Abstract
Biomechanical properties of calf muscles and Achilles tendon may be altered considerably in children with cerebral palsy (CP), contributing to childhood disability. It is unclear how muscle fascicles and tendon respond to rehabilitation and contribute to improvement of ankle-joint properties. Biomechanical properties of the calf muscle fascicles of both gastrocnemius medialis (GM) and soleus (SOL), including the fascicle length and pennation angle in seven children with CP, were evaluated using ultrasonography combined with biomechanical measurements before and after a 6-wk treatment of passive-stretching and active-movement training. The passive force contributions from the GM and SOL muscles were separated using flexed and extended knee positions, and fascicular stiffness was calculated based on the fascicular force-length relation. Biomechanical properties of the Achilles tendon, including resting length, cross-sectional area, and stiffness, were also evaluated. The 6-wk training induced elongation of muscle fascicles (SOL: 8%, P = 0.018; GM: 3%, P = 0.018), reduced pennation angle (SOL: 10%, P = 0.028; GM: 5%, P = 0.028), reduced fascicular stiffness (SOL: 17%, P = 0.128; GM: 21%, P = 0.018), decreased tendon length (6%, P = 0.018), increased Achilles tendon stiffness (32%, P = 0.018), and increased Young's modulus (20%, P = 0.018). In vivo characterizations of calf muscles and Achilles tendon mechanical properties help us better understand treatment-induced changes of calf muscle-tendon and facilitate development of more effective treatments.
Keywords: muscle fascicles, Achilles tendon, stiffness, pennation, ultrasonography
cerebral palsy (cp) is a common disorder of movement and posture caused by a nonprogressive injury to the developing brain (10, 33). The neurological disorder can cause secondary changes in the musculoskeletal system, such as muscle weakness, spasticity, and/or contractures around joints, which makes CP a leading cause of childhood disability (5, 34). Unlike adults with disabilities, children with CP face the risk of further losing functional mobility during adolescence because of ongoing secondary impairments, such as soft-tissue shortening, weakness, and bony deformity (7). As a result, children who are ambulatory may change the need for assistance over time and become reliant on walkers or wheelchairs. These changes are complex but are attributable to loss of strength, poor alignment, and motivation (11). Efficient walking has been one of the treatment goals for children with CP. The ability to translate the tibia over the foot decreases the work of walking and improves the gait-normalcy index. Of all of the modalities to decrease muscle tone and improve range of motion (ROM), a temporary decrease of motoneuron function through botulinum toxin injections, selective section of sensory roots involved in the hyperactive reflex pathway, orthopedic surgery, and serial casting can have a significant effect but are costly and invasive. Passive-stretching and active-movement training is readily available as a part of a physical therapy treatment session and home-exercise program, although its effect size may be moderate (e.g., <10° improvement through stretching) (31, 45). Clinically, a physical therapist (PT) manipulates the joint and moves it throughout the ROM to reduce spasticity/contracture. Despite therapy and home programs that include stretching, the effects of passive stretching may not persist. In addition, manual stretching by PTs is laborious, and the outcome depends on subjective assessments, such as the PT's “end feel”. Splints, casts, tilt-table, and some other external devices are also applied to assist treating the patients, among which, continuous passive motion (CPM) devices are widely used to prevent postoperative adhesion and reduce joint stiffness (24). However, CPM machines can only move the limb at a constant speed between two prescribed joint positions. The passive movement does not usually stretch into the extreme positions, where contracture/spasticity is significant.
Controlled, passive ankle stretching has been used to treat ankle contracture and spasticity in neurologically impaired patients (46). The stretching device is driven by a servomotor, controlled by a digital signal processor. The stretching velocity is controlled to be inversely proportional to the joint-resistance torque, so that near the ROM limits, the muscles/tendons involved are stretched slowly and safely. Once the specific peak-resistance torque is reached, the motor holds the joint at the extreme positions for a prescribed period of time, which can be conveniently adjusted. In addition to the passive-stretching mode, the intelligent ankle-stretching device can be operated in active-movement mode and provide assistance or resistance to the subject's voluntary ankle movement. Motivated by interactive games, the subject can actively move the ankle through the ROM, and the exercise intensity can be adjusted progressively by changing the training difficulty level and the assistance/resistance level. In addition, the intelligent ankle-stretching device is portable, which provides convenient and low-cost treatment for children with CP.
The functional outcome of treating ankle joints with contracture/spasticity in stroke survivors using controlled stretching has been investigated (35, 46). However, the studies were mainly focused on the biomechanical evaluations of the ankle joint, including stiffness, viscosity, and ROM. A detailed understanding of in vivo calf muscle fascicles and tendon adaptations may help us to assess the efficacy of such treatment. In vivo gastrocnemius (GS) muscle architecture in children with CP has been investigated using ultrasonography, and shortened muscle fascicles were reported (8, 23, 25, 26). Several studies have also been carried out to investigate the calf muscles or tendon mechanical changes induced by repeated stretching and resistant exercise in able-bodied subjects (17, 27).
The objective of the present study was to investigate changes in mechanical properties of calf muscle fascicles and Achilles tendon in children with CP before and after a 6-wk combined passive-stretching and active-movement training program. We hypothesized that the 6-wk combined passive-stretching and active-movement treatment would elongate the calf muscle fascicles and reduce fascicular stiffness and pennation angle on the one hand and reduce Achilles tendon length and increase its stiffness and Young's modulus on the other hand.
