Abstract
The causality of the associations between cellular and mechanical mechanisms of abdominal aortic aneurysm (AAA) formation has not been completely defined. Because reactive oxygen species are established mediators of AAA growth and remodeling, our objective was to investigate oxidative stress-induced alterations in aortic biomechanics and microstructure during subclinical AAA development. We investigated the mechanisms of AAA in an angiotensin II (ANG II) infusion model of AAA in apolipoprotein E-deficient (apoE−/−) mice that overexpress catalase in vascular smooth muscle cells (apoE−/−xTgSMC-Cat). At baseline, aortas from apoE−/−xTgSMC-Cat exhibited increased stiffness and the microstructure was characterized by 50% more collagen content and less elastin fragmentation. ANG II treatment for 7 days in apoE−/− mice altered the transmural distribution of suprarenal aortic circumferential strain (quantified by opening angle, which increased from 130 ± 1° at baseline to 198 ± 8° after 7 days of ANG II treatment) without obvious changes in the aortic microstructure. No differences in aortic mechanical behavior or suprarenal opening angle were observed in apoE−/−xTgSMC-Cat after 7 days of ANG II treatment. These data suggest that at the earliest stages of AAA development H2O2 is functionally important and is involved in the control of local variations in remodeling across the vessel wall. They further suggest that reduced elastin integrity at baseline may predispose the abdominal aorta to aneurysmal mechanical remodeling.
Keywords: hydrogen peroxide, reactive oxygen species, opening angle, arterial mechanics, extracellular matrix
despite a clear role for oxidative stress in the development of abdominal aortic aneurysms (AAA; Refs. 27, 44), the link between changes in oxidative stress and the local biomechanical response is not well understood. Animal models deficient in enzymatic sources of reactive oxygen species (ROS), such as inducible nitric oxide synthase, NOX1, and p47phox, clearly demonstrate preservation of aortic wall morphology and attenuated AAA development (12, 39, 44). A functional link between ROS and vascular mechanics has been previously demonstrated in nonaneurysmal human vascular tissues, which exhibit decreased aortic compliance in the presence of increased vascular superoxide (9). These studies illustrate that oxidative stress plays an important role in both the molecular and mechanical events associated with AAA. However, there is little evidence that links ROS directly to the biomechanical changes involved in AAA development.
ROS have been detected at all stages of aneurysm development (13, 29, 30) and are established mediators of extracellular matrix (ECM) degradation and remodeling (16, 34). AAA formation proceeds from localized remodeling and vessel dilation attributable to degeneration of elastin and alterations in collagen proteins within the aortic wall. Both events potentially reduce the compliance and tensile strength of aneurysmal aortas (19, 32, 41). Aneurysm formation is limited by inhibition of proteolytic enzymes, such as matrix metalloproteinases (MMPs; Ref. 26), and in animal models deficient in MMPs (Refs. 2, 20, 23, 37). These data suggest that proteolytic activity is necessary for AAA formation and that synergistic factors, such as spatial vulnerability to mechanical changes, are also relevant in the early stages of AAA formation.
We were motivated by the observation in a previous study from our group that transgenic apolipoprotein E-deficient (apoE−/−) mice with vascular smooth muscle cell (SMC)-specific overexpression of the human catalase gene (apoE−/−xTgSMC-Cat) were completely protected from AAA formation when treated with angiotensin II (ANG II; Ref. 28). ANG II infusion into apoE−/− mice has been shown by several groups to promote AAA formation (7, 8). We utilized this model to investigate H2O2-mediated alteration of aortic mechanics in the earliest stages of AAA formation, because previous studies in this model (45) demonstrated that aorta from WT mice showed significant ANG II-mediated aortic ROS production after 14 days, an event that was blunted in the TgSMC-Cat mice. The working hypothesis was that H2O2 is mechanistically and spatially linked to the biomechanical remodeling that promotes AAA. The data obtained in this study are the first to measure early changes in mechanical behavior in a mouse model of ANG II-induced AAA and demonstrate that local H2O2 is associated with the development of early alterations in local circumferential strain across the aortic wall.
METHODS
Animals.
Transgenic mice with smooth muscle-specific overexpression of catalase (apoE−/−xTgSMC-Cat) and littermate control (apoE−/−) were used in this study. ANG II (0.75 mg·kg−1·day−1) was dissolved in sterile saline and delivered subcutaneously via osmotic minipumps (Alzet, Cupertino, CA) for 7 days. Mice were fed either a standard chow diet (Certified Rodent Chow 5001; Purina) or a high-fat diet (atherogenic diet; Research Diets, New Brunswick, NJ). The generation and phenotypic characterization of the apoE−/−xTgSMC-Cat mice have been preformed previously (45). All procedures were approved by the Emory University Institutional Animal Care and Use Committee and were in compliance with the standards for the care and use of laboratory animals of the Institute of Laboratory Animal Resources, National Academy of Sciences (Bethesda, MD).
Aortic preparation and isolated vessel perfusion setup.
