Abstract
Rat vascular smooth muscle cells (VSMCs) from renal microvessels metabolize 2′,3′-cAMP to 2′-AMP and 3′-AMP, and these AMPs are converted to adenosine that inhibits microvascular VSMC proliferation via A2B receptors. The goal of this study was to test whether this mechanism also exists in VSMCs from conduit arteries and whether it is similarly expressed in human vs. rat VSMCs. Incubation of rat and human aortic VSMCs with 2′,3′-cAMP concentration-dependently increased levels of 2′-AMP and 3′-AMP in the medium, with a similar absolute increase in 2′-AMP vs. 3′-AMP. In contrast, in human coronary VSMCs, 2′,3′-cAMP increased 2′-AMP levels yet had little effect on 3′-AMP levels. In all cell types, 2′,3′-cAMP increased levels of adenosine, but not 5′-AMP, and 2′,3′-AMP inhibited cell proliferation. Antagonism of A2B receptors (MRS-1754), but not A1 (1,3-dipropyl-8-cyclopentylxanthine), A2A (SCH-58261), or A3 (VUF-5574) receptors, attenuated the antiproliferative effects of 2′,3′-cAMP. In all cell types, 2′-AMP, 3′-AMP, and 5′-AMP increased adenosine levels, and inhibition of ecto-5′-nucleotidase blocked this effect of 5′-AMP but not that of 2′-AMP nor 3′-AMP. Also, 2′-AMP, 3′-AMP, and 5′-AMP, like 2′,3′-cAMP, exerted antiproliferative effects that were abolished by antagonism of A2B receptors with MRS-1754. In conclusion, VSMCs from conduit arteries metabolize 2′,3′-cAMP to AMPs, which are metabolized to adenosine. In rat and human aortic VSMCs, both 2′-AMP and 3′-AMP are involved in this process, whereas, in human coronary VSMCs, 2′,3′-cAMP is mainly converted to 2′-AMP. Because adenosine inhibits VSMC proliferation via A2B receptors, local vascular production of 2′,3′-cAMP may protect conduit arteries from atherosclerosis.
Keywords: adenosine 2′,3′-cyclic monophosphate-adenosine pathway; 2′-adenosine monophosphate; 3′-adenosine monophosphate; 5′-adenosine monophosphate; vascular smooth muscle cells; coronary arteries; cardiovascular protection
our previous studies identify a biochemical mechanism, called the extracellular 3′,5′-cAMP-adenosine pathway, that involves 3′,5′-cAMP and contributes to the extracellular levels of adenosine (14–16, 19, 22, 27–31, 37–39). The extracellular 3′,5′-cAMP-adenosine pathway entails the following four spatially linked sequential steps: 1) receptor-mediated intracellular 3′,5′-cAMP production; 2) active transport of intracellular 3′,5′-cAMP to the cell surface; 3) metabolism of 3′,5′-cAMP to 5′-AMP in the extracellular compartment; and 4) conversion of 5′-AMP to adenosine in the extracellular compartment. Several independent laboratories corroborate the extracellular 3′,5′-cAMP-adenosine pathway in pial microvessels (26), skeletal muscle (8), and the gastrointestinal tract (24), and Müller et al. (41) report the existence of a 3′,5′-cAMP-adenosine pathway in which the sequential conversion of 3′,5′-cAMP to 5′-AMP and 5′-AMP to adenosine occurs on the outward-facing membrane surface of lipid droplets within adipocytes.
To study in more detail the extracellular 3′,5′-cAMP-adenosine pathway, we developed a high-performance liquid chromatography-tandem mass spectrometry assay to measure 3′,5′-cAMP and other purines (44); and, while applying this assay to measure 3′,5′-cAMP in the renal venous perfusate from isolated, perfused rat kidneys, we serendipitously discovered that intact organs make a positional isomer of 3′,5′-cAMP, namely 2′,3′-cAMP (43). This finding motivates our current hypothesis that there exists, in addition to the extracellular 3′,5′-cAMP-adenosine pathway, an extracellular 2′,3′-cAMP-adenosine pathway. The rationale for this hypothesis is that: 1) stimulation of mRNA turnover involves the actions of multiple RNases that first cleave the phosphodiester bonds in the polyadenine (poly-A) tail of mRNA (54); 2) 2′,3′-cAMP may be the intermediate adenine nucleotide formed by this process (50); 3) transporters, for example, multidrug resistance proteins 4 and 5, rapidly transport cyclic nucleotides out of cells (7, 9, 35); and 4) there exist enzymes that could serve as ecto-2′,3′-cAMP-phosphodiesterases and ecto-2′/3′-nucleotidases to hydrolyze extracellular 2′,3′-cAMP to 2′-AMP and 3′-AMP and extracellular 2′-AMP and 3′-AMP to adenosine, respectively (5, 47, 48, 56, 57). The extracellular 2′,3′-cAMP-adenosine pathway could be important whenever cells are exposed to stressful stimuli that enhance mRNA turnover, to provide the “retaliatory” metabolite, adenosine.
Our recent studies in isolated, perfused rat (33) kidneys corroborate the extracellular 2′,3′-cAMP-adenosine pathway hypothesis by showing that infusions of 2′,3′-cAMP in the renal artery increase levels of 2′-AMP, 3′-AMP, and adenosine in the renal vein, and infusions of 2′-AMP and 3′-AMP increase the concentrations of adenosine in the renal vein. Also, energy depletion increases renal venous secretion of endogenous 2′,3′-cAMP, 2′-AMP, 3′-AMP, and adenosine.
Because renal venous measurements likely reflect vascular metabolism of the 2′,3′-cAMP, the above findings suggest that the extracellular 2′,3′-cAMP-adenosine pathway exists in vascular smooth muscle cells (VSMCs). Indeed, our subsequent studies show that rat preglomerular vascular smooth muscle cells (PGVSMCs) express the extracellular 2′,3′-cAMP-adenosine pathway (32). Importantly, our investigations also demonstrate that, in rat PGVSMCs, 2′,3′-cAMP inhibits cell proliferation via generation of adenosine that stimulates A2B receptors (32).
If 2′,3′-cAMP inhibits proliferation of VSMCs from large conduit arteries, such as aortic or coronary VSMCs, then local production, release, and metabolism of 2′,3′-cAMP could protect the cardiovascular system form atherosclerosis and thrombosis. Accordingly, one goal of the present study was to determine whether VSMCs from conduit arteries metabolize 2′,3′-cAMP to 2′-AMP, 3′-AMP, and adenosine and whether such metabolism is associated with A2B receptor-mediated inhibition of VSMC proliferation. A second goal was to determine whether human VSMCs are similar to rat VSMCs in this regard.
METHODS
Animals.
Studies utilized adult (∼16 wk of age) male Wistar-Kyoto rats from Taconic Farms (Germantown, NY). The Institutional Animal Care and Use Committee approved all procedures. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH Publication no. 85–23, revised 1996).
Drugs.
