Abstract
A number of promising therapies for ischemic cardiomyopathy are emerging, and the role of translational research in testing the efficacy and safety of these agents in relevant clinical models has become important. The goal of this study was to develop a chronic model of ischemic cardiomyopathy in a large animal model. In this study, 40 consecutive pigs were initially enrolled. To induce progressive stenosis, a plastic occluder with a fixed diameter of 1.0 mm fitted with an 18-gauge copper wire was placed around the proximal left anterior descending (LAD) coronary artery. Coronary angiography, hemodynamic measurements, and echocardiography were performed at 2 wk and 1, 2, and 3 mo. Overall mortality was 26% at 3 mo, and up to 80% of the pigs showed total occlusion of LAD at 1 mo. A significant depression of peak LV pressure rate of rise (+dP/dtmax) was observed in the animals showing total artery occlusion throughout the study. Left ventricular ejection fraction was also impaired, and the left ventricular volumes tended to be larger in the pigs with occlusion. Approximately 10% of scar tissue was found in the LAD occluded pigs, whereas the coronary flow pattern in the rest of the area took the pattern of hibernating myocardium. At the same time, histological and protein analysis established the presence of fibrosis and ongoing apoptosis in the ischemic area. In this model, the timing and incidence of total occlusion and low mortality offer significant advantages over other ischemic cardiomyopathy models in conducting preclinical studies.
Keywords: chronic ischemia, translational research, total occlusion, hybernating myocardium
progress in percutaneous coronary interventions, cardiac surgeries, and implantable mechanical devices has improved the outcomes of ischemic cardiac diseases over the decades. However, patients who developed heart failure due to severe ischemia still have a 5-yr mortality of 40–50% (3, 6). Even with an intensive care with implantable cardioverter-defibrillator implantation, it could only reduce the rate to 37% (6). The development of novel therapies for these types of patients has accelerated in recent years, and novel targets have emerged at the cellular and molecular levels. There are many potential novel therapies, such as small molecules (17), gene therapy (18), and stem cell therapy (7), that are gaining popularity in cardiovascular disease treatment. Preclinical models of ischemic cardiomyopathy that closely mimic the clinical condition are important to develop to test the efficacy and safety of these new strategies of treatment.
The porcine heart exhibits similar coronary artery anatomy without inherent collaterals, as well as similar gross anatomic structure (14, 23), and resembles important characteristics of the human heart. Surgical or percutaneous occlusion of coronary arteries which mimics acute infarct and their sequelae (9, 21), and the implantation of the plastic occluders to the coronary arteries, which mimics hibernation (5, 11), are the current models of ischemic heart disease. However, patients with ischemic cardiomyopathy are more likely to have nontransmural infarcts with viable myocardium. A study (1) showed that 55% of ischemic cardiomyopathy patients with depressed cardiac function have viable myocardium. Thus the current models do not offer a reasonable model of ischemic cardiomyopathy. Accordingly, establishment of an ideal chronic ischemia model of swine brings in a significant advantage in translational research and eventually leads to future development of treatment in ischemic cardiac diseases. This study was aimed to test the hypothesis that the gradual occlusion of the coronary artery with appropriate duration could forge an ideal chronic ischemia model; that is, where myocardium is characterized by scar, dysfunctional, and intact myocardium, hibernating cardiomyocytes, apoptotic cardiomyocytes, and fibrosis.
MATERIALS AND METHODS
Experimental study.
The study was performed in accordance with the Guidelines for the Care and Use of Laboratory Animals and was approved by the Subcommittee on Research Animal Care at Mount Sinai School of Medicine. Yorkshire pigs (n = 40; body weight range: 9.4–20 kg) were premedicated using 0.04 mg/kg atropine and 6.0 mg/kg Telazol (tiletamine/zolazepam). Animals were intubated and ventilated with 100% oxygen. General anesthesia was maintained with 5–8 mg·kg−1·h−1 propofol throughout the procedure, except during the thoracotomy. A thoracotomy was performed while the animals were under isoflurane (1–2%) anesthesia. Surgical access was achieved through the third left intercostal space. A plastic occluder of fixed diameter and an 18-gauge copper wire were deployed around the proximal segment of the left anterior descending (LAD) coronary artery and fixed loosely with a cotton umbilical tape. A sham-operated group (control group: n = 6) was also included in the study. Heparin (2,000 IU) was administered after the operation followed by an oral administration of 10 mg/kg aspirin and 10 mg/kg clopidogrel for 10 days. Postoperational angiography was performed after 2 wk. All of the study animals underwent angiography, echocardiogram, and hemodynamic measurement at 1, 2, and 3 mo (1M, 2M, and 3M, respectively) after the occluder implantation. For the follow-up procedures, the femoral or cervical site was prepared with 70% isopropyl alcohol followed by providone iodine. A percutaneous puncture provided access to the artery and the vein for sheath placement. If the attempt failed, a cut-down was performed. After sheath insertion, 100 IU/kg iv of heparin was administered to maintain an activated coagulation time of 250–300 s. Some of the pigs were planned for death immediately after the occlusion of LAD to check the scar size, and the remaining pigs were euthanized at the end of the study period, which was set for 3M.
