Abstract
With the growth of genetic engineering, mice have become increasingly common as models of human diseases, and this has stimulated the development of techniques to assess the murine cardiovascular system. Our group has developed nonimaging and dedicated Doppler techniques for measuring blood velocity in the large and small peripheral arteries of anesthetized mice. We translated technology originally designed for human vessels for use in smaller mouse vessels at higher heart rates by using higher ultrasonic frequencies, smaller transducers, and higher-speed signal processing. With these methods one can measure cardiac filling and ejection velocities, velocity pulse arrival times for determining pulse wave velocity, peripheral blood velocity and vessel wall motion waveforms, jet velocities for the calculation of the pressure drop across stenoses, and left main coronary velocity for the estimation of coronary flow reserve. These noninvasive methods are convenient and easy to apply, but care must be taken in interpreting measurements due to Doppler sample volume size and angle of incidence. Doppler methods have been used to characterize and evaluate numerous cardiovascular phenotypes in mice and have been particularly useful in evaluating the cardiac and vascular remodeling that occur following transverse aortic constriction. Although duplex ultrasonic echo-Doppler instruments are being applied to mice, dedicated Doppler systems are more suitable for some applications. The magnitudes and waveforms of blood velocities from both cardiac and peripheral sites are similar in mice and humans, such that much of what is learned using Doppler technology in mice may be translated back to humans.
Keywords: peripheral vascular flow, pulse wave velocity, coronary flow reserve, transverse aortic constriction, pressure overload hypertrophy
this review article is part of a collection on Assessing Cardiovascular Function in Mice: New Developments and Methods. Other articles appearing in this collection, as well as a full archive of all Review collections, can be found online at http://ajpheart.physiology.org/.
The ability to alter the genotype of the mouse has produced numerous models for studying cardiovascular physiology and pathophysiology and has generated a need to evaluate the changes that occur in mice during phenotypic development and maturation (8). The genetic manipulations can alter the structure, anatomy, pathology, and physiology of cells, organs, or systems in ways that can be subtle and unpredictable and that often change with time (6, 33). The major problems in adapting existing methods for use in mice relate to the small size and high heart rates that place extreme demands on both spatial and temporal resolution (10). We will focus here on noninvasive ultrasonic Doppler methods, which our group and others have developed and used to evaluate cardiovascular physiology and function in anesthetized mice and which can be used serially to follow changes because of aging, remodeling, and/or the effects of surgical or pharmacological interventions (19, 66). The utility and value of the measurements will be illustrated using the widely used model of transverse aortic constriction (TAC) that was developed for producing pressure-overload cardiac hypertrophy and remodeling (51) but which produces numerous other measurable changes in peripheral vascular mechanics and function (23, 34).
Murine Doppler System
One of the most promising noninvasive methods available for use in mice is ultrasound, and Doppler methods are particularly attractive for sensing blood flow velocity in the heart and major blood vessels (19). Doppler instruments measure blood velocity by detecting the difference in frequency between an emitted burst of ultrasound and the returning echoes from moving blood. In a pulsed-Doppler system the sample volume can be adjusted in depth by varying the time delay between transmission of a short ultrasonic burst and sampling of the returning echoes. The resulting Doppler signal, which is usually in the audible range, is a summation of the Doppler-shifted echoes from many blood cells moving at different velocities, and the spectrum of frequencies represents the distribution of red cell velocities within the sample volume. The following equation relates the Doppler shift frequency (Δf) to the velocity (V) of a reflector:
| (1) |
where fo is the ultrasonic frequency (10 or 20 MHz for use in mice), c is the speed of sound in blood (∼1,540 m/s), and θ is the angle between the sound beam and the true direction of flow or motion. When the sound beam is parallel to the direction of flow (θ is zero), the conversion factors relating velocity to the Doppler shift are 7.5 (cm/s)/kHz at 10 MHz and 3.75 (cm/s)/kHz at 20 MHz (3). The murine pulsed-Doppler system was adapted from nonimaging technology originally designed by our group for use with catheter-tip and implantable probes to measure blood flow in small vessels of humans and larger animals (16). For noninvasive applications in mice, small handheld probes and a high-fidelity spectrum analyzer and signal processor were designed. The probes consist of a ∼1 mm square of 10- or 20-MHz piezoelectric material mounted at the end of a 2-mm-diameter, 10-cm-long stainless steel tube as shown in Fig. 1. A concave epoxy lens is molded to the front face of the piezoelectric element to focus the sound beam at a depth of 3–6 mm. The resulting sample volume is <0.02 μl (0.3 mm diameter × 0.3 mm long) at the focal point. The Doppler signal processor is a computer-based system that was designed in collaboration with Indus Instruments, Webster, TX (46, 49). The current generation system digitizes and stores the audio Doppler signals at 125 kHz from up to four probes, generates and displays a complex fast Fourier transform in real time, and also captures and displays auxiliary signals such as ECG and pressure. Imaging is not included. The Indus software can also calculate a waveform of the spectral peak velocity for export along with the auxiliary signals for further processing by other analysis packages such as Excel. The best velocity resolution is 5 mm/s at 20 MHz, the maximum measurable velocity is 9.3 m/s at 10 MHz, and the best temporal resolution is 0.1 ms (46). Most ultrasonic imaging systems also have spectral Doppler capability, and many of the measurements illustrated here can be made with those systems.
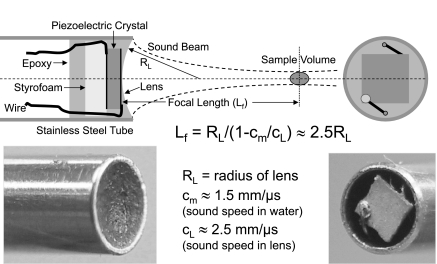
Fig. 1.
Drawing and photos of a 20-MHz Doppler probe designed to measure blood flow velocity noninvasively in mice. The probe consists of a ∼1-mm square piezoelectric crystal, air-backed using Styrofoam, and mounted into a 2-mm-diameter stainless-steel tube with epoxy. A concave epoxy lens is molded to the front face to focus the sound beam at a depth of ∼4 mm. A 10-MHz probe uses a 3-mm tube and is focused at ∼6 mm.
Doppler Displacement
Ultrasonic Doppler methods can also be used to measure cardiac motion (17, 31, 55) and artery diameter pulsation (20) in mice, which when combined with pressure provide an index of vessel compliance (29). Doppler devices actually measure the phase of blood echoes that advance (or recede) continuously to generate the Doppler shift frequency (3). Echoes from slower moving solid structures such as blood vessel walls that move back and forth generate phase signals which advance and recede with each cardiac cycle. One cycle of echo phase (360°) corresponds to reflector motion of 1/2 wavelength (because of the 2-way path) or about 38 μm at 20 MHz (18). Under optimal conditions, Doppler methods can resolve about 1° of phase or 0.1 μm of tissue motion and can display cardiac or vessel wall motion continuously throughout the cardiac cycle (20).
