Abstract
The pathophysiology of acute mountain sickness (AMS) is unknown. One hypothesis is that hypoxia induces biochemical changes that disrupt the blood-brain barrier (BBB) and, subsequently, lead to the development of cerebral edema and the defining symptoms of AMS. This study explores the relationship between AMS and biomarkers thought to protect against or contribute to BBB disruption. Twenty healthy volunteers participated in a series of hypobaric hypoxia trials distinguished by pretreatment with placebo, acetazolamide (250 mg), or dexamethasone (4 mg), administered using a randomized, double-blind, placebo-controlled, crossover design. Each trial included peripheral blood sampling and AMS assessment before (−15 and 0 h) and during (0.5, 4, and 9 h) a 10-h hypoxic exposure (barometric pressure = 425 mmHg). Anti-inflammatory and/or anti-permeability [interleukin (IL)-1 receptor agonist (IL-1RA), heat shock protein (HSP)-70, and adrenomedullin], proinflammatory (IL-6, IL-8, IL-2, IL-1β, and substance P), angiogenic, or chemotactic biomarkers (macrophage inflammatory protein-1β, VEGF, TNF-α, monocyte chemotactic protein-1, and matrix metalloproteinase-9) were assessed. AMS-resistant subjects had higher IL-1RA (4 and 9 h and overall), HSP-70 (0 h and overall), and adrenomedullin (overall) compared with AMS-susceptible subjects. Acetazolamide raised IL-1RA and HSP-70 compared with placebo in AMS-susceptible subjects. Dexamethasone also increased HSP-70 and adrenomedullin in AMS-susceptible subjects. Macrophage inflammatory protein-1β was higher in AMS-susceptible than AMS-resistant subjects after 4 h of hypoxia; dexamethasone minimized this difference. Other biomarkers were unrelated to AMS. Resistance to AMS was accompanied by a marked anti-inflammatory and/or anti-permeability response that may have prevented downstream pathophysiological events leading to AMS. Conversely, AMS susceptibility does not appear to be related to an exaggerated inflammatory response.
Keywords: high altitude, headache, hypoxia, cerebral edema, inflammatory
acute mountain sickness (AMS) is a transient syndrome primarily defined by a headache developing within hours after ascent to high altitude (12). The pathophysiology of AMS remains unclear (21, 39), as do the processes responsible for resistance to the condition. A popular theory, the “tight-fit hypothesis,” suggests that increased brain volume with hypobaric hypoxia elevates intracranial pressure and, when accompanied by impaired or diminished intracranial buffering capacity, contributes to the development of the symptoms that define AMS (50). Brain imaging studies show evidence of cerebral vasogenic edema in moderate to severe AMS and high-altitude cerebral edema; however, its presence and role in mild AMS is less clear (27). One decisive event for the onset of cerebral vasogenic edema is disruption of the blood-brain barrier (BBB). We consider it likely that compromised BBB function may also be involved in the pathophysiology of AMS, and, correspondingly, that protection of BBB integrity contributes to AMS resistance.
The capacity of the BBB to effectively regulate the flux of fluid and substances between the systemic circulation and the parenchyma depends not only on its structural components (e.g., tight junctions), but also on the biochemical behavior of astrocytes surrounding cerebral microvessels and biomarkers present in the systemic circulation (2, 20, 34). Given the potential for peripherally circulating factors to influence cerebral endothelial permeability (1, 18), we sought to determine the relationship between circulating biomarkers thought to influence BBB function and the development of AMS.
Numerous mediators of inflammation and angiogenesis are regulated, in part, by hypoxia (12, 15) and are known to be involved in BBB breakdown and vasogenic edema (4, 18). For these reasons, these processes are attractive pathological candidates for AMS. Proinflammatory mediators can directly weaken the tight junctions between cerebral endothelial cells, decrease the effectiveness of the BBB, and thereby increase the potential for vasogenic edema (reviewed in Ref. 42). Angiogenesis, including that induced by hypoxia, requires the degradation of underlying capillary basement membranes for vessel sprouting, thereby also increasing the potential for capillary leak (40, 41). Despite the strong biological plausibility that such biomarkers contribute to AMS, current literature does not provide conclusive evidence to support or refute this possibility. Notable limitations include the limited range of biomarkers investigated and the absence of serial measurements before and during AMS onset. Likewise, it is unclear whether individuals who remain healthy at high altitude have a unique, anti-inflammatory and/or anti-permeability response to hypobaric hypoxia relative to those who develop AMS.
This investigation, designed to identify whether circulating biomarkers known to influence BBB function are also related to AMS, has three advantages. First, sampling intervals were selected to capture biomarker differences between individuals who go on to develop AMS (AMS susceptible, “AMS-S”) compared with those who do not (AMS resistant, “AMS-R”) before altitude exposure, and, as AMS symptoms developed, to determine whether such variations were temporally related to AMS onset. This strategy also provided the opportunity to identify preexisting biomarker differences between AMS-S and AMS-R individuals. Second, a wide range of biomarkers was studied, including 1) proinflammatory mediators known to contribute to cerebral edema; 2) angiogenic or chemotactic substances known to participate in increased BBB permeability; and 3) biomarkers assumed to be protective against vascular permeability or inflammation. Finally, the combination of serial hypobaric hypoxia exposures and pretreatment with placebo or one of two highly effective drugs for AMS prevention [i.e., acetazolamide or dexamethasone (13, 35)] allowed for intraindividual comparisons to directly determine whether preventing AMS in AMS-S individuals also shifted biomarker levels toward values observed in AMS-R individuals.
The effectiveness of acetazolamide, a carbonic anhydrase enzyme inhibitor, to prevent AMS symptoms has primarily been attributed to its ability to stimulate ventilation and diuresis (reviewed in Ref. 25). Acetazolamide is also used to reduce blood-retina barrier leakage, an effect that has been ascribed to the ability of the drug to increase passive and unidirectional blood-retina barrier permeability (31); a similar mechanism may be responsible for its efficacy in AMS. Additionally, research suggests that acetazolamide has anti-inflammatory properties during inflammatory events and inhibits the release of tumor necrosis factor (TNF)-α and interleukin (IL)-8 from lipopolysaccharide stimulated cells (5, 22, 49). Dexamethasone, a glucocorticoid, is a first line treatment for AMS and high-altitude cerebral edema. Dexamethasone has anti-inflammatory properties, and enhances the effectiveness of tight junctions to maintain BBB integrity (7, 9). However, dexamethasone also increases levels of biomarkers thought to have positive cerebral hemodynamic, neuroprotective and/or diuretic effects (30, 48, 52, 53). It is not clear which of these properties contributes to dexamethasone's effectiveness in treating AMS.
We hypothesized that individuals who remained healthy with hypobaric hypoxia exposure would have lower levels of proinflammatory, angiogenic, or chemotactic mediators and higher levels of anti-permeability or anti-inflammatory biomarkers compared with individuals who developed AMS. By extension, we expected that pretreatment with acetazolamide or dexamethasone would reduce AMS symptoms by raising circulating levels of biomarkers with anti-inflammatory and/or anti-permeability properties in subjects who developed AMS on placebo.
