Abstract
Millions of older individuals consume acetaminophen or ibuprofen daily and these same individuals are encouraged to participate in resistance training. Several in vitro studies suggest that cyclooxygenase-inhibiting drugs can alter tendon metabolism and may influence adaptations to resistance training. Thirty-six individuals were randomly assigned to a placebo (67 ± 2 yr old), acetaminophen (64 ± 1 yr old; 4,000 mg/day), or ibuprofen (64 ± 1 yr old; 1,200 mg/day) group in a double-blind manner and completed 12 wk of knee extensor resistance training. Before and after training in vivo patellar tendon properties were assessed with MRI [cross-sectional area (CSA) and signal intensity] and ultrasonography of patellar tendon deformation coupled with force measurements to obtain stiffness, modulus, stress, and strain. Mean patellar tendon CSA was unchanged (P > 0.05) with training in the placebo group, and this response was not influenced with ibuprofen consumption. Mean tendon CSA increased with training in the acetaminophen group (3%, P < 0.05), primarily due to increases in the mid (7%, P < 0.05) and distal (8%, P < 0.05) tendon regions. Correspondingly, tendon signal intensity increased with training in the acetaminophen group at the mid (13%, P < 0.05) and distal (15%, P = 0.07) regions. When normalized to pretraining force levels, patellar tendon deformation and strain decreased 11% (P < 0.05) and stiffness, modulus, and stress were unchanged (P > 0.05) with training in the placebo group. These responses were generally uninfluenced by ibuprofen consumption. In the acetaminophen group, tendon deformation and strain increased 20% (P < 0.05) and stiffness (−17%, P < 0.05) and modulus (−20%, P < 0.05) decreased with training. These data suggest that 3 mo of knee extensor resistance training in older adults induces modest changes in the mechanical properties of the patellar tendon. Over-the-counter doses of acetaminophen, but not ibuprofen, have a strong influence on tendon mechanical and material property adaptations to resistance training. These findings add to a growing body of evidence that acetaminophen has profound effects on peripheral tissues in humans.
Keywords: stiffness, modulus, strain, cross-sectional area
tendons are an integral part of normal musculoskeletal function, and changes in tendon elastic properties can influence muscle force transmission to bone (4), energy conservation during locomotion, and joint position control (cf. 3, 22). Tendon strength is largely determined by the composition of the extracellular matrix (ECM), which consists mainly of type I collagen. In humans, acute exercise increases tendon collagen synthesis (23), which presumably contributes to chronic exercise adaptations such as tendon hypertrophy (10, 14, 32) and increased tendon stiffness (14, 17, 18, 29). These changes in tendon properties likely contribute to the improvements in physical function associated with exercise training in young and old individuals.
Interestingly, a substantial amount of data suggests that cyclooxygenase (COX)-inhibiting drugs may alter tendon ECM metabolism (1, 5, 19, 31, 40–42, 44), which could limit tendon adaptations to exercise. COX enzymes are the rate-limiting step in the conversion of arachidonic acid to various prostaglandins, which regulate a variety of processes including inflammation and pain (37). For this reason COX-inhibiting drugs such as ibuprofen and acetaminophen are consumed daily by millions of individuals for musculoskeletal pain and chronic arthritic conditions. Inhibition of COX activity decreases resting and postexercise prostaglandin production and blood flow in human Achilles tendon (19), inhibits collagen synthesis in animal tendon (6), and inhibits proliferation of tendon fibroblasts in in vitro cell culture models (31, 40–42). In nontendon connective tissue, prostaglandins influence ECM remodeling via regulating the activity of the same collagen degrading enzymes (matrix metalloproteinases) seen in tendon (24, 25) and COX inhibition increases expression of these enzymes (44). Therefore, these drugs, when taken during exercise training, may alter the tendon adaptations (increased stiffness and modulus, and decreased strain) previously demonstrated with chronic resistance exercise in older individuals (29), and ultimately might limit improvements in physical function. The potential effect of these drugs is especially important for older individuals, who are encouraged to exercise to improve health but are also commonly using COX-inhibiting drugs on a daily basis. Further, aging itself results in a change in ECM structure and a region-specific decline in patellar tendon size and signal intensity (1, 7, 9), changes which may be exacerbated by consumption of COX-inhibiting drugs during exercise training.
We evaluated the influence of daily ibuprofen and acetaminophen use on the in vivo properties of the patellar tendon using magnetic resonance imaging (MRI) and ultrasonography coupled with tendon force measurements before and after 12 wk of resistance training in older humans. We hypothesized that consumption of the COX-inhibiting drugs ibuprofen (1,200 mg/day) and acetaminophen (4,000 mg/day) during 12 wk of knee extensor resistance training would limit the increase in tendon stiffness and Young's modulus previously reported (29) with resistance training in individuals not consuming these drugs.
METHODS
Overall Study Design
This study was a randomized, placebo-controlled, double-blind 12-wk investigation. During the 12 wk subjects completed a progressive resistance-training program of the knee extensors three times per week and consumed a placebo, acetaminophen, or ibuprofen. The study was conducted at the Ball State University Human Performance Laboratory and Ball Memorial Hospital and approved by the Institutional Review Boards of both institutions. All of the procedures, risks, and benefits associated with the study were explained to the subjects before giving written consent to participate. This study was a part of another investigation (36) evaluating the influence of acetaminophen and ibuprofen consumption during chronic resistance training on skeletal muscle adaptations.