Descending aortas, from the sixth intercostal arteries to the left renal artery, were cleared of connective tissue, and the overall loaded length (l) was measured. Each vessel was excised and placed in sterile culture medium [DMEM (Sigma-Aldrich, St. Louis, MO) with HEPES at pH 7.4 containing 10 μM sodium nitroprusside] and the unloaded axial length (L) and unloaded suprarenal diameter (D) were measured. The intercostal arteries were individually tied off with suture, and the aortas were cannulated and placed in a vessel isolation chamber [Living Systems (LSI), Burlington, VT]. The vessels were extended to the in vivo axial stretch (λz = l/L) and bathed in sterile culture medium held at 37°C. The chamber was mounted on a Nikon DIAPHOT 200 inverted microscope and the vessels equilibrated for 30 min. Transmural pressure (P) was ramped from 0 to 130 mmHg at 0.5 mmHg/s using a perfusion pump and pressure controller (LSI). Outer aortic diameter (d) was measured with a video dimension analyzer (LSI).
Data analysis.
Thus, from recorded values (P, d, and λz), fixed length transmural pressure vs. aortic outer diameter (P-d) data were plotted for apoE−/−, apoE−/−xTgSMC-Cat, and apoE−/− + ANG II to compare the global mechanical response across groups. Local pressure-dependent compliance (CP) was calculated in 20 mmHg intervals as the derivative of the P-d data, where Cp = (rP + 10 mmHg − rP − 10 mmHg)/20 mmHg.
Because the diameter-pressure response depends on both material properties and geometry, it is difficult to quantify differences in the material properties between different vessels from these plots or compliance measures; e.g., vessels made of identical material, but with different thicknesses will exhibit different diameter-pressure curves and different values of compliance (the thicker vessel will be stiffer). Stress-strain plots, however, only depend on material properties; thus differences in the stress-strain response indicate differences in the material that constitutes the vessel. The mean circumferential stress, σθ, and mid-wall circumferential Green strain, E̅θ plots for each group were generated from these mechanical data as
where a is the inner radius, h is the vessel wall thickness, and λ̅θ is the mid-wall circumferential stretch, all in the loaded configuration and calculated from experimentally recorded measures are P, d, and λz. The unloaded inner radius, A, outer radius, B, and thickness, H (intima to outer adventitia), were measured from histology. Assuming material incompressibility, (where b = d/2 was the outer radius), h = b − a, and .
Measurement of aortic opening angle and strain distribution.
Suprarenal aortic opening angles, Φ, were measured following a published protocol (17) to quantify the distribution of circumferential strain across the aortic wall. Opening angles were measured in aortas not used for mechanical testing. Using the approach of Chuong and Fung (6), the distribution of the circumferential component of Green strain across the vessel wall is
| (1) |
where r ∈ [a, b] is an arbitrary position in the aorta wall in a loaded configuration, R ∈ (Ri, Ro) is the corresponding location in the stress free configuration and Ri and Ro are the inner and outer radii in the stress-free configuration, respectively. Note that the incompressibility constraint requires that
| (2) |
Following Rachev (33), changes in axial length and wall thickness between the unloaded configuration and the radially cut, stress-free configuration are typically small and may be neglected. For this case, for a given opening angle measurement, Ri and Ro may be calculated as
Thus, given the unloaded radius and thickness, the opening angle, and loaded outer diameter, the stress free inner and outer radius can be approximated. For all locations across the vessel wall (R in the stress-free location), from the inner wall location Ri to the outer wall location Ro, the radial location in the loaded configuration (r) may be calculated via Eq. 2 and the circumferential Green strain may be calculated via Eq. 1. The distribution of Eθ vs. radius can then be plotted.
Total aortic collagen content.
To quantify aortic collagen content, total aortic collagen per dry weight was measured by hydroxyproline assay as described by Woessner (43). Briefly, aortic tissue freed from fat was weighed and dehydrated in a speed vacuum for 2.5 h, and the dry weight was recorded. The dry tissue was digested in 500 μl 0.25 M sodium phosphate buffer with 0.0125 g protease K/g wet weight and then hydrolyzed into amino acid components in 6 N HCl at 120°C for 14 h. Fifty microliters of each sample and hydroxyproline standards were oxidized with Chloramine-T (Sigma). Oxidation was terminated with perchloric acid solution. A colormetric reaction was performed with p-dimethylaminobenzaldehyde at 60°C, and the plate was read at 540 nm. Total collagen content per dry weight was calculated on the assumption that collagen is 13% hydroxyproline.
Histological analysis.
Histological analysis was performed on the bisected aortic rings used for opening angle measurement, which were snap frozen in optimal cutting temperature medium after each experiment. Five-micrometer sections from each sample were stained with Verhoff van Geison Elastic stain (Sigma-Aldrich) for thickness measurement and quantification of elastin fragmentation or with picrosirius red (staining performed on intact aortic rings) to analyze collagen (3). Dimension analysis was performed using Image J software (NIH, Bethesda, MD). Elastin fragmentation was expressed as the number of elastin breaks per medial area, which was measured as the area between the inner and outer elastic laminae.
Statistical analysis.
Data are presented as means ± SE. Statistical analyses were performed using GraphPad Prism software. The pressure-diameter data were analyzed by ANOVA to examine the effect of mouse background on aortic compliance, and Bonferonni post tests were used for post hoc analyses. All other analysis between groups was performed with Mann-Whitney test. Analysis of covariance and an F-test to compare slopes were performed on the mean circumferential strain vs. radius data. P < 0.05 was considered significant.