2′,3′-cAMP, 2′-AMP, 3′-AMP, 5′-AMP, adenosine, α,β-methylene-adenosine-5′-diphosphate [AMPCP; ecto-5′-nucleotidase (CD73) inhibitor (55)], 1,3-dipropyl-8-cyclopentylxanthine [DPCPX; selective A1 receptor antagonist (34)], 7-(2-phenylethyl)-5-amino-2-(2-furyl)-pyrazolo-[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine [SCH-58261; selective A2A receptor antagonist (34)], 8-(4-{[(4-cyanophenyl)carbamoylmethyl]oxy}phenyl)-1,3-di(n-propyl)xanthine [MRS-1754; selective A2B receptor antagonist (34)], and N-(2-methoxyphenyl)-N′-[2-(3-pyridinyl)-4-quinazolinyl]-urea [VUF-5574; selective A3 receptor antagonist (52)] were obtained from Sigma-Aldrich (St. Louis, MO).
Culture of rat aortic vascular smooth muscle cells, human aortic vascular smooth muscle cells, and human coronary artery vascular smooth muscle cells.
Rat aortic vascular smooth muscle cells (AVSMCs) were cultured by explant from freshly isolated aorta as described by us previously (10). Human AVSMCs and human coronary artery vascular smooth muscle cells (CAVSMCs) were purchased from Cascade Biologics (Portland, OR). Cells were maintained in culture medium [DMEM-F-12 supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), NaHCO3 (13 mmol/l), and HEPES (25 mmol/l)] containing 10% fetal calf serum (FCS), plated in tissue-culture flasks (75 cm2), and incubated under standard tissue culture conditions. Confluent monolayers were dislodged by treatment with 0.25% trypsin-EDTA solution and passaged further. All experiments were performed in cells in the third to fifth passage.
Cell proliferation studies.
[3H]thymidine incorporation (index of DNA synthesis) and cell number (cell proliferation) studies were conducted to investigate the effects of 2′,3′-cAMP and AMPs on cell growth. Briefly, cells were plated at a density of 5 × 104 cells/well in 24-well tissue culture dishes and allowed to grow to subconfluence in DMEM-F-12 medium containing 10% FCS under standard tissue culture conditions. The cells were then growth-arrested by feeding DMEM containing 0.4% albumin for 48 h. For DNA synthesis, growth-arrested cells were treated with 2′,3′-cAMP, without or with adenosine receptor antagonists, in DMEM containing 2.5% FCS. After 20 h of incubation, the treatments were repeated with freshly prepared solutions but supplemented with [3H]thymidine (1 μCi/ml) for an additional 4 h. The experiments were terminated by washing the cells two times with Dulbecco's PBS and two times with ice-cold trichloroacetic acid (10%). The precipitate was solubilized in 500 μl of 0.3 N NaOH and 0.1% SDS (50°C for 2 h). Aliquots from each were placed in scintillation fluid and were counted in a liquid scintillation counter. For cell number experiments, cells were allowed to attach overnight, were growth-arrested for 48 h, and were treated with 2′,3′-cAMP, AMPs, or adenosine, with or without MRS-1754, in DMEM containing 2.5% FCS. Treatments were repeated every 24 h for 4 days. On day 5, cells were dislodged and counted with a Coulter counter.
Metabolism studies.
Cells were washed with HEPES-buffered Hanks' balanced salt solution and incubated with 0.5 ml of Dulbecco's PBS in the presence and absence of various treatments. After 1 h, the medium was collected, heated for 3 min at 100°C to denature enzymes, and then frozen at −80°C until assayed by mass spectrometry.
Analytical methods.
Purines were quantified using a triple quadrupole mass spectrometer (TSQ Quantum-Ultra; ThermoFisher Scientific, San Jose, CA) as previously described in detail (33).
Statistics.
Data from the metabolism studies were not normally distributed, so the results were analyzed statistically using the nonparametric Kruskal-Wallis one-way ANOVA on ranks test, with post hoc comparisons using the Kruskal-Wallis multiple-comparison Z-value test. Cell proliferation data were analyzed by one-factor or two-factor ANOVA, with post hoc comparisons using a Fisher's least-significant difference test or by Student's unpaired t-test. The criterion of significance was P < 0.05. All values in text and Figs. 1–10 are means ± SE.
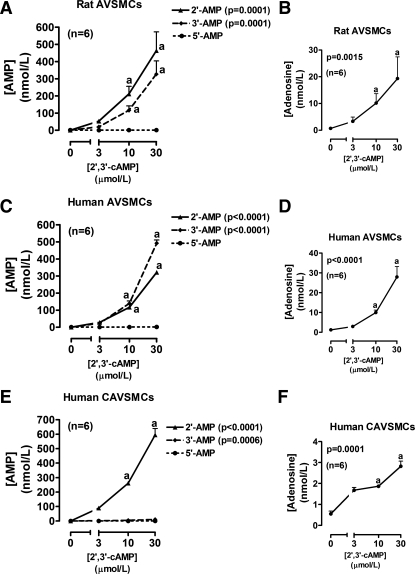
Fig. 1.
Line graphs illustrate the concentration-dependent effects of 2′,3′-cAMP on levels of 2′-AMP, 3′-AMP, 5′-AMP, and adenosine in the medium of cultures of rat aortic vascular smooth muscle cells (AVSMCs; A and B), human AVSMCs (C and D), and human coronary artery vascular smooth muscle cells (CAVSMCs; E and F). P values are from Kruskal-Wallis one-way ANOVA on ranks. aP < 0.05 (Kruskal-Wallis multiple-comparison Z-value test) compared with basal (0).
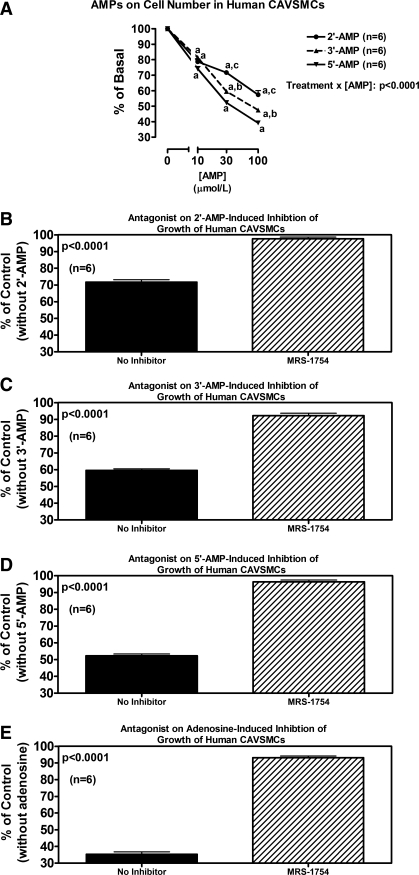
Fig. 10.
