Abstract
In developing countries, the chronic exposure to carbon monoxide (CO) from biomass-fueled cookstoves may pose a significant health risk for women who use these stoves, especially for those with underlying clinical conditions that impair tissue oxygenation, e.g., anemia and coronary artery disease. CO concentrations measured in the vicinity of these cookstoves often exceed World Health Organization (WHO) indoor air guidelines for an 8-h average (9 ppm) and a 1-h maximum (26 ppm). Carboxyhemoglobin levels, reported infrequently because they are difficult to obtain, often exceed the WHO threshold of 2.5%. Despite this evidence, specific adverse effects have not yet been linked with chronic CO exposures in these women. Furthermore, anemia, which is prevalent in populations that use biomass fuels, could exacerbate the adverse effects of chronic CO exposure. Because of the difficulties inherent in conducting prospective studies to address this issue, we used a mathematical model to calculate the effects of reported CO levels and exercise on carboxyhemoglobin for women living in 1) Guatemalan villages at altitudes of 4,429–4,593 ft, and 2) coastal villages in Pakistan. In addition, we used the model to calculate the effects of CO exposures in women with moderate to severe anemia on specific physiological parameters (carboxyhemoglobin, carboxymyoglobin, cardiac output, and tissue Po2) at exercise levels representing the activities in which these women would be engaged. Our results demonstrate the efficacy of using a mathematical model to predict the physiologic responses to CO and also demonstrate that chronic anemia is a critically important determinant of CO toxicity in these women.
Keywords: carboxyhemoglobin, carboxymyoglobin, cardiac output, tissue Po2, oxygen extraction ratio
of the roughly 3 billion people exposed on a daily basis to combustion products of biomass fuels, it is the women who are at greatest risk because they spend most of their day cooking over or working near the primitive stoves that burn these fuels (wood, grasses, and dung) exposing them to potentially injurious concentrations of respirable particulates and carbon monoxide (CO). The intensity of these exposures is often a function of the efficiency of the ventilation in the cooking area and the extent to which these primitive stoves are used for light and heating in addition to meal preparation and, therefore, varies considerably from one region to another. Published reports of cookstove exposures in developing countries indicate that in some settings the exposure levels are high enough to be of concern (30, 32, 34, 35, 41). Because of the large number of women exposed to biomass fuel fumes, there is an urgent need to gain a better understanding of the adverse effects of these exposures and the potential interactions between components of these fumes and conditions such as anemia and coronary artery disease that are prevalent in many of these women.
Epidemiologic studies conducted in Latin America, Sub-Saharan Africa, and Asia provide substantial evidence of a causal association between smoke exposure and respiratory disease (24, 29, 30, 32, 37, 40). A strong link has been documented between biomass fuel exposure and chronic obstructive pulmonary disease in women and acute lower respiratory tract infections in children (11, 15, 36). A mechanistic basis for the chronic pulmonary inflammation seen in those exposed to cigarette smoke was proposed recently by Snelgrove et al. (38), who reported that tripeptide proteolytic degradation products (PGPs) of collagen that are potent chemoattractants for polymorphonuclear neutrophils are normally degraded by leukotriene A4 hydroxylase (LTA4-H) soon after the onset of influenza or Strep infections but persist in the lungs of chronic obstructive pulmonary disease patients. Cigarette smoke extract was reported to inhibit the ability of LTA4-H to degrade PGPs and to increase the acetylation of PGPs, thus rendering these peptides less susceptible to degradation by LTA4-H and more chemoattractive to polymorphonuclear neutrophils. Acrolein, the cigarette smoke extract component thought to be responsible for the effects on LTA4-H and PGP (2), is also present in high concentrations in wood smoke. Thus chronic pulmonary inflammation is likely to be a persistent concern for women who cook with biomass fuels.
In contrast to the evidence linking cookstove particulate exposures to health risks, CO has not yet been linked to specific adverse health effects in these women. In fact, CO is sometimes monitored for the sole purpose of providing an indirect measure of particulate exposures, which are more difficult to assess (31). Many of the reported 22-, 24-, and 48-h time-weighted average (TWA) exposures exceed the recommended 8-h TWA for CO of 9 ppm (44) despite the fact that these measurements include long periods of time when fires were not in use for meal preparation. Although the 1- and 2-day TWAs (means ± SD) were often low in warmer climates, e.g., 3.8 ± 3.9 ppm CO in The Gambia (16), this was not the case in many other countries in the developing world. CO TWAs (24 or 48 h) of 9.1 ± 5.1, 11.0 ± 6.7, and 12.4 ppm (11, 30, 34) were reported in studies conducted in Guatemala, 28.3 ± 30.9 ppm in China (17), 29.5 ± 16.2 ppm in Pakistan (34); and 36.7 ± 27.1 ppm in Burundi (41). In view of the potentially injurious CO concentrations to which women and small children are continuously exposed, it seems likely that the adverse effects of the CO exposure are more subtle and thus more difficult to link with the exposure. Unfortunately, few studies have measured the actual carboxyhemoglobin COHb level(s) ([COHb]) in these women. Reported [COHb] range from 2.5 ± 3.6% in villages at moderate altitudes in Guatemala (14) to ∼13% in India (4). However, we are not aware of any studies in which [COHb] was measured in individual subjects at multiple times throughout the day. Thus there is a critical lack of information regarding the peak [COHb] in exposed women and the periods of time over which their [COHb] remain above acceptable levels, nor is there any information in the literature regarding changes in critical physiologic parameters, e.g., cardiac output and tissue Po2, that might be indicative of excessive demands on the heart and diminished O2 delivery to critical organs, respectively, as a result of chronic, intermittent exposure to concentrations of CO that exceed levels deemed acceptable by the World Health Organization (WHO).
While measurements of TWAs over an 8-h period or longer can provide useful information with regard to the estimation of body burden following inhalation exposure to particulates, which tends to be cumulative, the kinetics of CO uptake, storage, and washout differ substantially. Thus the reported TWA values for 8-, 24-, and 48-h exposures to CO can provide only limited insight into the range of [COHb] that would result from cookstove exposures. Unfortunately, it is not feasible to monitor changes in [COHb] throughout the day in women living in remote settings, nor is it possible to measure many of the physiologic changes associated with cookstove CO exposures. However, by using a mathematical modeling approach, we were able to calculate the time course and extent of changes in [COHb] in response to a range of CO concentrations and exposure durations reported in studies conducted in Guatemala by Dary et al. (14) and in Pakistan by Siddiqui et al. (34). We also calculated changes in specific physiological parameters, e.g., cardiac output, tissue Po2, carboxymyoglobin concentrations, minute ventilation, and partial pressures of oxygen in muscle venous blood, both at rest and at three levels of exercise, in women exposed to the fumes from biomass fuels, thus providing greater insight into the potential risks associated with chronic CO exposure for these women.
In addition to our analysis of the effects of biomass fuel-derived CO on relatively healthy women, we assessed the extent to which anemia, a condition prevalent in many of the developing countries in which biomass fuels are in use, increases the risks associated with chronic CO exposure in these women. These model simulations were based on a published report of CO exposure conditions in a coastal region of Pakistan (34). Women in this region were shown in a separate study to have a high incidence of anemia (12). By using subject-specific parameters from a study of anemic women whose hemoglobin concentration ([Hb]) ranged from 6.3 to 10.8 g/dl (7), we were able to predict the increase in cardiac output and the decrease in tissue Po2 that might be expected to occur in anemic women chronically exposed to CO. In addition to a modest increase in predicted [COHb] in anemic vs. normal women, the model simulations indicated a decrease in tissue Po2 levels below those predicted for women with normal [Hb] and a further increase in cardiac output, suggesting that chronic exposure to biomass fuel smoke has the potential for serious health risks for at least some anemic women.
METHODS
Description of the Model
The model used here is an enhancement of our previously published model of CO uptake and distribution (9), which included a multicompartment representation of myoglobin-containing (i.e., muscle) tissues. The muscle tissue compartment comprises two tissue subcompartments distinguished by their major vascular compartments (i.e., an arteriole-venule compartment and a capillary compartment). Except as noted below, the current model was identical to the previous model. As described in the Supplemental Material (Supplemental Material for this article is available online at the J Appl Physiol website), additions were made to the previous model to account for 1) changes in cardiac output, muscle blood flow, pulmonary shunt fraction (SF), and ventilation during moderate exercise; and 2) the effects of anemia on cardiac output, muscle blood flow, SF, ventilation, and the shape of the oxygen dissociation curve. Also, to simplify the calculations in this version of the model, dissolved CO is ignored in compartments containing Hb. Furthermore, to improve accuracy of computed partial pressures of oxygen when O2 content of venous blood compartments is small, the numerical approach for calculating these pressures was changed. Finally, formulas for estimating tidal volume and physiological dead space from minute ventilation were added to compare the model to published studies in which minute ventilation, but not alveolar ventilation, was measured. The details of these additions to the model are presented in the Supplemental Material.
