Abstract
Vascular tone, an important determinant of systemic vascular resistance and thus blood pressure, is affected by vascular smooth muscle (VSM) contraction. Key signaling pathways for VSM contraction converge on phosphorylation of the regulatory light chain (RLC) of smooth muscle myosin. This phosphorylation is mediated by Ca2+/calmodulin-dependent myosin light chain kinase (MLCK) but Ca2+-independent kinases may also contribute, particularly in sustained contractions. Signaling through MLCK has been indirectly implicated in maintenance of basal blood pressure, whereas signaling through RhoA has been implicated in salt-induced hypertension. In this report, we analyzed mice with smooth muscle-specific knockout of MLCK. Mesenteric artery segments isolated from smooth muscle-specific MLCK knockout mice (MLCKSMKO) had a significantly reduced contractile response to KCl and vasoconstrictors. The kinase knockout also markedly reduced RLC phosphorylation and developed force. We suggest that MLCK and its phosphorylation of RLC are required for tonic VSM contraction. MLCKSMKO mice exhibit significantly lower basal blood pressure and weaker responses to vasopressors. The elevated blood pressure in salt-induced hypertension is reduced below normotensive levels after MLCK attenuation. These results suggest that MLCK is necessary for both physiological and pathological blood pressure. MLCKSMKO mice may be a useful model of vascular failure and hypotension.
Keywords: vascular smooth muscle, regulatory light chain, contraction, knockout
systemic blood pressure is regulated by complex neurohumoral and mechanical signals that affect cardiac output, extracellular fluid volume, and vascular resistance, which arises from the contraction of arterial smooth muscle (3–5, 8, 21). Elevated blood pressure primarily occurs through a canonical mechanism that emphasizes a central role of the renin-angiotensin-aldosterone system in regulating volume expansion and systemic vascular resistance (8, 20, 23). Although it has been recognized for the past several decades that alteration in arterial wall structure, arterial smooth muscle hypersensitivity to vasoconstrictors, depressed responsiveness to vasodilators, and impaired endothelium function can increase vascular resistance and blood pressure, the contributions of vascular contractility to physiological blood pressure as well as hypertension have not been sufficiently tested (36). More recent reports suggest an important role of vascular contractility in high blood pressure with evidence that abnormalities of the vascular smooth muscle (VSM) contractile state are sufficient to cause disorders in the regulation of blood pressure, including hypertension (10, 23, 25, 26). However, the mechanisms regulating vascular contractility in relation to blood pressure are not well defined, and genetic models of impaired vascular contractility are rare.
VSM contractility is regulated by a network of signaling pathways directed to the molecular motor myosin (15, 16, 33, 34). Phosphorylation of the regulatory light chain (RLC) of smooth muscle myosin activates actomyosin Mg-ATPase resulting in cross-bridge cycling and force development in smooth muscle. The extent of RLC phosphorylation, regulated by multiple signaling pathways that impinge on both Ca2+/calmodulin-dependent myosin light chain kinase (MLCK) and myosin light chain phosphatase (MLCP), is thought to be the primary determinant for the production of force (16, 33, 34). However, other Ca2+-independent kinases such as integrin-linked kinase, Rho-associated kinase, and zipper-interacting protein kinase may also phosphorylate smooth muscle RLC during sustained contractions (6, 13, 29, 35). Additional regulation through thin-filament or focal adhesion proteins may participate, but the physiological role of these is unclear (7, 19, 27, 32). The loss-of-function evidence from our previous reports suggests that Ca2+/calmodulin-dependent MLCK phosphorylation of RLC is critical for gut and airway smooth muscle contraction (11, 40), and we here investigate its importance for VSM function.
Depolarization of the VSM cell membrane activates voltage-gated Ca2+-channels, resulting in Ca2+ influx; agonist stimulation may activate G protein-coupled receptors (GPCR), leading to inositol 1,4,5-trisphosphate (IP3) formation with subsequent Ca2+ release from the sarcoplasmic reticulum (SR) and Ca2+ entry across plasma membrane (12, 18, 31). The increase in cytosolic Ca2+ leads to MLCK activation and RLC phosphorylation (16, 17). Activation of GPCRs may also enhance RLC phosphorylation through Ca2+-sensitization mechanisms. In a typical smooth muscle contraction, stimuli induce an initial, robust contraction along with a subsequent, sustained contraction. In contrast with phasic smooth muscle, tonic smooth muscle displays a prolonged sustained contraction. The prevailing explanation for this two-phase contraction is that Ca2+-dependent MLCK initiates robust contraction by phosphorylating myosin RLC and that integrating signals produce sustained tension through Ca2+-independent inhibition of MLCP activity with additional activity of Ca2+-independent kinases (6, 13, 29, 33–35). To elucidate the role of VSM contractile regulation and its involvement in blood pressure, we focused on investigating the functional roles of Ca2+-dependent MLCK in VSM in vivo. We analyzed mice with tissue-specific attenuation of MLCK and revealed the importance of MLCK in VSM contraction. We also investigated the role of MLCK in the maintenance of basal blood pressure and salt-induced hypertension. We thus provide an animal model of impaired vascular contractility, which might be used to further investigate signaling mechanisms for blood pressure regulation.
MATERIALS AND METHODS
Chemicals and antibodies.
Norepinephrine (NE), phenylephrine (PE), ANG II, and endothelin-1 (ET-1) were purchased from Sigma (St. Louis, MO). Antibodies used in this study were to MLCK (K36; Sigma), Rho-associated coiled-coil-forming protein kinase II (ROCK-II; Santa Cruz Biotechnology, Santa Cruz, CA), regulatory light chain (RLC) (14), smooth muscle myosin heavy chain (SM-MHC; Abcam, Cambridge, MA), α-smooth muscle actin (α-SMA RB-9010; Thermo Scientific, Fremont, CA), myosin phosphatase targeting subunit of the RLC phosphatase (MYPT1; Upstate, Billerica, MA), and phospho-MYPT1[Thr-696] (Upstate), phospho-MYPT1[Thr-850] (Upstate), integrin-linked kinase (ILK; Sigma), and β-actin (Sigma).
