Abstract
There is strong evidence showing that aging is associated with vascular oxidative stress, which has been causally linked to the development of cardiovascular diseases. NF-E2-related factor-2 (Nrf2) is a transcription factor, which is activated by reactive oxygen species in the vasculature of young animals leading to the upregulation of various antioxidant genes. The present study was designed to elucidate age-related changes in the homeostatic role of Nrf2-driven free radical detoxification mechanisms in the vasculature. We found that in the aorta of Fischer 344 × Brown Norway rats, aging results in a progressive increase in O2·− production, and downregulates protein and mRNA expression of Nrf2, which is associated with a decreased nuclear Nrf2 activity and a decrease in the Nrf2 target genes NAD(P)H:quinone oxidoreductase 1, γ-glutamylcysteine synthetase, and heme oxygenase-1. There was an inverse relationship between vascular expression of Nrf2 target genes and age-related increases in the expression of the NF-κB target genes ICAM-1 and IL-6, which was significant by regression analysis. In cultured aorta segments of young (3 mo old) rats treatment with H2O2 and high glucose significantly increases nuclear translocation of Nrf2 and upregulates the expression of Nrf2 target genes. In contrast, in cultured aorta segments of aged (24 mo old) rats, the induction of Nrf2-dependent responses by H2O2 and high glucose are blunted. High glucose-induced vascular oxidative stress was more severe in aortas of aged rats, as shown by the significantly increased H2O2 production in these vessels, compared with responses obtained in aortas from young rats. Moreover, we found that aging progressively increases vascular sensitivity to the proapoptotic effects of H2O2 and high glucose treatments. Taken together, aging is associated with Nrf2 dysfunction in the vasculature, which likely exacerbates age-related cellular oxidative stress and increases sensitivity of aged vessels to oxidative stress-induced cellular damage.
Keywords: senescence, apoptosis, oxidative stress resistance, vascular injury
the oxidative stress theory of aging postulates that increased production of reactive oxygen species (ROS) with age induces a variety of macromolecular oxidative modifications and that accumulation of such oxidative damage is a major causal factor in organismic senescence. Although there is currently much debate over the importance of increased cellular oxidative stress in regulation of life span (4, 29), there is a consensus that increased ROS levels importantly contribute to the development of age-associated diseases (68). There is strong evidence that oxidative stress develops with age in the arterial system both in humans (19–21, 25, 28) and laboratory animals (16, 24, 26, 44, 61). Increased production of ROS in the aged vasculature results in endothelial dysfunction and promotes the development of atherosclerotic vascular diseases (including myocardial infarction, stroke, vascular dementia), which are responsible for the majority of age-related increases in morbidity and mortality in the Western world (for a recent review see Ref. 53).
Recent findings demonstrate that in the arteries of young animals in response to increased production of ROS (stimulated by proatherogenic conditions, including metabolic diseases, alterations in the hemodynamic environment, cigarette smoking) adaptive mechanisms are invoked that involve induction of NF-E2-related factor 2 (Nrf2)-driven antioxidant defense mechanisms (47, 58, 63, 64). Nrf2 is an evolutionarily highly conserved redox sensitive transcription factor that upregulates the expression of numerous genes for proteins that detoxify ROS, as well as those with other antioxidant properties. In young organisms this homeostatic response serves to attenuate vascular oxidative stress and limit the damage caused by the increased production of ROS induced by diabetic conditions (27, 63) and other stressors (1, 62). In the aged vasculature ROS production both by mitochondria (56) and by other sources [e.g., NADPH oxidases (9, 12, 16, 40)] is significantly increased. In cells of young animals a similar level of ROS would result in an adaptive induction of Nrf2-driven free radical detoxification mechanisms. Despite the current advances in understanding the pathophysiological role of oxidative stress in vascular aging, the role of Nrf2-mediated antioxidant response in the aged vasculature is not well understood.
The present study was designed to test the hypothesis that in aging vessels increased production of ROS fails to activate Nrf2 resulting in increased blood vessel sensitivity to the deleterious effects of ROS. To test our hypotheses, we assessed aging-induced changes in ROS production in arteries of Fischer 344 × Brown Norway (F344xBN) rats and correlated these changes with Nrf2 expression and activity and expression of Nrf2-driven antioxidant enzymes. Using cultured arteries isolated from young and aged rats we also determined whether aging impairs the ability of vascular cells to mount an effective antioxidant response to counteract the deleterious effects of oxidative stressors (H2O2 treatment, model hyperglycemia) by inducing Nrf2-regulated ROS detoxification systems.
METHODS
Animals.
F344xBN rats were used as a model of aging, since this strain has a lower incidence of age-specific pathology than other rat strains. Thus, in F344xBN rats, the primary effects of aging can be studied uncomplicated by compensatory effects caused by age-related pathology. Male 3-, 12-, 18-, 24-, and 28-mo-old F344xBN rats (n = 4–12 in each group) were obtained from the National Institute on Aging. All animals were disease free with no signs of systemic inflammation and/or neoplastic diseases. The rats were housed in an environmentally controlled vivarium under pathogen free conditions with unlimited access to food and water and a controlled photoperiod (12-h:12-h light/dark). All rats were maintained according to National Institutes of Health guidelines, and all animal use protocols were approved by the Institutional Animal Care and Use Committees of the participating institutions. The animals were euthanized with CO2, and the carotid arteries and aorta were isolated.
Measurement of vascular O2·− production.
Production of O2·− in the aortic wall was determined using dihydroethidium (DHE), an oxidative fluorescent dye, as previously reported (10, 51, 52). In brief, vessels were incubated with DHE (3 × 10−6 mol/l; at 37°C for 30 min). The vessels were then washed three times, embedded in optimum cutting temperature (OCT) medium, and cryosectioned. Fluorescent images were captured at 20 × magnification and analyzed using Metamorph imaging software as reported (11). Four entire fields per vessel were analyzed with one image per field. The mean fluorescence intensities of DHE-stained nuclei in the endothelium and medial layer were calculated for each vessel. Thereafter, the intensity values for each animal in the group were averaged.
Immunofluorescent labeling for Nrf2.
To assess nuclear translocation of Nrf2, immunolabeling for Nrf2 was performed using frozen vascular sections. In brief, vascular sections were incubated in ice-cold acetone (10 min) followed by washes in Tween-PBS (10 min) and then in PBS (3 × 5 min). Triton (0.5%; 10 min) was used for permeabilization. The sections were blocked with 10% fetal calf serum in PBS for 1 h, and immunolabeling was then performed using a rabbit polyclonal antibody directed against Nrf2 (Abcam ab31163; 1:50, overnight, at 4°C, in PBS containing 1% BSA). Thereafter, slides were washed with PBS (3 × 10 min) before adding the secondary antibody (Alexa Fluor 688 goat anti-rabbit IgG; for 1 h, at room temperature). After washing with PBS (3 × 5 min), Hoechst 33342 (5 μg/ml) was added for 5 min. The sections were coverslipped, and fluorescent images were obtained. Cells treated with the canonical Nrf2 activator sulforaphane (2 μmol/l) were also immunolabeled using the aforementioned protocol and were used as positive controls (data not shown).
