Abstract
Previous studies have demonstrated that chronic mechanical strain produced by continuous positive airway pressure (CPAP) reduces in vivo airway reactivity in rabbits and ferrets. For CPAP to potentially have a therapeutic benefit for asthmatic subjects, the reduction in airway responsiveness would need to persist for 12–24 h after its discontinuation, require application for only part of the day, and be effective in the presence of atopic airway inflammation. In the present study, airway responsiveness to acetylcholine or methacholine was measured during mechanical ventilation following three different protocols in which active, nonanesthetized, tracheotomized rabbits were treated with High vs. Low CPAP (6 vs. 0 cmH2O). 1) High CPAP was applied continuously for 4 days followed by 1 day of Low CPAP; 2) High CPAP was applied at night and Low CPAP during the daytime for 4 days, and 3) High CPAP was applied for 4 days in animals following ovalbumin (Ova) sensitization and challenge. For all three protocols, treatment with High CPAP resulted in significantly reduced airway responsiveness compared with treatment with Low CPAP. Cumulatively, our in vivo results in rabbits suggest that high CPAP, even when applied only at night, produces a persistent reduction of airway responsiveness. In addition, CPAP reduces airway responsiveness even in the presence of atopic airway inflammation.
Keywords: chronic mechanical strain, airway inflammation
our laboratory has previously demonstrated that prolonged application of mechanical strain to the lungs results in lower responsiveness to bronchoconstrictors in vivo (26, 27). Conscious, active, tracheotomized rabbits treated for a period of 4 days with continuous positive airway pressure (CPAP) of 6 cmH2O had significantly lower responsiveness to acetylcholine (ACh) compared with control tracheotomized animals maintained at 0 cmH2O. Furthermore, airways isolated from the lungs of animals subjected to the chronic application of high CPAP were also less responsive to ACh in vitro than airways excised from animals subjected to 0 cmH20 CPAP. The persistence of the lower responsiveness in the excised airway tissues suggests that the decreased respiratory system responsiveness observed in vivo was a direct result of changes in the airways per se, and that the effects of chronic mechanical strain are not transient.
Deep inspiration prior to bronchial challenge inhibits the subsequent degree of airway narrowing that occurs during bronchial challenge. The bronchoprotective effect of acute mechanical strain has been demonstrated to last for only 10–20 min (12, 13, 16). For the application of chronic mechanical strain to be effective as a therapeutic intervention for patients with airway hyperresponsiveness, its effects on airway responsiveness must be persistent enough that patients could use CPAP for only intermittent periods during a 24-h cycle. In addition, studies of the effects of deep inspiration on airway responsiveness have demonstrated that the bronchoprotective effect of deep inspirations is less effective in asthmatics than in healthy nonasthmatic subjects (1, 12, 19). Therefore, it is important to evaluate whether chronic mechanical strain can reduce airway responsiveness in an animal model of atopic airway inflammation.
The goals of our present study were to determine 1) whether the effects of chronic mechanical strain caused by CPAP on airway responsiveness persist for 24 h following discontinuation of the CPAP, 2) whether the intermittent use of chronic mechanical strain (nocturnal CPAP) also reduces airway responsiveness, and 3) whether chronic CPAP reduces airway responsiveness in a rabbit model of atopic airway hyperresponsiveness.
METHODS
Measurement of Airway Responsiveness
Rabbits were anesthetized with thiopental sodium (50 mg/kg iv) and mechanically ventilated using a computer-controlled volume ventilator (Flexivent, SCIREQ, Montreal, PQ, Canada) with a tidal volume of 7–8 ml/kg and a respiratory rate of 60 breaths/min at a positive end-expiratory pressure of 4–5 cmH2O. The study was approved by the Institutional Animal Care and Use Committee.
Before each measurement of resistance, the lungs were inflated four times to 30 cmH2O to establish a standard volume history. Respiratory system resistance was calculated using a multiple linear regression of the pressure, flow, and volume signals recorded during a 1-Hz volume oscillation signal (15 ml/kg) that interrupted mechanical ventilation for one cycle.
Bronchoconstrictor challenge.