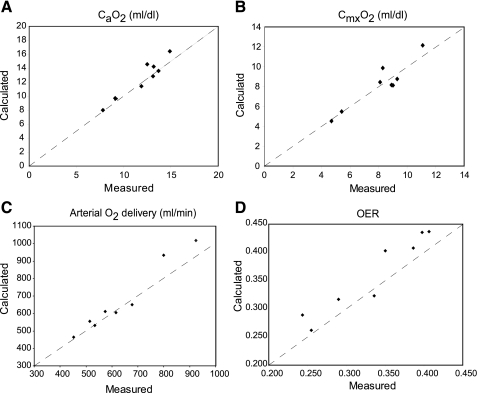
The ability of the expanded model to represent anemic subjects and exercising subjects was tested by comparisons to experimental data. For anemia, predicted arterial and mixed venous O2 contents in steady-state breathing on room air were compared with data from eight resting anemic subjects (7). Model predictions, based on the subject-specific parameter values provided in the study (Table 1) and default values for other parameters (9), are close to the measured values (Fig. 1).
Table 1.
Characteristics of anemic subjects
| Subject | Sex | Age | Weight, kg | V̇e, ml/min | MRO2, ml/min (STPD) | Q̇, l/min | [Hb], g/dl |
|---|---|---|---|---|---|---|---|
| LW | F | 19 | 41.7 | 5,406 | 166.95 | 5.7 | 8.8 |
| AH | F | 30 | 60.3 | 4,992 | 179.40 | 5.8 | 6.3 |
| LB | F | 45 | 59.0 | 5,460 | 168.48 | 4.7 | 9.9 |
| RH | F | 29 | 41.0 | 5,070 | 185.90 | 3.9 | 10.7 |
| OF | F | 41 | 52.2 | 9,765 | 198.40 | 3.9 | 10.8 |
| JM | M | 37 | 71.7 | 7,560 | 235.80 | 6.3 | 7.5 |
| HB | M | 19 | 81.2 | 8,600 | 264.00 | 6.4 | 11.2 |
| DH | M | 24 | 57.2 | 5,600 | 232.00 | 6.2 | 13.0 |
Data are from Brannon et al. (7). V̇e, minute ventilation; MRO2, metabolic rate of oxygen consumption; Q̇, cardiac output; [Hb], hemoglobin concentration.
Fig. 1.
Comparison of variables calculated by the model in the rest state with corresponding experimental measurements in 8 anemic subjects from the study of Brannon et al. (7). A: arterial oxygen concentration (CaO2; ml/dl). B: mixed venous oxygen concentration (CmxO2; ml/dl); C. total arterial oxygen delivery (ml/min), which equals cardiac output multiplied by CaO2. D: whole body oxygen extraction ratio (OER).
Predictions from the model during moderate exercise without anemia were evaluated by determining whether steady-state arterial Po2 remained approximately constant as muscle metabolic rate of O2 consumption increased to 10 times resting because the near constancy of arterial Po2 in conjunction with linear increases in minute ventilation (23) and cardiac output (26) is a feature of the normal response to exercise. We also ascertained that tissue Po2 levels remained above the lowest levels reported for resting skeletal muscle (i.e., ∼10 Torr; Ref. 43). Although this second criterion is conservative, it probably ensures that muscle metabolism remains below the anaerobic threshold. (Note that increasing muscle metabolism by a factor of 10 only increases whole body metabolic rate by a factor <3.) Subject-specific parameters for these simulations were taken from subject LB of Brannon et al. (7), except that [Hb] was set to a normal level of 13 g/dl. (It should be noted that the model calculates and removes the effects of anemia on cardiac output and ventilation and introduces no other effects of anemia in this case.) Results are shown in Table 2. Arterial Po2 remained within a 2-Torr range, and the predicted Po2 in the “capillary” tissue subcompartment of muscle tissues was >9.7 Torr at all exercise levels tested.
Table 2.
Predicted steady-state responses to exercise with normal [Hb]
| Exercise Level | MRO2wb, ml O2/min (BTPS) | MRO2m, O2/min (BTPS) | Q̇, ml/min | Q̇m, ml/min | V̇e, ml/min | VD, ml | V̇A, ml/min | PaO2 | Pcap,tO2, Torr |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 203.9 | 41.9 | 4,241 | 685 | 4,250 | 104 | 3,007 | 96.2 | 29.1 |
| 224.4 | 62.5 | 4,359 | 846 | 4,687 | 108 | 3,313 | 95.9 | 26.5 | |
| 1 | 245.8 | 83.8 | 4,476 | 1,008 | 5,124 | 112 | 3,617 | 95.6 | 24.7 |
| 266.8 | 104.8 | 4,596 | 1,169 | 5,561 | 117 | 3,919 | 95.3 | 23.3 | |
| 287.7 | 125.8 | 4,715 | 1,329 | 5,998 | 121 | 4,220 | 95 | 22.1 | |
| 308.7 | 146.7 | 4,833 | 1,488 | 6,435 | 126 | 4,519 | 94.8 | 21 | |
| 329.7 | 167.7 | 4,952 | 1,647 | 6,872 | 130 | 4,816 | 94.6 | 20 | |
| 371.6 | 209.6 | 5,188 | 1,964 | 7,746 | 139 | 5,406 | 94.3 | 18.1 | |
| 2 | 413.5 | 251.5 | 5,425 | 2,278 | 8,619 | 149 | 5,991 | 94.1 | 16.3 |
| 455.4 | 293.5 | 5,662 | 2,589 | 9,493 | 158 | 6,572 | 94 | 14.6 | |
| 476.4 | 314.4 | 5,781 | 2,744 | 9,930 | 163 | 6,861 | 94 | 13.7 | |
| 497.3 | 335.4 | 5,899 | 2,898 | 10,367 | 167 | 7,150 | 94 | 12.9 | |
| 3 | 539.3 | 377.3 | 6,136 | 3,204 | 11,241 | 177 | 7,728 | 94.2 | 11.3 |
| 581.2 | 419.2 | 6,372 | 3,507 | 12,115 | 186 | 8,305 | 94.4 | 9.75 |
MRO2wb, whole body oxygen consumption rate; Q̇m, muscle blood flow; VD, physiological dead space; V̇A, alveolar ventilation; Pcap,tO2, Po2 in second muscle tissue subcompartment.
The predicted effects of the combination of exercise with anemia were evaluated by repeating the above simulations at various levels of exercise using the same parameters except for [Hb], which was set to 9.9 g/dl. In this case the model calculates the effects of anemia on cardiac output, muscle blood flow, SF, oxygen dissociation curve, and ventilation. Detailed results, which provide the background for the main simulations related to CO exposures in (possibly) anemic subjects during moderate exercise, are presented in the Supplemental Material. In brief, with respect to Po2 in muscle tissue, the increases in cardiac output and muscle blood flow almost completely compensate for the diminished oxygen carrying capacity of the blood in an anemic subject. On the other hand, muscle venous Po2 falls in anemia due to increased oxygen extraction fraction.
RESULTS
Cookstove CO Exposures in Guatemalan Women
The first series of model simulations were based on a study by Dary et al. (14) of the CO exposures of women in 140 households in rural Guatemalan villages (14). The women used biomass fuel, primarily wood, in kitchens determined to be “poorly ventilated” based on the size of doors and windows, the presence or absence of chimneys, and the observer's estimate of the potential for air circulation in the kitchen (14). The homes were located at 4,429–4,593 ft above sea level at which elevations there would be a decrease in barometric pressure that was accounted for in the model. However, living at these altitudes would not be expected to result in significant changes in cardiac output or minute ventilation (3).
At sea level, the normal [Hb] for nonpregnant women 15–50 yr of age is 12.0 g/dl; thus lower levels would be indicative of anemia (28). For nonpregnant women living at 4,429–4,593 ft, [Hb] ≥12.5 g/dl is considered normal (39). For two groups of women in the study of Dary et al. (14), means ± SD [Hb] (g/dl) for young (13–20 yr; n = 13) and middle-aged women (21–49 yr; n = 43) were 12.4 ± 5.1 and 12.7 ± 8.1, respectively, suggesting that some of the women may have been anemic.
