Abstract
The objective of this study was to investigate vascular function at different ages in a transgenic murine model of fetal vascular programming using a model of uteroplacental insufficiency induced by lack of endothelial nitric oxide synthase. Homozygous NOS3 knockout (KO) and wild-type (WT) mice were cross bred to produce WT, KO, and heterozygous that developed in WT (KOP) or KO (KOM) mothers. Male/female offspring from the four groups were killed at 7, 14, and 21 wk of age (n = 5–10/group), and carotid arteries were used for in vitro vascular studies. Responses to phenylephrine (PE), with/without NG-nitro-l-arginine methyl ester (l-NAME), angiotensin (ANG), acetylcholine (ACh), sodium nitroprusside, and isoproterenol (ISO) were studied. At 7 wk, only KO offspring showed higher contractile response to PE, whereas, at 14 and 21 wk, both KO and KOM had a higher response. Incubation with l-NAME abolished these differences. ANG contraction was higher in male KO in all age groups and in 21-wk-old females. Relaxation to ACh and ISO was absent in KO, and significantly decreased in KOM offspring in all age groups compared with KOP and WT, independent of gender. Sodium nitroprusside was not different between groups. The effect of the altered intrauterine environment on the development of abnormal vascular function was limited at 7 wk of age and most evident at 14 wk; further deterioration was limited to ANG-mediated vascular contractility in KO offspring. Our findings provide some hope that at least the first seven postnatal weeks may be an appropriate therapeutic window to prevent cardiovascular disease later in life.
Keywords: fetal programming, age process, cardiovascular development, kidney development, vascular structure
the association between suboptimal intrauterine environment and adult diseases was initially suggested in the 1960s by Robert McCance and Elsie Widdowson (48, 49) in their original work on the influence of early neonatal nutrition on growth, body size, and development. Their work was later highlighted by the epidemiological studies of David Barker in the 1980s, which showed that low birth weight is an important risk factor for the future development of cardiovascular diseases. Barker's hypothesis, which was subsequently termed the “developmental origin of adult diseases,” suggests that insults to the fetus during critical periods of development lead to fetal programming and produce adaptive changes that have long-term consequences (1–4).
The milieu in which the fetus develops is determined by the interactions between the fetal genome and the intrauterine environment, which in turn may depend on the influences related to the maternal genetic information. Long-term health of the offspring is then determined by these intrauterine influences as well as by the genetic makeup of the individual, which is additionally dependent on inheritance of maternal genes. Therefore, a genetic trait that affects vascular function may influence uteroplacental perfusion if present in the mother, as well as influence long-term health of the offspring if inherited by her fetus. To study these complex interactions, we used a transgenic mouse model deficient in endothelial nitric oxide synthase (eNOS; or NOS3), the rate-limiting enzyme responsible for nitric oxide (NO) production from l-arginine in endothelial cells (15, 22). NO is a potent smooth muscle relaxant and an essential modulator of vascular tone (34, 38); it also contributes to the maintenance of adequate uteroplacental perfusion through its vasodilatory properties. Indeed, inhibition of NOS3 during pregnancy results in maternal hypertension and fetal growth restriction (37, 42, 47). Because it combines an adverse uterine environment with a genetic alteration affecting vascular function, this animal model is well-suited to study the contribution of each variable on the vascular function in adult life.
In prior work, transgenic male and female mice lacking a functional NOS3 gene [NOS3−/− or knockout (KO)] were crossbred with wild-type mice [NOS3+/+ or wild type (WT)] to generate heterozygous offspring (NOS3+/−) that developed either in a mother lacking a functional NOS3 (maternally derived heterozygous offspring; KOM) or in normal wild-type mothers (paternally derived heterozygous offspring; KOP).
In that study, we demonstrated that the abnormal uterine environment in which KOM offspring developed altered fetal and postnatal growth as well as resulted in abnormal adult vascular function compared with KOP offspring, which were genomically similar but developed in a normal uterine environment. Most importantly, the endothelium-dependent relaxation to acetylcholine (ACh) was absent in KOM offspring, similar to KO mice, whereas that of KOP mice was similar to the one observed in WT animals; this effect was evident in the carotid as well the mesenteric vascular bed (28). These striking differences were initially demonstrated in the offspring at 7–8 wk of age. Therefore, we hypothesized that the previously demonstrated differences in vascular profile in adult offspring progress over time and are gender specific. Our objective is thus to evaluate the progression of the vascular dysfunction in this animal model over time, and to characterize the differences existing between genders.
MATERIALS AND METHODS
Animals.
Mature cycling female and male mice that are homozygous for disruption of the NOS3 gene (NOS3 knockout, strain B6.129P2-Nos3tm1Unc, stock no. 002684, KO) and their age-matched wild-type controls (NOS3 wild type, strain C57BL/6J, stock no. 000664, WT) were purchased from Jackson Laboratory (Bar Harbor, ME). Approval for the study was obtained from the Institutional Animal Care and Use Committee (IACUC). The mice were housed separately in temperature- and humidity-controlled quarters with constant 12:12-h light-dark cycles in the animal care facility at the University of Texas Medical Branch. Female and male KO mice and their WT controls were crossbred to obtain KO, WT, KOM, and KOP litters. Six to 8 females and 6–10 males were used in each age group. First-generation offspring were killed at 7, 14, and 21 wk of life using CO2 inhalation according to the IACUC and the American Veterinary Medical Association guidelines. The carotid arteries were then dissected, isolated, and mounted on a wire myograph system for vascular reactivity studies. All surgical procedures were carried out by trained personnel according to the IACUC guidelines.