Subjects
Men and women were recruited from the greater Muncie, IN, area. Subjects completed a medical screening exam, which included routine blood and urine clinical chemistries, a resting and exercise electrocardiogram, and a detailed health and exercise history questionnaire. Subjects were excluded if they had any cardiac, orthopedic (e.g., patellar tendinopathy), or neuromuscular conditions that would preclude them from participating in a resistance exercise training program, abnormal blood or urine chemistries, arthritis, diabetes, uncontrolled hypertension, or any condition that would be a contraindication to taking acetaminophen or ibuprofen for 3 mo, if they were chronically consuming any prescription or non-prescription COX-inhibiting drugs, if they were involved in any formal aerobic or resistance exercise training program, if they smoked, or if they were <60 or >85 yr of age.
Sixty-one individuals consented to be screened for participation in this study, 17 were excluded due to medical reasons, and 7 decided not to participate for personal reasons. During training one individual sustained a back injury unrelated to the study and was unable to complete the resistance training. Subject characteristics of the 36 men and women included in the study are presented in Table 1.
Table 1.
Subject characteristics, intervention compliance, and resistance training parameters
| Subject Characteristics | Placebo | Acetaminophen | Ibuprofen |
|---|---|---|---|
| n | 12 | 11 | 13 |
| M/F | 8/4 | 7/4 | 9/4 |
| Agea, yr | 67 ± 2 | 64 ± 1 | 64 ± 1 |
| Age range, yr | 60–78 | 60–71 | 60–77 |
| Heighta, cm | 170 ± 3 | 172 ± 5 | 175 ± 2 |
| Weight, kg | 77.3 ± 4.4 | 93.1 ± 5.6* | 82.6 ± 2.8 |
| Intervention complianceb | |||
| Drug consumption, % | |||
| Observeda | 93 ± 2 | 93 ± 2 | 94 ± 2 |
| Reporteda | 100 ± 0.1 | 99 ± 1 | 99 ± 0.3 |
| Exercise traininga, % | 100 | 100 | 100 |
| Resistance training | |||
| Mean traininga, %1RM | 74 ± 1 | 71 ± 1 | 73 ± 1 |
| Mean training loada, kg | 58 ± 7 | 55 ± 6 | 56 ± 4 |
Data expressed as means ± SE; M, male; F, female; 1RM, one repetition maximum. Observed is that directly viewed by the research staff in person or on personal digital video. Reported is based on the number of doses remaining in the returned pillboxes. The 6–7% “noncompliance” with the observed compliance was primarily due to technical issues with the personal digital video cameras (8). %Exercise training compliance is the percentage of the 36 scheduled exercise training sessions completed by the subjects.
Significant difference from placebo, P < 0.05.
No significant difference among values for all three groups, P > 0.05.
%Drug consumption compliance is the percentage of the 252 scheduled doses consumed by the subjects.
Interventions
Resistance exercise training protocol.
All subjects completed a progressive resistance training program consisting of bilateral knee extension (36). Each subject was scheduled for resistance training on an isotonic knee extension device (Cybex Eagle, Medway, MA) three times per week for 12 wk for a total of 36 sessions. All sessions were supervised by a member of the research team. Each session was separated by at least 1 day and consisted of 5 min of light cycling (model 828E, Monark Exercise AB, Vansbro, Sweden), two sets of five knee extensions at a light weight, followed by three sets of 10 repetitions with 2 min of rest between sets. Training intensity was based on each individual's one repetition maximum (1RM) and was adjusted during the training based on each individual's training session performance and biweekly 1RM. Data on resistance training performance and compliance are presented in Table 1 and in Trappe et al. (36).
Cyclooxygenase-inhibitor consumption.
Drugs were administered over the 12 wk in double-blind, placebo-controlled fashion as we have previously described (8, 39). Each drug was administered in three doses per day (∼8 AM, ∼2 PM, ∼8 PM) corresponding to the maximal over-the-counter daily dose (acetminophen: 4,000 mg total; ibuprofen: 1,200 mg total). The placebo group was given an identical number of pills/dose (three), which were indistinguishable from the drug doses. Each subject was given their doses in weekly batches (21 doses) in pillboxes labeled with the date and consumption time. Subjects were instructed to not consume any other COX-inhibiting drugs outside of the study. Adherence to the study drug regimen was monitored as previously described (8) and compliance to study medication is reported in Table 1 and elsewhere (36).
Potential side effects of drug consumption were monitored via monthly blood draws for renal (creatinine), hepatic (alanine aminotransferase), and hematologic (hematocrit) measures, which were unchanged in all three groups (36).
Magnetic Resonance Imaging
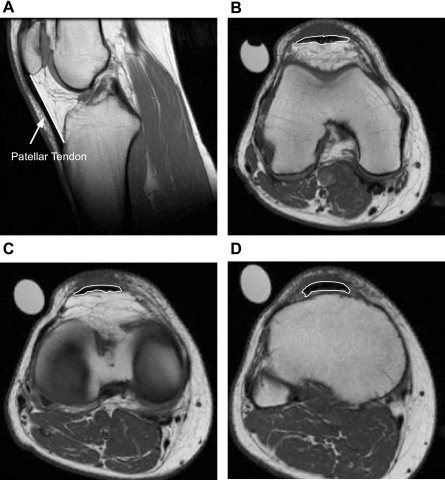
In conjunction with the thigh skeletal muscle scanning (36), axial and sagittal images of the patellar tendon were obtained using MRI as previously described (7). After 1 h of lying supine [to control for fluid shifts in skeletal muscle (36)], each subject's right knee was placed in an extremity coil (GE 1.5T, Quadrature Lower Extremity Coil 472GE-64, Invivo, Pewaukee, WI; Fig. 1). A plastic tube containing 1.0% CuSO4 was placed in the field of view for normalization of tendon signal intensity (tendon signal intensity/CuSO4 signal intensity). Sagittal images were obtained beginning on the lateral most portion of the lateral condyle of the tibia, then moving medially. Axial images of the patellar tendon were obtained beginning 8 mm (two slices) proximal of the distal pole of the patella and proceeding distally. For tendon length, sagittal images (Fig. 1A) were reviewed with OsiriX (version 2.7.5) and only slices with complete tendon from the distal pole of the patella to tibial insertion were chosen for analysis. ImageJ (version 1.34) was then used to determine tendon length via manual planimetry.
