Abstract
Amyotrophic lateral sclerosis and spinal muscular atrophy are devastating neurodegenerative diseases that lead to the specific loss of motor neurons. Recently, stem cell technologies have been developed for the investigation and treatment of both diseases. Here we discuss the different stem cells currently being studied for mechanistic discovery and therapeutic development, including embryonic, adult and induced pluripotent stem cells. We also present supporting evidence for the utilization of stem cell technology in the treatment of amyotrophic lateral sclerosis and spinal muscular atrophy, and describe key issues that must be considered for the transition of stem cell therapies for motor neuron diseases from bench to bedside. Finally, we discuss the first-in-human Phase I trial currently underway examining the safety and feasibility of intraspinal stem cell injections in amyotrophic lateral sclerosis patients as a foundation for translating stem cell therapies for various neurological diseases.
Keywords: amyotrophic lateral sclerosis, induced pluripotent stem cells, motor neuron disease, Phase I clinical trial, spinal muscular atrophy, stem cells, translational medicine
Stem cell technologies are becoming increasingly popular tools for the investigation and treatment of various diseases. In the current review, we describe the foundation supporting the utilization of stem cells for motor neuron disease treatment. We begin with an overview of stem cell biology and then discuss the rationale behind the utilization of stem cells for mechanistic discovery and treatment of amyotrophic lateral sclerosis (ALS) and spinal muscular atrophy (SMA). Finally, we describe the translation of stem cell therapy from the bench to the bedside, discussing the first clinical trial currently underway examining the safety of intraspinal neural stem cell injections in ALS patients.
Stem cells: the right tool for the right job
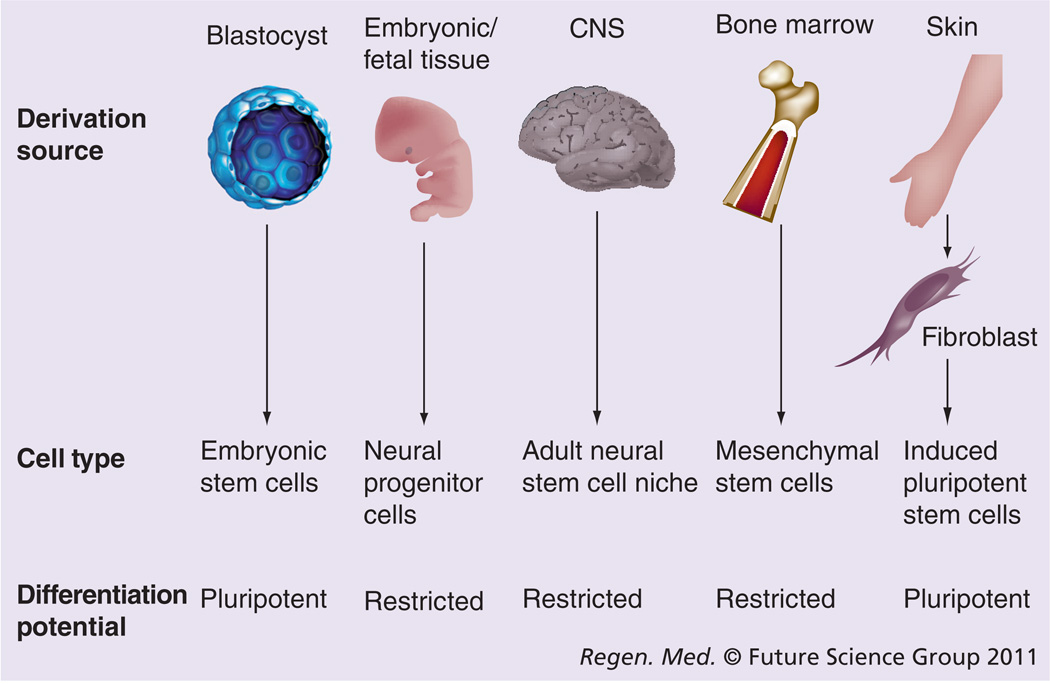
‘Stem cell’ is a term commonly used to describe several distinct cell populations that share specific cellular characteristics. The basic tenets of a stem cell are the ability to self-renew and differentiate into multiple cell types, and there are a range of cell lineages including embryonic stem (ES) cells [1,2], neural progenitor cells (NPCs) [3,4], adult neural stem cells [5], and adult non-neural mesenchymal stem cells (MSCs) [6,7] that differ in their source of derivation and differentiation potential (Figure 1). Pluripotent cells such as ES cells have unrestricted ability to differentiate into cells from all three germ layers, whereas the differentiation of NPCs and MSCs are inherently limited to their respective lineage. While the pluripotency of ES cells and the neuronal differentiation potential of NPCs and neural MSCs are appealing characteristics for the treatment of motor neuron diseases, adult non-neural MSCs are more abundant and more readily isolated than any of the other adult stem cells. These cells provide an option for the development of autologous cellular therapies to circumvent the immunoreactive issues of tissue grafting, but development for the treatment of neurological disorders relies in part on the ability to differentiate across lineages, from mesenchymal to neural. Many papers have described the neuronal differentiation of MSCs to varying degrees [8,9]; however, the generation of MSCs or any other stem cell from a patient with a degenerative disease carries the risk of the resultant population being compromised in some way.
Figure 1. Derivation of stem cells for the study of neurological disease and therapeutic development.
Embryonic stem cells are derived from the blastocyst inner cell mass and have an unrestricted ability to differentiate into cells from all three germ layers. Neural progenitors and neural stem cells are derived from fetal or adult tissue, respectively, and give rise to neural lineages. Mesenchymal stem cells are derived from bone marrow, but must differentiate across lineages to produce neural cells for autologous therapies. Fibroblasts are utilized for the derivation of induced pluripotent stem cells, which may be utilized for the generation of new models of neurological disease.