Organoid culture.
Aorta segments isolated from young and aged rats were maintained in organoid culture (for 24 h) as previously described (60). Vessels were treated with H2O2 (from 10−6 to 10−4 mol/l) or high glucose (30 mmol/l), which effectively increases mitochondrial H2O2 generation (33). At the end of the culture period nuclear Nrf2 binding activity, expression of Nrf2 targets, production of ROS, and apoptotic cell death were assayed as described below.
Nuclear extraction and Nrf2 binding activity assay.
Nuclei were isolated from carotid arteries using the Nuclear Extraction kit from Active Motif (Carlsbad, CA) as reported (15, 60). In brief, carotid arteries were homogenized with a dounce tissue homogenizer in 500 μl ice-cold hypotonic lysis buffer followed by two centrifugation steps (500 g, for 30 s, 4°C) to exclude tissue debris. Nuclear proteins (∼10 μg/vessel segment) were then extracted according to the manufacturer's protocol. Protein concentrations in samples were equalized using a Bradford protein assay (Bio-Rad). Using the nuclear extract obtained, Nrf2 binding activity was assayed using the TransAM Nrf2 ELISA kit (Active Motif) according to the manufacturer's guidelines.
Quantitative real-time RT-PCR.
A quantitative real-time RT-PCR technique was used to analyze mRNA expression of Nrf2, Keap-1, and the Nrf2/antioxidant response element (ARE) target genes NAD(P)H:quinone oxidoreductase 1 (Nqo1), heme oxygenase-1 (Hmox1), γ-glutamylcysteine synthetase (Gclc), and catalase in freshly isolated aortas as well as in aortas maintained in organoid cultures, as previously reported (12, 16, 59, 60). In brief, total RNA was isolated with a Mini RNA Isolation Kit (Zymo Research, Orange, CA) and was reverse transcribed using Superscript III RT (Invitrogen) as described previously (14, 16). A real-time RT-PCR technique was used to analyze mRNA expression using a Strategen MX3000, as reported (14). Amplification efficiencies were determined using a dilution series of a standard vascular sample. Quantification was performed using the efficiency-corrected ΔΔCq method. The relative quantities of the reference genes Gapdh, Hprt, and Actb were determined, and a normalization factor was calculated based on the geometric mean for internal normalization. Oligonucleotides used for quantitative real-time RT-PCR are listed in Table 1. Fidelity of the PCR reaction was determined by melting temperature analysis and visualization of the product on a 2% agarose gel.
Table 1.
Oligonucleotides for real-time RT-PCR
| mRNA Targets | Description | Sense | Antisense |
|---|---|---|---|
| Nrf2 | NF-E2-related factor-2, nuclear factor (erythroid-derived 2)-like 2 (NFE2L2) | ACGGTGGAGTTCAATGAC | GAAGAATGTGTTGGCTGTG |
| Keap1 | Kelch-like ECH-associated protein 1, cytosolic inhibitor of Nrf2 | GGACAGTGTGGAATGCTATG | GCCAGTGCTCAGGTAGTC |
| Nqo1 | NAD(P)H:quinone oxidoreductase 1 | TGGGATATGAATCAGGGAGAG | GAGAGGTAACTAATAGCAACAAG |
| Gclc | glutamate-cysteine ligase, catalytic subunit1 | GCTTTCTCCTACCTGTTTCTTG | TGGCAGAGTTCAGTTCCG |
| Hmox1 | heme oxygenase 1 (HO-1) | GGCTGTGAACTCTGTCTC | GGCATCTCCTTCCATTCC |
| Cat | catalase | GCCTCACCAGTAATCATCG | ATCCAAACAGAAGTCCTAAGC |
| Il6 | interleukin 6 | TACCCCAACTTCCAATGC | GATACCCATCGACAGGAT |
| Icam1 | intercellular adhesion molecule 1 | CACAGCCTGGAGTCTC | CCCTTCTAAGTGGTTGGAA |
| Hprt | hypoxanthine phosphoribosyltransferase 1 | AAGACAGCGGCAAGTTGAATC | AAGGGACGCAGCAACAGAC |
| Gapdh | glyceraldehyde-3-phosphate dehydrogenase | CCAAGGAGTAAGAAACCC | TTGATGGTATTCGAGAGAAGG |
| Actb | β-actin | GAAGTGTGACGTTGACAT | ACATCTGCTGGAAGGTG |
Western blotting.
To analyze protein expression of Nrf2 and the Nrf2 targets NQO1, and GCLC, Western blotting was performed as described (13), using the following primary antibodies: rabbit anti-Nrf2 (Abcam, ab31163; 1:1,000 5% milk), rabbit anti-Nrf2 (a generous gift from Dr. Scott M. Plafker, Oklahoma Medical Research Foundation, Oklahoma City, OK; 1:250 in 5% milk), rabbit anti-GCLC (Abcam, ab41463; 1 μg/ml in 5% milk), and rabbit anti-NQO1 (Abcam, ab34173; 1:2,000 in 5% milk). All polyvinylidene difluoride (PVDF) membranes were incubated in primary antibodies overnight at 4°C. A donkey anti-rabbit polyclonal secondary antibody was used (Abcam, ab16284; 1:2,000 in 5% milk). Mouse anti-β-actin (Abcam, ab6276; 1:10,000 in 5% milk) with a sheep anti-mouse IgG horseradish peroxidase linked secondary antibody (NA931V GE Healthcare UK, 1:10,000) and coomassie staining were used for normalization purposes.
Measurement of vascular H2O2 production.
H2O2 production in cultured vascular segments with or without treatment with 30 mmol/l glucose (for 24 h) was measured fluorometrically using the Amplex Red/horseradish peroxidase assay as described (13). The rate of H2O2 generation was assessed by measuring resorufin fluorescence for 60 min by a Tecan Infinite M200 plate reader. A calibration curve was constructed using H2O2, and the production of H2O2 in the samples was expressed as picomoles H2O2 released per minute, normalized to tissue wet weight.
Apoptotic cell death.
To compare cellular resistance with oxidative stress in arteries of young and aged rats, increases in the rate of apoptosis in response to treatment with high glucose or H2O2 (10−4 mol/l, for 24 h) were assessed. The vessels were homogenized in lysis buffer, and cytoplasmic histone-associated DNA fragments, which indicate apoptotic cell death, were quantified by the Cell Death Detection ELISAPlus kit (Roche Diagnostics, Indianapolis, IN) as described (9, 17). As an additional measure caspase 3 activity, which is also a useful measure of apoptosis, was measured as reported (17, 32, 57), using the Caspase-Glo 3/7 assay system (Promega). Luminescent intensity was measured using an Infinite M200 plate reader and was normalized to the sample protein concentration.
Data analysis.
Gene expression data were normalized to the respective control mean values. Statistical analyses of data were performed by Student's t-test or by two-way ANOVA followed by the Tukey post hoc test, as appropriate. Regression analysis was used to infer causal relationships between the independent and dependent variables. P < 0.05 was considered statistically significant. Data are expressed as means ± SE.