Fig. 1.
Sagittal (A) and axial (b–d) MRI images of the patellar tendon. Sagital images (TR/TE = 400/14, spin echo; ETL = 0; NEX = 2; PPFOV = 100; FOV = 16 × 16 cm, 256 × 256 matrix; slice thickness = 4 mm; spacing = 0 mm) were used for the tendon length measurement, represented by the solid white line. Axial images (TR/TE = 550/12.4, fast spin echo train; ETL = 3; NEX = 3; PPFOV = 100; FOV = 16 × 16 cm, 256 × 256 matrix; slice thickness = 4 mm; spacing = 0 mm) were used for the patellar tendon cross-sectional area (CSA) measurements at the proximal (B), mid (C), and distal (D) tendon, represented by the white line outlining the tendon in all 3 images. The knee joint was in slight flexion for all imaging.
Using the axial scans, tendon cross-sectional area (CSA) and signal intensity [mean gray value (MGV)] were determined by manually circumscribing the patellar tendon using ImageJ. The value of all slices from proximal to distal was averaged to determine mean tendon CSA and signal intensity. Region-specific CSA and signal intensity (Fig. 1, B–D) were determined as we have previously described (7). The reliability of this method in our laboratory has been reported previously (7). All measurements were performed by the same investigator.
MRI of the patellar tendon was performed on all 36 individuals (Table 1); however, two individuals were excluded from analysis because their knee would not adequately fit in the knee coil, another individual had a severe claustrophobic response and was unable to complete the tendon scans, and errors during data acquisition prevented the inclusion of tendon scans from two individuals. Additionally, due to an MRI software upgrade that occurred during the study, a different knee coil (GP Flex Coil) was temporarily used. Although this coil did not influence our tendon CSA and length measurements, it did alter the signal intensity. Therefore, we are only able to report CSA data on 31 individuals [placebo: n = 11, 7 males (M)/4 females (F); acetaminophen: n = 10, 6 M/4 F; ibuprofen: n = 10, 6 M/4 F] and signal intensity data on 21 individuals (placebo: n = 8, 6 M/2 F; acetaminophen: n = 8, 4 M/4 F; ibuprofen: n = 5, 3 M/2 F).
Patellar Tendon Mechanical Properties
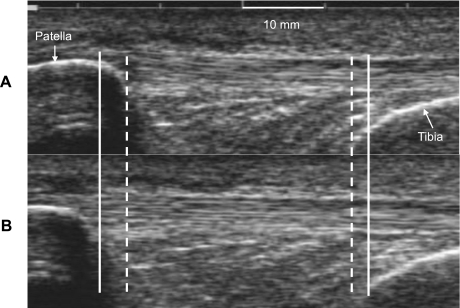
Patellar tendon mechanical properties were assessed as previously described (7, 9, 11, 14). Briefly, subjects were firmly strapped into a rigid aluminum chair with the knee joint at 90° of flexion. A minimally padded cuff was attached to their lower leg ∼3 cm proximal to the medial malleolus. Subjects performed several “ramped” 10-s isometric quadriceps contractions to maximal effort with 90-s rest between contractions (7, 9, 11, 14). Force was recorded via a strain-gauge load cell (Omegadyne, LC101–500) interfaced with a personal computer (Gateway E-4200). An ultrasound probe (7.5 MHz, 70-mm B-mode linear array, Sonoline Sienna, Siemens, Erlangen, Germany) was mounted sagittally on the skin overlying the patellar tendon to monitor displacement of both the patella and tibia during the “ramped” isometric effort [Fig. 2, (7, 9, 11, 14)]. Video feed from the ultrasound unit was captured on a personal computer (Matrox Inspector, Matrox Electronic Systems, Dorval, Quebec, Canada) in synchrony with recording of the instantaneous knee extensor moment. All participants completed three sessions, one familiarization and two testing sessions both pre- and posttraining. Displacement of the patella and tibia (Fig. 2) were determined with custom-designed software (21), and deformation was defined as the change in distance between the tibia and patella (7, 9, 11, 14). Tendon force was calculated by dividing the measured knee extension moment by the internal moment arm [estimated from femur length (7, 9, 11, 14, 43)].
Fig. 2.
Ultrasound image of the patellar tendon taken at rest (A) and during a 10-s isometric “ramped” contraction (B) with the knee joint at 90°. Dashed line indicates patella and tibia position before contraction, and solid line indicates patella and tibia position after contraction, the difference representing patellar tendon deformation.
Two comparisons of mechanical properties were made: 1) peak force: mechanical properties (pre- and posttraining) were determined from the two attempts from which the highest tendon force was achieved. The data generated (force, deformation, stiffness, modulus, stress, and strain) from these two attempts were then averaged; and 2) force-normalized: deformation for all “ramped” contractions was determined and the two contractions with the highest and lowest deformation within a given testing session were excluded. This approach was repeated for the posttraining data and then all attempts (pre and post) were analyzed to the greatest common force for each individual subject.
