Abstract
Coronary collateral vessels serve as a natural protective mechanism to provide coronary flow to ischemic myocardium secondary to critical coronary artery stenosis. The innate collateral circulation of the normal human heart is typically minimal and considerable variability occurs in extent of collateralization in coronary artery disease patients. A well-developed collateral circulation has been documented to exert protective effects upon myocardial perfusion, contractile function, infarct size, and electrocardiographic abnormalities. Thus therapeutic augmentation of collateral vessel development and/or functional adaptations in collateral and collateral-dependent arteries to reduce resistance into the ischemic myocardium represent a desirable goal in the management of coronary artery disease. Tremendous evidence has provided documentation for the therapeutic benefits of exercise training programs in patients with coronary artery disease (and collateralization); mechanisms that underlie these benefits are numerous and multifaceted, and currently under investigation in multiple laboratories worldwide. The role of enhanced collateralization as a major beneficial contributor has not been fully resolved. This topical review highlights literature that examines the effects of exercise training on collateralization in the diseased heart, as well as effects of exercise training on vascular endothelial and smooth muscle control of regional coronary tone in the collateralized heart. Future directions for research in this area involve further delineation of cellular/molecular mechanisms involved in effects of exercise training on collateralized myocardium, as well as development of novel therapies based on emerging concepts regarding exercise training and coronary artery disease.
Keywords: collateral-dependent, porcine, human, canine
coronary collateral arteries provide alternate pathways of blood flow between regional myocardial perfusion territories. Although the innate collateral circulation of the normal human heart is typically minimal, the collateral circulation develops in response to increasing severity of ischemic coronary disease. These anastomoses serve as a natural protective mechanism to provide coronary flow to ischemic myocardium secondary to coronary stenosis. Considerable variability occurs in extent of collateralization and the impact of genetic and cardiovascular risk factors on coronary collateral development (8, 43, 77). Irrespective, a well-developed collateral circulation has been documented to exert protective effects on myocardial perfusion, contractile function, infarct size, and electrocardiographic abnormalities of the ischemic myocardium (8, 9, 12, 21, 28, 52, 70, 73, 77, 79, 80, 85) as well as markedly improve long-term cardiac survival (52). Thus therapeutic augmentation of the extent and/or function of the collateral circulation likely represents a desirable goal in the management of coronary artery disease.
A large body of evidence has provided documentation for favorable protective and therapeutic benefits of exercise training programs in patients with coronary artery disease. These aspects are described in detail below and in the current series of minireviews on this topic. Reductions in the influences of major recognized risk factors are clearly involved as major preventative and therapeutic benefits of exercise training programs in both health and disease. However, the response of the collateralized heart to increases in physical activity represents additional complex and dynamic aspects. These include potential effects of exercise training on the degree of anatomical and functional collateralization, as well as potentially important effects on regulation of coronary tone in a complex hemodynamic circuitry involving both collateral and noncollateral (collateral-dependent) arteries and microvasculature. Analysis of exercise training programs on extent of collateralization in human patients with coronary disease has provided clinically relevant but often controversial findings. Experimental animal models of coronary artery disease and collateralization have been developed and utilized to assess effects of chronic exercise and address potential mechanisms involved. This review highlights effects of exercise training on the extent and function of enhanced collateralization in both patients and experimental models, as well as effects of exercise training on vascular smooth muscle and endothelial control of regional coronary tone in the collateralized heart.
EFFECT OF EXERCISE TRAINING ON CORONARY COLLATERALIZATION IN HUMAN CORONARY ARTERY DISEASE
It is well documented that inclusion of an exercise program in the therapeutic management of coronary artery disease reduces clinical symptoms, improves myocardial function, and reduces long-term cardiac mortality (7, 10, 18, 22, 23, 26, 28–30, 33, 35–37, 47–49, 57–59, 68, 72, 76, 83). In this setting, mechanisms that underlie the therapeutic advantages of exercise training are numerous (18, 47) and include enhancement of collateral development, increased perfusion of the collateralized myocardium, and improved endothelial vasomotor function of coronary vessels of the diseased heart (7, 30, 35, 37, 49). In a 2010 review, Fujita and Sasayama (28) concluded that a long-term exercise regimen is effective for collateral development in patients with coronary artery disease. They further suggested that exercise stress in the setting of coronary disease induces both arteriogenesis (enlargement and remodeling of preexisting collateral arterioles) as well as ischemia-induced angiogenesis (growth and proliferation of capillaries); however, arteriogenesis is more important in terms of the coronary collateral circulation and delivery of blood into the collateral-dependent regions (27, 28, 73).
Direct angiographic evidence of enhanced collateralization in response to exercise training is sparse in human patients with coronary disease (6, 7, 18, 22, 23, 26, 30, 37). Despite exercise-mediated improvements in myocardial function in regions compromised by ischemia, available methods for direct determination of collateral growth have significant limitations that render assessment of collateral development difficult. Belardinelli and colleagues (7) provided direct angiographic evidence of exercise-induced enhancement of collateral vasculature. In this study, effects of 8 wk of exercise training on collateral development in coronary disease patients were evaluated using retrograde filling and angiographic scoring techniques. At conclusion of the study, exercise-trained patients demonstrated significantly improved perfusion by collateral vessels that correlated with improvements in myocardial contractility and myocardial thallium uptake in response to low-dose dobutamine. Importantly, improvement in collateral growth was not accompanied by progression of severity of coronary artery stenosis. These investigators reported that training-induced development of collateral vessels was more pronounced in patients with higher collateral scores at baseline, indicating a predictive relationship of collateral development with initial levels of collateral perfusion (7). Similarly, a follow-up study by these authors indicated that exercise training potentiated coronary collateralization (assessed via angiography) in patients with ischemic cardiomyopathy treated with dipyridamole (6).
On the other hand, other studies using angiographic techniques have not provided support for exercise training-induced collateral development in the setting of coronary artery disease (22, 57, 60, 76). A 1979 angiographic analysis (60) reported the absence of training-induced improvements in the number, size, or extent of collaterals following a vigorous 7-mo exercise training program in patients following an initial myocardial infarction. In a series of studies (57–59, 76), effects of intensive physical exercise and a low-fat diet on coronary morphology, collateral development, myocardial perfusion, and progression/regression of coronary artery disease were evaluated over several years. The training regimen and low-fat diet significantly delayed the progression of coronary disease (lesion size) at one year (57, 76) and six years (59) of risk intervention. Multivariate analysis identified only physical activity as independently contributing to changes in progression of disease (59). The training-diet intervention decreased stress-induced myocardial ischemia (57) but did not significantly alter collateral artery formation as compared with control patients not exposed to these interventions (76). However, collateral formation was significantly related to progression of coronary artery disease (57). Similarly, in these studies, delayed progression of disease in these patients correlated with a decreased collateral formation, as confirmed by others (63). Taken together, these data suggest that collateral development was induced by progression of coronary disease rather than by the training program, and that exercise caused beneficial changes that appeared to be independent of angiographically visible collateral development. Despite the negative outcome regarding enhanced collateralizaton in response to exercise training and low-fat diet, the intervention group exhibited improved exercise stress-test performance, reduced myocardial ischemia on thallium imaging, and fewer cardiac symptoms (57–59, 76), supporting the results of Belardinelli et al. (7) and other investigators that exercise training improves functional performance of the compromised myocardial region(s).
Assessment of training-induced enhanced collateralization by coronary angiographic techniques is challenging. Angiography has inherent limitations regarding the level of resolution (sensitivity of discernment) of many vessels within the collateral network of the diseased heart (23, 26). Small microvascular caliber vessels (<100 μm) are difficult to visualize angiographically, and thus the full extent of collateralization cannot be accurately assessed or may be underestimated. Indeed, only half of patients with critical coronary stenosis develop angiographically visible collaterals, despite functional evidence for their presence (43). Nitrovasodilators, such as nitroglycerin, are typically present in patients undergoing coronary angiography, and collateral vessels have been shown to be responsive to the vasodilator actions of these agents (2, 5, 18, 31, 64). Although controversial (20) these actions may enhance the apparent recruitment and/or sensitivity of angiographic assessment of collaterals, although microvascular collaterals remain undetected. Importantly, typical angiographic assessment reflects vessels observable only under resting conditions. Thus, additional collateral recruitment (particularly microvascular collaterals) under conditions of high metabolic demand (myocardial stress or exercise) and optimal collateral flow is often not included for analysis in these studies.