Drugs and solutions.
Phenylephrine (PE), NG-nitro-l-arginine methyl ester (l-NAME), angiotensin (ANG), ACh, sodium nitroprusside (SNP), and isoproterenol (ISO) were purchased from Sigma-Aldrich Chemical (St. Louis, MO). Stock solutions of all of the drugs (10−2 mmol/l) were prepared in deionized water and stored at −20°C. The composition of Krebs solution was as follows (in mmol/l): 119 NaCl, 4.7 KCl, 1.2 NaH2PO4, 25 NaHCO3, 1.2 MgCl2, 2.5 CaCl2, 0.026 ethylenediaminetetraacetic acid, and 11.5 glucose.
In vitro experiments.
Two-millimeter segments of the carotid artery were mounted on a wire myograph (model 410A; J.P. Trading, Aarhus, Denmark) with 25-μm tungsten wires. The preparations were bathed in a physiological salt solution that was bubbled continuously with a mixture of 95% O2 and 5% CO2, with the temperature maintained at 37°C and the pH kept at 7.4. Vascular tension was recorded continuously by an isometric force transducer. Vessels were allowed to stabilize under a passive tension up to 3.5 mN and then contracted two times with 60 mmol/l KCl to enhance reproducibility of the responses. After 1 h equilibration, the α1-adrenergic agonist PE was added at a final concentration of 10−7 to 10−6 mmol/l to produce matching contractions in the study groups. Vascular responses to the endothelium-dependent vasorelaxant ACh (10−10 to 10−5 mmol/l), the endothelium-independent vasorelaxant SNP (10−10 to 10−5 mmol/l), and the β-adrenoreceptor agonist ISO (10−10 to 10−5 mmol/l) were studied. Contractile responses were evaluated for PE (10−10 to 10−5 mmol/l) in the presence or absence of the nonselective NOS inhibitor l-NAME (10−5 mmol/l) and ANG (10−10 to 10−5 mmol/l). The samples were washed with Krebs solution and left to recover for ≥30 min after each agent was tested.
Data analysis.
Vascular tension was recorded continuously by an isometric transducer, analyzed with Power laboratory data acquisition software (DataQ Instruments, Akron, OH), and presented as means ± SE. Dose-response curves were generated for each vasoactive agent. The maximal effect was calculated and expressed as the mean ± SE. The second response to KCl was used as a reference to calculate the percent vascular tension achieved by the contractile agents, whereas PE-induced contraction was used to measure the percent relaxation produced by the vasorelaxant agents. Continuous data were tested for normality using the Kolmogorov-Smirnov test and then compared using one-way ANOVA followed by the Newman-Keuls multiple-comparisons test. A P < 0.05 was considered statistically significant.
RESULTS
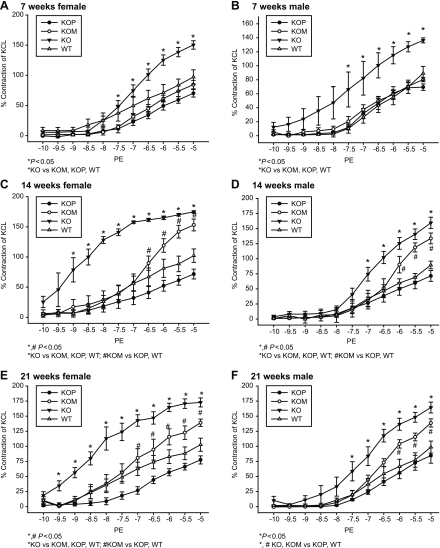
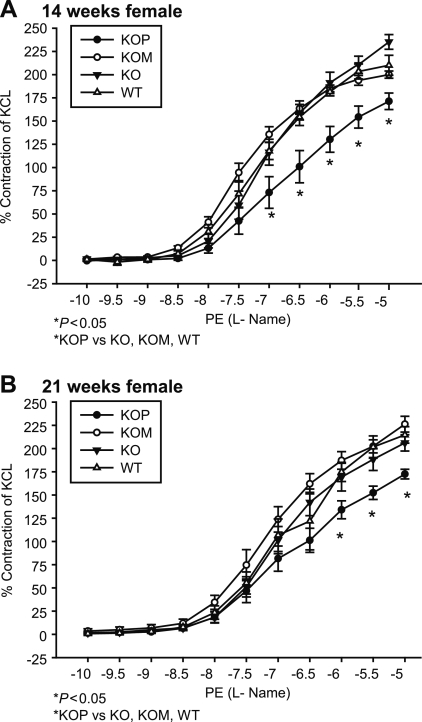
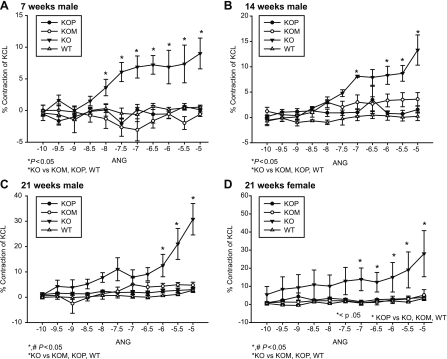