The exposure data provided by the study of Dary et al. (14) included the following: mean CO concentrations measured near the stoves at five times: 7 AM (40 ppm), 9 AM (39 ppm), 11 AM (50 ppm), 2:30 PM (27 ppm), and at 5:30 PM (29 ppm). In ∼60% of the homes, kitchen fires were reported to be in use for an average of 3–4 h/day. To estimate the effects of the exposure conditions in these Guatemalan kitchens, we used the mean [Hb] provided for women in each of the two age groups and three different CO-exposure scenarios, which included metabolic rates (see examples in Table 2.) consistent with standing (exercise level 1), standing while stirring a pot or walking slowly (exercise level 2), and walking while carrying a child on the back (exercise level 3) (25). For comparison with typical clinical measurements of [COHb] in blood from an antecubital vein, we used the percent COHb calculated by the model in the venous outflow from the muscle compartment.
Conditions for the lowest CO exposure reported in the Dary study.
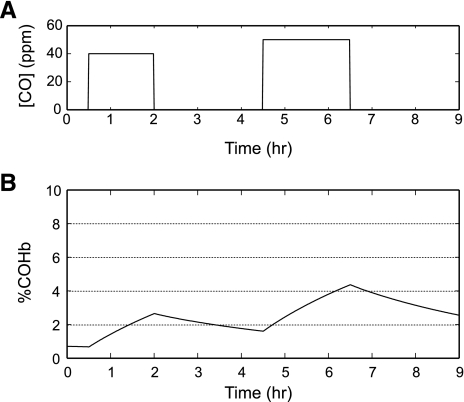
The conditions in the study of Dary et al. (14) were as follows: a 1.5-h exposure (6:30 to 8:00 AM) to 40 ppm CO (preparation of meal 1), a 2.5-h exposure (8:00 to 10:30 AM) to 0 ppm CO (fires were not burning in ∼75% of the households in the middle of the morning), and a 2-h exposure (10:30 to 12:30 PM) to 50 ppm CO (preparation of meal 2; Fig. 2). Simulation of this exposure regimen in young women at exercise level 1 resulted in [COHb] that closely approximated the mean levels reported by Dary et al. (14). The [COHb] was 2.65% after meal 1, decreased to 1.61% in the interval when fires were not burning, and gradually increased to 2.4% within 28 min of starting the preparations for the mid-day meal. These results are in close agreement with the report of Dary et al. of a mean of 2.4% COHb “at the hour of greatest smoke exposure,” presumably during the preparation of meal 2. However, because [COHb] continued to increase, it was much higher (4.36% COHb) at the end of meal 2. Thus the [COHb] reported by Dary et al. were almost certainly obtained at a time that underestimated the peak values that occurred in these women.
Fig. 2.
Model predictions of continuous carboxyhemoglobin (COHb) levels in a 17-yr-old Guatemalan female: hemoglobin (Hb) = 12.4 g/dl; weight = 49.5 kg; height = 1.494 m; metabolic rate of oxygen consumption (MRO2; rest) = 160 ml O2/min, exposed to 40 ppm carbon monoxide (CO) for 1.5 h (meal 1); 0 ppm CO for 2.5 h; 50 ppm CO for 2.0 h (meal 2); and 0 ppm CO for 2.5 h. A: CO level in the vicinity of the cookstove vs. time. B: %COHb vs. time.
Average exposure conditions based on the five CO measurements made by Dary.
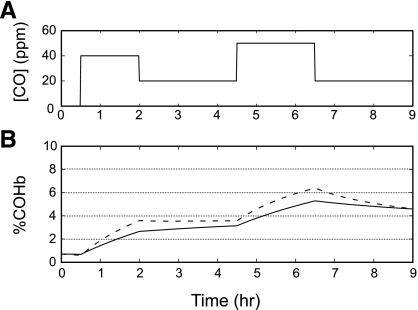
The conditions in the study of Dary et al. (14) were as follows: a 1.5-h exposure (6:30 to 8:00 AM) to 40 ppm CO (preparation of meal 1), a 2.5-h exposure (8:00 to 10:30 AM) to 20 ppm CO, and a 2-h exposure (10:30 to 12:30 PM) to 50 ppm CO (preparation of meal 2; Fig. 3A). Model simulations indicated that by 8 AM, [COHb] increased to 2.6% for a young woman who was standing (exercise level 1; Fig. 3B). During the 2.5-h period between the preparation of the first and second meals, the rate of increase in [COHb] was more gradual, reaching 3.1% at the end of this interval. A more rapid increase in [COHb] occurred during the preparation of the mid-day meal, reaching 5.3% at the end of the second cooking period. For a 30-yr-old woman [Hb = 12.7 g/dl; weight = 45.0 kg; height = 1.429 m; and metabolic rate of oxygen consumption (at rest) = 140 ml O2/min], [COHb] while standing were 2.8, 3.2, and 5.4% at 8 AM, immediately before, and immediately after preparation of meal 2, respectively (data not shown).
Fig. 3.
Model predictions of continuous COHb levels in response to changes in ambient CO concentrations. A: 40 ppm CO for 1.5 h (meal 1); 20 ppm CO for 2.5 h; 50 ppm CO for 2 h (meal 2); and 20 ppm CO for 2.5 h. B: %COHb for a 17-yr-old Guatemalan female: Hb = 12.4 g/dl Hb; weight = 49.5 kg; height = 1.494 m; MRO2 (rest) = 160 ml O2/min, while standing (exercise level 1, solid line) and walking slowly while carrying a child on her back (exercise level 3, dashed line).
These values, although higher than those seen with the previous CO exposure conditions, are still with the mean +1 SD of the values reported by Dary et al (14). Simulations of these CO exposure levels in a woman who was walking slowly while carrying a child on her back (exercise level 3) resulted in somewhat higher [COHb]. COHb values at 8 AM, 10:30 AM, and 12:30 PM were 3.6, 3.6, and 6.4%, respectively (Fig. 3B). For a 30-yr-old woman, values at these time points were 3.7, 3.6, and 6.5%, respectively (data not shown).
Highest exposure levels reported by Dary.
We also calculated the response to the highest CO concentration and longest exposure duration to which a subset of the women might have been exposed in Dary et al. (14). Although the majority (60%) used their cooking fires for a total of 3–4 h/day for the preparation of three meals, ∼25% of the kitchen fires were in use mid-morning and 15% were in use in the middle of the afternoon, presumably in addition to the three primary meal preparation times. For these women, the exposure to cookstove smoke may have been continuous for a period of up to 13 h, e.g., from as early as 6 AM until as late as 7 PM. After an exposure to 50 ppm CO (the highest CO concentration reported for women in these homes) for up to 13 h for a 30-yr-old woman standing (exercise level 1) or walking with a child on her back (exercise level 3), our model predicts that by 7 PM, her [COHb] would be 8.16 and 8.32%, respectively, considerably higher than the levels reported by Dary et al. (14), during the preparation of the mid-day meal (data not shown).
For each of these three exposure conditions, the model calculations provide greater insight regarding the body burden of CO than is possible from single COHb values. Although there was little or no effect of any of these exposures and exercise conditions on tissue Po2 levels or cardiac output (data not shown), in each of the three exposure simulations, [COHb] reached 2.5%, designated by WHO as the level that should not be exceeded (44), during the preparation of the first meal of the day. In the absence of a fire in the middle of the morning, the lowest of the likely exposure condition for these women, [COHb] would exceed 2.5% for ≥4 h in a woman at the lowest exercise level. At the higher exposure levels, [COHb] exceed the recommended threshold for a much longer period of time at both of the simulated exercise levels.
Cookstove CO Exposures in Normal Pakistani Women
CO exposure conditions used in this set of simulations were based on exposures in Rehri Goth, a semi-rural coastal community southeast of Karachi, Pakistan, reported by Siddiqui et al. (34). In this study, air sampling was conducted over an 8-h period that included “the majority of the cooking activities during the day” (34). The arithmetic means ± SD values reported were 29.5 ± 16.2 ppm CO for 8 h and 72.4 ± 32.6 ppm for the 1-h time-averaged peak CO concentration. With the exception of the mean age of the women in the study (28.6 ± 5.9 yr) who used wood for cooking, no other subject parameters were provided. Thus the female subject parameters (age, weight, cardiac output, metabolic rate, minute ventilation, etc.) used in the simulations were those reported for subject LB by Brannon et al. (7) (as described in methods) assuming a [Hb] of 13 g/dl.