In a large number of reports (78, 80, 81, 84, 85, 88), Seiler and colleagues have utilized hemodynamic, functional, vasomotor and angiographic approaches to assess effects of exercise training on collateral development and myocardial perfusion in patients with coronary artery disease. This group has developed and utilized a unique and quantifiable index of coronary collateral flow (collateral flow index; CFI) as a measurement of the functional presence of collateral channels during a transient balloon occlusion of the stenotic vessel. CFI is derived from intracoronary flow velocity (or pressure) measurements made in the recipient artery distal to occlusion. Therefore, CFI is a reflection of the flow velocity (or pressure) contributions from the collateral circulation as a fraction of flow during vessel patency, and includes changes in collateral flow resulting from resistance (and distal pressure) changes in the collateral-dependent microcirculation downstream to the occlusion. Indeed, in 2010, Seiler (80) indicated that the “gold standard” for collateral assessment is this collateral flow index. While collateral flow index obtained by this method accurately reflects collateral flow as an index of functional collateral development in human patients with stenotic coronary artery disease, it is also partially determined by vasomotor changes occurring in the distal microvascular bed supplied by the collateral circulation of these hearts.
Seiler and colleagues have performed long-term cross-sectional prospective studies using CFI (measured during percutaneous angioplasty) to assess effects of chronic increases in physical activity (determined by structured interview) upon the collateral circulation of patients with coronary disease (81). An increase in leisure-time physical activity in these patients was directly and independently associated with collateral flow to the occluded region of diseased hearts. More recently, Zbinden et al. (88) utilized CFI to assess changes in coronary collateral blood flow in coronary disease patients before and after a 3-mo exercise training regimen. CFI increased significantly in both normal and stenotic coronary artery regions after completion of the exercise program, but remained unchanged in the sedentary patients. These investigators documented that there was a direct correlation between the adaptations in CFI from baseline to postintervention (exercise or sedentary program), and the respective change in maximal oxygen consumption (V̇o2max) and exercise performance (88). Taken together, these data suggest that chronic endurance training augments coronary collateral blood flow to both normal and stenotic arteries. In addition, there appeared to be a correlation between coronary collateral flow augmentation and improvements in exercise capacity.
Improvements in perfusion of the ischemic myocardium after exercise training in patients with coronary artery disease may also reflect enhanced vasodilation responses of the vasculature of the collateralized heart as well as increases in the level of anatomical collateral development. In this regard, the role of training-induced improvements in coronary endothelial function and endothelium-dependent relaxation in the diseased coronary vasculature has been assessed by Hambrecht, Linke, and colleagues (30, 35, 37, 49). These investigators reported that exercise training has the potential to reverse endothelial dysfunction in patients with coronary artery disease (35, 37). In these studies, vigorous exercise training (bicycle ergometry) improved endothelial dysfunction in epicardial coronary vessels (angiography) and vasodilator reserve capacity of resistance vessels (adenosine-mediated responses) (37), as well as enhanced endothelial-dependent vasodilation and endothelial nitric oxide synthase (eNOS) phosphorylation in the left internal mammary artery (35). These combined data provide a mechanism whereby training may beneficially impact myocardial perfusion in the absence of morphological changes in collateral formation (30, 35, 37). However, the dominant role of improved endothelial function in training-induced enhancement of myocardial perfusion in these studies was questioned by Georgiou and Belardinelli (29), implicating the difficulties of angiographic assessment of enhanced collateralization. In important recent studies, Hambrecht and colleagues (36) reported that, compared with percutaneous coronary angioplasty (PCI), a 12-mo program of regular physical exercise (bicycle ergometry) resulted in superior event-free survival and exercise capacity, at lower costs and hospitalization rates.
In summary, while beneficial effects of training in patients with coronary artery disease and collateralized hearts are clearly numerous and diverse, considerable evidence has been generated for a significant role of improved endothelial function. However, differential roles of increased collateral development and enhanced coronary/collateral vasomotor responsiveness, in improvements in myocardial function and perfusion observed after exercise training in patients with coronary artery disease, have not been fully resolved. At the time of publication of this review, a clinical trial (Impact of Intensive Exercise Training on Coronary Collateral Circulation in Patients with Stable CAD; University of Leipzig; clinicaltrials.gov Identifier: NCT01209637) is in progress designed to further address this controversy. Continued clinical studies, in concert with results obtained from animal models (described below), are likely to provide significant information and more precise conclusions.
EFFECT OF EXERCISE TRAINING ON CORONARY COLLATERALIZATION IN ANIMAL MODELS OF CORONARY ARTERY DISEASE
Although evaluation of the effects exercise training programs on extent of collateralization in human coronary disease patients has provided controversial findings, animal models of critical stenosis or occlusion generally provide experimental support for beneficial effects of exercise training on collateral development and improved blood flow and myocardial function in the collateral-dependent region. However, there are limitations to the use of animal models. The induction of coronary artery disease occurs relatively abruptly in animal models, whereas human disease develops over decades. Additionally, animal models used for these studies typically lack other risk factor combinations (aging, hypertension, etc.) that are usually present in human patients presenting with coronary disease. As outlined below, there are also anatomical and physiological differences in the coronary and collateral vasculature of animals and humans, although collateral growth in the porcine model appears to be more applicable to that observed in humans. While most animal models lack the presence of multiple pharmacotherapeutic interventions being used by most patients with coronary artery disease, this difference also presents a unique advantage to independently assess effects of training in the absence of these confounding influences. Despite the limitations associated with animal models of coronary artery disease, there are other distinct advantages to the use of these animal models in the study of collateralization, including greater experimental control, more invasive evaluation of regional myocardial blood flow and function, as well as the ability to critically evaluate underlying adaptive mechanisms, including those at the cellular and molecular level.
Canine and porcine models of chronic coronary artery occlusion are commonly used for the experimental induction of collateral vessel growth; however, these species demonstrate differences in collateral development. The dog heart possesses an abundance of innate collaterals in the epicardial layer, which can be recruited rapidly under conditions of ischemia (15, 71). The combination of vessel recruitment and the growth of new collaterals (Fig. 1) in the dog are sufficient to restore adequate blood flow to the compromised myocardium during rest and often under conditions of increased myocardial O2 demand (4, 16, 45, 50). On the other hand, the porcine heart has limited innate collateral development and when subjected to gradual occlusion, growth of numerous small vessels occurs predominantly in the endocardial and midmyocardial layers (15, 86, 87). Collateral development in the porcine heart (Fig. 2) is only sufficient to restore resting blood flow to the collateral-dependent myocardium, whereas blood flow during exercise remains compromised and unable to support regional myocardial function (61, 67, 68, 87). Similarly, the human heart demonstrates few innate collaterals and the growth of new vessels typically occurs as an extensive network of functionally significant collaterals in the endocardial and midmyocardial layers (15). Furthermore, coronary artery disease patient populations often display persistent regional myocardial ischemia and contractile dysfunction during exercise (44, 57, 76) similar to that observed in pigs (61, 67, 87). However, most of our early understanding of the effects of exercise training on collateral vessel development in the heart evolved from studies using canine models of fixed stenosis or progressive coronary occlusion and thus contributed significantly to the body of literature in this area.
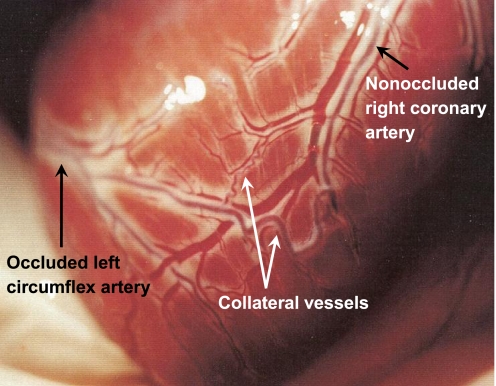
Fig. 1.
Representative photograph illustrating the apical view of a canine heart 4 mo following placement of an ameroid occluder around the proximal left circumflex coronary artery (entering from the left side of the photograph). Typical canine coronary collateral arteries are clearly visible on the epicardial surface, including both large (∼1 mm diameter) and smaller, tortuous arterial connections between a branch of the completely occluded left circumflex coronary artery and a branch of the nonoccluded right coronary artery. All animal protocols were in accordance with the “Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research and Training” and approved by the University of Missouri Animal Care and Use Committee.
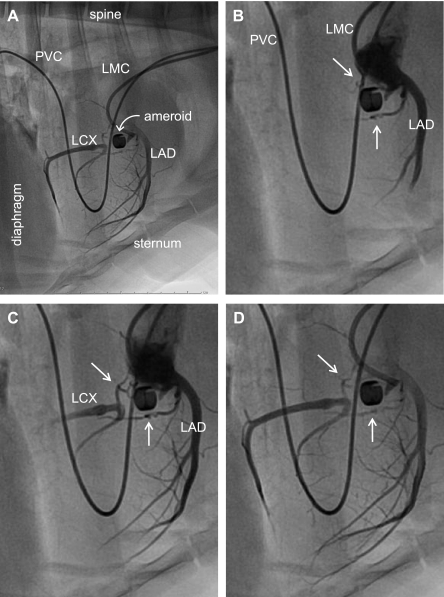
Fig. 2.