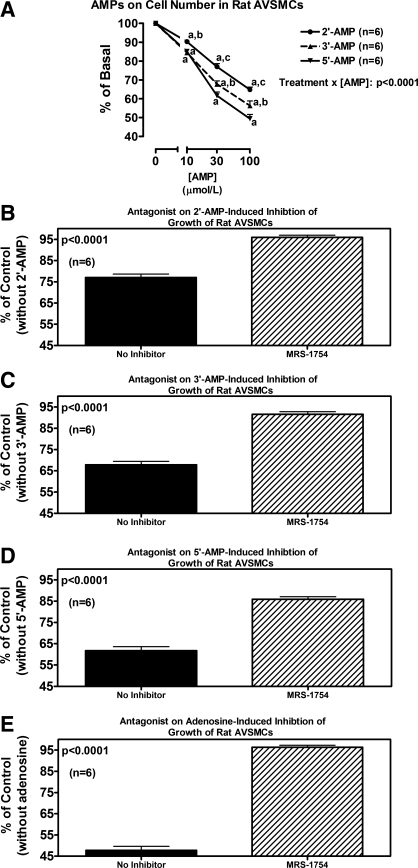
Line graph (A) illustrates the concentration-dependent effects of 2′-AMP, 3′-AMP, and 5′-AMP on cell number in human CAVSMCs. P value is interaction term from 2-factor ANOVA between type of AMP and concentration of AMP. aP < 0.05 (Fisher's LSD test) compared with corresponding basal (0). bP < 0.05 (Fisher's LSD test) compared with corresponding concentration of 5′-AMP. cP < 0.05 (Fisher's LSD test) compared with corresponding concentrations of 3′-AMP and 5′-AMP. Bar graphs illustrate effects of MRS-1754 (A2B antagonist; 0.1 μmol/l) on antiproliferative effects (cell number) of 2′-AMP (B), 3′-AMP (C), 5′-AMP (D), and adenosine (E) (each at 30 μmol/l) in human CAVSMCs. P values in B-E are from Student's unpaired t-test.
RESULTS
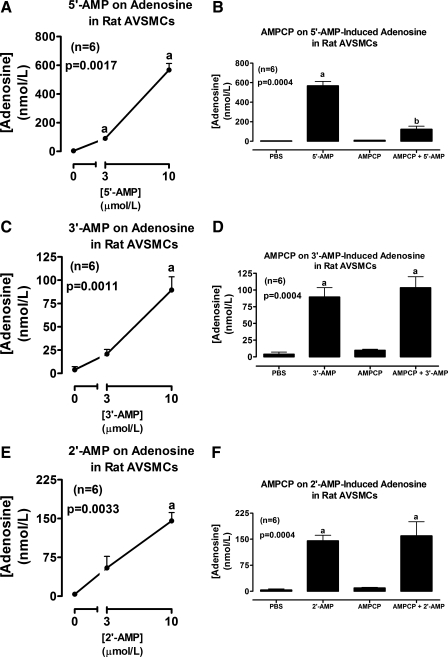
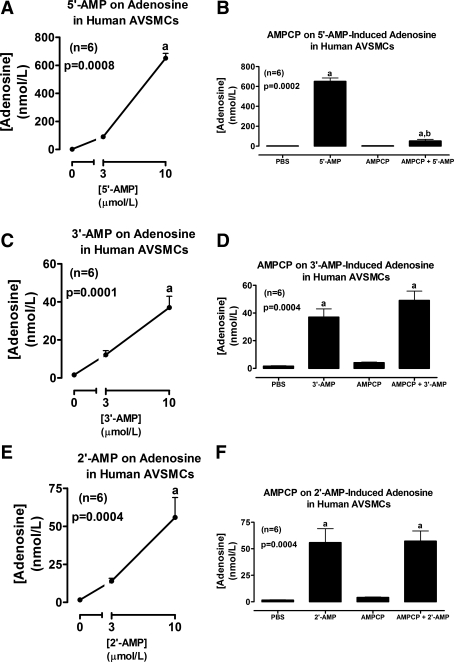
Incubation of rat AVSMCs with 2′,3′-cAMP significantly and concentration-dependently increased levels of 2′-AMP and 3′-AMP, but not 5′-AMP (Fig. 1A), in the medium. The increases in 2′-AMP and 3′-AMP were of similar magnitude and were accompanied by a significant increase in levels of adenosine in the medium (Fig. 1B). Incubation of rat AVSMCs with 5′-AMP (prototypical adenosine precursor) significantly and concentration-dependently increased levels of adenosine in the medium (Fig. 2A), and this effect was nearly abolished by AMPCP (100 μmol/l; ecto-5′-nucleotidase inhibitor) (Fig. 2B). 3′-AMP (Fig. 2C) also increased adenosine levels, but, unlike 5′-AMP, the effects of 3′-AMP were not affected by AMPCP (Fig. 2D). Likewise, 2′-AMP increased adenosine levels (Fig. 2E), and this increase was unaffected by AMPCP (Fig. 2F). Importantly, incubations of human AVSMCs with 2′,3′-cAMP (Fig. 1, C and D) and 5′-AMP, 3′-AMP, and 2′-AMP (Fig. 3) produced results that were qualitatively similar to those obtained in rat AVSMCs, indicating no species differences between rat and human cells with regard to the metabolism of 2′,3′-cAMPs or the AMPs.
Fig. 2.
Line graphs illustrate the concentration-dependent effects of 5′-AMP (A), 3′-AMP (C), and 2′-AMP (E) on levels of adenosine in the medium of cultures from rat AVSMCs. Bar graphs illustrate the effects of α,β-methylene-adenosine-5′-diphosphate [AMPCP; 100 μmol/l; ecto-5′-nucleotidase (CD73) inhibitor] on adenosine levels in the medium of rat AVSMCs incubated with 10 μmol/l of either 5′-AMP (B), 3′-AMP (D), or 2′-AMP (F). P values are from Kruskal-Wallis one-way ANOVA on ranks. aP < 0.05 (Kruskal-Wallis multiple-comparison Z-value test) compared with basal (0 or PBS) or AMPCP. bP < 0.05 (Kruskal-Wallis multiple-comparison Z-value test) compared with 5′-AMP in the absence of AMPCP.
Fig. 3.
Line graphs illustrate the concentration-dependent effects of 5′-AMP (A), 3′-AMP (C), and 2′-AMP (E) on levels of adenosine in the medium of cultures from human AVSMCs. Bar graphs illustrate the effects of α,β-methylene-adenosine-5′-diphosphate [AMPCP; 100 μmol/l; ecto-5′-nucleotidase (CD73) inhibitor] on adenosine levels in the medium of human AVSMCs incubated with 10 μmol/l of either 5′-AMP (B), 3′-AMP (D), or 2′-AMP (F). P values are from Kruskal-Wallis one-way ANOVA on ranks. aP < 0.05 (Kruskal-Wallis multiple-comparison Z-value test) compared with basal (0 or PBS) or AMPCP. bP < 0.05 (Kruskal-Wallis multiple-comparison Z-value test) compared with 5′-AMP in the absence of AMPCP.
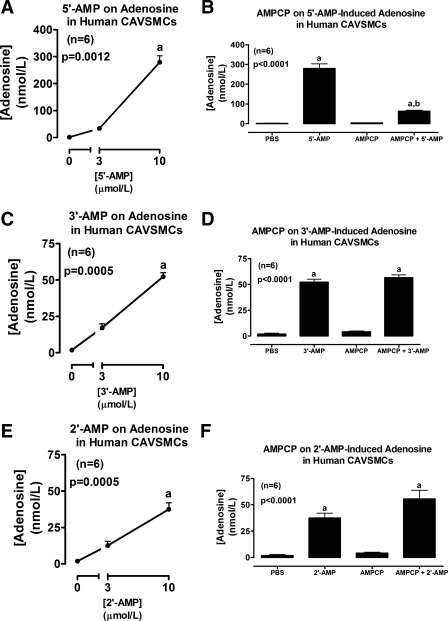
Incubation of human CAVSMCs with 2′3′-cAMP gave results that were different from both rat and human AVSMCs in one important aspect. Although 2′,3′-cAMP increased 2′-AMP levels in the medium (Fig. 1E), there was scant production of 3′-AMP (Fig. 1E). However, as with rat and human AVSMCs, 2′,3′-cAMP did not increase levels of 5′-AMP (Fig. 1E) but did significantly increase level of adenosine (Fig. 1F). As in rat and human AVSMCs, in human CAVSMCs, 5′-AMP, 3′-AMP, and 2′-AMP increased adenosine levels and AMPCP blocked this effect of 5′-AMP but not that of 3′-AMP and 2′-AMP (Fig. 4).