MATERIALS AND METHODS
Overall Study Approach
To evaluate the relationship between AMS and circulating biomarkers known to influence BBB function, subjects participated in a series of three hypobaric hypoxia trials, distinguished by pretreatment with placebo, acetazolamide (250 mg), or dexamethasone (4 mg), administered using a randomized, double-blind, placebo-controlled, crossover design. Drugs were administered in 8-h intervals beginning 24 h before hypobaric hypoxia (−24, −16, −8, and 0 h) with a final dose after ∼5 h of hypobaric hypoxia. Trials were separated by a minimum of 3 wk, and subjects were prohibited from travelling to high altitudes during this timeframe. These requirements reduced the potential for any residual effects of the previous treatment or exposure to affect measurements made during subsequent testing.
Each trial included peripheral blood sampling and AMS symptom assessment under baseline, normoxic conditions in Denver, Colorado [1,650 m; barometric pressure (Pb) = 625 mmHg] and at three points during a 10-h exposure to hypobaric hypoxia (4,875 m; Pb = 425 mmHg) in a hypobaric chamber. Blood samples were collected and AMS status evaluated 15 h (−15 h) and immediately before (0 h) ascent, as well as after 0.5, 4, and 9 h of hypobaric hypoxia. Thirty minutes after ascent (decompression) to 4,875 m, subjects completed four 30-min submaximal exercise bouts on a cycle ergometer at 50% of altitude-adjusted maximum O2 uptake with 15-min rest between each session to increase the likelihood of developing AMS (37). Subjects rested for the remainder of the hypobaric hypoxia exposure.
Subjects
Subjects included for analysis were 20 healthy volunteers (17 men and 3 women) living in the Denver metropolitan area (1,650 m). These 20 subjects were selected from 29 individuals who participated in the placebo trial and at least one of the drug trials and represent those with the highest (top 10) and lowest (bottom 10) AMS scores after 9 h of hypobaric hypoxia. Although this strategy reduced our overall sample size from 29 to 20, this approach is advantageous in that it maximizes differences between AMS-S and AMS-R subjects, avoids the inclusion of individuals with ambiguous AMS status, and ensures the possibility for intraindividual comparisons (i.e., placebo vs. drug pretreatment). Subjects were selected after all trials were completed and before any assays for this report were conducted. Subjects provided written, informed consent, as approved by the Colorado Multi-Institution Review Board (COMIRB) before participation. Subject recruitment, screening, and exclusions are described elsewhere (46).
Protocol
Sampling.
Blood samples were collected after ≥10 min in a supine position from an antecubital vein by venipuncture (−15 h) or an indwelling cannula (0, 0.5, 4, and 9 h). Samples were placed in EDTA-coated or SST Vacutainers, centrifuged, aliquoted, and stored at −80°C until analysis.
Biomarker quantification.
Anti-inflammatory or anti-permeability biomarkers that were assessed included IL-1 receptor agonist (IL-1RA), IL-4, IL-10, heat shock protein (HSP) 70, and adrenomedullin. Proinflammatory biomarkers included IL-6, IL-8, IL-2, IL-1β, and substance P. Angiogenic or chemotactic substances assayed were macrophage inflammatory protein-1β (MIP-1β), vascular endothelial growth factor (VEGF), TNF-α, monocyte chemotactic protein-1 (MCP-1), and serum matrix metalloproteinase-9 (MMP-9). Although the majority of the substances that were measured have more than one function, we elected to categorize them broadly for ease of presentation. Relevant functional overlap is presented in the text as appropriate.
Plasma levels of IL-4, IL-10, IL-6, IL-8, IL-2, IL-1β, MIP-1β, and MCP-1 were measured simultaneously (in a single assay) in duplicate using Bio-Plex (x-Plex) magnetic bead assays (Bio Rad Laboratories) and detected using Luminex multiplex scanning technologies (Luminex 100 system, Luminex). IL-4, IL-2, and IL-1β values fell below detection limits and could not be included for analysis. Intra- and interassay precision for luminex multiplex assays are 10–14% (8) and <10%, respectively.
Total serum MMP-9, plasma TNF-α, VEGF, IL-1RA, and substance P were measured in duplicate by ELISA (R&D Systems), as was total plasma HSP-70 (MesoScale Designs). Adrenomedullin was measured in duplicate by competitive radioimmunoassay (Peninsula Laboratories).
Intra- and interassay precision (% coefficient of variation) for each assay is as follows: MMP-9, 1.9–2.9 and 6.9–7.9; TNF-α, 4.2–5.2 and 4.6–7.4; VEGF, 4.5–6.7 and 6.2–8.8, IL-1RA, 3.7–7.3 and 6.7–11.0; substance P, 3.5–8.4 and 9.3–15.0.
AMS status.
Lake Louise Questionnaires (LLQ) were used to evaluate AMS symptoms. Subjects noted their degree of headache, fatigue, gastrointestinal upset, and dizziness on a four-point scale (i.e., 0 = no symptoms to 3 = severe) (36). AMS status assignment was determined after 9 h of hypobaric hypoxia during the placebo trial. AMS-S was defined by a cumulative LLQ score ≥3 with headache after 9 h of hypobaric hypoxia during the placebo trial, and AMS-R was defined by a LLQ score of ≤2 with headache or ≥3 without headache. Age, height, and weight were similar between AMS-S and AMS-R groups (Table 1). Throughout this report, AMS status designation as AMS-S or AMS-R refers to the subjects' condition after 9 h of hypobaric hypoxia during the placebo trial.
Table 1.
Subject characteristics
| Variable | AMS-S | AMS-R |
|---|---|---|
| Age, yr | 27.8 ± 2.3 | 29.4 ± 2.4 |
| Weight, kg | 73.0 ± 2.6 | 75.4 ± 3.1 |
| Height, cm | 178.4 ± 3.1 | 181.2 ± 2.1 |
| LLQ, 4 h | 3.5 ± 0.5‡ | 1.1 ± 0.5 |
| Placebo | ||
| Acetazolamide | 1.2 ± 0.5 | 0.9 ± 0.4 |
| Dexamethasone | 2.1 ± 0.4* | 0.6 ± 0.4 |
| LLQ, 9 h | 4.2 ± 0.2§ | 0.5 ± 0.2 |
| Placebo | ||
| Acetazolamide | 1.6 ± 0.5 | 0.7 ± 0.4 |
| Dexamethasone | 1.1 ± 0.3† | 0.5 ± 0.2 |
Values are means ± SE. AMS-S and AMS-R, acute mountain sickness susceptible and resistant, respectively; LLQ, Lake Louise Questionnaires. Significant differences between AMS·S and AMS·R are denoted by
P < 0.05,
P < 0.01,
P < 0.001, and trends by
0.05 < P < 0.10.