RESULTS
Age-related increase in vascular ROS production is not accompanied by increased Nrf2 activation and upregulation of Nrf2-driven antioxidant genes.
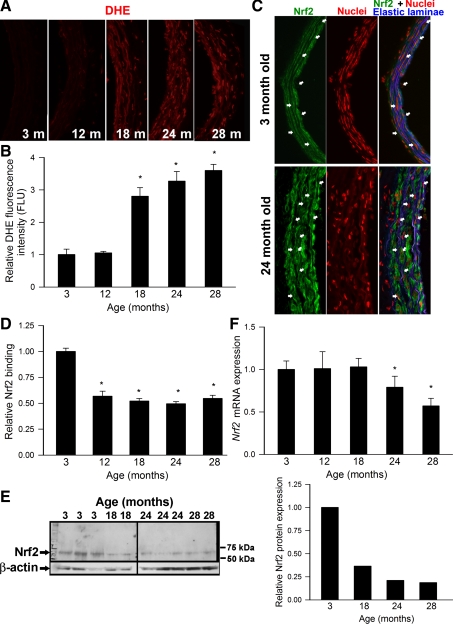
Representative fluorescent images of cross sections of DHE-stained aortas isolated from 3-, 12-, 18-, 24-, and 28-mo-old rats are shown in Fig. 1A. Analysis of nuclear DHE fluorescent intensities indicated that there was a significant age-related increase in vascular O2·− production in rat aortas (Fig. 1B). In young animals increased ROS levels are known to activate Nrf2, which translocates to the nucleus, where it binds to the ARE to activate transcription of phase II and antioxidant defense enzymes. Thus, to determine whether age-related oxidative stress is associated with Nrf2 activation, we tested nuclear translocation of Nrf2 and expression of known Nrf2 target genes. As shown in Fig. 1C, predominantly cytoplasmic labeling of Nrf2 with no significant nuclear staining was observed in arteries of aged rats, and this staining pattern did not differ from Nrf2 staining in arteries of young animals. To determine whether age-related vascular oxidative stress is associated with increased Nrf2 activation, we also assessed nuclear Nrf2 binding activity in aortas of rats from each age group. A significant, age-related decline in nuclear Nrf2 content was noted (Fig. 1D). Analysis of vascular Nrf2 protein expression revealed that age-related decline in nuclear Nrf2 content parallels a general age-related decline in cellular Nrf2 expression (Fig. 1E). Interestingly, our data show that vascular mRNA expression of Nrf2 also declines with age (Fig. 1F); however, the time course of age-related changes in mRNA and protein expression of Nrf2 occurred beginning at 24 mo of age. In contrast, mRNA expression of Keap-1 was significantly increased with advancing age (relative mRNA abundance: 1 ± 0.2, 1.2 ± 0.06, 1.5 ± 0.2, 1.8 ± 0.3, and 2.1 ± 0.5 in aorta of 3-, 12-, 18-, 24-, and 28-mo-old rats, respectively).
Fig. 1.
A: representative images showing red nuclear dihydroethidium (DHE) fluorescence, representing cellular O2·− production, in sections of aortas of 3-, 12-, 18-, 24-, and 28-mo-old Fischer 344 × Brown Norway (F344XBN) rats (A). The lumen is on the left of each image. Original magnification: 20×. B: summary data for nuclear DHE fluorescence intensities. Data are means ± SE. *P < 0.05 vs. 3 mo old. C: representative microscopic images showing immunofluorescent labeling for Nrf2 (green) in sections of aortas of 3- and 24-mo-old F344XBN rats. Blue autofluorescence of elastic laminae is shown for orientation purposes; propidium iodide (red) was used for nuclear staining (original magnification: 20×). Arrows point to the nuclei of endothelial cells and smooth muscle cells (at right: overlay images). Note cytoplasmic localization of Nrf2 in each image. D: ELISA-based demonstration of age-related decline of basal Nrf2 binding activity in nuclear extracts from carotid arteries of F344XBN rats. Data are means ± SE (n = 4–6 for each data point). *P < 0.05 vs. 3 mo old. E: representative Western blot showing an age-related decline in Nrf2 protein expression in the aorta of F344XBN rats. β-Actin was used as a loading control. Right: bar graphs are average normalized densitometric ratios. F: expression of Nrf2 mRNA in aortas isolated from 3-, 12-, 18-, 24-, and 28-mo-old F344XBN rats. Data are means ± SE (n = 4–6 for each data point). *P < 0.05 vs. 3 mo old.
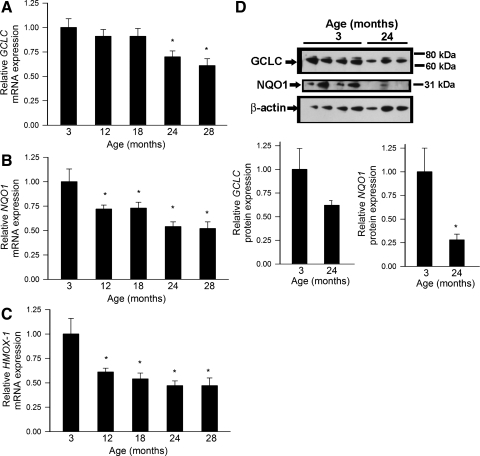
A quantitative real-time RT-PCR technique and Western blotting was used to analyze mRNA and protein expression of known Nrf2 targets in rat aortas. We found that vascular expression of GCLC (Fig. 2, A and D), NQO1 (Fig. 2, B and D), and heme oxygenase-1 (Fig. 2C) exhibited a significant age-related decline. The time course of the aforementioned changes was similar to the time course of age-related decline in nuclear Nrf2 content.
Fig. 2.
A: quantitative real-time RT-PCR data showing mRNA expression of γ-glutamylcysteine synthetase (GCLC; A), NAD(P)H:quinone oxidoreductase 1 (NQO1; B), and heme oxygenase-1 (HMOX1; C) in aorta segments isolated from 3-, 12-, 18-, 24-, and 28-mo-old F344XBN rats. Data are means ± SE (n = 4–6 for each data point). *P < 0.05 vs. 3 mo old. D: protein expression of GCLC and NQO1 also decreases with age (top: representative Western blots; bottom: bar graphs are normalized densitometric data). Data are means ± SE. *P < 0.05 vs. 3 mo old.
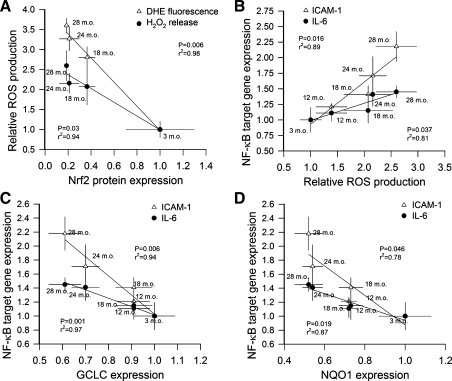
We found a significant inverse correlation between expression of Nrf2 protein and two independent markers of oxidative stress (mean nuclear DHE fluorescence and H2O2 production assessed by the Amplex Red/horseradish peroxidase assay) in aorta segments isolated from rats of various ages (Fig. 3A). We demonstrated that an inverse relationship exists between vascular H2O2 production and mRNA expression of the NF-κB target genes ICAM-1 and IL-6. The regression was significant for both targets (Fig. 3B). Our analysis showed a significant inverse relationship between vascular expression of GCLC (Fig. 3C) or NQO1 (Fig. 3D) and expression of the NF-κB target genes ICAM-1 and IL-6.