Seven-week-old KO offspring had significantly higher contractile responses to PE compared with WT, KOP, and KOM (Table 1 and Fig. 1, A and B; P < 0.05), whereas at 14 and 21 wk of age, both KO and KOM offspring had increased contractile responses to PE compared with WT and KOP (Table 1 and Fig. 1, C–F; P < 0.05). These responses were seen in both female and male offspring groups. Additionally, when compared with offspring of different ages or who had previously developed in a different intrauterine environment, both male and female KOM had increased vascular responses to PE at 14 and 21 wk that were even more prominent than the ones presented at 7 wk (P < 0.05). Incubation with l-NAME abolished the differences mediated by the α1-adrenergic agonist in the male offspring (Table 1). In contrast, vessels from female KOP preincubated with l-NAME showed decreased vascular contraction to PE compared with WT, KOM, and KO at 14 and 21 wk (Table 1 and Fig. 2, A and B; P < 0.05). The ANG-induced contractile response was highest among male KO, where it was directly related to the age of the offspring (Table 2 and Fig. 3, A–C; P < 0.05). In females, ANG-induced contractility was increased only in KO offspring at 21 wk compared with WT, KOM, and KOP (Table 2 and Fig. 3D; P < 0.05).
Table 1.
Maximal contractile effect to PE, with and without l-NAME, and angiotensin in female and male KO, KOM, KOP, and WT at 7, 14, and 21 wk of age
| Agents | Age, wk | KOP | KOM | KO | WT |
|---|---|---|---|---|---|
| Females | |||||
| PE | 7 | 70.1 ± 6.2 | 84.6 ± 7.0 | 150.3 ± 6.9* | 97.2 ± 11.9 |
| 14 | 71.7 ± 8.1 | 153 ± 9.8# | 174.8 ± 1.9* | 102.2 ± 11.1 | |
| 21 | 77.5 ± 6.7 | 139 ± 6.1# | 172.7 ± 7.5* | 102.7 ± 10.8 | |
| PE + l-NAME | 7 | 142.4 ± 7.7 | 201.03 ± 1.2 | 202.57 ± 17.1 | 155.8 ± 19.9 |
| 14 | 173.1 ± 8.8* | 201.89 ± 3.8 | 237.08 ± 7.9 | 211.9 ± 10.7 | |
| 21 | 172.4 ± 5.1* | 226.2 ± 8.4 | 206 ± 8.8 | 214.6 ± 8.8 | |
| ANG | 7 | 0.9 ± 0.5 | 2.0 ± 0.6 | 2.9 ± 1.4 | 0.6 ± 0.07 |
| 14 | 2.1 ± 0.7 | 5.5 ± 2.3 | 6.1 ± 2.5 | 4.5 ± 1.9 | |
| 21 | 3.8 ± 0.8 | 5.13 ± 3.01 | 2.8 ± 12.7* | 3.3 ± 0.7 | |
| Males | |||||
| PE | 7 | 69.4 ± 4.6 | 80.2 ± 3.1 | 136.3 ± 3.5* | 89.2 ± 9.8 |
| 14 | 71.4 ± 8.9 | 133.6 ± 8.9# | 159.4 ± 9.4* | 89.6 ± 6.3 | |
| 21 | 84.7 ± 12.6 | 138.7 ± 6.7# | 164.3 ± 8.9* | 98.7 ± 9.9 | |
| PE+l-NAME | 7 | 164.4 ± 7.2 | 184.1 ± 9.1 | 212.3 ± 5.4 | 192.1 ± 9.6 |
| 14 | 174.7 ± 3.1 | 205.1 ± 8.1 | 225.7 ± 5.9 | 186.9 ± 17.3 | |
| 21 | 170.3 ± 7.3 | 191.4 ± 10.4 | 219.3 ± 17.1 | 208.4 ± 10 | |
| ANG | 7 | 0.11 ± 0.56 | −0.57 ± 0.36 | 9.0 ± 2.42* | 0.53 ± 0.31 |
| 14 | 1.54 ± 0.57 | 3.59 ± 1.30 | 13.2 ± 2.98* | 0.21 ± 0.85 | |
| 21 | 3.01 ± 0.94 | 4.76 ± 1.16 | 30.7 ± 6.2* | 2.76 ± 0.90 | |
Results are expressed as percent of the reference KCl contraction (mean ± SE). PE, phenylephrine; l-NAME, NG-nitro-l-arginine methyl ester; KO, knockout, WT. wild type; KOM, maternally derived heterozygous; KOP, paternally derived heterozygous; ANG, angiotensin. P < 0.05 for KO vs. WT, KOM, and KOP (*) and for KOM vs. WT and KOP (#)).