Fig. 4.
Line graphs illustrate the concentration-dependent effects of 5′-AMP (A), 3′-AMP (C), and 2′-AMP (E) on levels of adenosine in the medium of cultures from human CAVSMCs. Bar graphs illustrate the effects of α,β-methylene-adenosine-5′-diphosphate [AMPCP; 100 μmol/l; ecto-5′-nucleotidase (CD73) inhibitor] on adenosine levels in the medium of human CAVSMCs incubated with 10 μmol/l of either 5′-AMP (B), 3′-AMP (D), or 2′-AMP (F). P values are from Kruskal-Wallis one-way ANOVA on ranks. aP < 0.05 (Kruskal-Wallis multiple-comparison Z-value test) compared with basal (0 or PBS) or AMPCP. bP < 0.05 (Kruskal-Wallis multiple-comparison Z-value test) compared with 5′-AMP in the absence of AMPCP.
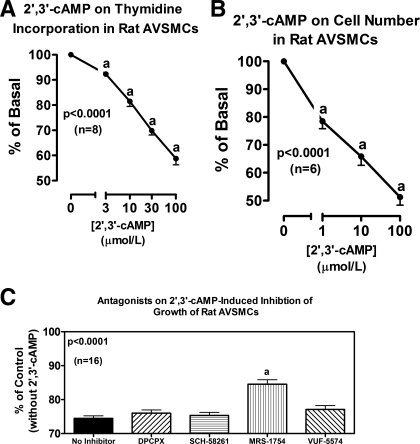
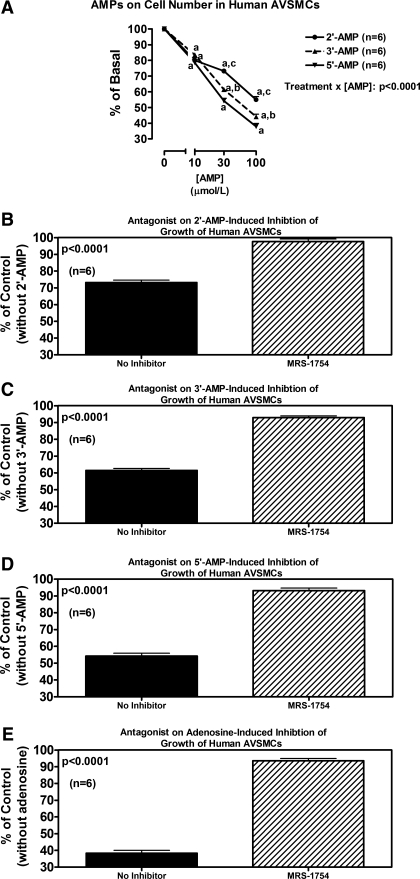
In rat AVSMCs, 2′,3′-cAMP significantly and concentration-dependently inhibited cell proliferation as assessed by either [3H]thymidine incorporation (Fig. 5A) or cell number (Fig. 5B). DPCPX, SCH-58261, and VUF-5574 (each at 0.1 μmol/l) did not antagonize the inhibitory effects of 2′,3′-cAMP (30 μmol/l) on [3H]thymidine incorporation (Fig. 5C). In contrast, MRS-1754 (0.1 μmol/l) significantly, but only partially, attenuated the reduction in [3H]thymidine incorporation induced by 2′,3′-cAMP (Fig. 5C). Similar to 2′,3′-cAMP, both 2′-AMP and 3′-AMP significantly inhibited proliferation (cell number) of rat AVSMCs (Fig. 6A). In this regard, 3′-AMP was nearly as potent as 5′-AMP (the prototypical adenosine precursor) and slightly more potent than 2′-AMP. Antagonism of A2B receptors with MRS-1754 (0.1 μmol/l) nearly completely inhibited the antiproliferative effects of 30 μmol/l of 2′-AMP (Fig. 6B), 3′-AMP (Fig. 6C), and 5′-AMP (Fig. 6D). Figure 6E confirms that adenosine (30 μmol/l) inhibits proliferation in rat AVSMCs and that this effect is fully prevented by MRS-1754 (0.1 μmol/l).
Fig. 5.
Line graphs illustrate the concentration-dependent effects of 2′,3′-cAMP on [3H]thymidine incorporation (A) and cell number (B) in rat AVSMCs. Bar graph (C) illustrates effects of 1,3-dipropyl-8-cyclopentylxanthine [DPCPX (A1 antagonist)], 7-(2-phenylethyl)-5-amino-2-(2-furyl)-pyrazolo-[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine [SCH-58261 (A2A antagonist)], 8-(4-{[(4-cyanophenyl)carbamoylmethyl]oxy}phenyl)-1,3-di(n-propyl)xanthine [MRS-1754 (A2B antagonist)], and N-(2-methoxyphenyl)-N′-[2-(3-pyridinyl)-4-quinazolinyl]-urea [VUF-5574 (A3 antagonist)] (each at 0.1 μmol/l) on inhibition of [3H]thymidine incorporation by 2′,3′-cAMP (30 μmol/l) in rat AVSMCs. P values are from 1-factor ANOVA. aP < 0.05 [Fisher's least-significant difference (LSD) test] compared with basal (0) (A and B) or compared with no inhibitor (C).
Fig. 6.
Line graph (A) illustrates the concentration-dependent effects of 2′-AMP, 3′-AMP, and 5′-AMP on cell number in rat AVSMCs. P value is interaction term from 2-factor ANOVA between type of AMP and concentration of AMP. aP < 0.05 (Fisher's LSD test) compared with corresponding basal (0). bP < 0.05 (Fisher's LSD test) compared with corresponding concentration of 5′-AMP. cP < 0.05 (Fisher's LSD test) compared with corresponding concentrations of 3′-AMP and 5′-AMP. Bar graphs illustrate effects of MRS-1754 (A2B antagonist; 0.1 μmol/l) on antiproliferative effects (cell number) of 2′-AMP (B), 3′-AMP (C), 5′-AMP (D), and adenosine (E) (each at 30 μmol/l) in rat AVSMCs. P values in B-E are from Student's unpaired t-test.
