Abstract
Uridine adenosine tetraphosphate (Up4A) was reported as a novel endothelium-derived contracting factor. Up4A contains both purine and pyrimidine moieties, which activate purinergic (P2)X and P2Y receptors. However, alterations in the vasoconstrictor responses to Up4A in hypertensive states remain unclear. The present study examined the effects of Up4A on contraction of isolated renal arteries (RA) and pulmonary arteries (PA) from DOCA-salt rats using isometric tension recording. RA from DOCA-salt rats exhibited increased contraction to Up4A versus arteries from control uninephrectomized rats in the absence and presence of NG-nitro-l-arginine (nitric oxide synthase inhibitor). On the other hand, the Up4A-induced contraction in PA was similar between the two groups. Up4A-induced contraction was inhibited by suramin (nonselective P2 antagonist) but not by diinosine pentaphosphate pentasodium salt hydrate (Ip5I; P2X1 antagonist) in RA from both groups. Furthermore, 2-thiouridine 5′-triphosphate tetrasodium salt (2-ThioUTP; P2Y2 agonist)-, uridine-5′-(γ-thio)-triphosphate trisodium salt (UTPγS; P2Y2/P2Y4 agonist)-, and 5-iodouridine-5′-O-diphosphate trisodium salt (MRS 2693; P2Y6 agonist)-induced contractions were all increased in RA from DOCA-salt rats. Protein expression of P2Y2-, P2Y4-, and P2Y6 receptors in RA was similar between the two groups. In DOCA-salt RA, the enhanced Up4A-induced contraction was reduced by PD98059, an ERK pathway inhibitor, and Up4A-stimulated ERK activation was increased. These data are the first to indicate that Up4A-induced contraction is enhanced in RA from DOCA-salt rats. Enhanced P2Y receptor signaling and activation of the ERK pathway together represent a likely mechanism mediating the enhanced Up4A-induced contraction. Up4A might be of relevance in the pathophysiology of vascular tone regulation and renal dysfunction in arterial hypertension.
Keywords: extracellular nucleotide, ERK, purinoceptor
extracellular nucleotides contribute to the local regulation of vascular tone and play important roles in pathophysiological processes, including hypertension, diabetes, atherosclerosis, and remodeling (7, 9, 16). In blood vessels, nucleotides, such as ATP and UTP, can be released from platelets or from adventitial nerves and endothelial cells to induce either vasoconstriction or vasodilation through cell surface purinergic (P2) receptors (1, 8, 15).
P2 receptors for nucleotides belong to two major families: ionotropic P2X receptors and metabotropic P2Y receptors (1, 8, 15). Several reports demonstrated that these purinoceptors are expressed in renal and pulmonary vascular beds (12, 25, 31, 38, 52, 53), contributing to the physiology of these specialized organ systems (4, 22, 30). However, the involvement of these purinoceptors on vascular dysfunction associated with arterial hypertension is largely unknown, partially because most of these purinoceptors are capable of mediating responses to several nucleotides, resulting in multiple receptors having overlapping ligand preferences (8, 50).
The dinucleotide uridine adenosine tetraphosphate (Up4A) was identified by Jankowski et al. (35) as a novel potent endothelium-derived contracting factor. Up4A is also the first dinucleotide found in living organisms that contains both purine and pyrimidine moieties, which are known to potentially activate both P2X and P2Y receptors. In the last five years since the identification of Up4A (35), several reports have indicated that Up4A modulates vascular tone in rat aorta (41), rat pulmonary arteries (23), mouse renal afferent arterioles (34), and mouse aorta (24). Moreover, Jankowski et al. (35) found that in isolated perfused kidney, Up4A stimulated vasoconstriction mainly through P2X1 receptors and probably also through P2Y2 and P2Y4 receptors. More recently, findings from this same group indicate that in the rat isolated perfused kidney, in addition to smooth muscle P2X1 receptor-mediated vasoconstriction, Up4A showed dose-dependent P2Y2 receptor-mediated, long-lasting vasoconstriction (58). Furthermore, they demonstrated that vasoconstriction by Up4A was followed by vasodilation mediated by P2Y1 and P2Y2 receptor activation on endothelial cells leading to the release of nitric oxide (NO) (58). So far, these studies have been carried out only in normal animals, and there is no study to indicate the vascular effects of Up4A under pathophysiological conditions, such as arterial hypertension.
There is evidence that Up4A might have implications in the pathogenesis of human hypertension (36). Plasma Up4A concentration is increased in human juvenile hypertensives compared with normotensive controls (33). Up4A concentration is significantly correlated with the left ventricular mass in these juvenile hypertensives (33). However, the vascular responsiveness to Up4A in hypertensive subjects remains unexplored/unknown.
The aim of the present study was to determine whether the vascular effects of Up4A are altered in DOCA-salt rats, a salt-dependent experimental model of arterial hypertension (19, 39, 40, 56). We hypothesized that Up4A-induced vasoconstriction is augmented in DOCA-salt hypertension. We used two different arteries [renal artery (RA) because the kidney is unarguably relevant to determination of blood pressure and extralobar pulmonary artery (PA), which is an important artery to determinate capacitance of pulmonary circulation (57)]. To test our hypothesis and to identify the mechanisms involved in Up4A-altered vasoconstriction, we performed functional studies on RA and PA from normotensive and DOCA-salt rats.
MATERIALS AND METHODS
Reagents.
ACh, DOCA, phenylephrine (PE), diinosine pentaphosphate pentasodium salt hydrate (Ip5I), suramin, and antibodies against β-actin, P2Y2, and P2Y4 were all purchased from Sigma Chemical (St. Louis, MO). The antibody against P2Y6 was obtained from Enzo Life Sciences (Plymouth Meeting, PA), whereas antibodies against ERK1/2 and phosphorylated ERK1/2 (pT202/pY204) were obtained from Cell Signaling Technology (Danvers, MA). PD98059 and NG-nitro-l-arginine (l-NNA) were obtained from Calbiochem (San Diego, CA). Up4A was obtained from Biolog Life Science Institute (Bremen, Germany). 2-Thiouridine 5′-triphosphate tetrasodium salt (2-ThioUTP), uridine-5′-(γ-thio)-triphosphate trisodium salt (UTPγS), and 5-iodouridine-5′-O-diphosphate trisodium salt (MRS 2693) were obtained from Tocris Bioscience (Ellisville, MO). Drugs were dissolved in sterile HPLC grade water. Horseradish peroxidase-linked secondary anti-mouse and anti-rabbit antibodies were purchased from GE Healthcare (Piscataway, NJ).