Angiography of porcine model of chronic coronary artery occlusion and collateral perfusion. Twenty-two weeks after surgical placement of an ameroid constrictor around the proximal left circumflex coronary artery, the animal was placed under general anesthesia. Hemodynamics were monitored throughout the procedure. Arterial access was obtained by surgical cutdown of the carotid artery. The left main coronary artery was catheterized with a 6F guiding catheter (Vista Brite Tip; Cordis) introduced over a 0.035-in. guidewire. Selective coronary angiography was performed with nonionic contrast (Oxilan 350; Guerbet). A: image of lateral view displaying the majority of the thoraci structure for perspective. B–D: regionally enhanced serial images emphasizing the cardiac silhouette to provide additional detail of coronary vasculature. White arrows in B–D identify collateral vessels supplying the left circumflex artery (LCX) distal to occlusion. LAD, left anterior descending artery; LMC, left main coronary artery catheter; PVC, pulmonary vein catheter for procedure not related to the data associated with this study. [Reproduced from Zhou et al. (89).]
Eckstein (19) examined the effects of a 6- to 8-wk exercise training regimen on collateral vessel development in a canine model of surgically induced chronic narrowing (fixed stenosis) of the left circumflex coronary artery. The extent of collateral blood flow, as determined by measurement of retrograde flow into the distal left circumflex artery, was proportional to the degree of arterial narrowing, as determined by blood flow through the proximal constricted left circumflex artery. Importantly, exercise training markedly increased the extent of collateral vessel development above that observed in sedentary dogs. Furthermore, mild arterial narrowing failed to elicit collateral vessel development in sedentary animals, although exercise training stimulated collateral growth even under conditions of mild stenosis. This seminal paper also provided evidence that production of a pressure gradient across innate collateral vessels, as well as myocardial hypoxia, provided the underlying mechanisms for collateral growth following arterial narrowing, mechanisms that continue to be proposed to stimulate collateral vessel growth in current literature. Despite the apparent positive influence of exercise training on collateral development, the experimental design of this study was limited, since data were obtained only after completion of the sedentary or exercise training regimens and did not include baseline collateral flow measurements for each experimental subject. However, the relatively large number of animals used for this study (46 sedentary and 44 exercise trained) likely strengthens the value of the postintervention comparisons between these groups and the conclusions derived from this study.
In a subsequent report, Cohen et al. (17) also utilized experimentally induced critical narrowing of the left circumflex coronary artery and subsequently measured blood flow (microspheres) to the ischemic region prior to and following completion of a 12-wk exercise training or sedentary protocol. Findings from this study confirmed the results of Eckstein (19) demonstrating that exercise-trained dogs had significantly enhanced blood flow to the ischemic myocardial region after completion of the exercise training regimen compared with pretraining values both at rest and during exercise stress. Blood flow to the ischemic myocardium in sedentary dogs was not significantly increased after 12 wk of cage confinement. Notably, the increased blood flow into the ischemic region after exercise training was associated with attenuation of myocardial dysfunction during transient complete occlusion of the left circumflex artery.
Using a more severe intervention, Heaton et al. (42) instrumented dogs with an ameroid constrictor around the left anterior descending artery and also created a fixed stenosis resulting in 60–90% obstruction at the left circumflex artery. Blood flow (microspheres) was measured at rest and during treadmill exercise before and after completion of a 6-wk exercise training or sedentary protocol. Exercise training resulted in significantly enhanced blood flow to the endocardial region of collateral-dependent myocardium during treadmill exercise, an improvement that was not observed in sedentary control dogs. In contrast, Neill and Oxendine (56) exercise-trained dogs that had been subjected to progressive left circumflex coronary artery occlusion with an ameroid constrictor. Exercise training did not appear to stimulate collateral vessel development beyond that observed in response to chronic coronary artery occlusion alone, assessed using angiography, microspheres, and retrograde flow. However, heart rate at submaximal workloads and height-to-body weight ratio were not altered significantly by exercise training, suggesting that the training regimen was not sufficiently strenuous to elicit exercise-induced adaptations. Furthermore, animals did not serve as their own control and the number of dogs utilized was small (4–8 dogs per treatment group).
In additional studies, Scheel et al. (74) examined the effects of 6–8 wk of exercise training on collateral resistance in both control dogs and those that had been subjected to progressive left circumflex coronary artery occlusion with an ameroid constrictor. After completion of the experimental protocols, collateral resistance was determined in isolated heart preparations. Chronic occlusion significantly decreased collateral resistance to the collateral-dependent myocardial region compared with control animals. Importantly, exercise training doubled collateral conductance compared with the sedentary group in the ameroid-constricted dogs. Exercise training had no effect on collateral resistance in the control animals. Taken together, these data indicate that exercise training has no effect on collateral development in control hearts but contributes significantly to collateralization in chronically occluded hearts (74).
In a canine model of two-vessel (left circumflex and right coronary arteries) ameroid occlusion, Schaper (72) determined the effect of exercise training on coronary and collateral blood flows (microspheres) in a Langendorff heart preparation. Dogs were exercise trained for approximately 2 mo before surgical placement of the ameroid constrictors. Treadmill training resumed 2 wk postoperatively and continued for 12 wk. Despite relatively high intensity of exercise training, heart weight and heart-to body weight ratio were not significantly elevated in trained compared with sedentary animals, although heart rate at submaximal exercise intensity decreased with training. After completion of the experimental protocol, maximal blood flow (adenosine infusion) into the collateral-dependent region was ∼40% of that into the control myocardial region and was not altered by exercise training. Collateral resistance, determined from the relationship between total collateral blood flow and the pressure difference across the entire collateral bed (aortic pressure − peripheral coronary pressure), also was not altered by exercise training. Potentially, baseline blood flow to the collateral-dependent region due to the chronic occlusion in this study (prior to training) may have been at a level sufficient to minimize the need for further effects of exercise training on collateralization (18).
This pioneering work using canine models of chronic stenosis/occlusion was instrumental in our early understanding of collateral development in coronary artery disease. However, as discussed above, similarities between pigs and humans in collateral development and persistent regional myocardial dysfunction under conditions of increased myocardial O2 demand make the porcine a more appropriate model for studies of coronary occlusion or critical stenosis. Bloor and colleagues (10, 68) recognized the importance of the pig model in this area and research from their group has contributed extensively to the characterization of adaptations in the collateral circulation of the pig model in response to chronic coronary artery occlusion and exercise training.
Utilizing the porcine model of chronic coronary artery occlusion, Bloor assessed the effect of exercise training on blood flow (microspheres) to both the proximal and distal portions of the ameroid-occluded left circumflex coronary artery (10). Blood flow in the proximal left circumflex artery was very similar to that measured in the nonoccluded left anterior descending artery. Collateral blood flow to the distal left circumflex artery was increased to a significantly greater extent after exercise training compared with occlusion alone. Collateral-dependent flow in exercise-trained pigs increased to 79% of control flow in the proximal left circumflex, which was significantly greater than the increase to 63% of control flows in the sedentary pigs. However, collateral blood flow was determined only under resting conditions in these studies and thus the effect of exercise training on collateral blood flow during increased myocardial demand was not assessed. The effects of exercise training on myocardial function of the collateral-dependent region also were not assessed, and therefore the importance of the increase in collateral blood flow as contributing to improved myocardial function was not determined.
In a subsequent study, these investigators (68) studied the effects of exercise training on coronary collateral development and regional myocardial function at rest and during moderate and severe exercise after gradual occlusion of the left circumflex artery. Myocardial function and collateral blood flow were determined before and after completion of the exercise training or sedentary protocols. In the exercise training group, blood flow ratios of the collateral-dependent region to the normally perfused regions were significantly enhanced in the endocardium, midmyocardium, and epicardium during severe exercise and statistically increased only in the endocardium during moderate exercise. In the sedentary animals, the blood flow ratio was increased significantly only to the endocardium during moderate exercise and less so than that observed in the exercise-trained pigs. Correspondingly, exercise training significantly improved systolic wall thickening in the collateral-dependent region at both moderate and severe exercise levels compared with the preexercise training stress test. The sedentary group also displayed an improvement in systolic wall thickening at moderate exercise levels, although not as pronounced as that observed in the exercise-trained animals, with no significant change in wall thickening during severe exercise. Thus findings from this study revealed an increase in blood flow into the collateral-dependent region as well as corresponding improvements in regional myocardial function primarily under conditions of high-intensity exercise. Taken together, these data suggest that differences in collateral blood flow between exercise-trained and sedentary groups may be best revealed during periods of intense exercise where maximal collateral conductance or maximal collateral-dependent vasodilator reserve are attained.