Fig. 3.
A: inverse relationship between expression of Nrf2 protein and vascular reactive oxygen species (ROS) production in aorta segments isolated from 3-, 18-, 24-, and 28-mo-old F344XBN rats. As a measure of vascular ROS production nuclear DHE fluorescence and release of H2O2 from the vascular tissues (assessed by the Amplex Red/horseradish peroxidase method) were quantified. The regression is significant for both indexes. B: relationship between vascular H2O2 production and mRNA expression of the NF-κB target genes ICAM-1 and IL-6. The regression is significant for both targets. C and D: inverse relationship between expression of GCLC (C) or NQO-1 (D) and expression of the NF-κB target genes ICAM-1 and IL-6 in aorta segments isolated from 3-, 12-, 18-, 24-, and 28-mo-old F344XBN rats. The regression is significant for each target.
Oxidative stressors elicit a blunted Nrf2-driven antioxidant response in aortas of aged rats.
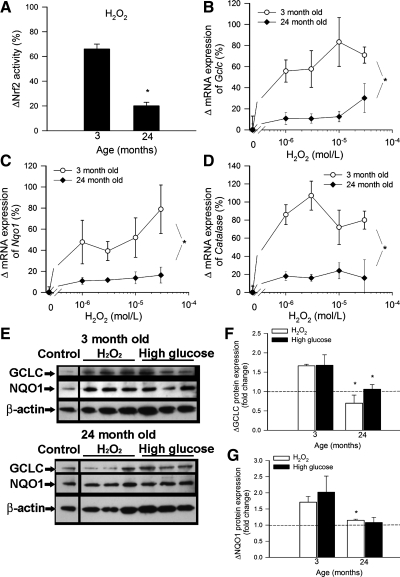
To determine whether age-related Nrf2 dysregulation impairs the ability of vascular cells to mount an effective antioxidant response to oxidative stressors, we treated cultured aorta segments isolated from 3-mo-old (young) and 24-mo-old (aged) rats with H2O2 (10−6 to 3 × 10−5 mol/l) and high glucose (30 mmol/l). We found that H2O2 significantly increased Nrf2 binding activity in nuclear extracts from carotid arteries of young rats, whereas H2O2-induced Nrf2 translocation to the nuclei was significantly less in aortas of aged rats (Fig. 4A). In a dose-dependent manner, H2O2 elicited substantial upregulation of mRNA and protein expression of Nrf2 target genes in young aortas (Fig. 4, B–G). In contrast, H2O2-induced increases in mRNA and protein expression of GCLC, NQO1, and/or catalase were significantly attenuated in aortas of aged rats, compared with young vessels (Fig. 4, B–G).
Fig. 4.
A: H2O2-induced increases in Nrf2 binding activity in nuclear extracts from carotid arteries of 3- and 24-mo-old F344XBN rats. Data are means ± SE. *P < 0.05 vs. 3 mo old (n = 6). B: quantitative real-time RT-PCR data showing H2O2-induced increases in mRNA expression of GCLC (B), NQO1 (C), and catalase (D) in aorta segments isolated from 3- and 24-mo-old F344xBN rats. Data are means ± SE (n = 4–6 for each data point). *P < 0.05 vs. 3 mo old. E: representative Western blots showing H2O2 (10−5 mol/l) and high glucose (30 mmol/l)-induced increases in protein expression of GCLC and NQO1 in aorta segments isolated from 3- and 24-mo-old F344XBN rats. β-Actin was used for normalization purposes. Bar graphs (F: GCLC; G: NQO1) are normalized densitometric ratios. *P < 0.05 vs. 3 mo old.
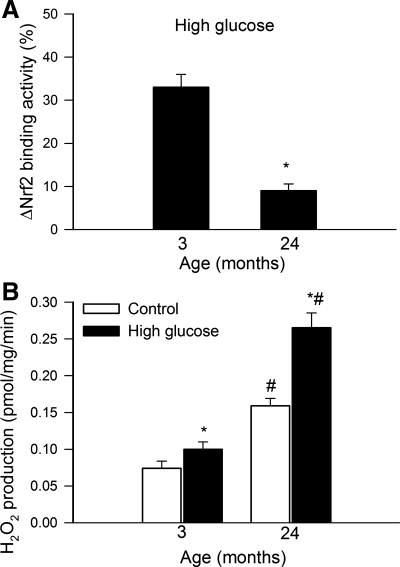
Model hyperglycemia significantly increased Nrf2 binding activity in nuclear extracts from carotid arteries of young rats, whereas Nrf2 translocation to the nuclei in response to 30 mmol/l glucose was significantly blunted in aortas of aged rats (Fig. 5A). High glucose treatment also elicited significantly greater increases in GCLC mRNA expression in aortas of young rats (50 ± 4%) than in aged vessels (18 ± 6%; P < 0.05).
Fig. 5.
A: high glucose-induced increases in Nrf2 binding activity in nuclear extracts from carotid arteries of 3- and 24-mo-old F344xBN rats. Data are means ± SE (n = 6). *P < 0.05 vs. 3 mo old. B: high glucose-induced increases in H2O2 production in aortas of 3- and 24-mo-old F344xBN rats. Data are means ± SE (n = 6). *P < 0.05 vs. no treatment; #P < 0.05 vs. 3 mo old.
Model hyperglycemia elicits increased oxidative stress in aortas of aged rats.
In aortas of aged rats baseline H2O2 production was significantly increased compared with young vessels (Fig. 5B). High glucose significantly increased H2O2 production in aortas isolated from young rats (Fig. 5B). The magnitude of high glucose-induced H2O2 production was significantly greater in aortas of aged rats than on young vessels (Fig. 5B).
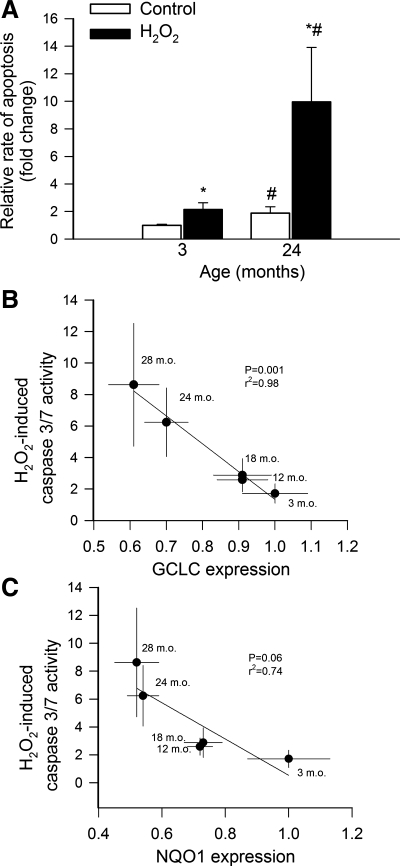
Age-related decline in cellular resistance to oxidative stress-induced apoptosis in rat aortas.