Animals.
Male Wistar rats (8 wk old, 230–250 g; Harlan Laboratories, Indianapolis, IN) were used in this study. All procedures were performed in accordance with the Guiding Principles in the Care and Use of Animals and approved by the Medical College of Georgia Committee on the Use of Animals in Research and Education. The animals were housed three per cage on a 12-h:12-h light/dark cycle and fed a standard chow diet with water ad libitum.
DOCA-salt hypertension, systolic blood pressure measurements, and assessment of left ventricular hypertrophy.
DOCA-salt hypertension was induced in rats as previously described (19, 39, 40). Briefly, all animals were uninephrectomized under anesthesia and were given no further treatment (Uni rats) or received 1% NaCl plus 0.2% KCl in the drinking water and DOCA silastic pellets (0.2 g/kg), which were implanted subcutaneously in the scapular region (DOCA-salt rats). The duration of treatment was 4 to 5 wk.
One week before euthanasia, systolic blood pressure (SBP) was measured by the tail-cuff method using a CODA system (Kent Scientific). At the end of 4 to 5 wk of treatment, RA and PA arteries were removed and they were submitted to experimental procedures.
Left-ventricular hypertrophy was assessed by left-to-right ventricular ratio and left ventricular body weight index after removing the atria and the right ventricle free wall and separating them from the left ventricle, including the septum.
Arterial isolation and functional studies.
Vascular isometric force was recorded by myograph technique as previously described (19, 20, 27, 39, 40). After euthanasia, the left and right PA and the main branch of the RA were isolated and placed in an ice-cold physiological salt solution (PSS). This solution consisted of (in mM) 130.0 NaCl, 4.7 KCl, 14.9 NaHCO3, 5.5 dextrose, 1.18 KH2PO4, 1.17 MgSO47H2O, 1.6 CaCl22H2O, and 0.026 EDTA. The artery was carefully cleaned of all fat and connective tissue under a stereomicroscope. The arteries were then cut into 2-mm rings (2–4 rings were obtained from each animal) and mounted on a wire myograph (Danish MyoTech; Aarhus, Denmark) filled with 5 ml PSS and continuously gassed with 95% O2-5% CO2 while maintaining temperature at 37°C. The rings were stretched until an optimal resting tension of 10 mN (PA) or 3 mN (RA) and then allowed to equilibrate for at least 45 min. The optimal basal tensions were established from preliminary length-active tension curves, and these were similar in arteries from DOCA-salt and Uni groups (data not shown). Arterial integrity was assessed by contracting the segments with a depolarizing concentration of potassium chloride (KCl; 120 mM) and subsequently with phenylephrine (PE; 10−5 M) followed by relaxation with ACh (10−5 M).
After washing and equilibrating for 1 h, Up4A (10−9 − 3 × 10−5 M) or PE (10−10 − 10−4 M) was added cumulatively to the bath until a maximal response was achieved. After the addition of sufficient aliquots of the agonist to produce the chosen concentration, a plateau response was allowed to develop before the addition of the next concentration of the same agonist. To investigate the effects of NO on Up4A-induced contraction, rings were incubated with 10−4 M l-NNA [NO synthase (NOS) inhibitor] for 30 min before administration of Up4A. Concentration-response curves for Up4A-induced contraction in RA were also generated in the combined presence of 10−4 M l-NNA and either 10−4 M suramin [non-selective P2 antagonist (1, 7)] or 10−4 M Ip5I [P2X1 antagonist (37, 48)]. To investigate P2Y receptor-mediated contraction in RA, concentration-response curves for 2-ThioUTP [10−9 − 3 × 10−5 M; P2Y2 selective agonist (14)], UTPγS [10−9 − 3 × 10−5 M; P2Y2/P2Y4 selective agonist (60)], or MRS 2693 [10−9 − 3 × 10−5 M; P2Y6 selective agonist (42)] in the presence of 10−4 M l-NNA were performed.
In another set of experiments, performed to investigate a role of the ERK pathway on Up4A-induced contraction in RA, concentration-response curves for Up4A-induced contraction in RA were generated in the combined presence of 10−4 M l-NNA and 10−5 M PD98059 [ERK inhibitor (19, 20, 43)].
Western blotting.
The protein levels of P2Y2, P2Y4, and P2Y6 receptors and of phosphorylated ERK1/2 and total ERK1/2 were quantified using immunoblotting procedures, essentially as described before (19, 20, 39, 40). RA from DOCA-salt and Uni rats were isolated, cleaned from fat, dissected, and frozen in liquid nitrogen. To investigate the expression of phospho-ERK1/2 in RA upon Up4A stimulation, the RA rings were incubated with 10−4 M l-NNA or with 10−4 M l-NNA plus 10−5 M PD98059 for 30 min. After incubation, the rings were exposed to 3 × 10−6 M Up4A for 5 min. These rings were removed rapidly and rinsed with ice-cold Ca2+-free PSS containing 1 mM sodium orthovanadate and frozen in liquid nitrogen. Proteins (20 μg for P2Y receptor or 10 μg for phospho-ERK1/2 and total ERK1/2) extracted from the arteries were separated by electrophoresis on a 10% polyacrylamide gel and transferred to a nitrocellulose membrane. Nonspecific binding sites were blocked with 5% skim milk in Tris-buffered saline solution with Tween (0.1%) for 1 h at 24°C. Membranes were then incubated with antibodies overnight at 4°C. Antibodies were as follows: anti-P2Y2 (42 kDa; 1:200), anti-P2Y4 (78 kDa; 1:500), anti-P2Y6 (100 kDa; 1:500), anti-phospho-ERK1/2 (pT202/pY204; 44 and 42 kDa; 1:1,000), anti-ERK1/2 (44 and 42 kDa; 1:1,000), and β-actin (42 kDa; 1:15,000). After incubation with secondary antibodies, signals were revealed with chemiluminesence, visualized by autoradiography, and quantified densitometrically. Results were normalized by β-actin expression and expressed as a percentage of control.