Thus data from experimental animal studies have provided generally promising findings which demonstrate that exercise training results in increased collateral development and improved function of the collateral-dependent myocardial region in the presence of critical stenosis or complete occlusion. While occlusive coronary artery disease is adequate to stimulate sufficient collateral development to restore adequate blood flow to the compromised myocardium during rest, findings from the studies above indicate that exercise training increases collateral development to an even greater extent and thus provide a greater level of blood flow to the collateral-dependent region during periods of increased myocardial demand.
EFFECT OF EXERCISE TRAINING ON CORONARY VASCULATURE REACTIVITY IN THE COLLATERAL-DEPENDENT MYOCARDIAL REGION
The effects of exercise training on cellular and molecular adaptations in collateral-dependent vasculature have been studied exclusively by our group (Parker, Heaps, and colleagues: 24, 25, 33, 34, 38–41, 66, 83, 89) using the well-established porcine model of chronic coronary artery occlusion. We have reported exercise training-induced adaptations in the collateral-dependent vasculature across the vascular tree that reveal improvements in endothelial function mediated primarily by the nitric oxide pathway, as well as unexpected exercise training-induced smooth muscle adaptations of increased basal tone and agonist-mediated vasoconstrictor responses. Many of these adaptations are summarized in Table 1.
Table 1.
Vascular adaptations in coronary vasculature in response to chronic coronary artery occlusion and/or exercise training in ameroid occluded pigs
| Vessel size | Experimental Method | Occlusion | Nonoccluded + Exercise | Occlusion + Exercise |
|---|---|---|---|---|
| Epicardial arteries (approximately 1 mm) | Isometric tension | ↔ Relaxation to BK and ADPa | ↑ Relaxation to BK and ADPc | ↑ Relaxation to BK and ADPc |
| Fura-2 | ↔ Basal endothelial cell Ca2 + a | ↓ Basal endothelial cell Ca2 + c | ↓ Basal endothelial cell Ca2 + c,d | |
| Fura-2 | ↔ BK-stimulated endothelial cell Ca2 + a | ↑ BK-stimulated endothelial cell Ca2 + c | ↑ BK-stimulated endothelial cell Ca2 + c | |
| HPLC | ↔ BK-stimulated nitric oxidea | ↑ BK-stimulated nitric oxidee | ↑ BK-stimulated nitric oxidee | |
| Immunoblot | ↑ Total eNOS proteind | ↔ Total eNOS proteinb | ↑ Total eNOS proteinc | |
| Immunoblot | ↔ p-eNOS (Ser1179) proteina | ↔ p-eNOS (Ser1179) proteinb | ↑ p-eNOS (Ser1179) proteind | |
| LSCM | ↔ eNOS:caveolin-1 PM distributiona | ↑ eNOS:caveolin-1 PM distributionc | ↑ eNOS:caveolin-1 PM distributionc | |
| Isometric tension | ↑ NO-dependent basal tonef | ↔ NO-dependent basal toneb | ↑ NO-dependent basal tonef | |
| Isometric tension | ↓ Relaxation to adenosined | ↔ Relaxation to adenosineb | ↔ Relaxation to adenosinea | |
| Isometric tension | ↓ Relaxation to isoproterenold | ↔ Relaxation to isoproterenolb | ↔ Relaxation to isoproterenola | |
| Combined tension + fura-2 | ↓ Relaxation, Ca2 + Removal to adenosined | ↔ Relaxation, Ca2 + removal to adenosineb | ↔ Relaxation, Ca2 + removal to adenosinea | |
| Small arteries (approximately 150–400 μm) | Isometric tension | ↔ Ca2 + -dependent basal tonea | ↔ Ca2 + -dependent basal toneb | ↑ Ca2 + -dependent basal tonec,d |
| Isometric tension | ↔ Nitric oxide contribution to basal tonea | ↔ Nitric oxide contribution to basal toneb | ↑ Nitric oxide contribution to basal tonec,d | |
| Isometric tension | ↔ Kv channel contribution to basal tonea | ↔ Kv channel contribution to basal toneb | ↑ Kv channel contribution to basal toned | |
| Immunoblot | ↔ Total eNOS proteina | ↔ Total eNOS proteinb | ↑ Total eNOS proteinc | |
| Immunoblot | ↑ p-eNOS (Ser1179) proteind | ↑ p-eNOS (Ser1179) proteinc | ↑ p-eNOS (Ser1179) proteinc | |
| Isometric tension | ↔ Constriction to ET-1a | ↔ Constriction to ET-1b | ↑ Constriction to ET-1d | |
| Combined tension + fura-2 | ↔ ET-1-mediated Ca2 + sensitizationa | ↔ ET-1-mediated Ca2 + sensitizationb | ↑ ET-1-mediated Ca2 + sensitizationd | |
| Isometric tension | ↔ PKC-mediated ET-1 constrictiona | ↔ PKC-mediated ET-1 constrictionb | ↑ PKC-mediated ET-1 constrictiond | |
| Isometric tension | ↔ Constriction to PDBUa | ↔ Constriction to PDBUb | ↑ Constriction to PDBUd | |
| Arterioles (approximately 100 μm) | Cannulated microvessel | ↓ Dilation to bradykinind | ↔ Dilation to bradykininb | ↔ Dilation to bradykinina |
| Cannulated microvessel | ↓ NO-dependent dilation to bradykinind | ↔ NO-dependent dilation to bradykininb | ↔ NO-dependent dilation to bradykinina | |
| Polymerase chain reaction | ↓ eNOS mRNAd | ↔ eNOS mRNAb | ↔ eNOS mRNAa | |
| Cannulated microvessel | ↔ Dilation to VEGF165a | ↔ Dilation to VEGF165b | ↑ Dilation to VEGF165c,d | |
| Cannulated microvessel | ↔ NO-dependent dilation to VEGF165a | ↔ NO-dependent dilation to VEGF165b | ↑ NO-dependent dilation to VEGF165c,d | |
| Immunoblot | ↔ VEGFR1 proteina | ↔ VEGFR1 proteinb | ↑ VEGFR1 proteinc,d | |
| Immunoblot | ↔ VEGFR2 proteina | ↔ VEGFR2 proteinb | ↑ VEGFR2 proteinc,d | |
| Immunoblot | ↔ Neuropilin-1 proteina | ↔ Neuropilin-1 proteinb | ↑ Neuropilin-1 proteinc,d | |
| Cannulated microvessel | ↓ Dilation to adenosined | ↔ Dilation to adenosineb | ↔ Dilation to adenosinea | |
| Cannulated microvessel | ↓ NO-dependent dilation to adenosined | ↔ NO-dependent dilation to adenosineb | ↑ NO-dependent dilation to adenosinec | |
| Cannulated microvessel | ↓ Dilation to ionomycind | ↔ Dilation to ionomycinb | ↔ Dilation to ionomycina | |
| Cannulated microvessel | ↓ NO-dependent dilation to ionomycind | ↔ NO-dependent dilation to ionomycinb | ↑ NO-dependent dilation to ionomycinc | |
| Cannulated microvessel | ↑ H2O2-dependent dilation to adenosineg |
Occlusion, vascular adaptations in collateral-dependent region; Nonoccluded + Exercise, vascular adaptations with exercise training in nonoccluded region; Occlusion + Exercise, vascular adaptations with exercise training in collateral-dependent region; BK, bradykinin; ADP, adenosine diphosphate; eNOS, endothelial nitric oxide synthase; p-eNOS, phosphorylated eNOS; LSCM, laser-scanning confocal microscopy; PM, plasma membrane; ET-1, endothelin-1; PDBU, phorbol 12,13-dibutyrate; NO, nitric oxide; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; H2O2, hydrogen peroxide.
Not different from respective nonoccluded artery.
Not different from respective artery of sedentary pig.
Significantly different from respective artery of sedentary pig.
Significantly different from respective nonoccluded artery.
Significant exercise training effect independent of occlusion.
Significant occlusion effect independent of exercise training.
Significantly different from all other vessel treatment groups.