Coronary flow measurement.
Regional perfusion was quantified using colored microspheres that were analyzed as previously described by Etz et al. (10). Briefly, 1–2 × 107 polystyrene fluorescent microspheres (15 um; Interactive Medical Technologies, Irvine, CA) were injected into the left ventricle (LV). Reference blood was withdrawn from a femoral artery sheath using a specialized pump for 2 min at a rate of 2.9 ml/min (Harvard Apparatus, Holliston, MA). After the measurement of baseline flow with Purple-Low colored microspheres, a second microsphere measurement was performed using Coral-High colored microspheres. This second collection was executed to measure coronary artery reserve during adenosine vasodilatation (0.9 mg·kg−1·min−1) with phenylephrine (10 mg·kg−1·min−1) infusion to maintain arterial pressure. Distribution of fluorescent microspheres in the central region of the stenotic LAD was cut into three layers and quantified by flow cytometric analysis (Interactive Medical Technologies). Normally perfused right and left circumflex (LCX) coronary artery regions were used for comparison.
Regional coronary flow (CF) was calculated using the formula: CF, ml·min−1·g−1 = (R × lt)/(Ibr × Wt), where R is blood reference withdrawal rate (2.9 ml/min); lt and Ibr are fluorescent counts in the tissue and the blood reference sample, respectively; and Wt is the weight of the tissue sample (g). Comparison was performed between ischemic (anterior) and nonischemic (inferior) regions.
Hemodynamic analysis.
Through the femoral artery sheath, a Millar catheter (Millar Instruments, Houston, TX) was advanced to measure the following hemodynamic parameters: systolic pressure, LV end diastolic pressure, peak LV pressure rate of rise (+dP/dtmax) and decline (−dP/dtmin), and τ-value (time constant of isovolumic relaxation). Power lab/4sp (AD Instruments, Colorado Springs, CO) was used to acquire analog data and convert them to digital data. Data analysis was performed using Chart 5 v. 5.2.2 (AD Instruments). All the measurements were performed during a breath hold to avoid the fluctuation of pressure. After hemodynamic measurement, a guiding catheter was advanced and coronary angiography was performed to confirm the status of coronary arteries. Dobutamine stress test was carried out after the baseline measurement with a starting rate of 2.5 μg·kg−1·min−1 and increased to 40 μg·kg−1·min−1 in a stepwise fashion.
Echocardiography.
Complete Doppler transthoracic echocardiographic studies were performed using a Philips iE-33 ultrasound system equipped with a multifrequency imaging transducer. With the use of high frame rate, two-dimensional long axis and cross-sectional images of the LV were obtained. At least three consecutive heartbeats were acquired and stored as digital images. End diastolic and end systolic volumes were obtained using Simpson's method, and stroke volume was calculated by subtracting the end systolic volume from the end diastolic volume. Peak early (E) and late (A) transmitral filling velocities and their ratio (E/A) were measured from the mitral valve inflow profile. Wall motion of anterior region was scored as follows: 1, normal or hyperkinetic; 2, hypokinetic; 3, akinetic; and 4, dyskinetic.
Postmortem histology.
Hearts were explanted and sectioned into six slices. To quantify the infarction size by digital planimetry, slices of heart tissue were immersed in triphenyl tetrazolium chloride. One of the slices was used for histological and protein analyses, and the samples from sections of the LV were separated into three layers, the endomyocardium, midmyocardium, and epimyocardium, and then snap frozen both directly and in the optimum cutting temperature (OCT) compound. To inquire further into the mechanism of the occlusion, a sample from the occlusion site was taken out. The copper wire and the occluder were removed for the cutting process, and the sample was embedded in an OCT compound. After being frozen down to −80°C, the samples in OCT compound were sliced into sections measuring 20 μm, and Masson's trichrome staining was performed.