Data analyses and statistics.
Arterial contractions recorded from the myograph were expressed as changes in the displacement from baseline in millinewtons and were represented as percent maximum contraction to 120 mM KCl (percent contraction). The contractions induced by 120 mM KCl were similar between DOCA-salt and Uni groups in RA [12.80 ± 0.96 mN (n = 57) and 15.03 ± 0.88 mN (P > 0.05; n = 54), respectively] and PA [9.31 ± 0.75 mN (n = 18) and 8.86 ± 0.36 mN (P > 0.05; n = 18), respectively]. Agonist concentration-response curves were fitted using a nonlinear interactive fitting program (Graph Pad Prism 5.0; GraphPad Software, San Diego, CA). Data are expressed as means ± SE. Statistical analysis of the concentration-response curves was performed by using two-way ANOVA for comparison between the groups. When compared with the control Uni group, data were analyzed using ANOVA with post hoc Bonferroni testing or Student's t-test. Western blot data were analyzed by one-sample t-test or ANOVA with post hoc Bonferroni testing. The values of P < 0.05 were considered statistically significant.
RESULTS
SBP and body weight of the rats.
At 3 or 4 wk, DOCA-salt rats displayed higher SBP (in mmHg), compared with Uni rats (186 ± 4, n = 35 vs. 134 ± 2, n = 35, P < 0.001, respectively). At the time of the experiment, the body weight of the DOCA-salt rats was lower than that of Uni rats (351.1 ± 6.2 g, n = 35 vs. 428.1 ± 5.2 g, n = 3, P < 0.001, respectively).
To evaluate left ventricular hypertrophy, we measured left ventricle-to-body weight ratio and left-to-right ventricular ratio, which are important indexes of left ventricular hypertrophy (13, 32). These parameters of the DOCA-salt rats were significantly higher than that of Uni rats (left ventricle-to-body weight ratio, 2.77 ± 0.05 mg/g, n = 35 vs. 1.83 ± 0.02 mg/g, n = 35, P < 0.001, respectively; left-to-right ventricular ratio, 4.48 ± 0.12, n = 35 vs. 3.45 ± 0.08, n = 35, P < 0.001, respectively).
Up4A and PE reactivity in RA and PA.
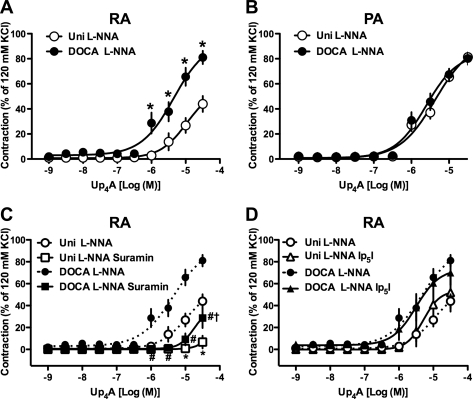
Cumulative administration of Up4A (10−9 − 3 × 10−5 M)-induced concentration-dependent contractions in RA (Fig. 1A) and PA (Fig. 1B) rings from both DOCA-salt and Uni rats. The Up4A-induced concentration-dependent contractile response was significantly greater in RA rings from DOCA-salt rats than in those from Uni rats (Fig. 1A). On the other hand, in the PA rings, the Up4A-induced contraction was similar between DOCA-salt and Uni rats (Fig. 1B). Exposure of RA (Fig. 1C) or PA (Fig. 1D) to PE (10−10-10−4 M) led to a concentration-dependent increase in tension in the DOCA-salt and Uni groups. Although PE-induced maximal response (Emax; percentage of 120 mM KCl-induced contraction) was similar between DOCA-salt and Uni rats [110.7 ± 6.1% (n = 7) and 115.6 ± 3.6% (n = 7), P > 0.05, respectively], the concentration-response curve was significantly leftward shifted in RA rings from DOCA-salt rats versus Uni rats [−log EC50: 6.51 ± 0.13 (n = 7) and 5.76 ± 0.05 (P < 0.01, n = 7), respectively]. On the other hand, PE-induced contractile responses were similar in PA rings from DOCA-salt [Emax and −log EC50: 84.1 ± 2.4% and 7.44 ± 0.09 (n = 6)] and Uni rats [Emax and −log EC50: 73.7 ± 2.5% and 7.54 ± 0.10 (P > 0.05, n = 6)].
Fig. 1.
Contractile responses to uridine adenosine tetraphosphate (Up4A) and phenylephrine (PE) are augmented in renal arteries (RA) but not pulmonary arteries (PA)from DOCA-salt hypertensive rats. Concentration-response curves for Up4A (A, B) and PE (C, D) in RA (A, C) and PA (B, D) arterial rings obtained from DOCA-salt (DOCA) and normotensive control [uninephrectomized (Uni)] rats are shown. Data are means ± SE from 6 to 8 experiments; the SE is included only when it exceeds the dimension of the symbol used. *P < 0.05, DOCA vs. Uni.
l-NNA, an inhibitor of NOS, and Up4A-induced contraction of RA and PA.
NO plays an important role in the regulation of vascular tone, both under basal conditions and when tone is augmented by various vasoconstrictor agonists (43–46). Indeed, Up4A-induced NO-dependent relaxation has been observed in rat aorta (41) and in rat perfused kidney (58). To mask any putative NO component in Up4A-induced contraction in the RA and PA, we used a representative NOS inhibitor. In the presence of l-NNA (10−4 M), which inhibits both basal and agonist-induced NOS activity, Up4A-induced contraction was increased in RA and PA from both the DOCA-salt and Uni groups (Fig. 1 vs. Fig. 2). In the presence of l-NNA, Up4A-induced contraction was significantly greater in RA rings from DOCA-salt than in those from Uni (Fig. 2A) but was similar in PA rings between DOCA-salt and Uni (Fig. 2B).
Fig. 2.