RESULTS
Overexpression of catalase alters aortic mechanical behavior.
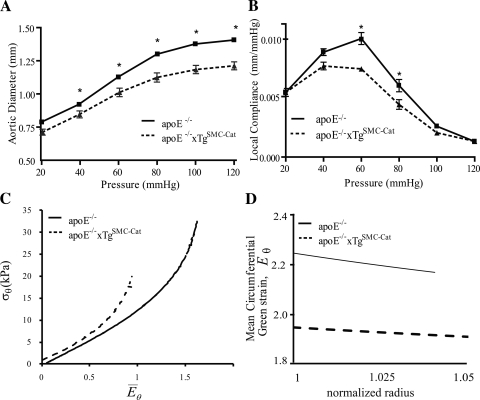
To determine whether AAA protection may derive from endogenous differences in mean systolic blood pressure and descending aortic mechanical behavior, unloaded and loaded dimensions (Table 1) and P-d response were measured in apoE−/− and apoE−/−xTgSMC-Cat mice. Mean systolic blood pressure for each group was the same (96 ± 9.3 vs. 102 ± 10 mmHg; P = NS). At baseline, descending aortas from apoE−/−xTgSMC-Cat mice demonstrated reduced in vivo axial stretch, λz, compared with aortas from apoE−/− mice (Table 1; 1.26 ± 0.008 vs. 1.48 ± 0.005, respectively), indicating altered axial loading. The P-d data (Fig. 1A) of both groups exhibited biphasic behavior. The apoE−/−xTgSMC-Cat P-d curve was shifted downward from apoE−/−, indicating that apoE−/−xTgSMC−Cat aortas had smaller diameters and were less distensible. Pressure-dependent compliance (Cp) curves (Fig. 1B) further demonstrated that apoE−/−xTgSMC-Cat aortas were stiffer than apoE−/− aortas at mid-range pressure. Mean circumferential stress-strain ( ) curves (Fig. 1C) of each group were nonlinear and exhibited increased stiffening at high strain. The curves indicate that apoE−/−xTgSMC-Cat aortas had higher material stiffness compared with apoE−/− aortas at baseline.
Table 1.
Mechanical and geometry results of aortas from apoE−/− and apoE−/−xTgSMC-Cat mice with and without ANG II treatment
| ANG II | λz | d, mm | B, mm | H, μm | O.A., ° | |
|---|---|---|---|---|---|---|
| apoE−/− | − | 1.48 ± 0.005 | 1.37 ± 0.03 | 0.343 ± 0.02 | 89 ± 2.1 | 130 ± 1 |
| + | 1.38 ± 0.04* | 1.39 ± 0.08 | 0.342 ± 0.06 | 89 ± 1.9 | 198 ± 8* | |
| apoE−/−xTgSMC-Cat | − | 1.26 ± 0.008* | 1.16 ± 0.01*† | 0.347 ± 0.005 | 98 ± 2.1*† | 130 ± 8† |
| + | 1.23 ± 0.05* | – | – | 88 ± 1.6‡ | 130 ± 5† |
Values are means ± SE. Stretch ratio and loaded diameter data were acquired during isolated aortic inflation. Unloaded morphology was acquired from histological analysis of bisected aortic rings used in opening angle (O.A.) measurement. apoE−/−, apolipoprotein E-deficient mice; apoE−/−xTgSMC-Cat, apoE−/− mice that overexpress catalase in vascular smooth muscle cells; λz, axial stretch ratio (l/L), where l is loaded length and L is unloaded length; d, ex vivo loaded diameter; B, unloaded outer radius; H, unloaded thickness.
P < 0.05 vs. apoE−/−.
P < 0.05 vs. apoE−/− + ANG II.
P < 0.05 vs. apoE−/−xTgSMC-Cat.
Fig. 1.
Mechanical behavior of aortas of apolipoprotein E-deficient (apoE−/−) mice vs. apoE−/− mice that overexpress catalase in vascular smooth muscle cells (apoE−/−xTgSMC-Cat) at baseline as acquired by fixed length aortic inflation and opening angle measurement. A and B: pressure vs. diameter and pressure-dependent compliance vs. pressure behavior showed that aortas from apoE−/− mice are more compliant than aortas from apoE−/−xTgSMC-Cat mice. C: mean circumferential stress (σθ) vs. mid-wall circumferential Green strain (E̅θ) behavior of apoE−/−xTgSMC-Cat aortas shifted to the left of apoE−/− aortas, indicating that the aortic material properties of the groups differed at baseline. Mean circumferential stress and strain were calculated using ex vivo and histological mean morphological measurements provided in Table 1. D: mean circumferential Green strain vs. normalized radius [Eθ(r)] showed that the distribution of strain across the aortic wall of both groups was constant and that the strain magnitude in the apoE−/− aortas was greater than in apoE−/−xTgSMC-Cat. Data were analyzed by ANOVA using a Bonferroni's post hoc analysis. *P < 0.05.