Examples and Applications
Although spectral (57), tissue (31, 45), color (57), and power (32) Doppler modalities are available and have been used to assess cardiovascular function and flow in mice, we will concentrate here on spectral Doppler measurements of blood flow velocity and vessel wall motion in peripheral vessels of anesthetized mice. Blood flow velocity can be measured from the left ventricular inflow and outflow tracts and from peripheral arteries smaller than ∼100 μm diameter. The protocols from which the following examples were obtained were approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine and conform with the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996).
An example of cardiac Doppler signals from a mouse using a 10-MHz transducer placed at the base of the sternum and pointed toward the heart is shown to the right in Fig. 2 along with the ECG. A photo of a mouse on an ECG/heater board is shown to the left. From this approach cardiac timing and several indexes of left ventricular systolic function (peak velocity and acceleration), diastolic function (peak-early and late-filling velocities and their ratio), and the Tei index (55) can be measured (59). In this example, which was done to illustrate the technique, the relatively large sample volume included both the inflow and outflow tracts of the left ventricle. With a focused probe and proper positioning, signals from the inflow tract and the aortic root can be isolated. Although Doppler signals can provide measures of cardiac timing and function (41, 55), imaging of cardiac structures and their motion is often required to put the velocity indexes into proper context in the estimation of ventricular function (57).
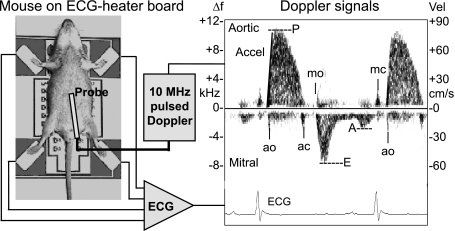
Fig. 2.
Illustration of the setup used to make noninvasive ECG and Doppler measurements showing an anesthetized mouse taped to electrodes on a temperature-controlled circuit board, Doppler and ECG signal processors, and a display of ECG and Doppler signals from the left ventricular inflow and outflow tracts. The 23 × 30-cm-printed circuit board contains 4 stainless-steel ECG electrodes, an array of 50 surface-mount resistors, a temperature sensor, a large ground plane, electrical connections to an ECG amplifier, and a temperature controller. Vel, velocity; Δf, change in frequency. Labeled on the Doppler tracing are the opening (o) and closing (c) of the mitral (m) and aortic (a) valves, peak ejection velocity (P) and acceleration (Accel), and peak-early (E) and late-filling (A) velocities. From these signals, one can obtain accurate timing of cardiac events such as preejection time, filling and ejection times, and isovolumic contraction and relaxation times as indexes of systolic and diastolic ventricular function [from Hartley et al. (25)].
With the use of a 20-MHz probe, Doppler signals can be obtained from many peripheral arterial sites as shown in Fig. 3. All of the signals except for coronary flow were obtained from the same mouse. By the measurement of peak and mean velocity and the evaluation of the shapes of the waveforms from several arterial sites, changes in regional peripheral vascular impedance can be detected (49). Other groups have used duplex echo-Doppler systems to measure spectral Doppler velocity signals from the aortic arch (12), the renal artery (4, 58), the hepatic artery (5), the coronary artery (54), and the umbilical artery (38) of anesthetized mice.
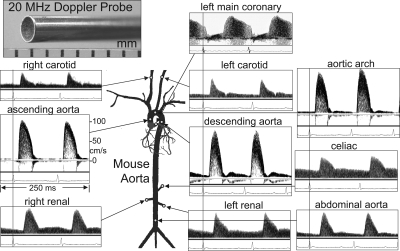
Fig. 3.
Doppler velocity signals from several peripheral arterial sites in an anesthetized mouse using a 2-mm-diameter 20-MHz probe. All signals were obtained with the mouse supine except for the renal signals, which were obtained with the mouse prone and the probe placed lateral to the spine. The coronary signals were obtained later from a second mouse. The vertical lines correspond to the R wave of the ECG and are used to measure velocity pulse arrival times for calculation of pulse wave velocity (PWV) [from Hartley, et al. (25)].
Pulse Wave Velocity
One of the applications for peripheral measurements of blood velocity is the determination of arterial pulse wave velocity (PWV) as an index of arterial stiffness (2, 68). Stiffer vessels propagate pressure and velocity waves faster than more compliant vessels. The vertical lines in Fig. 3 are aligned with the R wave of the ECG, and it can be seen that the time to the upstroke of velocity increases in proportion to the distance from the heart. By recording velocity signals from two sites, separated by a known distance, and measuring the difference in pulse arrival times, one can determine the pulse transit time and calculate PWV (21, 24). Velocity can be measured sequentially from the two sites with a high-fidelity ECG used as a timing reference, or velocity can be measured from two sites simultaneously using two Doppler probes (48). Typically, we determine aortic PWV from velocities measured in the aortic arch and in the descending aorta about 4 cm distal. Aortic arch velocity signals are obtained by placing a focused 20-MHz Doppler probe to the right of the upper sternum and angling the sound beam down and to the left to align with the direction of the aortic arch and looking for “aortic shape” velocity signals moving away from the probe. The sample volume depth is typically set to ∼3.0 mm. After recording aortic arch signals, the probe position is marked and another mark is placed 4 cm distal on the abdomen. At this location the probe is angled toward the head and moved laterally looking for strong signals moving toward the probe. After the signals are recorded and stored, the fast Fourier transform display is adjusted to maximize temporal resolution (0.1 ms) and the time delay from the R wave of the ECG to the upstroke of velocity is measured and averaged over several cardiac cycles at each site. Aortic PWV (on the order of 4 m/s in mice) (24) is then calculated by dividing the distance (4 cm) by the difference in pulse arrival times (on the order of 10 ms) at each site. In previous studies, we have found that apolipoprotein E-deficient (ApoE−/−) mice have elevated PWV compared with age-matched wild-type mice (21, 62), but in other models the changes are not always apparent during rest. For instance, in α-smooth muscle actin knockout (α-SMA−/−) mice (56), the resting PWV is only slightly lower than in wild-type mice. However, the response to a bolus intravenous injection of phenylephrine, which doubles PWV (from 4.6 to 9.9 m/s) in wild-type mice, is absent in α-SMA−/− mice (49). In contrast, matrix GLA protein knockout mice (37), which have calcified arteries, have significantly elevated PWV (10.4 m/s) even at rest (25).