Data Analyses and Statistics
All 20 subjects completed the placebo trial (10 AMS-S and 10 AMS-R), 16 completed the dexamethasone trial (8 AMS-S and 8 AMS-R), and 12 completed the acetazolamide trial (5 AMS-S and 7 AMS-R). Independent sample t-tests with correction for multiple comparisons (Benjamin-Hochberg correction) were used to determine whether the biomarkers of interest varied between AMS-S vs. AMS-R individuals at single points of measure (e.g., after 4 h of hypobaric hypoxia). One-level linear mixed models were used to determine the effect of time on biomarker levels within each AMS group on a given treatment (e.g., did HSP remain constant over time in AMS-S subjects on placebo?). Three-level linear mixed models were used to assess the relationship between biomarker levels, AMS status (AMS-S and AMS-R), drug (placebo, acetazolamide, or dexamethasone), and/or hypobaric hypoxia exposure (time). Main effects were considered for discussion if there was strong evidence in the literature for biological and/or pharmacological effects relevant to AMS. Two preexposure measurements (−15 and 0 h) were made to ensure that differences attributed to the effect of hypobaric hypoxia were not due to circadian effects. For example, if differences were identified between 0 and 9 h, but not between −15 and 9 h (both of which were at ∼4 PM), it would be assumed that the time of day rather than the hypoxic exposure itself was responsible. For this reason, all five points were analyzed for the placebo arm. The only exception was adrenomedullin, which was not measured at 0 h, since it does not appear to be influenced by circadian effects (51). In the acetazolamide and dexamethasone arms, −15, 4, and 9 h samples were included for study, given that no marked differences between −15 and 0 h were identified. Statistical analyses were conducted using SPSS 17.0 (Chicago, IL). Data are expressed as means ± SE, or as the estimated marginal means ± SE, in Tables 2 and 3, as well as in Figs. 1–3. Criteria for significance was set a priori at two-tailed P < 0.05; trends were considered at P < 0.10.
Table 2.
Effect of time at altitude and AMS on biomarkers during placebo
| Time at Altitude |
||||||||
|---|---|---|---|---|---|---|---|---|
| Preascent (1,609 m) |
Altitude (4,875 m) |
|||||||
| AMS Status | −15 h | 0 h | 0.5 h | 4 h | 9 h | Time | AMS | |
| Proinflammatory biomarkers | ||||||||
| IL-6, pg/ml | AMS·S | 16.9 ± 13.2 | 18.1 ± 17.1 | 20.0 ± 13.2 | 59.6 ± 11.2† | 33.0 ± 10.5 | † | NS |
| AMS·R | 17.6 ± 6.9 | 19.2 ± 5.4 | 16.1 ± 6.9 | 23.9 ± 4.2 | 16.3 ± 4.2 | NS | ||
| IL-8, pg/ml | AMS·S | 43.4 ± 6.1 | 33.7 ± 5.8 | 38.1 ± 6.1 | 49.0 ± 6.5 | 36.4 ± 6.1 | NS | NS |
| AMS·R | 39.3 ± 5.6 | 37.1 ± 5.6 | 30.7 ± 5.6 | 33.5 ± 5.6 | 33.7 ± 5.6 | NS | ||
| Angiogenic and chemotactic biomarkers | ||||||||
| VEGF, pg/ml | AMS·S | 90.8 ± 34.5 | 102.1 ± 34.5 | 75.1 ± 34.5 | 77.5 ± 32.5 | 88.3 ± 32.5 | NS | NS |
| AMS·R | 73.5 ± 52.8 | 111.7 ± 52.8 | 128.8 ± 52.8 | 60.0 ± 52.8 | 62.4 ± 49.8 | NS | ||
| TNF-α, pg/ml | AMS·S | 12.8 ± 3.5 | 14.3 ± 3.9 | 15.4 ± 3.9 | 15.1 ± 3.9 | 18.9 ± 3.7 | NS | NS |
| AMS·R | 15.2 ± 3.7 | 14.6 ± 3.7 | 16.2 ± 3.7 | 16.2 ± 3.7 | 19.6 ± 3.7 | NS | ||
| MCP-1, pg/ml | AMS·S | 185.4 ± 24.7 | 222.7 ± 24.7 | 191.9 ± 24.7 | 263.1 ± 27.6 | 169.4 ± 24.7 | NS | NS |
| AMS·R | 154.3 ± 19.2 | 197.4 ± 18.2 | 180.1 ± 20.4 | 195.4 ± 19.2 | 205.7 ± 18.2 | NS | ||
| MIP-1β, pg/ml | AMS·S | 487.9 ± 65.5 | 500.3 ± 65.5 | 455.1 ± 65.5 | 700.8 ± 73.2* | 457.9 ± 65.5 | NS | NS† |
| AMS·R | 418.9 ± 53.7 | 514.9 ± 53.7 | 479.3 ± 53.7 | 436.4 ± 56.6 | 396.3 ± 53.7 | NS | ||
| MMP-9, ng/ml | AMS·S | 332.2 ± 57.0 | 321.3 ± 57.0 | 197.5 ± 57.0 | 331.0 ± 60.0 | 502.2 ± 57.0 | * | NS |
| AMS·R | 299.9 ± 59.0 | 283.0 ± 52.0 | 199.2 ± 55.0 | 342.5 ± 55.2 | 430.1 ± 49.3 | * | ||
| Anti-inflammatory or anti-permeability biomarkers | ||||||||
| IL-1RA, pg/ml | AMS·S | 110.0 ± 10.9 | 101.8 ± 12.4 | 89.7 ± 12.4† | 134.6 ± 11.6* | 135.2 ± 10.9* | * | § |
| AMS·R | 127.7 ± 25.2 | 157.6 ± 25.2 | 147.0 ± 25.2 | 194.4 ± 25.2 | 195.4 ± 25.2 | NS | ||
| HSP-70, ng/ml | AMS·S | 56.9 ± 5.8 | 39.8 ± 6.1* | 36.6 ± 6.1‡ | 48.8 ± 6.1 | 40.9 ± 5.8 | † | * |
| AMS·R | 65.9 ± 8.8 | 77.6 ± 8.8 | 50.3 ± 9.4 | 46.8 ± 8.8 | 36.8 ± 8.8 | * | ||
| Adrenomedullin, ng/ml | AMS·S | 0.22 ± 0.03 | 0.24 ± 0.03 | 0.26 ± 0.03† | 0.27 ± 0.03 | § | * | |
| AMS·R | 0.25 ± 0.04 | 0.26 ± 0.04 | 0.39 ± 0.04 | 0.32 ± 0.03 | § | |||
| IL-10, pg/ml | AMS·S | 52.5 ± 25.1 | 52.4 ± 27.1 | 54.9 ± 29.7 | 61.8 ± 27.1 | 54.3 ± 29.7 | NS | § |
| AMS·R | 11.3 ± 5.0 | 16.8 ± 4.2 | 16.7 ± 5.0 | 10.7 ± 6.4 | 18.5 ± 6.4 | NS | ||
Values are estimated marginal means ± SE. IL, interleukin; VEGF, vascular endothelial growth factor; TNF-α, tumor necrosis factor-α; MCP-1, monocyte chemotactic protein-1; MIP-1β, macrophage inflammatory protein-1β; MMP-9, metalloproteinase-9; IL-1RA, IL-1 receptor agonist; HSP-70, heat shock protein-70; NS, nonsignificant. Significant differences between AMS-S and AMS-R at individual points or across time are denoted by
P = 0.05,
P < 0.05,
P < 0.01, and trends by
0.05 < P < 0.10.
Table 3.