Opening angles were measured to quantify variations in strain across the wall of the suprarenal aorta at the site of AAA formation. At baseline, apoE−/− and apoE−/−xTgSMC-Cat aortic rings exhibited comparable opening angles (Table 1), with magnitudes in agreement with published values from apoE−/− mice (18). Mean circumferential strain E̅θ across the wall was nearly uniform for both groups (Fig. 1D), although the strain magnitude of apoE−/− aortas was higher than apoE−/−xTgSMC-Cat aortas. Thus, at baseline, the apoE−/−xTgSMC-Cat abdominal aortas had different axial strain, diameter-pressure behavior, mean circumferential strain magnitude, and material properties compared with the apoE−/− aortas. Furthermore, both groups exhibited comparable opening angle and nearly uniform distribution of circumferential strain across the vessel wall.
Overexpression of catalase alters aortic microstructure.
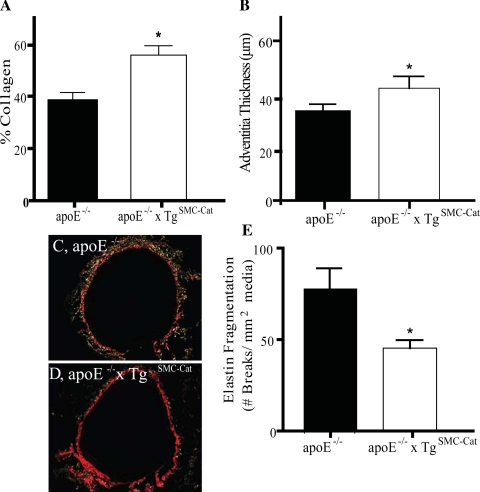
To investigate the structural basis for the mechanical differences between the two groups, matrix composition of the aortic walls was examined. At baseline, collagen content in the apoE−/− group was 50% lower than the apoE−/−xTgSMC-Cat group (Fig. 2A). Measurement of unloaded wall thickness (Table 1), adventitial thickness (Fig. 2B), and picrosirius red staining of untreated aortic cross sections imaged under polarized light (Fig. 2, C and D) confirmed that apoE−/− aortas were thinner and had less adventitial collagen than apoE−/−xTgSMC-Cat aortas. Elastin fragmentation was significantly greater in aortas from apoE−/− mice compared with apoE−/−xTgSMC-Cat (Fig. 2E), indicating less functional elastin in apoE−/− aortas. Thus, at baseline, both the collagen content and elastin integrity of apoE−/− aortas were reduced from apoE−/−xTgSMC-Cat aortas.
Fig. 2.
Morphology of apoE−/− and apoE−/−xTgSMC-Cat aortas at baseline. A: total collagen content per aortic dry weight decreased by 41% in apoE−/− compared with apoE−/−xTgSMC-Cat. B: apoE−/− adventitia thickness was 32% less than apoE−/−xTgSMC-Cat. C and D: representative images of the aortic wall stained with picrosirius red and imaged under polarized light. ApoE−/− image shows red and green illumination indicating presence of type I and type III collagen, respectively. ApoE−/−xTgSMC-Cat aortic cross section showed intense red illumination indicating a greater abundance of type I collagen. E: elastin fragmentation in the abdominal aortic wall was greater in apoE−/− compared with apoE−/−xTgSMC-Cat aortas. Elastin fragmentation was expressed as the number of breaks in elastic laminae per medial area. Medial area was defined as the area enclosed by the inner and outer elastic laminae measured from aortic sections. *P < 0.05; n = 5 for apoE−/−; n = 3 for apoE−/−xTgSMC-Cat.
ANG II infusion alters the circumferential strain distribution of aortas from apoE−/− mice but does not affect aortic microstructure.
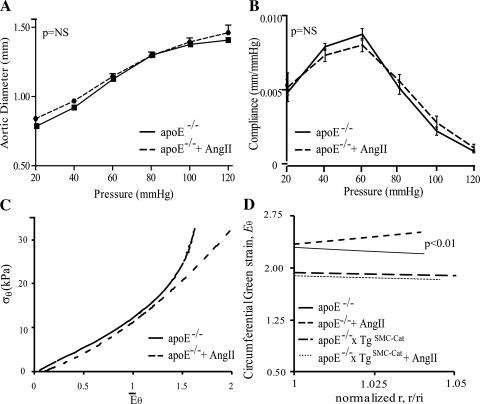
To determine if H2O2 is causally associated with the modulation of aortic mechanics during AAA progression, apoE−/− mice were treated with ANG II and an atherogenic diet for 7 days. This time point captured the earliest stages of ANG II-mediated AAA formation. The P-d behavior and compliance were unchanged in aortas from ANG II-treated apoE−/− mice compared with untreated apoE−/− mice (Fig. 3, A and B), and axial stretch was reduced (Table 1). The aortic mean circumferential stress-strain (E̅θ) behavior (Fig. 3C) in both groups was comparable at low and mid-range strain, but ANG II-treated aortas reached greater strain values. Abdominal aortic opening angle increased significantly with ANG II treatment (Table 1 and Fig. 4A). In addition, after a 7-day ANG II treatment, mean circumferential wall strain change with radius Eϴ(r), which was fairly uniform across the vessel wall at baseline, was higher at the outer vessel wall compared with the inner wall (Fig. 3D). Note also that the circumferential strain at the inner wall was similar before and after ANG II treatment. These data indicate that short-term ANG II treatment alters the opening angle and circumferential strain distribution in the apoE−/− abdominal aorta.