Contractile responses to Up4A in the presence of nitric oxide synthase (NOS) inhibitor are augmented in RA but not PA from DOCA-salt hypertensive rats. Concentration-response curves for Up4A in isolated rings of RA (A) or PA (B) in the presence of 10−4 M NG-nitro-l-arginine (l-NNA) are shown. C, D: contractile response to Up4A in the presence of NOS inhibitor is suppressed by purinergic (P2)Y antagonist but not by P2X antagonist in RA. Effects of 10−4 M suramin (C) or 10−4 M diinosine pentaphosphate pentasodium salt hydrate (Ip5I; D) on concentration-response curves for Up4A in RA obtained from DOCA-salt and Uni rats in the presence of 10−4 M l-NNA. Data are means ± SE from 3 to 8 experiments; the SE is included only when it exceeds the dimension of the symbol used. The concentration-response curves for Up4A depicted in A are replotted in C and D. *P < 0.05 vs. Uni l-NNA group; #P < 0.05 DOCA l-NNA vs. DOCA l-NNA suramin group; †P < 0.05, Uni l-NNA suramin vs. DOCA l-NNA suramin group.
Effects of P2 receptor antagonism on Up4A-induced contraction in RA.
To investigate which P2 receptors are responsible for the augmented Up4A-induced contraction in RA from DOCA-salt rats, we determined the effects of P2 receptor antagonists on Up4A-induced contractions in the presence of 10−4 M l-NNA. Pretreatment of RA with the nonselective P2 receptor antagonist suramin (10−4 M) markedly reduced Up4A-induced contractions in both DOCA-salt and Uni rats (Fig. 2C). On the other hand, pretreatment with a P2X1 receptor antagonist Ip5I (10−4 M) did not affect Up4A-induced contraction either in DOCA-salt or Uni rats (Fig. 2D).
Selective P2Y receptor agonist-induced contraction in RA.
To investigate the possible mechanisms underlying the alterations in Up4A-mediated responses in RA from DOCA-salt rats, we next examined the effects of selective P2Y receptor agonists in the presence of 10−4 M l-NNA. Interestingly, 2-ThioUTP [P2Y2 selective agonist (Fig. 3A)]-, UTPγS [P2Y2/P2Y4 selective agonist (Fig. 3B)]-, and MRS 2693 [P2Y6 selective agonist (Fig. 3C)]-induced contractions were all significantly increased in RA from DOCA-salt rats versus Uni rats.
Fig. 3.
Contractile responses to P2Y agonists in the presence of NOS inhibitor are augmented in RA from DOCA-salt hypertensive rats. Concentration-response curves for selective P2Y receptor agonist [2-thiouridine 5′-triphosphate tetrasodium salt (2-ThioUTP; P2Y2 agonist; A), uridine-5′-(γ-thio)-triphosphate trisodium salt (UTPγS; P2Y2/P2Y4 agonist; B), and 5-iodouridine-5′-O-diphosphate trisodium salt (MRS 2693; P2Y6 agonist; C)] in isolated rings of RA obtained from DOCA-salt and Uni rats in the presence of 10−4 M l-NNA are shown. Data are means ± SE from 4 or 5 experiments; the SE is included only when it exceeds the dimension of the symbol used. *P < 0.05, DOCA vs. Uni.
Protein expression of P2Y receptors in RA.
Conceivably, an increased expression of P2Y receptors in RA could underlie the enhanced Up4A vasoconstriction observed in DOCA-salt rats. Accordingly, we examined the protein expression of P2Y receptors in RA by immunoblotting (Fig. 4). Expression levels of P2Y2 (Fig. 4A) and P2Y4 (Fig. 4B) receptors were similar between arteries from DOCA-salt and Uni rats. The vascular expression of P2Y6 receptor tended to be higher in DOCA-salt than in Uni rats; however, statistical significance was not reached (P = 0.23; Fig. 4C).
Fig. 4.
The receptor expressions of P2Y2, P2Y4, and P2Y6 are similar between RA from DOCA-salt and Uni rats. Analysis of P2Y2 (A), P2Y4 (B), and P2Y6 (C) protein expression in RA obtained from DOCA-salt and Uni rats is shown. Left: representative Western blots for P2Y2, P2Y4, P2Y6, and β-actin. Proteins were subjected to 10% SDS-PAGE and then transferred to nitrocellulose membranes. They were then incubated with a primary antibody specific for P2Y2 (42 kDa), P2Y4 (78 kDa), P2Y6 (100 kDa), or β-actin (42 kDa) and also with a secondary anti-rabbit or anti-mouse antibody. Right: bands were quantified as described in materials and methods. Ratios were calculated for the optical density of each receptor over that of β-actin. Data are means ± SE from 4 experiments.
Effect of ERK pathway inhibition on Up4A-induced contraction in RA.
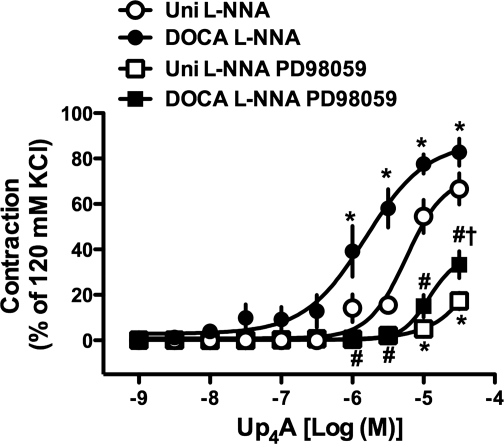
The ERK pathway plays important roles in the regulation of vascular tone (19, 20, 43). Pretreatment of RA with an ERK inhibitor (PD98059; 10−5 M) markedly reduced Up4A-induced contractions in the presence of l-NNA (10−4 M) in both DOCA-salt and Uni rats (Fig. 5).
Fig. 5.
Contractile response to Up4A in the presence of NOS inhibitor is suppressed by ERK inhibition in RA. Effects of 10−5 M PD98059 on concentration-response curves for Up4A in RA obtained from DOCA-salt and Uni rats in the presence of 10−4 M l-NNA are shown. Data are means ± SE from 5 experiments; the SE is included only when it exceeds the dimension of the symbol used. *P < 0.05 vs. Uni l-NNA group; #P < 0.05 DOCA l-NNA vs. DOCA l-NNA PD98059 group; †P < 0.05, Uni l-NNA PD98059 vs. DOCA l-NNA PD98059 group.