In early studies, we reported that exercise training significantly enhanced both bradykinin- and ADP-mediated relaxation in nonoccluded and collateral-dependent arteries (∼1 mm luminal diameter), with no significant effect of occlusion (33). Findings from these studies also suggested that the exercise training-enhanced, endothelium-dependent relaxation was partially dependent on an increased contribution of nitric oxide (33). In support of these findings, we recently established that exercise training significantly enhanced bradykinin-stimulated intracellular free calcium levels in endothelial cells and increased bradykinin-stimulated nitric oxide levels in both nonoccluded and collateral-dependent coronary arteries (89). Occlusion alone did not alter bradykinin-stimulated nitric oxide or calcium responses (89). Exercise training also significantly enhanced the bradykinin-stimulated distribution of eNOS/caveolin-1 ratio at the plasma membrane in endothelial cells of control and collateral-dependent arteries (89). Bradykinin-stimulated phosphorylation of pSer1179-eNOS and pSer473-Akt and dephosphorylation of pThr497-eNOS proteins were not altered by chronic occlusion or exercise training (89). It is also interesting to note that under resting conditions, treatment with a NOS inhibitor increased basal tension to a greater extent in collateral-dependent compared with nonoccluded arteries in both sedentary and exercise-trained pigs (89). Consistent with these findings, basal protein levels of total eNOS and pSer1179-eNOS were increased in collateral-dependent arteries of sedentary and exercise-trained pigs, providing a mechanism by which nitric oxide contributes to a greater extent in collateral-dependent arteries under resting conditions (89). Taken together, these findings provide novel insight into exercise training-induced adaptations in cellular mechanisms of nitric oxide regulation that contribute to enhanced nitric oxide production and agonist-mediated relaxation in arteries of occluded/stenosed hearts.
Studies in epicardial vessels also revealed that adenosine-mediated relaxation was impaired in collateral-dependent compared with nonoccluded arteries from sedentary pigs (41). Exercise training restored adenosine-induced relaxation in collateral-dependent arteries to levels observed in nonoccluded arteries. Correspondingly, adenosine-mediated reductions in simultaneous measures of tension and intracellular free calcium were impaired in collateral-dependent compared with nonoccluded arteries isolated from sedentary pigs but corrected after exercise training (41). The impact of alterations in the vasomotor reactivity of epicardial collateral-dependent arteries has potential physiological and clinical significance. The presence of disease has been reported to enhance the contribution of epicardial arteries to total coronary vascular resistance, and therefore blood flow regulation (32). Thus the observed exercise training-induced adaptations in epicardial coronary arteries in the underlying setting of coronary artery disease may give rise to enhanced blood flow into the collateral-dependent region.
Studies in small coronary arteries (∼150–400 μm luminal diameter) have revealed novel experimental findings that exercise training enhances both vasodilation and vasoconstriction responses in small coronary arteries distal to occlusion. Collateral-dependent coronary arteries of exercise trained pigs exhibited significantly enhanced Ca2+-dependent basal active tone compared with arteries from nonoccluded regions of exercise-trained pigs and arteries from the collateral-dependent and nonoccluded regions of sedentary animals (38). Endothelin-1-mediated concentration-response curves were also significantly increased in collateral-dependent arteries of exercise-trained animals compared with the other artery treatment groups and appeared to be mediated in part by PKC-mediated calcium sensitization of the contractile apparatus (66). We also reported that arteries from the collateral-dependent region of exercise-trained animals displayed significantly enhanced constriction in response to NOS inhibition (nitro-l-arginine methyl ester) compared with arteries from the other treatment groups (38), suggesting that enhanced nitric oxide availability may mask the increased Ca2+-dependent tone observed in these arteries under basal conditions. Immunoblot analyses revealed that protein content of eNOS and pSer1179-eNOS was elevated in arteries from exercise-trained animals with the greatest effect in collateral-dependent vasculature (38). Thus the observed increase in eNOS and p-eNOS protein levels in the collateral-dependent coronary arteries following exercise training suggests that the enhanced constrictor response to NOS inhibition with occlusion and exercise training may be attributable to increased nitric oxide production. Finally, our studies in small coronary arteries have also revealed significantly enhanced K+ channel contribution to basal tone in collateral-dependent arterioles of exercise-trained pigs (38). We propose that these parallel, although seemingly contradictory, adaptations to exercise training are necessary to maintain adequate basal coronary tone via vasoconstriction under resting conditions, as well as stimulate marked vasodilation when cardiac metabolic demand is high. Thus these adaptations potentially provide greater intrinsic capacity (range) of local vascular control mechanisms to regulate blood flow to collateral-dependent myocardium, and subsequently contribute to the improved perfusion observed after exercise training as documented by others (10, 68).
Functional alterations have also been reported in coronary arterioles (∼100 μm luminal diameter) in the porcine model of chronic coronary artery occlusion and exercise training. In these studies, endothelium-dependent relaxation in response to bradykinin was significantly attenuated in arterioles isolated from the collateral-dependent region of chronically occluded hearts compared with arterioles isolated from a nonoccluded region of the same heart (34). Because the differences in bradykinin-mediated relaxation were eliminated in the presence of NOS inhibition (l-NMMA), we attributed the impaired vasodilation to a reduction in nitric oxide bioavailability in collateral-dependent arterioles (34). Importantly, following 14 wk of exercise training, bradykinin-induced vasodilatation in collateral-dependent arterioles was not different from that observed in control, nonoccluded arterioles and nitric oxide appeared to contribute similarly to relaxation in both collateral-dependent and nonoccluded arterioles after exercise training (34). In association with the finding that exercise training restores bradykinin-mediated, nitric oxide-dependent relaxation in collateral-dependent coronary arterioles, eNOS mRNA expression was significantly reduced in coronary arterioles isolated from the collateral-dependent myocardial region compared with that observed in arterioles from the nonoccluded region (34). Following the exercise training program, eNOS mRNA expression in arterioles from the collateral-dependent region was restored to that observed in the nonoccluded region (34). These findings suggest that impaired endothelium-mediated, nitric oxide-dependent relaxation in coronary arterioles distal to chronic occlusion may be attributable to reduced nitric oxide production subsequent to diminished eNOS mRNA expression. However, future studies examining components of the eNOS signaling pathway as well as basal and agonist-stimulated nitric oxide levels in the microcirculation are necessary to determine the effects of chronic occlusion and exercise training on nitric oxide bioavailability in these vessels. These studies are currently underway in our laboratory. More recently, hydrogen peroxide (H2O2), in addition to nitric oxide, has been shown to contribute to training-induced restoration of endothelium-dependent vasodilation in collateral-dependent coronary arterioles distal to chronic occlusion (83), implicating a role for additional endothelium-dependent influences in effects of exercise training in collateralized hearts.
Interestingly, exercise training increased VEGF165-mediated dilation in collateral-dependent coronary arterioles; enhanced VEGF responses were nitric oxide-dependent (25). Subsequent studies revealed that enhanced VEGF165-induced dilation was dependent on neuropilin-1 and associated with increased VEGFR-2 and neuropilin-1 protein levels in collateral-dependent arterioles of exercise-trained pigs (24). Neuropilin-1 is a coreceptor that enhances the signaling of VEGF165 through VEGFR-2. Furthermore, VEGF121-mediated dilation was not altered by occlusion or exercise training (24). Increased protein levels of these VEGF receptors with exercise training provide evidence of an additional potential mechanism by which exercise training may contribute to arteriogenesis and collaterogenesis in the setting of coronary artery disease.
Taken together, these experimental studies indicate that in the underlying setting of chronic coronary artery occlusion, exercise training produces adaptations of the coronary vasculature that generally involve enhanced agonist-mediated relaxation/dilation and thus potentially contribute to improvements observed in regional myocardial blood flow into the collateral-dependent region (10, 68). More recently, our lab has revealed important cellular and molecular mechanisms that underlie exercise training-enhanced dilation of collateral-dependent vasculature. These studies have supported important adaptations in the nitric oxide signaling pathway, as well as more recently, the superoxide/H2O2 signaling as contributors to enhanced dilation after exercise training. Furthermore, enhanced VEGF165-mediated dilation, as well as increased VEGF receptor protein levels in the collateral-dependent microcirculation with exercise training, suggests that chronic exercise may augment or extend the opportunity for neovascularizaton associated with occlusion or critical stenosis.
Interestingly, exercise training increases both vasodilation and vasoconstriction responses in small coronary arteries of the collateral-dependent region. Small arteries typically contribute about 20–25% of total coronary vascular resistance (13, 14) and may take on a greater fraction of the total coronary vascular resistance under conditions of myocardial ischemia when arterioles undergo metabolic vasodilation. Although seemingly paradoxical, these novel training-induced adaptations in small collateral-dependent coronary arteries potentially provide a greater intrinsic ability for vascular control mechanisms to regulate blood flow to collateral-dependent myocardium (enhanced reserve), and subsequently contribute to improved perfusion.
FUTURE DIRECTIONS: MECHANISMS OF CORONARY COLLATERALIZATION AND THERAPEUTIC APPLICATIONS
Mechanisms that underlie collateral development in response to coronary artery disease, as well as potentially enhanced collateralization subsequent to exercise training, are extraordinarily complex and beyond the scope of this review. Arteriogenesis, including collaterogenesis, involves an intricate, balanced and coordinated expression of many growth factors, cellular and tissue remodeling, as well as modification of cardiovascular risk factors that adversely affect the intrinsic capacity for collateral development (28, 43, 73, 75). Kinnaird et al. (43) recently reviewed the potential for impaired collaterogenic capacity by many classical risk factors (hyperlipidemia, diabetes, etc.). Known effects of exercise training on reduction of these cardiovascular risk factors encourage speculation that chronic exercise may exert a positive effect on collateral development in the diseased heart, in part by diminishing the detrimental effects of risk factors on the host response to ischemia.