Force-deformation curves were fit with a second-order polynomial and any curves with r2 < 0.97 were excluded from analysis. Tendon variables (stiffness, modulus, stress, strain) were calculated as previously described (7). The reliability of this method in our laboratory has been reported previously (7). The same investigator performed all mechanical analyses. Testing of in vivo patellar tendon mechanical properties was conducted on a subset of individuals (placebo: n = 7, 6 M/1 F; acetaminophen: n = 7, 4 M/3 F; ibuprofen: n = 8, 5 M/3 F) who participated in the 12 wk of resistance training.
Statistics
All data were first tested for normality using a Shapiro-Wilk test (P < 0.05). Pre- to posttraining data that fit a normal distribution were evaluated using a two-way (drug and time) repeated-measures ANOVA. A one-way ANOVA was used to evaluate any differences in subject characteristics, training intensity, drug compliance, and percent change in tendon CSA, signal intensity, and mechanical properties with training. The Student-Newman-Keuls post hoc test was used to explore differences when a significant interaction was detected.
For pre-post training data that failed normality testing, a rank or log10 transformation was performed, and the transformed data were assessed with a two-way (drug and time) repeated-measures ANOVA. A Kruskal-Wallis one-way ANOVA on Ranks with Dunn's post hoc test was used to evaluate any other data that failed normality testing. Values were considered significant at an alpha level of P < 0.05. All data are expressed as means ± SE. All data were analyzed using SigmaPlot Version 11 (Systat Software).
RESULTS
Patellar Tendon CSA and MRI Signal Intensity
Mean patellar tendon CSA and CSA at the proximal and mid regions of the tendon were unchanged (P > 0.05) from pre- to post-training in the placebo group, and this response was not influenced with ibuprofen consumption (Tables 2 and 3, Fig. 3). Distal tendon CSA increased with training across all three groups but this increase was driven primarily by the acetaminophen group (Fig. 3 and Table 3). Mean tendon CSA was increased with training in the acetaminophen group (3 ± 1%, P < 0.05), primarily due to increases in the mid (7 ± 3%, P < 0.05) and distal (8 ± 2%, P < 0.05) regions of the tendon (Tables 2 and 3, Fig. 3). Correspondingly, patellar tendon signal intensity increased from pre- to posttraining in the acetaminophen group at the mid (13 ± 5%, P < 0.05) and distal (15 ± 4%, P = 0.07) regions of the tendon (Table 4). There was no change from pre- to posttraining in tendon signal intensity at any region in the placebo or ibuprofen groups (Table 4). Resting patellar tendon length did not change (P > 0.05) from pre- to posttraining in any group (placebo: pre 45.1 ± 1.5, post 45.1 ± 1.4 mm; acetaminophen: pre 45.6 ± 2.3, post 45.1 ± 2.2 mm; ibuprofen: pre 45.1 ± 1.4, post 44.5 ± 1.4 mm).
Table 2.
Mean tendon CSA and signal intensity
| Placebo |
Acetaminophen |
Ibuprofen |
||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| CSA, mm2 | 117.1 ± 10.1 | 115.1 ± 9.6 | 124.4 ± 13.1 | 127.9 ± 13.0* | 122.8 ± 8.5 | 124.2 ± 8.5 |
| Signal intensity, MGV | 18.4 ± 1.0 | 18.3 ± 1.2 | 18.9 ± 1.5 | 20.2 ± 1.5 | 20.4 ± 0.6 | 20.8 ± 1.6 |
Data expressed as means ± SE; CSA, cross-sectional area; Pre, pretraining; Post, posttraining; MGV, mean gray value in arbitrary units.
P < 0.05, increase with training.
Table 3.
Regional tendon CSA
| Placebo |
Acetaminophen |
Ibuprofen |
||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Proximal | 139.0 ± 14.0 | 135.5 ± 14.1 | 145.8 ± 11.0 | 146.2 ± 11.1 | 140.1 ± 9.8 | 143.9 ± 10.1 |
| Mid | 111.4 ± 9.1 | 109.8 ± 8.7 | 121.0 ± 13.5 | 128.7 ± 14.3* | 119.8 ± 9.7 | 115.7 ± 9.4 |
| Distal | 107.5 ± 8.3 | 107.9 ± 8.7† | 110.8 ± 15.1 | 118.1 ± 14.9† | 131.3 ± 9.1 | 133.4 ± 7.7† |
Data expressed as means ± SE of CSA in mm2.
P < 0.05, increase with training.
P < 0.05, increase with training independent of drug group.
Fig. 3.
Percent change in patellar tendon CSA at the proximal, mid, and distal regions of the patellar tendon. *P < 0.05, acetaminophen vs. placebo and ibuprofen. †P = 0.05, acetaminophen vs. placebo.
Table 4.
Regional tendon signal intensity (MGV)
| Placebo |
Acetaminophen |
Ibuprofen |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | %Δ | Pre | Post | %Δ | Pre | Post | %Δ | |
| Proximal | 22.9 ± 0.6 | 22.7 ± 0.7 | −1 ± 3 | 23.9 ± 2.0 | 24.5 ± 1.8 | 4 ± 3 | 22.7 ± 0.9 | 22.9 ± 1.6 | 1 ± 7 |
| Mid | 17.8 ± 1.1 | 17.6 ± 1.6 | −3 ± 3 | 17.8 ± 2.1 | 20.0 ± 2.4* | 13 ± 5† | 20.3 ± 0.7 | 19.0 ± 1.5 | −6 ± 7 |
| Distal | 13.0 ± 1.1 | 12.9 ± 1.2 | −1 ± 5 | 13.0 ± 1.4 | 14.6 ± 1.3‡ | 15 ± 4 | 16.9 ± 1.5 | 18.5 ± 3.5 | 8 ± 14 |
Data expressed as means ± SE of MGV in arbitrary units.