Western blotting.
Cardiac tissue was minced and subsequently homogenized in radioimmunoprecipitation assay buffer containing a protease inhibitor cocktail (Sigma-Aldrich). Protein extracts were separated on 10% SDS-PAGE and transferred onto PVDF membrane (Millipore). Antibody binding was visualized by ECL (Immobilon Western, Chemiluminiscent HRP Substrate; Millipore). MAb used in Western blotting included anti-collagen I, anti-collagen III, and TGF-β (all from Abcam) and caspase-3 (Cell Signaling). Protein loading was controlled by GAPDH (Sigma-Aldrich) expression. Peroxidase-conjugated secondary antibodies (Sigma-Aldrich) were used.
Statistical analysis.
Continuous variables were reported as means ± SD. The Student's t-test was used to analyze differences within the same group, whereas the two-sample t-test was used for differences between two groups. A P value < 0.05 was considered statistically significant.
RESULTS
Mortality.
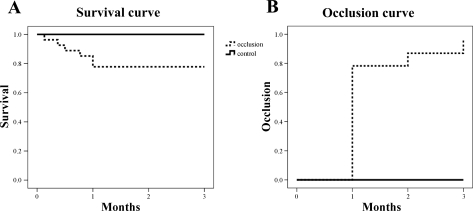
Forty consecutive pigs were initially enrolled in the study. One animal was excluded because it had an oversized LAD to implant the occluder. A total of 39 pigs received this new occluder. Two pigs died from procedural failure, and two pigs with significant ST change during the operation died within 12 h after the procedure most likely from arrhythmias due to acute large myocardial infarctions. Overall mortality was 26% (6 deaths after 24 h postoperation). However, sudden death was only seen in two pigs and four other pigs were procedure-related deaths during the follow-up. Immediately after the confirmation of LAD occlusion, six pigs at 1M and two pigs at 2M were euthanized to check the scar size. Survival curve of the pigs without planned deaths are shown in Fig. 1A.
Fig. 1.
A: survival curve of the pigs successfully implanted the occluder (dashed line) and sham operated pigs (solid line). Planned deaths are excluded. B: time course of pigs showing total occlusion. Up to 80% of the pigs presented total occlusion at 1 mo follow-up.
Occlusion.
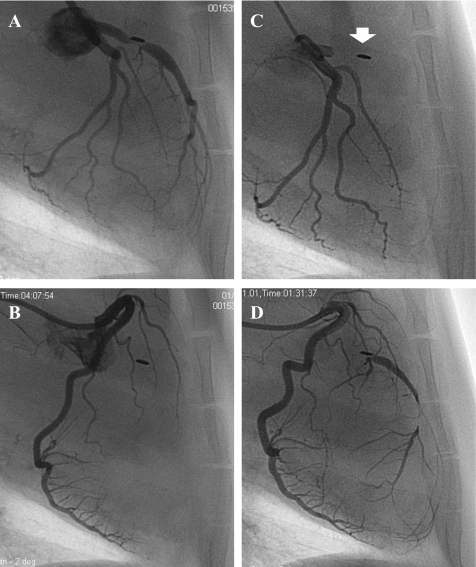
Two pigs had total occlusion of the LAD at 2 wk, and almost 80% of the pigs showed total occlusion at 1M (Fig. 1B). After 1M, gradual increase of occlusion rate was seen at each follow-up point. Only one pig did not show total occlusion throughout the study. A representative coronary angiogram before and after the total occlusion of the LAD is shown in Fig. 2.
Fig. 2.
Representative coronary angiography of the pigs with occluder. Only stenosis shaped by the occluder was found at 2 wk follow-up (A) without any collaterals from right coronary artery (RCA; B). Left anterior descending coronary artery (LAD) showed total occlusion at the proximal at 1-mo follow-up (1M; C). Arrow indicates the occlusion site with copper wire placed on the LAD. Rich collateral from RCA was seen (D).
Coronary flow measurement.