When faced with the question of which stem cells are correct for the treatment of disease, it is likely that there is no right answer. Better questions are what do these particular stem cells offer for a particular disease, and what is the stem cell expected to do? There are multiple ways a stem cell can interact with its environment in the context of motor neuron diseases. Stem cells can offer cellular replacement, augment a cellular population, or provide an enriched extracellular microenvironment [10]. In motor neuron diseases it is unlikely that direct replacement of the motor neuron populations will be a viable option. While there are established protocols to purify and enrich stem cells for motor neuron differentiation, there is no evidence that cellular replacement would lead to target muscle innervation [11–15]. In a disease like ALS where the non-neuronal component of disease is perpetuated by glial dysfunction, addition of ‘fresh’ glia to augment the resident glial population may alleviate the stress on the endogenous population [16]. Trophic support by grafted cells through the production of growth factors may also provide first aid to diseased motor neurons [10,17]. Many growth factors have very potent neuroprotective properties and may enrich the microenvironment of the spinal cord [10]. Each individual stem cell line will have a unique signature with respect to its secretion profile and its terminal differentiation capabilities. The strength of stem cell therapy is that the potentially beneficial effects are not mutually exclusive, and both cellular and trophic interventions are afforded by a single treatment.
Exploration of motor neuron disease mechanisms
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis is an adult-onset, rapidly progressing neurodegenerative disease characterized by the selective loss of both upper and lower motor neurons. ALS has an estimated incidence of 3–5 cases per 100,000 in the USA [18], with approximately 10% of patients affected by familial ALS (fALS). Autosomal dominant fALS is clinically and pathologically indistinguishable from sporadic ALS and both forms are lethal within 3–5 years of onset. Typically, ALS diagnosis occurs very late in the disease course after symptoms present, at a point when a large number of motor neurons are already lost. In 1993, mutations were reported in cytosolic Cu2+/Zn2+ superoxide dismutase (SOD1) in several fALS families [19] and currently more than 150 missense mutations in SOD1 have been identified [20,21]. Despite over a decade of study, however, the etiology of the toxic gain-of-function associated with mutant SOD1 has remained elusive. Studies have described a plethora of mechanisms including mitochondrial dysfunction, protein transport defects, aggregation, reactive oxygen species, excitotoxic stress, glial and astrocyte dysfunction, and many others [22]. More recently, mutations discovered in the DNA/RNA binding proteins TDP-43 and FUS/TLS have also added a new dimension to the genetic component of ALS and proposed potential mechanisms [23–26]. The growing consensus, however, is that the complex ALS disease phenotype is likely due to contributions from multiple mechanisms.
Traditionally, mutations associated with fALS have been utilized in both rats and mice to develop models for ALS. These rodent models display the same loss of motor neurons observed in human forms of ALS [27,28]. The SOD1-G93A mouse shows disease onset at 180 days, progressive motor defects and muscle weakness, and endstage at approximately 240 days [28]. The SOD1-G93A rat presents a very aggressive disease course, with disease onset around 115 days followed by rapid progression to endstage within 11 days, with associated symptomatic deficits in muscle strength throughout the disease course and motor neuron loss [27]. Finally, TDP-43 mutant mice develop progressive motor neuron, weight and muscle loss and provide an additional model to study ALS pathogenesis [29].
Research over the last decade has produced a single US FDA-approved drug for ALS, riluzole [30]. Since that time, several novel therapeutic approaches with a strong potential to treat ALS have also been investigated. Silencing of mutant SOD1 using a lentivirus-delivered short hairpin to SOD1 delays disease onset and disease progression when given prior to symptom onset in SOD1-G93A mice [31]. The β-lactam antibiotic ceftriaxone delays motor neuron loss, improves muscle strength and increases survival in the SOD1-G93A mouse, either through increased glutamate transporter expression [32] or by acting as a metal ion chelator [33]. Pioglitazone, an anti-inflammatory/antidiabetic agent, also improves muscle strength and motor neuron survival in SOD1-G93A mice [34]. Both manganese porphyrin and pyruvate treatments have shown beneficial effects on disease progression of the SOD1-G93A mouse [35,36]. Finally, growth factors have been increasingly described for their potential as therapeutic agents. Changes in cerebrospinal fluid levels of growth factors such as IGF-I, VEGF and others have lead to the hypothesis that upregulating and maintaining growth factor levels may provide neuroprotection for motor neurons [37,38]. Indeed, growth factor treatment demonstrates neuroprotective properties on motor neuron survival in vitro and in vivo [39–43]; however, translation to larger animals and to humans has been slow and not yielded the expected outcomes [44–48]. These results could potentially be related to the limited accessibility of such treatments to the motor neurons residing within the spinal cord [43].
Spinal muscular atrophy
Spinal muscular atrophy is an inherited autosomal disease that presents clinically with a broad range of onset and severity associated with the selective loss of motor neurons within the spinal cord and muscle weakness. Currently there is no effective treatment available for SMA and care options are based either around palliative care, respiratory protocols using Bipap machines, or a tracheotomy to ventilate the patient to assist in breathing. Clinically, there are four distinct forms of SMA [49]. SMA type I is the leading genetic cause of infantile mortality, and is the most severe and common form of SMA with an incidence of 1 in 6000. SMA type I is typically diagnosed within the first 6 months of life and has a poor prognosis, commonly associated with respiratory failure and death within 2 years. Infants present with proximal weakness, poor muscle tone and the inability to support themselves or hold their head up. SMA type II is less severe than type I with a slightly later onset and longer life expectancy, while SMA types III and IV both present with slow, mild muscle weakness and patients exhibit a normal lifespan. Because of the severity of SMA type I and its presentation in such young infants, it is a key disease to target for the development of stem cell therapies.