Smooth muscle-specific Mlck knockout mice.
The generation of tamoxifen inducible smooth muscle-specific Mlck knockout mice (MLCKSMKO) has been described previously (11). Mlckflox/flox; SM-CreERT2 mice received 5-day consecutive tamoxifen injections for generating MLCKSMKO mice. Mlck+/flox; SM-CreERT2 mice with tamoxifen injection were used as controls (CTR). All animal experiments were approved by the Animal Care and Use Committee of Model Animal Research Center of Nanjing University.
Blood pressure recordings.
Blood pressure was measured by using a validated pulse-based tail-cuff method as described (37). Mice were fixed in restrainers and placed in a warming chamber (32°C). Each tail was placed into a tail cuff, and pulsations were detected by a pulse sensor (ALC-NIBP system; Shanghai Alcott Biotech). The pressure at the point of disappearance of pulses upon inflation of the occlusion cuff was recorded to estimate blood pressure. All mice (10 wk old) received 5 days of training before data collection. Measurements were performed at 10 to 11 am. Daily measurements started on the first day of tamoxifen treatment. To measure the blood pressure in response to vasoconstrictors, each vasoconstrictor was dissolved in 200 μl of physiological saline buffer and injected into the tail vein in a bolus dose. The measurements of blood pressure were taken for 15 min before and 90 min after drug application at 1-min intervals. The responses to the vasoconstrictors were estimated by the peak value of the blood pressure.
Echocardiography.
Left ventricular cardiac function was assessed by echocardiography with a Vevo 770 High-Resolution Imaging System (VisualSonics, Toronto, ON, Canada). After mice were anesthetized with avertin (250 mg/kg ip injection), the mice were maintained on a platform at 37°C and transthoracic M-mode echocardiography was performed with a 30-MHz RMV-707B scanning head (2).
Measurement of the plasma renin, ANG II, and aldosterone values.
Blood samples were collected from the retrobulbar venous plexus using Na2-EDTA as an anticoagulant. After centrifugation for 15 min at 10,000 rpm at 4°C, the separated plasma was divided in aliquots and stored at −80°C. The plasma renin, ANG II, and aldosterone levels were measured with ELISA (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Mesenteric artery contractility.
The second-order branches of mesenteric artery were cleaned of adipose and connective tissue, cut into segments (1.40 mm in length), and mounted on a wire myograph (610-M; Danish Myo Technology, Aarhus, Denmark). The arteries were kept in HEPES-Tyrode (H-T) buffer containing (in mM) 137.0 NaCl, 2.7 KCl, 1.0 MgCl2, 1.8 CaCl2, 10 HEPES, and 5.6 glucose (pH 7.4) aerated with oxygen at 37°C. Isometric tension was recorded by a PowerLab recording device (ADInstruments). The normalized passive resting force and the corresponding diameter were determined for each artery segment from its own length-pressure curve (28). The arteries were progressively stretched until the wall tension was equivalent to a transmural pressure of 100 mmHg. IC100 is the internal circumference corresponding to a transmural pressure of 100 mmHg. The IC1 was set as 90% of IC100 where the active force production of the vessel is maximal. H-T buffer containing 124 mM KCl and (in mM) 15.7 NaCl, 124.0 KCl, 1.0 MgCl2, 1.8 CaCl2, 10 HEPES, and 5.6 glucose (pH 7.4) was used to achieve membrane depolarization. Agonists including NE, PE, ANG II, and ET-1 were used to examine the GPCR-mediated contractile responses of the arterial smooth muscle. START.
DOCA-salt-induced hypertension model.
An animal model of DOCA-salt-induced hypertension was produced as described (38). The animals were anesthetized with avertin (250 mg/kg ip injection). The left kidney was exteriorized through a 1-cm flank incision. The left renal artery and vein and the left ureter were tied off, and the kidney was removed. Mice were allowed to recover for at least 1 wk. DOCA pellets (50 mg DOCA, 21-day release time; Innovative Research of America, Sarasota, FL) were implanted subcutaneously on the lateral side of the neck in anesthetized mice. Mice received 1% NaCl in the drinking water after DOCA administration. Blood pressure was measured by the tail-cuff method.
Histology and immunohistochemistry.
Aortic, femoral, coronary, and mesenteric arteries were fixed with 4% formalin, embedded in paraffin, and transversely sectioned at a thickness of 6 μm. Tissue sections were stained with hematoxylin and eosin to examine artery histology. For immunohistochemistry, cryosections (10 μm) were fixed in cold acetone, and the nonspecific binding of primary antibody was blocked with 5% bovine serum albumin in phosphate-buffered saline. After washing with the same buffer, overnight incubation with antibodies to MLCK or α-smooth muscle actin was performed at 4°C. FITC-labeled rabbit-anti-mouse IgG (Sigma) or RITC-labeled goat-anti-mouse IgG (Pierce, Rockford, IL) was used individually as secondary antibodies. Images of immunofluorescence were acquired with a confocal microscope (Leica, TCS-SP2, Wetzlar, Germany).
Western blot.