Fig. 3.
Mechanical response of apoE−/− aortas after ANG II treatment for 7 days. A and B: ANG II treatment had no effect on the pressure vs. diameter and compliance vs. pressure behavior of aortas from apoE−/− mice (n = 5–6). C: mean circumferential stress (σθ) vs. mean mid-wall circumferential Green strain (E̅θ) changed only at high E̅θ values, where aortas from treated apoE−/− mice showed reduced circumferential stress. D: mean circumferential Green strain [Eθ(r)] vs. normalized radius showed the distribution of strain across the aortic wall in apoE−/− aortas changed after ANG II treatment to reach higher strain at the outer wall radius. ANG II treatment did not affect the strain distribution of aortas from apoE−/−xTgSMC-Cat mice; n = 3; for apoE−/−; n = 5–8 for apoE−/− + ANG II; n = 4 for apoE−/−xTgSMC-Cat; n = 5 for apoE−/−xTgSMC-Cat + ANG II.
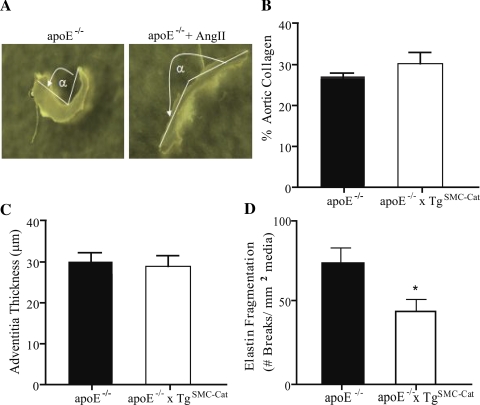
Fig. 4.
Opening angle of apoE−/− and apoE−/− + ANG II aortas and morphology of apoE−/− and apoE−/−xTgSMC-Cat aortas after ANG II treatment for 7 days. A: apoE−/− abdominal aortic opening angle (α) increased after ANG II treatment. B: collagen content decreased in the apoE−/−xTgSMC-Cat group and was unchanged in the apoE−/− after ANG II treatment. C: adventitial thickness in apoE−/−xTgSMC-Cat aortas decreased to the same level as apoE−/−. D: elastin fragmentation in the abdominal aortic wall of apoE−/− mice remained higher than in aortas from apoE−/−xTgSMC-Cat mice, with no change from baseline in either group. *P < 0.05; n = 3 for apoE−/−; n = 5–8 for apoE−/− + ANG II; n = 4 for apoE−/−xTgSMC-Cat; n = 5 for apoE−/−xTgSMC-Cat + ANG II.
To determine if catalase overexpression in apoE−/−xTgSMC-Cat aortas protected against ANG II-induced changes in wall strain, blood pressure and opening angle were measured after treatment with ANG II for 7 days. ANG II infusion elevated mean systolic blood pressure equally in both apoE−/− and apoE−/−xTgSMC-Cat mice (150 ± 13 vs. 147 ± 10 mmHg; P = NS). Importantly, catalase overexpression blunted the ANG II-induced changes in opening angle (Table 1) and in mean circumferential strain distribution (Fig. 3D).
To determine if ANG II promoted matrix changes that altered opening angle and circumferential strain in aortas from apoE−/− mice, total collagen protein and elastin fragmentation were measured in aortas from ANG II-treated apoE−/− and apoE−/−xTgSMC-Cat mice. Total collagen content (Fig. 4B) and adventitial thickness (Fig. 4C) in the ANG II-treated apoE−/−xTgSMC-Cat group decreased to the same level as aortas from apoE−/− mice. ANG II treatment had no effect on elastin fragmentation, which remained higher in apoE−/− aortas compared with apoE−/−xTgSMC-Cat aortas (Fig. 4D). Thus the data suggest that ANG II-induced increases in apoE−/− mean circumferential wall strain and opening angle were not attributed to changes in collagen content or elastin fragmentation measured in the aortic ECM.
DISCUSSION
In this study, we examined the contribution of H2O2 to baseline vascular mechanics and to associated biomechanical changes in the setting of AAA formation in apoE−/− mice. The data show, that early in ANG II-induced AAA formation, mean circumferential strain is increased in the outer abdominal aortic wall, while global aortic pressure-diameter mechanics are conserved. We further showed that SMC-specific catalase overexpression in the aortas of these mice prevented ANG II-induced mechanical alterations. Thus these data support an association between ROS production and early aneurysmal remodeling and mechanical adaptation.
AAA formation results from local aortic mechanical failure, but whether the spatial vulnerability of aneurysmal dilation is dominated by external hemodynamic forces or by local aortic weakness is not fully understood. Our study suggests that aortic microstructure in apoE−/− mice may confer mechanical vulnerability to AAA formation. At baseline, the mechanical and material properties of apoE−/− aortas were different from apoE−/−xTgSMC-Cat aortas, in that apoE−/− aortas had greater circumferential strain magnitude, i.e., had reduced circumferential stiffness, contained less adventitial collagen, and had less functional elastin. As elastic laminae rupture has been shown to reduce the stiffness of the rat abdominal aorta (31), elastin fragmentation in apoE−/− aortas likely contributes to low stiffness compared with apoE−/−xTgSMC−Cat. The critical role of elastin to vessel stability is well established in AAA, as emphasized by other models of AAA development that directly degrade elastin fibers to cause aortic dilation (1). In this case, the basal increase in elastin fragmentation in apoE−/− aortas may be due to the advanced age (16–18 wk) of the mice, as loss of elastin fiber structural integrity with aging is well established (35). Thus, given the baseline differences in elastin fragmentation between aortas from apoE−/− and apoE−/−xTgSMC-Cat mice, reduced elastin integrity may be a predisposing factor in the ANG II-infusion model of AAA.