Over 90% of SMA cases are caused by a homozygous deletion of the survival motor neuron (SMN)1 gene. Despite the fact that most cases of SMA are associated with the lack of a functional SMN1 gene, the lost function of SMN1 that causes disease still remains in debate. In humans, a second copy of SMN exists, SMN2 [50]. SMN2 is identical to SMN1 with the exception of a single nucleotide mutation that prompts alternative splicing of the transcript, resulting in a nonfunctional truncated protein in which exon 7 is deleted. A small proportion of SMN2-derived transcripts do not get alternatively spliced, however, leading to a very small amount of functional full length protein that can partially compensate for the loss of SMN1 [51,52]. Thus, modulating the proportion of SMN2 that gives rise to full-length protein as a means to treat SMA is the subject of multiple studies [51].
Original attempts to create animal models of SMA relied on a knockout approach; however, other animals do not have the same SMN duplication as humans and knockout of SMN results in embryonic lethality [53]. On the other hand, Smn+/− mice that exhibit approximately a 50% reduction in SMN protein levels in the spinal cord resemble the less severe SMA type III with a slow disease progression and later-onset motor neuron loss [54]. Models based on the introduction of human SMN2 on a SMN knockout background (SMN2tg/Smn−/−), however, have been developed that mimic the genetic basis of human SMA type I and circumvent the embryonic lethality of traditional SMN knockout models [55]. The severity of these models is dependent on the dosage of SMN2; mice expressing 1–2 copies survive to postnatal day 5, whereas more than six copies abrogate the SMA phenotype in the mice [56,57]. These models further validate the feasibility of targeting SMN2 expression levels for SMA therapeutic development.
Induced pluripotent stem cells: the future of disease modeling & therapeutic development
Patient-specific induced pluripotent stem (iPS) cells provide ideal new models for the study of diseases such as SMA and ALS, as they essentially link cell behavior to donor disease phenotypes. iPS cells, which are developed by reprogramming somatic cells back to a pluripotent state, offer numerous advantages for studying disease mechanisms and discovering and developing novel therapies [58–61]. For diseases like ALS where the majority of cases have no known genetic etiology, iPS cells provide a means to develop models of both familial and sporadic disease. Patient-specific iPS cell lines can then be used to examine disease mechanisms, for drug discovery, or as a means to provide cells for cellular replacement therapy. To generate iPS cells, fibroblasts are isolated after a skin punch biopsy (Figure 1). After fibroblast expansion, multiple methods exist to generate iPS lines. The original reported methodology involves retroviral delivery of a cocktail of four transcription factors known as the Yamanaka factors, consisting of oct3/4, Sox2, c-Myc and Klf4, which are sufficient to change the phenotype of the fibroblasts, reprogramming them back to a stem cell state [62]. iPS cells can then be subsequently differentiated into neurons to generate a new human model of disease. Since the first reports of iPS cells, many groups have worked to improve the technology using different combinations of up to six factors [60,62–69]. Various viral delivery mechanisms, or alternatively direct protein treatments, have also been examined to circumvent some concerns with genomic incorporation and silencing of the factors, and methods utilizing nonintegrating protocols now exist [60,62–70]. Some concerns still remain regarding the appropriate protocols for reprogramming factor combinations and method of gene transfer; however, iPS technology is progressing rapidly for the modeling of human diseases and drug discovery.
Induced pluripotent stem cell lines have recently been established from patients with multiple neurodegenerative diseases including Huntington’s disease [71], Parkinson’s disease [72] and Freidreich ataxia [73], as well as from ALS [74] and SMA [75] patients. Dimos et al. described the first iPS cells generated from an 82-year-old ALS patient in 2008 [74]. The patient demonstrated a rare, slowly progressing disease with clear clinical symptoms. This study demonstrated that neither age nor disease state perturbs the ability to generate iPS cells. They further showed that iPS cells could generate motor neurons, although additional assessment of the motor neurons for ALS disease characteristics was not presented. The first iPS cells from a patient with SMA was described a year later when iPS cells were derived from a patient with SMA type I, the rapidly progressing infantile form of SMA [75]. This study revealed the power of SMA iPS cells as a novel disease model, demonstrating multiple SMA characteristics including lowered SMN1 transcript levels and selective reduction of the protein in motor neurons. In addition, these SMA iPS cells reacted to drug treatments that increased SMN levels, thus validating the iPS platform for drug discovery in motor neuron disease. Based on these findings using SMA iPS cell lines, the field is poised for novel drug discovery efforts to identify much-needed treatments for both ALS and SMA.
While iPS cells are an exciting development for disease modeling and potential autologous treatments, there are cautionary notes that need to be considered for such a new technology. The true equivalence of iPS to ES cells is still under investigation, and while genomic analysis has demonstrated highly similar profiles for certain lines [76], some lines appear to only be partially reprogrammed. This is of high importance given that many lines are described only for their morphological appearance, with minimal description of their capability to differentiate into germ layers. There are also differences in the efficiency and variability of neuronal differentiation between lines, making line selection an important consideration for modeling and therapeutic studies [77]. Other potential issues such as genetic stability, point mutations, Yamanaka factor insertion sites, incomplete transgene silencing and global epigenetic modifications highlight the need for full characterization of individual cell lines for further advancement of the field [67,78–82].