Fig. 1.
A–F: concentration-response curves to phenylephrine (PE, 10−10 to 10−5 mmol/l) in the carotid artery of female and male knockout (KO), wild-type (WT), maternally derived heterozygous (KOM), and paternally derived heterozygous (KOP) offspring at 7, 14, and 21 wk of age (female, n = 6–8/age group; male, n = 6–10/age group).
Fig. 2.
A and B: concentration-response curves to PE (10−10 to 10−5 mmol/l) in the presence of NG-nitro-l-arginine methyl ester (L-NAME) in the carotid artery of female KO, WT, KOM, and KOP offspring at 14 and 21 wk of age (female, n = 6–8/age group; male, n = 6–10/age group).
Table 2.
Maximal relaxant effect to ACh, ISO, and SNP in female and male KO, KOM, KOP, and WT at 7, 14, and 21 wk of age
| Agents | Age, wk | KOP | KOM | KO | WT |
|---|---|---|---|---|---|
| Females | |||||
| ACh | 7 | 101.1 ± 3.2 | 70.6 ± 4.4# | −7.9 ± 5.7* | 111.5 ± 5.0 |
| 14 | 114.9 ± 9.1 | 23.6 ± 5.0# | −35.6 ± 7.4* | 109.7 ± 4.9 | |
| 21 | 98.1 ± 2.6 | 39.9 ± 6.8# | −24.0 ± 2.6* | 109.±6.3 | |
| ISO | 7 | 98.9 ± 2.8 | 91.3 ± 2.7# | 29.1 ± 6.4* | 92.9 ± 2.9 |
| 14 | 100.2 ± 6.0 | 45.2 ± 8.90# | −1.09 ± 6.0* | 108.2 ± 3.5 | |
| 21 | 97 ± 2.3 | 85.9 ± 2.3 | 21.32 ± 6.8* | 105.9 ± 5.0 | |
| SNP | 7 | 110.2 ± 4.7 | 101.0 ± 4.5 | 105.2 ± 3.0 | 99.5 ± 2.2 |
| 14 | 101.5 ± 1.7 | 98.7 ± 4.7 | 101.2 ± 3.9 | 102.8 ± 0.3 | |
| 21 | 100.6 ± 1.7 | 103.06 ± 3.5 | 106.2 ± 1.1 | 109.0 ± 2.7 | |
| Males | |||||
| ACh | 7 | 103.9 ± 1.6 | 65.7 ± 4.9# | −8.8 ± 5.6* | 108.4 ± 6.7 |
| 14 | 99.5 ± 3.4 | 15.9 ± 2.1# | −49.1 ± 12.8* | 101.4 ± 3.6 | |
| 21 | 88.9 ± 15.3 | 49.6 ± 4.6# | −35.9 ± 2.87* | 100.2 ± 4.5 | |
| ISO | 7 | 90.8 ± 6.9 | 85.01 ± 4.4# | 30.8 ± 8.93* | 113.7 ± 8.9 |
| 14 | 102.1 ± 3.4 | 57.9 ± 5.07# | 5.5 ± 1.94* | 112.8 ± 8.1 | |
| 21 | 102.7 ± 1.6 | 88.3 ± 4.06 | 27.7 ± 6.05* | 107.2 ± 8.7 | |
| SNP | 7 | 104.6 ± 1.6 | 106.2 ± 4.8 | 103.08 ± 2.40 | 104.6 ± 5.1 |
| 14 | 101.2 ± 1.3 | 104.7 ± 1.7 | 106.1 ± 3.26 | 96.8 ± 1.5 | |
| 21 | 105.7 ± 1.2 | 99.9 ± 3.09 | 103.8 ± 1.41 | 102.6 ± 1.1 | |
Results express as percent of the reference PE contraction (mean ± SE). ACh, acetylcholine; ISO, isoproterenol; SNP, sodium nitroprusside. P < 0.05 for KO vs. WT, KOM, and KOP (*) and for KOM vs. WT and KOP (#).
Fig. 3.
A–D: concentration-response curves to angiotensin (ANG, 10−10 to 10−5 mmol/l) in the carotid artery of female and male KO, WT, KOM, and KOP offspring at 7, 14, and 21 wk of age (female, n = 6–8/age group; male, n = 6–10/age group).