Respiratory system resistance was measured every 3 s for 60 s after the ACh challenge to ensure that the maximal response was recorded. Resistance was measured at baseline and then every 8 min after each stepwise increase in the concentration of ACh (1.0, 3.3, 10, 20, 33 and 50 mg/ml). ACh was aerosolized into the inspiratory circuit with an ultrasonic nebulizer (Aeroneb, FV-AN-A45, SCIREQ,Montreal, PQ, Canada) during 15 s of mechanical ventilation.
In ovalbumin (Ova)-sensitized animals, resistance was measured every 13 s for 60 s following each intravenous MCh dose (0.0025, 0.005, 0.01, 0.025, 0.05 and 0.25 mg/kg) delivered via a jugular vein catheter.
Administration of CPAP
New Zealand White male rabbits were anesthetized (Isoflurane) and tracheotomized, and a custom 4.0-mm-ID tracheostomy cannula (Bivona Medical Technologies, INC, CL27585) was sutured into the trachea. The standard respiratory connection port to the cannula was modified; flexible tubing was inserted into the walls to create a T-connection for the inspiratory and expiratory circuit with minimal dead space and minimal protrusion of the cannula from the neck. In addition, the standard opening of the tracheostomy cannula was fitted with a cap that could be removed to suction the airway. The flexible tubing from the tracheostomy connection was secured in place with a custom-made vest worn by each animal. Warmed and humidified air as well adjusted to a level of continuous positive airway pressure (CPAP: Healthdyne Tranquility Plus) entered an inspiratory circuit attached to the vest from the top of the cage (Fisher and Paykel Healthcare, MR 730). The connection at the top of the cage swiveled to provide tethering of the tubing and mobility for the animal within the cage. Tracheostomy care included instillation of 1 ml of sterile saline and suctioning every 6–10 h to assist in removal of secretions that could block the cannula (26, 27).
Bronchoalveolar Lavage Cell Differentiation
Immediately following the bronchial challenge, animals were euthanized, the lungs were excised, and bronchoalveolar lavage (BAL) fluid was collected by intratracheal flushing of 30 ml normal saline. Following centrifugation of BAL fluid and reconstitution of the cell pellet, total cell counts were performed manually using a hemocytometer, and cell differential counts for eosinophils, neutrophils, and lymphocytes were determined manually from Wright-Giemsa stained smears (26).
Immunohistochemistry
Excised lungs were inflated with 10% formalin to a distending pressure of 20 cmH2O for 24 h fixation and then sectioned and embedded in paraffin. Five-micrometer tissue slices were stained for Mucin 5AC (mouse monoclonal antibody, Abcam, Cambridge, MA) (8).
Experimental Protocols
Protocol 1: effect of High CPAP on airway responsiveness after 24 h.
Rabbits (males; 2.0–2.2 kg) were randomly assigned to receive either CPAP of 6 cmH2O (High CPAP) or 0 cmH2O (Low CPAP) continuously for 4 days. On day 5, CPAP was reduced to 0 cmH2O for 24 h in the High CPAP group, while rabbits in the Low CPAP group were maintained at CPAP of 0 cmH2O for another 24 h. On day 6, in vivo airway responsiveness was assessed in both groups.
Protocol 2: effect of Nocturnal High CPAP on airway responsiveness.
Rabbits (males; 2.0–2.2 kg) were randomly assigned to receive CPAP of 6 cmH2O for 12 h during the night followed by CPAP of 0 cmH2O for 12 h during the day for 5 days (Nocturnal High CPAP) or Low CPAP of 0 cmH2O during both the night and the day for 5 days. On day 6, in vivo airway responsiveness was assessed in both groups.
Protocol 3: effect of High CPAP on Ova-induced airway hyperresponsiveness.
Rabbits (males; 1.6–1.8 kg) were actively sensitized with ovalbumin (0.125 mg Ova vs. normal saline; 10 mg aluminum hydroxide ip; once per 2 wk for 4 wk) and then challenged with Ova (10 mg/ml Ova vs. normal saline nebulized for 20 min daily for 5 days).