Stem cell efficacy for motor neuron diseases
Stem cell efficacy for ALS
Recently, several studies have addressed the potential of stem cells to modulate ALS disease progression (Table 1). Endogenous NPCs, as well as direct spinal injection of MSCs and NPCs, have been examined for their potential to provide cellular protection, growth factor delivery and cellular augmentation. MSCs provide an easily accessible source for autologous cellular replacement therapies in ALS. Systemic delivery and intrathecal injection of MSCs in rodent models of ALS demonstrate integration of a small proportion of cells into the spinal cord [83–86]. Characterization of these MSCs indicate that they are capable of differentiating into both neurons and astrocytes [83,84,87]. Furthermore, MSC transplantation in SOD1-G93A mice delays disease progression and motor neuron loss, and also improves lifespan [16,83,84,86–88]. NPCs have also been extensively studied for efficacy in ALS. During the progression of ALS, the endogenous population of NPCs residing beside the central canal exit quiescence, proliferate, and migrate to the ventral horn of the spinal cord [89]. While this event provides support and cells to the diseased spinal cord, it is likely that there are insufficient cells to mount a viable defense against disease progression. Direct implantation of exogenous stem cells into the diseased spinal cord, however, is likely to have the greatest impact on disease. Studies have demonstrated the ability of astrocytes derived from human NPCs to protect motor neurons from degeneration in SOD1-G93A rats [90], and neurons derived from human NPCs to prevent motor neuron degradation and prolong survival [4,10]. NPCs grafted directly into the ventral horn of the spinal cord of SOD1-G93A rats delayed disease onset by 7 days and prolonged the lifespan by 11 days [4]. The NPCs produced detectable levels of the growth factors glial cell-derived neurotrophic factor (GDNF) and brain-derived neurotrophic factor (BDNF) within the cerebrospinal fluid and spinal cord. Furthermore, additional characterization demonstrated that the NPCs grafted into the spinal cord and form symmetrical synapses with α-motor neurons and transneuronal transfer between host and grafted neurons was observed, demonstrating functional neural circuitry [91].
Table 1.
Stem cell technology utilization in motor neuron disease.
| Cell type | Model | Outcome | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Species | Type | Modification | Species | Genetic modification |
Delivery; treatment age | Disease onset |
Survival increase |
Additional notes | |
| ALS | |||||||||
| Mouse | ES | MN differentiated | Rat | SOD1-G93A | Direct spinal implant; 10 weeks | None | None | Loss of grafted cells by end stage | [115] |
| Mouse | BM | N/A | Mouse | SOD1-G93A | Intraperitoneal injection; 4 weeks | Delay | 13 days | MN protection; differentiation into neurons and contribution to non-neuronal tissue | [83] |
| Mouse | BM | GFP tag | Mouse | SOD1-G93A | Intra-bone marrow transplantation; 12 weeks | None | ~10 days | No MN protection; GFP-positive cells in spinal cord; differentiation into glia; no neuronal differentiation | [86] |
| Mouse | BM | Lin-c-kit + specific cells | Mouse | SOD1-G93A | Intravenous injection; 70 days | 4-day delay | 17 days | MN protection, slowed progression; neuronal differentiation | [84] |
| Mouse | NPC | Le + CX + specific cells | Mouse | SOD1-G93A | Direct spinal implantation; 70 days | Delay | 23 days | MN protection; neuronal differentiation | [116] |
| Rat | Primary NPC | GFP | Rat | SOD1-G93A | Intravenous injection; 14, 26 weeks | N/A | N/A | NPC migration to the CNS and differentiation | [85] |
| Rat | GRP | N/A | Rat | SOD1-G93A | Direct spinal implantation; 90 days | None | 17 days | MN protection; astrocyte differentiation; decreased microgliosis | [16] |
| Human | UBC | N/A | Mouse | SOD1-G93A | Intravenous injection; 7–8 weeks | Delay | Various | Dose-dependent changes in survival; cytokine regulation | [88] |
| Human | UBC | N/A | Mouse | SOD1-G93A | Intravenous injection; 66 days | Delay | 2–3 weeks | Migration to the CNS; neuronal differentiation | [87] |
| Human | UBC | VEGF, FGF2 | Mouse | SOD1-G93A | Retro-orbital injection; 24–28 weeks | N/A | N/A | Migration to the CNS; differentiation to migroglia and astrocytes | [117] |
| Human | MSC | GDNF producing | Rat | SOD1-G93A | Intramuscular transplantation; 80 days | None | 28 days | Protection of MN in the spinal cord and NMJ integrity | [93] |
| Human | MSC | Derived from ALS patients | Mouse | SOD1-G93A | Injection into cisterna magna; 60 days | None | 7 days | Dose-dependent effect on survival; slowed progression | [118] |
| Human | NPC | VEGF producing | Mouse | SOD1-G93A | Intrathecal injection; 70 days | Delay | 12 days | Regulation of apoptosis pathway | [119] |
| Human | NPC | BDNF, IGF-I, VEGF, NT-3, GDNF | Mouse | SOD1-G93A | Injection into cisterna magna; 75 days | None | None | Astrocyte differentiation and MN protection with production of GDNF or IGF-I | [92] |
| Human | NPC | N/A | Rat | SOD1-G93A | Direct spinal implantation; 62 days | Delay | 10 days | Neuronal differentiation, MN protection, delayed progression, growth factor production | [4] |
| Human | NPC | N/A | Rat | SOD1-G93A | Direct spinal implantation; 56 days | N/A | N/A | Integration and formation of synaptic contacts between graft and host | [91] |
| Human | NPC | GDNF producing | Rat | SOD1-G93A | Direct spinal implantation; 90/100 days | None | None | [120] | |
| Human | NPC | GDNF producing | Rat | SOD1-G93A | Direct spinal implantation; 70 days | None | None | MN protection | [90] |
| SMA | |||||||||
| Mouse | ES | GFP, NPC differentiation | Mouse | Smn−/− SMN2+/+SMNΔ7+/+ | Intrathecal injection; 1 day | N/A | 6.75–8.17 days | Neural (including MN) and astrocyte differentiation, MN and NMJ protection, growth factor production, axon projection toward ventral root | [95] |
| Mouse | ES | HB9-GFP, ALDHhiSSClo specific, neural ‘primed’ | Mouse | Smn−/− SMN2+/+SMNΔ7+/+ | Intrathecal injection; 1 day | N/A | 5.12 days | Neural (including MN) and astrocyte differentiation, MN and NMJ protection. Growth factor production | [94] |
ALS: Amyotrophic lateral sclerosis; BDNF: Bone-derived neurotrophic factor; BM: Bone marrow; GDNF: Glial-derived neurotrophic factor; GFP: Green fluorescent protein; GRP: Glial restricted precursor; ES: Embryonic stem; MN: Motor neuron; MSC: Mesenchymal stem cell; N/A: Not applicable; NMJ: Neuromuscular junction; NPC: Neural progenitor cell; NT: Neurotrophin; UBC: Umbilical cord cell.