As in rat AVSMCs, in human AVSMCs, 2′,3′-cAMP significantly and concentration-dependently inhibited cell proliferation as assessed by either [3H]thymidine incorporation (Fig. 7A) or cell number (Fig. 7B). Likewise, DPCPX, SCH-58261, and VUF-5574 did not antagonize the inhibitory effects of 2′,3′-cAMP on [3H]thymidine incorporation (Fig. 7C), yet MRS-1754 (0.1 μmol/l) partially, but significantly, attenuated the reduction in [3H]thymidine incorporation induced by 2′,3′-cAMP (Fig. 7C). Also as in rat AVSMCs, in human AVSMCs, 2′-AMP and 3′-AMP significantly inhibited proliferation (cell number) (Fig. 8A), again with 3′-AMP being nearly as potent as 5′-AMP and slightly more potent than 2′-AMP. As in rat AVSMCs, in human AVSCMs, antagonism of A2B receptors with MRS-1754 abrogated the antiproliferative effects of 2′-AMP (Fig. 8B), 3′-AMP (Fig. 8C), and 5′-AMP (Fig. 8D). Figure 8E confirms that adenosine inhibits proliferation in human AVSMCs and that this effect is fully prevented by MRS-1754.
Fig. 7.
Line graphs illustrate the concentration-dependent effects of 2′,3′-cAMP on [3H]thymidine incorporation (A) and cell number (B) in human AVSMCs. Bar graph (C) illustrates effects of DPCPX (A1 antagonist), SCH-58261 (A2A antagonist), MRS-1754 (A2B antagonist), and VUF-5574 (A3 antagonist) (each at 0.1 μmol/l) on inhibition of [3H]thymidine incorporation by 2′,3′-cAMP (30 μmol/l) in human AVSMCs. P values are from 1-factor ANOVA. aP < 0.05 (Fisher's LSD test) compared with basal (0) (A and B) or compared with no inhibitor (C).
Fig. 8.
Line graph (A) illustrates the concentration-dependent effects of 2′-AMP, 3′-AMP, and 5′-AMP on cell number in human AVSMCs. P value is interaction term from 2-factor ANOVA between type of AMP and concentration of AMP. aP < 0.05 (Fisher's LSD test) compared with corresponding basal (0). bP < 0.05 (Fisher's LSD test) compared with corresponding concentration of 5′-AMP. cP < 0.05 (Fisher's LSD test) compared with corresponding concentrations of 3′-AMP and 5′-AMP. Bar graphs illustrate effects of MRS-1754 (A2B antagonist; 0.1 μmol/l) on antiproliferative effects (cell number) of 2′-AMP (B), 3′-AMP (C), 5′-AMP (D), and adenosine (E) (each at 30 μmol/l) in human AVSMCs. P values in B–E are from Student's unpaired t-test.
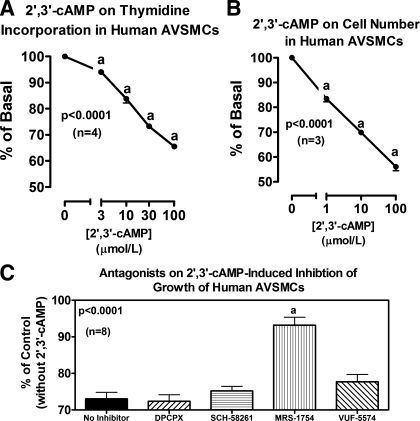
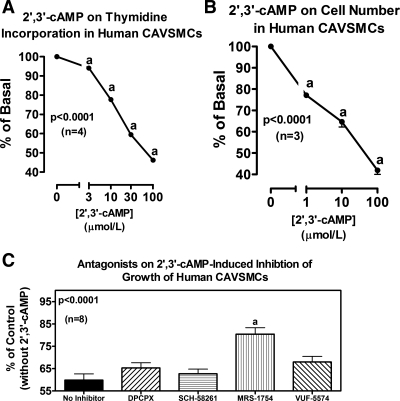
The results with 2′,3′-cAMP (Fig. 9) and with 2′-AMP, 3′-AMP, 5′-AMP, and adenosine (Fig. 10) on proliferation of human CAVSMCs were qualitatively similar to the results for both rat and human AVSMCs.
Fig. 9.
Line graphs illustrate the concentration-dependent effects of 2′,3′-cAMP on [3H]thymidine incorporation (A) and cell number (B) in human CAVSMCs. Bar graph (C) illustrates effects of DPCPX (A1 antagonist), SCH-58261 (A2A antagonist), MRS-1754 (A2B antagonist), and VUF-5574 (A3 antagonist) (each at 0.1 μmol/l) on inhibition of [3H]thymidine incorporation by 2′,3′-cAMP (30 μmol/l) in human CAVSMCs. P values are from 1-factor ANOVA. aP < 0.05 (Fisher's LSD test) compared with basal (0) (A and B) or compared with no inhibitor (C).
DISCUSSION
The present study demonstrates that VSMCs from conduit arteries metabolize extracellular 2′,3′-cAMP to 2′-AMP or 3′-AMP, which are subsequently metabolized to adenosine. Therefore, this investigation confirms the existence of the 2′,3′-cAMP-adenosine pathway in VSMCs from conduit arteries. Moreover, this study verifies that this biochemical mechanism exists in conduit VSMCs obtained from both rats and humans and occurs in VSMCs from both the aorta and coronary arteries.
Importantly, 2′,3′-cAMP does not increase the levels of 5′-AMP in the medium, as would be expected since the 5′-hydroxy position in 2′,3′-cAMP is not involved in the phosphodiester bond of this cAMP isomer. Nonetheless, this is a noteworthy finding because this anticipated negative result increases confidence that the observed effects of 2′,3′-cAMP on 2′-AMP and 3′-AMP levels are not merely procedural or analytical artifacts unrelated to the metabolism of 2′,3′-cAMP. Also, because 5′-AMP does not increase with application of 2′,3′-cAMP, even though in these cell types 5′-AMP is converted to adenosine more efficiently than are 2′-AMP and 3′-AMP, 5′-AMP cannot be the source of the increased levels of adenosine with application of 2′,3′-cAMP.
Recent studies by Rao et al. (42) reveal at least six different enzymes that hydrolyze 2′,3′-cAMP to 3′-AMP, and decades of research confirm the existence of 2′,3′-cAMP-3′-phosphodiesterase, an enzyme that metabolizes 2′,3′-cAMP to 2′-AMP (49, 53). Whether these enzymes are involved in the metabolism of extracellular 2′,3′-cAMP to its corresponding AMPs is unknown, and there are currently no selective inhibitors to test this hypothesis. The fact that 2′,3′-cAMP is metabolized to both 2′-AMP and 3′-AMP in rat and human AVSMCs but is converted predominantly to 2′-AMP in human CAVSMCs suggests that the metabolism of extracellular 2′,3′-cAMP is a highly regulated process.
With regard to the metabolism of the AMPs, ecto-5′-nucleotidase, a well-characterized ectoenzyme also known as CD73, metabolizes extracellular 5′-AMP, the prototypical adenosine precursor, to adenosine. The present study demonstrates that rat AVSMCs, human AVSMCs, and human CAVSMCs robustly metabolize extracellular 5′-AMP to adenosine and that AMPCP, a selective inhibitor of CD73, practically abolishes the conversion of 5′-AMP to adenosine. However, unlike 5′-AMP, AMPCP does not affect the metabolism of either 2′-AMP or 3′-AMP to adenosine. These findings indicate that, in these cell types, CD73 mediates the conversion of extracellular 5′-AMP to adenosine but does not catalyze the conversion of extracellular 2′-AMP or 3′-AMP to adenosine. This suggests the existence of ectonucleotidases distinct from CD73 that catalyze the metabolism of 2′-AMP and 3′-AMP to adenosine. Importantly, in both AVSMCs and CAVSMCs, 5′-AMP is metabolized to adenosine more rapidly than are 2′-AMP or 3′-AMP. Thus, during cellular injury, CD73 may function to supply adenosine rapidly from 5′-AMP, and other ectonucleotidases may afford adenosine more slowly but sustainably from 2′-AMP and 3′-AMP.