Practical considerations and pitfalls.
When PWV is calculated, the ability to accurately time the upstroke of velocity with respect to the R wave of the ECG is more important than measuring the magnitude of velocity, and this requires clean waveforms for both velocity and ECG. A major source of error is in measuring the distance between the measurement sites along the curved path of the aorta. This requires careful measurements of the probe locations along the skin surface, an estimate of the sample volume positions based on the probe angles and sample volume depths, and an estimate of the curved path length. When sequential velocity measurements with a single probe are used, any variability in the preejection time or changes in PWV between the velocity measurements will also add to the measurement error. Consistency in measurement sites and probe orientations minimizes the variability in PWV measurements and maximizes the ability to detect differences in groups of mice.
Pressure Drop at a Stenosis
A common method for stressing the cardiovascular system in wild-type and mutant mice is a chronic pressure overload model produced by TAC between the origins of the innominate and left carotid arteries (51). This model produces cardiac hypertrophy and heart failure in 1–4 wk, but it is difficult to measure the degree of stenosis or the pressure drop or to predict the resulting hypertrophy. At the time of death, one can cannulate (and occlude) both carotid arteries to measure the pressure difference, but this act is expected to alter flow (and the pressure drop) significantly. We have found that it is possible to measure the velocity of the jet produced by the stenosis and employ the simplified Bernoulli equation developed by cardiologists (26) to estimate the pressure drop:
| (2) |
where ΔP is the pressure drop (in mmHg) and V is the peak jet velocity (in m/s). We validated this equation in mice as illustrated in Fig. 4 by cannulating both carotid arteries in five mice after TAC and measuring the pressures and the jet velocities as the pressure drops and flows were varied over a large range (35). The mouse shown in Fig. 4 had an arrhythmia such that the jet velocities and pressure drops changed with each cardiac cycle and each cardiac cycle produced one data point on the graph. There is an excellent agreement (R2 = 0.94) between the measured and calculated pressure drops.
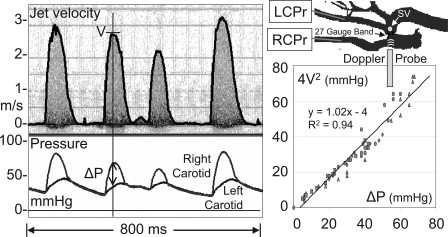
Fig. 4.
Velocity signals from the high-velocity jet in a mouse with a transverse aortic constriction (TAC) along with pressure signals from the right (RCPr) and left (LCPr) carotid arteries. The mouse shown had an arrhythmia such that the jet velocity (V) and the pressure drop (ΔP) varied with each cardiac cycle. The graph to the right shows data from the 5 pulses shown (including the very small one near the center) plus data from 4 other mice where the velocity was varied over a large range. SV, sample volume. As shown, the maximum jet velocity (in m/s) measured noninvasively can be used with the simplified Bernoulli equation (4V2) to estimate the maximum pressure drop (in mmHg) across the aortic constriction [from Li et al. (35)].
Practical considerations and pitfalls.
When the pressure drop across a stenosis estimated, it is important to align the sound beam parallel to the direction of flow and to search for the maximum velocity signal that is usually found just distal to the stenosis. The Doppler system must be able to resolve velocities as high as 5 m/s, and because the flow distal to the stenosis is often turbulent with velocity components traveling in many directions at once, it is not advisable to use a nonparallel probe orientation with angle correction to estimate the peak jet velocity.
Coronary Flow Reserve
As shown in Fig. 3, it is also possible to measure left main coronary flow velocity in mice (13, 15, 52, 53, 66). However, coronary flow velocity by itself is not as useful or diagnostic as coronary flow reserve (CFR), which is the ratio of hyperemic flow during maximal vasodilation to baseline resting flow (H/B) (7, 14, 30). In humans and other animals, adenosine administered intravenously is often used as a maximal coronary vasodilator (39, 67). While we were attempting to develop methods to administer adenosine effectively to mice, we discovered that isoflurane, which we use as an anesthetic, had an effect on coronary flow similar to that of adenosine (22, 50) but with a smaller change in heart rate. Our protocol for measuring CFR in mice is to place the mouse anesthetized with 2% isoflurane in oxygen on the procedure board to which a micromanipulator has been attached to hold the Doppler probe. The Doppler probe is placed horizontally to the left of the sternum with the sample volume depth set at 2 to 3 mm and the sound beam pointing toward the aortic root and the origin of the left main coronary artery (22). In the absence of imaging, coronary velocity signals are identified as flow moving toward the probe during diastole with a characteristic shape. The probe holder is then clamped into position, and the sample volume and probe positions are adjusted to maximize the magnitude, intensity, and stability of the signal. The isoflurane level is then set to 1% to obtain and record a baseline level of coronary velocity and then increased to 2.5% to obtain a hyperemic level of coronary velocity (9). The sample volume position is reoptimized at each level of anesthesia, and it typically takes about 2 min for coronary velocity to stabilize at each level.
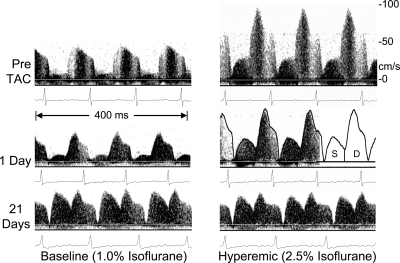
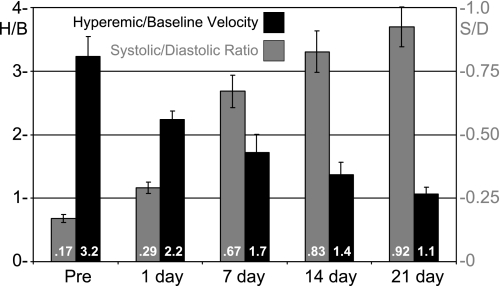
Using the above procedure, we estimated left main CFR in 10 mice before and at several time points for 21 days after TAC (23). Figure 5 shows waveforms at baseline and at hyperemia from one mouse before, 1 day after, and 21 days after TAC. In addition to the expected decrease in the amount of hyperemia at 21 days, there is also a significant increase in the amount of flow that occurs during systole. We calculated the hyperemic/baseline (H/B) ratio from the temporal average spectral peak velocity at baseline and hyperemia and the systolic-to-diastolic ratio (S/D) from the areas under the systolic and diastolic parts of the spectral peak velocity as shown in Fig. 5. Figure 6 shows a summary of H/B and S/D at baseline for all mice at the five time points shown. We found that CFR as estimated by H/B progressively decreases as the heart adapts and hypertrophies after TAC and that the amount of flow during systole progressively increases after TAC. After 21 days, CFR is nearly abolished (1.1), and the amount of left main coronary flow occurring during systole is nearly equal to that occurring during diastole (S/D = 0.92).