On the day following the final Ova challenge, group A was maintained without tracheostomy or CPAP (no CPAP) for 5 days and then in vivo airway responsiveness was assessed using intravenous MCh. Group B underwent tracheostomy on the day following the final Ova challenge and was then randomly assigned to be administered either continuous CPAP of 6 cmH2O (Sens/Challenge-High CPAP) or 0 cmH2O (Sens/Challenge-Low CPAP) for 5 days and then in vivo airway responsiveness was assessed using intravenous MCh.
Statistical Analysis
Dose-response curves for groups were compared using repeated-measures ANOVA. In addition, differences in dose-response curves were compared using nonparametric analysis, which included an overall group effect using the average response for all of the agonist doses, as well as the slope of the dose-response curve. P values < 5% were considered statistically significant. Analysis was performed using StatView software (Windows version 5.0.1, SAS Institute).
RESULTS
Protocol 1: Persistence of Effect of High CPAP on Airway Responsiveness after 24 h
Baseline resistances did not differ for animals treated with High CPAP × 4 days followed by Low CPAP for 1 day (n = 4) compared with Low CPAP for 5 days (n = 3) (means ± SE: 0.023 ± 0.001 vs. 0.024 ± 0.001 cmH2O·s·ml−1; P = 0.72). Inhaled ACh caused a progressive increase in respiratory system resistance for both treatment groups; however, the group treated with High and then Low CPAP exhibited lower airway responsiveness with smaller increases in respiratory system resistances than the Low CPAP group (Fig. 1). Nonparametric analysis also demonstrated a greater average resistance and a greater slope for the Low compared with High CPAP group (P < 0.034).
Fig. 1.
Resistance (cmH2O·ml−1·s) vs. concentration of inhaled acetylcholine (mg/ml). Treatment with High continuous positive airway pressure (High CPAP; 6 cmH2O) for 4 days followed by Low CPAP (0 cmH2O) for 1 day (thin dashed line and open circles) produced a persistent reduction in airway responsiveness compared with Low CPAP for 5 days (solid black line and closed diamonds). Animals treated with Nocturnal High CPAP for 4 days (thick dashed line and open squares) had reduced airway responsiveness compared with animals treated with Low CPAP for 5 days (solid black line and closed symbols). There was no difference in airway responsiveness for the 2 groups treated with High CPAP.
Protocol 2: Effect of Nocturnal High CPAP on Airway Responsiveness
Baseline respiratory system resistances were not significantly different for animals treated with Nocturnal High CPAP and daytime Low CPAP (n = 3) vs. animals treated continuously with Low CPAP (n = 3) (means ± SE: 0.023 ± 0.002 vs. 0.024 ± 0.001 cmH2O·s·ml−1; P = 0.51). Inhaled ACh caused a progressive increase in respiratory system resistance for both treatment groups; however, the Nocturnal High CPAP group exhibited lower airway responsiveness with smaller increases in respiratory system resistances than the Low CPAP group (Fig. 1). Nonparametric analysis also demonstrated a greater average resistance and a greater slope for the Low compared with High CPAP group (P < 0.05).
We also compared the dose-response curves for Nocturnal High CPAP from this protocol (protocol 2) to the dose-response curves for High CPAP for 4 days followed by Low CPAP for 1 day in protocol 1. There was no significant difference (P > 0.67) in the dose-response curves for two different High CPAP protocols, thus indicating that both continuous and intermittent CPAP equally suppressed airway responsiveness compared with Low CPAP.
Protocol 3: Effect of High CPAP on Ova-Induced Airway Hyperresponsiveness
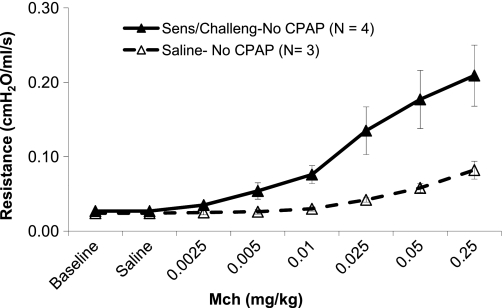
Group A (Ova sensitization and Ova challenge or saline sensitization and challenge; No CPAP).