Analysis of cytoplasmic histone-associated DNA fragments (Fig. 6A) showed that rate of apoptosis in aortic segments under baseline conditions increases as a function of age. Exposure to H2O2 (Fig. 6A) significantly increased rate of apoptosis in each age group (P < 0.05). The magnitude of H2O2-induced DNA fragmentation significantly increased with age. Analysis of caspase 3/7 activity, which also indicates apoptotic cell death, yielded similar results. Figure 6, B and C, shows that an inverse relationship exists between expression of Nrf2 target genes (GCLC, NQO1) and H2O2-induced caspase 3/7 activation. GCLC and NQO1 expression was used as an index of Nrf2 activity in this analysis.
Fig. 6.
A: H2O2 (10−4 mol/l)-induced increases in cytoplasmic histone-associated DNA fragments in aorta segments isolated from 3- and 24-mo-old F344XBN rats, indicating an increased rate of apoptosis in aged vessels. Data are means ± SE (n = 6). *P < 0.05 vs. no treatment; #P < 0.05 vs. 3 mo old. B: inverse relationship between expression of GCLC and H2O2-induced caspase 3/7 activation in aorta segments isolated from 3-, 12-, 18-, 24-, and 28-mo-old F344XBN rats. The regression is significant (P = 0.001; r2 = 0.98). C: inverse relationship between expression of NQO1 and H2O2-induced caspase 3/7 activation. The regression approaches significance (P = 0.06; r2 = 0.74). Data are means ± SE (n = 4–6 for each data point).
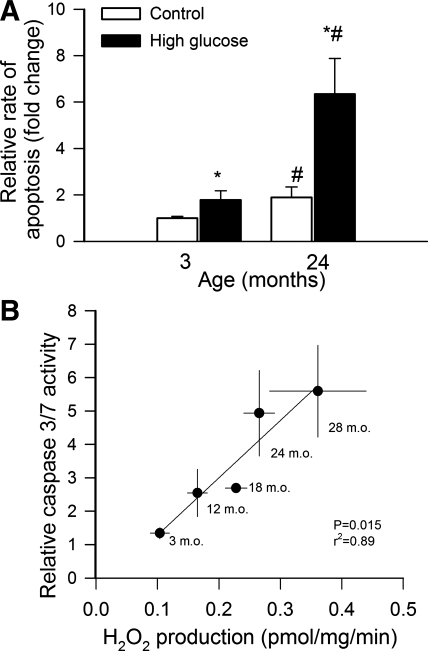
We also found that high glucose treatment also significantly increased rate of apoptosis in each age group and that the magnitude of high glucose-induced DNA fragmentation significantly increased with age (Fig. 7A). There was a significant positive correlation between high glucose-induced H2O2 production and high glucose-induced caspase 3/7 activation, demonstrating that in aortas of aged rats the same metabolic stress elicits significantly greater oxidative stress and more cellular damage than in aortas from young rats.
Fig. 7.
A: high glucose (30 mmol/l)-induced increases in cytoplasmic histone-associated DNA fragments in aorta segments isolated from 3- and 24-mo-old F344XBN rats, indicating an increased rate of apoptosis in aged vessels. Data are means ± SE (n = 6). *P < 0.05 vs. no treatment; #P < 0.05 vs. 3 mo old. B: relationship between high glucose-induced H2O2 production and H2O2-induced caspase 3/7 activation in aorta segments isolated from 3-, 12-, 18-, 24-, and 28-mo-old F344XBN rats. The regression is significant (P = 0.015; r2 = 0.89). Data are means ± SE (n = 4–6 for each data point).
DISCUSSION
Several lines of evidence suggest that stress-activated cap‘n’collar transcription factors, including Nrf2, play an important role in regulating the aging process by orchestrating the transcriptional response of cells to oxidative stress (34). Homologues of Nrf2 are evolutionarily highly conserved (35), and studies on invertebrate model organisms demonstrate that knockdown of Nrf2 homologs in Caenorhabditis elegans (30) and Drosophila melanogaster (45) significantly shortens life span. In mammals, Nrf2-driven pathways confer cytoprotection by activating the transcription of more than 200 genes that are crucial in protection against oxidative stress. Previous studies suggest that genetic depletion of Nrf2 also affects the aging process in mice, increasing age-related cancer morbidity and abrogating the anti-cancer effects of caloric restriction (41). Recent studies have demonstrated that Nrf2-driven pathways are functional in endothelial cells and they confer important antioxidative, anti-inflammatory, and anti-apoptotic effects (6, 22, 23, 31, 63, 65).
Here we show for the first time that development of vascular oxidative stress in a rodent model of aging is associated with a homeostatic failure due to dysregulation of the Nrf2-mediated antioxidant response. Our present findings (Fig. 1, A and B) extend the results of previous studies (10, 12, 16) and provide evidence that vascular ROS generation in rats substantially increases after mid-life. In young animals adaptive activation of the Nrf2/ARE pathway has a critical role in endothelial protection in response to increases in vascular O2·− and H2O2 production. In young animals, upon ROS-induced activation, Nrf2 translocates to the nucleus, where it binds to the ARE to activate transcription of phase II and antioxidant defense enzymes, including NQO1 (a key component of the plasma membrane redox system), heme oxygenase-1, and γ-glutamylcysteine synthetase (the rate-limiting enzyme for glutathione synthesis). Here we report that in aged rats despite the presence of significant oxidative stress, Nrf2 is not activated (Fig. 1, C and D) and Nrf2-driven gene expression is not induced (Fig. 2). The mechanisms underlying dysregulation of Nrf2-mediated antioxidant response in aged cells are likely multifaceted. Our findings suggest that aging is associated with a downregulation of vascular Nrf2 expression both at the mRNA and protein level (Fig. 1, E and F), which likely contribute to the age-related decline in nuclear Nrf2 content and the consequential decline in transcriptional activity of Nrf2 in aged rats. Because age-related changes in Nrf2 content show a close inverse correlation with age-related increases in cellular ROS levels we propose that age-related Nrf2 dysregulation contributes to vascular oxidative stress in aging (Fig. 3A). Aging may also impair the pathways that regulate Nrf2 activation and nuclear translocation. An important factor regulating Nrf2 activity is Keap1, a cytosolic repressor protein, which interacts with Nrf2, preventing its nuclear translocation. Importantly, Keap1 expression is upregulated in aging, which likely contributes to the dysregulation of Nrf2 activity by oxidative stressors. This view is supported by our recent findings demonstrating that overexpression of Keap1 in endothelial cells can abolish the adaptive antioxidant response in response to hyperglycemia (48).