We first calculated the peak [COHb] that would result from the following: 1) the reported mean TWA value (29.5 ppm); 2) the mean +1 SD (45.7 ppm); and 3) the mean +2 SD (61.9 ppm) to evaluate this approach to risk assessment. Model calculations of the effects of exercise using two intensity levels relevant to these women, e.g., standing (exercise level 1) and standing while stirring a pot or walking slowly (exercise level 2) indicated that exercise intensity was a significant factor (Table 3). [COHb] did not plateau but continued to increase throughout each of the three 8-h CO exposure concentrations at each of the two exercise levels. By the end of the 8-h exposures, all of the [COHb] exceeded the WHO (44) recommendation that indoor air exposures for those engaged in “light to moderate exercise” should not result in [COHb] levels >2.5%. Model calculations of carboxymyoglobin concentration(s) in the muscle tissue (skeletal and cardiac) compartment perfused by capillaries [COMbcap] (muscle subcompartment 2; Ref. 9) increased 16- and 27-fold, at exercise levels 1 and 2, respectively, after the 8-h exposure to 61.9 ppm. The increase in exercise intensity from exercise level 1 to level 2 had a greater impact on both cardiac output and tissue Po2 levels than did the CO exposures simulated. For any two CO exposure levels, the percent increase in cardiac output and the percent decrease in tissue Po2 were roughly the same for each exercise level.
Table 3.
Model predictions for three 8-h TWA CO exposures in a female subject
| CO, ppm | 0 | 0 | 29.5 | 29.5 | 45.7 | 45.7 | 61.9 | 61.9 |
|---|---|---|---|---|---|---|---|---|
| Exercise level | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 |
| COHb | 0.56 | 0.39 | 4.42 | 5.15 | 6.48 | 7.64 | 8.48 | 10.06 |
| COMbcap | 0.18 | 0.19 | 1.43 | 2.54 | 2.14 | 3.88 | 2.863 | 5.23 |
| Cardiac output, ml · min−1 · kg−1 | 75.82 | 91.79 | 77.98 | 95.16 | 79.55 | 97.78 | 81.49 | 101.1 |
| Pcap,tO2, Torr | 24.74 | 16.35 | 23.4 | 14.98 | 22.7 | 14.27 | 22.03 | 13.61 |
| O2 delivery, ml/muscle | 9.15 | 20.67 | 9 | 20.25 | 8.921 | 20.04 | 8.844 | 19.84 |
Carbon monoxide (CO): mean time-weighted average (TWA) = 29.5; mean TWA ± 1 SD = 45.7; and mean TWA ± 2 SD = 61.9 (36). Exercise level: 1, standing; 2, standing while stirring a pot or walking slowly. CO, carbon monoxide. COHb, carboxyhemoglobin; COMbcap, carboxymyoglobin concentration(s) in the muscle tissue (skeletal and cardiac) compartment perfused by capillaries.
Because the women in the study of Siddiqui et al. (34) are likely to have been exposed to CO concentrations higher than the reported 8-h TWA when preparing the meals, but lower at other times, the simulated exposure conditions were revised to reflect a more realistic exposure regimen that included three meal preparation periods that totaled 4 h in duration (59% of households reported burning fuel for ≥4 h) over a 9-h period extending from 8:30 AM until 5:30 PM. The simulated CO exposure resulting from the preparation of the meal 1 was 50 ppm CO for 1 h; for meal 2 the exposure was 110 ppm for 2 h; and for meal 3 the exposure was 50 ppm for 1 h. This revised protocol would result in a 9-h TWA of 35.6 ppm CO, only slightly higher than the 8-h TWA (29.5 ppm) reported by Siddiqui et al. (34) and considerably less than the mean +1 SD (45.7 ppm).
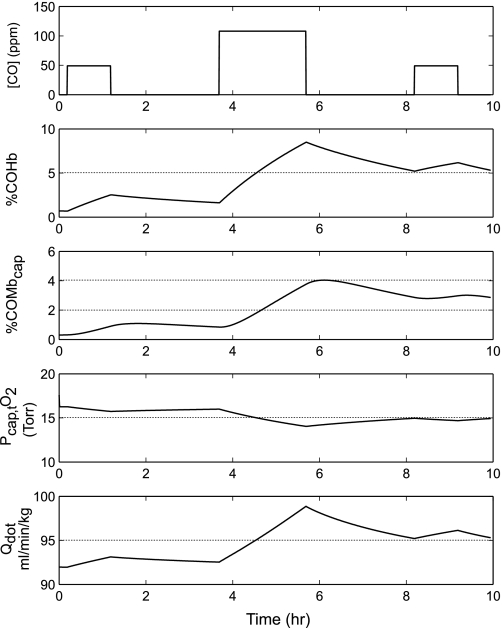
Using these three-meal exposure conditions at exercise level 2 (Fig. 4), [COHb] increased during the first hour of cooking to 2.53% and remained at approximately this level until the onset of the 2-h preparation of the second meal. By the end of the second meal, [COHb] increased to 8.48%, well above the 5.15% COHb predicted for the end of the 8-h day when the [COHb] was based on the 8-h TWA reported by Siddiqui et al. (34). [COHb] decreased slightly thereafter to 6.10% 1 h after the end of the third meal. [COMbcap] was also considerably higher when the exposure simulation was based on three cooking periods. Compared with a level of 2.54% at the end of the 8-h TWA exposure, COMbcap was 4.04% after the second cooking period. There was a modest increase in cardiac output (ml·min−1·kg−1) from 92 before the onset of the CO exposure to a peak value of 98.9 after the preparation of meal 2. Cardiac output did not return to preexposure levels in the interval between meals 2 and 3, and by the end of meal 3 it was higher than it had been at the end of the preparation of meal 1, i.e., 96.1 vs. 93.1 ml·min−1·kg−1, respectively. The partial pressure of O2 in compartment 2 of muscle tissue fell from a preexposure level of 16.35 to 14.04 Torr after the preparation of meal 2 and did not return to preexposure levels by the end of the 10-h period simulated.
Fig. 4.
Model predictions for Siddiqui et al. (34) exposure conditions of %COHb, %carboxymyoglobin in muscle compartment 2 (COMbcap), partial pressure of O2 in compartment 2 of muscle tissue (Pcap,tO2), and cardiac output (Qdot) for a normal woman, based on subject LB (7) [49 yr; Hb = 13 g/dl; weight = 59 kg; MRO2 (rest) = 168 ml O2/min] standing while stirring a pot (exercise level 2) in response to the following CO exposures during the preparation of 3 meals: 50 ppm CO for 1 h (meal 1); 0 ppm CO for 2.5 h; 110 ppm CO for 2 h (meal 2); 0 ppm for 2.5 h; and 50 ppm CO for 1 h (meal 3).
Cookstove Exposures in Pakistani Women Who Are Anemic
The extent to which anemia, which is prevalent in many impoverished rural communities including those in the study of Siddiqui et al. (34), could exacerbate the adverse effects of cookstove exposures to CO was of particular interest, as the influence of these combined risk factors would be difficult to assess experimentally. Because the response to anemia varies from one individual to another, Hb level alone is a weak predictor in individual subjects of changes in cardiac output, heart rate, or stroke volume (32). Therefore, to evaluate responses that might occur in anemic women during cookstove CO exposures, simulations of exposures in the study of Siddiqui et al. (34) at each of two exercise levels (level 2: walking slowly or standing, stirring a pot) and (level 3: walking slowly while carrying a child on the back) were conducted using measured parameter values described in a study of Brannon et al. (7) from five chronically anemic women, whose Hb concentrations ranged from 10.8 to 6.3 g Hb/dl and for whom other critical parameter values (age, weight, basal metabolic rate, cardiac output, minute ventilation, and arterial O2 content) were available (7) (details presented in methods). Results were compared with those of a simulated control subject [subject LB from the study of Brannon et al. (7), who was assigned a normal Hb concentration of 13 g/dl as explained in the Supplemental Material].
