GA, Cox BF, Fryer RM. Comparative effects of levosimendan, OR-1896, OR-1855, dobutamine, and milrinone on vascular resistance, indexes of cardiac function, and O2 consumption in dogs. Am J Physiol Heart Circ Physiol 294: H238–H248, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Bavendiek U, Brixius K, Munch G, Zobel C, Muller-Ehmsen J, Schwinger RH. Effect of inotropic interventions on the force-frequency relation in the human heart. Basic Res Cardiol 92, Suppl 1: 76–85, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Bristow MR. beta-Adrenergic receptor blockade in chronic heart failure. Circulation 101: 558–569, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Brixius K, Reicke S, Schwinger RH. Beneficial effects of the Ca2+ sensitizer, levosimendan, in human myocardium. Am J Physiol Heart Circ Physiol 282: H131–H137, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Cheng CP, Suzuki M, Ohte N, Ohno M, Wang ZM, Little WC. Altered ventricular and myocyte response to angiotensin II in pacing-induced heart failure. Circ Res 78: 880–892, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Cheng CP, Ukai T, Onishi K, Ohte N, Suzuki M, Zhang ZS, Cheng HJ, Tachibana H, Igawa A, Little WC. The role of ANG II and endothelin-1 in exercise-induced diastolic dysfunction in heart failure. Am J Physiol Heart Circ Physiol 280: H1853–H1860, 2001 [DOI] [PubMed] [Google Scholar]
- 9.De Luca L, Colucci WS, Nieminen MS, Massie BM, Gheorghiade M. Evidence-based use of levosimendan in different clinical settings. Eur Heart J 27: 1908–1920, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Dernellis J, Panaretou M. Effects of levosimendan on restrictive left ventricular filling in severe heart failure: a combined hemodynamic and Doppler echocardiographic study. Chest 128: 2633–2639, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Edes I, Kiss E, Kitada Y, Powers FM, Papp JG, Kranias EG, Solaro RJ. Effects of levosimendan, a cardiotonic agent targeted to troponin C, on cardiac function and on phosphorylation and Ca2+ sensitivity of cardiac myofibrils and sarcoplasmic reticulum in guinea pig heart. Circ Res 77: 107–113, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Endoh M. Mechanisms of action of novel cardiotonic agents. J Cardiovasc Pharmacol 40: 323–338, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Endoh M. Force-frequency relationship in intact mammalian ventricular myocardium: physiological and pathophysiological relevance. Eur J Pharmacol 500: 73–86, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Follath F, Cleland JG, Just H, Papp JG, Scholz H, Peuhkurinen K, Harjola VP, Mitrovic V, Abdalla M, Sandell EP, Lehtonen L, and Steering Committee and Investigators of the Levosimendan Infusion versus Dobutamine (LIDO) Study Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO Study): a randomised double-blind trial. Lancet 360: 196–202, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Freeman GL, Little WC, O'Rourke RA. Influence of heart rate on the left ventricular performance in conscious dogs. Circ Res 61: 455–464, 1987 [DOI] [PubMed] [Google Scholar]
- 16.Givertz MM, Andreou C, Conrad CH, Colucci WS. Direct myocardial effects of levosimendan in humans with left ventricular dysfunction: alteration of force-frequency and relaxation-frequency relationships. Circulation 115: 1218–1224, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Haikala H, Linden IB. Mechanisms of action of calcium-sensitizing drugs. J Cardiovasc Pharmacol 26, Suppl 1: S10–S19, 1995 [PubMed] [Google Scholar]
- 18.Haikala H, Nissinen E, Etemadzadeh E, Levijoki J, Linden IB. Troponin C-mediated calcium sensitization induced by levosimendan does not impair relaxation. J Cardiovasc Pharmacol 25: 794–801, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Harkin CP, Pagel PS, Tessmer JP, Warltier DC. Systemic and coronary hemodynamic actions and left ventricular functional effects of levosimendan in conscious dogs. J Cardiovasc Pharmacol 26: 179–188, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Hasenfuss G, Pieske B, Castell M, Kretschmann B, Maier LS, Just H. Influence of the novel inotropic agent, levosimendan, on isometric tension and calcium cycling in failing human myocardium. Circulation 98: 2141–2147, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Higashiyama A, Watkins MW, Chen Z, LeWinter MM. Effects of EMD 57033 on contraction and relaxation in isolated rabbit hearts. Circulation 92: 3094–3104, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Higgins CB, Vatner SF, Eckberg DL, Braunwald E. Alterations in the baroreceptor reflex in conscious dogs with heart failure. J Clin Invest 51: 715–724, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho JE, Bittner V, DeMicco DA, Breazna A, Deedwania PC, Waters DD. Usefulness of heart rate at rest as a predictor of mortality, hospitalization for heart failure, myocardial infarction, and stroke in patients with stable coronary heart disease (data from the Treating New Targets [TNT] Trial). Am J Cardiol 105: 905–911, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Izawa H, Yokota M, Takeichi Y, Inagaki M, Nagata K, Iwase M, Sobue T. Adrenergic control of the force-frequency and relaxation-frequency relations in patients with hypertrophic cardiomyopathy. Circulation 96: 2959–2968, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Janssen PM, Datz N, Zeitz O, Hasenfuss G. Levosimendan improves diastolic and systolic function in failing human myocardium. Eur J Pharmacol 404: 191–199, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Janssen PML, Stull LB, Marban E. Myofilament properties comprise the rate-limiting step for cardiac relaxation at body temperature in the rat. Am J Physiol Heart Circ Physiol 282: H499–H507, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Kaheinen P, Pollesello P, Levijoki J, Haikala H. Levosimendan increases diastolic coronary flow in isolated guinea-pig heart by opening ATP-sensitive potassium channels. J Cardiovasc Pharmacol 37: 367–374, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Kass DA. Force-frequency relation in patients with left ventricular hypertrophy and failure. Basic Res Cardiol 1, Suppl 93: 108–116, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Kass DA, Solaro RJ. Mechanisms and use of calcium-sensitizing agents in the failing heart. Circulation 113: 305–315, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Kivikko M, Lehtonen L, Colucci WS. Sustained hemodynamic effects of intravenous levosimendan. Circulation 107: 81–86, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Lamberts RR, Hamdani J, Soekhoe TW, Boontje NM, Zaremba R, Walker LA, de Tombe PP, van der Velden J, Stienen GJM. Frequency-dependent myofilament Ca2+ desensitization in failing rat myocardium. J Physiol 582: 695–709, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lehtonen L, Poder P. The utility of levosimendan in the treatment of heart failure. Ann Med 39: 2–17, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Lenfant C. Report of the task force on research in heart failure. Circulation 90: 1118–1123, 1994 [DOI] [PubMed] [Google Scholar]
- 34.Li L, Chu G, Kranias EG, Bers DM. Cardiac myocyte calcium transport in phospholamban knockout mouse: relaxation and endogenous CaMKII effects. Am J Physiol Heart Circ Physiol 274: H1335–H1347, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Little WC. The left ventricular dP/dtmax-end-diastolic volume relation in closed-chest dogs. Circ Res 56: 808–815, 1985 [DOI] [PubMed] [Google Scholar]
- 36.Little WC, Cheng CP. Effect of exercise on left ventricular-arterial coupling assessed in the pressure-volume plane. Am J Physiol Heart Circ Physiol 264: H1629–H1633, 1993 [DOI] [PubMed] [Google Scholar]
- 37.Masutani S, Cheng HJ, Hyttila-Hopponen M, Levijoki J, Heikkila A, Vuorela A, Little WC, Cheng CP. Orally available levosimendan: dose-related positive inotropic and lusitropic effect in conscious, chronically-instrumented normal and heart failure dogs. J Pharmacol Exp Ther 325: 236–247, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Masutani S, Little WC, Hasegawa H, Cheng HJ, Cheng CP. Restrictive left ventricular filling pattern does not result from increased left atrial pressure alone. Circulation 117: 1550–1554, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Miura T, Miyazaki S, Guth BD, Kambayashi M, Ross J., Jr Influence of the force-frequency relation on left ventricular function during exercise in conscious dogs. Circulation 86: 563–571, 1992 [DOI] [PubMed] [Google Scholar]
- 40.Moiseyev VS, Poder P, Andrejevs N, Ruda MY, Golikov AP, Lazebnik LB, Kobalava ZD, Lehtonen LA, Laine T, Nieminen MS, Lie KI, Study Investigators RUSSLAN Safety and efficacy of a novel calcium sensitizer, levosimendan, in patients with left ventricular failure due to an acute myocardial infarction. A randomized, placebo-controlled, double-blind study (RUSSLAN). Eur Heart J 23: 1422–1432, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Morimoto A, Hasegawa H, Cheng HJ, Little WC, Cheng CP. Endogenous β3-adrenoreceptor activation contributes to left ventricular and cardiomyocyte dysfunction in heart failure. Am J Physiol Heart Circ Physiol 286: H2425–H2433, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Nozawa T, Cheng CP, Noda T, Little WC. Effect of exercise on left ventricular mechanical efficiency in conscious dogs. Circulation 90: 3047–3054, 1994 [DOI] [PubMed] [Google Scholar]