Fig. 5.
Baseline and hyperemic left main coronary velocity signals taken noninvasively from a mouse before, 1 day, and 21 days after TAC. The relative contributions of systolic (S) and diastolic (D) flow vary between baseline and hyperemia and with time after constriction [from Hartley et al. (23)].
Fig. 6.
Hyperemic/baseline (H/B) coronary velocity and systolic/diastolic (S/D) area ratios at 5 time points before and after TAC. H/B is an index of coronary flow reserve and is progressively reduced and nearly abolished (1.1) after 21 days of aortic constriction. Similarly, the amount of coronary flow that occurs during systole increases progressively and significantly after aortic constriction.
We have found that left main CFR as estimated by isoflurane increases from 2.4 at 6 wk to 3.6 at 2 yr of age but is reduced from 3.6 to 2.5 in ApoE−/− (atherosclerotic) mice (22), from 3.2 to 2.0 in mice with chronic left anterior descending coronary artery occlusions, and from 3.0 to 1.4 in mice with chronic infusions of angiotensin. The systolic component of coronary flow is more difficult to measure and is often ignored by coronary physiologists because systolic flow cannot perfuse actively contracting myocardium (54, 61). Thus the significant increase in systolic coronary flow following TAC may indicate a redistribution of flow away from contracting myocardium or from endocardium to epicardium as the heart hypertrophies (27, 28, 60). CFR is relatively easy to measure in mice, appears to be reduced by most forms of heart disease, and could be used in place of ejection fraction as an index of global cardiac reserve in mice. The coronary S/D ratio, which does not require the use of a vasodilator, may also be a useful index of myocardial perfusion status.
Practical considerations and pitfalls.
The Doppler sample volume is slightly larger than the left main coronary artery, but because of cardiac motion, the spectral intensity often varies slightly during the cardiac cycle as shown in Fig. 3. In the absence of imaging, the proper probe position is verified by temporarily advancing the sample volume to check for the presence of aortic velocity signals. Proper measurements of CFR require that the baseline flow be “normal,” that the coronary vasodilator be maximal with no other cardiovascular effects, and that the diameter of the proximal coronary artery where velocity is sensed be constant. It is unlikely that any of these conditions are actually met using isoflurane or even adenosine (67), but it is also unlikely for coronary flow to go above maximal or below minimal values during estimations of CFR. The result would be an underestimation of CFR. Nevertheless, our estimates of CFR using isoflurane are similar to those reported in humans (39) and are higher than those reported by several other groups in mice using adenosine as a coronary vasodilator and isoflurane as an anesthetic agent (52, 67).
Carotid Velocities Diameter Pulsations
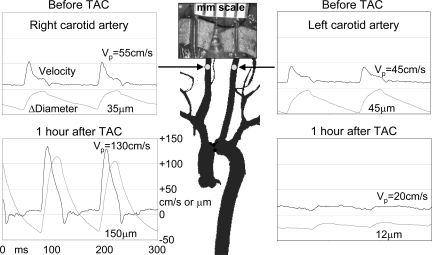
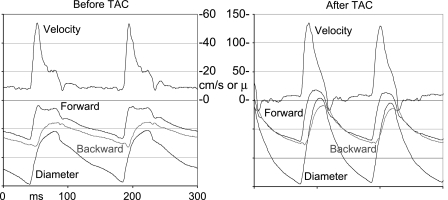
We find that the pulsations in flow velocity are increased in the right carotid artery proximal to the stenosis and decreased in the left carotid artery distal to the stenosis after TAC as shown in Fig. 7. Also shown in Fig. 7 are carotid artery diameter signals measured before and after TAC using the same probe held at a 60° angle to the vessel (20). The signals were obtained using a modified Doppler module with two additional sample volumes that were designed to measure tissue displacement rather than blood velocity and that could be placed over the near and far vessel walls to measure their motion during the cardiac cycle. The diameter waveforms shown were calculated by subtracting the displacement signals from the near and far vessel walls. Before TAC, the peak velocities and diameter pulsations are similar in the right and left carotid arteries. One hour after TAC, both the blood velocity and diameter pulsations are increased in the right carotid artery and decreased in the left carotid artery. However, the mean velocities in each vessel before and after TAC are not significantly different after 1 h. In a normal mouse (or human) the total arterial compliance must be sufficient to absorb one stroke volume per cardiac cycle or the heart cannot eject blood fully. After TAC, this task must be accomplished by the ascending aorta and the innominate artery and its branches, which includes the right carotid artery either by increasing diameter pulsations or by increasing heart rate, which decreases stroke volume. The average diameter of the right carotid artery also increases as shown in Fig. 7 such that the fourfold increase in diameter pulsations operating on a larger vessel results in a significant increase (∼8×) in volume pulsations. The ability of the vessels originating proximal to the TAC to remodel and increase compliance is one of the reasons that mice can survive TAC but cannot survive a more proximal aortic constriction (51).
Fig. 7.
Velocity (in cm/s) and diameter change (in μm) signals taken noninvasively from the right and left carotid arteries of a mouse before and 1 h after TAC. After TAC, the pulsations in both velocity and diameter are increased in the right carotid artery proximal to the band and decreased in the left carotid artery distal to the band. The photo taken at death 2 wk later shows dilation of the right carotid artery because of increased pressure and/or wall shear stress. VP, peak velocity.
Practical considerations and pitfalls.
When using tissue Doppler to measure diameter pulsations, only the pulsations and not the absolute diameter can be sensed with precision (∼0.1 μm) (20). The absolute diameter can be estimated from the distance between the sample volumes positioned over the near and far artery walls, but the accuracy is poor given the ∼300 μm length of the sample volumes. The absolute diameter and the magnitude, but not a high-fidelity waveform, of arterial pulsations can be estimated with a high-resolution (VisualSonics) imaging system (68).