The effect of dexamethasone and acetazolamide on biomarkers
| Time at Altitude |
||||||
|---|---|---|---|---|---|---|
| Preascent (1,609 m) |
Altitude (4,875 m) |
|||||
| Variable | Drug | AMS Status | −15 h | 4 h | 9 h | Time |
| Anti-inflammatory or anti-permeability factors | ||||||
| IL-10, pg/ml | Dexamethasone | AMS·S | 67.5 ± 22.9 | 81.5 ± 27.1 | 59.8 ± 24.8 | NS |
| AMS·R | 51.5 ± 20.2 | 20.3 ± 22.6 | 13.0 ± 26.1 | NS | ||
| Acetazolamide | AMS·S | 123.6 ± 64.3 | 104.6 ± 52.5 | 69.5 ± 45.5 | NS | |
| AMS·R | 13.4 ± 4.0 | 18.8 ± 4.6 | 12.0 ± 4.6 | NS | ||
| Proinflammatory biomarkers | ||||||
| IL-6, pg/ml | Dexamethasone | AMS·S | 21.3 ± 9.5 | 26.7 ± 7.4 | 25.1 ± 8.2 | NS |
| AMS·R | 11.1 ± 6.0 | 22.3 ± 4.3 | 15.9 ± 5.2 | NS | ||
| Acetazolamide | AMS·S | 30.6 ± 31.0 | 51.0 ± 17.9 | 34.9 ± 15.5 | NS | |
| AMS·R | 21.7 ± 10.5 | 36.3 ± 7.9 | 44.5 ± 8.5* | NS | ||
| IL-8, pg/ml | Dexamethasone | AMS·S | 41.0 ± 9.1 | 51.8 ± 9.7 | 46.7 ± 9.7 | NS |
| AMS·R | 33.1 ± 5.9 | 41.0 ± 5.9 | 33.6 ± 6.3 | NS | ||
| Acetazolamide | AMS·S | 34.3 ± 14.5 | 48.3 ± 14.5 | 48.9 ± 14.5 | NS | |
| AMS·R | 35.4 ± 8.3 | 45.8 ± 8.3 | 38.6 ± 8.3 | NS | ||
| Angiogenic and chemotactic factors | ||||||
| VEGF, pg/ml | Dexamethasone | AMS·S | 153.6 ± 51.4 | 163.6 ± 51.4 | 112.3 ± 44.5 | NS |
| AMS·R | 33.7 ± 11.3 | 47.1 ± 12.4 | 38.6 ± 11.3 | NS | ||
| Acetazolamide | AMS·S | 89.5 ± 32.6 | 113.5 ± 32.6 | 106.4 ± 31.4 | NS | |
| AMS·R | 69.9 ± 17.0 | 53.8 ± 17.0 | 54.6 ± 17.0 | NS | ||
| TNF-α, pg/ml | Dexamethasone | AMS·S | 4.1 ± 1.2* | 5.0 ± 1.2* | 6.2 ± 1.2* | NS |
| AMS·R | 6.2 ± 0.7* | 5.3 ± 0.7* | 4.1 ± 0.9* | NS | ||
| Acetazolamide | AMS·S | 4.4 ± 0.5* | 5.5 ± 7.4 | 4.2 ± 0.5* | NS | |
| AMS·R | 5.4 ± 1.1* | 4.7 ± 1.2* | 5.4 ± 1.1* | NS | ||
| MCP-1, pg/ml | Dexamethasone | AMS·S | 107.7 ± 13.6* | 92.1 ± 14.5* | 83.8 ± 13.6* | NS |
| AMS·R | 113.5 ± 15.6* | 106.7 ± 15.6* | 114.8 ± 15.6* | NS | ||
| Acetazolamide | AMS·S | 139.1 ± 36.3 | 166.1 ± 41.9 | 217.8 ± 36.3 | NS | |
| AMS·R | 189.2 ± 32.9 | 197.5 ± 32.9 | 152.7 ± 32.9 | NS | ||
| MIP-1, pg/ml | Dexamethasone | AMS·S | 448.3 ± 50.9 | 436.4 ± 54.4* | 381.9 ± 50.9 | NS |
| AMS·R | 376.0 ± 64.4 | 340.5 ± 60.2 | 373.3 ± 60.2 | NS | ||
| Acetazolamide | AMS·S | 448.3 ± 50.9 | 436.4 ± 54.4 | 381.9 ± 50.9 | NS | |
| AMS·R | 412.2 ± 63.3 | 418.1 ± 63.3 | 407.2 ± 63.3 | NS | ||
| MMP-9, ng/ml | Dexamethasone | AMS·S | 522.9 ± 86.8* | 752.1 ± 86.8 | 760.5 ± 86.8* | NS |
| AMS·R | 434.8 ± 105.9 | 554.1 ± 91.7 | 755.0 ± 92.0* | † | ||
| Acetazolamide | AMS·S | 392.3 ± 102.3 | 483.9 ± 114.4 | 654.6 ± 102.3 | NS | |
| AMS·R | 284.2 ± 89.5 | 271.7 ± 89.5 | 606.8 ± 89.5 | * | ||
Values are estimated marginal means ± SE. Significant difference or trends between placebo and drug at each sampling interval are denoted by
P < 0.05,
0.05 < P < 0.10.
Fig. 1.
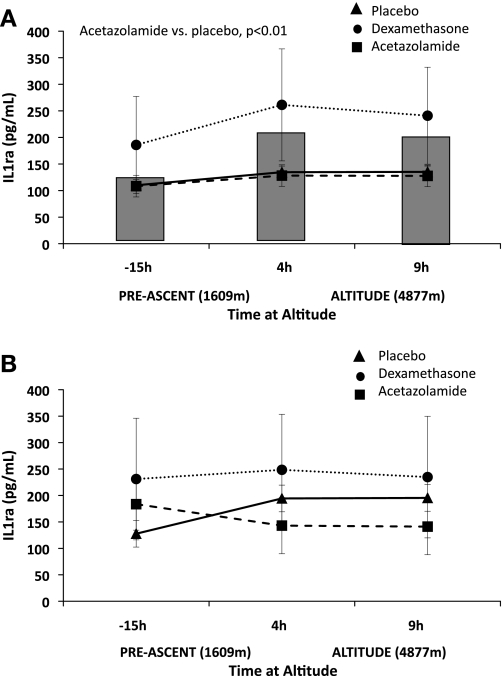
Comparison of acetazolamide and dexamethasone pretreatment on interleukin-1 receptor agonist (IL-1RA). Acetazolamide pretreatment increased IL-1RA compared with placebo in acute mountain sickness-susceptible subjects (AMS-S; P < 0.01; A), such that values were equivalent to the acute mountain sickness-resistant (AMS-R) group during hypoxia (P = nonsignificant; B). Dexamethasone had no effect in AMS-S. Neither drug influenced IL-1RA in AMS-R, with the exception of a tendency for dexamethasone to decrease IL-1RA relative to placebo at 4 and 9 h. The shaded bars behind the AMS-S figure (A) represent placebo IL-1RA values for AMS-R subjects to better visualize placebo comparisons between AMS-S and AMS-R subjects. Placebo, solid triangle with solid line; acetazolamide, solid circle, dotted line; dexamethasone, solid square, dashed line. Significant comparisons between placebo and drug pretreatment are designated in the figure by placebo vs. acetazolamide, or placebo vs. dexamethasone. Values are estimated marginal means ± SE.
Fig. 2.