The metabolism of extracellular 2′,3′-cAMP to 2′-AMP, 3′-AMP, and adenosine may be more than just a biological curiosity. Importantly, in all three cell types, 2′,3′-cAMP, 2′-AMP, and 3′-AMP potently and efficaciously inhibit cell proliferation. Most likely, the antiproliferative effects of 2′,3′-cAMP, 2′-AMP, and 3′-AMP are mediated, at least in part, by metabolism to adenosine. The evidence for this conclusion is that MRS-1754 attenuates the antiproliferative effects of 2′,3′-cAMP, 2′-AMP, and 3′-AMP. Because MRS-1724 is a selective A2B antagonist (34), these results imply that adenosine produced by metabolism of 2′,3′-cAMP and its corresponding AMPs inhibits cell proliferation in VSMCs from conduit arteries via A2B receptors. The observation that blockade of A1, A2A, and A3 receptors does not affect the antiproliferative effects of these compounds further supports a role for the A2B receptor. The dissociation constants (Ki) of DPCPX, SCH-58261, MRS-1754, and VUF-5574 for their respective receptor subtypes are very similar and between 0.5 and 4 nmol/l (34, 52). In our study, we used a concentration of 100 nmol/l, which is at least 25-fold greater than the Ki for each antagonist. Therefore, the concentrations were more than adequate. This was confirmed by the fact that MRS-1754 blocked all of the effects of a high concentration of adenosine. These results are consistent with our previous findings that adenosine, via A2B receptors, exerts antiprolifertive effects in a number of other cell types (10, 12, 13, 17, 18, 20, 21) and the very recent findings by Mayer et al. (36) that adenosine, again via the A2B receptor, induces antiproliferative effects in human CAVSMCs (as also observed in our study). In contrast to the effects of adenosine in human CAVMSCs, adenosine stimulates proliferation of pig CAVSMCs via activation of A1 receptors (46). Most likely, this represents an important species difference in adenosine receptor subtype expression in human vs. pig CAVSMCs.
Although 100 nmol/l of MRS-1754 attenuates the antiproliferative effects of 2′,3′-cAMP, this concentration of MRS-1754 does not completely prevent these effects of 2′,3′-cAMP on cell proliferation. This cannot be due to the use of insufficiently high concentrations of MRS-1754 because 100 nmol/l of MRS-1754 abolishes the antiproliferative effects of 2′-AMP, 3′-AMP, 5′-AMP, and adenosine. Consistent with our previous findings in microvascular VSMCs (32), we must conclude that, in conduit VSMCs, 2′,3′-cAMP inhibits cell proliferation not only by an adenosine/A2B receptor mechanism but also by a mechanism not involving adenosine or adenosine receptors.
It is noteworthy that the antiproliferative effects of 2′,3′-cAMP are profound, generally inhibiting proliferation by ∼50% at a concentration of 100 μmol/l. Importantly, even very low concentrations of 2′,3′-cAMP can inhibit cell proliferation (levels <1 μmol/l). Degradation of mRNA (the source of 2′,3′-cAMP) likely could achieve such concentrations of 2′,3′-cAMP in the local cellular environment. Partial support for this conclusion is provided by the following calculation. In general, mRNAs have a poly-A tail of ∼150 adenine repeats (2). Moreover, mRNA degradation begins with RNase-mediated hydrolysis of the poly-A tail (54). Because a typical mammalian cell contains 363,000 molecules of mRNA (2), a typical mammalian cell would house 5.445 × 107 potential molecules of 2′,3′-cAMP stored in the poly-A tails of mRNA. Dividing this potential store of 2′,3′-cAMP by Avogadro's number (6.022 × 1023) gives 0.9042 × 10−16 mol/cell. Because the average mammalian cell has a water volume of 2.800 × 10−12 l (3), the potential concentration of 2′,3′-cAMP achieved in a mammalian cell by mobilizing the 2′,3′-cAMP stored in the poly-A tails of mRNA would be 32.29 μmol/l. Furthermore, the average mRNA is 1,500 bases in length (2), and ∼25% of these bases are adenine. Thus there would likely be 1,500 × 0.25 × 363,000 = 1.361 × 108 potential molecules of 2′,3′-cAMP in the non-poly-A tail portion of mRNAs. If these potential stores of 2′,3′-cAMP were also released, then the concentration of 2′,3′-cAMP would increase by another 80.71 μmol/l. Each cell then has the capacity to produce a total concentration of ∼113.0 μmol/l of 2′,3′-cAMP by degrading mRNA.
Although a cell may have the capacity to produce >100 μmol/l of 2′,3′-cAMP, most likely only a fraction of this level would be produced because degradation of mRNA would only be partial, and salvage pathways may consume some of the mRNA degradation products. On the other hand, because adenosine formation from 2′,3′-cAMP would occur in the biophase of the external cell membrane, levels of adenosine generated from this pathway could be quite high at the site of action. Although the ideal way to test our hypothesis would be to measure 2′,3′-cAMP (and adenosine generation by 2′,3′-cAMP metabolism) in the biophase next to the external surface of cell membranes, such experiments are currently beyond our technical reach. However, based on pharmacological evidence, efflux of 3′,5′-cAMP from VSMCs during stimulation of adenylyl cyclase is sufficient to inhibit VSMC proliferation via conversion of 3′,5′-cAMP to adenosine with activation of A2 receptors (19). Also, exogenous 2′,3′-cAMP (via conversion to adenosine with activation of A2 receptors) is more potent than exogenous 3′,5′-cAMP with regard to inhibiting VSMC proliferation (32), and tissue injury can increase the concentration of 2′,3′-cAMP exiting a vascular bed to levels that are 100-fold greater than the levels of 3′,5′-cAMP (33). Because the cellular efflux of endogenous 3′,5′-cAMP can inhibit VSMC proliferation and because extracellular 2′,3′-cAMP is more potent than extracellular 3′,5′-cAMP with regard to inhibiting proliferation and tissue injury can increase extracellular 2′,3′-cAMP to 100-fold the levels of 3′,5′-cAMP, we conclude that the biophase levels of 2′,3′-cAMP near the cell membrane during cellular injury are very likely high enough to exert an effect. Nonetheless, the pathophysiological significance of our findings will remain uncertain until an approach is devised to block the formation of 2′,3′-cAMP or its metabolism to adenosine.