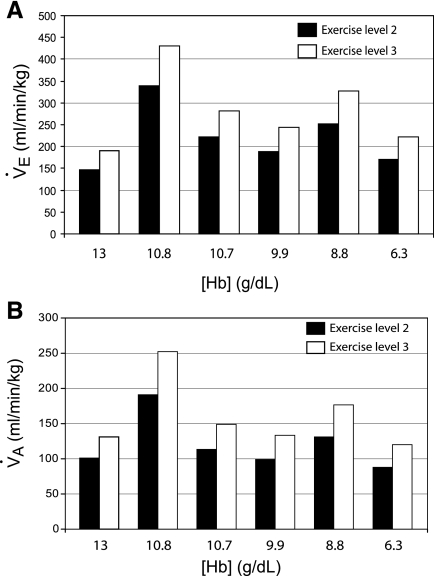
Values for minute ventilation (ml·min−1·kg−1) at rest, as reported by Brannon et al. (7) and shown in Table 1 were higher in each of the anemic women than in the control, consistent with the fact that alveolar dead space is often increased in those with moderate to severe anemia (Fig. 5A). Model calculations of values for alveolar ventilation (ml·min−1·kg−1) at rest were also higher in the anemic women vs. the control (Fig. 5B). In addition to predicting alveolar ventilation in these subjects, the model also showed that Po2 levels were “about normal” at rest. Although CO exposure does not influence model predictions of either minute or alveolar ventilation, both increased as a function of exercise level (data not shown). When compared with the control subject, model predictions of baseline alveolar and minute ventilation at exercise level 2 or 3, before CO exposure, were greater in three of the five anemic women, in one of whom alveolar and minute ventilation were almost twofold greater than the control. Neither alveolar ventilation (calculated by the model) nor minute ventilation (measured by Brannon et al.) was closely correlated with [Hb]; it was highest in the anemic subjects with [Hb] of 10.8 and 8.8 g/dl and lowest in the subject with 6.3 g/dl in whom alveolar ventilation was also slightly lower than that in the control subject.
Fig. 5.
Model predictions of minute ventilation (A; V̇e) and alveolar ventilation (B; V̇A) for a woman with a normal Hb concentration (13 g/dl) and for anemic women [from Brannon et al. (7)], who had Hb levels of 10.8, 10.7, 9.9, 8.8, and 6.3 g/dl, who are standing while stirring a pot (exercise level 2, shaded bars) or walking slowly while carrying a child (exercise level 3, patterned bars).
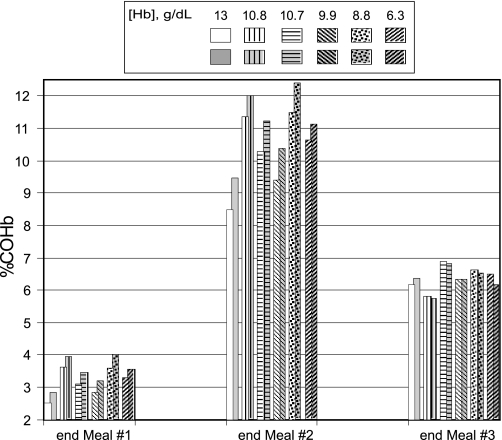
After preparation of meal 1 (50 ppm CO for 1 h), [COHb] at each exercise level was greater in the anemic women than the control (Fig. 6). After meal 2 (110 ppm CO for 2 h) the disparity between [COHb] for the anemic women vs. the normal woman increased further. Peak [COHb] at exercise level 2 was as much as 35% higher in the anemic women (9.40–11.49% COHb) when compared with the normal subject (8.48% COHb). [COHb] increased further in each subject with the increase to exercise level 3. There was no evidence of an inverse correlation between [Hb] and [COHb] in these women. The highest [COHb] occurred in subjects who had [Hb] of 10.8 and 8.8 g/dl, whereas the severely anemic subject (Hb = 6.3 g/dl) had a lower [COHb] than either of these women. As baseline alveolar ventilation (ml·min−1·kg−1) was substantially higher in the subject with 10.8 g/dl Hb, the inhaled CO dose would also be greater in this subject than the others and could explain the high [COHb] in this subject despite her relatively high [Hb]. In the subject with 8.8 g/dl, the relatively high [COHb] could be due, in part, to the fact that cardiac output was markedly higher in this subject than in the others, leading to more rapid uptake and distribution of CO. In contrast to meal 1 and 2 exposures, after meal 3 (50 ppm CO for 1 h) [COHb] was slightly lower at the higher vs. the lower exercise level in four of the five anemic women. Before the onset of meal 3 [COHb] was lower at exercise level 3 vs. 2 in each of the anemic women but not in the control, suggesting that increased minute ventilation and cardiac output at the higher exercise level may have contributed to more rapid removal of CO from the blood after meal 2.
Fig. 6.
Model predictions of %COHb for a woman with a normal Hb concentration (13 g/dl) and for anemic women [from Brannon et al. (7)], who had Hb levels of 10.8, 10.7, 9.9, 8.8, and 6.3 g/dl at exercise level 2 (unshaded, patterned bars) and at exercise level 3 (shaded, patterned bars) in response to the following CO exposures during the preparation of 3 meals: 50 ppm CO for 1 h (meal 1); 0 ppm CO for 2.5 h; 110 ppm CO for 2 h (meal 2); 0 ppm for 2.5 h; and 50 ppm CO for 1 h (meal 3).
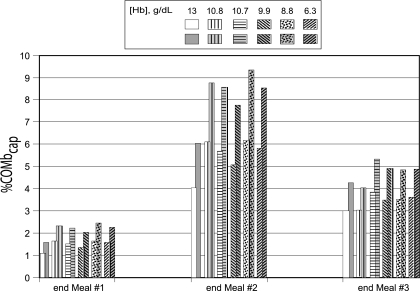
As was the case with the [COHb], [COMbcap] was consistently higher in the anemic women than in the normal woman but was not inversely correlated with [Hb] (Fig. 7). In contrast to the other calculated parameter values, [COMbcap] did not reach maximal levels by the end of the cooking periods. Instead, at exercise level 2, [COMbcap] in anemic women peaked between 24 and 34.5 min after the first meal vs. 38.5 min in the normal woman; between 13 to 22 min after the second meal in anemics vs. 25 min in the normal; and between 9 to 11 min after the third meal in anemics vs. 12 min in the normal woman. At exercise level 3, the peak [COMbcap] occurred from 58 to 84 min after meal 1 in anemic women vs. 92 min in the normal; from 34 to 42 min after the second meal in anemic women vs. 62 min in the normal; and from 24 to 26 min after the last meal in anemic women vs. 28 min in the normal woman. In addition, [COMbcap] remained elevated for a longer period of time in the anemic women.
Fig. 7.
Model predictions of %COMbcap for a woman with a normal Hb concentration (13 g/dl) and for anemic women [from Brannon et al. (7)], who had Hb levels of 10.8, 10.7, 9.9, 8.8, and 6.3 g/dl at exercise level 2 (unshaded, patterned bars) and at exercise level 3 (shaded, patterned bars) in response to the following CO exposures during the preparation of 3 meals: 50 ppm CO for 1 h (meal 1); 0 ppm CO for 2.5 h; 110 ppm CO for 2 h (meal 2); 0 ppm for 2.5 h; and 50 ppm CO for 1 h (meal 3).
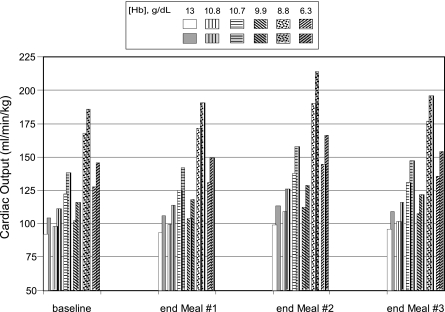
Before the onset of the CO exposure, baseline cardiac output at rest varied considerably among the anemic subjects, ranging from 74.7 to 136.7 ml·min−1·kg−1. Compared with the control subject, cardiac output [as reported by Brannon et al (7)] was consistently higher in the anemic women and substantially higher in the subject with 8.8 g/dl Hb. After adjustment for exercise in the model, cardiac output was higher in the anemics vs. the normal at both exercise levels (Fig. 8). At exercise level 2, cardiac output was only slightly higher in two of the anemic subjects (97.8 and 102.4) but much higher in the other three (126.9, 127.6, and 167.9) than in the normal subject in whom the cardiac output was 92. The increase in cardiac output associated with the increase in exercise level from level 2 to 3 was somewhat higher in the two subjects with the lowest [Hb] and ranged from 11.6 to 18.3 ml·min−1·kg−1 in the anemic women vs. 12.2 ml·min−1·kg−1 in the control.
Fig. 8.
Model predictions of cardiac output for a woman with a normal Hb concentration (13 g/dl) and for anemic women [from Brannon et al. (7)], who had Hb levels of 10.8, 10.7, 9.9, 8.8, and 6.3 g/dl at exercise level 2 (unshaded, patterned bars) and at exercise level 3 (shaded, patterned bars) before CO exposure and in response to the following CO exposures during the preparation of 3 meals: 50 ppm CO for 1 h (meal 1); 0 ppm CO for 2.5 h; 110 ppm CO for 2 h (meal 2); 0 ppm for 2.5 h; and 50 ppm CO for 1 h (meal 3).