Western blot analyses were performed for measurements of MLCK and other proteins (11). Briefly, tissue samples were collected and frozen quickly in 10% trichloroacetic acid and 10 mM dithiothreitol in acetone precooled to slush at −80°C. After thorough homogenization, the sample pellet was washed three times with ether for 5 min each and dried to remove residual ether. The protein was dissolved completely in 8 M urea solution. Protein concentration was measured with bicinchoinic acid (BCA) protein assay reagent. Aliquots were added to SDS sample buffer and boiled briefly. Equal amounts of protein were loaded for 6% SDS-PAGE followed by protein transfer to a nitrocellulose membrane. The membrane was probed with primary antibody and appropriate secondary antibody sequentially and then visualized by incubation in Super Signal West Dura substrate (Pierce) before exposure to film.
Measurement of myosin RLC phosphorylation.
Urea/glycerol-PAGE was used for measurement of RLC phosphorylation where the nonphosphorylated RLC is separated from the monophosphorylated RLC (14). RLC protein was visualized by Western blot assay with an antibody to RLC. The percentage of phosphorylated RLC relative to total RLC was quantified with a Jieda 801 Image Analysis system 3.3.2 (JEDA Science-Technology Development, Nanjing, China).
Data analysis.
Data are presented as means ± SE. Significant differences between groups were determined by Student's t-test.
RESULTS
Attenuation of MLCK and phenotypic characterization of VSMs.
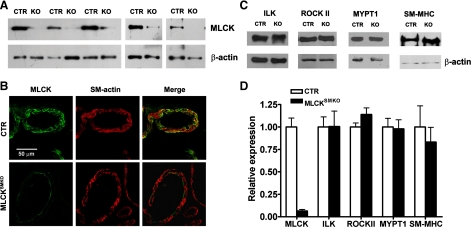
MLCK was disrupted specifically in smooth muscle cells of MLCKSMKO (Mlckflox/flox; SM-CreERT2) mice by using a tamoxifen-inducible Cre-loxP approach (11). To examine the knockout efficiency of MLCK in arterial smooth muscle, we focused on mesenteric arteries. Western blot assays showed a variable reduction of MLCK protein in mesenteric arteries from different MLCKSMKO mice (Fig. 1A). The number of MLCK-deficient arteries, which expressed <5, 5–10, and >10 percent of residual MLCK protein, compared with CTR, is in the ratio of 5:3:2. The average value of retained MLCK is 6.5 ± 1.6% in mesenteric arteries from MLCKSMKO mice (N=10; Fig. 1D). Immunohistochemical staining with anti-MLCK antibody also showed a marked reduction of MLCK protein. Figure 1B shows representative images for staining of MLCK in mesenteric arteries from CTR and MLCKSMKO mice where arterial MLCK contents are less than 5% of CTR. The smooth muscle layers of the artery wall showed significantly reduced MLCK expression, whereas the adventitia and intima showed positive staining (Fig. 1B).
Fig. 1.
Targeted deletion of myosin light chain kinase (Mlck) gene in mesenteric arteries. A: Western blot of MLCK in mesenteric arteries from smooth muscle-specific MLCK knockout [MLCKSMKO; knockout (KO)] and control (CTR) mice collected at day 16 after tamoxifen injection. β-Actin was used as a loading control. B: immunofluorescence analysis of MLCK expression in mesenteric arteries from MLCKSMKO and CTR mice. At day 16 after tamoxifen injection, arteries were embedded in optimum cutting temperature and sectioned. Frozen sections were fixed with acetone and then stained with K36 antibody and α-smooth muscle actin antibody. Scale bar=50 μm. C: representative Western blots of contractile and regulatory proteins [integrin-linked kinase (ILK), Rho-associated coiled-coil-forming protein kinase II (ROCK II), myosin phosphatase targeting subunit of the RLC phosphatase (MYPT1), smooth muscle myosin heavy chain (SM-MHC)] in mesenteric arteries from MLCKSMKO and CTR mice collected at day 16 after tamoxifen injection. β-Actin was used as a loading control. D: expression of MLCK (N=10) and other proteins (N=3) in KO arteries is expressed as the ratio relative to that of CTR.
To assess possible compensatory effects on myosin signaling, we evaluated the expression of ILK, ROCK II, MYPT1, and SM-MHC by Western blotting mesenteric arteries from CTR and MLCKSMKO mice. Results showed no significant differences in amounts of these proteins (Fig. 1, C and D), similar to observations from gut and airway smooth muscles (11, 40). Phosphorylation of MYPT1 in response to NE was robust in arteries from both CTR and MLCKSMKO mice (Supplemental Fig. S1, A–C). To test whether RhoA/Rho kinase-mediated inhibition of MLCP was impaired in MLCK-attenuated arteries, Rho kinase-specific inhibitor Y27632 was cumulatively added to mesenteric arteries contracted by KCl. As shown in Supplemental Fig. S1, D–F, 10 μm of Y27632 almost completely inhibited the sustained contraction in MLCK-attenuated arteries. Interestingly, arteries from these mice were even more sensitive to Y27632 compared with those from control mice. These results suggest a greater dependence on the RhoA/Rho kinase system when MLCK expression is attenuated.