Effect of Up4A on ERK activation in RA.
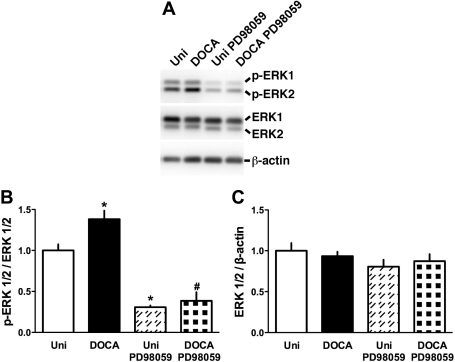
Collectively, the present data (Fig. 5) and previous reports (19, 20, 43) suggest that the ERK pathway modulates contractile responses of vascular smooth muscle. As shown in Fig. 6, Up4A (3 × 10−6 M)-stimulated ERK1/2 phosphorylation (activation), as detected by means of Western blotting, was significantly greater in RA from DOCA-salt rats than in those from Uni rats. The phosphorylated ERK1/2 level was greatly suppressed by PD98059 (10−5 M) treatment. The total ERK1/2 expression was similar among these groups (Fig. 6C).
Fig. 6.
ERK1/2 phosphorylation is increased in Up4A-treated RA from DOCA-salt hypertensive rats. A: representative Western blots for phosphorylated ERK1/2 (p-ERK1/2), total ERK1/2, and β-actin. B and C: bands were quantified as described in materials and methods. Ratios were calculated for the optical density of p-ERK1/2 over that of the corresponding total ERK1/2 (B) or for the optical density of total ERK1/2 over that of the corresponding β-actin (C). Data are means ± SE from 5 to 8 experiments. *P < 0.05 vs. Uni; #P < 0.05 vs. DOCA.
DISCUSSION
The main conclusion to be drawn from the present study is that enhanced Up4A-induced contraction in RA, but not in PA, of DOCA-salt hypertensive rats is due to increased activation of P2Y receptor signaling, and the mechanisms underlying this enhancement may be related to increases in ERK1/2 activity.
Up4A is the first dinucleotide containing one purine and one pyrimidine moiety found in living organisms (35). Although Up4A has been characterized as a potent constrictor in rat aorta (41), rat pulmonary arteries (23), rat perfused kidney (35, 58), mouse aorta (24), and mouse renal afferent arterioles (34), there are no studies on its vascular effects in vessels from animals with vascular disease. The present study is the first to show enhancement of Up4A-induced contraction in RA in a salt-dependent model of hypertension, the DOCA-salt rats.
In general, NO negatively modulates vasoconstrictor responses in smooth muscle (4, 43, 44). Indeed, Up4A-induced NO-dependent relaxation has been observed in rat aorta (41) and in rat perfused kidney (58). Moreover, Up4A-induced contraction is increased by treatment with NOS inhibitor in PA (23) and in perfused kidney (34, 58). In the present study, we found that Up4A-induced contraction was augmented in the presence of a NOS inhibitor in both the DOCA-salt and Uni groups. However, it should be noted that under such condition, of masking NO component, Up4A-induced contraction was still greater in RA from DOCA-salt rats versus Uni group. These results suggest that the hyperreactivity to Up4A in RA from DOCA-salt rats may be attributable to alterations on smooth muscle signaling rather than a defect in NO activity/signaling upon Up4A stimulation.
The purine and pyrimidine moieties of Up4A potentially activate P2X and P2Y receptors (35, 36). Several reports suggest that upregulation of P2 receptors is associated with the development of cardiovascular diseases (10, 29, 55). The activation of both P2X and P2Y receptors on smooth muscle cells leads to vasoconstriction in several arteries (9, 16, 30). Gui et al. (23) suggested that Up4A leads to contraction of rat isolated PA probably via both suramin-sensitive and suramin-resistant P2Y receptors. In rat perfused kidney, Up4A-induced vasoconstriction depends not only on the activation of the P2X1 receptor (35) but also on the activation of the P2Y2 receptor (58). In the present study, the increased Up4A-induced contraction in RA from DOCA-salt rats was not due to the activation of P2X receptors since Up4A-mediated responses were unaffected by Ip5I. Four subtypes of P2Y receptors have been found to be coexpressed in vascular smooth muscle cells: P2Y1, P2Y2, P2Y4, and P2Y6 receptors (17). P2Y2 and P2Y6 receptors appear to be the most abundant. P2Y1 receptor expression is very low or undetectable in contractile vascular smooth muscle cells (17, 49). All P2Y receptor subtypes are coupled to phospholipase C activation and cause an elevation in the intracellular Ca2+ concentration (21, 49). Moreover, using P2Y6 receptor knockout mice, Bar et al. (3) demonstrated that the P2Y6 receptor is responsible for the contractile action of both UDP and UTP and that P2Y6 receptor is expressed and functional in vascular smooth muscle cells. In the present study, we found that in isolated RA: 1) Up4A-induced contraction is inhibited (but not abolished) by suramin, which contrasts with the findings that P2Y6 receptor is usually insensitive to suramin (11, 51); 2) increased contractions are induced by the selective agonists for P2Y2, P2Y2/P2Y4, or P2Y6 in DOCA-salt rats; and 3) these arteries express P2Y2-, P2Y4-, and P2Y6 receptors; however, these expressions did not change between two groups. Taken together, the evidence and our results indicate that the increased Up4A-induced contraction in RA from DOCA-salt rats is due to activation of P2Y receptors and activation of downstream component(s) of P2Y receptor. However, the possibility of P2Y6 receptor activation contributing to augmented Up4A-induced contraction in RA from DOCA-salt rats cannot be completely ruled out considering that suramin-resistant contraction induced by Up4A is higher in DOCA-salt rats; P2Y6 agonist-induced contraction was very weak, but specifically unmasked in DOCA-salt rats; and P2Y6 receptor expression was slightly increased in DOCA-salt rats. However, further studies will be required to determine how and to what extent abnormal/augmented activation of P2Y receptors are involved in the increased Up4A-induced vasoconstriction in this hypertensive model. Currently, it is not possible to study single P2Y receptor subtypes due to the lack of selective antagonists.