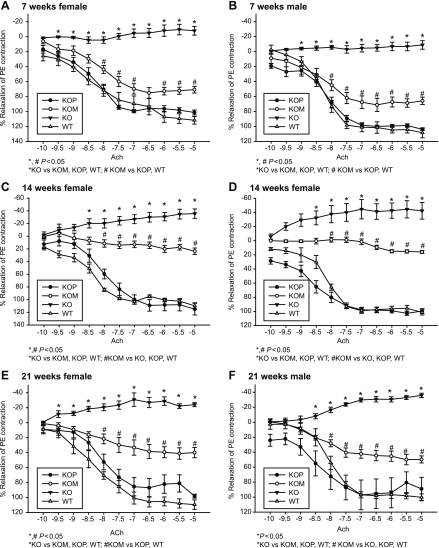
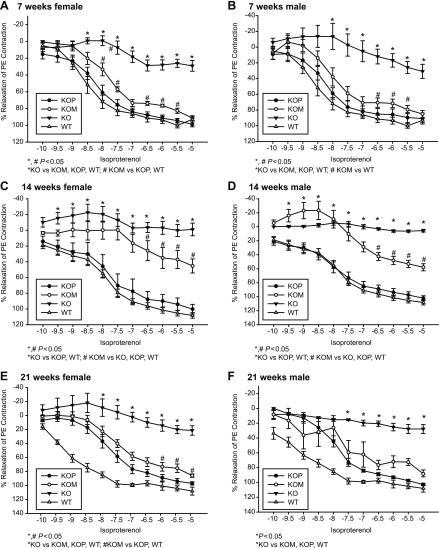
Vascular relaxation induced by ACh was higher in both WT and KOP offspring compared with KOM and KO (Tables 1 and 2 and Fig. 4, A–F; P < 0.05). These findings were demonstrated in both male and female offspring within each of the three age groups. When evaluating the effect of both postnatal age and intrauterine environment on ACh relaxation, the highest response was detected in 7-wk-old KOM mice (P < 0.05), with no significant differences observed between the other groups (Table 2). Relaxation to ISO was higher in WT and KOP compared with KO and KOM offspring, independent of age and gender (Table 2 and Fig. 5, A–F; P < 0.05). Similar to the findings with ACh, the highest response to ISO was detected at 7 wk within KOM offspring (Table 2; P < 0.05).
Fig. 4.
A–F: concentration-response curves to acetylcholine (ACh, 10−10 to 10−5 mmol/l) in the carotid artery of female and male KO, WT, KOM, and KOP offspring at 7, 14, and 21 wk of age (female/male n = 5–10/age group).
Fig. 5.
A–F: concentration-response curves to isoproterenol (10−10 to 10−5 mmol/l) in the carotid artery of female and male KO, WT, KOM, and KOP offspring at 7, 14, and 21 wk of age (female/male, n = 5–10/age group).
No differences were observed in the response to the endothelium-independent vasorelaxant SNP (Table 2).
DISCUSSION
At 7 wk of age, only KO offspring showed higher contractile response to PE, whereas at 14 and 21 wk of age, both KO and KOM (male and female) had significantly higher PE-induced vascular contractility compared with WT and KOP offspring. Incubation with l-NAME abolished the differences seen in response to PE in all groups, especially in male offspring, indirectly suggesting that the exaggerated PE responses are mediated by the nitric oxide pathway. On the other hand, ANG-induced vascular contractility was higher in male KO offspring in all age groups and in 21-wk-old female offspring compared with WT, KOM, and KOP. The relaxation induced by ACh and ISO was absent in KO and significantly decreased in KOM offspring in all age groups compared with KOP and WT, independent of gender. ACh is an endothelium-dependent vasorelaxant. The lack of relaxation observed in the eNOS-knockout group (KO) provides further evidence that ACh-mediated vascular relaxation is NO dependent, and irrespective of age and gender. The altered vascular response detected in KOM at 14 wk and the normal response observed in KOP provide evidence of the timing of the altered endothelial function (being age dependent) and that only one copy of the eNOS gene is sufficient for normal responses to ACh in carotid arteries (12, 21).
From these data, we conclude that the effects of the altered intrauterine environment on the development of abnormal vascular function were limited at 7 wk of age in both male and female offspring and became well established at 14 wk, and further deterioration was limited to ANG-mediated vascular contractility in KO offspring at 21 wk. Our findings provide insight about a potential window in the first seven postnatal weeks when interventions may be directed to prevent cardiovascular disease later in life.
In an attempt to explain our findings of different vascular profile between KOP and KOM offspring, we previously looked at eNOS gene expression in the vasculature of these offspring and found no differences between KOP and KOM (8). Additionally, we have shown that the abnormal vascular phenotype of the adult KOM offspring (born to a KO mother) was abolished when KOM embryos were transferred to develop in wild-type (NOS3+/+) surrogate mothers (28). These findings suggest that the differences in vascular profile between heterozygous offspring are related to the uterine environment in which the pups develop, rather than genetics. Although transmission of epigenetic traits across both maternal and paternal lines has been described, our data do not support such a mechanism in our animal model. Indeed, in support of the presence of abnormal uterine environment, it has been demonstrated that structural and cellular changes characteristic of uterine artery remodeling during pregnancy are markedly reduced in NOS3-deficient mice, contributing to poorer pregnancy outcome (43).
Our findings support the importance of in utero effects on vascular programming as one of the mechanisms by which growth-restricted infants are at increased risk of hypertension, cardiovascular morbidity, and mortality (3, 4). Our study also suggests a time frame for the development of these adverse outcomes. Identifying such a window in infants at risk of adverse outcomes when adults may help define when interventions may succeed the most in preventing the development of these morbidities.