ANG II infusion stimulates the progression of AAA in apoE−/− mice, and short-term infusion allowed examination of early aneurysmal changes in the descending aorta and in the local abdominal aorta where AAA develop. Acute ANG II treatment promoted increased aortic opening angle and redistribution of mean circumferential strain across the abdominal aortic wall, despite conservation of global pressure-diameter behavior. Opening angle is a manifestation of residual stresses in an unloaded aortic ring. Residual stress is thought to be a consequence of achieving uniform stress distribution across the medial thickness and, likely a different, uniform stress distribution across the adventitial thickness under physiologic loading. Opening angle is affected by multiple factors, including changes in geometry (e.g., radius-to-thickness ratio) and changes in material properties, which arise through changes in the content and organization of cells and ECM. Evidence that opening angles are larger in aortic rings containing intimal atheromas compared with autologous nonatherosclerotic regions (40) suggests that opening angle enlargement is indicative of preferential growth and/or increased compressive forces in the vessel interior (11). The converse, however, has been demonstrated by Greenwald et al. (14), wherein enzymatic degradation of medial elastin and collagen affected an opening angle decrease. ANG II-treated apoE−/− aortas in our study, however, did not have atheromas or significant changes in elastin integrity or collagen content. A possible difference between the characterization by Greenwald et al. and our study is that, in our model, degraded elastin fibers remained embedded within an intact medial collagen. Because other factors not measured here, such as collagen undulation and recruitment or ECM cross-linking, may underlie the microstructural basis for opening angle changes, a mechanical evaluation of strain distribution across the abdominal vessel was performed.
After ANG II treatment, mean circumferential strain distribution in the apoE−/− aorta outer wall adjusted to higher strain. Physiologic strain redistribution toward the adventitia has been explored as a protective mechanism to offload stress from the intima during hypertension (42) and may underlie the changes in mean strain observed here, especially given that ANG II infusion induced hypertensive blood pressure in apoE−/− mice. However, ANG II treatment raised blood pressure in apoE−/−xTgSMC-Cat mice as well without affecting opening angle. Importantly, the key finding in our study was the dramatic change in local circumferential mechanics in the absence of overt ECM changes, as measured by collagen content, wall thickness, and elastin fragmentation, in the apoE−/− abdominal aorta after ANG II infusion. It should be noted that other ultrastructural alterations in ECM cross-linking or cellular composition not measured here may ultimately underlie the biomechanical events observed. Dysfunctional elastin and collagen cross-linking in lysyl oxidase-deficient mice promotes AAA (25), and deficiency of the cystein protease inhibitor cystatin C is linked to AAA formation in humans (22). Aneurysm microstructure is also complicated by infiltrating inflammatory cells (10), reduced proteoglycans (38), and defects in collagen microarchitecture, as demonstrated by Lindeman et al. (21). Given the absence of ANG II-induced mechanical changes in mice with overexpression of catalase before the development of AAA, the current data suggest that there are additional ROS-associated changes in the vessel wall that remain to be elucidated.
SMC-specific overexpression of catalase in apoE−/− mice allowed us to determine if H2O2 is a critical molecular mediator in the subclincal mechanism of AAA pathogenesis. Aortas from these mice had thicker walls, greater functional elastin, and increased collagen content, which clearly accounted for the increase in mechanical stiffness detected at baseline compared with the apoE−/− group. However, after ANG II treatment, the collagen content and adventitial thickness of both apoE−/− and apoE−/−xTgSMC-Cat aortas were equivalent. Thus, given that overexpression of catalase-blunted ANG II-mediated changes in opening angle and mean circumferential strain distribution across the aorta, our data suggest that H2O2 is functionally important and is involved in the control of local variations in remodeling across the vessel wall. ROS are shown to be significantly upregulated in established AAA (13, 29, 30), promoting ECM degradation by upregulating MMP activity via the NAD(P)H oxidase (16, 34). Our data establish H2O2 as a mediator of subclinical AAA and support the pivotal role of ROS at all stages of AAA pathogenesis. Antioxidant studies in animal models also support the link between H2O2 and AAA. Catalase deficiency was associated with AAA formation in a study utilizing the elastase infusion model (15), supporting our finding that catalase participates in mitigating AAA formation. It is plausible that hydrogen peroxide-scavenging by catalase blunted AAA formation by activating mechanism to compensate for ANG II-induced collagen degradation. Indeed, our investigation showed a trend for increased procollagen type I mRNA expression in apoE−/−xTgSMC-Cat aortas. We note that in the transgenic model of SMC-specific catalase overexpression studied here, we cannot exclude the possibility that catalase was a “sink” for freely diffusible H2O2 secreted by other cell types within the vessel wall.