METHODS
Participants
Seven children with spastic CP participated in the study (four hemiplegic and three diplegic; one female and six male). Mean age was 9.3 ± 3.2 (range 5∼15) yr. All of the children could follow instructions, and they were able to walk independently, with two of them at Gross Motor Function Classification System (GMFCS) Level II and five of them at GMFCS Level I.
Experimental Setup
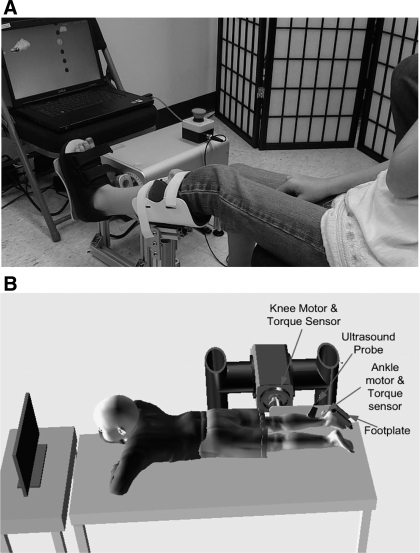
The participants were treated over a 6-wk period of time using the intelligent ankle-stretching device shown in Fig. 1A. The device was attached to a chair, where the child was seated comfortably with the leg supported by a brace and fixed at a prescribed position. The foot of the patient was secured to a footplate with the center of rotation of the ankle aligned with the motor-rotation axis. The ankle-joint torque and position were sampled by a digital signal processor, which controlled the joint movement and communicated with the display computer to show the interactive games.
Fig. 1.
A: ankle intelligent stretching device used for the combined passive-stretching and active-movement training over 6 wk. B: ultrasound and biomechanical evaluations of the soleus (SOL) and gastrocnemius (GS) muscle fascicles and Achilles tendon. The subject lay prone with the ankle and knee aligned with the custom knee–ankle-joint driving device with the joint torque and position measured synchronously with the ultrasound images.
Before and after the 6-wk treatment, ultrasound evaluations were performed. The setup was comprised of a custom-made knee–ankle-joint driving device, a GE LOGIQ 9 ultrasound imaging system with a 12-MHz high-resolution matrix probe M12L (GE Healthcare, Waukesha, WI) and a data acquisition computer (Fig. 1B). Ultrasound and biomechanical properties of the soleus (SOL) and GS muscle fascicles and Achilles tendon were evaluated. The subject lay prone with the ankle and knee aligned with the custom knee–ankle-joint driving device and the joint torque and position measured synchronously with the ultrasound images of calf fascicles and Achilles tendon. Further information about the evaluation setup can be found in ref. (47), except that the chair in the knee-ankle device was replaced by a bed with the subject lying down in a prone position.
Experimental Design
Passive-stretching and active-movement training.
The patients went through a three-sessions/wk, 6-wk program of combined passive ankle-stretching and active-movement training (45). Each training session consisted of 20-min passive-stretching, 30-min active-movement training, followed by 10-min passive stretching on the subject's impaired (for hemiplegic CP) or more impaired (in diplegic CP) side (45). The participants sat upright comfortably and were asked to relax during the passive stretching; the predetermined torque was applied to strenuously stretch the calf muscle toward extreme dorsiflexion. During the active-movement training, the participants used the ankle rehabilitation robot and actively engaged themselves in computer games to exercise the ankle joint in both dorsiflexion and plantar flexion. The robot provided assistance if the subject had difficulty finishing the movement task, and it provided resistance if the subject could perform the task easily (45).
Outcome Evaluations Using Ultrasonic and Biomechanical Measurements
Before and after the 18 training sessions, biomechanical properties of the calf muscles and Achilles tendon on the impaired (for hemiplegic CP) or more impaired (in diplegic CP) side were evaluated using ultrasonography combined with biomechanical measurements. The participant lay down in a prone position with the thigh and trunk secured to the bed using Velcro straps. The leg and foot were securely attached to the leg linkage and footplate, respectively, and the knee and ankle joints aligned with the motor-rotation axes. To evaluate muscle fascicle properties, the ankle was fixed at various positions (at 10° intervals) across the participant's ankle ROM. All measurements were obtained with the knee in 90° flexion and full extension to separate ankle-joint torque contributions from the SOL and GS muscles. The ankle-joint torque and the ultrasound image of the SOL and GS medialis (GM) muscle, at every knee-ankle configuration, were recorded, and the subject was asked to relax during the measurement.
GM and SOL Fascicle Images
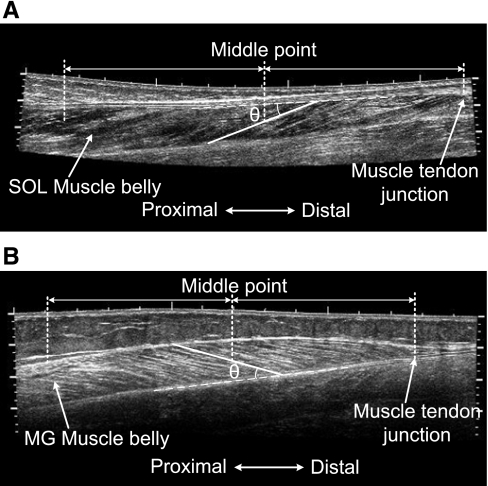
To improve the accuracy of the GM fascicle measurement, the probe was moved, scanning the transverse plane of the distal muscle belly of GM to locate the fascicle plane. Once the middle of the ultrasound image at the deep aponeurosis of GM was parallel to the bottom of the image, the probe was rotated 90°, and the image plane was regarded as being aligned with the fascicle plane (2). The probe was then moved proximally within the fascicle plane to cover the full longitudinal image of the GM muscle using an extended field-of-view technique called LOGIQView (41). To obtain the SOL fascicle image, the probe was moved along the posterior lower leg, scanning the sagittal plane using LOGIQView. The representative fascicle images of GM and SOL muscles are shown in Fig. 2, A and B.