Given that the combination of both cellular and trophic intervention provides added benefit in ALS model systems, the efficacy of cellular therapies engineered to produce increased levels of growth factors has also been examined. The protective effects of NPCs derived from human cortical tissue, which were engineered to produce increased amounts of GDNF and primarily give rise to astrocytes upon differentiation, have been examined for their ability to protect motor neurons in the ALS rat [90]. While nonengineered cells did not provide protection, production of GDNF enriched the spinal cord environment and protected motor neurons from cell death. Neuromuscular contacts, however, were still lost and disease onset and survival were not improved. An additional study also supports the transplantation of human NPCs producing either GDNF or IGF-I into the SOD1-G93A mouse, and demonstrates attenuation of motor neuron loss, but did not affect overall survival [92]. To address the potential for distal intervention, however, Suzuki et al. utilized human MSCs as a shuttle to deliver GDNF to the neuromuscular contacts in SOD1-G93A rats [93]. They demonstrated that GDNF delivery to muscle maintained neuromuscular contacts, which not only promoted increased motor neuron survival, but also an increase in lifespan of up to 28 days. Overall, these results support the need for both cellular and axonal motor neuron treatments for ALS, and provide evidence for the potential efficacy of stem cell therapies for ALS.
Stem cell efficacy for SMA
Stem cells have recently been examined for their potential for the treatment of SMA (Table 1). Mouse ES cell-derived NPCs were examined in mice for efficacy on SMA disease progression [94,95]. ES cells, which were predifferentiated into NPCs and motor neuron progenitors, incorporated into the ventral horn of SMN2tg/Smn−/−/SMNΔ7+/+ SMA mice after intrathecal injection. The integrated NPCs produced an array of growth factors including GDNF, BDNF, TGF-α and neurotrophin-3. Engrafted animals demonstrated a 39% increase in lifespan which coincided with improved body weight and grip strength, decreased motor neuron and muscle loss and maintained neuromuscular contacts. Given the short time the stem cells have to integrate and mature in the spinal cord, it is somewhat surprising they had such a dramatic effect. These promising results bode well for the extrapolation of stem cell therapies to larger animals and eventually humans.
Stem cell intervention for motor neuron diseases is most likely to provide support when the cellular therapy can be administered earlier in the disease course. Supporting the existing motor neurons is likely the most critical component to halt or decrease disease progression. In 2009, California Stem Cell, Inc. (CA, USA) announced the completion of a pre-IND meeting with the FDA for a Phase I/II trial to inject human-grade motor neurons derived from human ES cells as a therapy for SMA. ES-derived motor neurons are proposed to alleviate disease by cell replacement and muscle innervation, effects which may be more viable in younger patients, and through cellular support. Any potential stem cell treatment for SMA faces similar issues to those described for ALS with respect to motor neuron replacement; however, neuronal support combined with upregulation of SMN2 may provide an effective avenue for therapy development. Given the genetic background of the disease, treatment combining stem cells and gene therapy may enhance stem cell function and improve therapeutic efficacy.
Translating stem cell therapy from bench to bedside
Given the increasing body of in vitro and in vivo data supporting the beneficial nature of NPCs to protect motor neurons in both ALS and SMA, we are at the cusp of the development of new cellular therapies for both diseases. But what does it take to get neuroprotective stem cells from bench to bedside? Here we describe the steps required to make that leap.
Hurdles to cellular transplantation therapy
While there are abundant data supporting the potential efficacy of cellular replacement therapies in motor neuron disease, there are certain issues that must be considered before designing a trial and translating therapies to patients [96–98]. Selection of the appropriate cell type is imperative to achieve the desired neuroprotective outcomes; appropriate amounts of cells must be accessible and available, and they must exhibit the characteristics that are predicted to confer neuroprotection while avoiding the potential for tumor formation. Furthermore, issues regarding cell graft survival must be examined within the transplanted microenvironment, as well as immune rejection potential. Though some transplantation options such as MSCs and iPS cells involve the use of autologous cells and could circumvent the possibility of rejection, immunosuppression must be utilized for most cellular transplantation therapy options to prevent cellular rejection. Finally, careful attention must be paid to the technical aspects of cell delivery within the nervous system to avoid adverse reactions and surgical complications.
Supporting data & rationale
Human NPCs are currently being utilized in a Phase I safety trial in ALS patients. The NPCs are derived from a single fetus donated after elective abortion. The upper thoracic region of the spinal cord was dissected out and the cells were expanded to provide a population of human spinal cord stem cells (HSSCs). These HSSCs have been shown to effectively enhance recovery of lost motor function in rats after spinal cord ischemic injury [99] and improve survival up to 20 days in SOD1-G93A rats [4]. Approximately 70% of the HSSCs injected in to the ventral horn of the spinal cord are of a neural lineage and express both glutamatergic and GABAergic neurotransmitter markers [100]. Procedural and HSSC safety was also assessed in a larger animal, the Gottingen-Minnesota minipig [98,101]. Using the minipig, a dose of 30,000 cells/2–6 µl for injection into the spinal cord was determined to be well tolerated. Delivery of the cells into the spinal cord was accomplished utilizing an innovative device designed to deliver HSSCs that reduces surgical complexity while providing advanced safety and accuracy [98, 102].