The increase in cardiac output by the end of the first meal of the day was quite small, <4 ml·min−1·kg−1 for each subject at each exercise level (Fig. 8). However, when compared with baseline preexposure values, cardiac output at exercise level 2 increased 9.6–13.3% in the anemic women by the end of the preparation of the mid-day meal, vs. an increase of 7.6% in the control subject. At the higher exercise level, cardiac output increased 13–15% in the anemic subjects vs. an increase of 8.8% in the control by the end of the second meal. Cardiac output did not return to baseline levels in the interval between the end of meal 2 and the beginning of preparations for meal 3, and by the end of meal 3, cardiac output was still greater in all subjects at both exercise levels than it was after the first meal of the day.
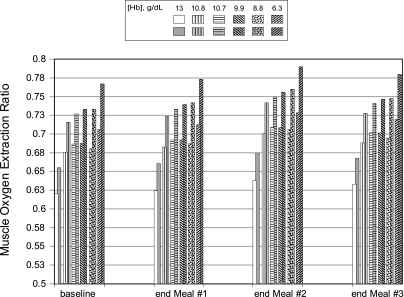
Before the onset of the CO exposures, values for the oxygen extraction ratio(s) (OER) for muscle blood flow were similar for four of five anemic subjects at both exercise levels but markedly higher in the subject with the lowest [Hb] (Fig. 9). The normal subject had an OER much lower than those of the anemic subjects. After meal 1, the increase in muscle OER was approximately the same in all of the anemic subjects and somewhat less in the normal woman. There was a larger increase in OER by the end of the mid-day meal preparation in all subjects, but again the increase was smaller in the control vs. the anemic women. After the last meal of the day, muscle OER had dropped somewhat but were still greater than those predicted for the end of the meal 1. Throughout the period simulated, muscle OER was consistently lower in the subject with a normal [Hb] whereas the subject with the lowest [Hb] always had a higher OER than the other subjects.
Fig. 9.
Model predictions of oxygen extraction ratios for the muscle compartment for a woman with a normal Hb concentration (13 g/dl) and for anemic women [from Brannon et al. (7)], who had Hb levels of 10.8, 10.7, 9.9, 8.8, and 6.3 g/dl at exercise level 2 (unshaded, patterned bars) and at exercise level 3 (shaded, patterned bars) before CO exposure and in response to the following CO exposures during the preparation of 3 meals: 50 ppm CO for 1 h (meal 1); 0 ppm CO for 2.5 h; 110 ppm CO for 2 h (meal 2); 0 ppm for 2.5 h; and 50 ppm CO for 1 h (meal 3).
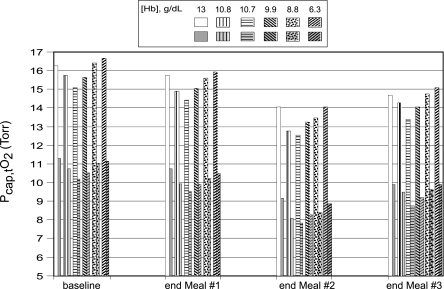
The partial pressures of oxygen in the muscle (merged skeletal and cardiac) subcompartment perfused by capillaries decreased markedly, and to approximately the same extent, with the increase in exercise level from level 2 to 3 in all subjects, regardless of [Hb] (Fig. 10). At exercise level 2, Po2 was slightly higher in the control subject vs. the anemic women after each of the meals. However, at the higher exercise level (level 3), the muscle Po2 levels in the two women with the lowest [Hb] were almost as high as, or greater than, control levels, likely due in part to the increased cardiac output in one of the subjects and to the high OER in the other anemic subject.
Fig. 10.
Model predictions of partial pressure of O2 in compartment 2 of muscle tissue (Pcap,tO2) for a woman with a normal Hb concentration (13 g/dl) and for anemic women [from Brannon et al. (7)], who had Hb levels of 10.8, 10.7, 9.9, 8.8, and 6.3 g/dl at exercise level 2 (unshaded, patterned bars) and at exercise level 3 (shaded, patterned bars) before CO exposure and in response to the following CO exposures during the preparation of 3 meals: 50 ppm CO for 1 h (meal 1); 0 ppm CO for 2.5 h; 110 ppm CO for 2 h (meal 2); 0 ppm for 2.5 h; and 50 ppm CO for 1 h (meal 3).
DISCUSSION
We have used a mathematical model to calculate the uptake and whole body distribution of CO, and the physiologic responses to decreased O2 delivery, in response to a range of CO-exposure regimens and activity levels in women living in a Guatamalan village at moderate altitudes (14) and in a coastal region of Pakistan (34). The agreement between the [COHb] reported by Dary et al. (14) and our simulation results confirms the ability of the model to predict the [COHb] resulting from their cookstove CO exposures. Simulations of exposure conditions based on the five ambient CO concentrations provided by the authors (14), indicated that [COHb] exceeded 2.5% for most of the exposure period, indicative of a greater risk than Dary et al. suggested. These observations emphasize the fact that the period of time over which [COHb] exceeds “safe” levels is at least as important as a single [COHb] measurement that is assumed to represent the peak value because sustained increases in [COHb] could increase the risk for abnormal fetal development in pregnant women and could also exacerbate any preexisting cardiovascular disease.
We also used the model to evaluate the responses of five moderately or severely anemic women to multiple CO exposure conditions at exercise levels relevant to their probable activities to determine the extent to which anemia could potentiate cookstove-related hypoxia, an issue thus far ignored in the literature. Our simulations predict generally higher [COHb] and lower tissue Po2 levels in anemic women; however, the differences from the woman with a normal [Hb] are not simple functions of their [Hb] levels.
Although a few studies (32, 34) have reported ambient CO concentrations on a minute-to-minute basis, the majority report 24- or 48-h TWAs, which are of limited value unless the cookstoves are in continuous use as a source of heat, which is rarely the case, even in colder climates. Although [COHb] has the potential to be a more reliable measure of CO exposure than the TWA, published reports of venous [COHb] associated with cookstove exposures are often of limited value because blood samples are rarely obtained at more than one time during the meal preparation periods. However, our results have shown that even in the absence of multiple COHb measurements, frequent measures of ambient CO concentrations make it possible to estimate both the peak [COHb] and the period of time that [COHb] exceeds recommended threshold levels.
Also of concern is the fact that venous [COHb] does not accurately reflect total body burden. Although CO is rapidly absorbed into the blood via the lungs, the uptake by muscle is delayed (8, 9). Furthermore, CO washout from the body is a biexponential process (10). The initial phase represents the rapid removal from the blood while the second and slower phase represents release from COMb in the skeletal and cardiac muscle compartments into the blood. Thus, after a relatively short exposure to a high concentration of CO, such as those reported by Siddiqui et al. (34; 1 h peak value of 72 ppm), [COHb] would initially decrease more rapidly than [COMb], in which case myocardial [COMb] could be of greater concern than might be expected based on the [COHb] alone (8, 18).
Exercise intensity enhances CO toxicity due to the attendant increases in metabolic rate and minute ventilation, yet the activities of women who use biomass fuels are rarely discussed in the cookstove literature. However, by adjusting the metabolic rate and minute ventilation in the model to reflect the various activities of these women while preparing meals, we were able to determine both the time course and the extent of changes in [COHb], cardiac output, merged (skeletal and cardiac) muscle COMb concentration, and tissue Po2 levels during and after the exposure period. A limitation of the current simulations is that the exercise level was held constant throughout each simulation, whereas in practice the level of exercise varies throughout the day. Although we have no specific information in this regard, it is likely that the qualitative behaviors reported here would be preserved if such variations were taken into account.
To better understand the physiologic implications of these exposure conditions, the model was also used to assess changes in [COMbcap], cardiac output, tissue Po2, and O2 delivery to muscle, factors not addressed thus far in the cookstove literature. The predicted increase in cardiac output and [COMbcap], together with the decrease in tissue Po2, could be a cause for concern, even for those without preexisting conditions. The calculated increases in [COMbcap] and decreases in tissue Po2 resulting from cookstove CO exposures suggest the potential for hypoxic episodes, especially when there is an abrupt increase in the exercise level and metabolic rate or when there is a spike in ambient CO concentrations, as reported by Siddiqui et al. (34) and Rinne et al. (32). Furthermore, because CO uptake by muscle is slow rather than rapid as initially proposed (13, 27), and because CO continues to leave the blood and enter the muscle compartment after the end of the CO exposure (8), the time course of the increase in COMb is influenced both by the CO exposure conditions and by muscle mass. Thus, for an undernourished woman with a lower-than-average muscle mass, there is an increased likelihood that the MgbO2 stores in the myocardium could be inadequate when these women are exposed to higher levels of CO or during a sudden increase in metabolic rate.