As described in prior sections of this review, a number of clinical studies and experimental models of coronary artery disease reveal unique adaptations of collateral and collateral-dependent vasculature in response to exercise training. Numerous studies from our lab and others have documented impairments of endothelial or smooth muscle regulation of coronary tone that are improved or reversed by exercise training. Of particular interest are the observations of training-induced improved endothelial function and enhanced influence of endothelium-derived vasomotor factors (e.g., nitric oxide; hydrogen peroxide) in the collateralized heart (24, 25, 33–35, 37, 38, 83, 89). Increased bioactivity of nitric oxide after training in the diseased heart has numerous implications including reductions in platelet adhesion and aggregation, thrombogenesis, and coronary vasospasm, as well as increases in angiogenesis and collaterogenesis. Training also appears to exert synergistic actions with certain endothelial growth factors on coronary cellular pathways involved in the synthesis and/or bioavailability of nitric oxide (24, 25). Indeed, many effects of vascular endothelial growth factor (VEGF) on vasodilation, angiogenesis, and collateral development involve nitric oxide (51); training-induced bioactivity of nitric oxide would theoretically enhance these activities and promote collateralization (25). Current and future studies in these areas are likely to advance our understanding of the cellular mechanisms that underlie beneficial adaptations to exercise training in coronary disease, and as a result may implicate future new approaches for novel cellular targets and discoveries with important implications for the management of ischemic heart disease.
Experimental models (discussed above) have indicated that while narrowing of a coronary artery may initiate collateral growth in proportion to vessel narrowing (15), addition of exercise promotes the collateral circulation. These results provide evidence that a pressure gradient across collateral vessels, as well as myocardial hypoxia, are both involved as underlying mechanisms for collateral growth following arterial narrowing. This basic concept has been reinforced by a huge number of clinical and experimental studies. Occlusion of a major coronary artery causes development of a steep pressure gradient across preexisting anastomoses, increasing blood flow and fluid shear stress in these connections (73). Numerous investigators have further investigated the role of increased pulsatile shear stress, activation of the endothelial cytoskeleton and endothelial gene expression, isoforms of nitric oxide synthase, and secretion of endothelial growth factors in the outward remodeling of preexisting interconnecting arterioles (arteriogenesis) following an arterial occlusion (43, 73, 75). Increased levels of coronary and collateral flow, as well as fluid shear stress, occurring during bouts of exercise are therefore likely to contribute to training-induced increases in vascular remodeling, arteriogenesis, and collateralization. Interestingly, whole body periodic acceleration has been recently developed as a “passive exercise device” for improvement of endothelial function via increased fluid shear stress to vascular endothelium (53). This intervention with heparin pretreatment has recently been found to reduce myocardial ischemia and improve exercise capacity, potentially through shear stress-induced nitric oxide production (53).
Isolation of endothelial progenitor cells (EPCs) in 1997 (3) has resulted in increased awareness of the role of EPCs in exercise training and collaterogenesis (1, 46, 65, 69, 82), as well as their potential use as a therapeutic adjunct to improve the microcirculation in coronary artery disease (1, 54, 55). Increased levels of EPCs are observed following bouts of exercise (46, 65) and exercise-induced limb ischemia (69). EPCs are recognized promoters of angiogenesis (46) and also contribute to the increase in cross-sectional area of the vascular tree in response to physical training (82); these effects appear to be at least partly dependent on nitric oxide (46). EPCs are responsive to fluid shear stress; cultured EPCs increase gene expression of arterial endothelial markers under shear stress conditions, indicating differentiation into mature arterial endothelial cells under shear stress conditions (62). The relationship of these findings to training-induced arteriogenesis remains debatable; however, these studies implicate a role for EPC mobilization and maturation under physiological conditions of increased flow, increased shear stress, and increased synthesis/release of nitric oxide, such as exercise. Interestingly, clinical studies have reported a positive effect on EPC mobilization from bone marrow by exercise-based cardiac rehabilitation programs in patients with coronary disease (1, 11, 54, 55, 82). Ongoing observational and interventional trials are likely to reveal exciting information regarding the potential role of EPCs in the effects of exercise training on collateral development and improved myocardial perfusion in patients with coronary artery disease.
GRANTS
Support was provided by research funds from the National Institutes of Health (R01-HL-064931).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Adams V. Role of endothelial progenitor cells in the beneficial effects of physical activity on atherosclerosis and coronary artery disease. J Appl Physiol (February 24, 2011). doi:10.1152/japplphysiol.01464.2010 [DOI] [PubMed] [Google Scholar]
- 2. Aoki M, Sakai K, Koyanagi S, Takeshita A, Nakamura M. Effect of nitroglycerin on coronary collateral function during exercise evaluated by quantitative analysis of thallium-201 single photon emission computed tomography. Am Heart J 121: 1361–1366, 1991 [DOI] [PubMed] [Google Scholar]
- 3. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964–966, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Bache RJ, Schwartz JS. Myocardial blood flow during exercise after gradual coronary occlusion in the dog. Am J Physiol Heart Circ Physiol 245: H131–H138, 1983 [DOI] [PubMed] [Google Scholar]
- 5. Bache RJ, Tockman BA. Effect of nitroglycerin and nifedipine on subendocardial perfusion in the presence of a flow-limiting coronary stenosis in the awake dog. Circ Res 50: 678–687, 1982 [DOI] [PubMed] [Google Scholar]
- 6. Belardinelli R, Belardinelli L, Shryock JC. Effects of dipyridamole on coronary collateralization and myocardial perfusion in patients with ischaemic cardiomyopathy. Eur Heart J 22: 1205–1213, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Belardinelli R, Georgiou D, Ginzton L, Cianci G, Purcaro A. Effects of moderate exercise training on thallium uptake and contractile response to low-dose dobutamine of dysfunctional myocardium in patients with ischemic cardiomyopathy. Circulation 97: 553–561, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Berry C, Balachandran KP, L'Allier PL, Lesperance J, Bonan R, Oldroyd KG. Importance of collateral circulation in coronary heart disease. Eur Heart J 28: 278–291, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Billinger M, Kloos P, Eberli FR, Windecker S, Meier B, Seiler C. Physiologically assessed coronary collateral flow and adverse cardiac ischemic events: a follow-up study in 403 patients with coronary artery disease. J Am Coll Cardiol 40: 1545–1550, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Bloor CM, White FC, Sanders TM. Effects of exercise on collateral development in myocardial ischemia in pigs. J Appl Physiol 56: 656–665, 1984 [DOI] [PubMed] [Google Scholar]
- 11. Cesari F, Sofi F, Caporale R, Capalbo A, Marcucci R, Macchi C, Lova RM, Cellai T, Vannucci M, Gensini GF, Abbate R, Gori AM. Relationship between exercise capacity, endothelial progenitor cells and cytochemokines in patients undergoing cardiac rehabilitation. Thromb Haemost 101: 521–526, 2009 [PubMed] [Google Scholar]
- 12. Charney R, Cohen M. The role of the coronary collateral circulation in limiting myocardial ischemia and infarct size. Am Heart J 126: 937–945, 1993 [DOI] [PubMed] [Google Scholar]
- 13. Chilian WM, Eastham CL, Marcus ML. Microvascular distribution of coronary vascular resistance in beating left ventricle. Am J Physiol Heart Circ Physiol 251: H779–H788, 1986 [DOI] [PubMed] [Google Scholar]
- 14. Chilian WM, Layne SM, Klausner EC, Eastham CL, Marcus ML. Redistribution of coronary microvascular resistance produced by dipyridamole. Am J Physiol Heart Circ Physiol 256: H383–H390, 1989 [DOI] [PubMed] [Google Scholar]