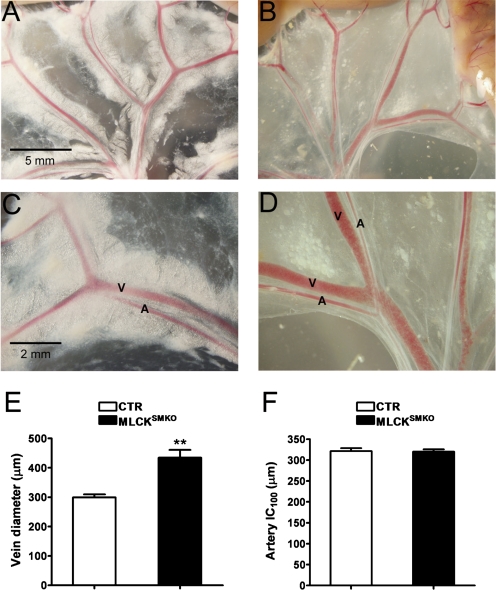
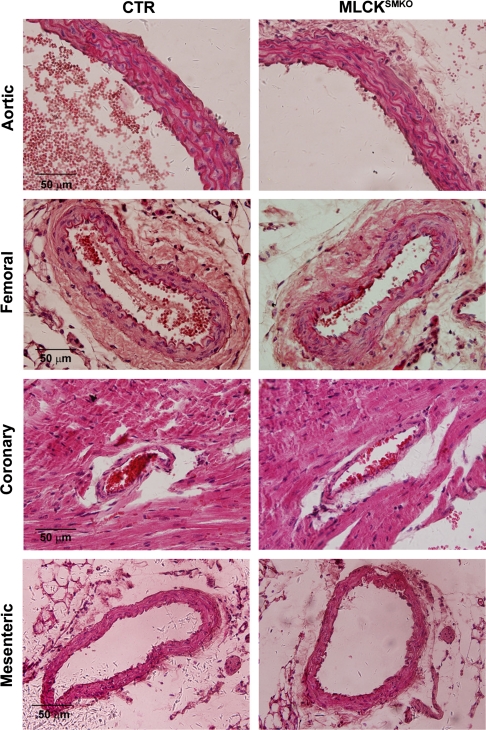
No striking differences were observed in the morphology of arteries from CTR and MLCKSMKO mice at day 16 after tamoxifen injection except for decreased perivascular fat content in MLCKSMKO mice (Fig. 2 and 3). Whereas the gross morphology of the mesenteric arterial bed in vivo appeared similar, mesenteric veins from MLCKSMKO mice exhibited an enlarged lumen compared with CTR mice (Fig. 2, A–D). Diameter measurements of the secondary branches of mesenteric veins showed a significant increase in MLCKSMKO mice (299 ± 10 μm CTR and 434 ± 27 μm MLCKSMKO, P < 0.01; Fig. 2E). The dilation of mesenteric veins may result from contractile dysfunction associated with MLCK attenuation in venous smooth muscle cells. In fixed tissues, there were no gross differences in the appearance of several arteries including aorta, femoral artery, coronary artery, and mesenteric artery (Fig. 3). In a functional assessment of arterial diameter and compliance, the internal circumferences of relaxed, MLCK-deficient mesenteric arteries were compared with controls in a procedure that estimates the circumference corresponding to a transmural pressure of 100 mmHg (IC100, automatically calculated by myograph normalization procedure) (28). IC100 values were comparable between MLCKSMKO and CTR vessels (321 ± 12 μm vs. 320 ± 18 μm, P > 0.05; Fig. 2F). This result suggests that the mesenteric arteries in MLCKSMKO mice have normal elastic properties.
Fig. 2.
Gross morphology of mesenteric vessels of MLCKSMKO mice and CTR mice. The mesenteric vasculature from a CTR mouse (A, C) and a MLCKSMKO mouse (B, D) was photographed at 16 days after tamoxifen treatment. Images were obtained at comparable anatomical sites (A, B, scale bar=5 mm; C, D, scale bar=2 mm). Arteries (A) and veins (V) are indicated. E: diameters of secondary branches of the mesenteric vein were measured from photographs as in C and D. Values are means ± SE of CTR (N=4) and MLCKSMKO (N=4); **P < 0.01. F: internal circumferences of second-order branches of mesenteric arteries of CTR and MLCKSMKO mice were calculated by the Myograph normalization procedure. IC100 is the internal circumference corresponding to a transmural pressure of 100 mmHg. Values are means ± SE for CTR (N=4) and MLCKSMKO (N=12).
Fig. 3.
Histological sections of control and MLCK-deficient arteries. Fresh tissues of aorta, femoral artery, heart, and mesenteric artery from CTR and MLCKSMKO mice were fixed with 4% formalin and dehydrated in a graded series of ethanol solutions followed by standard paraffin section and hematoxylin and eosin staining. Scale bars=50 μm.
Impaired VSM contraction in MLCKSMKO mice.
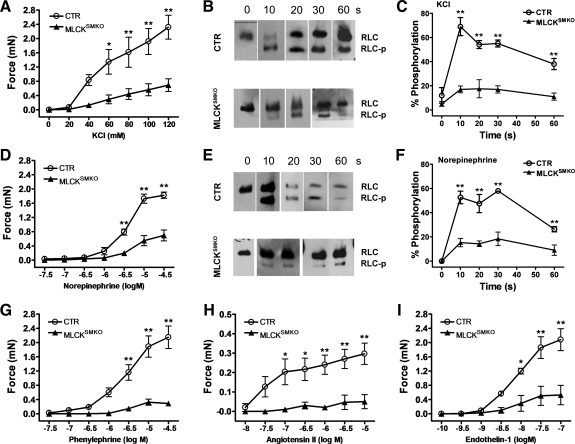
Contractile properties of MLCK-deficient VSM were measured on segments of mesenteric artery by myography. With depolarization by 124 mM KCl, both the transient and sustained phases of force in mesenteric arteries prepared from MLCKSMKO mice were significantly smaller than that from control, but the contraction showed a typical force pattern (transient: CTR, 3.0 ± 0.2 mN; MLCKSMKO, 1.2 ± 0.1 mN, P < 0.01; sustained: CTR, 2.8 ± 0.3 mN; MLCKSMKO, 0.9 ± 0.1 mN, P < 0.01). Dose-response curves also showed reduced ability of KCl to induce contraction in MLCK-attentuated arteries (Fig. 4A). The potency as well as efficacy of NE (Fig. 4D), PE, ANG II, and ET-1 (Fig. 4, G–I) were also reduced in MLCK-deficient arteries compared with CTR arteries. These results collectively suggested that attenuation of MLCK led to a significant impairment of VSM contraction evoked by membrane depolarization and vasoconstrictors acting on distinct receptors.