The encouraging results of these and other supporting in vitro and in vivo studies provide the rationale and foundation for the first translational trial of HSSCs in ALS patients. The current trial is a Phase I trial with the overall objective of validating the feasibility and safety of direct intraspinal transplantation of stem cells for ALS. While the safety of intraspinal injection and examination of the toxicity of HSSC transplantation are the primary objectives of the current trial, validation of this approach will pave the way for future studies examining the translational efficacy of stem cell transplantation therapy for ALS, and potentially other motor neuron and neurological diseases. We hypothesize, based on the supporting studies, that engrafted cells will provide neuroprotection to diseased motor neurons in ALS patients through direct cellular support, as a source of neurotrophic support, and by maintaining a suitable spinal cord microenvironment for remaining motor neurons (Figure 2B).
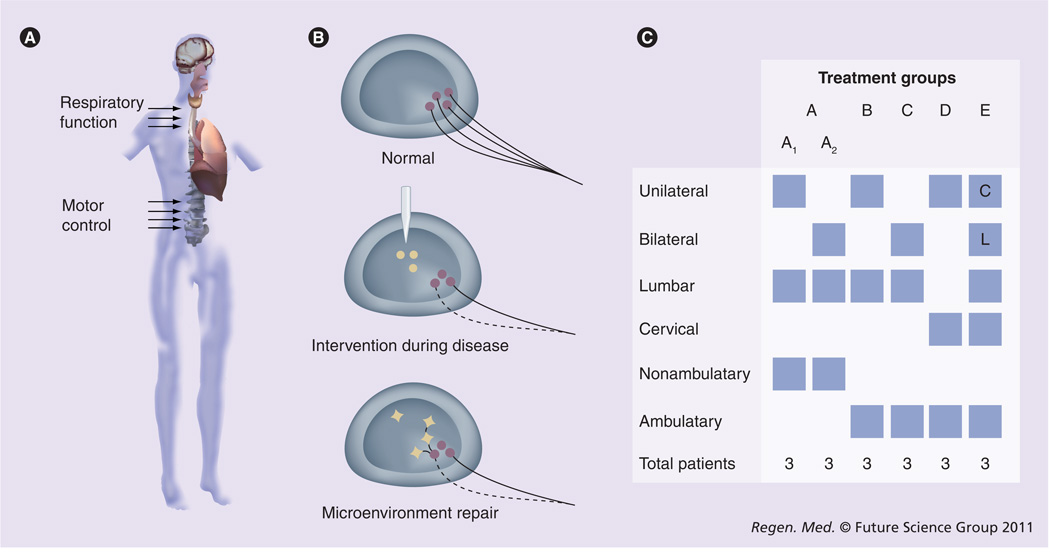
Figure 2. Clinical trial design examining intraspinal transplantation of human spinal cord stem cells in amyotrophic lateral sclerosis patients.
(A) Amyotrophic lateral sclerosis (ALS) patients will receive unilateral or bilateral human spinal cord stem cell injections in the lumbar or cervical enlargements of the spinal cord (arrows). (B) Proposed mechanism of neuroprotection conferred by grafted cells in ALS patients. As motor neurons normally residing in the spinal cord of ALS patients start to degenerate, injected human spinal cord stem cells are hypothesized to integrate into the spinal cord to provide a source of cellular support, maintain a healthy microenvironment within the spinal cord, and provide neurotrophic support to the remaining motor neurons. (C) The trial will follow a ‘risk escalation’ paradigm, which reflects the gradual increase in risk between the different cohorts. ALS patients are divided into groups A–E based on ambulatory ability, number of injections (unilateral vs bilateral), and injection location (lumbar [L] vs cervical [C]).
Trial design & surgical procedures
In total, 18 ALS patients with varying degrees of disease will receive unilateral or bilateral injections of HSSCs into the ventral horn of the lumbar or cervical enlargements of the spinal cord (Figure 2A). Patients are divided into five cohorts (Groups A–E) according to a paradigm we term ‘Risk Escalation’, which maintains the most conservative path with respect to risk to patients as the trial progresses (Figure 2C). Subjects undergo surgery to receive direct intraspinal transplantation of HSSCs following a carefully optimized protocol based on collective results of large animal and human data [98,101,103–107]. Briefly, the surgical procedure includes a laminectomy, mounting of an innovative apparatus designed by our group to stabilize the injection device, controlled injection of HSSCs, and then regimented postoperative recovery and evaluation. At this point, FDA approval has been obtained for the first 12 patients and progression through the trial groups will occur as subsequent subjects continue to present without major adverse side effects.
Outcomes & expectations
To date, the first eight patients have successfully received intraspinal HSSC transplants. The first six patients in Group A, while nonambulatory, did not experience transplant-related loss of sensory or remaining motor function. Similarly, the two patients in Group B did not experience any major adverse effects of the surgery and maintained ambulatory ability. These results are promising, and support the safety of the procedural technique and intraspinal cellular transplantation. While multiple questions remain to be addressed with advancement of the field, we are optimistic that progression through the trial groups and successful completion of the trial will support the utilization of cellular transplantation for therapeutic efficacy in ALS.