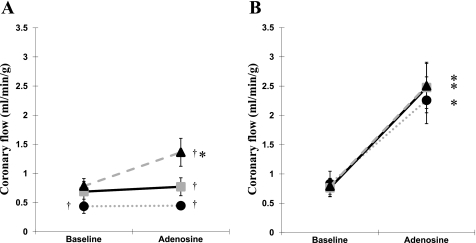
Figure 3 shows regional perfusion measured by colored microspheres from three pigs at 3M. The resting coronary flow was impaired only in the endocardial layer of the anterior area and not in the inferior area (0.44 ± 0.12 vs. 0.83 ± 0.22 ml·min−1·g−1; P < 0.05). The significant increase of coronary flow during the adenosine infusion was observed in all layers of the inferior area (inferior end: 0.83 ± 0.22 vs. 2.26 ± 0.40 ml·min−1·g−1, P < 0.05; inferior mid: 0.76 ± 0.16 vs. 2.48 ± 0.86 ml·min−1·g−1, P < 0.05l; inferior epi: 0.79 ± 0.14 vs. 2.50 ± 0.36 ml·min−1·g−1, P < 0.05) but only in the epicardial layer of the anterior area (0.78 ± 0.13 vs. 1.36 ± 0.24 ml·min−1·g−1, P < 0.05). There was a significantly lower coronary flow during the adenosine infusion in all three layers of the anterior area compared with those of inferior area (in end: 0.45 ± 0.05 vs. 2.26 ± 0.40 ml·min−1·g−1, respectively, P < 0.05; in mid: 0.77 ± 0.16 vs. 2.48 ± 0.43 ml·min−1·g−1, respectively, P < 0.05; in epi: 1.36 ± 0.24 vs. 2.50 ± 0.36 ml·min−1·g−1, P < 0.05).
Fig. 3.
Regional coronary perfusion measured by colored microspheres. A: anterior area. B: inferior area. Triangle, epicardium; square, midmyocardium; circle, endocardium. *P < 0.05, compared with baseline. †P < 0.05, compared with inferior counterpart.
Cardiac function.
Hemodynamic parameters of the pigs with total occlusion at each follow-up point were compared with that of control animals (Table 1). There were no significant differences in heart rate, systolic blood pressure, end diastolic pressure, −dP/dtmin, and τ-value between the groups during the study. However, the pigs with total occlusion exhibited a significantly decreased +dP/dtmax throughout the study compared with that of the control group (Table 1).
Table 1.
Body weight and hemodynamic parameters of the pigs with total occlusion
| 1 mo |
2 mo |
3 mo |
||||
|---|---|---|---|---|---|---|
| Occluder (n = 18) | Control (n = 6) | Occluder (n = 11) | Control (n = 6) | Occluder (n = 12) | Control (n = 6) | |
| Body weight, kg | 18 ± 4 | 17 ± 3 | 25 ± 5 | 24 ± 2 | 31 ± 5 | 31 ± 4 |
| Heart rate, beats/min | 95 ± 28 | 91 ± 28 | 80 ± 18 | 78 ± 13 | 78 ± 10 | 79 ± 17 |
| Systolic arterial pressure, mmHg | 99 ± 15 | 100 ± 12 | 109 ± 21 | 112 ± 17 | 112 ± 13 | 119 ± 15 |
| End diastolic pressure, mmHg | 14.8 ± 6.4 | 12.7 ± 3.1 | 13.4 ± 3.1 | 10.7 ± 1.4 | 13.8 ± 3.6 | 11.3 ± 1.1 |
| +dP/dtmax, mmHg/s | 1,553 ± 488 | 2,200 ± 510* | 1,722 ± 654 | 2,393 ± 321* | 1,742 ± 456 | 2,336 ± 535* |
| −dP/dtmin, mmHg/s | −1,080 ± 856 | −1,584 ± 450 | −1,619 ± 452 | −1,796 ± 496 | −1,687 ± 457 | −1,948 ± 199 |
| τ, s | 0.06 ± 0.02 | 0.08 ± 0.10 | 0.06 ± 0.01 | 0.05 ± 0.02 | 0.06 ± 0.02 | 0.05 ± 0.02 |
Values are means ± SD. +dP/dtmax and −dP/dtmin, peak left ventricular pressure rate of rise and decline, respectively; τ, time constant of isovolumic relaxation.
P > 0.05.