An even greater challenge is that of determining the effects of biomass fuel smoke exposure on women who have a specific health problem. To address one such problem, we used our model to estimate the range of responses that might occur in a population of anemic women. In this context, anemia is defined as a decrease in the capacity to deliver oxygen to the tissues due to a decrease in [Hb] and/or a decrease in the number of red blood cells. We considered this issue to be of particular importance because severe anemia is prevalent in economically disadvantaged areas that are often the same regions as those in which women use biomass fuels. For women between the ages of 15 and 50 yr who live at sea level, the Hb threshold is 12.0 g/dl for anemia and 7.0 g/dl for severe anemia. (28, 39). Worldwide, 468.4 million nonpregnant women are anemic (28). In Southeast Asia, the incidence of anemia (Hb <12 g/dl) in nonpregnant women is 68% (27). Of 6,288 pregnant women in five sites in “peri-urban Karachi,” one of which was Rehri Goth, the site of the study of Siddiqui et al. (34), 10.5% of the women were found to be severely anemic with [Hb] ≤7.0 g/dl (12). Although malaria, intestinal Geohelminths, chronic infection, and hemoglobinopathies resulting from genetic mutations are the primary causes of anemia in some populations, iron-deficiency resulting from chronic malnutrition is responsible for the vast majority of the cases of anemia in the developing world (28). For the Pakastani women in the study of Siddiqui et al. (34), anemia was largely due to iron deficiency, as their diets were likely to consist primarily of cereals with few animal products. A possible contributory factor was the inclusion in their diets of significant amounts of phytate, fiber, and tannins, all of which inhibit the absorption of iron (5).
Compensatory physiologic changes in response to anemia vary considerably. Thus in individual subjects the severity of these adaptive responses to a decreased O2 supply is difficult to predict on the basis of [Hb] alone. Compensatory changes include but are not limited to the following: 1) an increase in cardiac output, due primarily to an increase in stroke volume; 2) a decrease in vascular resistance; 3) an increase in minute ventilation; and 4) an increase in the OER due to an increase in capillary density. Coronary and cerebral blood flows are increased at the expense of blood flow to other tissues. Thus, although tissue Po2 levels are generally maintained near those of a nonanemic woman, this is accomplished at the expense of an increased demand on the heart.
The increases in minute ventilation observed in the anemic women in our simulations were associated with higher [COHb], due in part to the increase in volume of CO inhaled. Accordingly, one would also expect to see an increase in inhaled particulate material in these women, an issue that has not, to our knowledge, been addressed in the literature, but one that is important to understand to better protect these women from chronic respiratory disease.
When anemic women with compensatory increases in cardiac output are exposed to CO, our model simulations indicate that cardiac output is likely to increase further, and for prolonged periods of time, resulting in even greater demands on the heart. Furthermore, our simulations are likely to underestimate the potential effects on myocardial oxygenation because we included the heart mass in a single compartment representing all tissues containing myoglobin. We have shown previously that cardiac and skeletal muscle Po2 respond differently to CO poisoning and that myocardial oxygenation may be at greater risk in situations involving, in addition to CO, a sudden increase in local oxygen consumption (18).
Another concern for women exposed daily to the CO in cookstove smoke is the potential for exacerbation of coronary artery disease, which is prevalent in south east Asia (22). In Pakistan, women were found to be at greater risk than men in a recent study in which major ECG and ischemic changes were twice as prevalent in women as in men (20). [COHb] as low as 2.0 and 3.9%, levels well within the range of those reported in cookstove exposures, have been linked with an increased risk for myocardial ischemia during moderate exercise in patients with stable coronary artery disease (1). Within-subjects comparisons indicated a decrease in the time required to develop ECG ST-segment changes suggestive of myocardial ischemia of 5.1% (at 2.0% COHb) and 12.1% (at 3.9% COHb). Although the authors did not conclude that the ST-segment changes detected at these [COHb] were life threatening, they cited a possible risk for myocardial necrosis as a result of repeated ischemic episodes (19) and a predisposition to ventricular arrythmias (6).
To date, there is no evidence of a direct link between cookstove CO exposures and cardiac injury in these women. However, because they often lack access to adequate health care, cardiac problems are unlikely to be diagnosed, decreasing the probability that a causal relationship between CO exposure and myocardial injury would be detected. Left ventricular hypertrophy, which can be caused by chronic anemia, has been studied in this population, but no explanation has been offered for the fact that the incidence is much higher in women than in men (21). The higher incidence of anemia in women vs. men is consistent with the possibility that anemia is a factor, but the extent to which chronic CO exposure either causes or exacerbates left ventricular hypertrophy has not yet been explored and merits further consideration.
Despite the absence of compelling evidence that chronic exposure to CO from biomass fueled cookstoves has an adverse effect on the health of the women who use the stoves, the CO levels measured in the vicinity of the cookstoves often exceed levels deemed acceptable in the 1999 WHO guidelines (44). Perhaps more relevant to cookstove CO exposures are the recent (2010) WHO guidelines that recommend that, for 24-h exposures, CO not exceed 6.11 ppm for someone who is “awake and alert, but not exercising” and that [COHb] should be ≤2.0% (44). Our model simulations indicate that after a 24-h exposure to 6.11 ppm at exercise level 1, [COHb] would be 1.62%, a value only marginally greater than endogenous levels for a nonsmoker. In the absence of data linking adverse health effects with specific CO exposure levels, setting a safe threshold level for healthy subjects presents a significant challenge. An alternative approach, and the one we used in this study, is to use a mathematical model to determine the extent of the physiologic changes that can occur in response to a range of CO exposure conditions.
In conclusion, our results demonstrate the value of using a mathematical modeling approach to evaluate the potential for health risks associated with chronic exposure to one of the combustion products of biomass fuels. These results emphasize the importance of obtaining an accurate assessment of exposure conditions and activity levels, e.g., by using a personal activity monitor, to estimate the CO dose inhaled and the body burden. [COHb], which is rarely measured in cookstove exposure studies (4, 40), was also shown to be an important variable. In addition, we have shown that our model can be used to investigate the responses of anemic women to cookstove CO exposures, an issue that would be both difficult and expensive to address experimentally. Of particular relevance to recent international initiatives to address the adverse effects of biomass fuel combustion, e.g., the Global Alliance for Clean Cookstoves, is the fact that anemia, a condition prevalent in women in developing countries, could substantially increase the health risks associated with cookstove exposures to CO. While it is critically important to reduce the use of biomass fuels for cooking and heating, our results suggest that this initiative be accompanied by a concerted effort to identify and treat, and ultimately prevent, anemia in these women.