Calculation of Vascular Indexes
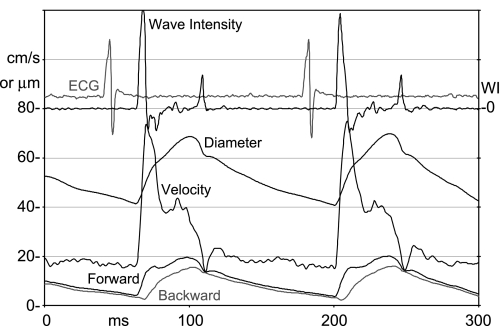
In many of the murine models we use, including TAC, PWV, vascular mechanics, and vascular impedance are altered in measurable ways, and velocity and diameter measurements allow for the calculation of several indexes of vascular function (40). When the heart generates pressure and ejects blood, the impulse created by the heart propagates into the arterial system as a forward-traveling wave, and whenever this wave passes a branch or other discontinuity in vascular impedance, part of the wave is reflected back toward the heart (65). If there were no reflections, pressure and flow waveforms would be identical, related by the characteristic impedance of the vessel, and travel at the PWV. The pressure measured at any site is the sum of forward and backward pressure waves, and the flow measured at any site is the difference in the forward and backward flow waves. This gives us enough information to calculate forward and backward waves from the measured pressure and flow waves and the characteristic impedance of the vessel (65), and if we are allowed to use diameter in place of pressure and velocity in place of flow, this can be done from the measured Doppler signals (43). Figure 8 shows carotid velocity and diameter signals and calculated wave intensity (43) and forward and backward waves (65) from the common carotid artery of a mouse. Wave intensity is calculated by multiplying the derivatives of pressure and flow (or diameter and velocity). Although the units are wrong when using diameter and velocity, the shapes of the waves and their relative magnitudes are largely correct (43). The first positive peak in intensity is the forward-traveling acceleration wave generated at the opening of the aortic valve, and the second positive peak is the forward-traveling deceleration wave generated by the closing of the aortic valve. The first negative intensity waves occur because of reflected waves that decelerate flow while pressure is still increasing. The shapes of the velocity, diameter, and wave intensity signals from mice as shown in Fig. 8 look remarkably similar to those measured in humans using similar methods (43).
Fig. 8.
Doppler velocity and diameter change signals from the carotid artery of a mouse showing some of the sophisticated analyses that can be done. Wave intensity (WI) is calculated from the product of the derivatives of pressure and flow (or diameter and velocity) and indicates the net magnitude and direction of energy transfer along the vessel. Forward and backward-traveling waves are calculated from the measured pressure and flow (or diameter and velocity) waves and can be used to estimate the magnitude and timing of wave reflections.
The backward wave is the sum of the reflections from all sites including the terminal capillaries distal to the measurements site (65). The initial part of the forward wave is generated by the heart, but the timing of the second peak during late systole suggests that it is caused by the reflected wave from the descending aorta returning to the heart and then traveling forward in the carotid artery. We hypothesized that TAC, which adds significant resistance, would reduce or eliminate the second peak in the forward wave. Figure 9 shows diameter, velocity, and forward and backward waves in the right carotid artery of a mouse before and after TAC. Note that the scale on the right after TAC is 2.5 times the scale on the left before TAC and that the second peak in the forward wave before TAC is indeed eliminated after TAC. Although the ratio of the magnitudes of backward to forward waves are similar before and after TAC, the delay to the start of the backward wave is a little shorter after TAC, suggesting that PWV is higher. We have not attempted to do a full analysis of the changes in vascular indexes before and after TAC or in mice with other vascular abnormalities, but these examples show the potential of Doppler methodology in quantifying peripheral vascular mechanics in mice and the similarity of the signals to those recorded by others from comparable but larger vessels in humans.
Fig. 9.
Velocity, diameter change, and forward and backward waves from the right carotid artery of a mouse before and after TAC. Note that the scales are different before and after constriction and that the second peak seen in the forward wave is missing after constriction.
General Discussion
We and others typically use the spectral peak or maximum velocity waveform because it is the most robust parameter in the Doppler signal (11). Thus the measured peak velocity represents the highest component of velocity within the sample volume in the direction of the sound beam. If one knows the true direction of flow (only reliable in a straight, unbranching vessel) and the direction of the sound beam, angle correction can be used to estimate the true velocity.
Where possible, we always align the Doppler transducer such that the sound beam is parallel to the axis of the vessel (and the normal direction of flow) and the Doppler angle (θ) is close zero. At 0°, a 10% error in angle produces a 1.6% error in velocity, whereas at 60°, a 10% angle error produces a 32% error in velocity. When the spectral Doppler mode of most ultrasonic imaging systems is used, the larger transducer size and configuration often prevent an optimal alignment of the sound beam with the direction of flow, and a larger Doppler angle is necessary. When velocity ratios are being measured (as in estimating CFR), angle errors will cancel, but when absolute values of velocity are important (as in estimating the pressure drop at a stenosis) or where the flow may be turbulent and/or in an unknown direction, angle correction must be used with caution. In our experience, angle correction often results in an overestimation of the true velocity (44).
Because the distance required for a laminar velocity profile to develop (entrance length) is on the order of 2.0 mm in mice (42), the velocity profile becomes fully developed a short distance along the aorta, and the measured peak velocity is about twice the luminal average velocity (47). In humans and larger animals, the entrance length is much longer and the velocity profile in central vessels is more blunt (11, 36, 42) such that the peak velocity is closer to the average velocity. These differences are important to recognize if one attempts to calculate the luminal average velocity or volume flow from the measured (and often angle corrected) peak velocity and diameter. Because the blood velocities are similar and the vessel diameters are smaller, the calculated wall shear stresses in most arteries are much higher in mice than in humans (12).
The size of the Doppler sample volume where velocity is sensed is also important in interpreting signals from flowing blood or moving tissue (3). Most ultrasonic imaging systems have Doppler sample volumes that are adjustable in the axial direction along the sound beam. The lateral dimensions are fixed by the transducer geometry, beam forming, and focusing and are difficult to determine with precision. If too small, the sample volume may not remain centered on a moving vessel of interest (such as a coronary artery) throughout the cardiac cycle. If too large, the sample volume may include motion from vessel walls or other structures that are not of interest and that may generate interfering signals. However, the use of the spectral peak in analyzing Doppler velocities allows flow to be sensed in small vessels embedded in slowly moving muscle (coronary arteries) or other tissues and mitigates many of these potential problems.
Duplex Doppler/Imaging Systems
Many of the peripheral vascular Doppler measurements illustrated here can be made with clinical or duplex echo-Doppler systems (4, 5, 52) or with one of the VisualSonics mouse ultrasonic systems (1, 12, 58). However, the optimal ultrasonic frequencies, probe configurations, and display modalities for imaging and Doppler are different such that some trade-offs are necessary with duplex systems. The major limitations when making Doppler measurements with duplex systems are the footprint of the probe that limits access at the optimal Doppler angle to some sites (left main coronary arteries), limited temporal resolution (needed for PWV and cardiac timing), and limited resolution of higher velocities (needed for estimating the pressure drop at a stenosis). For instance, we have measured jet velocities as high as 4.5 m/s in TAC mice and coronary velocities as high as 3.6 m/s in atherosclerotic mice during hyperemia (21). From Eq. 1, the maximum velocity limit using a 30-MHz probe at a pulse repetition frequency of 120 kHz is ∼3 m/s. Imaging is important and necessary when measuring velocity in cardiac chambers or near the heart where there are many sites with complex velocity patterns, but in locations where imaging is not required, dedicated Doppler systems can employ more optimal ultrasonic parameters and smaller transducers.