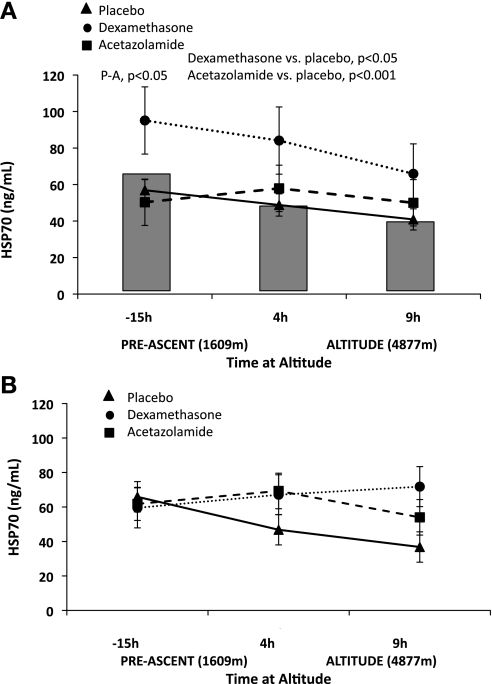
Comparison of acetazolamide and dexamethasone pretreatment on heat shock protein (HSP)-70. A: acetazolamide (P < 0.001) and dexamethasone (P < 0.05) pretreatment increased HSP-70 in AMS-S compared with placebo. Acetazolamide increased HSP at −15 h and tended to do so at both 4 and 9 h in AMS-S. The shaded bars behind the figure represent placebo HSP-70 values for AMS-R subjects to better visualize placebo comparisons between AMS-S than AMS-R. B: dexamethasone and acetazolamide tended to increase HSP-70 at 9 h in AMS-R; no other significant drug effects were noted. Symbols are as defined in Fig. 1 legend. Significant comparisons between placebo and drug pretreatment are designated in the figure by placebo vs. acetazolamide (P-A), or placebo vs. dexamethasone. Values are estimated marginal means ± SE.
Fig. 3.
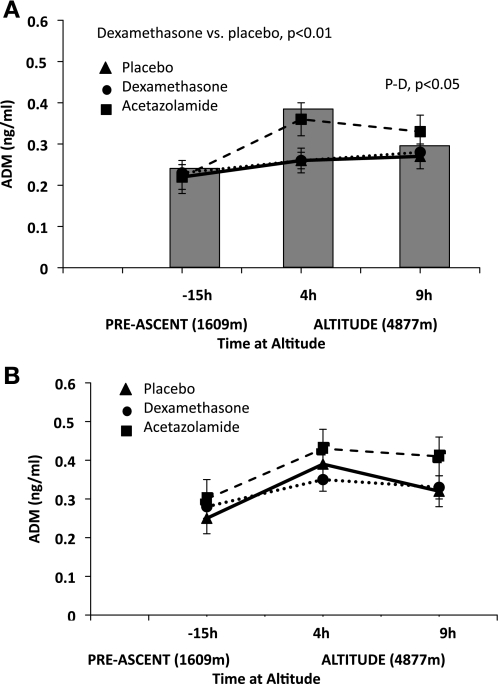
Comparison of acetazolamide and dexamethasone pretreatment on adrenomedullin (ADM). A: pretreatment with dexamethasone increased ADM values in AMS-S subjects during hypoxia (4 h, P < 0.10; and 9 h P < 0.05). The shaded bars behind the figure represent placebo ADM values for AMS-R subjects to better visualize placebo comparisons between AMS-S and AMS-R. B: dexamethasone tended to increase ADM at baseline in AMS-R subjects at baseline only. Symbols are as defined in Fig. 1 legend. Significant comparisons between placebo and drug pretreatment are designated in the figure by placebo vs. acetazolamide, or placebo vs. dexamethasone (P-D). Values are estimated marginal means ± SE.
RESULTS
Effectiveness of Drug Pretreatment to Lower LLQ Score
LLQ scores were higher in AMS-S than AMS-R at 4 and 9 h of hypobaric hypoxia while on placebo (Table 1). Pretreatment with dexamethasone or acetazolamide was effective for reducing AMS symptoms in AMS-S such that 9-h AMS scores on either drug were at least 62% lower than on placebo (acetazolamide, 62%; dexamethasone, 74%; both P < 0.001; Table 1). Neither drug influenced AMS scores in AMS-R.
Effect of Time Under Hypoxic Conditions, AMS Status, and Drug Pretreatment on Biomarkers
Proinflammatory biomarkers.
Exposure to hypobaric hypoxia had no marked effect on circulating levels of the proinflammatory biomarkers included for study in AMS-S or AMS-R (Table 2). Notably, levels of proinflammatory biomarkers were also independent of AMS status and were unaffected by pretreatment with acetazolamide or dexamethasone (Tables 2 and 3). The only exception was IL-6, which was greater after 9 h of hypobaric hypoxia in AMS-R on acetazolamide compared with placebo (Table 3). IL-2 and IL-1β fell below detection limits in both groups at all time points and were not included for analysis.
Angiogenic and chemotactic biomarkers.
Of the angiogenic or chemotactic substances included for study, only MMP-9 was affected by hypobaric hypoxia, rising similarly with hypobaric hypoxia exposure in both AMS-S and AMS-R (Table 2). Comparisons between AMS-S and AMS-R revealed that, after 4 h of hypobaric hypoxia, AMS-S had higher MIP-1β values than AMS-R, but this difference did not persist with continued hypobaric hypoxia exposure (Table 2). MIP-1β was the only angiogenic or chemotactic substance related to AMS status, albeit only at one point of measure (4 h). Notably, however, this short-lived MIP-1β difference between AMS-S and AMS-R was abolished with dexamethasone pretreatment (Table 3; 4 h, P < 0.05).
Although TNF-α and MCP-1 levels were independent of hypobaric hypoxia exposure and AMS status, drug pretreatment reduced TNF-α and MCP-1 compared with placebo. Specifically, both drugs lowered TNF-α in AMS-S and AMS-R at all points of measure (−15, 4, and 9 h; all P < 0.05), with the exception of the 4-h measurement in AMS-S, at which time levels were unchanged. Dexamethasone pretreatment also reduced MCP-1 values after −15, 4, and 9 h of hypobaric hypoxia compared with placebo in both AMS-S and AMS-R (Table 3; P < 0.05). However, given that TNF-α and MCP-1 were unrelated to AMS, it is unlikely that these effects of acetazolamide and dexamethasone are important to their role in AMS prevention.
Anti-inflammatory and anti-permeability biomarkers.
IL-1RA rose 18.6 and 34.6% from baseline to 9 h of hypobaric hypoxia in AMS-S and AMS-R, respectively. This effect was statistically significant only in AMS-S due to substantial variation of values in AMS-R (Table 2). IL-1RA levels were similar between AMS-S and AMS-R before hypobaric hypoxia exposure, but higher in AMS-R than AMS-S after 4 or 9 h of hypobaric hypoxia (Table 2C; both P < 0.05) and overall (P < 0.01). Pretreatment with acetazolamide reduced LLQ scores and increased IL-1RA in AMS-S (P < 0.01), such that values were equivalent to AMS-R also pretreated with acetazolamide (Fig. 1). Dexamethasone had no effect on IL-1RA levels in either AMS-R or AMS-S.