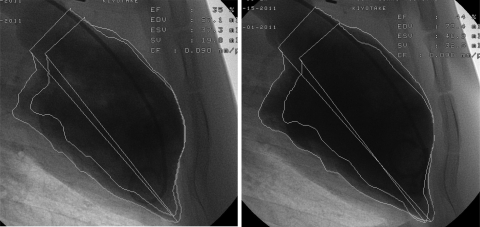
Table 2 shows the results of the echocardiographic study. Although stroke volume was comparable between the groups, ejection fraction was significantly impaired in the pigs with total occlusion at every time point. In the same group, both end diastolic volume and end systolic volume tended to be larger with the significant differences in end diastolic volume at 3M and in end systolic volume at 2M and 3M. Anterior wall motion score ranged from two to three in the pigs with total occlusion, whereas all the sham-operated pigs presented normal wall motion. Dobutamine stress test showed improved motion of the anterior wall in pigs with total occlusion (Fig. 4 and Supplemental Videos; Supplemental Material for this article is available online at the Am J Physiol Heart Circ Physiol website).
Table 2.
Echocardiographic parameters of the pigs with total occlusion
| 1 mo |
2 mo |
3 mo |
||||
|---|---|---|---|---|---|---|
| Occluder (n = 18) | Control (n = 6) | Occluder (n = 11) | Control (n = 6) | Occluder (n = 12) | Control (n = 6) | |
| Ejection fraction, % | 47 ± 14 | 63 ± 4* | 49 ± 13 | 67 ± 6* | 51 ± 8 | 66 ± 5* |
| End diastolic volume, ml | 58 ± 24 | 41 ± 7 | 67 ± 20 | 48 ± 8* | 72 ± 20 | 55 ± 9 |
| End systolic volume, ml | 32 ± 23 | 15 ± 2 | 36 ± 18 | 16 ± 3* | 35 ± 9 | 19 ± 5* |
| Stroke volume, ml | 26 ± 8 | 26 ± 6 | 32 ± 7 | 33 ± 7 | 37 ± 13 | 36 ± 5 |
| E/A ratio | 1.03 ± 0.24 | 1.11 ± 0.28 | 1.41 ± 0.52 | 1.56 ± 0.31 | 1.17 ± 0.26 | 1.20 ± 0.11 |
| Anterior wall motion score | 2.7 ± 0.5 | 1.0 ± 0* | 2.6 ± 0.5 | 1.0 ± 0* | 2.4 ± 0.5 | 1.0 ± 0* |
Values are means ± SD. E, peak transmitral flow velocities at early filling phase; A, peak transmitral flow velocities at late filling phases.
P > 0.05.
Fig. 4.
Left ventriculography before (left; Supplemental Video S1) and after (right; Supplemental Video S2) dobutamine infusion. Anterior wall motion was improved by the injection of dobutamine (2.5 μg·kg−1·min−1). EF, ejection fraction; EDV and ESV, end diastolic and end systolic volumes; SV, stroke volume; CF, calibration factor.
Postmortem analysis.
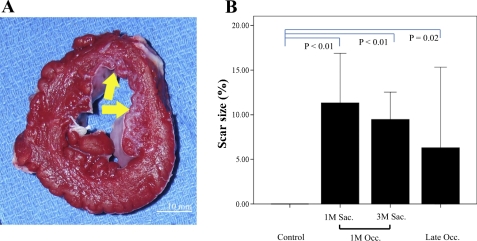
Figure 5 shows the typical example of the heart cross section stained with tetrazolium chloride. Although a nontransmural scar was present in the anterior region, the scar showed a patchy pattern and the wall thickness was maintained. Mean scar size of the pigs with 1M occlusion did not differ between the death time point (1M: 11.3 ± 3.5%; 3M: 9.5 ± 3.7%, P = NS). The scar size of the four pigs that presented an occlusion after 1M took a wide range between the animals (0.5–13%). Although it was not significant, the average of the scar size was smaller than that of pigs with 1M occlusion (6.3 ± 5.7%).
Fig. 5.
A: cross section of the papillary muscle level from one of the pigs that received the occluder implantation. Tetrazolium chloride staining was performed to delineate the scar area. Arrow shows the nontransmural scar with a patchy appearance predominantly seen in the endocardium. B: scar size of the pigs classified by the time of occlusion and the death time point. Scar size did not change from 1M to 3-mo follow-up (3M) in the pigs with 1M occlusion. Pigs showing total occlusion after 1M had varied scar size. Sac., death; Occ.; occlusion.