Fig. 4.
Reduced contraction and regulatory light chain (RLC) phosphorylation in mesenteric arteries from MLCKSMKO mice. Dose-response curves of KCl (A), norepinephrine (NE; D), phenylephrine (PE), ANG II, and endothelin-1 (ET-1; G–I) in fresh mesenteric artery segments from MLCKSMKO (KO) and CTR mice analyzed by wire myography are shown. Bars are means ± SE (N=4); *P < 0.05, **P < 0.01. After stimulation with 124 mM KCl (B, C) or 1 μM NE (E, F), mesenteric arteries were quickly frozen at indicated times for sample preparation. RLC phosphorylation (RLC-p) was measured by Western blots of glycerol/urea PAGE gels (B, E). RLC-p level was expressed as percentage of total RLC (C, F; N=4). All values represent means ± SE; *P < 0.05, **P < 0.01 compared with MLCKSMKO values at the indicated time.
Diminished myosin light chain phosphorylation of MLCKSMKO VSMs.
We measured RLC phosphorylation in mesenteric arteries from MLCKSMKO mice in response to KCl-induced depolarization. In CTR arteries, RLC phosphorylation increased to 72 ± 8% by 10 s after KCl stimulation and was then maintained at a high level for more than 1 min. However, MLCKSMKO artery segments displayed less than 20% of RLC phosphorylation compared with CTR segment at all time points after stimulation (Fig. 4, B and C). The reduced RLC phosphorylation in response to KCl was consistent with the prominent attenuation of force development by VSM from MLCKSMKO mice. RLC phosphorylation values with NE stimulation were also significantly reduced in MLCKSMKO artery (Fig. 4, E and F). Thus MLCK appears to be the predominant kinase for RLC phosphorylation in VSM contractions.
Attenuation of MLCK in smooth muscle causes decreased physiological blood pressure and response to vasoconstrictors.
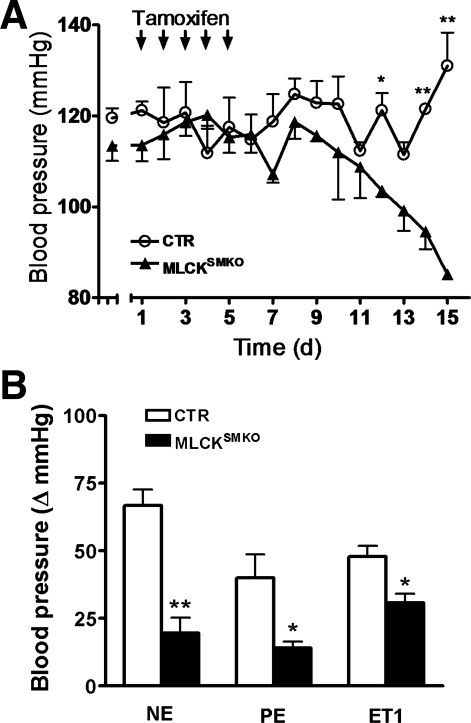
We made daily measurements of systolic blood pressure (SBP) in Mlckflox/flox; SM-CreERT2 mice during and after tamoxifen treatment. SBP of MLCKSMKO mice gradually decreased beginning 5–7 days after the last injection (Fig. 5A). By 16 days after starting tamoxifen injections, SBP decreased from 114 ± 4 to 85 ± 1 mmHg (P < 0.05; Fig. 5A). Thus attenuation of MLCK leads to a lower basal blood pressure.
Fig. 5.
Reduced physiological blood pressures of MLCKSMKO mice. A: systolic blood pressures in CTR and MLCKSMKO mice were measured by the tail-cuff method. Daily measurements were performed for 15 days following the initial tamoxifen injection. Each arrow indicates an injection of tamoxifen; N=3 (female mice). B: effect of intravenous injection of NE (25 μg/kg), PE (10 μg/kg), and ET-1 (1 nmol/kg) on blood pressures of CTR and MLCKSMKO mice. Responses are plotted as the maximum change in pressure (ΔmmHg) after injection. Values are means ± SE; N=3. *P < 0.05, **P < 0.01 compared with CTR values.
To determine the response to agonists in vivo, mice were injected intravenously with various vasopressors, and the responses were expressed as the maximum increase in blood pressure (Fig. 5B). When compared with CTR, the response to NE was significantly less in MLCKSMKO mice (change of SBP: CTR, 67 ± 6 mmHg; MLCKSMKO, 20 ± 6 mmHg, P < 0.05). The responses to PE and ET-1 were also decreased in MLCKSMKO mice (Fig. 5B). This result shows that MLCKSMKO mice have significantly lower vascular responses to agonists, suggesting a general impairment of GPCR-mediated signaling to elevate blood pressure.
To assess possible cardiac and renal contributions to blood pressure regulation in MLCKSMKO mice, we measured cardiac function by echocardiography and renin, angiotensin, and aldosterone concentrations by ELISA. Measurements were made on mice at 15 days after initiating tamoxifen treatment. Two-dimensional-color Doppler ultrasound imaging revealed comparable cardiac functional parameters in CTR and MLCKSMKO mice including fractional shortening, ejection fraction, and ventricular volume (Table 1). Biochemical measurements of renin, ANG II, and aldosterone in peripheral blood were also comparable in CTR and MLCKSMKO mice (Table 2). Thus the MLCKSMKO and CTR mice appear to have comparable cardiac function and renal endocrine performance.
Table 1.