GRANTS
Support for this work was provided by National Institute of Occupational Safety and Health Grant OH-008651.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
REFERENCES
- 1. Allred EN, Bleecker ER, Chaitman BR, Dahms TE, Gottlieb SO, Hackney JD, Pagano M, Selvester RH, Walden SM, Warren J. Effects of carbon monoxide on myocardial ischemia. Environ Health Perspect 91: 89–132, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barnes Medicine PJ. Neutrophils find smoke attractive. Science 330: 40–41, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Beall CM, Strohl KP, Blangero J, Williams-Blangero S, Almasy LA, Decker MJ, Worthman CM, Goldstein MC, Vargas E, Villena M, Soria R, Alarcon AM, Gonzales C. Ventilation and hypoxic ventilatory response of Tibetan and Aymara high altitude natives. Am J Phys Anthropol 104: 427–447, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Behera D, Dash S, Malik SK. Blood carboxyhaemoglobin levels following acute exposure to smoke of biomass fuel. Indian J Med Res 88: 522–524, 1988 [PubMed] [Google Scholar]
- 5. Bhutta ZA. Iron and zinc intake from complementary foods: some issues from Pakistan. Pediatrics 106: 1295–1297, 2000 [PubMed] [Google Scholar]
- 6. Bolli R, Fisher DJ, Entman ML. Factors that determine the occurrence of arrhythmias during acute myocardial ischemia. Am Heart J 111: 261–270, 1986 [DOI] [PubMed] [Google Scholar]
- 7. Brannon ES, Merrill AJ, Warren JV, Stead EA. The cardiac output in patients with chronic anemia as measured by the technique of right atrial catheterization. J Clin Invest 24: 332–336, 1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bruce EN, Bruce MC. A multicompartment model of carboxyhemoglobin and carboxymyoglobin responses to inhalation of carbon monoxide. J Appl Physiol 95: 1235–1247, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Bruce EN, Bruce MC, Erupaka K. Prediction of the rate of uptake of carbon monoxide from blood by extravascular tissues. Respir Physiol Neurobiol 161: 142–159, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bruce MC, Bruce EN. Analysis of factors that influence rates of carbon monoxide uptake, distribution, and washout from blood and extravascular tissues using a multicompartment model. J Appl Physiol 100: 1171–1180, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Bruce N, Perez-Padilla R, Albalak R. Indoor air pollution in developing countries: a major environmental and public health challenge. Bull World Health Organ 78: 1078–1092, 2000 [PMC free article] [PubMed] [Google Scholar]
- 12. Christian P, Shahid F, Rizvi A, Klemm RD, Bhutta ZA. Treatment response to standard of care for severe anemia in pregnant women and effect of multivitamins and enhanced anthelminthics. Am J Clin Nutr 89: 853–861, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Coburn RF, Blakemore WS, Forster RE. Endogenous carbon monoxide production in man. J Clin Invest 42: 1172–1178, 1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dary O, Pineda O, Belizán JM. Carbon monoxide contamination in dwellings in poor rural areas of Guatemala. Bull Environ Contam Toxicol 26: 24–30, 1981 [DOI] [PubMed] [Google Scholar]
- 15. Desai M, Mehta S, Smith K. Indoor Smoke from Solid Fuels: Assessing the Environmental Burden of Disease at National and Local Levels. Geneva, Switzerland: World Health Organization; 2004 [Google Scholar]
- 16. Dionisio KL, Howie S, Fornace KM, Chimah O, Adegbola RA, Ezzati M. Measuring the exposure of infants and children to indoor air pollution from biomass fuels in The Gambia. Indoor Air 18: 317–327, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Edwards RD, Liu Y, He G, Yin Z, Sinton J, Peabody J, Smith KR. Household CO and PM measured as part of a review of China's National Improved Stove Program. Indoor Air 17: 189–203, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Erupaka K, Bruce EN, Bruce MC. Prediction of extravascular burden of carbon monoxide (CO) in the human heart. Ann Biomed Eng 38: 403–438, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Geft IL, Fishbein MC, Ninomiya K, Hashida J, Chaux E, Yano J, YRit J, Genov T, Shell W, Ganz W. Intermittent brief periods of ischemia have a cumulative effect and may cause myocardial necrosis. Circulation 66: 1150–1153, 1982 [DOI] [PubMed] [Google Scholar]
- 20. Jafar TH, Qadri Z, Chaturvedi N. Coronary artery disease epidemic in Pakistan: more electrocardiographic evidence of ischaemia in women than in men. Heart 94: 408–413, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jafary FH, Jafar TH. Disproportionately high risk of left ventricular hypertrophy in Indo-Asian women: a call for more studies. Echocardiography 25: 812–819, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Joshi P, Islam S, Pais P, Reddy S, Dorairaj P, Kazmi K, Pandey MR, Haque S, Mendis S, Rangarajan S, Yusuf S. Risk factors for early myocardial infarction in South Asians compared with individuals in other countries. JAMA 297: 286–294, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Kay JD, Petersen ES, Vejby-Christensen H. Breathing in man during steady-state exercise on the bicycle at two pedalling frequencies, and during treadmill walking. J Physiol 251: 645–656, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kilabuko JH, Matsuki H, Nakai S. Air quality and acute respiratory illness in biomass fuel using homes in Bagamoyo, Tanzania. Int J Environ Res Public Health 4: 39–44, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lawrence M, Singh J, Lawrence F, Whitehead RG. The energy cost of common daily activities in African women: increased expenditure in pregnancy? Am J Clin Nutr 42: 753–763, 1985 [DOI] [PubMed] [Google Scholar]
- 26. Lewis SF, Taylor WF, Graham RM, Pettinger WA, Schutte JE, Blomqvist CG. Cardiovascular responses to exercise as functions of absolute and relative work load. J Appl Physiol 54: 1314–1323, 1983 [DOI] [PubMed] [Google Scholar]
- 27. Luomanmäki K, Coburn RF. Effects of metabolism and distribution of carbon monoxide on blood and body stores. Am J Physiol 217: 354–363, 1969 [DOI] [PubMed] [Google Scholar]
- 28. McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr 12: 444–454, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Mengersen K, Morawska L, Wang H, Murphy N, Tayphasavanh F, Darasavong K, Holmes NS. Association between indoor air pollution measurements and respiratory health in women and children in Lao PDR. Indoor Air 21: 25–35, 2011 [DOI] [PubMed] [Google Scholar]
- 30. Naeher LP, Brauer M, Lipsett M, Zelikoff JT, Simpson CD, Koenig JQ, Smith KR. Woodsmoke health effects: a review. Inhal Toxicol 19: 67–106, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Naeher LP, Smith KR, Leaderer BP, Neufeld L, Mage DT. Carbon monoxide as a tracer for assessing exposures to particulate matter in wood and gas cookstove households of highland Guatemala. Environ Sci Technol 35: 575–581, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Rinne ST, Rodas EJ, Rinne ML, Simpson JM, Glickman LT. Use of biomass fuel is associated with infant mortality and child health in trend analysis. Am J Trop Med Hyg 76: 585–591, 2007 [PubMed] [Google Scholar]
- 33. Roy SB, Bhatia ML, Mathur VS, Virmani S. Hemodynamic effects of chronic severe anemia. Circulation 28: 346–356, 1963 [DOI] [PubMed] [Google Scholar]
- 34. Siddiqui AR, Lee K, Bennett D, Yang X, Brown KH, Bhutta ZA, Gold EB. Indoor carbon monoxide and PM2.5 concentrations by cooking fuels in Pakistan. Indoor Air 19: 75–82, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Smith KR, McCracken JP, Thompson L, Edwards R, Shields KN, Canuz E, Bruce N. Personal child and mother carbon monoxide exposures and kitchen levels: methods and results from a randomized trial of woodfired chimney cookstoves in Guatemala (RESPIRE). J Expo Sci Environ Epidemiol 20: 406–416, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smith KR, Mehta S, Maeusezahl-Feuz M. Indoor smoke from household solid fuels. In: Comparative Quatification of Health Risks: Global and Regional Burden of Disease Due To Selected Major Risk Factors, edited by Ezzati M, Rodgers AD, Lopez AD, Murray CJL. Geneva: World Health Organization, vol 2, 2004, p. 1435–1493 [Google Scholar]
- 37. Smith-Sivertsen T, Díaz E, Pope D, Lie RT, Díaz A, McCracken J, Bakke P, Arana B, Smith KR, Bruce N. Effect of reducing indoor air pollution on women's respiratory symptoms and lung function: RESPIRE Randomized Trial, Guatemala. Am J Epidemiol 170: 211–220, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Snelgrove RJ, Jackson PL, Hardison MT, Noerager BD, Kinloch A, Gaggar A, Shastry S, Rowe SM, Shim YM, Hussell T, Blalock JE. A critical role for LTA4H in limiting chronic pulmonary neutrophilic inflammation. Science 330: 90–94, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sullivan KM, Mei Z, Grummer-Strawn L, Parvanta I. Haemoglobin adjustments to define anaemia. Trop Med Int Health 13: 1267–1271, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Torres-Dosal A, Pérez-Maldonado IN, Jasso-Pineda Y, Martínez Salinas RI, Alegría-Torres JA, Díaz-Barriga F. Indoor air pollution in a Mexican indigenous community: evaluation of risk reduction program using biomarkers of exposure and effect. Sci Total Environ 390: 362–368, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Viau C, Hakizimana G, Bouchard M. Indoor exposure to polycyclic aromatic hydrocarbons and carbon monoxide in traditional houses in Burundi. Int Arch Occup Environ Health 73: 331–338, 2000 [DOI] [PubMed] [Google Scholar]
- 42. WHO Guidelines for Indoor Air Quality: Selected Pollutants. Copenhagen: World Health Organization, p. 55–101, 2010 [PubMed] [Google Scholar]
- 43. Wilson DF, Lee WMF, Makonnen S, Finikova O, Apreleva S, Vinogradov SA. Oxygen pressures in the interstitial space and their relationship to those in the blood plasma in resting skeletal muscle. J Appl Physiol 101: 1648–1656, 2006 [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization. Environmental Health Criteria 213: Carbon Monoxide. 1999. Considering Chronic CO Effects: WHO's CO Exposure Guidelines. (Online). www.who.int/indoorair/interventions/antiguamod21.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.