Fig. 2.
Representative LOGIQView images of (A) SOL and (B) GS medialis (GM) muscles, demonstrating fascicle length and pennation angle measurement. Some landmarks, including muscle-tendon junction and muscle belly, are also marked on the images. MG, medial GS.
Measurement of Muscle Fascicle Length and Pennation Angle
In this study, the average fascicle length was defined as the length of the fascicle halfway between the middle-muscle belly and the distal muscle-tendon junction (MTJ). Usually, the clearest fascicle in the region was selected for length measurement. For GM, the pennation angle was defined as the angle between the fascicle selected for length measurement and deep aponeurosis of GM. For SOL, the pennation angle was defined as the angle between the fascicle selected for length measurement and superficial aponeurosis of SOL. ImageJ (National Institutes of Health, Bethesda, MD) software was used to measure the fascicle length and pennation angles displayed in Fig. 2, A and B.
Achilles Tendon Mechanical Properties
The Achilles tendon was scanned in the sagittal plane at rest using a special function of LOGIQ 9, called LOGIQView, and the tendon resting length was measured using ImageJ. The transverse plane of the Achilles tendon was scanned to measure the cross-sectional area (CSA). The tendon moment arm was measured using a physical measurement and ultrasonography combined method. Then, the subject performed a controlled isometric plantar flexion effort, following the target and actual torques displayed in real-time on the monitor, with the ultrasound video and ankle-joint torque recorded synchronously. The tendon-length change was calculated by tracking the displacement of MTJ between frames in the ultrasound video. The tendon force was computed from the joint torque and tendon moment arm. The tendon stiffness was further calculated based on the tendon elongation and force. The detailed methods were described in ref. (47).
SOL and GM Muscle Fascicular Stiffness
The Achilles tendon moment arm at 0° dorsiflexion was measured for each subject. The values of the moment arm at other ankle-joint angles were computed based on the moment arm at 0° dorsiflexion and the moment arm–ankle-joint angle relation curve obtained from Software for Interactive Muskuloskeletal Modeling (SIMM; Motion Analysis, Santa Rosa, CA). It was assumed that the passive tension of the GS muscle was negligible at 90° knee flexion (9, 27a). Therefore, most of the ankle-resistance torque at 90° knee flexion came from the SOL muscle. The SOL passive force was then calculated in the range of 20° plantar flexion to 10° dorsiflexion. The corresponding SOL fascicular force was scaled by 1/cos(θSOL), with θSOL as the pennation of SOL fascicles. The GS contribution to the ankle passive-resistance torque was estimated as the difference of the passive ankle-resistance torque between full knee extension and 90° knee flexion, as GS muscles are major ankle plantar flexors crossing the knee joint. The GS passive force was also calculated in the range of 20° plantar flexion to 10° dorsiflexion. The passive force from GM was determined as 61% of the total passive GS force based on the method described in refs. (6, 9). The corresponding GM fascicular force was scaled by 1/cos(θGM), with θGM as the pennation of GM fascicles. The fascicular stiffness of GM and SOL was determined as the slope of their force–fascicle length relation curves.
Statistical Analysis
Nonparametric analysis (Friedman test and Wilcoxon's signed ranks test) was used to analyze the response variables (fascicle length and stiffness, pennation angle, and Achilles tendon length) with respect to the factors (pre- and post-treatment, ankle position, knee position). Spearman's rank correlation was used to test the monotonic trend (e.g., if one variable is increased, then the other one also follows). The significance level was set at 0.05. The results were presented as means ± SD unless specified otherwise.
RESULTS
SOL and GM Fascicle Length and Pennation Angle
As a uniarticular muscle, the SOL muscle fascicle length varied with ankle angle (P < 0.001). The fascicle length increased monotonically as the ankle dorsiflexed (P = 0.003).
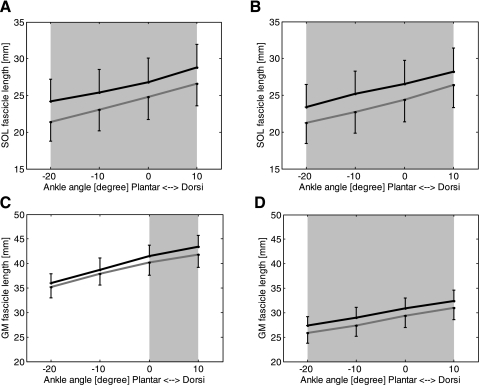
After the 6-wk treatment, SOL fascicle length increased significantly at both full knee extension (Fig. 3A) and 90° knee flexion (Fig. 3B; P < 0.001). For example, at full knee extension and 0° ankle dorsiflexion, SOL fascicle length increased significantly (P = 0.018) from 24.8 ± 8.2 mm to 26.8 ± 8.6 mm (Fig. 3A). At 90° knee flexion and 0° ankle dorsiflexion, SOL fascicle length increased significantly (P = 0.018) from 24.4 ± 8.0 mm to 26.5 ± 8.5 mm (Fig. 3).