Several mechanisms have been previously proposed and are thought to lead to abnormal fetal vascular programming, although are speculative when applied to our model and require further testing. One mechanism involves structural changes in resistant vessels. Arterial wall stiffness is associated with adult hypertension and has been recognized as an early marker of cardiovascular disease. Vessel wall development is affected by blood flow and elastin synthesis, which in fetuses peaks in the last weeks of development (24, 27, 33). Vascular dysfunction and hypertension in the adult may therefore be the result of decreased arterial compliance originating in fetal life secondary to reduced elastin deposition (6, 16, 17). In fact, elastin knockout mice manifest high blood pressure and stiff stenotic arteries leading to impaired ventricular function (11, 46, 47). Adverse structural vascular changes are known to occur with aging, and this may explain the limited vascular dysfunction at 7 wk but established dysfunction at 14 wk in our animal model.
An adverse fetal environment may also lead to altered renal structure and function, leading to hypertension in adulthood (2, 10, 17, 51). Human studies also show that low-birth-weight infants have lower kidney weight, fewer nephrons (39), and higher rates of progressive glomerular sclerosis predisposing them to renal insufficiency and hypertension (7, 36). Animal models support this hypothesis as well.
Different animal models of growth restriction, such as protein restriction, decreased placenta perfusion, or those using corticosteroids, show that fetal growth restriction was associated with permanent nephron deficit (5, 25). Specifically, in the NOS3 knockout mouse model, we have shown that the glomeruli of 14-wk-old KOM and KO offspring are decreased and have altered structure compared with KOP and WT offspring (29). Others have shown that KO mice have altered renal function associated with accelerated glomerular and tubulointerstitial injury with a loss of glomerular and peritubular capillaries (35). These findings associated with the endothelial dysfunction seen in these KO and KOM mice lead to hypertension. Because nephrogenesis in mice is not completed until 2 wk after birth, further hemodynamic changes related to the postnatal environment may contribute to the abnormal renal and vascular development occurring later in life.
Studies have shown that treatment with NO inhibitors results in increased tissue angiotensin-converting enzyme expression in the perivascular areas, as well as cardiac superoxide production, thereby contributing to the long-term vascular effects of NO inhibition (18, 19, 20). In our animal model, KO offspring had significant contractile responses to ANG, but no differences were detected between KOM and KOP. Therefore, the renin-angiotensin system may play a role in the regulation of vascular tone homeostasis during fetal kidney development; its role later in life is unclear and needs further investigation.
Important gender differences in vascular reactivity profiles have been observed, since KO male offspring had higher responses to contractile and relaxant agents at any age, whereas the differences in female offspring became apparent only later in life. Such variation may be related to differences in estrogen concentrations between the different groups and as these female offspring age. This is, however, speculative and requires further investigation. Estrogen improves vascular tone by decreasing myointimal smooth muscle cell proliferation (40), inhibiting tumor necrosis factor-α-induced apoptosis in endothelial cells, (32), and inducing NOS3 in different tissues (45, 50).
Another mechanism is the response to stress later in life. We (9) have previously demonstrated that KOM offspring had significantly higher blood pressure compared with KOP after the introduction of stressful stimuli. Stress is believed to be a major risk factor in the development of cardiovascular disease in mice, since it alters the cardiovascular function (13, 14). The cardiovascular effects of stress are mediated by increased sympathetic activity and baroreflex sensitivity, leading to a reduction in blood pressure buffering (23, 41). As previously mentioned, KOM offspring had better vascular reactivity profiles as KO at 7 wk of age but not later in life. This could be related to an increased baseline sympathetic tone that accounts for worsening vascular function in response to shear stress as it is upregulated from 7 to 14 wk of age. Such findings are consistent with previous reports investigating the same animal model at a different age (16 wk) (44).
This study has limitations. Our findings in the carotid artery may not apply to other vascular beds. However, we have previously shown similar vascular responses to contractile and relaxant agents between carotid and mesenteric vessels (30). Others have demonstrated that, although the downstream vasculature contributes to the resistance in their vascular beds because of their small caliber, the differences when compared with larger vessels such as carotids are mostly quantitative (magnitude of effect) and not qualitative (nature of response) (26).