Echocardiographic assessment of CTR and MLCKSMKO mice
| Parameter | CTR | MLCKSMKO | P Value |
|---|---|---|---|
| LV | |||
| Fractional shortening, % | 32.6 ± 1.6 | 33.5 ± 1.2 | >0.05 |
| Ejection fraction, % | 61.4 ± 2.0 | 63.4 ± 1.8 | >0.05 |
| Mass, mg | 73.2 ± 4.8 | 67.2 ± 7.1 | >0.05 |
| LV volume, μl | |||
| Diastole | 55.8 ± 4.7 | 46.3 ± 5.3 | >0.05 |
| Systole | 21.5 ± 2.4 | 16.9 ± 2.2 | >0.05 |
| Interventricular septum, mm | |||
| Diastole | 0.74 ± 0.05 | 0.75 ± 0.06 | >0.05 |
| Systole | 0.83 ± 0.06 | 0.83 ± 0.06 | >0.05 |
| LV inner distance, mm | |||
| Diastole | 3.61 ± 0.10 | 3.35 ± 0.19 | >0.05 |
| Systole | 2.43 ± 0.10 | 2.21 ± 0.12 | >0.05 |
| LV posterior wall, mm | |||
| Diastole | 0.79 ± 0.03 | 0.76 ± 0.05 | >0.05 |
| Systole | 1.04 ± 0.05 | 1.00 ± 0.06 | >0.05 |
| Heart rate, beats/min | 386 ± 10 | 384 ± 20 | >0.05 |
Values are means ± SE; N=12 for control (CTR) mice and N=8 for smooth muscle-specific MLCK knockout mice (MLCKSMKO) mice. LV, left ventricular. Measurements were made on mice at 15 days after initiating tamoxifen treatment.
Table 2.
Plasma renin, ANG II, and aldosterone in CTR and MLCKSMKO mice
| Parameter, pg/ml | CTR | MLCKSMKO | P Value |
|---|---|---|---|
| Renin | 21.6 ± 3.2 | 18.7 ± 2.5 | >0.05 |
| ANG II | 212 ± 20 | 265 ± 40 | >0.05 |
| Aldosterone | 753 ± 55 | 637 ± 95 | >0.05 |
Values are means ± SE; N=9–14. Samples were collected at 15 days after initiating tamoxifen treatment.
Attenuation of MLCK attenuates DOCA-salt-induced hypertension.
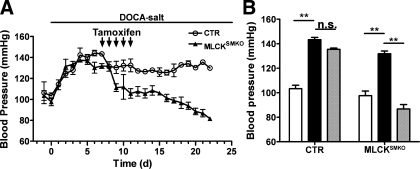
GPCR-mediated signaling pathways differentially regulate physiological and pathological blood pressure through MLCK activation and/or MLCP inactivation, coordinated with other signaling modules (38). Dietary salt intake and a tendency toward salt retention are important in the pathogenesis of hypertension (1, 24). We here analyzed the effect of MLCK knockout on elevated blood pressure induced by DOCA and NaCl to assess the role of MLCK in hypertension. We selected the DOCA-salt model since this animal model is a well-established, clinically relevant model of systemic hypertension. In CTR mice, DOCA-salt treatment produced a robust increase in blood pressure within 5 days that was subsequently maintained at a high level (SBP: elevated from 103 ± 3 mmHg to 143 ± 2 mmHg; Fig. 6). A similar effect was observed in Mlckflox/flox; SM-CreERT2 mice without tamoxifen induction (SBP: from 98 ± 4 mmHg to 132 ± 2 mmHg). These results show salt-induction of hypertension. The elevated blood pressure of CTR mice was transiently and slightly reduced during the period of tamoxifen injections, but then returned to a high level (∼140 mmHg; Fig. 6). Mlckflox/flox; SM-CreERT2 mice displayed a progressive decrease in blood pressure after tamoxifen treatment, reaching values below normotensive levels (SBP decreased from 132 ± 2 mmHg to 87 ± 4 mmHg). Thus attenuation of MLCK expression led to a failure to maintain DOCA-salt-induced hypertension.
Fig. 6.
Attenuation of Mlck abolished salt-induced hypertension. CTR and MLCKSMKO mice received sequential treatments of uninephrectomy and implantation of a DOCA pellet for induction of hypertension. MLCK attenuation was induced by 5 consecutive daily injections of tamoxifen (arrows). A: daily systolic blood pressure was measured with the tail-cuff method for 23 days (d). B: statistical analysis of systolic blood pressure in CTR and MLCKSMKO mice (KO) before (white bars) and 7 days after (black bars) DOCA-salt treatment and 2 wk after induction of DOCA-salt-treated mice with tamoxifen (gray bars). NS, no statistical significance. Values of blood pressure are means ± SE; N=3 (female mice). **P < 0.01.
DISCUSSION
In response to vasoconstrictors, VSM produces robust and prolonged sustained force (30). The established regulatory scheme for this process involves initiation of robust contraction through RLC phosphorylation by Ca2+-dependent MLCK and maintenance of sustained contraction through Ca2+-sensitization by Gα12-Gα13/Rho/Rho kinase-mediated inhibition of MLCP (15, 22). Additionally, Ca2+-independent kinases and thin filament-based regulation have been suggested to play a role in regulation of contraction (6, 7, 13, 19, 27, 29, 32, 35). We found in this study that profound reduction of Ca2+-dependent MLCK impaired both the robust contraction and force maintenance in response to depolarization as well as vasoconstrictors. In addition, RhoA/ROCK phosphorylation of MYPT1 was not affected in arteries from MLCKSMKO mice. Thus MLCK appears to be required both for initiation and maintenance of arterial contraction. RLC phosphorylation was also accordingly inhibited after MLCK attenuation, indicating that MLCK was the predominant kinase for RLC phosphorylation in VSM. Similar to results with intestinal and airway smooth muscles from MLCKSMKO mice, mesenteric arteries retained a small contractile response to both depolarization and agonist that was associated with RLC phosphorylation (11, 40). Although the origins of the contraction are not defined, we consider the possibility that residual MLCK is available in an amount sufficient to phosphorylate a small fraction of RLC leading to initiation of contraction, which would be sustained by inhibition of MLCP. Whereas we previously showed that the fractional activation of a biosensor MLCK is only 0.25 for maximal agonist-induced contraction, the 6.5% remaining kinase in the MLCKSMKO vessels may be sufficient for a limited contraction with enhanced MLCP inhibition (14). The apparent greater sensitivity to the inhibitory effects of the ROCK inhibitor in arteries from MLCKSMKO mice supports this idea. He et al. (11) also showed that the limited residual contraction was calcium dependent, ruling out a role for calcium-independent kinases. Further experiments are required to define the properties of the residual contraction in smooth muscles from these mice.