Fig. 3.
Fascicle length before (gray lines) and after (black lines) the 6-wk treatment of passive-stretching and active-movement training. A: SOL fascicle length measured at full knee extension; B: SOL fascicle length measured at 90° knee flexion. The error bars represent the SEM. C: GM fascicle length measured at full knee extension. D: GM fascicle length measured at 90° knee flexion. The error bars represent SEM. Shading indicates a significant difference between before and after the 6-wk treatment.
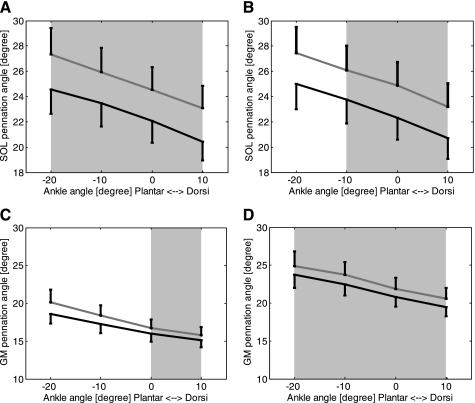
Together with elongation of SOL fascicles after the 6-wk treatment, the SOL pennation angle decreased significantly at the extended (Fig. 4A) and flexed (Fig. 4B) knee positions (P < 0.001). For example, at 0° ankle dorsiflexion and full knee extension, the pennation angle decreased significantly (P = 0.028) from 24.5 ± 4.8° to 22.1 ± 4.5°, and at 90° knee flexion and 0° ankle dorsiflexion, SOL pennation angle decreased significantly (P = 0.028) from 24.9 ± 4.9° to 22.3 ± 4.6°.
Fig. 4.
Pennation angle before (gray lines) and after (black lines) the 6-wk treatment. A: SOL pennation angle measured at full knee extension; B: SOL pennation angle measured at 90° knee flexion. The error bars represent the SEM. C: GM pennation angle measured at full knee extension; D: GM pennation angle measured at 90° knee flexion, with the error bars also representing the SEM. Shading indicates a significant difference between before and after the 6-wk treatment.
As a biarticular muscle, the GM muscle fascicle length varied with both ankle (P < 0.001) and knee flexion (P < 0.001). The fascicle length increased monotonically as the ankle dorsiflexed (P = 0.004) but decreased as the knee flexed (P < 0.0001).
Similar to the SOL muscle, the GM muscle showed significantly increased fascicle length (Fig. 3, C and D, P < 0.001) and decreased pennation angle (Fig. 4, C and D, P < 0.001) at both full knee extension and 90° knee flexion after the 6-wk training. For example, at 0° ankle dorsiflexion and full knee extension, GM fascicle length increased significantly (P = 0.018) from 40.2 ± 6.6 mm to 41.5 ± 5.9 mm, and pennation angle decreased significantly (P = 0.028) from 16.7 ± 2.9° to 16.0 ± 2.8°. At 90° knee flexion and 0° ankle dorsiflexion, GM fascicle length increased significantly (P = 0.018) from 29.3 ± 6.2 mm to 30.9 ± 5.5 mm, and pennation angle decreased significantly (P = 0.043) from 21.9 ± 4.0° to 20.8 ± 3.3°.
SOL and GM Muscle Fascicular Stiffness
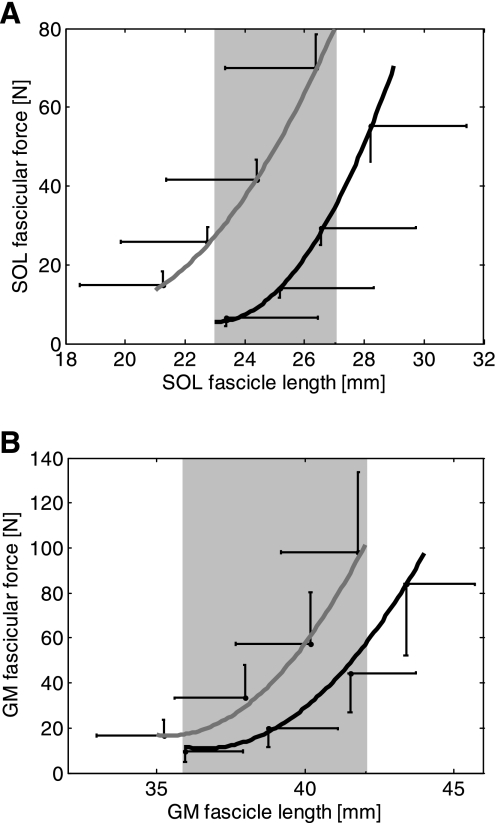
With the SOL and GM fascicular force at four ankle positions, calculated from the ankle-joint torque using the method previously described (47), the mean results over the subjects were plotted with respect to the fascicle length and curve fitted using second order polynomial as shown in Fig. 5, A and B. For both SOL and GM, the force-length curves shifted to the right and became less steep after the 6-wk training, indicating that the fascicles became longer and less stiff. For example, at 0° ankle dorsiflexion and full knee extension, the passive SOL fascicle stiffness decreased slightly (P = 0.128) from 33.3 ± 10.4 N/mm before training to 27.6 ± 12.5 N/mm after training, whereas the passive stiffness of GM fascicles reduced significantly (P = 0.018) from 12.2 ± 3.8 N/mm to 9.6 ± 3.6 N/mm.