We demonstrated that altered vascular development starts early in life, and a window for potential interventions may exist. Our findings worsened with aging and were also gender dependent. This emphasizes the complexity of mechanisms involved in fetal cardiovascular development. Several pathways have been proposed and may explain our findings. These include vessel structure, endothelial function, kidney development, and central vascular tone regulation. These are speculative and require further research, however; identifying putative mechanisms is fundamental for any prevention strategy.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01 HL-080558-02.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1. Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet 2: 577–580, 1989 [DOI] [PubMed] [Google Scholar]
- 2. Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. Br Med J 298: 564–567, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barker DJ. Maternal nutrition, fetal nutrition, and disease in later life. Nutrition 13: 807–813, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Barker DJ, Shiell AW, Barker EW, Law M. Growth in utero, and blood pressure levels in the next generation, J. Hypertens 18: 843–846, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Bassan H, Trejo LL, Kariv N, Bassan M, Berger E, Fattal A, Gozes I, Harel S. Experimental intrauterine growth retardation alters renal development. Pediatr Nephrol 15: 192–195, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Bendeck MP, Keeley FW, Langille BL. Perinatal accumulation of arterial wall constituents: relation to hemodynamic changes at birth. Am J Physiol Heart Circ Physiol 267: H2268–H2279, 1994 [DOI] [PubMed] [Google Scholar]
- 7. Brenner BM, Chertow GM. Congenital oligonephropathy and the etiology of adult hypertension and progressive renal injury. Am J Kidney Dis 23: 171–175, 1994 [PubMed] [Google Scholar]
- 8. Costantine M, Yin H, Tamayo E, Makhlouf M, Ghulmiyyah L, Mateus J, Saade GR, Longo M. Fetal origin of adult diseases: genetic imprinting versus developmental programming (Abstract). Am J Obstet Gynecol 199: S21, 2008 [Google Scholar]
- 9. Costantine MM, Ferrari F, Chiossi G, Tamayo E, Hankins GD, Saade G, Longo M. Effect of intrauterine fetal programming on response to postnatal shaker stress in endothelial nitric oxide knockout mouse model. Am J Obstet Gynecol 201: 301.e1–301.e6, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ. Birth weight, and adult hypertension, diabetes mellitus, and obesity in U.S men. Circulation 94: 3246–3250, 1996 [DOI] [PubMed] [Google Scholar]
- 11. Davis EC. Smooth muscle cell to elastic lamina connections in developing mouse aorta. Role in aortic medial organization. Lab Invest 68: 89–99, 1993 [PubMed] [Google Scholar]
- 12. Faraci FM, Sigmund CD, Shesely EG, Maeda N, Heistad DD. Responses of carotid artery in mice deficient in expression of the gene for endothelial NOS. Am J Physiol Heart Circ Physiol 274: H564–H570, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Farah VM, Joaquim LF, Bernatova I, Morris M. Acute and chronic stress influence blood pressure variability in mice. Physiol Behav 83: 135–142, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Farah VM, Joaquim LF, Morris M. Stress cardiovascular/autonomic interactions in mice. Physiol Behav 89: 569–575, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Furchgott RF. The role of endothelium in the responses of vascular smooth muscle to drugs. Annu Rev Pharmacol Toxicol 24: 175–197, 1984 [DOI] [PubMed] [Google Scholar]
- 16. Geelhoed JJ, Steegers EA, van Osch-Gevers L, Verburg BO, Hofman A, Witteman JC, van der Heijden AJ, Helbing WA, Jaddoe VW. Cardiac structures track during the first 2 years of life and are associated with fetal growth and hemodynamics: the Generation R Study. Am Heart J 158: 71–77, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Geelhoed JJ, Verburg BO, Nauta J, Lequin M, Hofman A, Moll HA, Witteman JC, van der Heijden AJ, Steegers EA, Jaddoe VW. Tracking and determinants of kidney size from fetal life until the age of 2 years: the Generation R Study. Am J Kidney Dis 53: 248–258, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury. Part II. animal and human studies. Circulation 108: 2034–2040, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Gubler MC, Antignac C. Renin-angiotensin system in kidney development: renal tubular dysgenesis. Kidney Int 77: 400–406, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Hou J, Kato H, Cohen RA, Chobanian AV, Brecher P. Angiotensin II-induced cardiac fibrosis in the rat is increased by chronic inhibition of nitric oxide synthase. J Clin Invest 96: 2469–2477, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 377: 239–242, 1995 [DOI] [PubMed] [Google Scholar]
- 22. Ignarro LJ. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Pharmacol Toxicol 30: 535–560, 1990 [DOI] [PubMed] [Google Scholar]
- 23. Joaquim LF, Farah VM, Bernatova I, Fazan JR, Jr, Grubbs R, Morris M. Enhanced heart rate variability and baroreflex index after stress and cholinesterase inhibition in mice Am J Physiol Heart Circ Physiol 287: H251–H257, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Kelleher CM, McLean SE, Mecham RP. Vascular extracellular matrix and aortic development. Curr Top Dev Biol 62: 153–188, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Langley-Evans SC, Welham SJ, Jackson AA. Fetal exposure to a maternal low protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sci 64: 965–974, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Laughlin MH, Turk JR, Schrage WG, Woodman CR, Price EM. Influence of coronary artery diameter on eNOS protein content. Am J Physiol Heart Circ Physiol 284: H1307–H1312, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, Eichwald E, Keating MT. Elastin is an essential determinant of arterial morphogenesis. Nature 393: 276–280, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Longo M, Langenveld J, Vedernikov Y, Anderson G, Bukowski R, Garfield R, Saade GR. Abnormal vascular function in offspring of endothelial nitric oxide synthase knockout mice: genetic factors or fetal programming by uterine environment? (Abstract) Am J Obstet Gynecol 189: S91, 2003 [Google Scholar]