Various signal transduction pathways (which are associated with vasoconstriction) activated by P2Y receptor stimulation have been proposed, including MAPK, Rho A/Rho kinase, and EGF receptor (EGFR) transactivation (47, 54). Moreover, several reports have demonstrated that these signal transduction pathways are altered in various animal models, including mineralocorticoid hypertension (5, 18, 19, 59). In the present study, we observed that Up4A-induced contraction was markedly suppressed by ERK inhibition. Furthermore, Up4A-stimulated ERK activation was greater in RA from DOCA-salt rats than in those from Uni rats. These results strongly indicate that the ERK pathway makes a substantial contribution to the differences in Up4A-induced contraction observed between DOCA-salt and Uni rats. However, other signaling pathway(s) may also contribute to the enhanced Up4A-induced contraction because the difference in RA Up4A-induced contraction between two groups could not be completely abolished by treatment with ERK inhibitor. Therefore, future research will need to focus on the changes in signal transduction pathways activated by Up4A in animals in a hypertensive state.
Up4A has been proposed as a circulating, endothelium-derived vasoconstrictor since it is released from human endothelial cells stimulated by agonists such as ACh and endothelin-1 (ET-1) or by mechanical stress. Furthermore, these stimulated levels of Up4A are sufficient to produce vasoconstriction and increase mean arterial blood pressure (35). In previous reports, the circulating level of Up4A is in the nanomolar range (33, 35), and such lower concentrations of Up4A can lead to vasoconstriction in perfused systems (34, 35, 58). Considering a report that suggested the circulating Up4A level is correlated with the left ventricular mass (33) and that DOCA-salt rats exhibit left ventricular hypertrophy, it is possible that the circulating Up4A level is increased in DOCA-salt hypertensive rats. With the use of the myograph technique, higher concentrations of Up4A (i.e., in the micromolar range) were required to achieve adequate vasoconstriction (even though Up4A sensitivity is increased in RA rings from DOCA-salt rats), and this is consistent with previous reports using other arterial rings in the myograph system (23, 24, 41). The discrepancy between these studies may be explained by differences between these methodologies. Although previous studies reported that the circulating Up4A level is in nanomolar range (33, 35), the local level of Up4A may transiently reach higher concentrations (i.e., over the nanomolar range) leading to vasoconstriction. Endothelial cell activation and release of Up4A from the endothelium (or unknown sources) may be different in various regions and conditions. However, future experiments need to be performed to address this question.
In our DOCA-salt model, the hyperreactivity to Up4A was specifically seen in RA but not in PA. This suggests that Up4A may be important for the regulation of systemic vascular tone and have a functional role in the regulation of renal and pulmonary blood flow in hypertensive states. There are several reports suggesting that the basal renal perfusion pressure does not differ between DOCA-salt rats and controls. However, the responsiveness to various drugs (e.g., ET-1, ACh) on renal perfusion pressure is altered in DOCA-salt hypertensive rats (26, 28). On the other hand, the PA is expected to be less affected by hypertension, since the blood pressure in this artery is known to be much lower than the blood pressure in systemic arteries (2, 6). Moreover, in small mesenteric arteries, which are important resistance vessels (27), Up4A-induced contraction was smaller in DOCA-salt rats than in Uni rats (unpublished observation). All together, these data suggest that the Up4A-mediated contraction may be heterogenous among various vascular beds in systemic hypertension. However, to establish our hypothesis, future studies are needed to investigate the vasoconstrictor effects of Up4A in various arteries.
In conclusion, the present findings indicate that increased activation of P2Y receptors and ERK1/2 pathway represents a likely mechanism mediating the enhancement of Up4A responses in DOCA-salt hypertensive rats. Because it was shown in young hypertensive patients that the Up4A concentration correlates with blood pressure (33), results of the present study indicate that abnormal vascular responsiveness to Up4A may contribute to the development of arterial hypertension and/or aggravate renal dysfunction associated with hypertension. Understanding the signal transduction and regulation of vascular tone by Up4A may be of extreme value to understanding the pathophysiology and treatment of hypertension.
GRANTS
This study was supported in part by the National Institutes of Health (Grants R01 HL-071138 and R01 DK-083685) and by the Naito Foundation Japan.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International union of pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58: 281–341, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aharinejad S, Schraufnagel DE, Bock P, MacKay CA, Larson EK, Miksovsky A, Marks SC., Jr Spontaneously hypertensive rats develop pulmonary hypertension and hypertrophy of pulmonary venous sphincters. Am J Pathol 148: 281–290, 1996 [PMC free article] [PubMed] [Google Scholar]
- 3.Bar I, Guns PJ, Metallo J, Cammarata D, Wilkin F, Boeynams JM, Bult H, Robaye B. Knockout mice reveal a role for P2Y6 receptor in macrophages, endothelial cells, and vascular smooth muscle cells. Mol Pharmacol 74: 777–784, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Barnes PJ, Liu SF. Regulation of pulmonary vascular tone. Pharmacol Rev 47: 87–131, 1995 [PubMed] [Google Scholar]