P < 0.05, increased with training.
P < 0.05, acetaminophen vs. placebo and ibuprofen.
P = 0.07, increase with training.
Patellar Tendon Mechanical Properties
Patellar tendon mechanical properties at peak tendon force are presented in Table 5 and Fig. 4. Patellar tendon stress at peak tendon force increased with training across all three groups (P < 0.05). Patellar tendon deformation and strain were unchanged (P > 0.05) from pre- to posttraining in the placebo group, and this response was not influenced with ibuprofen consumption. Conversely, in the acetaminophen group patellar tendon deformation and strain at peak force increased (P < 0.05) with training by 24 ± 8% and 31 ± 13%, respectively.
Table 5.
Patellar tendon mechanical properties at peak tendon force
| Placebo |
Acetaminophen |
Ibuprofen |
||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Tendon force, N | 3622 ± 387 | 4271 ± 371* | 3464 ± 756 | 4010 ± 963* | 2422 ± 233 | 2829 ± 255* |
| Deformation, mm | 2.82 ± 0.31 | 2.83 ± 0.29 | 2.46 ± 0.35 | 2.95 ± 0.42† | 2.81 ± 0.20 | 2.57 ± 0.19 |
| Stiffness, N/mm | 2,928 ± 381 | 3,335 ± 385‡ | 3,313 ± 417 | 3,078 ± 480 | 2,053 ± 213 | 2,590 ± 310† |
| Modulus, GPa | 1.03 ± 0.12 | 1.22 ± 0.13† | 1.23 ± 0.06 | 1.07 ± 0.09 | 0.73 ± 0.07 | 0.89 ± 0.10§ |
| Stress, MPa | 30.4 ± 3.9 | 37.1 ± 3.6* | 26.8 ± 3.4 | 27.4 ± 4.5* | 18.9 ± 2.4 | 21.4 ± 1.9* |
| Strain, % | 6.5 ± 0.7 | 6.5 ± 0.6 | 5.2 ± 0.5 | 6.5 ± 0.7† | 6.1 ± 0.4 | 5.6 ± 0.4 |
Data expressed as means ± SE.
P < 0.05, increase with training independent of drug group;
P ≤ 0.05, increase with training,
P = 0.08, increase with training,
P = 0.07, increase with training.
Fig. 4.
Percent change in patellar tendon mechanical properties at peak tendon force. *P ≤ 0.05, acetaminophen vs. placebo and ibuprofen. †P < 0.05, acetaminophen vs. ibuprofen. §P = 0.057, acetaminophen vs. placebo.
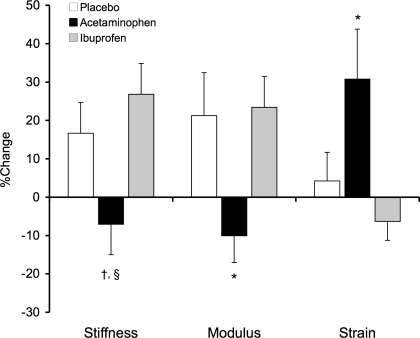
Force-normalized patellar tendon mechanical properties are presented in Table 6 and Fig. 5. Deformation decreased with training in the placebo (11 ± 5%, P < 0.05) and ibuprofen groups (10 ± 5%, P = 0.07). Strain also decreased in the placebo group (11 ± 5%, P < 0.05) but not in the group consuming ibuprofen. Patellar tendon stiffness and modulus were unchanged by resistance training in the placebo and ibuprofen groups. In contrast, stiffness (−17 ± 8%) and modulus (−20 ± 7%) decreased (P < 0.05) with training in the group consuming acetaminophen. Patellar tendon deformation and strain increased (P < 0.05) with training by 20 ± 8% and 20 ± 9%, respectively, in the acetaminophen group.
Table 6.
Patellar tendon mechanical properties normalized to a common tendon force
| Placebo |
Acetaminophen |
Ibuprofen |
||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Deformation, mm | 2.62 ± 0.31 | 2.29 ± 0.25* | 2.14 ± 0.24 | 2.56 ± 0.32† | 2.51 ± 0.21 | 2.25 ± 0.18‡ |
| Stiffness, N/mm | 2,668 ± 362 | 2,723 ± 379 | 2,948 ± 315 | 2,480 ± 406* | 1,796 ± 219 | 1,988 ± 192 |
| Modulus, GPa | 0.93 ± 0.11 | 0.96 ± 0.11 | 1.11 ± 0.05 | 0.88 ± 0.07* | 0.64 ± 0.08 | 0.66 ± 0.07 |
| Stress, MPa | 25.1 ± 2.8 | 25.4 ± 2.5 | 21.6 ± 1.8 | 20.9 ± 1.8* | 14.9 ± 1.9 | 14.8 ± 1.8 |
| Strain, % | 6.0 ± 0.7 | 5.2 ± 0.5* | 4.6 ± 0.4 | 5.5 ± 0.6† | 5.4 ± 0.4 | 4.9 ± 0.3 |
Data expressed as means ± SE;
P < 0.05, decrease with training.
P < 0.05, increase with training.
P = 0.07, decrease with training.
Fig. 5.
Percent change in patellar tendon mechanical properties compared at a common tendon force. *P < 0.05, acetaminophen vs. placebo and ibuprofen, †P < 0.05, acetaminophen vs. ibuprofen.