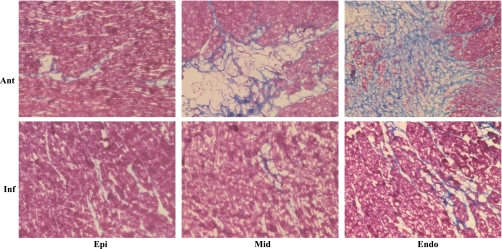
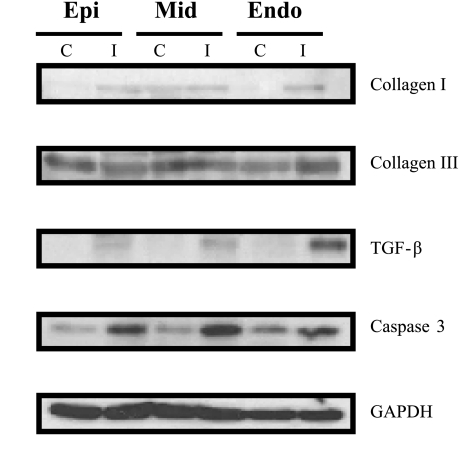
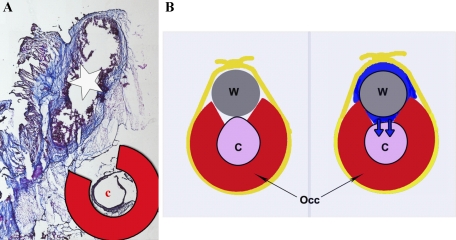
Consistent with the macroscopic appearance, Masson's trichrome staining revealed an interlaced fibrosis inside the normal myocardium, with more enhancement toward the endomyocardium (Fig. 6). To further look into the fibrosis and the apoptosis in this model, protein analysis was performed. In correspondence to the histological staining, both fibrosis and apoptotic markers were found to be increased in the anterior midmyocardium and endomyocardium of chronic ischemia pigs (Fig. 7). The occluder implanted site was collected from one of the pigs that died at 1M follow-up. Fibrosis was demonstrated around the copper wire by Masson's trichrome staining (Fig. 8).
Fig. 6.
Histological assessment of cardiac fibrosis. An increased fibrosis (blue area) was found predominantly toward the endomyocardium (Endo) by Masson's trichrome staining. There was only a minor change found in the inferior (Inf) area. Ant, anterior; Epi, epimyocardium; Mid, midmyocardium.
Fig. 7.
Cardiac fibrosis and apoptosis in the chronic ischemia model. Analysis of collagen I, collagen III, TGF-β, and caspase-3 expression markers by Western blotting in cardiac lysates from controls (C) and chronic ischemia (I). An accumulation of collagen I, collagen III, and TGF-β was observed in the anterior Mid and Endo of chronic ischemia pigs. Similarly, an increase of apoptosis activity detected by caspase-3 expression was also detected in the anterior Mid and Endo of the chronic ischemia pigs. GAPDH expression was used as a loading control.
Fig. 8.
A: Masson's trichrome staining of the tissue around the occluder (Occ) site. Copper wire (w) next to the coronary artery (c) was surrounded by the fibrosis (star indicates the space where wire was in). B: schema shows the presumed mechanism of occlusion. First, occluder was implanted around the coronary artery, and a copper wire was placed on the gap and loosely fixed by an umbilical tape (left). Inflammation (blue) around the wire induces the fibrotic tissue proliferation and gradually compresses the artery resulting in total occlusion at moderate time point.
DISCUSSION
We describe a new swine model of chronic ischemia that presents total occlusion of the LAD with rich collaterals. This model has a number of advantages for preclinical studies targeting ischemic heart disease. Low mortality, cardiac dysfunction with nontransmural scar, ideal time frame with minimal increase in the body weight, and easily reproducible technique were demonstrated. A number of studies have reported large animal ischemic cardiomyopathy models using various induction methods. Implanting ameroid constrictors (2, 4, 14–15, 20, 22) induces inflammation and gradual occlusion of the coronary artery. However, the ameroid constrictors have been mainly used for LCX as a target (4, 14–15, 20, 22), due to a high mortality, as high as 80%, when implanted on LAD (19–20). The LCX supplies a small area (14–15) compared with the other coronary arteries, and the depression of cardiac function in these models may be small. Candidates for cutting edge therapy in humans are those with severe heart dysfunction. With such a perspective, LCX chronic ischemia is not the optimal model for preclinical studies. By inducing a total occlusion at the proximal LAD, we have developed a model of chronic ischemia in LAD with <30% of mortality. Both hemodynamic and echocardiographic analysis confirmed the depression of cardiac function with a more dilated heart in our model.