In addition to the spectral Doppler methods illustrated here to measure peripheral and cardiac blood velocities in mice, other Doppler modalities are also being applied to mice (57). Tissue Doppler and strain imaging have been used to measure cardiac timing (55) and ventricular function (31), color Doppler is used to locate the direction and timing of flow across valves and in cardiac chambers (57), and power Doppler with contrast agents is used to assess perfusion in organs such as the brain and kidney (58) and in tumors (32).
Summary
We have shown that high-resolution velocity and diameter measurements can be made noninvasively and serially from large and small peripheral vessels in mice. An advantage to measuring blood velocity rather than volume flow is that the values do not have to be normalized to body size (10, 21). Because the lengths and time constants of the vascular systems scale with the cardiac period (63, 64), reflected waves return at similar times during the cardiac cycle, and the magnitudes and waveforms of pressure and velocity in mice are almost indistinguishable from signals recorded from similar sites in humans and other species (42). Aortic PWV, percent arterial diameter pulsation, ejection fraction, and CFR also have similar values, and many of the indexes (59) used to evaluate cardiac and vascular function in mice are derived from human indexes. Importantly, the use of familiar ultrasonic Doppler methods and the similarity of velocity, pressure, and diameter waveforms may permit much of what we learn about blood flow and vascular mechanics in mice to be translated directly to humans.
GRANTS
This work was supported in part by National Institutes of Health Grants HL-22512, HL-42550, HL-57068, HL-52364, HL-42550, HL-42267, AG-15568, HL-42313, HL-13870, HL-89792, and AG-13251.
DISCLOSURES
Drs. C. J. Hartley and A. K. Reddy collaborated extensively with Dr. S. Madala of Indus Instruments on the design of the Doppler signal processing, and much of the technology described in this review is available commercially from Indus Instruments (Webster, TX). Indus also supplies procedure boards, temperature controllers, and ECG amplifiers to VisualSonics for use with mouse ultrasonic imaging systems.
REFERENCES
- 1. Aristizabal O, Christopher DA, Foster FS, Turnbull DH. 40-MHz echocardiographic scanner for cardiovascular assessment of mouse embryos. Ultrasound Med Biol 24: 1407–1417, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Asmar R, Benetos A, Topouchian J, Laurent P, Pannier B, Brisac AM, Target R, Levy BI. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical studies. Hypertension 26: 485–490, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Baker DW. Pulsed ultrasonic Doppler blood flow sensing. IEEE Trans Sonics Ultrason SU-17: 170–185, 1970 [Google Scholar]
- 4. Bonnin P, Sabaa N, Flamant M, Debbabi H, Tharaux PL. Ultrasound imaging of renal vaso-occlusive events in transgenic sickle mice exposed to hypoxic stress. Ultrasound Med Biol 34: 1076–1084, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Bonnin P, Villemain A, Vincent F, Debbabi H, Silvestre JS, Contreses JO, Levy BI, Tobelem G, Dupuy E. Ultrasonic assessement of hepatic blood flow as a marker of mouse hepatocarcinoma. Ultrasound Med Biol 33: 561–570, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Chien KR. Genes and physiology: molecular physiology in genetically engineered animals. J Clin Invest 97: 901–909, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cole JS, Hartley CJ. The pulsed Doppler coronary artery catheter: preliminary report of a new technique for measuring rapid changes in coronary artery flow velocity in man. Circulation 56: 18–25, 1977 [DOI] [PubMed] [Google Scholar]
- 8. Cowley AW., Jr Genomics to physiology and beyond: how do we get there. Physiologist 40: 205–211, 1997 [PubMed] [Google Scholar]
- 9. Crystal GJ, Czinn EA, Silver JM, Ramez SM. Coronary vasodilation by isoflurane: abrupt versus gradual administration. Anesthesiology 82: 542–549, 1995 [DOI] [PubMed] [Google Scholar]
- 10. Dawson TH. Engineering Design of the Cardiovascular System of Mammals. Englewood Cliffs: Prentice Hall, 1991 [Google Scholar]
- 11. Evans DH, McDicken WN, Skidmore R, Woodcock JP. Doppler Ultrasound: Physics, Instrumentation, and Clinical Applications. New York: John Wiley & Sons, 1989 [Google Scholar]
- 12. Fientuch A, Ruengsakularach P, Lin A, Zhang J, Bishop J, Davidson L, Courtman D, Foster FS, Steinman DA, Henkelman RM, Ethier CR. Hemodynamics in the mouse aortic arch as assessed by MRI, ultrasound, and numerical modeling. Am J Physiol Heart Circ Physiol 292: H884–H892, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Gan LM, Wikstrom J, Bergstrom G, Wandt B. Non-invasive imaging of coronary arteries using high-resolution echocardiography. Scand Cardiovasc J 38: 121–126, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Gould LK, Lipscomb K, Hamilton GW. Physiologic basis for assessing critical coronary stenosis: Instantaneous flow response and regional distribution during coronary hyperemia as measures of coronary flow reserve. Am J Cardiol 33: 87–94, 1974 [DOI] [PubMed] [Google Scholar]
- 15. Gronros J, Wikstrom J, Hagg U, Wandt B, Gan LM. Proximal to middle left coronary artery flow velocity ratio, as assessed using color Doppler echocardiography, predicts coronary artery atherosclerosis in mice. Arterioscler Thromb Vasc Biol 26: 1126–1131, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Hartley CJ, Cole JS. An ultrasonic pulsed Doppler system for measuring blood flow in small vessels. J Appl Physiol 37: 626–629, 1974 [DOI] [PubMed] [Google Scholar]
- 17. Hartley CJ, Latson LA, Michael LH, Seidel CL, Lewis RM, Entman ML. Doppler measurement of myocardial thickening with a single epicardial transducer. Am J Physiol Heart Circ Physiol 245: H1066–H1072, 1983 [DOI] [PubMed] [Google Scholar]