DISCUSSION
To our knowledge this is the first investigation to study the influence of ibuprofen (1,200 mg/day) or acetaminophen (4,000 mg/day) consumption during resistance training on in vivo tendon properties in older humans. The main findings from the study were that 12 wk of knee extensor resistance training only (placebo group) had a modest impact on the mechanical properties of the patellar tendon and no impact on the size or MRI signal intensity. Similar responses to the resistance training were observed in the ibuprofen group, suggesting that ibuprofen (1,200 mg/day) did not interfere with the patellar tendon adaptations to resistance training. In contrast, daily acetaminophen consumption during resistance training resulted in patellar tendon hypertrophy and MRI signal intensity changes, along with changes in tendon mechanical properties, none of which were consistent with the placebo group.
Our initial impetus to examine the patellar tendon properties in conjunction with our study on the effects of COX-inhibiting drugs on skeletal muscle (36) was based on the results of Reeves et al. (29) that demonstrated significant changes in the mechanical properties of the patellar tendon after 14 wk of knee extensor resistance training in older men and women. Their findings (29) of a decrease in deformation and strain and no change in stress or tendon CSA are in general agreement with our common force data in the placebo group [maximal tendon force did not change significantly from pre- to posttraining in their study (29)]. However, they (29) did show a substantial increase in tendon stiffness and modulus (65–69%), which were unchanged in the present study. These differences may be due to methodological differences in the ultrasound measurements that have been outlined previously (10, 11, 26).
The placebo group data should be considered in the context of what happens to the patellar tendon with aging (7, 9) and with resistance exercise training in young individuals (13–16, 20, 34). In general it appears that with aging patellar tendon mechanical properties do not change (7, 9), but resistance exercise training in young individuals does seem to have some impact, albeit sometimes minor, on patellar tendon mechanical properties (13–16, 20, 34). The degree of training impact may be related to the duration (weeks, months, or years) or type of training employed. Further, aging appears to have little effect on the average patellar tendon CSA (7, 9), but may cause region-specific tendon atrophy (7). Resistance training does cause region-specific hypertrophy of the patellar tendon in young individuals (14, 34), but this finding is not universal (15, 16). Finally, the material properties, estimated with MRI signal intensity (7) and measured directly with biopsy measurements of collagen and cross-linking (9), are altered with aging. Studies in healthy young individuals show chronic resistance training does not alter the patellar tendon collagen content or cross-linking (20). Based on the aforementioned data and those of Reeves et al. (29) our findings from the placebo group of a modest change in some of the patellar tendon mechanical properties and lack of change in the size and material properties (MRI signal intensity) are reasonable and for the most part expected.
We have previously shown profound effects of both acetaminophen and ibuprofen on skeletal muscle prostaglandin production, protein synthesis, and hypertrophy following resistance exercise (36, 38, 39). These findings are in contrast to the findings from the present study in which ibuprofen did not appear to have any substantial impact on the tendon mechanical properties or any of the MRI measurements of CSA and signal intensity, as the responses in the ibuprofen group basically mirrored the placebo group. The only difference between the placebo and ibuprofen groups was a lack of significant reduction in strain in the ibuprofen group following training (Tables 5 and 6). Given the overall similar responses observed in both groups, it is unlikely this reflects a significant influence of ibuprofen on the tendon adaptations to training and suggests the effects of ibuprofen and acetaminophen may be tissue specific (tendon vs. skeletal muscle), a potentially important consideration for physicians when prescribing these medications.
The acetaminophen group, however, was impacted by the daily consumption of acetaminophen during the 12 wk of resistance training. Patellar tendon CSA increased with training, which would be expected to increase tendon stiffness and reduce strain. In contrast, we observed a large increase in strain with decreased stiffness. The magnitude of this effect was substantial considering a similar increase in gastrocnemius tendon strain was reported after 90 days of unloading and inactivity due to bed rest (28). A corresponding reduction in tendon elastic modulus in the acetaminophen group suggests that the internal structure of the tendon may have been altered, a conclusion supported by the change in MRI signal intensity observed in the acetaminophen group (Table 4). We can only speculate as to the changes in the tendon that may have occurred in the acetaminophen group; however, changes in MRI signal intensity have been correlated to histological findings and may indicate changes in the ECM tissue hydration, or collagen fiber structure (35). In support of this notion, we have shown that changes in biopsy-derived skeletal muscle tissue protein content corresponded with changes in skeletal muscle MRI signal intensity over a 3-mo exercise-training program in older individuals (12). Whatever the cause of the altered tendon mechanical properties, it is clear that the increased CSA is not improving mechanical properties in the acetaminophen group, as would be expected if changes in tendon material properties were proportional to the increase in tendon CSA. Based on the current findings, it could be suggested that long-term acetaminophen consumption might predispose individuals to tendinopathy (30, 33) or impact the rate of torque development during muscle contraction (4). In contrast, some of the adaptations, such as a reduction in tendon stiffness and modulus, might be beneficial for diabetics where contracture of the Achilles tendon may contribute to foot deformities and ulcer formation (2, 27). Finally, if acetaminophen is altering tendon ECM metabolism then it seems likely that these effects would not be limited to the tendon. Many tissues rely on an extensive collagen matrix for support and effective force transfer (e.g., bone). Thus future investigations should consider the possible impact of acetaminophen on this and other tissues.