Previous studies indicated that age-related oxidative stress promotes vascular inflammation in aged animals by activating the redox sensitive transcription factor NF-κB (56). In line with these findings we found that there is a close correlation between vascular ROS production and expression of NF-κB target genes during vascular aging (Fig. 3B). We posit that Nrf2 dysfunction exerts proinflammatory effects by exacerbating ROS-mediated NF-κB activation in aging. This concept is supported by the significant inverse correlation between age-related upregulation of NF-κB target genes and decreases in the expression of Nrf2 and Nrf2 targets (Fig. 3, C and D). Furthermore, our recent data show that decreased Nrf2 expression and activity in smooth muscle cells derived from aged nonhuman primates is associated with an increased transcriptional activity of NF-κB (49).
It is unlikely that the threshold for ROS-induced Nrf2 activation is increased in aging since results from the present study show that exogenous administration of H2O2, which elicits significant induction of Nrf2-dependent genes in arteries of young rats, fails to substantially upregulate Nrf2-dependent free radical detoxification pathways in vessels of aged rats (Fig. 4). Impaired H2O2-induced Nrf2 activation was recently also demonstrated in smooth muscle cells derived from aged nonhuman primates (49). The impaired ability of aged cells to mount an effective Nrf2/ARE-mediated antioxidant response (2, 5, 7, 43) potentially renders the aged vascular system vulnerable to the deleterious effects of increased ROS production associated with various pathological conditions including type 2 diabetes.
Recent studies have shown that in young animals adaptive activation of the Nrf2/ARE pathway confers endothelial protection in response to oxidative stress associated with type 2 diabetes (47, 48). In isolated blood vessels and endothelial cells from young animals, high glucose also elicits significant mitochondrial ROS production (37, 55), which significantly increases the transcriptional activity of Nrf2 (48). In contrast, genetic lack of a functional Nrf2/ARE pathway results in significant increases in vascular ROS levels and a more severe endothelial functional impairment in arteries of young type 2 diabetic Nrf2−/− mice compared with vessels of young wild-type controls (47). Our present studies provide evidence that aging impairs the ability of vascular cells to mount an effective Nrf2-dependent antioxidant defense in response to metabolic stress in vitro (Fig. 5A). As a result, diabetic conditions induce significantly more robust oxidative stress in the arteries of aged rats than in those of young animals (Fig. 5B). Of note, type 2 diabetes is a disease of aging, affecting almost one in five of people over age 65. Because aging appears to sensitize the vasculature to the deleterious effects of diabetes, future studies should investigate the interaction of aging and type 2 diabetes, with special emphasis on the role of Nrf2 dysfunction in exaggerated microvascular complications observed in elderly patients with diabetes mellitus (3). Both aging (16) and diabetes mellitus (39) increase the production of reactive nitrogen species in the vasculature; thus future studies should also determine whether Nrf2 dysfunction sensitizes the blood vessels of aged animals to the deleterious effects of diabetes-related nitrosative stress (46).
There are multiple mechanisms by which dysregulation of Nrf2 and exaggerated oxidative stress may promote the development of cardiovascular diseases in aging. Recent findings suggest that induction of Nrf2-driven free radical detoxification pathways confers significant anti-apoptotic effects in cultured endothelial cells (38), similar to other cell types (27). Previous studies and our present findings suggest that in aged vessels increased ROS production, coupled with Nrf2 dysregulation, is associated with an increased basal rate of endothelial apoptosis (12, 17, 40). Our present studies extend these findings showing that Nrf2 dysregulation in aging is also associated with exaggerated cellular damage in response to H2O2 or metabolic stress induced by model hyperglycemia (Figs. 6 and 7). Age-related Nrf2 dysregulation and the increases in oxidative stress-induced NF-κB activation are also likely to contribute to increased vascular inflammation in aged animals (Fig. 3) (8, 18, 56). The intimate link among aging, Nrf2 activation, and vascular health is also underscored by the observations that arteries of extremely long-lived muroid rodents (P. leucopus, maximal lifespan: ∼8 years) exhibit an increased expression of Nrf2-driven antioxidant enzymes, decreased cellular and mitochondrial levels of ROS, and increased resistance to the proinflammatory and proapoptotic effects of hyperglycemia compared with vessels of shorter-lived M. musculus (13, 33, 50, 54).
Limitations of the study.
We measured Nrf2 binding activity in carotid arteries, whereas oxidative stress and Nrf2-driven gene expression was determined in the rat aorta. Yet, we have good reason to believe that age-related phenotypic and functional alterations in the aorta and the carotid arteries are similar. We base this assumption on the following evidence. First, previous studies by us and others have shown that aging results in similar endothelial dysfunction as well as similar increases in oxidative stress in the aorta, carotid artery and coronary arteries in laboratory rodents (reviewed in Ref. 53). Finally, both the aorta and the carotid arteries are conduit vessels, which exhibit increased sensitivity to atherosclerosis in the elderly.
Conclusions
In conclusion, our studies provide evidence that aging impairs the ability of vascular cells to mount an effective Nrf2-dependent antioxidant defense, which likely renders the aged vasculature vulnerable to oxidative stress associated with metabolic disease and other pathophysiological conditions. There is a clear opportunity for pharmacological intervention to facilitate the efficiency of Nrf2-driven homeostatic mechanisms in aging. In that regard it is significant that in endothelial cells Nrf2 can be activated pharmacologically by the polyphenol resveratrol (47). Importantly, resveratrol has been previously shown to upregulate Nrf2-driven cellular antioxidant systems and increase GSH levels with a consequential reduction of vascular oxidative stress, endothelial apoptosis, and inflammation in animal models of aging [independent of its effects on life span (36, 42)]. Resveratrol was also shown to confer similar vasoprotective effects in animal models of accelerated vascular aging (40, 55–57, 66, 67). Further studies involving animals with genetic depletion of specific Nrf2 targets (i.e., NQO1, heme oxygenase, glutathione peroxidase) are warranted to understand the role of individual antioxidant mechanisms in the vasoprotective effects of resveratrol and other Nrf2 activators in aging.