HSP-70 levels declined with exposure to hypobaric hypoxia in AMS-R and tended to do so in AMS-S as well. HSP-70 was higher in AMS-R compared with AMS-S overall (P < 0.05), as well as both before hypoxic exposure (0 h, P < 0.05; Table 2). Notably, acetazolamide increased HSP-70 before hypoxic exposure in AMS-S compared with placebo (−15 h, P < 0.05; Fig. 2) and tended to have a similar effect at 4 and 9 h. Dexamethasone also increased HSP-70 in AMS-S compared with placebo (Fig. 2; P < 0.05); however, the effect was much less pronounced compared with that of acetazolamide.
Circulating adrenomedullin increased with time under hypoxic conditions in AMS-S and AMS-R on placebo (Table 2; both P < 0.01); however, absolute levels were greater in AMS-R than AMS-S (Table 2; P < 0.05). Acetazolamide had no effect on adrenomedullin in AMS-R or AMS-S. Dexamethasone increased adrenomedullin in AMS-S during hypoxia (Fig. 3; 9 h, P < 0.05).
Hypobaric hypoxia did not affect IL-10 levels in either AMS group (Table 2). IL-10 was higher in AMS-S overall. Given that neither drug influenced IL-10 it is unlikely that this anti-inflammatory contributes to AMS.
DISCUSSION
Taken together, our findings provide the first evidence to suggest that resistance to AMS may be driven, in part, by the ability to mount an adequate “defensive” anti-inflammatory or anti-permeability response during acute exposure to hypobaric hypoxia. This relationship is supported by our observation that peripherally circulating IL-1RA, HSP-70, and adrenomedullin are elevated in untreated individuals who do not develop AMS compared with those who do. It is notoriously difficult to attribute altered levels of a single biomarker to the development of a complex disorder such as AMS, given that the regulation of inflammation and vascular permeability involves numerous mediators, each with multiple biological effects. For this reason, one of the greatest strengths of this study was that we were able to pharmacologically prevent AMS in AMS-S individuals, allowing us to directly observe whether preventing AMS also abolished changes in biomarkers that appeared to be associated with the condition. Using this strategy, we consider our observation that pretreatment with acetazolamide or dexamethasone simultaneously reduced AMS symptoms, and narrowed IL-1RA, HSP-70, and adrenomedullin differences between AMS-R and AMS-S subjects strengthens the possibility that these biomarkers are directly involved in AMS resistance.
This study tested the novel idea that proteins thought to promote inflammation and endothelial permeability would be reduced, and those with anti-inflammatory or anti-permeability characteristics would be higher in individuals who remained healthy throughout a 10-h exposure to hypobaric hypoxia compared with those who developed AMS. Our hypothesis was based on the hallmark effect of acute inflammation to increase endothelial barrier dysfunction (reviewed in Refs. 28, 44) and the popular theory that compromised BBB integrity contributes to increased brain volume and AMS. The underlying logic of our approach relies on the fact that biomarkers in the peripheral circulation can influence endothelial permeability in cerebral as well as peripheral vessels, although higher concentrations of such biomarkers are generally required to have such effects in the cerebral compared with the systemic circulation (1, 18). For example, proteins such as adrenomedullin can “tighten” the BBB and effectively minimize abnormal movement of fluid and substances from the circulation into the brain parenchyma (2). It is important to acknowledge that, although variable levels of peripherally circulating proteins are certainly not a direct measure of BBB function, they do reflect the microenvironment to which the BBB is exposed and, by extension, the microenvironment that directly affects BBB function (2, 20, 34).
One of our most intriguing observations was that IL-1RA, a highly selective competitive antagonist of the potent inflammatory cytokine IL-1, was similar between AMS-S and AMS-R before exposure, but greater in AMS-R than AMS-S after 4 or 9 h of hypobaric hypoxia and overall. The identification of such differences after only 4 h of hypobaric hypoxia (before AMS onset) suggests that IL-1RA may serve to prevent the development of AMS via its anti-inflammatory actions. Previous reports indicate that hypoxia increases (15) or has no effect (24) on IL-1RA levels in humans, but no study, to our knowledge, found a correlation between IL-1RA and AMS. In further support of a protective role for IL-1RA against AMS, pretreatment with acetazolamide increased circulating IL-1RA in AMS-S individuals (P < 0.01), such that values were equivalent to AMS-R subjects also pretreated with acetazolamide (Fig. 1). These observations are in agreement with the hypothesis that IL-1RA contributes to protection against AMS, a suggestion that is supported by reports that IL-1RA minimizes edema and neuronal injury under experimental ischemic, traumatic, or hemorrhagic insult (32, 38).
HSP-70 is understood to protect against cellular stress induced by inflammation and hypoxia (10, 54). In this study, HSP-70 levels were higher in AMS-R compared with AMS-S individuals before hypoxic exposure. In light of a recent report that induction of HSP-70 before hypoxic exposure protects against hypoxia-related brain injury in rats (55), we consider that our findings may indicate preexisting hypoxia tolerance in AMS-R subjects. Suggesting a possible mechanism for acetazolmide's effectiveness to prevent AMS, pretreatment with the drug increased HSP-70 before hypoxic exposure in AMS-S subjects compared with placebo (Fig. 2) and tended to have a similar effect at 4 and 9 h. Dexamethasone also increased HSP-70 in AMS-S compared with placebo (Fig. 2; overall P < 0.05); however, the effect appeared less pronounced compared with that of acetazolamide. It is notable that HSP-70 appeared to drop from −15 to 0 h in AMS-S, but not AMS-R, subjects. Since HSP-70 is subject to diurnal variation, it may be that AMS-S individuals have a wider daily HSP-70 variation than AMS-R subjects. To our knowledge, the relationship between acetazolamide and HSPs has not been described previously; however, dexamethasone has been reported to raise HSP levels (reviewed in Ref. 11).
Adrenomedullin is induced, in part, by hypoxia (6, 16, 33) and is thought to have positive cerebral hemodynamic and/or neuroprotective effects during cerebral events, including vascular headache (30, 48, 52, 53). Moreover, in vitro studies indicate that adrenomedullin has potent anti-inflammatory properties and improves BBB endothelial function (17, 23, 26). In this study, we found circulating adrenomedullin levels to be greater in untreated AMS-R compared with AMS-S subjects overall (Table 2). In combination with the observation that dexamethasone pretreatment raised adrenomedullin levels in AMS-S subjects during hypoxia, our findings suggest that adrenomedullin may provide some level of protection against the development of AMS. In addition to its purported role as a protective factor against BBB permeability, adrenomedullin also has potent diuretic properties. High-altitude diuresis, considered to be beneficial for AMS, has been partially attributed to the effect of hypoxia to increase adrenomedullin as measured by urinary excretion (14). Whether a similar relationship exists between serum adrenomedullin levels and diuresis is not clear. Together, the hemodynamic, neuroprotective, anti-inflammatory, and diuretic properties of adrenomedullin may contribute to AMS resistance. Notably, our and others' findings indicate that dexamethasone, a reliable treatment and prophylaxis for AMS, upregulates adrenomedullin (19, 29), presenting another possible mechanism by which dexamethasone may prevent AMS.
Chemokines and chemoattractants, such as MCP-1 and MIP-1β, are thought to increase endothelial permeability during inflammation by altering tight junction composition and morphology and to drive leukocytes into the brain parenchyma (43). Of the angiogenic or chemotactic substances included for study in this report, only MIP-1β was related to AMS, and even then only at a single point of measure (4 h, Table 2). However, coupled with the reduced MIP-1β levels with dexamethasone pretreatment, our finding renders this an interesting biomarker for further investigation.