Baseline resistance 5 days following Ova sensitization and challenge (n = 4) was not significantly different compared with saline controls (n = 3) (means ± SE: 0.027 ± 0.001 vs. 0.024 ± 0.004 cmH2O·s·ml−1; P = 0.45). Analysis by repeated ANOVA demonstrated that increasing doses of intravenous MCh produced a progressive increase in respiratory system resistance for both groups. In addition, the Ova group exhibited larger increases in respiratory system resistance than the normal saline controls (Fig. 2). Nonparametric analysis also demonstrated a greater average resistance and a greater slope for the Low compared with High CPAP group (P < 0.034).
Fig. 2.
Resistance (cmH2O·ml−1·s) vs. concentration of intravenous methacholine (mg/kg) for animals sensitized and challenged with Ova and then treated with High CPAP (dashed line and open symbols) or Low CPAP (solid black line and closed symbols). In Ova-sensitized and challenged animals, High CPAP reduced airway responsiveness compared with Low CPAP.
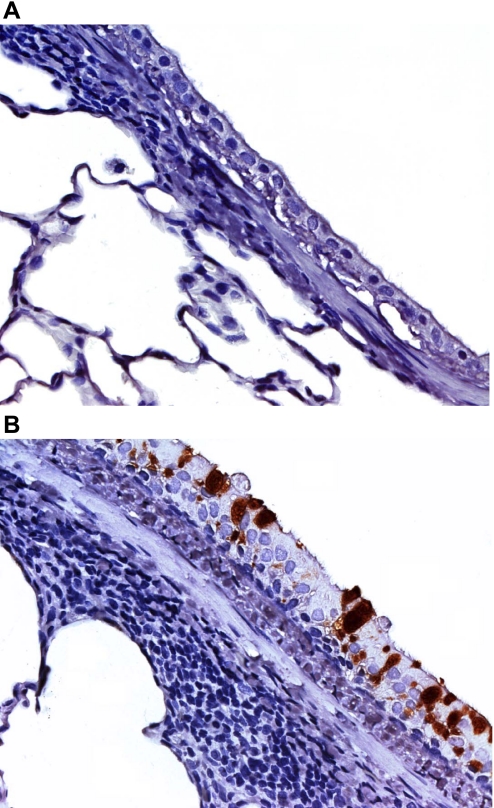
The Ova-sensitized and -challenged animals had a significantly higher number of eosinophils in their BAL fluid compared with saline controls (14.3% vs. 0%; P < 0.001). In addition, immunohistochemical staining for Mucin 5AC was very positive in airway epithelium from the animals sensitized and challenged with Ova, but not very prominent in airway epithelium from saline-treated control animals (Fig. 3).
Fig. 3.
Mucin 5AC immunohistochemical staining of airways from nonsensitized and nonchallenged animal (A) and Ova-sensitized and challenged animals (B).
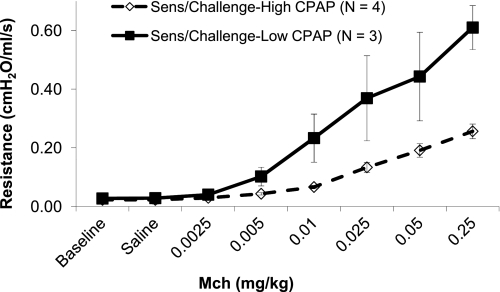
Group B (Ova sensitization and Ova challenge; Low or High CPAP).
Baseline resistance was significantly higher for rabbits treated with Low CPAP (n = 3) compared with High CPAP (n = 4) (means ± SE: 0.027 ± 0.001 vs. 0.022 ± 0.002 cmH2O·s·ml−1; P < 0.03). When analyzed by repeated ANOVA, increasing MCh resulted in a progressive increase in resistance for both groups. In addition, there was a significant interaction term for group by MCh; the High CPAP group had a smaller increase in resistance with increasing MCh than the Low CPAP group (P < 0.016) (Fig. 4). Nonparametric analysis also demonstrated a greater average resistance and a greater slope for the Low compared with High CPAP group (P < 0.034).
Fig. 4.
Resistance (cmH2O·ml−1·s) vs. concentration of intravenous methacholine (mg/kg) for animals sensitized and challenged with Ova and then treated with High CPAP (dashed line and open symbols) or Low CPAP (solid black line and closed symbols). In Ova-sensitized and challenged animals, High CPAP reduced airway responsiveness compared with Low CPAP.