In conclusion, the present study suggests that the earliest local mechanical changes in ANG II-mediated AAA development are associated with the local production of ROS. The ANG II-mediated increase in abdominal aorta opening angle was blunted by overexpression of catalase, suggesting that H2O2 is a pivotal molecular signal in the pathogenesis of AAA. Furthermore, our data suggest that H2O2 initially impacts arterial wall biomechanics via a mechanism independent of overt changes in the aortic microstructure metrics that we examined. Thus the data provide a critical links between oxidative stress, matrix composition, and biomechanical behavior.
GRANTS
We acknowledge funding from the American Heart Association Southeast Affiliate for Predoctoral Fellowship Support (to K. Maiellaro-Rafferty) and National Heart, Lung, and Blood Institute Grants P01-HL-095070, RO1-HL-70531, and RO1-HL-090584.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Anidjar S, Salzmann J, Gentric D, Lagneau P, Camilleri J, Michel J. Elastase-induced experimental aneurysms in rats. Circulation 82: 973–981, 1990 [DOI] [PubMed] [Google Scholar]
- 2. Basalyga DM, Simionescu DT, Xiong W, Baxter BT, Starcher BC, Vyavahare NR. Elastin degradation and calcification in an abdominal aorta injury model: role of matrix metalloproteinases. Circulation 110: 3480–3487, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burke JM, Balian G, Ross R, Bornstein P. Synthesis of types I and III procollagen and collagen by monkey aortic smooth muscle cells in vitro. Biochemistry 16: 3243–3249, 1977 [DOI] [PubMed] [Google Scholar]
- 4. Chatelain RE, D BN. Increased DNA replication in the arterial adventitia after aortic ligation. Hypertension 11: I130–I134, 1988 [DOI] [PubMed] [Google Scholar]
- 5. Chiquet M, Renedo AS, Huber F, Flück M. How do fibroblasts translate mechanical signals into changes in extracellular matrix production? Matrix Biol 22: 73–80, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Chuong CJ, Fung YC. On residual stresses in arteries. J Biomech Eng 108: 189–192, 1986 [DOI] [PubMed] [Google Scholar]
- 7. Daugherty A, Cassis LA. Mouse models of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 24: 429–434, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest 105: 1605–1612, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Delles CZL, McGrane DJ, Koh-Tan CH, Pathi VL, McKay AJ, Steedman T, Dargie HJ, Hamilton CA, Dominiczak AF. Vascular stiffness is related to superoxide generation in the vessel wall. J Hypertens 26: 946–955, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Freestone T, Turner RJ, Coady A, Higman DJ, Greenhalgh RM, Powell JT. Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol 15: 1145–1151, 1995 [DOI] [PubMed] [Google Scholar]
- 11. Fung YC. What are the residual stresses doing in our blood vessels? Ann Biomed Eng 19: 237–249, 1991 [DOI] [PubMed] [Google Scholar]
- 12. Gavazzi G, Deffert C, Trocme C, Schappi M, Herrmann FR, Krause KH. NOX1 deficiency protects from aortic dissection in response to angiotensin II. Hypertension 50: 189–196, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Gavrila D, Li WG, McCormick ML, Thomas M, Daugherty A, Cassis LA, Miller FJ, Jr, Oberley LW, Dellsperger KC, Weintraub NL. Vitamin E inhibits abdominal aortic aneurysm formation in angiotensin II-infused apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 25: 1671–1677, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Greenwald SE, Moore J JE, Rachev A, Kane TPC, Meister JJ. Experimental investigation of the distribution of residual strains in the artery wall. J Biomech Eng 119: 438–444, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Grigoryants V, Hannawa KK, Pearce CG, Sinha I, Roelofs KJ, Ailawadi G, Deatrick KB, Woodrum DT, Cho BS, Henke PK, Stanley JC, Eagleton MJ, Upchurch J, Gilbert R. Tamoxifen up-regulates catalase production, inhibits vessel wall neutrophil infiltration, and attenuates development of experimental abdominal aortic aneurysms. J Vasc Surg 41: 108–114, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Grote K, Flach I, Luchtefeld M, Akin E, Holland SM, Drexler H, Schieffer B. Mechanical stretch enhances mrna expression and proenzyme release of matrix metalloproteinase-2 (MMP-2) via NAD(P)H oxidase-derived reactive oxygen species. Circ Res 92: 80e–86, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Guo X, Lu X, Kassab GS. Transmural strain distribution in the blood vessel wall. Am J Physiol Heart Circ Physiol 288: H881–H886, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Hayashi K, Imai Y. Tensile property of atheromatous plaque and an analysis of stress in atherosclerotic wall. J Biomech 30: 573–579, 1997 [DOI] [PubMed] [Google Scholar]
- 19. He CM, Roach MR. The composition and mechanical properties of abdominal aortic aneurysms. J Vasc Surg 20: 6–13, 1994 [DOI] [PubMed] [Google Scholar]