Fig. 5.
A: SOL fascicular force-length relation curve before (gray line) and after (black line) the 6-wk treatment of combined passive-stretching and active-movement training. B: GM fascicular force-length relation curve before (gray line) and after (black line) the treatment. The error bars represent the SEM. Shading indicates a significant difference between before and after the 6-wk treatment.
Achilles Tendon Mechanical Properties
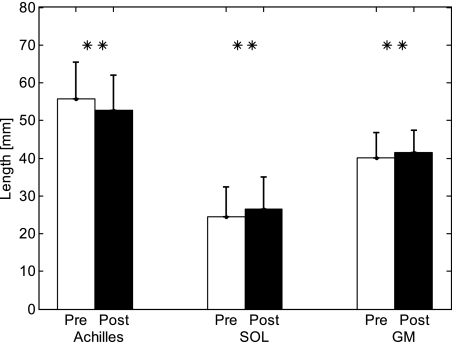
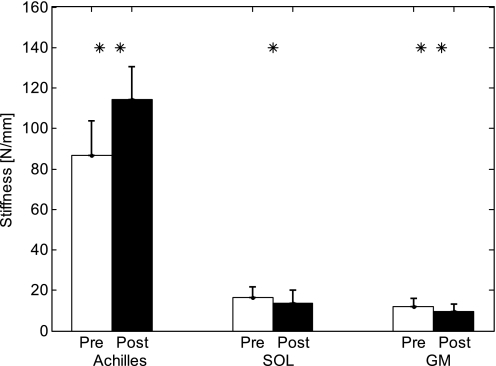
After training, Achilles tendon length decreased (P = 0.018) from 55.7 ± 9.9 mm to 52.6 ± 9.4 mm, as opposed to a length increase of SOL and GM fascicles measured at 0° dorsiflexion and full knee extension (Fig. 6), whereas no significant change of CSA was found (P = 0.063). Achilles tendon stiffness increased significantly from 86.8 ± 16.9 N/mm to 114.4 ± 16.0 N/mm (P = 0.018), and the Young's modulus increased significantly (P = 0.018) from 113.6 ± 34.9 megapascals (MPa) to 136.8 ± 38.8 MPa, as opposed to stiffness decrease of SOL and GM fascicles, measured at 0° dorsiflexion and full knee extension (Fig. 7).
Fig. 6.
Comparisons of the Achilles tendon resting length and SOL and GM fascicle lengths measured at full knee extension and 0° ankle dorsiflexion before and after the 6-wk treatment. ** Significant difference with P < 0.01.
Fig. 7.
Comparisons of the Achilles tendon stiffness and SOL and GM fascicular stiffness lengths measured at full knee extension and 0° ankle dorsiflexion before and after the 6-wk treatment. *Significant difference with P < 0.05; **significant difference with P < 0.01.
DISCUSSION
In the present study, changes of GM and SOL muscle fascicles and Achilles tendon mechanical properties induced by passive-stretching and active-movement training were evaluated in vivo and noninvasively using ultrasound in children with CP. Results showed significant changes of physical dimension and biomechanical properties in calf muscle fascicles and Achilles tendon after training, including increased calf muscle fascicle length, decreased pennation angle, decreased fascicular stiffness, shortened Achilles tendon, and increased tendon stiffness, indicating the adaptability and responsiveness of the children's growing muscles and tendons to the treatment.
The results on GM muscle architecture, such as fascicle length (ranged from 25.87 ± 5.61 to 41.78 ± 6.89 mm across the tested ankle and knee positions) and pennation angle (ranged from 15.76 ± 2.82° to 24.90 ± 5.05°; 20.17 ± 4.36° at ankle resting position and full knee extension), in children with CP agree with previous studies (1, 23, 25, 26, 37, 38, 42a). The Achilles tendon length quantified at neutral ankle and 90° knee flexion changed from 55.7 ± 9.9 mm to 52.6 ± 9.4 mm postintervention, which was in agreement with a previous study (8), in which Achilles tendon length was measured across larger ankle and knee range (45.7 ± 3.8 mm to 64.1 ± 10.3 mm). There is a lack of published results on SOL muscle fascicle length in children with CP for relevant comparisons, but in young adults, SOL muscle fascicle length ranged from 29 ± 5 mm to 43 ± 5 mm across various ankle and knee positions (12), which were considerably longer than those in the children with CP in the current study (ranged from 21.3 ± 7.4 mm to 26.6 ± 7.9 mm and from 24.2 ± 7.9 mm to 28.2 ± 8.5 mm pre- and postintervention, respectively).