- 5.Benter IF, Canatan H, Benboubetra M, Yousif MH, Akhtar S. Global upregulation of gene expression associated with renal dysfunction in DOCA-salt-induced hypertensive rats occurs via signaling cascades involving epidermal growth factor receptor: a microarray analysis. Vascul Pharmacol 51: 101–109, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Bieger D, Duggan JA, Tabrizchi R. Effects of chloride substitution on electromechanical responses in the pulmonary artery of Dahl normotensive and hypertensive rats. Br J Pharmacol 141: 1068–1076, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev 58: 58–86, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci 64: 1471–1483, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnstock G. Purinergic regulation of vascular tone and remodelling. Auton Autacoid Pharmacol 29: 63–72, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Carpenter RC, Miao L, Miyagi Y, Bengten E, Zhang JH. Altered expression of P2 receptor mRNAs is the basilar artery in a rat double hemorrhage model. Stroke 32: 516–522, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Chang K, Hanaoka K, Kumada M, Takuwa Y. Molecular cloning and functional analysis of a novel P2 nucleotide receptor. J Biol Chem 270: 26152–26158, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Churchill PC, Ellis VR. Pharmacological characterization of the renovascular P2 purinergic receptors. J Pharmacol Exp Ther 265: 334–338, 1993 [PubMed] [Google Scholar]
- 13.Ebrahim Z, Yellon DM, Baxter GF. Attenuated cardioprotective response to bradykinin, but not classical ischaemic preconditioning, in DOCA-salt hypertensive left ventricular hypertrophy. Pharmacol Res 55: 42–48, 2007 [DOI] [PubMed] [Google Scholar]
- 14.El-Tayeb A, Qi A, Muller CE. Synthesis and structure-activity relationships of uracil nucleotide derivatives and analogues as agonists at human P2Y2, P2Y4, and P2Y6 receptors. J Med Chem 49: 7076–7087, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Erb L, Liao Z, Seye CI, Weisman GA. P2 receptors: intracellular signaling. Pflügers Arch 452: 552–562, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal 4: 1–20, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erlinge D, Hou M, Webb TE, Barnard EA, Moller S. Phenotype changes of the vascular smooth muscle cell regulate P2 receptor expression as measured by quantitative RT-PCR. Biochem Biophys Res Commun 248: 864–870, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Florian JA, Watts SW. Epidermal growth factor: a potent vasoconstrictor in experimental hypertension. Am J Physiol Heart Circ Physiol 276: H976–H983, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Giachini FR, Sullivan JC, Lima VV, Carneiro FS, Fortes ZB, Pollock DM, Carvalho MH, Webb RC, Tostes RC. Extracellular signal-regulated kinase 1/2 activation, via downregulation of mitogen-activated protein kinase phosphatase 1, mediated sex differences in deoxycorticosterone acetate-salt hypertension vascular reactivity. Hypertension 55: 172–179, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giachini FR, Zemse SM, Carneiro FS, Lima VV, Carneiro ZN, Callera GE, Ergul A, Webb RC, Tostes RC. Interleukin-10 attenuates vascular responses to endothelin-1 via effects on ERK1/2-dependent pathway. Am J Physiol Heart Circ Physiol 296: H489–H496, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Govindan S, Taylor EJ, Taylor CW. Ca2+ signalling by P2Y receptors in cultured rat aortic smooth muscle cells. Br J Pharmacol 160: 1953–1962, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guan Z, Osmond DA, Inscho EW. P2X receptors as regulators of the renal microvasculature. Trends Pharmacol Sci 28: 646–652, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Gui Y, Walsh MP, Jankowski V, Jankowski J, Zheng XL. Up4A stimulates endothelium-independent contraction of isolated rat pulmonary artery. Am J Physiol Lung Cell Mol Physiol 294: L733–L738, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Hansen PB, Hristovska A, Wolff H, Vanhoutte P, Jensen BL, Bie P. Uridine adenosine tetraphosphate affects contractility of mouse aorta and decrease blood pressure in conscious rats and mice. Acta Physiologica 200: 171–179, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Hassessian H, Burnstock G. Interacting roles of nitric oxide and ATP in the pulmonary circulation of the rat. Br J Pharmacol 114: 846–850, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayakawa H, Hirata Y, Suzuki E, Kimura K, Kikuchi K, Nagano T, Hirobe M, Omata M. Long-term administration of l-arginine improves nitric oxide release from kidney in deoxycorticosterone acetate-salt hypertensive rats. Hypertension 23: 752–756, 1994 [DOI] [PubMed] [Google Scholar]
- 27.Hilgers RH, Todd J, Jr, Webb RC. Regional heterogeneity in acetylcholine-induced relaxation in rat vascular bed: role of calcium-activated K+ channels. Am J Physiol Heart Circ Physiol 291: H216–H222, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Hirata Y, Hayakawa H, Suzuki E, Kimura K, Kikuchi K, Nagano T, Hirobe M, Omata M. Direct measurements of endothelium-derived nitric oxide release by stimulation of endothelin receptors in rat kidney and its alteration in salt-induced hypertension. Circulation 91: 1229–1235, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Hou M, Harden TK, Kuhn CM, Baldetorp B, Lazarowski E, Pendergast W, Moller S, Edvinsson L, Erlinge D. UDP acts as a growth factor for vascular smooth muscle cells by activation of P2Y6 receptors. Am J Physiol Heart Circ Physiol 282: H784–H792, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Inscho EW. ATP, P2 receptors and the renal microcirculation. Purinergic Signal 5: 447–460, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inscho EW, Ohishi K, Navar LG. Effects of ATP on pre- and postglomerular juxtamedullary microvasculature. Am J Physiol Renal Fluid Electrolyte Physiol 263: F886–F893, 1992 [DOI] [PubMed] [Google Scholar]
- 32.Jadhav A, Torlakovic E, Ndisang JF. Interaction among heme oxygenase, nuclear factor-kappaB, and transcription activating factors in cardiac hypertrophy in hypertension. Hypertension 52: 910–917, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Jankowski V, Meyer AA, Schlattmann P, Gui Y, Zheng XL, Stamcou I, Radtke K, Tran TN, van der Giet M, Tolle M, Zidek W, Jankowski J. Increased uridine adenosine tetraphosphate concentrations in plasma of juvenile hypertensives. Arterioscler Thromb Vasc Biol 27: 1776–1781, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Jankowski V, Patzak A, Herget-Rosenthal S, Tran TN, Lai EY, Gunthner T, Buschmann I, Zidek W, Jankowski J. Uridine adenosine tetraphosphate acts as an autocrine hormone affecting glomerular filtration rate. J Mol Med 86: 333–340, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Jankowski V, Tolle M, Vanholder R, Schonfelder G, van der Giet M, Henning L, Schluter H, Paul M, Zidek W, Jankowski J. Uridine adenosine tetraphosphate: a novel endothelium-derived vasoconstrictive factor. Nat Med 11: 223–227, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Jankowski V, van