- 20. Lemaitre V, Soloway PD, D'Armiento J. Increased medial degradation with pseudo-aneurysm formation in apolipoprotein e-knockout mice deficient in tissue inhibitor of metalloproteinases-1. Circulation 107: 333–338, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Lindeman JHN, Ashcroft BA, Beenakker JWM, van Es M, Koekkoek NBR, Prins FA, Tielemans JF, Abdul-Hussien H, Bank RA, Oosterkamp TH. Distinct defects in collagen microarchitecture underlie vessel-wall failure in advanced abdominal aneurysms and aneurysms in Marfan syndrome. Proc Natl Acad Sci USA 107: 862–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lindholt JSEE, Henneberg EW. Cystatin C deficiency is associated with the progression of small abdominal aortic aneurysms. Br J Surg 88: 1472–1475, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Longo G, Buda SJ, Fiotta N, Xiong W, Griener T, Shapiro S, Baxter BT. MMP-12 has a role in abdominal aortic aneurysms in mice. Surgery 137: 457–462, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Maki JM, Rasanen J, Tikkanen H, Sormunen R, Makikallio K, Kivirikko KI, Soininen R. Inactivation of the lysyl oxidase gene lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation 106: 2503–2509, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Manning MW, Cassis LA, Daugherty A. Differential effects of doxycycline, a broad-spectrum matrix metalloproteinase inhibitor, on angiotensin II-induced atherosclerosis and abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 23: 483–488, 2003 [DOI] [PubMed] [Google Scholar]
- 27. McCormick ML, Gavrila D, Weintraub NL. Role of oxidative stress in the pathogenesis of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 27: 461–469, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Mendez JI, Weiss D, Gupta D, Taylor WR. Smooth muscle-specific overexpression of catalase reduces atherosclerosis and inflammatory gene expression in ApoE-deficient mice. In: Arteriosclerosis Thorombosis and Vascular Biology Conference. Denver, CO: American Heart Association, 2006 [Google Scholar]
- 29. Miller FJ, Jr, Sharp WJ, Fang X, Oberley LW, Oberley TD, Weintraub NL. Oxidative stress in human abdominal aortic aneurysms: a potential mediator of aneurysmal remodeling. Arterioscler Thromb Vasc Biol 22: 560–565, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Miller FJ., Jr Aortic aneurysms: it's all about the stress. Arterioscler Thromb Vasc Biol 22: 1948–1949, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Obsborne-Pellegrin M, Labat C, Marcier N, Challande P, PL Changes in aortic stiffness related to elastic fiber network anomalies in the Brown Norway rat maturation and aging. Am J Physiol Heart Circ Physiol 299: H144–H152, 2010 [DOI] [PubMed] [Google Scholar]
- 32. Okamoto RJ, Wagenseil JE, DeLong WR, Peterson SJ, Kouchoukos NT, Sundt TM., III Mechanical properties of dilated human ascending aorta. Ann Biomed Eng 30: 624–635, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Rachev A, Greenwald SE. Residual strains in conduit arteries. J Biomech 36: 661–670, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest 98: 2572–2579, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Santelices LC, Rutman SJ, Prantil-Baun R, Vorp DA, JMA Relative contributions of age and atherosclerosis to vascular stiffness. Clin Transl Sci 1: 62–66, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shi YOBJ, Fard A, Zalewski A. Transforming growth factor-beta1 expression and myofibroblast formation during arterial repair. Arterioscler Thromb Vasc Biol 16: 1298–1305, 1996 [DOI] [PubMed] [Google Scholar]
- 37. Silence J, Collen D, Lijnen HR. Reduced atherosclerotic plaque but enhanced aneurysm formation in mice with inactivation of the tissue inhibitor of metalloproteinase-1 (TIMP-1) gene. Circ Res 90: 897–903, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Tamarina NA, Grassi MA, Johnson DA, Pearce WH. Proteoglycan gene expression is decreased in abdominal aortic aneurysms. J Surg Res 74: 76–80, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Thomas M, Gavrila D, McCormick ML, Miller FJ, Jr, Daugherty A, Cassis LA, Dellsperger KC, Weintraub NL. Deletion of p47phox attenuates angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-deficient mice. Circulation 114: 404–413, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Valenta J, Svoboda J, Valerianova D, Vitek K. Residual strain in human atherosclerotic coronary arteries and age related geometrical changes. Biomed Mater Eng 9: 311–317, 1999 [PubMed] [Google Scholar]
- 41. Vorp DA, Raghavan ML, Muluk SC, Makaroun MS, Steed DL, Shapiro R, Webster MW. Wall strength and stiffness of aneurysmal and nonaneurysmal abdominal aorta. Ann NY Acad Sci 800: 274–276, 1996 [DOI] [PubMed] [Google Scholar]
- 42. Wang C, Kassab GS. Increase in opening angle in hypertension off-loads the intimal stress: a simulation study. J Biomech Eng 131: 114502, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Woessner J. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys 93: 440–447, 1961 [DOI] [PubMed] [Google Scholar]
- 44. Xiong W, Mactaggart J, Knispel R, Worth J, Zhu Z, Li Y, Sun Y, Baxter BT, Johanning J. Inhibition of reactive oxygen species attenuates aneurysm formation in a murine model. Atherosclerosis 202: 128–134, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang Y, Griendling KK, Dikalova A, Owens GK, Taylor WR. Vascular hypertrophy in angiotensin II-induced hypertension is mediated by vascular smooth muscle cell-derived H2O2. Hypertension 46: 732–737, 2005 [DOI] [PubMed] [Google Scholar]