- 15. Cohen MV. Functional significance of coronary collaterals in man. In: Coronary Collaterals: Clinical and Experimental Observation. New York: Futura, 1985, p. 93–185 [Google Scholar]
- 16. Cohen MV, Yipintsoi T. Restoration of cardiac function and myocardial flow by collateral development in dogs. Am J Physiol Heart Circ Physiol 240: H811–H819, 1981 [DOI] [PubMed] [Google Scholar]
- 17. Cohen MV, Yipintsoi T, Scheuer J. Coronary collateral stimulation by exercise in dogs with stenotic coronary arteries. J Appl Physiol 52: 664–671, 1982 [DOI] [PubMed] [Google Scholar]
- 18. Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev 88: 1009–1086, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Eckstein RW. Effect of exercise and coronary artery narrowing on coronary collateral circulation. Circ Res 5: 230–235, 1957 [DOI] [PubMed] [Google Scholar]
- 20. Eriksen UH, Nielsen TT, Egeblad H, Bagger JP. Coronary collaterals during single-vessel coronary angioplasty: Effects of nitroglycerin. Clin Cardiol 25: 340–344, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eritsland J, Fossum E. Chronic occlusion of the left main coronary artery: importance of collaterals. Cardiology 117: 128–130, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Ferguson RJ, Petitclerc R, Choquette G, Chaniotis L, Gauthier L, Huot P, Allard C, Jankowski L, Campeau L. Effect of physical training on treadmill exercise capacity, collateral circulation and progression of coronary disease. Am J Cardiol 34: 764–769, 1974 [DOI] [PubMed] [Google Scholar]
- 23. Firoozan S, Forfar JC. Exercise training and the coronary collateral circulation: is its value underestimated in man? Eur Heart J 17: 1791–1795, 1996 [DOI] [PubMed] [Google Scholar]
- 24. Fogarty JA, Delp MD, Muller-Delp JM, Laine GA, Parker JL, Heaps CL. Neuropilin-1 is essential for enhanced VEGF165-mediated vasodilatation in collateral-dependent coronary arterioles of exercise trained pigs. J Vasc Res 46: 152–161, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fogarty JA, Muller-Delp JM, Delp MD, Mattox ML, Laughlin MH, Parker JL. Exercise training enhances vasodilation responses to vascular endothelial growth factor in porcine coronary arterioles exposed to chronic coronary occlusion. Circulation 109: 664–670, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Franklin BA. Exercise training and coronary collateral circulation. Med Sci Sports Exer 23: 648–653, 1991 [PubMed] [Google Scholar]
- 27. Fujita M, Tambara K. Recent insights into human coronary collateral development. Heart 90: 246–250, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fujita M, Sasayama S. Coronary collateral growth and its therapeutic application to coronary artery disease. Circ J 74: 1283–1289, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Georgiou D, Belardinelli R. Exercise and coronary endothelial function. N Engl J Med 343: 147–148, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Gielen S, Schuler G, Hambrecht R. Exercise training in coronary artery disease and coronary vasomotion. Circulation 103: e1–e6, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Goldstein RE, Stinson EB, Scherer JL, Seningen RP, Grehl TM, Epstein SE. Intraoperative coronary collateral function in patients with coronary occlusive disease: nitroglycerin responsiveness and angiographic correlations. Circulation 49: 298–308, 1974 [DOI] [PubMed] [Google Scholar]
- 32. Gordon JB, Ganz P, Nabel EG, Fish RD, Zebede J, Mudge GH, Alexander RW, Selwyn AP. Atherosclerosis influences the vasomotor response of epicardial coronary arteries to exercise. J Clin Invest 83: 1946–1952, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Griffin KL, Laughlin MH, Parker JL. Exercise training improves endothelium-mediated vasorelaxation after chronic coronary occlusion. J Appl Physiol 87: 1948–1956, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Griffin KL, Woodman CR, Price EM, Laughlin MH, Parker JL. Endothelium-mediated relaxation of porcine collateral-dependent arterioles is improved by exercise training. Circulation 104: 1393–1398, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Hambrecht R, Adams V, Erbs S, Linke A, Krankel N, Shu Y, Baither Y, Gielen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation 107: 3152–3158, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Hambrecht R, Walther C, Mobius-Winkler S, Gielen S, Linke A, Conradi K, Erbs S, Kluge R, Kendziorra K, Sabri O, Sick P, Schuler G. Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease: a randomized trial. Circulation 109: 1371–1378, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Hambrecht R, Wolf A, Gielen S, Linke A, Hofer J, Erbs S, Schoene N, Schuler G. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med 342: 454–460, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Heaps CL, Mattox ML, Kelly KA, Meininger CJ, Parker JL. Exercise training increases basal tone in arterioles distal to chronic coronary occlusion. Am J Physiol Heart Circ Physiol 290: H1128–H1135, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Heaps CL, Parker JL, Sturek M, Bowles DK. Altered calcium sensitivity contributes to enhanced contractility of collateral-dependent coronary arteries. J Appl Physiol 97: 310–316, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Heaps CL, Sturek M, Price EM, Laughlin MH, Parker JL. Sarcoplasmic reticulum Ca2+ uptake is impaired in coronary smooth muscle distal to coronary occlusion. Am J Physiol Heart Circ Physiol 281: H223–H231, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Heaps CL, Sturek M, Rapps JA, Laughlin MH, Parker JL. Exercise training restores adenosine-induced relaxation in coronary arteries distal to chronic occlusion. Am J Physiol Heart Circ Physiol 278: H1984–H1992, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Heaton WH, Marr KC, Capurro NL, Goldstein RE, Epstein SE. Beneficial effect of physical training on blood flow to myocardium perfused by chronic collaterals in the exercising dog. Circulation 57: 575–581, 1978 [DOI] [PubMed] [Google Scholar]
- 43. Kinnaird T, Stabile E, Zbinden S, Burnett MS, Epstein SE. Cardiovascular risk factors impair native collateral development and may impair efficacy of therapeutic interventions. Cardiovasc Res 78: 257–264, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Kolibash AJ, Bush CA, Wepsic RA, Schroeder DP, Tetalman MR, Lewis RP. Coronary collateral vessels: spectrum of physiologic capabilities with respect to providing rest and stress myocardial perfusion, maintenance of left ventricular function and protection against infarction. Am J Cardiol 50: 230–238, 1982 [DOI] [PubMed] [Google Scholar]
- 45. Lambert PR, Hess DS, Bache RJ. Effect of exercise on perfusion of collateral-dependent myocardium in dogs with chronic coronary artery occlusion. J Clin Invest 59: 1–7, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Laufs U, Werner N, Link A, Endres M, Wassmann S, Jurgens K, Miche E, Bohm M, Nickenig G. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation 109: 220–226, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Laughlin MH, Korthuis RJ, Duncker DJ, Bache RJ. Control of Blood Flow to Cardiac and Skeletal Muscle During Exercise. New York: Wiley, 2010 [Google Scholar]
- 49. Linke A, Erbs S, Hambrecht R. Effects of exercise training upon endothelial function in patients with cardiovascular disease. Front Biosci 13: 424–432, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Longhurst JC, Motohara S, Atkins JM, Ordway GA. Function of mature coronary collateral vessels and cardiac performance in the exercising dog. J Appl Physiol 59: 392–400, 1985 [DOI] [PubMed] [Google Scholar]
- 51. Matsunaga T, Warltier DC, Weihrauch DW, Moniz M, Tessmer J, Chilian WM. Ischemia-induced coronary collateral growth is dependent on vascular endothelial growth factor and nitric oxide. Circulation 102: 3098–3103, 2000 [DOI] [PubMed] [Google Scholar]
- 52. Meier P, Gloekler S, Zbinden R, Beckh S, de Marchi SF, Zbinden S, Wustmann K, Billinger M, Vogel R, Cook S, Wenaweser P, Togni M, Windecker S, Meier B, Seiler C. Beneficial effect of recruitable collaterals: a 10-year follow-up study in patients with stable coronary artery disease undergoing quantitative collateral measurements. Circulation 116: 975–983, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Miyamoto S, Fujita M, Inoko M, Oba M, Hosokawa R, Haruna T, Izumi T, Saji Y, Nakane E, Abe T, Ueyama K, Nohara R. Effect on treadmill exercise capacity, myocardial ischemia, and left ventricular function as a result of repeated whole-body periodic acceleration with heparin pretreatment in patients with angina pectoris and mild left ventricular dysfunction. Am J Cardiol 107: 168–174, 2011 [DOI] [PubMed] [Google Scholar]