GRANTS
This work was supported by grants from the American Diabetes Association (to Z. Ungvari), American Federation for Aging Research (to A. Csiszar), the American Heart Association (to A. Csiszar), the University of Oklahoma College of Medicine Alumni Association (to A. Csiszar), The Oklahoma Center for the Advancement of Science and Technology (to A. Csiszar and Z. Ungvari), the National Institutes of Health (NIH; AG-031085 to A. Csiszar; AT-006526 and HL-077256 to Z. Ungvari; 1R01-AG-038747, 5R01-NS-056218, and P01 AG-11370 to W. E. Sonntag), and the Intramural Research Program of NIH (to R. D. Cabo).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Afonyushkin T, Oskolkova OV, Philippova M, Resink TJ, Erne P, Binder BR, Bochkov VN. Oxidized phospholipids regulate expression of ATF4 and VEGF in endothelial cells via NRF2-dependent mechanism: novel point of convergence between electrophilic and unfolded protein stress pathways. Arterioscler Thromb Vasc Biol 30: 1007–1013, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Bloomer SA, Zhang HJ, Brown KE, Kregel KC. Differential regulation of hepatic heme oxygenase-1 protein with aging and heat stress. J Gerontol A Biol Sci Med Sci 64: 419–425, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bruce DG, Davis WA, Casey GP, Starkstein SE, Clarnette RM, Almeida OP, Davis TM. Predictors of cognitive decline in older individuals with diabetes. Diabetes care 31: 2103–2107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Campisi J, Vijg J. Does damage to DNA and other macromolecules play a role in aging? If so, how? J Gerontol A Biol Sci Med Sci 64: 175–178, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen CN, Brown-Borg HM, Rakoczy SG, Ferrington DA, Thompson LV. Aging impairs the expression of the catalytic subunit of glutamate cysteine ligase in soleus muscle under stress. J Gerontol A Biol Sci Med Sci 65: 129–137, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen XL, Varner SE, Rao AS, Grey JY, Thomas S, Cook CK, Wasserman MA, Medford RM, Jaiswal AK, Kunsch C. Laminar flow induction of antioxidant response element-mediated genes in endothelial cells. A novel anti-inflammatory mechanism. The Journal of biological chemistry 278: 703–711, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Collins AR, Lyon CJ, Xia X, Liu JZ, Tangirala RK, Yin F, Boyadjian R, Bikineyeva A, Pratico D, Harrison DG, Hsueh WA. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res 104: e42–e54, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Csiszar A, Labinskyy N, Orosz Z, Xiangmin Z, Buffenstein R, Ungvari Z. Vascular aging in the longest-living rodent, the naked mole-rat. Am J Physiol Heart Circ Physiol 293: H919–H927, 2007. [DOI] [PubMed] [Google Scholar]
- 11. Csiszar A, Labinskyy N, Perez V, Recchia FA, Podlutsky A, Mukhopadhyay P, Losonczy G, Pacher P, Austad SN, Bartke A, Ungvari Z. Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. Am J Physiol Heart Circ Physiol 295: H1882–H1894, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-TNFalpha treatment in aging. The American journal of pathology 170: 388–398, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Csiszar A, Labinskyy N, Zhao X, Hu F, Serpillon S, Huang Z, Ballabh P, Levy RJ, Hintze TH, Wolin MS, Austad SN, Podlutsky A, Ungvari Z. Vascular superoxide and hydrogen peroxide production and oxidative stress resistance in two closely related rodent species with disparate longevity. Aging Cell 6: 783–797, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates TNF-α-induced activation of coronary arterial endothelial cells: role of NF-κB inhibition. Am J Physiol Heart Circ Physiol 291: H1694–H1699, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Csiszar A, Smith KE, Koller A, Kaley G, Edwards JG, Ungvari Z. Regulation of bone morphogenetic protein-2 expression in endothelial cells: role of nuclear factor-kappaB activation by tumor necrosis factor-alpha, H2O2, and high intravascular pressure. Circulation 111: 2364–2372, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Csiszar A, Ungvari Z, Edwards JG, Kaminski PM, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 90: 1159–1166, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics 17: 21–30, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Csiszar A, Wang M, Lakatta EG, Ungvari ZI. Inflammation and endothelial dysfunction during aging: role of NF-κB. J Appl Physiol 105: 1333–1341, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circulation research 100: 1659–1666, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Eskurza I, Kahn ZD, Seals DR. Xanthine oxidase does not contribute to impaired peripheral conduit artery endothelium-dependent dilatation with ageing. The Journal of physiology 571: 661–668, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. The Journal of physiology 556: 315–324, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fledderus JO, Boon RA, Volger OL, Hurttila H, Yla-Herttuala S, Pannekoek H, Levonen AL, Horrevoets AJ. KLF2 primes the antioxidant transcription factor Nrf2 for activation in endothelial cells. Arterioscler Thromb Vasc Biol 28: 1339–1346, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Fledderus JO, Boon RA, Volger OL, Hurttila H, Yla-Herttuala S, Pannekoek H, Levonen AL, Horrevoets AJ. KLF2 primes the antioxidant transcription factor Nrf2 for activation in endothelial cells. Arterioscler Thromb Vasc Biol 28: 1339–1346, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Francia P, delli Gatti C, Bachschmid M, Martin-Padura I, Savoia C, Migliaccio E, Pelicci PG, Schiavoni M, Luscher TF, Volpe M, Cosentino F. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation 110: 2889–2895, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Gates PE, Boucher ML, Silver AE, Monahan KD, Seals DR. Impaired flow-mediated dilation with age is not explained by l-arginine bioavailability or endothelial asymmetric dimethylarginine protein expression. J Appl Physiol 102: 63–71, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension 37: 529–534, 2001 [DOI] [PubMed] [Google Scholar]
- 27. He X, Kan H, Cai L, Ma Q. Nrf2 is critical in defense against high glucose-induced oxidative damage in cardiomyocytes. J Mol Cell Cardiol 46: 47–58, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Jablonski KL, Seals DR, Eskurza I, Monahan KD, Donato AJ. High-dose ascorbic acid infusion abolishes chronic vasoconstriction and restores resting leg blood flow in healthy older men. J Appl Physiol 103: 1715–1721, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Jang YC, Perez VI, Song W, Lustgarten MS, Salmon AB, Mele J, Qi W, Liu Y, Liang H, Chaudhuri A, Ikeno Y, Epstein CJ, Van Remmen H, Richardson A. Overexpression of Mn superoxide dismutase does not increase life span in mice. J Gerontol A Biol Sci Med Sci 64: 1114–1125, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jasper H. SKNy worms and long life. Cell 132: 915–916, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Jyrkkanen HK, Kansanen E, Inkala M, Kivela AM, Hurttila H, Heinonen SE, Goldsteins G, Jauhiainen S, Tiainen S, Makkonen H, Oskolkova O, Afonyushkin T, Koistinaho J, Yamamoto M, Bochkov VN, Yla-Herttuala S, Levonen AL. Nrf2 regulates antioxidant gene expression evoked by oxidized phospholipids in endothelial cells and murine arteries in vivo. Circ Res 103: e1–e9, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Labinskyy N, Csiszar A, Orosz Z, Smith K, Rivera A, Buffenstein R, Ungvari Z. Comparison of endothelial function, O2·−, and H2O2 production, and vascular oxidative stress resistance between the longest-living rodent, the naked mole rat, and mice. Am J Physiol Heart Circ Physiol 291: H2698–H2704, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Labinskyy N, Mukhopadhyay P, Toth J, Szalai G, Veres M, Losonczy G, Pinto JT, Pacher P, Ballabh P, Podlutsky A, Austad SN, Csiszar A, Ungvari Z. Longevity is associated with increased vascular resistance to high glucose-induced oxidative stress and inflammatory gene expression in Peromyscus leucopus. Am J Physiol Heart Circ Physiol 296: H946–H956, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lewis KN, Mele J, Hayes JD, Buffenstein R. Nrf2, a guardian of healthspan and gatekeeper of species longevity. Integr Comp Biol 50: 829–843, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miller RA. Cell stress and aging: new emphasis on multiplex resistance mechanisms. J Gerontol A Biol Sci Med Sci 64: 179–182, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci 66: 191–201, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mukhopadhyay P, Rajesh M, Hasko G, Hawkins BJ, Madesh M, Pacher P. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat Protoc 2: 2295–2301, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Okouchi M, Okayama N, Alexander JS, Aw TY. NRF2-dependent glutamate-L-cysteine ligase catalytic subunit expression mediates insulin protection against hyperglycemia-induced brain endothelial cell apoptosis. Curr Neurovasc Res 3: 249–261, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87: 315–424, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 8: 157–168, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pearson KJ, Lewis KN, Price NL, Chang JW, Perez E, Cascajo MV, Tamashiro KL, Poosala S, Csiszar A, Ungvari Z, Kensler TW, Yamamoto M, Egan JM, Longo DL, Ingram DK, Navas P, de Cabo R. Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc Natl Acad Sci USA 105: 2325–2330, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ryan MJ, Jackson JR, Hao Y, Williamson CL, Dabkowski ER, Hollander JM, Alway SE. Suppression of oxidative stress by resveratrol after isometric contractions in gastrocnemius muscles of aged mice. J Gerontol A Biol Sci Med Sci 65: 815–831, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, Hagen TM. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci USA 101: 3381–3386, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sun D, Huang A, Yan EH, Wu Z, Yan C, Kaminski PM, Oury TD, Wolin MS, Kaley G. Reduced release of nitric oxide to shear stress in mesenteric arteries of aged rats. Am J Physiol Heart Circ Physiol 286: H2249–H2256, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sykiotis GP, Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell 14: 76–85, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Szabo C, Zanchi A, Komjati K, Pacher P, Krolewski AS, Quist WC, LoGerfo FW, Horton ES, Veves A. Poly(ADP-Ribose) polymerase is activated in subjects at risk of developing type 2 diabetes and is associated with impaired vascular reactivity. Circulation 106: 2680–2686, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol 299: H18–H24, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ungvari Z, Bailey-Downs L, Gautam T, Jimenez J, Losonczy G, Zhang C, Ballabh P, Recchia FA, Wilkerson DC, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Adaptive induction of NF-E2-Related Factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am J Physiol Heart Circ Physiol 300: H1133–H1140, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ungvari Z, Bailey-Downs L, Gautam T, Sosnowska D, Wang M, Monticone RE, Telljohann R, Pinto JT, de Cabo R, Sonntag WE, Lakatta E, Csiszar A. Age-associated vascular oxidative stress, Nrf2 dysfunction and NF-kB activation in the non-human primate Macaca mulatta. J Gerontol Biol Med Sci. 2011, doi:10.1093/gerona/glr092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ungvari Z, Buffenstein R, Austad SN, Podlutsky A, Kaley G, Csiszar A. Oxidative stress in vascular senescence: lessons from successfully aging species. Front Biosci 13: 5056–5070, 2008 [DOI] [PubMed] [Google Scholar]
- 51. Ungvari Z, Csiszar A, Huang A, Kaminski PM, Wolin MS, Koller A. High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase. Circulation 108: 1253–1258, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Ungvari Z, Gautam T, Koncz P, Henthorn JC, Pinto JT, Ballabh P, Yan H, Mitschelen M, Farley J, Sonntag WE, Csiszar A. Vasoprotective effects of life span-extending peripubertal GH replacement in Lewis dwarf rats. J Gerontol A Biol Sci Med Sci 65: 1145–1156, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci 65: 1028–1041, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ungvari Z, Krasnikov BF, Csiszar A, Labinskyy N, Mukhopadhyay P, Pacher P, Cooper AJL, Podlutskaya N, Austad SN, Podlutsky A. Testing hypotheses of aging in long-lived mice of the genus Peromyscus: association between longevity and mitochondrial stress resistance, ROS detoxification pathways and DNA repair efficiency. Age (Dordr) 30: 121–133, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ungvari Z, Labinskyy N, Mukhopadhyay P, Pinto JT, Bagi Z, Ballabh P, Zhang C, Pacher P, Csiszar A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol 297: H1876–H1881, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-κB activation in aged rat arteries. Am J Physiol Heart Circ Physiol 293: H37–H47, 2007 [DOI] [PubMed] [Google Scholar]
- 57. Ungvari Z, Orosz Z, Rivera A, Labinskyy N, Xiangmin Z, Olson S, Podlutsky A, Csiszar A. Resveratrol increases vascular oxidative stress resistance. Am J Physiol Heart Circ Physiol 292: H2417–H2424, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res 102: 519–528, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ungvari ZI, Labinskyy N, Gupte SA, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol 294: H2121–H2128, 2008 [DOI] [PubMed] [Google Scholar]
- 60. Ungvari ZI, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith KE, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-κB activation in aged rat arteries. Am J Physiol Heart Circ Physiol 293: H37–H47, 2007 [DOI] [PubMed] [Google Scholar]
- 61. van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Lüscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med 192: 1731–1744, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Warabi E, Takabe W, Minami T, Inoue K, Itoh K, Yamamoto M, Ishii T, Kodama T, Noguchi N. Shear stress stabilizes NF-E2-related factor 2 and induces antioxidant genes in endothelial cells: role of reactive oxygen/nitrogen species. Free radical biology & medicine 42: 260–269, 2007 [DOI] [PubMed] [Google Scholar]
- 63. Xue M, Qian Q, Antonysunil A, Rabbani N, Babaei-Jadidi R, Thornalley PJ. Activation of NF-E2-related factor-2 reverses biochemical dysfunction of endothelial cells induced by hyperglycemia linked to vascular disease. Diabetes 57: 2809–2817, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yoh K, Hirayama A, Ishizaki K, Yamada A, Takeuchi M, Yamagishi S, Morito N, Nakano T, Ojima M, Shimohata H, Itoh K, Takahashi S, Yamamoto M. Hyperglycemia induces oxidative and nitrosative stress and increases renal functional impairment in Nrf2-deficient mice. Genes Cells 13: 1159–1170, 2008 [DOI] [PubMed] [Google Scholar]
- 65. Zakkar M, Van der Heiden K, Luong LA, Chaudhury H, Cuhlmann S, Hamdulay SS, Krams R, Edirisinghe I, Rahman I, Carlsen H, Haskard DO, Mason JC, Evans PC. Activation of Nrf2 in endothelial cells protects arteries from exhibiting a proinflammatory state. Arterioscler Thromb Vasc Biol 29: 1851–1857, 2009 [DOI] [PubMed] [Google Scholar]
- 66. Zhang H, Morgan B, Potter BJ, Ma L, Dellsperger KC, Ungvari ZI, Zhang C. Resveratrol improves left ventricular diastolic relaxation in type 2 diabetes by inhibiting oxidative/nitrative stress. Am J Physiol Heart Circ Physiol 299: H985–H994, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhang H, Zhang J, Ungvari Z, Zhang C. Resveratrol improves endothelial function: role of TNFα and vascular oxidative stress. Arterioscler Thromb Vasc Biol 29: 1164–1171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhang Y, Ikeno Y, Qi W, Chaudhuri A, Li Y, Bokov A, Thorpe SR, Baynes JW, Epstein C, Richardson A, Van Remmen H. Mice deficient in both Mn superoxide dismutase and glutathione peroxidase-1 have increased oxidative damage and a greater incidence of pathology but no reduction in longevity. J Gerontol A Biol Sci Med Sci 64: 1212–1220, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]