Future perspective
With the recent advances in stem cell biology, we are poised to move safely, yet rapidly, towards cellular therapies for neurodegenerative disorders. The development of new human models for disease via the generation of patient-specific iPS cells will allow us to more closely relate cellular dysfunction with disease mechanisms. Given the multiple complex mechanisms that can contribute to disease progression in ALS, comparison of individual patient iPS cells may reveal hierarchical targets or subdivisions of disease that can be differentially targeted for pharmacological drug development. Furthermore, safety trials are already complete for MSCs in ALS [108], and with the completion of further safety trials using different cell lines, we will begin to distill which components of stem cell therapies are most advantageous for targeting different aspects of disease. Given what we know regarding the complex mechanisms and consequences of motor neuron loss in ALS, combinational therapies with additional factors or reagents and cell cocktails including NPCs with a propensity to give rise to both glia and neurons will likely afford maximal effectiveness. We must also consider systemic treatment to maintain neuromuscular contacts in conjunction with spinal treatments, since both may be essential for a more complete efficacious therapy. Finally, we must develop better ways of monitoring and visualizing stem cells in situ within the human body to fully understand long-term effects. With a greater understanding of the process of cellular transplantation for motor neuron diseases, we will develop streamlining methods for translational stem cell therapies.
Convincing results in animal models and safety trials for stem cell therapy in humans currently underway are fueling the drive to translate stem cell therapies from the bench to the bedside for multiple neurodegenerative diseases. While the current review discusses the development of stem cell therapies specifically for motor neuron diseases, it should be noted that the principles discussed also extend to the development of stem cell treatments for other neurological disorders and diseases as well. The progress of stem cell technology for neurodegenerative diseases in general has been reviewed elsewhere [61,109,110] and stem cells have been extensively studied for Alzheimer’s disease, multiple sclerosis, Parkinson’s disease and spinal cord trauma [111–113]. In all cases, the cells impact the same three rationales outlined above: to replace lost cells, augment and support cells, or enrich the cellular microenvironment. Indeed, ES-derived oligodendrocytes are currently being utilized in a human trial for spinal cord injury where the proposed mechanisms include cell replacement and environmental enrichment for neuronal growth [114]. Overall, we have made great strides in the translation of stem cell therapies from the bench to patients for motor neuron diseases, and the field of cellular transplantation technology for multiple diseases is primed for continued advancement.
Executive summary.
Stem cells: the right tool for the right job
-
▪
Individual stem cell lines may have different innate abilities to provide support, and growth factor production may be critical to their function.
-
▪
The strength of stem cell therapy lies in its ability to combat multiple aspects of disease treatment in a single therapy.
Exploration of motor neuron disease mechanisms
-
▪
The amyotrophic lateral sclerosis (ALS) disease phenotype is complex and motor neuron loss is likely the result of contributions from multiple mechanisms including loss of neurotrophic support and glial dysfunction.
-
▪
In spinal muscular atrophy, motor neuron loss is associated with significant decreases in survival motor neuron (SMN)1 protein levels that could potentially be restored by altering the levels of compensatory SMN2 expression.
-
▪
Induced pluripotent stem cells provide a system to examine disease mechanisms and test potential therapeutics in a patient-specific manner.
Stem cell efficacy for motor neuron diseases
-
▪
A combination of maintenance in the spinal cord and at neuromuscular junctions may be required for a systems approach for the treatment of ALS.
-
▪
Stem cell therapy in combination with gene therapy to upregulate SMN2 protein levels may provide cellular support in spinal muscular atrophy.
Translating stem cell therapy from bench to bedside
-
▪
In vitro and in vivo data support the efficacy of stem cells for the treatment of ALS, and data from large animals and humans provide an innovative means for human spinal cord stem cell transplantation.
-
▪
A Phase I trial is currently underway examining the feasibility and safety of direct intraspinal transplantation of human spinal cord stem cells into ALS patient spinal cords.
-
▪
Results to date on the initial patient groups are promising, and with continued success, the trial paves the way for the translation of stem cell therapies for motor neuron diseases and other neurodegenerative diseases as well.
Acknowledgements
We thank M Marsala, M Hefferan, J Riley, B Raore and J Taub for their technical assistance with the preclinical studies for the trial and we acknowledge the valuable input of K Johe and the Data Safety Monitoring Board of the current trial. We thank M Polak and C Kelly for their clinical assistance and collection of clinical data. We are grateful to the Emory Amyotrophic Lateral Sclerosis Center, patients, and families for their participation.
Preclinical studies were funded in part by the A Alfred Taubman Medical Research Institute, the Amyotrophic Lateral Sclerosis Association and Neuralstem, Inc. SA Sakowski is supported by the NIH (T32 NS007222-28).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
NM Boulis is the inventor of devices to enable safe and accurate injection of the human spinal cord. NeuralStem, Inc. has purchased an exclusive license to this technology. NM Boulis recieved an inventors share of this fee, and has the rights to royalty payments for distribution of this technology.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Vazin T, Freed WJ. Human embryonic stem cells: derivation, culture, and differentiation: a review. Restor. Neurol. Neurosci. 2010;28(4):589–603. doi: 10.3233/RNN-2010-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132(4):661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Kim SU. Human neural stem cells genetically modified for brain repair in neurological disorders. Neuropathology. 2004;24(3):159–171. doi: 10.1111/j.1440-1789.2004.00552.x. [DOI] [PubMed] [Google Scholar]
- 4. Xu L, Yan J, Chen D, et al. Human neural stem cell grafts ameliorate motor neuron disease in SOD-1 transgenic rats. Transplantation. 2006;82(7):865–875. doi: 10.1097/01.tp.0000235532.00920.7a. ▪▪ Demonstrates the therapeutic efficacy of human neural stem cells in the amyotrophic lateral sclerosis rat model, which provides support for the use of these cells in the current trial.