- 54. Moebius-Winkler S, Schuler G, Adams V. Endothelial progenitor cells and exercise-induced redox-regulation. Antioxid Redox Signal doi:10.1089/ars.2010.3734:2011 [DOI] [PubMed] [Google Scholar]
- 55. Napoli C, Hayashi T, Cacciatore F, Casamassimi A, Casini C, Al-Omran M, Ignarro LJ. Endothelial progenitor cells as therapeutic agents in the microcirculation: An update. Atherosclerosis 215: 9–22, 2011 [DOI] [PubMed] [Google Scholar]
- 56. Neill WA, Oxendine JM. Exercise can promote coronary collateral development without improving perfusion of ischemic myocardium. Circulation 60: 1513–1519, 1979 [DOI] [PubMed] [Google Scholar]
- 57. Niebauer J, Hambrecht R, Marburger C, Hauer K, Velich T, von Hodenberg E, Schlierf G, Kubler W, Schuler G. Impact of intensive physical exercise and low-fat diet on collateral vessel formation in stable angina pectoris and angiographically confirmed coronary artery disease. Am J Cardiol 76: 771–775, 1995 [DOI] [PubMed] [Google Scholar]
- 58. Niebauer J, Hambrecht R, Schlierf G, Marburger C, Kalberer B, Kubler W, Schuler G. Five years of physical exercise and low fat diet: effects on progression of coronary artery disease. J Cardiopulm Rehab 15: 47–64, 1995 [DOI] [PubMed] [Google Scholar]
- 59. Niebauer J, Hambrecht R, Velich T, Hauer K, Marburger C, Kalberer B, Weiss C, von Hodenberg E, Schlierf G, Schuler G, Zimmermann R, Kubler W. Attenuated progression of coronary artery disease after 6 years of multifactorial risk Intervention: role of physical exercise. Circulation 96: 2534–2541, 1997 [DOI] [PubMed] [Google Scholar]
- 60. Nolewajka AJ, Kostuk WJ, Rechnitzer PA, Cunningham DA. Exercise and human collateralization: an angiographic and scintigraphic assessment. Circulation 60: 114–121, 1979 [DOI] [PubMed] [Google Scholar]
- 61. O'Konski MS, White FC, Longhurst JC, Roth DM, Bloor CM. Ameroid constriction of the proximal left circumflex coronary artery in swine. Am J Cadiovasc Path 1: 69–77, 1986 [PubMed] [Google Scholar]
- 62. Obi S, Yamamoto K, Shimizu N, Kumagaya S, Masumura T, Sokabe T, Asahara T, Ando J. Fluid shear stress induces arterial differentiation of endothelial progenitor cells. J Appl Physiol 106: 203–211, 2009 [DOI] [PubMed] [Google Scholar]
- 63. Pohl T, Seiler C, Billinger M, Herren E, Wustmann K, Mehta H, Windecker S, Eberli FR, Meier B. Frequency distribution of collateral flow and factors influencing collateral channel development: Functional collateral channel measurement in 450 patients with coronary artery disease. J Am Coll Cardiol 38: 1872–1878, 2001 [DOI] [PubMed] [Google Scholar]
- 64. Rapps JA, Myers PR, Zhong Q, Parker JL. Development of endothelium-dependent relaxation in canine coronary collateral arteries. Circulation 98: 1675–1683, 1998 [DOI] [PubMed] [Google Scholar]
- 65. Rehman J, Li J, Parvathaneni L, Karlsson G, Panchal VR, Temm CJ, Mahenthiran J, March KL. Exercise acutely increases circulating endothelial progenitor cells and monocyte-/macrophage-derived angiogenic cells. J Am Coll Cardiol 43: 2314–2318, 2004 [DOI] [PubMed] [Google Scholar]
- 66. Robles JC, Sturek M, Parker JL, Heaps CL. Ca2+ sensitization and PKC contribute to exercise training-enhanced contractility in porcine collateral-dependent coronary arteries. Am J Physiol Heart Circ Physiol 300: H1201–H1209, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Roth DM, Maruoka Y, Rogers J, White FC, Longhurst JC, Bloor CM. Development of coronary collateral circulation in left circumflex ameroid-occluded swine myocardium. Am J Physiol Heart Circ Physiol 253: H1279–H1288, 1987 [DOI] [PubMed] [Google Scholar]
- 68. Roth DM, White FC, Nichols ML, Dobbs SL, Longhurst JC, Bloor CM. Effects of chronic exercise on regional myocardial function and coronary collateral development after gradual coronary artery occlusion in pigs. Circulation 82: 1778–1789, 1990 [DOI] [PubMed] [Google Scholar]
- 69. Sandri M, Beck EB, Adams V, Gielen S, Lenk K, Hlriegel R, Mangner N, Linke A, Erbs S, Möbius-Winkler S, Scheinert D, Hambrecht R, Schuler G. Maximal exercise, limb ischemia, and endothelial progenitor cells. Eur J Cardiovasc Prev Rehab 18: 55–64, 2011 [DOI] [PubMed] [Google Scholar]
- 70. Sasayama S. Effect of coronary collateral circulation on myocardial ischemia and ventricular dysfunction. Cardiovasc Drugs Ther 8: Suppl 34, 1994 [DOI] [PubMed] [Google Scholar]
- 71. Schaper W. The morphology of collaterals and anastomoses in human, canine and porcine hearts. In: The Collateral Circulation of the Heart, edited by Black DAK. Amsterdam: North Holland Publishing, 1971, p. 5–18 [Google Scholar]
- 72. Schaper W. Influence of physical exercise on coronary collateral blood flow in chronic experimental two-vessel occlusion. Circulation 65: 905–912, 1982 [DOI] [PubMed] [Google Scholar]
- 73. Schaper W. Collateral circulation: past and present. Basic Res Cardiol 104: 5–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Scheel KW, Ingram LA, Wilson JL. Effects of exercise on the coronary and collateral vasculature of beagles with and without coronary occlusion. Circ Res 48: 523–530, 1981 [DOI] [PubMed] [Google Scholar]
- 75. Schirmer SH, van Nooijen FC, Piek JJ, van Royen N. Stimulation of collateral artery growth: travelling further down the road to clinical application. Heart 95: 191–197, 2009 [DOI] [PubMed] [Google Scholar]
- 76. Schuler G, Hambrecht R, Schlierf G, Niebauer J, Hauer K, Neumann J, Hoberg E, Drinkmann A, Bacher F, Grunze M. Regular physical exercise and low-fat diet. Effects on progression of coronary artery disease. Circulation 86: 1–11, 1992 [DOI] [PubMed] [Google Scholar]
- 77. Seiler C. Collateral Circulation of the Heart. London: Springer-Verlag, 2009 [Google Scholar]
- 78. Seiler C, Fleisch M, Billinger M, Meier B. Simultaneous intracoronary velocity- and pressure-derived assessment of adenosine-induced collateral hemodynamics in patients with one- to two-vessel coronary artery disease. J Am Coll Cardiol 34: 1985–1994, 1999 [DOI] [PubMed] [Google Scholar]
- 79. Seiler C. The human coronary collateral circulation. Heart 89: 1352–1357, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Seiler C. The human coronary collateral circulation. Eur J Clin Invest 40: 465–476, 2010 [DOI] [PubMed] [Google Scholar]
- 81. Senti S, Fleisch M, Billinger M, Meier B, Seiler C. Long-term physical exercise and quantitatively assessed human coronary collateral circulation. J Am Coll Cardiol 32: 49–56, 1998 [DOI] [PubMed] [Google Scholar]
- 82. Shantsila E, Lip GY. Endothelial function and endothelial progenitors: possible mediators of the benefits from physical exercise? Eur J Cardiovasc Prev Rehabil 16: 401–403, 2009 [DOI] [PubMed] [Google Scholar]
- 83. Thengchaisri N, Shipley R, Ren Y, Parker JL, Kuo L. Exercise training restores coronary arteriolar dilation to NOS activation distal to coronary artery occlusion: role of hydrogen peroxide. Arterioscler Thromb Vasc Biol 27: 791–798, 2007 [DOI] [PubMed] [Google Scholar]
- 84. Togni M, Gloekler S, Meier P, de Marchi SF, Rutz T, Steck H, Traupe T, Seiler C. Instantaneous coronary collateral function during supine bicycle exercise. Eur Heart J 31: 2148–2155, 2010 [DOI] [PubMed] [Google Scholar]
- 85. Traupe T, Gloekler S, de Marchi SF, Werner GS, Seiler C. Assessment of the human coronary collateral circulation. Circulation 122: 1210–1220, 2010 [DOI] [PubMed] [Google Scholar]
- 86. White FC, Bloor CM. Coronary vascular remodeling and coronary resistance during chronic ischemia. Am J Cardiovasc Pathol 4: 193–202, 1992 [PubMed] [Google Scholar]
- 87. White FC, Carroll SM, Magnet A, Bloor CM. Coronary collateral development in swine after coronary artery occlusion. Circ Res 71: 1490–1500, 1992 [DOI] [PubMed] [Google Scholar]
- 88. Zbinden R, Zbinden S, Meier P, Hutter D, Billinger M, Wahl A, Schmid JP, Windecker S, Meier B, Seiler C. Coronary collateral flow in response to endurance exercise training. Eur J Cardiovasc Prev Rehabil 14: 250–257, 2007 [DOI] [PubMed] [Google Scholar]
- 89. Zhou M, Widmer RJ, Xie W, Widmer AJ, Miller MW, Schroeder F, Parker JL, Heaps CL. Effects of exercise training on cellular mechanisms of endothelial nitric oxide synthase regulation in coronary arteries after chronic occlusion. Am J Physiol Heart Circ Physiol 298: H1857–H1869, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]