Our findings also suggest that acetazolamide and dexamethasone likely act via different mechanisms to prevent AMS. In this study, both drugs were effective in reducing AMS symptoms, but had different effects on biomarkers that were associated with AMS. Both treatments raised HSP-70 in AMS-S subjects. Acetazolamide also increased IL-1RA, but not adrenomedullin, whereas the opposite was true for dexamethasone. If BBB integrity is of key importance for resistance to AMS, our findings suggest that acetazolamide and dexamethasone operate via unique pathways to achieve the same result. Our laboratory's recent report that the two drugs have distinct cerebral hemodynamic effects under hypoxic conditions (45) supports the proposition that the physiological effects of acetazolamide and dexamethasone in the context of high-altitude hypoxia are distinct, despite having similar clinical results (i.e., prevention of AMS).
In summary, our findings support the possibility that resistance to AMS may involve the ability to mount an adequate “defensive” anti-inflammatory or anti-permeability response during hypoxic exposure. We consider it likely that such effects may have prevented downstream pathophysiological events leading to AMS. Conversely, AMS susceptibility does not appear to be related to an exaggerated inflammatory response. Similar to previous reports (3, 15, 47), there was little evidence that the proinflammatory, angiogenic, or chemotactic biomarkers included for study were associated with the development of AMS. This investigation provides rationale for further research to explore factors that determine and regulate circulating levels of IL-1RA, HSP-70, and adrenomedullin, and how such effects directly influence BBB integrity and the likelihood of developing AMS under hypoxic conditions. It is also of clinical interest to assess whether such agents participate in the resolution of other cerebral conditions, such as stroke, that are marked by vasogenic edema, inflammation, and tissue hypoxia.
GRANTS
Funding was provided by a National Heart, Lung, and Blood Institute Grant (HL-070362), the Maren Foundation, and the Altitude Research Center, University of Colorado Denver.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank the research subjects for participation, and Alison Anderson, Jason Chapman, Maggie Crawford, Ruth Johnson, Barbara Lommen, Nick Robbins, and Janet Uhde for technical and administrative expertise. In addition, we thank Dr. Peter H. Hackett for thoughtful review of this manuscript.
REFERENCES
- 1. Abbott NJ. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell Mol Neurobiol 20: 131–147, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7: 41–53, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Bailey DM, Kleger GR, Holzgraefe M, Ballmer PE, Bartsch P. Pathophysiological significance of peroxidative stress, neuronal damage, and membrane permeability in acute mountain sickness. J Appl Physiol 96: 1459–1463, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Basnyat B, Murdoch DR. High-altitude illness. Lancet 361: 1967–1974, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Coakley RJ, Taggart C, Greene C, McElvaney NG, O'Neill SJ. Ambient pCO2 modulates intracellular pH, intracellular oxidant generation, and interleukin-8 secretion in human neutrophils. J Leukoc Biol 71: 603–610, 2002 [PubMed] [Google Scholar]
- 6. Cormier-Regard S, Nguyen SV, Claycomb WC. Adrenomedullin gene expression is developmentally regulated and induced by hypoxia in rat ventricular cardiac myocytes. J Biol Chem 273: 17787–17792, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Cucullo L, Hallene K, Dini G, Dal Toso R, Janigro D. Glycerophosphoinositol and dexamethasone improve transendothelial electrical resistance in an in vitro study of the blood-brain barrier. Brain Res 997: 147–151, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Dossus L, Becker S, Achaintre D, Kaaks R, Rinaldi S. Validity of multiplex-based assays for cytokine measurements in serum and plasma from “non-diseased” subjects: comparison with ELISA. J Immunol Methods 350: 125–132, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Fischer S, Renz D, Schaper W, Karliczek GF. In vitro effects of dexamethasone on hypoxia-induced hyperpermeability and expression of vascular endothelial growth factor. Eur J Pharmacol 411: 231–243, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Giffard RG, Yenari MA. Many mechanisms for hsp70 protection from cerebral ischemia. J Neurosurg Anesthesiol 16: 53–61, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Guerrero JA, Vicente V, Corral J. Dexamethasone induction of a heat stress response. Methods Enzymol 490: 121–135 [DOI] [PubMed] [Google Scholar]
- 12. Hackett P, Roach RC. High-altitude illness. N Engl J Med 345: 107–114, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Hackett PH, Roach RC, Wood RA, Foutch RG, Meehan RT, Rennie D, Mills WJJ. Dexamethasone for prevention and treatment of acute mountain sickness. Aviat Space Environ Med 59: 950–954, 1988 [PubMed] [Google Scholar]
- 14. Haditsch B, Roessler A, Hinghofer-Szalkay HG. Renal adrenomedullin and high altitude diuresis. Physiol Res 56: 779–787, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Hartmann G, Tschop M, Fischer R, Bidlingmaier C, Riepl R, Tschop K, Hautmann H, Endres S, Toepfer M. High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine 12: 246–252, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Hofbauer KH, Jensen BL, Kurtz A, Sandner P. Tissue hypoxygenation activates the adrenomedullin system in vivo. Am J Physiol Regul Integr Comp Physiol 278: R513–R519, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Honda M, Nakagawa S, Hayashi K, Kitagawa N, Tsutsumi K, Nagata I, Niwa M. Adrenomedullin improves the blood-brain barrier function through the expression of claudin-5. Cell Mol Neurobiol 26: 109–118, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci 24: 719–725, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Imai T, Hirata Y, Iwashina M, Marumo F. Hormonal regulation of rat adrenomedullin gene in vasculature. Endocrinology 136: 1544–1548, 1995 [DOI] [PubMed] [Google Scholar]
- 20. Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature 325: 253–257, 1987 [DOI] [PubMed] [Google Scholar]
- 21. Kallenberg K, Bailey DM, Christ S, Mohr A, Roukens R, Menold E, Steiner T, Bartsch P, Knauth M. Magnetic resonance imaging evidence of cytotoxic cerebral edema in acute mountain sickness. J Cereb Blood Flow Metab 27: 1064–1071, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Kawaai H, Seino H, Yamazaki S, Taki K. Changes in leukocyte migration during carbonic anhydrase activity inhibition. Burns 35: 397–404, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Kis B, Snipes JA, Deli MA, Abraham CS, Yamashita H, Ueta Y, Busija DW. Chronic adrenomedullin treatment improves blood-brain barrier function but has no effects on expression of tight junction proteins. Acta Neurochir Suppl (Wien) 86: 565–568, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Klausen T, Olsen NV, Poulsen TD, Richalet JP, Pedersen BK. Hypoxemia increases serum interleukin-6 in humans. Eur J Appl Physiol Occup Physiol 76: 480–482, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Leaf DE, Goldfarb DS. Mechanisms of action of acetazolamide in the prophylaxis and treatment of acute mountain sickness. J Appl Physiol 102: 1313–1322, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Macmanus CF, Campbell EL, Keely S, Burgess A, Kominsky DJ, Colgan SP. Anti-inflammatory actions of adrenomedullin through fine tuning of HIF stabilization. FASEB J 25: 1856–1864, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matsuzawa Y, Kobayashi T, Fujimoto K, Shinozaki S, Yoshikawa S. Cerebral edema in acute mountain sickness. In: High Altitude Medicine, edited by Ueda G, Reeves JT, Sekiguchi M. Matsumoto, Japan: Shinshu University Press, 1992, p. 300–304 [Google Scholar]