What might be the physiological, pathophysiological, or pharmacological significance of 2′,3′-cAMP-induced inhibition of cell proliferation? Atherosclerosis is associated with increased proliferation of VSMCs in large arteries, particularly in the coronary circulation. Thus processes that inhibit proliferation of VSMCs would reduce the progression of macrovascular disease. It is conceivable that injury to blood vessels releases sufficient 2′,3′-cAMP to inhibit cell proliferation. Such locally formed 2′,3′-cAMP could afford protection against vascular lesion formation. From a pharmacological perspective, it is important to note that one class of drug-eluting stents employs rapamycin. Rapamycin accelerates mRNA breakdown (4, 6, 25) and increases the formation of 2′,3′-cAMP (33). It is conceivable that the ability of rapamycin to inhibit the formation of neointimal hyperplasia following balloon angioplasty with stenting is in part via local vascular formation of 2′,3′-cAMP, which would inhibit proliferation of VSMCs in the intima. In support of this hypothesis, the results of the present study suggest that human CAVSMCs are the most sensitive to the antiproliferative effects of 2′,3′-cAMP.
If the antiproliferative effect of the 2′,3′-cAMP-adenosine pathway acts to inhibit atherosclerosis, what would this mean for conditions where angiogenesis/vasculogenesis might be desirable (e.g., myocardial ischemia, peripheral arterial disease)? Importantly, in addition to protecting against abnormal/harmful accumulation of VSMCs in injured blood vessels by inhibiting VSMC proliferation, adenosine also promotes angiogenesis (1) and vasculogenesis (40). Regarding the proangiogenic effects of adenosine, this nucleoside increases VEGF production by vascular cells (23) and stimulates vascular endothelial cell proliferation (11). With regard to new blood vessel formation, adenosine facilitates vasculogenesis by promoting adhesion of endothelial progenitor cells to vascular endothelium (45). Finally, adenosine enhances the vascular endothelial cell barrier (51). Thus the fact that adenosine inhibits abnormal VSMC proliferation does not translate into compromised angiogenesis/vasculogenesis. Rather, the inhibition of VSMC proliferation may facilitate angiogenesis/vasculogenesis by allowing endothelial tube formation to proceed without being impaired by premature proliferation of VSMCs.
In summary, 2′,3′-cAMP inhibits proliferation of VSMCs from rats and humans and from the aorta and coronary arteries. This effect is due in part to metabolism of 2′,3′-cAMP to adenosine (via 2′-AMP or 3′-AMP), with engagement of the A2B receptor. However, only a portion of the effects of 2′,3′-cAMP on VSMC proliferation is mediated by adenosine. Endogenous 2′,3′-cAMP may play an important role to protect against macrovascular disease, and drugs that augment 2′,3′-cAMP formation may find utility in the prevention and treatment of vascular disorders.
GRANTS
This work was supported by National Institutes of Health Grants HL-069846, DK-068575, and DK-079307.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1.Adair TH. An emerging role for adenosine in angiogenesis. Hypertension 44: 618–620, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD. The Cell Nucleus. In: Molecular Biology of The Cell (2nd ed.). New York, NY: Garlan, 1989, p. 481–550 [Google Scholar]
- 3. Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD. Macromolecules: structure, shape, information. In: Molecular Biology of The Cell. New York, NY: Garland, 1989, p. 87–134 [Google Scholar]
- 4.Albig AR, Decker CJ. The target of rapamycin signaling pathway regulates mRNA turnover in the yeast Saccharomyces cerevisiae. Mol Biol Cell 12: 3428–3438, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkinson B, Dwyer K, Enjyoji K, Robson SC. Ecto-nucleotidases of the CD39/NTPDase family modulate platelet activation and thrombus formation: potential as therapeutic targets. Blood Cells Mol Dis 36: 217–222, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Banholzer R, Nair AP, Hirsch HH, Ming XF, Moroni C. Rapamycin destabilizes interleukin-3 mRNA in autocrine tumor cells by a mechanism requiring an intact 3′ untranslated region. Mol Cell Biol 17: 3254–3260, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borst P, de Wolf C, van de Wetering K. Multidrug resistance-associated proteins 3, 4, and 5. Pflugers Arch 453: 661–673, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Chiavegatti T, Costa VL, Jr, Araujo MS, Godinho RO. Skeletal muscle expresses the extracellular cyclic AMP-adenosine pathway. Br J Pharmacol 153: 1331–1340, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deeley RG, Westlake C, Cole SPC. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev 86: 849–899, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Dubey RK, Gillespie D, Osaka K, Suzuki F, Jackson EK. Adenosine inhibits growth of rat aortic smooth muscle cells: possible role of A2b receptor. Hypertension 27: 786–793, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Dubey RK, Gillespie DG, Jackson EK. A(2B) adenosine receptors stimulate growth of porcine and rat arterial endothelial cells. Hypertension 39: 530–535, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Dubey RK, Gillespie DG, Jackson EK. Adenosine inhibits collagen and protein synthesis in cardiac fibroblasts: potential role of A2B receptors. Hypertension 31: 943–948, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Dubey RK, Gillespie DG, Mi Z, Jackson EK. Adenosine inhibits growth of human aortic smooth muscle cells via A2B receptors. Hypertension 31: 516–521, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Dubey RK, Gillespie DG, Mi Z, Jackson EK. Cardiac fibroblasts express the cAMP-adenosine pathway. Hypertension 36: 337–342, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Dubey RK, Gillespie DG, Mi Z, Jackson EK. Cyclic AMP-adenosine pathway inhibits glomerular mesangial cell growth (Abstract). Hypertension 30: 506, 1997 [Google Scholar]
- 16.Dubey RK, Gillespie DG, Mi Z, Jackson EK. Endogenous cyclic AMP-adenosine pathway regulates cardiac fibroblast growth. Hypertension 37: 1095–1100, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Dubey RK, Gillespie DG, Mi Z, Jackson EK. Exogenous and endogenous adenosine inhibits fetal calf serum-induced growth of rat cardiac fibroblasts: role of A2B receptors. Circulation 96: 2656–2666, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Dubey RK, Gillespie DG, Mi Z, Jackson EK. Exogenous and endogenous adenosine inhibits fetal calf serum-induced growth of rat glomerular mesangial cells via A2b receptors (Abstract). Hypertension 30: 509, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Dubey RK, Gillespie DG, Mi Z, Rosselli M, Keller PJ, Jackson EK. Estradiol inhibits smooth muscle cell growth in part by activating the cAMP-adenosine pathway. Hypertension 35: 262–266, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Dubey RK, Gillespie DG, Shue H, Jackson EK. A2B receptors mediate antimitogenesis in vascular smooth muscle cells. Hypertension 35: 267–272, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Dubey RK, Gillespie DG, Zacharia LC, Mi Z, Jackson EK. A2B receptors mediate the antimitogenic effects of adenosine in cardiac fibroblasts. Hypertension 37: 716–721, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Dubey RK, Mi Z, Gillespie DG, Jackson EK. Cyclic AMP-adenosine pathway inhibits vascular smooth muscle cell growth. Hypertension 28: 