- 18. Hartley CJ, Litowitz H, Rabinovitz RS, Zhu WX, Chelly JE, Michael LH, Bolli R. An ultrasonic method for measuring tissue displacement: technical details and validation for measuring myocardial thickening. IEEE Trans Biomed Eng 38: 735–747, 1991 [DOI] [PubMed] [Google Scholar]
- 19. Hartley CJ, Michael LH, Entman ML. Noninvasive measurement of ascending aortic blood velocity in mice. Am J Physiol Heart Circ Physiol 268: H499–H505, 1995 [DOI] [PubMed] [Google Scholar]
- 20. Hartley CJ, Reddy AK, Madala S, Entman ML, Michael LH, Taffet GE. Noninvasive ultrasonic measurement of arterial wall motion in mice. Am J Physiol Heart Circ Physiol 287: H1426–H1432, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Hartley CJ, Reddy AK, Madala S, Martin-McNulty B, Vergona R, Sullivan ME, Halks-Miller M, Taffet GE, Michael LH, Entman ML, Wang YX. Hemodynamic changes in apolipoprotein E-knockout mice. Am J Physiol Heart Circ Physiol 279: H2326–H2334, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Hartley CJ, Reddy AK, Madala S, Michael LH, Entman ML, Taffet GE. Effects of isoflurane on coronary blood flow velocity in young, old, and ApoE−/− mice measured by Doppler ultrasound. Ultrasound Med Biol 33: 512–521, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hartley CJ, Reddy AK, Madala S, Michael LH, Entman ML, Taffet GE. Doppler estimation of reduced coronary flow reserve in mice with pressure overload cardiac hypertrophy. Ultrasound Med Biol 34: 892–901, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hartley CJ, Taffet GE, Michael LH, Pham TT, Entman ML. Noninvasive determination of pulse-wave velocity in mice. Am J Physiol Heart Circ Physiol 273: H494–H500, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Hartley CJ, Taffet GE, Reddy AK, Entman ML, Michael LH. Noninvasive cardiovascular phenotyping in mice. ILAR J 43: 147–158, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Hatle L, Brubakk A, Tromsdal A, Angelsen BA. Noninvasive assessment of pressure drop in mitral stenosis by Doppler ultrasound. Br Heart J 40: 131–140, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hess DS, Bache RJ. Transmural distribution of myocardial blood flow during systole in the awake dog. Circ Res 38: 5–15, 1976 [DOI] [PubMed] [Google Scholar]
- 28. Hittinger L, Mirsky I, Shen YT, Patrick TA, Bishop SP, Vatner SF. Hemodynamic mechanisms responsible for reduced subendocardial coronary reserve in dogs with severe left ventricular hypertrophy. Circulation 92: 978–986, 1995 [DOI] [PubMed] [Google Scholar]
- 29. Hoeks AP, Brands PJ, Willigers JM, Reneman RS. Non-invasive measurement of mechanical properties of arteries in health and disease. Proc Inst Mech Eng H 213: 195–202, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Hoffman JI. Maximal coronary flow and the concept of coronary vascular reserve. Circulation 70: 153–159, 1984 [DOI] [PubMed] [Google Scholar]
- 31. Jassal DS, Han SY, Hans C, Sharma A, Fang T, Ahmadie R, Lytwyn M, Walker JR, Bhalla RS, Czarnecki A, Moussa T, Singal PK. Utility of tissue Doppler and strain rate imaging in the early detection of trastuzumab and anthracycline mediated cardiomyopathy. J Am Soc Echocardiogr 22: 418–424, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Jugold M, Palmowski M, Huppert J, Woenne EC, Mueller MM, Semmler W, Kiessling F. Volumetric high-frequency Doppler ultrasound enables the assessment of early antiangiogenic therapy effects on tumor xenografts in nude mice. Eur Radiol 18: 753–758, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Kass DA, Hare JM, Georgakopoulos D. Murine cardiac function: a cautionary tail. Circ Res 82: 519–522, 1998 [DOI] [PubMed] [Google Scholar]
- 34. Li YH, Reddy AK, Ochoa LN, Pham TT, Hartley CJ, Michael LH, Entman ML, Taffet GE. Effect of age on peripheral vascular response to transverse aortic banding in mice. J Gerontol Biol Sci 58A: 895–899, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Li YH, Reddy AK, Taffet GE, Michael LH, Entman ML, Hartley CJ. Doppler evaluation of peripheral vascular adaptations to transverse aortic banding in mice. Ultrasound Med Biol 29: 1281–1289, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Lucas CL, Keagy BA, Hsiao HS, Johnson TA, Henry GW, Wilcox BR. The velocity profile in the canine ascending aorta and its effects on the accuracy of pulsed Doppler determinations of mean blood velocity. Cardiovasc Res 18: 282–293, 1985 [DOI] [PubMed] [Google Scholar]
- 37. Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 386: 78–81, 1997 [DOI] [PubMed] [Google Scholar]
- 38. Mai W, Le Floc'h J, Vray D, Samarut J, Barthez P, Janier M. Evaluation of cardiovascular flow characteristics in the 129Sv mouse fetus using color-Doppler-guided spectral Doppler ultrasound. Vet Radiol Ultrasound 45: 568–573, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Marcus ML. The Coronary Circulation in Health and Disease. New York: McGraw-Hill, 1983 [Google Scholar]
- 40. Meinders JM, Hoeks AP. Simultaneous assessment of diameter and pressure waveforms in the carotid artery. Ultrasound Med Biol 30: 147–154, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Michael LH, Ballantyne CM, Zachariah JP, Gould KE, Pocius JS, Taffet GE, Hartley CJ, Pham TT, Daniel SL, Funk E, Entman ML. Myocardial infarction and remodeling in mice: effect of reperfusion. Am J Physiol Heart Circ Physiol 277: H660–H668, 1999 [DOI] [PubMed] [Google Scholar]
- 42. Nichols WW, O'Rourke MF. McDonald's Blood Flow in Arteries: Theoretical, Experimental, and Clinical Principles. London: Edward Arnold, 1998 [Google Scholar]
- 43. Niki K, Sugawara M, Chang D, Harada A, Okada T, Sakai R, Uchida K, Tanaka R, Mumford CE. A new noninvasive measurement system for wave intensity: evaluation of carotid arterial wave intensity and reproducibility. Heart Vessels 17: 12–21, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Phillips DJ, Beach KW, Primozich J, Strandness DE., Jr Should results of ultrasound Doppler studies be reported in units of frequency or velocity? Ultrasound Med Biol 15: 205–212, 1989 [DOI] [PubMed] [Google Scholar]