Basal blood pressure is dependent on multiple factors, including circulating volume, cardiac output, renal function, and hormone-, paracrine-, or myogenic-regulated vascular tone. Our results showed that vasorelaxation mediated by attenuation of MLCK was sufficient to reduce basal blood pressure. The SBP of MLCKSMKO mice fell to 85 mmHg at day 16 after initiating tamoxifen treatment, having begun to drop rapidly over preceding days. MLCKSMKO mice succumb at day 17 from a general failure of smooth muscle contraction that includes paralytic ileus (11). A SBP of 85 mmHg would not be expected to cause lethality (26), so it is unlikely that the animals succumb from severe hypotension.
The importance of the kidney for the pathogenesis of hypertension is well established where the renin-angiotensin system plays a major role (8, 21). Molecular genetic studies have identified mutations in several genes linked to hypertension or hypotension, and these generally act in the physiological pathway that alters net renal salt reabsorption (21). However, it has also been shown that, during the development of hypertension, MLCK expression may be upregulated in blood vessels, implying a functional role for MLCK in progression of hypertension (9). In the present study, we found that attenuation of MLCK was able to abolish salt-induced hypertension. This effect does not appear to be due to primary cardiac and renal dysfunction or secondary compensatory effects after MLCK attenuation, because the cardiac functional parameters and serum renin/ANG II/aldosterone levels in MLCKSMKO mice are not obviously altered. Because MLCK is a common module of the signaling pathway regulating smooth muscle contraction, the conclusion that MLCK supports salt-induced hypertension might be considered for other kinds of hypertension. Additionally, our results emphasize the importance of vascular contractility in hypertension.
Phenotypic comparison among mice with smooth muscle-specific knockouts of Cav1.2, GPCR, and MLCK sheds light on the role of VSM signaling cascades in blood pressure regulation. Deletion of Cav1.2, an essential L-type calcium channel for influx of calcium and subsequent MLCK activation during depolarization-induced contraction, causes reductions in VSM contraction and basal blood pressure similar to the phenotype of MLCK-deficient mice (26). Thus the Cav1.2/MLCK pathway in VSM appears necessary for regulation of basal blood pressure. Smooth muscle-specific knockout of Gαq-Gα11 in mice impairs basal blood pressure maintenance and development of salt-induced hypertension, whereas deletion of Gα12-Gα13 inhibits salt-induced hypertension without affecting basal blood pressure (38, 39). In this study, we found that attenuation of MLCK not only impaired the maintenance of physiological blood pressure but also abolished salt-induced hypertension. Collectively, Gαq-Gα11/MLCK signaling appears essential for physiological maintenance of blood pressure, whereas the Gα12-Gα13/MLCP signaling pathway may be required for salt-induced hypertension in addition to MLCK activation.
In summary, we established an animal model of impaired vascular contractility, demonstrating the essential role of MLCK in VSM contraction and blood pressure regulation in vivo. Because MLCK is central to RLC phosphorylation and myosin cross bridge cycling, its absence impairs contractile responses to a wide variety of agonists with markedly different transduction pathways.
GRANTS
This work was supported by the National Health, Lung, and Blood Institute (HL-26043), National Basic Research Program of China (973) (2009CB942602; 2005CB522501; 2007CB947100), and National Natural Science Funding of China (30570911).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Robert Feil of Interfakultäres Institut für Biochemie Signaltransduktion for providing the SM-CreERT2 mice.