Canty and colleagues (5, 11–13, 16) have also established an elegant swine model of chronic ischemia using plastic occluder on LAD. They allowed young pigs to grow >80 kg during period of 100 days and were enabled to produce ideal hibernating myocardium with minimum scar formation (16). The model has contributed to elucidate the mechanism (5, 11), protein change (12), energy metabolism (13), etc., in hibernating myocardium. Although it is suitable for studying the nature of hibernating myocardium, chronic ischemia without any infarct is farther apart from the patients who need to be treated in the clinical settings. In addition, this model requires the animals to grow large and takes time to attain total occlusion. Consequently, a large facility would be required to conduct preclinical studies with multiple animals. We managed to accomplish total occlusion in 80% of the pigs within 1M by adding the copper wire on the occluder. This high probability of occlusion within 1M would help reduce the length of the study and ascertains that the myocardium will have areas of infarction.
We assume that less mortality with high rate of total occlusion was achieved by the mechanism shown in Fig. 8. Inflammation around the copper wire induces fibrosis, and it gradually fills the space surrounded by the occluder, wire, and the umbilical tape. Thereby, the coronary artery becomes slowly compressed and causes occlusion. This gradual compression allows for faster occlusion of the artery, than only implanting the plastic occluder, but slower than using ameroid constrictors. This moderate speed of artery compression seems to be the best for both survival and reproducibility of the total occlusion.
Although ∼10% of scar was found in postmortem study, wall thickness was preserved. Most of the pigs had akinetic anterior wall motion, and the coronary flow pattern of our model resembled the results from Canty's group (11, 13). These data, along with the wall motion improvement with dobutamine, support that the remaining myocardium is in the stage of hibernation described by these investigators. Furthermore, histological and protein analysis established the presence of fibrosis and ongoing apoptosis in this model. This mixture of various stages of myocardium from normal to hibernating, to apoptotic, and to fibrotic myocardium, closely mimics the state of human chronic ischemic cardiomyopathy.
Clinical implications.
Our model, which is characterized by patchy scars, viable myocardium, fibrotic area, and hibernating myocardium, indicates that there are various types of cardiomyocytes within the myocardium (8). We believe this mimics closely the state of myocardium in patients with ischemic cardiomyopathy. Thus our model would be very suitable to test therapies, such as small molecule delivery, gene transfer, and stem cell therapy, targeting specifically ischemic cardiomyopathy.
In summary, our model combines useful characteristics that are present in chronic ischemic cardiomyopathy in humans, and it will be a useful experimental model to evaluate novel therapies.
GRANTS
This work is supported by Leducq Foundation through the Caerus network (to R. J. Hajjar) and by National Heart, Lung, and Blood Institute Grants R01-HL-093183, HL-088434, HL-071763, HL-080498, HL-083156, and P20-HL-100396 (to R. J. Hajjar). This work is also supported by the National Heart, Lung, and Blood Institute, as a Program of Excellence in Nanotechnology Award, Contract No. HHSN268201000045C. D. Ladage was supported by the German Research Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Catherine McMahon and James Lough for providing technical expertise.
REFERENCES
- 1. Auerbach MA, Schoder H, Hoh C, Gambhir SS, Yaghoubi S, Sayre JW, Silverman D, Phelps ME, Schelbert HR, Czernin J. Prevalence of myocardial viability as detected by positron emission tomography in patients with ischemic cardiomyopathy. Circulation 99: 2921– 2926, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Barandon L, Calderon J, Reant P, Caillaud D, Lafitte S, Roques X, Couffinhal T, Dos Santos P. Adjustment and characterization of an original model of chronic ischemic heart failure in pig. Cardiol Res Pract 2010: pii: 542451, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med 341: 1882– 1890, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Caillaud D, Calderon J, Reant P, Lafitte S, Dos Santos P, Couffinhal T, Roques X, Barandon L. Echocardiographic analysis with a two-dimensional strain of chronic myocardial ischemia induced with ameroid constrictor in the pig. Interact Cardiovasc Thorac Surg 10: 689– 693, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Canty JM, Jr, Suzuki G, Banas MD, Verheyen F, Borgers M, Fallavollita JA. Hibernating myocardium: chronically adapted to ischemia but vulnerable to sudden death. Circ Res 94: 1142– 1149, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Chan P, Hayward R. Mortality reduction by implantable cardioverter-defibrillators in patients with ischemic heart disease, congestive heart failure, and ventricular arrhythmias. Circulation 110: 503– 503, 2004 [Google Scholar]