- 45. Popovic ZB, Sun JP, Yamada H, Drinko J, Mauer K, Greenberg NL, Cheng Y, Moravec CS, Penn MS, Mazgalev TN, Thomas JD. Differences in left ventricular long-axis function from mice to humans follow allometric scaling to ventricular size. J Physiol 568: 255–265, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reddy AK, Jones AD, Martino C, Caro WA, Madala S, Hartley CJ. Pulsed Doppler signal processing for use in mice: design and evaluation. IEEE Trans Biomed Eng 52: 1764–1770, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Reddy AK, Li YH, Pham TT, Ochoa LN, Trevino MT, Hartley CJ, Michael LH, Entman ML, Taffet GE. Measurement of aortic input impedance in mice: effects of age on aortic stiffness. Am J Physiol Heart Circ Physiol 285: H1464–H1470, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Reddy AK, Madala S, Jones AD, Caro WA, Eberth JF, Pham TT, Taffet GE, Hartley CJ. Multichannel pulsed Doppler signal processing for vascular measurements in mice. Ultrasound Med Biol 35: 2042–2054, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reddy AK, Taffet GE, Li YH, Lim SW, Pham TT, Pocius J, Entman ML, Michael LH, Hartley CJ. Pulsed Doppler signal processing for use in mice: applications. IEEE Trans Biomed Eng 52: 1771–1783, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Reiz S, Balfors E, Sorensen MB, Ariola S, Friedman A, Truedsson H. Isoflurane—a powerful coronary vasodilator in patients with coronary artery disease. Anesthesiology 59: 91–97, 1983 [DOI] [PubMed] [Google Scholar]
- 51. Rockman HA, Knowlton KU, Ross J, Jr, Chien KR. In vivo murine cardiac hypertrophy: a novel model to identify genetic signaling mechanisms that activate an adaptive physiological response. Circulation 8, Suppl VII: VII-14–VII-21, 1993 [Google Scholar]
- 52. Saraste A, Kyto V, Laitinen I, Saraste M, Leppanen P, Yla-Herttuala S, Saukko P, Hartiala J, Knuuti J. Severe coronary artery stenoses and reduced coronary flow velocity reserve in atherosclerotic mouse model, Doppler echocardiography validation study. Atherosclerosis 200: 89–94, 2008 [DOI] [PubMed] [Google Scholar]
- 53. Saraste A, Kyto V, Saraste M, Vuorinen T, Hartiala J, Saukko P. Coronary flow reserve and heart failure in experimental coxsackievirus myocarditis. A transthoracic Doppler echocardiography study. Am J Physiol Heart Circ Physiol 291: H871–H875, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Saraste M, Koskenvuo JW, Knuuti J, Toikka JO, Laine H, Niemi P, Sakuma H, Hartiala JJ. Coronary flow reserve: measurement with transthoracic Doppler echocardiography is reproducible and comparable with positron emission tomography. Clin Physiol 21: 114–122, 2001 [DOI] [PubMed] [Google Scholar]
- 55. Schaefer A, Meyer GP, Hilfiker-Kleiner D, Brand B, Drexler H, Klein G. Evaluation of tissue Doppler Tei index for global left ventricular function in mice after myocardial infarction: comparison with pulsed Doppler Tei index. Eur J Echocardiogr 6: 367–375, 2005 [DOI] [PubMed] [Google Scholar]
- 56. Schildmeyer LA, Braun R, Taffet GE, De Biasi M, Burns AE, Bradley A, Schwartz RJ. Impaired vascular contractility and blood pressure homeostasis in the smooth muscle a-actin null mouse. FASEB J 14: 2213–2220, 2000 [DOI] [PubMed] [Google Scholar]
- 57. Styomann J. Doppler ultrasound in mice. Echocardiography 24: 97–112, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Sullivan JC, Wang B, Boesen EI, D'Angelo G, Pollock DM. Novel use of ultrasound to examine regional blood flow in the mouse kidney. Am J Physiol Renal Physiol 297: F228–F235, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Taffet GE, Hartley CJ, Wen X, Pham TT, Michael LH, Entman ML. Noninvasive indexes of cardiac systolic and diastolic function in hyperthyroid and senescent mouse. Am J Physiol Heart Circ Physiol 270: H2204–H2209, 1996 [DOI] [PubMed] [Google Scholar]
- 60. Vatner SF, Hittinger L. Coronary vascular mechanisms involved in decompensation from hypertrophy to heart failure. J Am Coll Cardiol 22: 34A–40A, 1993 [DOI] [PubMed] [Google Scholar]
- 61. Voci P, Pizzuto F, Romeo F. Coronary flow: a new asset for the echo lab. Eur Heart J 25: 1867–1879, 2004 [DOI] [PubMed] [Google Scholar]
- 62. Wang YX, Halks-Miller M, Vergona R, Sullivan ME, Fitch R, Mallari C, Martin-McNulty B, Da Cunha V, Freay A, Rubanyi GM, Kauser K. Increased aortic stiffness by pulse wave velocity in apolipoprotein E - deficient mice. Am J Physiol Heart Circ Physiol 278: H428–H434, 2000 [DOI] [PubMed] [Google Scholar]
- 63. Westerhof N. Heart period is related to the time constant of the arterial system and not to the minimum of impedance modulus. In: Recent Progress in Cardiovascular Mechanics, edited by Hosoda S, Yaginuma T, Sugawara M, Taylor MG, Caro CG. New York: Harwood Academic, 2002, p. 115–127 [Google Scholar]
- 64. Westerhof N, Elzinga G. Normalized input impedance and arterial decay time over heart period are independent of animal size. Am J Physiol Regul Integr Comp Physiol 261: R126–R133, 1991 [DOI] [PubMed] [Google Scholar]
- 65. Westerhof N, Sipkema P, Van Den Bos GC, Elzinga G. Forward and backward waves in the arterial system. Cardiovasc Res 6: 648–656, 1972 [DOI] [PubMed] [Google Scholar]
- 66. Wikstrom J, Gronros J, Bergstrom G, Gan LM. Functional and morphologic imaging of coronary atherosclerosis in living mice using high-resolution color Doppler echocardiography and ultrasound biomicroscopy. J Am Coll Cardiol 46: 720–727, 2005 [DOI] [PubMed] [Google Scholar]
- 67. Wikstrom J, Gronros J, Gan LM. Adenosine induces dilation of epicardial coronary arteries in mice - Relationship between coronary flow velocity reserve and coronary flow reserve in vivo using transthoracic echocardiography. Ultrasound Med Biol 34: 1053–1062, 2008 [DOI] [PubMed] [Google Scholar]
- 68. Williams R, Needles A, Cherin E, Zhou YQ, Henkelman RM, Adamson SL, Foster FS. Noninvasive ultrasonic measurement of regional and local pulse-wave velocity in mice. Ultrasound Med Biol 33: 1368–1375, 2007 [DOI] [PubMed] [Google Scholar]