REFERENCES
- 1. Adrogue HJ, Madias NE. Sodium and potassium in the pathogenesis of hypertension. N Engl J Med 356: 1966–1978, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Chang Z, Zhang Q, Feng Q, Xu J, Teng T, Luan Q, Shan C, Hu Y, Hemmings BA, Gao X, Yang Z. Deletion of Akt1 causes heart defects and abnormal cardiomyocyte proliferation. Dev Biol 347: 384–391: [DOI] [PubMed] [Google Scholar]
- 3. Cowley AW, Jr The genetic dissection of essential hypertension. Nat Rev Genet 7: 829–840, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79: 387–423, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Davis MJ, Wu X, Nurkiewicz TR, Kawasaki J, Davis GE, Hill MA, Meininger GA. Integrins and mechanotransduction of the vascular myogenic response. Am J Physiol Heart Circ Physiol 280: H1427–H1433, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Ganitkevich V, Hasse V, Pfitzer G. Ca2+-dependent and Ca2+-independent regulation of smooth muscle contraction. J Muscle Res Cell Motil 23: 47–52, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Gunst SJ, Zhang W. Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am J Physiol Cell Physiol 295: C576–C587, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hall JE. The kidney, hypertension, and obesity. Hypertension 41: 625–633, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Han YJ, Hu WY, Chernaya O, Antic N, Gu L, Gupta M, Piano M, de Lanerolle P. Increased myosin light chain kinase expression in hypertension: regulation by serum response factor via an insertion mutation in the promoter. Mol Biol Cell 17: 4039–4050, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harris DM, Cohn HI, Pesant S, Zhou RH, Eckhart AD. Vascular smooth muscle Gq signaling is involved in high blood pressure in both induced renal and genetic vascular smooth muscle-derived models of hypertension. Am J Physiol Heart Circ Physiol 293: H3072–H3079, 2007 [DOI] [PubMed] [Google Scholar]
- 11. He WQ, Peng YJ, Zhang WC, Lv N, Tang J, Chen C, Zhang CH, Gao S, Chen HQ, Zhi G, Feil R, Kamm KE, Stull JT, Gao X, Zhu MS. Myosin light chain kinase is central to smooth muscle contraction and required for gastrointestinal motility in mice. Gastroenterology 135: 610–620, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hirano K, Hirano M, Kanaide H. Regulation of myosin phosphorylation and myofilament Ca2+ sensitivity in vascular smooth muscle. J Smooth Muscle Res 40: 219–236, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Ihara E, Moffat L, Ostrander J, Walsh MP, MacDonald JA. Characterization of protein kinase pathways responsible for Ca2+ sensitization in rat ileal longitudinal smooth muscle. Am J Physiol Gastrointest Liver Physiol 293: G699–G710, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Isotani E, Zhi G, Lau KS, Huang J, Mizuno Y, Persechini A, Geguchadze R, Kamm KE, Stull JT. Real-time evaluation of myosin light chain kinase activation in smooth muscle tissues from a transgenic calmodulin-biosensor mouse. Proc Natl Acad Sci USA 101: 6279–6284, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ito M, Nakano T, Erdodi F, Hartshorne DJ. Myosin phosphatase: structure, regulation and function. Mol Cell Biochem 259: 197–209, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Kamm KE, Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem 276: 4527–4530, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Kamm KE, Stull JT. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol 25: 593–620, 1985 [DOI] [PubMed] [Google Scholar]
- 18. Karaki H, Ozaki H, Hori M, Mitsui-Saito M, Amano K, Harada K, Miyamoto S, Nakazawa H, Won KJ, Sato K. Calcium movements, distribution, and functions in smooth muscle. Pharmacol Rev 49: 157–230, 1997 [PubMed] [Google Scholar]
- 19. Kim HR, Appel S, Vetterkind S, Gangopadhyay SS, Morgan KG. Smooth muscle signalling pathways in health and disease. J Cell Mol Med 12: 2165–2180, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59: 251–287, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell 104: 545–556, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Matsumura F, Hartshorne DJ. Myosin phosphatase target subunit: many roles in cell function. Biochem Biophys Res Commun 369: 149–156, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mendelsohn ME. In hypertension, the kidney is not always the heart of the matter. J Clin Invest 115: 840–844, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev 85: 679–715, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Michael SK, Surks HK, Wang Y, Zhu Y, Blanton R, Jamnongjit M, Aronovitz M, Baur W, Ohtani K, Wilkerson MK, Bonev AD, Nelson MT, Karas RH, Mendelsohn ME. High blood pressure arising from a defect in vascular function. Proc Natl Acad Sci USA 105: 6702–6707, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moosmang S, Schulla V, Welling A, Feil R, Feil S, Wegener JW, Hofmann F, Klugbauer N. Dominant role of smooth muscle L-type calcium channel Cav1.2 for blood pressure regulation. EMBO J 22: 6027–6034, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morgan KG, Gangopadhyay SS. Invited review: cross-bridge regulation by thin filament-associated proteins. J Appl Physiol 91: 953–962, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res 41: 19–26, 1977 [DOI] [PubMed] [Google Scholar]
- 29. Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol 68: 345–374, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Ogut O, Brozovich FV. Regulation of force in vascular smooth muscle. J Mol Cell Cardiol 35: 347–355, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Sakurada S, Takuwa N, Sugimoto N, Wang Y, Seto M, Sasaki Y, Takuwa Y. Ca2+-dependent activation of Rho and Rho kinase in membrane depolarization-induced and receptor stimulation-induced vascular smooth muscle contraction. Circ Res 93: 548–556, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Somara S, Gilmont RR, Bitar KN. Role of thin-filament regulatory proteins in relaxation of colonic smooth muscle contraction. Am J Physiol Gastrointest Liver Physiol 297: G958–G966, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature 372: 231–236, 1994 [DOI] [PubMed] [Google Scholar]
- 35. Sward K, Mita M, Wilson DP, Deng JT, Susnjar M, Walsh MP. The role of RhoA and Rho-associated kinase in vascular smooth muscle contraction. Curr Hypertens Rep 5: 66–72, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Touyz RM. Molecular and cellular mechanisms regulating vascular function and structure—implications in the pathogenesis of hypertension. Can J Cardiol 16: 1137–1146, 2000 [PubMed] [Google Scholar]
- 37. Whitesall SE, Hoff JB, Vollmer AP, D′Alecy LG. Comparison of simultaneous measurement of mouse systolic arterial blood pressure by radiotelemetry and tail-cuff methods. Am J Physiol Heart Circ Physiol 286: H2408–H2415, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Wirth A, Benyo Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, Orsy P, Horvath B, Maser-Gluth C, Greiner E, Lemmer B, Schutz G, Gutkind JS, Offermanns S. G12--G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med 14: 64–68, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Worzfeld T, Wettschureck N, Offermanns S. G12/G13-mediated signalling in mammalian physiology and disease. Trends Pharmacol Sci 29: 582–589, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Zhang WC, Peng YJ, Zhang GS, He WQ, Qiao YN, Dong YY, Gao YQ, Chen C, Zhang CH, Li W, Shen HH, Ning W, Kamm KE, Stull JT, Gao X, Zhu MS. Myosin light chain kinase is necessary for tonic airway smooth muscle contraction. J Biol Chem 285: 5522–5531, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]