Achilles tendon stiffness (86.8 ± 16.9 N/mm and 114.4 ± 16.0 N/mm pre- and postinterventions, respectively) in the current study falls into the wide range of tendon stiffness reported in the literature. For example, in adult human subjects, the tendon stiffness was reported as 30 N/mm (17), 150 N/mm (18a), 180 N/mm (18), and 330 N/mm (28). Young's modulus of the Achilles tendon was measured in vivo as 788 MPa in adult male subjects (22) and in vitro on adult cadaver specimens as 820 MPa (43), whereas the modulus in rats was reported as 33–53 MPa (4). In the current study, Young's modulus of the Achilles tendon included 113.6 ± 34.9 MPa and 136.8 ± 38.8 MPa pre- and postinterventions, respectively. The tendon stiffness and Young's modulus from kids in the current study were lower than those reported on adults. The difference might be attributed to factors including aging (30), test protocol (e.g., loading rate) (43), and specimen condition (i.e., in vivo or in vitro) (39), as well as experimental errors in the different studies. It was reported that with growing and aging, tendons become much stronger and stiffer than at birth (36). Differences in Achilles tendon stiffness and Young's modulus between data in literature and our results might also be attributed to overall nonlinear tendon stress-strain properties. In the present study, Young's modulus and stiffness were calculated under submaximal contractions within the manageable force range of children with CP, whereas some other studies used much higher forces with muscles under maximal voluntary contraction (19–21).
As shown in results, the Achilles tendon was much stiffer than GM and SOL fascicles under passive condition, which reflects the difference in stiffness between muscles and tendons in general (Fig. 7). Furthermore, GM fascicles were less stiff than SOL fascicles at both sides, which could be related to the different functional roles played by the biarticular GM (mostly fast-twitch fibers with strong contractions and rapid development of large tension) and uniarticular SOL (mostly slow-twitch fibers and long, sustaining contractions) muscles (40).
Significant fascicle elongation, reduction in pennation, and decrease in fascicular stiffness were observed in both SOL and GS muscles after the 6-wk intensive treatment of combined passive-stretching and active-movement training, which indicates that the passive-stretching and active-movement treatment loosened up the stiff muscles and reduced the tension in muscle fascicles. It was reported that stretching of muscles may delay atrophy and can even cause growth (3, 13, 14). The passive-stretching and active-movement training may be especially effective in reducing muscle stiffness and preventing contracture for growing children with highly adaptive muscles and tendons. The treatment-induced tension reduction could be due to tension reduction in muscle fascicles themselves and/or in connective tissues around the muscle fascicles and the whole muscle. Furthermore, the results showed relatively greater changes in fascicles/tendon stiffness than the fascicles/tendon length changes, which may be due to the combined effect of the fascicle/tendon length and force changes on the stiffness changes.
Biomechanical properties of the Achilles tendon were changed significantly after the 6-wk combined passive-stretching and active-movement training, indicating that the growing tendons of the children were responsive to the treatment. It was reported that the combined stiffness of tendon and aponeurosis increased significantly after 8-wk, 4 sessions/wk, resistance training (18.8%) or resistance and static-stretching training (15.3%) (17), whereas 3-wk static stretching at 35° dorsiflexion with the ankle at the standing position induced reduction in tendon-aponeurosis viscosity but no effect on its elasticity (16). Achilles tendon mechanical properties were shown degraded in the elderly, characterized as reduced stiffness, and a three times/wk over 14-wk resistance exercise training reversed the process and increased tendon stiffness significantly by ∼65% (32). In an animal study, 21-day passive stretching on the ankle significantly increased the tendon maximum stress (∼21%) and modulus of elasticity (∼55%) (4). The increase in tendon stiffness could be attributed to adaptation and remodeling of tendon structure subject to mechanical stimuli at the cellular level, and evidences have been shown in both animal and human experimental (4, 15) and theoretical studies (42). Further studies need to be carried out to differentiate the changes induced by the passive-stretching and active-movement training.
There were limitations with the study. The fascicular force-length relation might be affected by the simplified model of calculating the fascicular force from the ankle-joint dorsiflexion-resistance torque. The model assumed that the torque contribution from SOL did not change with knee flexion angle, and most ankle torque at 90° knee flexion came from SOL. Moreover, the model did not take into account the torque contribution from other muscles and soft tissues, the intramuscular force, and the complicated relation between muscle force and fascicular force. Nevertheless, according to Fig. 3, A and B, the fascicle length of SOL did not change much from 90° knee flexion to full knee extension, indicating the resistance torque from SOL did not change with knee flexion angle. In addition, the sample size of the present study was relatively small. More subjects should be included in future studies.
In the present study, architectural and mechanical properties of GM and SOL muscle fascicles and Achilles tendon in children with CP were evaluated before and after a 6-wk program of passive-stretching and active-movement training. Increased calf muscle fascicle length and decreased fascicular stiffness, decreased tendon resting length, and increased tendon stiffness were induced by the 6-wk training. The combined ultrasonic and biomechanical evaluations help us understand calf muscle and Achilles tendon functions and gain insight into the closely related biomechanical changes of calf muscles and Achilles tendon in neurological disorders, provide quantitative assessment for rehabilitation treatment, and conduct CP rehabilitation more effectively.
GRANTS
The authors acknowledge the support of the National Institutes of Health and National Institute on Disability and Rehabilitation Research.
DISCLOSURES
The rehabilitation robotic device used in the 6-wk treatment part in this study is developed by Rehabtek LLC (Wilmette, IL) under federal Small Business Technology Transfer grant support. L-Q. Zhang and Y. Ren have equity positions in Rehabtek.