- 28. Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 86: 279–367, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Minamino N, Shoji H, Sugo S, Kangawa K, Matsuo H. Adrenocortical steroids, thyroid hormones and retinoic acid augment the production of adrenomedullin in vascular smooth muscle cells. Biochem Biophys Res Commun 211: 686–693, 1995 [DOI] [PubMed] [Google Scholar]
- 30. Miyashita K, Itoh H, Arai H, Suganami T, Sawada N, Fukunaga Y, Sone M, Yamahara K, Yurugi-Kobayashi T, Park K, Oyamada N, Taura D, Tsujimoto H, Chao TH, Tamura N, Mukoyama M, Nakao K. The neuroprotective and vasculo-neuro-regenerative roles of adrenomedullin in ischemic brain and its therapeutic potential. Endocrinology 147: 1642–1653, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Moldow B, Sander B, Larsen M, Lund-Andersen H. Effects of acetazolamide on passive and active transport of fluorescein across the normal BRB. Invest Ophthalmol Vis Sci 40: 1770–1775, 1999 [PubMed] [Google Scholar]
- 32. Mulcahy NJ, Ross J, Rothwell NJ, Loddick SA. Delayed administration of interleukin-1 receptor antagonist protects against transient cerebral ischaemia in the rat. Br J Pharmacol 140: 471–476, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nakayama M, Takahashi K, Murakami O, Shirato K, Shibahara S. Induction of adrenomedullin by hypoxia in cultured human coronary artery endothelial cells. Peptides 20: 769–772, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Prat A, Biernacki K, Wosik K, Antel JP. Glial cell influence on the human blood-brain barrier. Glia 36: 145–155, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Reid L, Carter K, Ellsworth A. Acetazolamide or dexamethasone for prevention of acute mountain sickness: a meta-analysis. J Wilderness Med 5: 34–48, 1994 [Google Scholar]
- 36. Roach RCBP, Hackett P, Oelz O. The Lake Louise acute mountain sickness scoring system. In: Hypoxia and Molecular Medicine. Burlington, VT: Queen City Printers, 1993, p. 272–274 [Google Scholar]
- 37. Roach RC, Icenogle M, Hinghofer-Szalkay H, Maes D, Sandoval D, Robergs R, Lium D, Loeppky JA. Exercise exacerbates acute mountain sickness at simulated high altitude. J Appl Physiol 88: 581–585, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Rothwell N. Interleukin-1 and neuronal injury: mechanisms, modification, and therapeutic potential. Brain Behav Immun 17: 152–157, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Schoonman GG, Sandor PS, Nirkko AC, Lange T, Jaermann T, Dydak U, Kremer C, Ferrari MD, Boesiger P, Baumgartner RW. Hypoxia-induced acute mountain sickness is associated with intracellular cerebral edema: a 3 T magnetic resonance imaging study. J Cereb Blood Flow Metab 28: 198–206, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359: 843–845, 1992 [DOI] [PubMed] [Google Scholar]
- 41. Sirovskiy E, Kornienko V, Moshkin A, Amcheslavskiy V, Ingorokva G, Glazman L. VPF and interstitial fluid pressure in brain oedema. Acta Neurochir Suppl (Wien) 51: 411–413, 1990 [DOI] [PubMed] [Google Scholar]
- 42. Stamatovic SM, Dimitrijevic OB, Keep RF, Andjelkovic AV. Inflammation and brain edema: new insights into the role of chemokines and their receptors. Acta Neurochir Suppl (Wien) 96: 444–450, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Stamatovic SM, Shakui P, Keep RF, Moore BB, Kunkel SL, Van Rooijen N, Andjelkovic AV. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J Cereb Blood Flow Metab 25: 593–606, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Stevens T, Garcia JG, Shasby DM, Bhattacharya J, Malik AB. Mechanisms regulating endothelial cell barrier function. Am J Physiol Lung Cell Mol Physiol 279: L419–L422, 2000 [DOI] [PubMed] [Google Scholar]
- 45. Subudhi AW, Dimmen AC, Julian CG, Wilson MJ, Panerai RB, Roach RC. Effects of acetazolamide and dexamethasone on cerebral hemodynamics in hypoxia. J Appl Physiol 110: 1219–1225, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Subudhi AW, Panerai RB, Roach RC. Effects of hypobaric hypoxia on cerebral autoregulation. Stroke 41: 641–646 [DOI] [PubMed] [Google Scholar]
- 47. Swenson ER, MacDonald A, Vatheuer M, Maks C, Treadwell A, Allen R, Schoene RB. Acute mountain sickness is not altered by a high carbohydrate diet nor associated with elevated circulating cytokines. Aviat Space Environ Med 68: 499–503, 1997 [PubMed] [Google Scholar]
- 48. Watanabe K, Takayasu M, Noda A, Hara M, Takagi T, Suzuki Y, Yoshia J. Adrenomedullin reduces ischemic brain injury after transient middle cerebral artery occlusion in rats. Acta Neurochir (Wien) 143: 1157–1161, 2001 [DOI] [PubMed] [Google Scholar]
- 49. West MA, LeMieur TL, Hackam D, Bellingham J, Claire L, Rodriguez JL. Acetazolamide treatment prevents in vitro endotoxin-stimulated tumor necrosis factor release in mouse macrophages. Shock 10: 436–441, 1998 [DOI] [PubMed] [Google Scholar]
- 50. Wilson MH, Milledge J. Direct measurement of intracranial pressure at high altitude and correlation of ventricular size with acute mountain sickness: Brian Cummins' results from the 1985 Kishtwar expedition. Neurosurgery 63: 970–974; discussion 974–975, 2008 [DOI] [PubMed] [Google Scholar]
- 51. Wolk R, Svatikova A, Otto ME, Hoffmann MS, Duenwald CJ, Somers VK. Plasma adrenomedullin and obstructive sleep apnea. Am J Hypertens 17: 74–76, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Xia CF, Yin H, Borlongan CV, Chao J, Chao L. Adrenomedullin gene delivery protects against cerebral ischemic injury by promoting astrocyte migration and survival. Hum Gene Ther 15: 1243–1254, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Xia CF, Yin H, Borlongan CV, Chao J, Chao L. Postischemic infusion of adrenomedullin protects against ischemic stroke by inhibiting apoptosis and promoting angiogenesis. Exp Neurol 197: 521–530, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Yenari MA, Liu J, Zheng Z, Vexler ZS, Lee JE, Giffard RG. Antiapoptotic and anti-inflammatory mechanisms of heat-shock protein protection. Ann N Y Acad Sci 1053: 74–83, 2005 [DOI] [PubMed] [Google Scholar]
- 55. Zhang K, Zhao T, Huang X, Liu ZH, Xiong L, Li MM, Wu LY, Zhao YQ, Zhu LL, Fan M. Preinduction of HSP70 promotes hypoxic tolerance and facilitates acclimatization to acute hypobaric hypoxia in mouse brain. Cell Stress Chaperones 14: 407–415, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]