- 7. Charwat S, Gyongyosi M, Lang I, Graf S, Beran G, Hemetsberger R, Nyolczas N, Sochor H, Glogar D. Role of adult bone marrow stem cells in the repair of ischemic myocardium: current state of the art. Exp Hematol 36: 672– 680, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Chen C, Ma L, Linfert DR, Lai T, Fallon JT, Gillam LD, Waters DD, Tsongalis GJ. Myocardial cell death and apoptosis in hibernating myocardium. J Am Coll Cardiol 30: 1407– 1412, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Dixon JA, Spinale FG. Large animal models of heart failure: a critical link in the translation of basic science to clinical practice. Circ Heart Fail 2: 262– 271, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Etz CD, Homann TM, Luehr M, Kari FA, Weisz DJ, Kleinman G, Plestis KA, Griepp RB. Spinal cord blood flow and ischemic injury after experimental death of thoracic and abdominal segmental arteries. Eur J Cardiothorac Surg 33: 1030– 1038, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fallavollita JA, Canty JM., Jr Differential 18F-2-deoxyglucose uptake in viable dysfunctional myocardium with normal resting perfusion: evidence for chronic stunning in pigs. Circulation 99: 2798– 2805, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Fallavollita JA, Jacob S, Young RF, Canty JM., Jr Regional alterations in SR Ca2+-ATPase, phospholamban, and HSP-70 expression in chronic hibernating myocardium. Am J Physiol Heart Circ Physiol 277: H1418– H1428, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Fallavollita JA, Malm BJ, Canty JM., Jr Hibernating myocardium retains metabolic and contractile reserve despite regional reductions in flow, function, and oxygen consumption at rest. Circ Res 92: 48– 55, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Hughes GC, Post MJ, Simons M, Annex BH. Translational physiology: porcine models of human coronary artery disease: implications for preclinical trials of therapeutic angiogenesis. J Appl Physiol 94: 1689– 1701, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Ikonen TS, Patila T, Virtanen K, Lommi J, Lappalainen K, Kankuri E, Krogerus L, Harjula A. Ligation of ameroid-stenosed coronary artery leads to reproducible myocardial infarction–a pilot study in a porcine model. J Surg Res 142: 195– 201, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Lim H, Fallavollita JA, Hard R, Kerr CW, Canty JM., Jr Profound apoptosis-mediated regional myocyte loss and compensatory hypertrophy in pigs with hibernating myocardium. Circulation 100: 2380– 2386, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Poller W, Hajjar R, Schultheiss HP, Fechner H. Cardiac-targeted delivery of regulatory RNA molecules and genes for the treatment of heart failure. Cardiovasc Res 86: 353– 364, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prunier F, Kawase Y, Gianni D, Scapin C, Danik SB, Ellinor PT, Hajjar RJ, Del Monte F. Prevention of ventricular arrhythmias with sarcoplasmic reticulum Ca2+ ATPase pump overexpression in a porcine model of ischemia reperfusion. Circulation 118: 614– 624, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Ramo BW, Peter RH, Ratliff N, Kong Y, McIntosh HD, Morris JJ., Jr The natural history of right coronary arterial occlusion in the pig. Comparison with left anterior descending arterial occlusion. Am J Cardiol 26: 156– 161, 1970 [DOI] [PubMed] [Google Scholar]
- 20. Roth DM, Maruoka Y, Rogers J, White FC, Longhurst JC, Bloor CM. Development of coronary collateral circulation in left circumflex ameroid-occluded swine myocardium. Am J Physiol Heart Circ Physiol 253: H1279– H1288, 1987 [DOI] [PubMed] [Google Scholar]
- 21. Suzuki Y, Lyons JK, Yeung AC, Ikeno F. In vivo porcine model of reperfused myocardial infarction: in situ double staining to measure precise infarct area/area at risk. Catheter Cardiovasc Interv 71: 100– 107, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Tuzun E, Oliveira E, Narin C, Khalil H, Jimenez-Quevedo P, Perin E, Silva G. Correlation of ischemic area and coronary flow with ameroid size in a porcine model. J Surg Res 164: 38– 42, 2009 [DOI] [PubMed] [Google Scholar]
- 23. White FC, Carroll SM, Magnet A, Bloor CM. Coronary collateral development in swine after coronary artery occlusion. Circ Res 71: 1490– 1500, 1992 [DOI] [PubMed] [Google Scholar]