REFERENCES
- 1. Barrett RS, Lichtwark GA. Gross muscle morphology and structure in spastic cerebral palsy: a systematic review. Dev Med Child Neurol 52: 794–804, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Benard MR, Becher JG, Harlaar J, Huijing PA, Jaspers RT. Anatomical information is needed in ultrasound imaging of muscle to avoid potentially substantial errors in measurement of muscle geometry. Muscle Nerve 39: 652–665, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Buschbacher RM, Porter CD. Deconditioning, conditioning, and the benefits of exercise. In: Physical Medicine and Rehabilitation (2nd ed.), edited by Braddom RL. Philadelphia, PA: W. B. Sauders, 2000, p. 702–726 [Google Scholar]
- 4. de Almeida F, Tomiosso T, Nakagaki W, Gomes L, Matiello-Rosa S, Pimentel E. Effects of passive stretching on the biochemical and biomechanical properties of calcaneal tendon of rats. Connect Tissue Res 50: 279–284, 2009 [PubMed] [Google Scholar]
- 5. Engsberg JR, Ross SA, Olree KS, Park TS. Ankle spasticity and strength in children with spastic diplegic cerebral palsy. Dev Med Child Neurol 42: 42–47, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Fukunaga T, Roy RR, Shellock FG, Hodgson JA, Day MK, Lee PL, Wong-Fu H, Edgerton VR. Physiological cross-sectional area of human leg muscles based on magnetic resonance imaging. J Orthop Res 10: 926–934, 1992 [DOI] [PubMed] [Google Scholar]
- 7. Gajdosik CG, Cicirello N. Secondary conditions of the musculoskeletal system in adolescents and adults with cerebral palsy. Phys Occup Ther Pediatr 21: 49–68, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Gao F, Zhao H, Gaebler-Spira D, Zhang L-Q. In vivo evaluations of morphological changes of gastrocnemius muscle fascicles and Achilles tendon in children with cerebral palsy. Am J Phys Med Rehab 90: 364–371, 2011 [DOI] [PubMed] [Google Scholar]
- 9. Gao F, Grant TH, Roth EJ, Zhang LQ. Changes in passive mechanical properties of the gastrocnemius muscle at the muscle fascicle and joint levels in stroke survivors. Arch Phys Med Rehabil 90: 819–826, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Hagberg B, Hagberg G, Olow I. The changing panorama of cerebral palsy in Sweden. IV. Epidemiological trends 1959–78. Acta Paediatr Scand 73: 433–440, 1984 [DOI] [PubMed] [Google Scholar]
- 11. Hanna SE, Rosenbaum PL, Bartlett DJ, Palisano RJ, Walter SD, Avery L, Russell DJ. Stability and decline in gross motor function among children and youth with cerebral palsy aged 2 to 21 years. Dev Med Child Neurol 51: 295–302, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Kawakami Y, Ichinose Y, Fukunaga T. Architectural and functional features of human triceps surae muscles during contraction. J Appl Physiol 85: 398–404, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Kemp TJ, Sadusky TJ, Saltisi F, Carey N, Moss J, Yang SY, Sassoon DA, Goldspink G, Coulton GR. Identification of Ankrd2, a novel skeletal muscle gene coding for a stretch-responsive ankyrin-repeat protein. Genomics 66: 229–241, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Kemp TJ, Sadusky TJ, Simon M, Brown R, Eastwood M, Sassoon DA, Coulton GR. Identification of a novel stretch-responsive skeletal muscle gene (Smpx). Genomics 72: 260–271, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Kjaer M, Langberg H, Heinemeier K, Bayer ML, Hansen M, Holm L, Doessing S, Kongsgaard M, Krogsgaard MR, Magnusson SP. From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scand J Med Sci Sports 19: 500–510, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Kubo K, Kanehisa H, Fukunaga T. Effect of stretching training on the viscoelastic properties of human tendon structures in vivo. J Appl Physiol 92: 595–601, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Kubo K, Kanehisa H, Fukunaga T. Effects of resistance and stretching training programmes on the viscoelastic properties of human tendon structures in vivo. J Physiol 538: 219–226, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lichtwark GA, Wilson AM. In vivo mechanical properties of the human Achilles tendon during one-legged hopping. J Exp Biol 208: 4715–4725, 2005 [DOI] [PubMed] [Google Scholar]
- 18a. Maganaris CN. Tensile properties of in vivo human tendinous tissue. J Biomech 35: 1019–1027, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Maganaris CN, Paul JP. In vivo human tendon mechanical properties. J Physiol 521: 307–313, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Magnusson SP, Aagaard P, Dyhre-Poulsen P, Kjaer M. Load-displacement properties of the human triceps surae aponeurosis in vivo. J Physiol 531: 277–288, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Magnusson SP, Hansen P, Aagaard P, Brond J, Dyhre-Poulsen P, Bojsen-Moller J, Kjaer M. Differential strain patterns of the human gastrocnemius aponeurosis and free tendon, in vivo. Acta Physiol Scand 177: 185–195, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Malaiya R, McNee AE, Fry NR, Eve LC, Gough M, Shortland AP. The morphology of the medial gastrocnemius in typically developing children and children with spastic hemiplegic cerebral palsy. J Electromyogr Kinesiol 17: 657–663, 2007 [DOI] [PubMed] [Google Scholar]