- 29. Longo M, Lu F, Snyder R, Anderson GD, Hankins GDV, Saade GR. Abnormal renal development in a mouse model of fetal vascular programming (Abstract). Am J Obstet Gynecol 191: S31, 2004 [Google Scholar]
- 30. Longo M, Jain V, Vedernikov YP, Bukowski R, Garfield RE, Hankins GDV, Anderson GD, Saade GR. Fetal origins of adult vascular dysfunction in mice lacking endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol 288: R1114–R1121, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Longo M, Bytautiene E, Lu F, Tamayo E, Hankins GDV, Anderson G, Saade GR. Blood pressure regulation and uterine environment in conscious unrestrained NOS3 knockout mice (Abstract). Am J Obstet Gynecol 193: S10, 2005 [Google Scholar]
- 32. Mabley JG, Horváth EM, Murthy KG, Zsengellér Z, Vaslin A, Benkö R, Kollai M, Szabó C. Gender differences in the endotoxin-induced inflammatory and vascular responses: potential role of poly(ADP-ribose) polymerase activation. J Pharmacol Exp Ther 315: 812–820, 2005 [DOI] [PubMed] [Google Scholar]
- 33. McLean SE, Mecham BH, Kelleher CM, Mariani TJ, Mecham RP. Extracellular matrix gene expression in the developing mouse aorta. In: Extracellular Matrices and Development, edited by Miner JH. New York, NY: Elsevier, 2005, vol. 15, p. 82–128 [Google Scholar]
- 34. Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 43: 109–142, 1991 [PubMed] [Google Scholar]
- 35. Nakayama T, Sato W, Kosugi T, Zhang L, Campbell-Thompson M, Yoshimura A, Croker BP, Johnson RJ, Nakagawa T. Endothelial injury due to eNOS deficiency accelerates the progression of chronic renal disease in the mouse. Am J Physiol Renal Physiol 296: F317–F327, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nyengaard JR, Bendtsen TF. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec 232: 194–201, 1992 [DOI] [PubMed] [Google Scholar]
- 37. Pallares P, Gonzalez-Bulnes A. Intrauterine growth retardation in endothelial nitric oxide synthase-deficient mice is established from early stages of pregnancy. Biol Reprod 78: 1002–1006, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Palmer RMJ, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327: 524–526, 1987 [DOI] [PubMed] [Google Scholar]
- 39. Reyes L, Herrera M, Melendi C, Fundora I. Relationship between weight at birth, and the number and size of renal glomeruli in humans: a histomorphometric study. Kidney Int 58: 770–773, 2000 [DOI] [PubMed] [Google Scholar]
- 40. Sader MA, Celermajer DS. Endothelial function, vascular reactivity and gender differences in the cardiovascular system. Cardiovasc Res 53: 597–604, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Stauss HM, Persson PB. Role of nitric oxide in buffering short-term blood pressure fluctuations. News Physiol Sci 15: 229–233, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Tsukimori K, Komatsu H, Fukushima K, Kaku T, Nakano H, Wake N. Inhibition of nitric oxide synthetase at mid-gestation in rats is associated with increases in arterial pressure, serum tumor necrosis factor-alpha, and placental apoptosis. Am J Hypertens 21: 477–481, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Van der Heijden OWH, Essers YPG, Fazzi G, Peeters LLH, De Mey GR, Van Eys JM. Uterine artery remodeling and reproductive performance are impaired in endothelial nitric oxide synthase-deficient mice. Biol Reprod 72: 1161–1168, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Van Vliet BN, Chafe LL. Maternal endothelial nitric oxide synthase genotype influences offspring blood pressure and activity in mice. Hypertension 49: 556–562, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Villar IC, Hobbs AJ, Ahluwalia A. Sex differences in vascular function: implication of endothelium-derived hyperpolarizing factor. J Endocrinol 197: 447–462, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Wagenseil JE, Ciliberto CH, Knutsen RH, Levy MA, Kovacs A, Mecham RP. Reduced vessel elasticity alters cardiovascular structure and function in newborn mice. Circ Res 104: 1217–1224, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wagenseil JE, Ciliberto CH, Knutsen RH, Levy MA, Kovacs A, Mecham RP. The importance of elastin to aortic development in mice. Am J Physiol Heart Circ Physiol 299: H257–H264, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Widdowson EM, McCance RA. Some effects of accelerating growth. I. General somatic development. Proc R Soc Lond B Biol Sci 152: 188–206, 1960 [DOI] [PubMed] [Google Scholar]
- 49. Widdowson EM, McCance RA. The effects of finite periods of undernutrition at different ages on the composition and subsequent development of the rat. Proc R Soc Lond B Biol Sci 972: 329–342, 1963 [DOI] [PubMed] [Google Scholar]
- 50. Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Hodgin J, Shaul PW, Thoren P, Smithies O, Gustafsson JA, Mendelsohn ME. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science 18: 505–508, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Zidar N, Avgustin Cavic M, Kenda RB, Ferluga D. Unfavorable course of minimal change nephrotic syndrome in children with intrauterine growth retardation. Kidney Int 54: 1320–1323, 1998 [DOI] [PubMed] [Google Scholar]