In conclusion, we have demonstrated that acetaminophen (4,000 mg/day) but not ibuprofen (1,200 mg/day) taken orally during 12 wk of knee extensor resistance training in humans results in tendon hypertrophy but decreased tendon stiffness and increased strain. The significance of these findings is wide ranging and could impact how acetaminophen it utilized clinically. Our findings further highlight the profound effect of acetaminophen on peripheral tissues in vivo (36, 38, 39) and have important implications for the millions of individuals who consume acetaminophen daily for painful musculoskeletal and arthritic conditions, tendinopathy, and other soft tissue injuries. Although further studies are needed to evaluate why acetaminophen altered tendon mechanical properties in the present study, our data add to a growing body of literature challenging the traditional view that acetaminophen has little effect on peripheral tissues.
GRANTS
Funding for this study was provided by National Institutes of Health Grant R01-AG-020532 to T. A. Trappe and by American Physiological Society Postdoctoral Initiative Award to C. C. Carroll.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank the subjects for their participation, Bridget Sullivan and Jonah Lee for assistance with subject training, Beverly Slye, Sharon Meece, and Jamie Roberts of the Ball Memorial Hospital Radiology Department for their expert assistance with the MRI procedures, the staff at Medical Consultants, Ball Memorial Hospital for screening of the older participants, and Gary Lee for assistance fabricating the equipment for testing tendon mechanical properties.
REFERENCES
- 1. Almekinders LC, Deol G. The effects of aging, antiinflammatory drugs, and ultrasound on the in vitro response of tendon tissue. Am J Sports Med 27: 417–421, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Aronow MS, Diaz-Doran V, Sullivan RJ, Adams DJ. The effect of triceps surae contracture force on plantar foot pressure distribution. Foot Ankle Int 27: 43–52, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Biewener AA, Roberts TJ. Muscle and tendon contributions to force, work, and elastic energy savings: a comparative perspective. Exerc Sport Sci Rev 28: 99–107, 2000 [PubMed] [Google Scholar]
- 4. Bojsen-Moller J, Magnusson SP, Rasmussen LR, Kjaer M, Aagaard P. Muscle performance during maximal isometric and dynamic contractions is influenced by the stiffness of the tendinous structures. J Appl Physiol 99: 986–994, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Carlstedt CA. Mechanical and chemical factors in tendon healing. Effects of indomethacin and surgery in the rabbit. Acta Orthop Scand Suppl 224: 1–75, 1987 [DOI] [PubMed] [Google Scholar]
- 6. Carlstedt CA, Madsen K, Wredmark T. The influence of indomethacin on collagen synthesis during tendon healing in the rabbit. Prostaglandins 32: 353–358, 1986 [DOI] [PubMed] [Google Scholar]
- 7. Carroll CC, Dickinson JM, Haus JM, Lee GA, Hollon CJ, Aagaard P, Magnusson SP, Trappe TA. Influence of aging on the in vivo properties of human patellar tendon. J Appl Physiol 105: 1907–1915, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carroll CC, Trappe TA. Personal digital video: a method to monitor drug regimen adherence during human clinical investigations. Clin Exp Pharm Physiol 33: 1125–1127, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Couppe C, Hansen P, Kongsgaard M, Kovanen V, Suetta C, Aagaard P, Kjaer M, Magnusson SP. Mechanical properties and collagen cross-linking of the patellar tendon in old and young men. J Appl Physiol 107: 880–886, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Couppe C, Kongsgaard M, Aagaard P, Hansen P, Bojsen-Moller J, Kjaer M, Magnusson SP. Habitual loading results in tendon hypertrophy and increased stiffness of the human patellar tendon. J Appl Physiol 105: 805–810, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Hansen P, Bojsen-Moller J, Aagaard P, Kjaer M, Magnusson SP. Mechanical properties of the human patellar tendon, in vivo. Clin Biomech (Bristol, Avon) 21: 54–58, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Harber MP, Konopka AR, Douglass MD, Minchev K, Kaminsky LA, Trappe TA, Trappe S. Aerobic exercise training improves whole muscle and single myofiber size and function in older women. Am J Physiol Regul Integr Comp Physiol 297: R1452–R1459, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kongsgaard M, Qvortrup K, Larsen J, Aagaard P, Doessing S, Hansen P, Kjaer M, Magnusson SP. Fibril morphology and tendon mechanical properties in patellar tendinopathy. Am J Sports Med 38: 749–756, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Kongsgaard M, Reitelseder S, Pedersen TG, Holm L, Aagaard P, Kjaer M, Magnusson SP. Region specific patellar tendon hypertrophy in humans following resistance training. Acta Physiol (Oxf) 191: 111–121, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Kubo K, Ikebukuro T, Yaeshima K, Yata H, Tsunoda N, Kanehisa H. Effects of static and dynamic training on the stiffness and blood volume of tendon in vivo. J Appl Physiol 106: 412–417, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Kubo K, Ikebukuro T, Yata H, Tsunoda N, Kanehisa H. Time course of changes in muscle and tendon properties during strength training and detraining. J Strength Cond Res 24: 322–331, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Kubo K, Kanehisa H, Fukunaga T. Effects of resistance and stretching training programmes on the viscoelastic properties of human tendon structures in vivo. J Physiol 538: 219–226, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kubo K, Kanehisa H, Ito M, Fukunaga T. Effects of isometric training on the elasticity of human tendon structures in vivo. J Appl Physiol 91: 26–32, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Langberg H, Boushel R, Skovgaard D, Risum N, Kjaer M. Cyclo-oxygenase-2 mediated prostaglandin release regulates blood flow in connective tissue during mechanical loading in humans. J Physiol 551: 683–689, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lemoine JK, Lee JD, Trappe TA. Impact of sex and chronic resistance training on human patellar tendon dry mass, collagen content, and collagen cross-linking. Am J Physiol Regul Integr Comp Physiol 296: R119–R124, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Magnusson SP, Hansen P, Aagaard P, Brond J, Dyhre-Poulsen P, Bojsen-Moller J, Kjaer M. Differential strain patterns of the human gastrocnemius aponeurosis and free tendon, in vivo. Acta Physiol Scand 177: 185–195, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Magnusson SP, Hansen P, Kjaer M. Tendon properties in relation to muscular activity and physical training. Scand J Med Sci Sports 13: 211–223, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj JA, Smith K, Rennie MJ. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol 567: 1021–1033, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Noguchi K, Maeda M, Ruwanpura SM, Ishikawa I. Prostaglandin E2 (PGE2) downregulates interleukin (IL)-1alpha-induced IL-6 production via EP2/EP4 subtypes of PGE2 receptors in human periodontal ligament cells. Oral Dis 11: 157–162, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Noguchi K, Ruwanpura SM, Yan M, Yoshida N, Ishikawa I. Down-regulation of interleukin-1alpha-induced matrix metalloproteinase-13 expression via EP1 receptors by prostaglandin E2 in human periodontal ligament cells. Oral Microbiol Immunol 20: 56–59, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Onambele GN, Burgess K, Pearson SJ. Gender-specific in vivo measurement of the structural and mechanical properties of the human patellar tendon. J Orthop Res 25: 1635–1642, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care 13: 513–521, 1990 [DOI] [PubMed] [Google Scholar]
- 28. Reeves ND, Maganaris CN, Ferretti G, Narici MV. Influence of 90-day simulated microgravity on human tendon mechanical properties and the effect of resistive countermeasures. J Appl Physiol 98: 2278–2286, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Reeves ND, Maganaris CN, Narici MV. Effect of strength training on human patella tendon mechanical properties of older individuals. J Physiol 548: 971–981, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Riley G. Tendinopathy—from basic science to treatment. Nat Clin Pract Rheumatol 4: 82–89, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Riley GP, Cox M, Harrall RL, Clements S, Hazleman BL. Inhibition of tendon cell proliferation and matrix glycosaminoglycan synthesis by non-steroidal anti-inflammatory drugs in vitro. J Hand Surg [Br] 26: 224–228, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Rosager S, Aagaard P, Dyhre-Poulsen P, Neergaard K, Kjaer M, Magnusson SP. Load-displacement properties of the human triceps surae aponeurosis and tendon in runners and non-runners. Scand J Med Sci Sports 12: 90–98, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Schmid MR, Hodler J, Cathrein P, Duewell S, Jacob HA, Romero J. Is impingement the cause of jumper's knee? Dynamic and static magnetic resonance imaging of patellar tendinitis in an open-configuration system. Am J Sports Med 30: 388–395, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Seynnes OR, Erskine RM, Maganaris CN, Longo S, Simoneau EM, Grosset JF, Narici MV. Training-induced changes in structural and mechanical properties of the patellar tendon are related to muscle hypertrophy but not to strength gains. J Appl Physiol 107: 523–530, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Shalabi A, Kristoffersen-Wiberg M, Papadogiannakis N, Aspelin P, Movin T. Dynamic contrast-enhanced MR imaging and histopathology in chronic Achilles tendinosis. A longitudinal MR study of 15 patients. Acta Radiol 43: 198–206, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Trappe TA, Carroll CC, Dickinson JM, LeMoine JK, Haus JM, Sullivan BE, Lee JD, Jemiolo B, Weinheimer EM, Hollon CJ. Influence of acetaminophen and ibuprofen on skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol Regul Integr Comp Physiol 300: R655–R662, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Trappe TA, Carroll CC, Jemiolo B, Trappe SW, Dossing S, Kjaer M, Magnusson SP. Cyclooxygenase mRNA expression in human patellar tendon at rest and after exercise. Am J Physiol Regul Integr Comp Physiol 294: R192–R199, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Trappe TA, Fluckey JD, White F, Lambert CP, Evans WJ. Skeletal muscle PGF(2)(alpha) and PGE(2) in response to eccentric resistance exercise: influence of ibuprofen acetaminophen. J Clin Endocrinol Metab 86: 5067–5070, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Trappe TA, White F, Lambert CP, Cesar D, Hellerstein M, Evans WJ. Effect of ibuprofen and acetaminophen on postexercise muscle protein synthesis. Am J Physiol Endocrinol Metab 282: E551–E556, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Tsai WC, Hsu CC, Chen CP, Chen MJ, Lin MS, Pang JH. Ibuprofen inhibition of tendon cell migration and down-regulation of paxillin expression. J Orthop Res 24: 551–558, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Tsai WC, Hsu CC, Chou SW, Chung CY, Chen J, Pang JH. Effects of celecoxib on migration, proliferation and collagen expression of tendon cells. Connect Tissue Res 48: 46–51, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Tsai WC, Tang FT, Hsu CC, Hsu YH, Pang JH, Shiue CC. Ibuprofen inhibition of tendon cell proliferation and upregulation of the cyclin kinase inhibitor p21CIP1. J Orthop Res 22: 586–591, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Visser JJ, Hoogkamer JE, Bobbert MF, Huijing PA. Length and moment arm of human leg muscles as a function of knee and hip-joint angles. Eur J Appl Physiol Occup Physiol 61: 453–460, 1990 [DOI] [PubMed] [Google Scholar]
- 44. Wang XM, Wu TX, Lee YS, Dionne RA. Rofecoxib regulates the expression of genes related to the matrix metalloproteinase pathway in humans: implication for the adverse effects of cyclooxygenase-2 inhibitors. Clin Pharmacol Ther 79: 303–315, 2006 [DOI] [PubMed] [Google Scholar]