- 24. McNair PJ, Dombroski EW, Hewson DJ, Stanley SN. Stretching at the ankle joint: viscoelastic responses to holds and continuous passive motion. Med Sci Sports Exerc 33: 354–358, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Mohagheghi AA, Khan T, Meadows TH, Giannikas K, Baltzopoulos V, Maganaris CN. Differences in gastrocnemius muscle architecture between the paretic and non-paretic legs in children with hemiplegic cerebral palsy. Clin Biomech (Bristol, Avon) 22: 718–724, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Mohagheghi AA, Khan T, Meadows TH, Giannikas K, Baltzopoulos V, Maganaris CN. In vivo gastrocnemius muscle fascicle length in children with and without diplegic cerebral palsy. Dev Med Child Neurol 50: 44–50, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Morse CI, Degens H, Seynnes OR, Maganaris CN, Jones DA. The acute effect of stretching on the passive stiffness of the human gastrocnemius muscle tendon unit. J Physiol 586: 97–106, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27a. Muraoka T, Chino K, Muramatsu T, Fukunaga T, Kanehisa H. In vivo passive mechanical properties of the human gastrocnemius muscle belly. J Biomech 38: 1213–1219, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Muraoka T, Muramatsu T, Fukunaga T, Kanehisa H. Geometric and elastic properties of in vivo human Achilles tendon in young adults. Cells Tissues Organs 178: 197–203, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Nakagawa Y, Hayashi K, Yamamoto N, Nagashima K. Age-related changes in biomechanical properties of the Achilles tendon in rabbits. Eur J Appl Physiol Occup Physiol 73: 7–10, 1996 [DOI] [PubMed] [Google Scholar]
- 31. Pin T, Dyke P, Chan M. The effectiveness of passive stretching in children with cerebral palsy. Dev Med Child Neurol 48: 855–862, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Reeves ND. Adaptation of the tendon to mechanical usage. J Musculoskelet Neuronal Interact 6: 174–180, 2006 [PubMed] [Google Scholar]
- 33. Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, Dan B, Jacobsson B. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol 109: 8–14, 2007 [PubMed] [Google Scholar]
- 34. Sanger TD, Delgado MR, Gaebler-Spira D, Hallett M, Mink JW. Classification and definition of disorders causing hypertonia in childhood. Pediatrics 111: e89–e97, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Selles RW, Li X, Lin F, Chung SG, Roth EJ, Zhang LQ. Feedback-controlled and programmed stretching of the ankle plantarflexors and dorsiflexors in stroke: effects of a 4-week intervention program. Arch Phys Med Rehabil 86: 2330–2336, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Shadwick RE. Elastic energy storage in tendons: mechanical differences related to function and age. J Appl Physiol 68: 1033–1040, 1990 [DOI] [PubMed] [Google Scholar]
- 37. Shortland AP, Harris CA, Gough M, Robinson RO. Architecture of the medial gastrocnemius in children with spastic diplegia. Dev Med Child Neurol 43: 796–801, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Shortland AP, Harris CA, Gough M, Robinson RO. Architecture of the medial gastrocnemius in children with spastic diplegia. Dev Med Child Neurol 44: 158–163, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Smith C, Young I, Kearney J. Mechanical properties of tendons: changes with sterilization and preservation. J Biomech Eng 118: 56–61, 1996 [DOI] [PubMed] [Google Scholar]
- 40. Staron RS, Kraemer WJ, Hikida RS, Fry AC, Murray JD, Campos GER. Fiber type composition of four hindlimb muscles of adult Fisher 344 rats. Histochem Cell Biol 111: 117–123, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Weng L, Tirumalai AP, Lowery CM, Nock LF, Gustafson DE, Von Behren PL, Kim JH. US extended-field-of-view imaging technology. Radiology 203: 877–880, 1997 [DOI] [PubMed] [Google Scholar]
- 42. Wren TA, Beaupre GS, Carter DR. Tendon and ligament adaptation to exercise, immobilization, and remobilization. J Rehabil Res Dev 37: 217–224, 2000 [PubMed] [Google Scholar]
- 42a. Wren TA, Cheatwood AP, Rethlefsen SA, Hara R, Perez FJ, Kay RM. Achilles tendon length and medial gastrocnemius architecture in children with cerebral palsy and equinus gait. J Pediatr Orthop 30: 479–484, 2010 [DOI] [PubMed] [Google Scholar]
- 43. Wren TA, Yerby SA, Beaupre GS, Carter DR. Mechanical properties of the human Achilles tendon. Clin Biomech (Bristol, Avon) 16: 245–251, 2001 [DOI] [PubMed] [Google Scholar]
- 45. Wu YN, Hwang M, Ren Y, Gaebler-Spira DJ, Zhang LQ. Combined passive stretching and active movement rehabilitation of lower-limb impairments in children with cerebral palsy using a portable robot. Neurorehabil Neural Repair 25: 378–385, 2011 [DOI] [PubMed] [Google Scholar]
- 46. Zhang LQ, Chung SG, Bai Z, Xu D, van Rey EM, Rogers MW, Johnson ME, Roth EJ. Intelligent stretching of ankle joints with contracture/spasticity. IEEE Trans Neural Syst Rehabil Eng 10: 149–157, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Zhao H, Ren Y, Wu YN, Liu SQ, Zhang LQ. Ultrasonic evaluations of Achilles tendon mechanical properties post stroke. J Appl Physiol 106: 843–849, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]