The airway responsiveness in the Ova-challenged and tracheostomized animals treated with CPAP (Fig. 4) was greater than that observed in the Ova-challenged nontracheostomized animals (Fig. 2). This overall heightened airway responsiveness was most likely secondary to additional airway inflammation associated with the chronic presence of a tracheostomy tube.
DISCUSSION
Our current findings demonstrate that chronic mechanical distension of the respiratory system of rabbits with CPAP causes a reduction in airway responsiveness that persists for at least 24 h after removal of the CPAP. Furthermore, the reduction in airway responsiveness can still be produced if CPAP is applied to the respiratory system only at night. Lastly, CPAP suppressed the airway hyperresponsiveness caused by allergen-induced airway inflammation. Cumulatively, these results significantly advance our previous findings in rabbits that the administration of CPAP results in a reduction in airway responsiveness in vivo that persists in isolated airway segments in vitro. These observations in rabbits suggest that chronic nocturnal CPAP may provide a new approach for decreasing airway hyperresponsiveness in subjects with asthma.
Our previous study demonstrated that continuous use of High CPAP could decrease airway reactivity to ACh in tracheotomized rabbits, and that airways isolated from rabbits subjected to chronic CPAP were hyporesponsive in vitro. We found no effect of the chronic CPAP on the amount of airway smooth muscle within the airway wall (27). This suggested that the chronic mechanical strain caused by the CPAP had a direct effect on airway smooth muscle contractility, although it may also have an effect on contractility by remodeling the noncontractile tissues in both the inflamed and noninflamed airways. In the present study, we have demonstrated that when CPAP is administered chronically in vivo, it results in a persistent decrease in airway responsiveness that lasts for at least 24 h following removal of the CPAP (26, 27). These effects of chronic strain on airway smooth muscle responsiveness may result from mechanosensitive signaling to the airway smooth muscle cells that alter their cytoskeletal organization, modulate their structure or arrangement within the smooth muscle tissue in the airway wall, and/or affect signaling to the contractile apparatus (3, 9, 10, 17, 23–25, 29). Our observations that the effects of CPAP persist for at least 24 h after the CPAP is discontinued contrast to the transient effects of deep inspiration on airway responsiveness, which persist for only 10–20 min (12, 13, 16). Deep inspiration also causes mechanical stretch of the airways, but in contrast to CPAP, the airways are restored to an unstrained state between each inspiratory breath. The decreases in airway responsiveness induced by deep inspiration and by CPAP may result from different mechanisms.
As the decrease in airway reactivity with chronic CPAP persisted for at least 24 h following removal of the mechanical strain, we evaluated whether applying intermittent chronic strain would still suppress airway responsiveness in vivo. Our results indicate that the application of chronic nocturnal CPAP results in a suppression of airway responsiveness comparable to that obtained with chronic continuous CPAP (Figs. 1 and 2).
We used an Ova sensitization and challenge in rabbits to generate allergic pulmonary inflammation and airway hyperresponsiveness as a model of asthma. Our protocol resulted in increased eosinophils in the BAL fluid, positive MUC5AC staining in the airway epithelium (6), and increased in vivo airway responsiveness to MCh. We demonstrated that chronic CPAP caused a marked reduction in the in vivo airway responsiveness to MCh using this model of atopic airway inflammation and airway hyperresponsiveness. Our observations of the effects of chronic CPAP on airway responsiveness contrast with the very limited effect of deep inspiration on airway responsiveness, as well as the limited bronchodilating effect of deep inspiration in patients with asthma (7, 12, 20, 21). Therefore, the application of deep inspiration and chronic CPAP may have different effects on airway responsiveness during allergic inflammation. Slats and coworkers (22) reported that asthmatics that did not demonstrate bronchodilation with deep inspiration did exhibit bronchodilation when acute lung inflation was produced by positive airway pressure rather than deep inspiration. Using HRCT imaging, Brown and Mitzner (4) found that a deep inspiration of short duration (5–10 s) resulted in airway constriction in dogs challenged with MCh, while a deep inspiration of more prolonged duration (30–60 s) resulted in airway dilation. These findings suggest that the method of applying mechanical strain and the duration of the strain may be important determinants of their effects on airway responsiveness.