765–771, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Feoktistov I, Ryzhov S, Zhong H, Goldstein AE, Matafonov A, Zeng D, Biaggioni I. Hypoxia modulates adenosine receptors in human endothelial and smooth muscle cells toward an A2B angiogenic phenotype. Hypertension 44: 649–654, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Giron MC, Bin A, Brun P, Etteri S, Bolego C, Florio C, Gaion RM. Cyclic AMP in rat ileum: evidence for the presence of an extracellular cyclic AMP-adenosine pathway. Gastroenterology 134: 1116–1126, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Hashemolhosseini S, Nagamine Y, Morley SJ, Desrivieres S, Mercep L, Ferrari S. Rapamycin inhibition of the G1 to S transition is mediated by effects on cyclin D1 mRNA and protein stability. J Biol Chem 273: 14424–14429, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Hong KW, Shin HK, Kim HH, Choi JM, Rhim BY. Metabolism of cAMP to adenosine: role in vasodilation of rat pial artery in response to hypotension. Am J Physiol Heart Circ Physiol 276: H376–H382, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Jackson EK. Adenosine: a physiological brake on renin release. Annu Rev Pharmacol Toxicol 31: 1–35, 1991 [DOI] [PubMed] [Google Scholar]
- 28.Jackson EK, Dubey RK. Role of the extracellular cAMP-adenosine pathway in renal physiology. Am J Physiol Renal Physiol 281: F597–F612, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Jackson EK, Mi Z. Preglomerular microcirculation expresses the cAMP-adenosine pathway. J Pharmacol Exp Ther 295: 23–28, 2000 [PubMed] [Google Scholar]
- 30.Jackson EK, Mi Z, Gillespie DG, Dubey RK. Metabolism of cAMP to adenosine in the renal vasculature. J Pharmacol Exp Ther 283: 177–182, 1997 [PubMed] [Google Scholar]
- 31.Jackson EK, Raghvendra DK. The extracellular cyclic AMP-adenosine pathway in renal physiology. Annu Rev Physiol 66: 571–599, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Jackson EK, Ren J, Gillespie DG, Dubey RK. Extracellular 2′,3′-cyclic adenosine 5′-monophosphate is a potent inhibitor of preglomerular vascular smooth muscle cell and mesangial cell growth. Hypertension 56: 151–158, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson EK, Ren J, Mi Z. Extracellular 2′,3′-cAMP is a source of adenosine. J Biol Chem 284: 33097–33106, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobson KA, Knutsen LJS. P1 and P2 purine and pyrimidine receptor ligands. In: Purinergic and Pyrmidinergic Signalling I, edited by Abbracchio MP, Williams M. Berlin, Germany: Springer-Verlag, 2001, p. 129–175 [Google Scholar]
- 35.Kruh GD, Zeng H, Rea PA, Liu G, Chen ZS, Lee K, Belinsky MG. MRP subfamily transporters and resistance to anticancer agents. J Bioenerg Biomembr 33: 493–501, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Mayer P, Hinze AV, Harst A, von Kügelgen I. A2B receptors mediate the induction of early genes and inhibition of arterial smooth muscle cell proliferation via Epac. Cardiovasc Res 90: 148–156, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Mi Z, Herzer WA, Zhang Y, Jackson EK. 3-Isobutyl-1-methylxanthine decreases renal cortical interstitial levels of adenosine and inosine. Life Sci 54: 277–282, 1994 [DOI] [PubMed] [Google Scholar]
- 38.Mi Z, Jackson EK. Evidence for an endogenous cAMP-adenosine pathway in the rat kidney. J Pharmacol Exp Ther 287: 926–930, 1998 [PubMed] [Google Scholar]
- 39.Mi Z, Jackson EK. Metabolism of exogenous cyclic AMP to adenosine in the rat kidney. J Pharmacol Exp Ther 273: 728–733, 1995 [PubMed] [Google Scholar]
- 40.Montesinos MC, Shaw JP, Yee H, Shamamian P, Cronstein BN. Adenosine A(2A) receptor activation promotes wound neovascularization by stimulating angiogenesis and vasculogenesis. Am J Pathol 164: 1887–1892, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Müller G, Wied S, Over S, Frick W. Inhibition of lipolysis by palmitate, H2O2 and the sulfonylurea drug, glimepiride, in rat adipocytes depends on cAMP degradation by lipid droplets. Biochemistry 47: 1259–1273, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Rao F, Qi Y, Murugan E, Pasunooti S, Ji Q. 2′,3′-cAMP hydrolysis by metal-dependent phosphodiesterases containing DHH, EAL, and HD domains is non-specific: Implications for PDE screening. Biochem Biophys Res Commun 398: 500–505, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Ren J, Mi Z, Stewart NA, Jackson EK. Identification and quantification of 2′,3′-cAMP release by the kidney. J Pharmacol Exp Ther 328: 855–865, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ren J, Mi ZC, Jackson EK. Assessment of nerve stimulation-induced release of purines from mouse kidneys by tandem mass spectrometry. J Pharmacol Exp Ther 325: 920–926, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Ryzhov S, Solenkova NV, Goldstein AE, Lamparter M, Fleenor T, Young PP, Greelish JP, Byrne JG, Vaughan DE, Biaggioni I, Hatzopoulos AK, Feoktistov I. Adenosine receptor-mediated adhesion of endothelial progenitors to cardiac microvascular endothelial cells. Circ Res 102: 356–363, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen J, Halenda SP, Sturek M, Wilden PA. Cell-signaling evidence for adenosine stimulation of coronary smooth muscle proliferation via the A1 adenosine receptor. Circ Res 97: 574–582, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Sorrentino S. Human extracellular ribonucleases: multiplicity, molecular diversity and catalytic properties of the major RNase types. Cell Mol Life Sci 54: 785–794, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sorrentino S, Libonati M. Structure-function relationships in human ribonucleases: main distinctive features of the major RNase types. FEBS Lett 404: 1–5, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Sprinkle TJ. 2′,3′-Cyclic nucleotide 3′-phosphodiesterase, an oligodendrocyte-Schwann cell and myelin-associated enzyme of the nervous system. Crit Rev Neurobiol 4: 235–301, 1989 [PubMed] [Google Scholar]
- 50.Thompson JE, Venegas FD, Raines RT. Energetics of catalysis by ribonucleases: fate of the 2′,3′-cyclic phosphodiester intermediate. Biochemistry 33: 7408–7414, 1994 [DOI] [PubMed] [Google Scholar]
- 51.Umapathy NS, Fan Z, Zemskov EA, Alieva IB, Black SM, Verin AD. Molecular mechanisms involved in adenosine-induced endothelial cell barrier enhancement. Vasc Pharmacol 52: 199–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Muijlwijk-Koezen JE, Timmerman H, van der Goot H, Menge WM, Frijtag Von DrabbeKunzel J, deGroote M, Ijzerman AP. Isoquinoline and quinazoline urea analogues as antagonists for the human adenosine A3 receptor. J Med Chem 43: 2227–2238, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Vogel US, Thompson RJ. Molecular structure, localization, and possible functions of the myelin-associated enzyme 2′,3′-cyclic nucleotide 3′-phosphodiesterase. J Neurochem 50: 1667–1677, 1988 [DOI] [PubMed] [Google Scholar]
- 54.Wilusz CJ, Wormington M, Peltz SW. The cap-to-tail guide to mRNA turnover. Nat Rev Mol Cell Biol 2: 237–246, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Zimmermann H. 5′-Nucleotidase: molecular structure and functional aspects. Biochem J 285: 345–365, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zimmermann H. Ectonucleotidases in the nervous system. Novartis Found Symp 276: 113–128, 2006 [PubMed] [Google Scholar]
- 57.Zimmermann H, Braun N. Ecto-nucleotidases–molecular structures, catalytic properties, and functional roles in the nervous system. Prog Brain Res 120: 371–385, 1999 [PubMed] [Google Scholar]