der Giet M, Mischak H, Morgan M, Zidek W, Jankowski J. Dinucleoside polyphosphates: strong endogenous agonists of the purinergic system. Br J Pharmacol 157: 1142–1153, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.King BF, Liu M, Pintor J, Gualix J, Miras-Portugal MT, Burnstock G. Diinosine pentaphosphate (IP5I) is a potent antagonist at recombinant rat P2X1 receptors. Br J Pharmacol 128: 981–988, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knight GE, Oliver-Redgate R, Burnstock G. Unusual absence of endothelium-dependent or -independent vasodilatation to purines or pyrimidines in the rat renal artery. Kidney Int 64: 1389–1397, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Lima VV, Giachini FR, Carneiro FS, Carneiro ZN, Saleh MA, Pollock DM, Fortes ZB, Carvalho MH, Ergul A, Webb RC, Tostes RC. O-GlcNAcylation contributes to augmented vascular reactivity induced by endothelin-1. Hypertension 55: 180–188, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lima VV, Giachini FR, Choi H, Carneiro FS, Carneiro ZN, Fortes ZB, Carvalho MH, Webb RC, Tostes RC. Impaired vasodilator activity in deoxycorticosterone acetate-salt hypertension is associated with increased protein O-GlcNAcylation. Hypertension 53: 166–174, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linder AE, Tumbri M, Linder FF, Webb RC, Leite R. Uridine adenosine tetraphosphate induces contraction and relaxation in rat aorta. Vascul Pharmacol 48: 202–207, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mamedova LK, Wang R, Besada P, Liang BT, Jacobson KA. Attenuation of apoptosis in vitro and ischemia/reperfusion injury in vivo in mouse skeletal muscle by P2Y6 receptor activation. Pharmacol Res 58: 232–239, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsumoto T, Ishida K, Nakayama N, Kobayashi T, Kamata K. Involvement of NO and MEK/ERK pathway in enhancement of endothelin-1-induced mesenteric artery contraction in later-stage type 2 diabetic Goto-Kakizaki rat. Am J Physiol Heart Circ Physiol 296: H1388–H1397, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Matsumoto T, Kakami M, Noguchi E, Kobayashi T, Kamata K. Imbalance between endothelium-derived relaxing and contracting factors in mesenteric arteries from aged OLETF rats, a model of Type 2 diabetes. Am J Physiol Heart Circ Physiol 293: H1480–H1490, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Matsumoto T, Noguchi E, Ishida K, Kobayashi T, Yamada N, Kamata K. Metformin normalizes endothelial function by suppressing vasoconstrictor prostanoids in mesenteric arteries from OLETF rats, a model of type 2 diabetes. Am J Physiol Heart Circ Physiol 295: H1165–H1176, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Michel FS, Man RYK, Vanhoutte PM. Increased spontaneous tone in renal arteries of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 293: H1673–H1681, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Norambuena A, Palma F, Poblete MI, Donoso MV, Pardo E, Gonzalez A, Huidobro-Toro JP. UTP controls cell surface distribution and vasomotor activity of the human P2Y2 receptor through an epidermal growth factor receptor-transregulated mechanism. J Biol Chem 285: 2940–2950, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Osmond DA, Inscho EW. P2X1 receptor blockade inhibits whole kidney autoregulation of renal blood flow in vivo. Am J Physiol Renal Physiol 298: F1360–F1368, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pacaud P, Malam-Souley R, Loirand G, Desgranges C. ATP raises [Ca2+]i via different P2-receptor subtypes in freshly isolated and cultured aortic myocytes. Am J Physiol Heart Circ Physiol 269: H30–H36, 1995 [DOI] [PubMed] [Google Scholar]
- 50.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 50: 413–492, 1998 [PubMed] [Google Scholar]
- 51.Robaye B, Boeynaems JM, Communi D. Slow desensitization of the human P2Y6 receptor. Eur J Pharmacol 329: 231–236, 1997 [PubMed] [Google Scholar]
- 52.Rubino A, Burnstock G. Evidence for a P2-purinoceptor mediating vasoconstriction by UTP, ATP and related nucleotides in the isolated pulmonary vascular bed of the rat. Br J Pharmacol 118: 1415–1420, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rump LC, Oberhauser V, non Kugelgen I. Purinoceptors mediate renal vasodilation by nitric oxide dependent and independent mechanisms. Kidney Int 54: 473–481, 1998 [DOI] [PubMed] [Google Scholar]
- 54.Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Vaillant N, Gadeau AP, Desgranges C, Scalbert E, Chardin P, Pacaud P, Loirand G. P2Y1, P2Y2, P2Y4, and P2Y6 receptors are coupled to Rho and Rho kinase activation in vascular myocytes. Am J Physiol Heart Circ Physiol 278: H1751–H1761, 2000 [DOI] [PubMed] [Google Scholar]
- 55.Seye CI, Kong Q, Erb L, Garrad RC, Krugh B, Wang M, Turner JT, Sturek M, Gonzalez FA, Weisman GA. Functional P2Y2 nucleotide receptors mediate uridine 5′-triphosphate-induced intimal hyperplasia in collared rabbit carotid arteries. Circulation 106: 2720–2726, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Szasz T, Watts SW. Uric acid does not affect the acetylcholine-induced relaxation of aorta from normotensive and deoxycorticosterone acetate-salt hypertensive rats. J Pharmacol Exp Ther 333: 758–763, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tabima DM, Chesler NC. The effects of vasoactivity and hypoxic pulmonary hypertension on extralobar pulmonary artery biomechanics. J Biomech 43: 1864–1869, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tolle M, Schuchardt M, Wiedon A, Huang T, Kkockel L, Jankowski J, Jankowski V, Zidek W, van der Giet M. Differential effects of uridine adenosine tetraphosphateon purinoceptors in the rat isolated perfused kidney. Br J Pharmacol 161: 530–540, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wehrwein EA, Northcott CA, Loberg RD, Watts SW. Rho/Rho kinase and phosphoinositide 3-kinase are parallel pathways in the development of spontaneous arterial tone in deoxycorticosterone acetate-salt hypertension. J Pharmacol Exp Ther 309: 1011–1019, 2004 [DOI] [PubMed] [Google Scholar]
- 60.Wihlborg AK, Balogh J, Wang L, Borna C, Dou Y, Joshi BV, Lazarowski E, Jacobson KA, Arner A, Erlinge D. Positive inotropic effects by uridine triphosphate (UTP) and uridine diphosphate (UDP) via P2Y2 and P2Y6 receptors on cardiomyocytes and release of UTP in man during myocardial infarction. Circ Res 98: 970–976, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]