Patients with obstructive sleep apnea (OSA) have evidence for heightened systemic inflammation with elevated levels of several systemic inflammatory markers (CRP, IL-6, 8-isoprostane) and lower levels of circulating nitrate and nitrite levels, which have been reported to normalize with CPAP therapy (2, 11, 18, 28). Therefore, chronic CPAP could decrease airway responsiveness both through its direct effects on airway smooth muscle contractility and by its potential to decrease inflammatory responses that might be contributing to heightened airway responsiveness. In patients with OSA and asthma, Lafond and coworkers (14) reported that nocturnal CPAP did not decrease airway responsiveness to methacholine, but that it did improve the asthma quality of life score. Chan and coworkers (5) evaluated the effects of 2-wk periods nocturnal CPAP of 8 cmH2O on peak airway flows in 9 patients with OSA and asthma who used only inhaled beta agonists. They reported that during the period of CPAP, patients had improvement in their pre- and postbronchodilator peak expiratory flows measured in the morning and evening compared with similar patients with no CPAP. The improvement in pre-post peak flows would suggest a decrease in airway responsiveness; however, this was not assessed. Lin and colleagues (15) reported that nonasthmatic subjects with OSA demonstrated a decrease in airway reactivity to MCh following use of CPAP for 2 mo. Although all of these observations are consistent with a potential suppressive effect of chronic CPAP on airway responsiveness, it is difficult to directly translate our results in rabbits to asthmatic human adults. Rabbits have a more compliant respiratory system compared with humans, so higher levels of CPAP may be required in humans to obtain comparable degrees of mechanical strain and airway distension.
In summary, our study demonstrates that the administration of chronic CPAP to rabbits results in a persistent reduction of in vivo airway responsiveness, and that the positive effects of CPAP can also be obtained by using only nocturnal CPAP for the same time period. In addition, chronic CPAP can suppress in vivo airway reactivity in the presence of allergic airway inflammation. Our findings in rabbits suggest that future controlled studies should evaluate the potential of CPAP to decrease airway responsiveness in patients with asthma.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-48522 and HL-29289.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Allen ND, Davis BE, Cockcroft DW. Correlation between airway inflammation and loss of deep-inhalation bronchoprotection in asthma. Ann Allergy Asthma Immunol 101: 413–418, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Alonso-Fernández A, García-Río F, Arias MA, Hernanz Á, de la Peña M, Piérola J, Barceló A, López-Collazo E, Agustí A. Effects of CPAP on oxidative stress and nitrate efficiency in sleep apnoea: a randomised trial. Thorax 64: 581–586, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Bosse Y, Sobieszek A, Pare PD, Seow CY. Length adaptation of airway smooth muscle. Proc Am Thoracic Soc 5: 62–67, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Brown R, Mitzner W. Duration of deep inspiration and subsequent airway constriction in vivo. J Asthma 40: 119–124, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Chan KN, Noble-Jamieson CM, Elliman A, Bryan EM, Aber VR, Silverman M. Airway responsiveness in low birthweight children and their mothers. Arch Dis Child 63: 905–910, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fahy JV. Goblet cell and mucin gene abnormalities in asthma. Chest 122: 320S–326S, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Fish JE, Ankin MG, Kelly JF, Peterman VI. Regulation of bronchomotor tone by lung inflation in asthmatic and nonasthmatic subjects. J Appl Physiol 50: 1079–1086, 1981 [DOI] [PubMed] [Google Scholar]
- 8. Groneberg DA, Eynott PR, Lim S, Oates T, Wu R, Carlstedt I, Roberts P, McCann B, Nicholson AG, Harrison BD, Chung KF. Expression of respiratory mucins in fatal status asthmaticus and mild asthma. Histopathology 40: 367–373, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Gunst SJ, Tang DD, Opazo SA. Cytoskeletal remodeling of the airway smooth muscle cell: a mechanism for adaptation to mechanical forces in the lung. Respir Physiol Neurobiol 137: 151–168, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Gunst SJ, Wu MF. Selected contribution: Plasticity of airway smooth muscle stiffness and extensibility: role of length-adaptive mechanisms. J Appl Physiol 90: 741–749, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Ip MS, Lam B, Chan LY, Zheng L, Tsang KW, Fung PC, Lam WK. Circulating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am J Respir Crit Care Med 162: 2166–2171, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Kapsali T, Permutt S, Laube B, Scichilone N, Togias A. Potent bronchoprotective effect of deep inspiration and its absence in asthma. J Appl Physiol 89: 711–720, 2000 [DOI] [PubMed] [Google Scholar]