- 43.Nozawa T, Yasumura Y, Futaki S, Tanaka N, Uenishi M, Suga H. Efficiency of energy transfer from pressure-volume area to external mechanical work increases with contractile state and decreases with afterload in the left ventricle of the anesthetized closed-chest dog. Circulation 77: 1116–1124, 1988 [DOI] [PubMed] [Google Scholar]
- 44.Ohte N, Cheng CP, Little WC. Tachycardia exacerbates abnormal left ventricular-arterial coupling in heart failure. Heart Vessels 18: 136–141, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Ohte N, Cheng CP, Suzuki M, Little WC. Effects of atrial natriuretic peptide on left ventricular performance in conscious dogs before and after pacing-induced heart failure. J Pharmacol Exp Ther 291: 589–595, 1999 [PubMed] [Google Scholar]
- 46.Pagel PS, McGough MF, Hettrick DA, Lowe D, Tessmer JP, Jamali IN, Warltier DC. Levosimendan enhances left ventricular systolic and diastolic function in conscious dogs with pacing-induced cardiomyopathy. J Cardiovasc Pharmacol 29: 563–573, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Pieske B, Kretschmann B, Meyer M, Holubarsch C, Weirich J, Posival H, Minami K, Just H, Hasenfuss G. Alterations in intracellular calcium handling associated with the inverse force-frequency relation in human dilated cardiomyopathy. Circulation 92: 1169–1178, 1995 [DOI] [PubMed] [Google Scholar]
- 48.Poder P, Eha J, Sundberg S, Antila S, Heinpalu M, Loogna I, Planken U, Rantanen S, Lehtonen L. Pharmacokinetic-pharmacodynamic interrelationships of intravenous and oral levosimendan in patients with severe congestive heart failure. Int J Clin Pharmacol Ther 41: 365–373, 2003 [PubMed] [Google Scholar]
- 49.Sato S, Talukder MAH, Sugawara H, Sawada H. Effects of levosimendan on myocardial contractility and Ca2+ transients in aequorin-loaded right-ventricular papillary muscles and indo-1-loaded single ventricular cardiomyocytes of the rabbit. J Mol Cell Cardiol 30: 1115–1128, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Schwinger RH, Brixius K, Bavendiek U, Hoischen S, Muller-Ehmsen J, Bolck B, Erdmann E. Effect of cyclopiazonic acid on the force-frequency relationship in human nonfailing myocardium. J Pharmacol Exp Ther 283: 286–292, 1997 [PubMed] [Google Scholar]
- 51.Sunagawa K, Maughan WL, Burkhoff D, Sagawa K. Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol Heart Circ Physiol 245: H773–H780, 1983 [DOI] [PubMed] [Google Scholar]
- 52.Tachibana H, Cheng HJ, Ukai T, Igawa A, Zhang ZS, Little WC, Cheng CP. Levosimendan improves LV systolic and diastolic performance at rest and during exercise after heart failure. Am J Physiol Heart Circ Physiol 288: H914–H922, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Todaka K, Wang J, Yi GH, Stennett R, Knecht M, Packer M, Burkhoff D. Effects of levosimendan on myocardial contractility and oxygen consumption. J Pharmacol Exp Ther 279: 120–127, 1996 [PubMed] [Google Scholar]
- 54.Travill CM, Williams TD, Pate P, Song G, Chalmers J, Lightman SL, Sutton R, Noble MI. Haemodynamic and neurohumoral response in heart failure produced by rapid ventricular pacing. Cardiovasc Res 26: 783–790, 1992 [DOI] [PubMed] [Google Scholar]
- 55.Ukkonen H, Saraste M, Akkila J, Knuuti J, Karanko M, Iida H, Lehikoinen P, Nagren K, Lehtonen L, Voipio-Pulkki LM. Myocardial efficiency during levosimendan infusion in congestive heart failure. Clin Pharmacol Ther 68: 522–531, 2000 [DOI] [PubMed] [Google Scholar]
- 56.Varian KD, Janssen PML. Frequency-dependent acceleration of relaxation involves decreased myofilament calcium sensitivity. Am J Physiol Heart Circ Physiol 292: H2212–H2219, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Wachter R, Schmidt-Schweda S, Westermann D, Post H, Edelmann F, Kasner M, Luers C, Steendijk P, Hasenfub G, Tschope C, Pieske B. Blunted frequency-dependent upregulation of cardiac output is related to impaired relaxation in diastolic heart failure. Eur Heart J 30: 3027–3036, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu X, Bers DM. Sarcoplasmic reticulum and nuclear envelope are one highly interconnected Ca2+ store throughout cardiac myocyte. Circ Res 99: 283–291, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Yokoshiki H, Katsube Y, Sunagawa M, Sperelakis N. Levosimendan, a novel Ca2+ sensitizer, activates the glibenclamide-sensitive K+ channel in rat arterial myocytes. Eur J Pharmacol 333: 249–259, 1997 [DOI] [PubMed] [Google Scholar]
- 60.Yokoshiki H, Katsube Y, Sunagawa M, Sperelakis N. The novel calcium sensitizer, levosimendan, activates the ATP-sensitive K+ channel in rat ventricular cells. J Pharmacol Exp Ther 283: 375–383, 1997 [PubMed] [Google Scholar]