- 13. King GG, Moore BJ, Seow CY, Pare PD. Time course of increased airway narrowing caused by inhibition of deep inspiration during methacholine challenge. Am J Respir Crit Care Med 160: 454–457, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Lafond C, Series F, Lemiere C. Impact of CPAP on asthmatic patients with obstructive sleep apnoea. Eur Respir J 29: 307–311, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Lin CC, Lin CY. Obstructive sleep apnea syndrome and bronchial hyperreactivity. Lung 173: 117–126, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Malmberg P, Larsson K, Sundblad BM, Zhiping W. Importance of the time interval between FEV1 measurements in the methacholine provocation test. Eur Respir J 6: 680–686, 1993 [DOI] [PubMed] [Google Scholar]
- 17. Mehta D, Wu MF, Gunst SJ. Role of contractile protein activation in the length-dependent modulation of tracheal smooth muscle force. Am J Physiol Cell Physiol 270: C243–C252, 1996 [DOI] [PubMed] [Google Scholar]
- 18. Minoguchi K, Yokoe T, Tanaka A, Ohta S, Hirano T, Yoshino G, O'Donnell CP, Adachi M. Association between lipid peroxidation and inflammation in obstructive sleep apnoea. Eur Respir J 28: 378–385, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Pyrgos G, Kapsali T, Permutt S, Togias A. Absence of deep inspiration-induced bronchoprotection against inhaled allergen. Am J Respir Crit Care Med 167: 1660–1663, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Salome CM, Thorpe CW, Diba C, Brown NJ, Berend N, King GG. Airway re-narrowing following deep inspiration in asthmatic and nonasthmatic subjects. Eur Respir J 22: 62–68, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Scichilone N, Permutt S, Togias A. The lack of the bronchoprotective and not the bronchodilatory ability of deep inspiration is associated with airway hyperresponsiveness. Am J Respir Crit Care Med 163: 413–419, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Slats AM, Janssen K, de Jeu RC, van der Plas DT, Schot R, van den Aardweg JG, Sterk PJ. Enhanced airway dilation by positive-pressure inflation of the lungs compared with active deep inspiration in patients with asthma. J Appl Physiol 105: 1725–1732, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Tang DD, Gunst SJ. Selected contribution: roles of focal adhesion kinase and paxillin in the mechanosensitive regulation of myosin phosphorylation in smooth muscle. J Appl Physiol 91: 1452–1459, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Wang L, Liu HW, McNeill KD, Stelmack G, Scott JE, Halayko AJ. Mechanical strain inhibits airway smooth muscle gene transcription via protein kinase C signaling. Am J Respir Cell Mol Biol 31: 54–61, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Wu Y, Huang Y, Herring BP, Gunst SJ. Integrin-linked kinase regulates smooth muscle differentiation marker gene expression in airway tissue. Am J Physiol Lung Cell Mol Physiol 295: L988–L997, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xue Z, Zhang L, Liu Y, Gunst SJ, Tepper RS. Chronic inflation of ferret lungs with CPAP reduces airway smooth muscle contractility in vivo and in vitro. J Appl Physiol 104: 610–615, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Xue Z, Zhang L, Ramchandani R, Liu Y, Antony VB, Gunst SJ, Tepper RS. Respiratory system responsiveness in rabbits in vivo is reduced by prolonged continuous positive airway pressure. J Appl Physiol 99: 677–682, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Yokoe T, Minoguchi K, Matsuo H, Oda N, Minoguchi H, Yoshino G, Hirano T, Adachi M. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation 107: 1129–1134, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Zhao R, Du L, Huang Y, Wu Y, Gunst SJ. Actin depolymerization factor/cofilin activation regulates actin polymerization and tension development in canine tracheal smooth muscle. J Biol Chem 283: 36522–36531, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]