- 5.Garzon-Muvdi T, Quinones-Hinojosa A. Neural stem cell niches and homing: recruitment and integration into functional tissues. ILAR J. 2009;51(1):3–23. doi: 10.1093/ilar.51.1.3. [DOI] [PubMed] [Google Scholar]
- 6.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell Physiol. 2007;213(2):341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 7.Mezey E, Mayer B, Nemeth K. Unexpected roles for bone marrow stromal cells (or MSCs): a real promise for cellular, but not replacement, therapy. Oral Dis. 2010;16(2):129–135. doi: 10.1111/j.1601-0825.2009.01605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson AP, Foraker JE, Ylostalo J, Prockop DJ. Human stem/progenitor cells from bone marrow enhance glial differentiation of rat neural stem cells: a role for transforming growth factor-β and Notch signaling. Stem Cells Dev. 2010;20(2):289–300. doi: 10.1089/scd.2009.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li JM, Zhu H, Lu S, et al. Migration and differentiation of human mesenchymal stem cells in the normal rat brain. Neurol. Res. 2010;33(1):84–92. doi: 10.1179/016164110X12670144737819. [DOI] [PubMed] [Google Scholar]
- 10.Lunn JS, Hefferan MP, Marsala M, Feldman EL. Stem cells: comprehensive treatments for amyotrophic lateral sclerosis in conjunction with growth factor delivery. Growth Factors. 2009;27(3):133–140. doi: 10.1080/08977190902814855. [DOI] [PubMed] [Google Scholar]
- 11.Hu BY, Zhang SC. Directed differentiation of neural-stem cells and subtype-specific neurons from hESCs. Methods Mol. Biol. 2010;636:123–137. doi: 10.1007/978-1-60761-691-7_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo X, Johe K, Molnar P, Davis H, Hickman J. Characterization of a human fetal spinal cord stem cell line, NSI-566RSC, and its induction to functional motoneurons. J. Tissue Eng. Regen. Med. 2010;4(3):181–193. doi: 10.1002/term.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu BY, Zhang SC. Differentiation of spinal motor neurons from pluripotent human stem cells. Nat. Protoc. 2009;4(9):1295–1304. doi: 10.1038/nprot.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thonhoff JR, Ojeda L, Wu P. Stem cell-derived motor neurons: applications and challenges in amyotrophic lateral sclerosis. Curr. Stem Cell Res. Ther. 2009;4(3):178–199. doi: 10.2174/157488809789057392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jordan PM, Ojeda LD, Thonhoff JR, et al. Generation of spinal motor neurons from human fetal brain-derived neural stem cells: role of basic fibroblast growth factor. J. Neurosci. Res. 2009;87(2):318–332. doi: 10.1002/jnr.21856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lepore AC, Rauck B, Dejea C, et al. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat. Neurosci. 2008;11(11):1294–1301. doi: 10.1038/nn.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki M, Svendsen CN. Combining growth factor and stem cell therapy for amyotrophic lateral sclerosis. Trends Neurosci. 2008;31(4):192–198. doi: 10.1016/j.tins.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Feldman EL, Goldman L, Ausiello D. Cecil Textbook of Medicine. Saunders, PA, USA: 2004. Amyotrophic lateral sclerosis and other motor neuron diseases; pp. 2316–2319. [Google Scholar]
- 19.Rosen DR, Siddique T, Patterson D, et al. Mutations in Cu/Zn superoxide dismutase are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez DE, Aguilar JL, Echaniz-Laguna A, et al. Amyotrophic lateral sclerosis: all roads lead to Rome. J. Neurochem. 2007;101(5):1153–1160. doi: 10.1111/j.1471-4159.2006.04408.x. [DOI] [PubMed] [Google Scholar]
- 21.Turner BJ, Talbot K. Transgenics, toxicity and therapeutics in rodent models of mutant SOD1-mediated familial ALS. Prog. Neurobiol. 2008;85(1):94–134. doi: 10.1016/j.pneurobio.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J. Cell Biol. 2009;187(6):761–772. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kabashi E, Valdmanis PN, Dion P, et al. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat. Genet. 2008;40(5):572–574. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- 24.Kwiatkowski TJ, Jr, Bosco DA, Leclerc AL, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323(5918):1205–1208. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- 25.Lagier-Tourenne C, Cleveland DW. Rethinking ALS: the FUS about TDP-43. Cell. 2009;136(6):1001–1004. doi: 10.1016/j.cell.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sreedharan J, Blair IP, Tripathi VB, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319(5870):1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howland DS, Liu J, She Y, et al. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS) Proc. Natl Acad. Sci. USA. 2002;99:1604–1609. doi: 10.1073/pnas.032539299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gurney Me, Pu H, Chiu Ay, et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science. 1994;264(5166):1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 29.Stallings NR, Puttaparthi K, Luther CM, Burns DK, Elliott JL. Progressive motor weakness in transgenic mice expressing human TDP-43. Neurobiol. Dis. 2010;40(2):404–414. doi: 10.1016/j.nbd.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 30.Festoff BW, Suo Z, Citron BA. Prospects for the pharmacotherapy of amyotrophic lateral sclerosis: old strategies and new paradigms for the third millennium. CNS Drugs. 2003;17:699–717. doi: 10.2165/00023210-200317100-00002. [DOI] [PubMed] [Google Scholar]
- 31.Raoul C, Bbas-Terki T, Bensadoun JC, et al. Lentiviral-mediated silencing of SOD1 through RNA interference retards disease onset and progression in a mouse model of ALS. Nat. Med. 2005;11:423–428. doi: 10.1038/nm1207. [DOI] [PubMed] [Google Scholar]
- 32.Rothstein JD, Patel S, Regan MR, et al. β-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 33.Ji HF, Shen L, Zhang HY. β-lactam antibiotics are multipotent agents to combat neurological diseases. Biochem. Biophys. Res. Commun. 2005;333:661–663. doi: 10.1016/j.bbrc.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 34.Schutz B, Reimann J, Dumitrescu-Ozimek L, et al. The oral antidiabetic pioglitazone protects from neurodegeneration and amyotrophic lateral sclerosis-like symptoms in superoxide dismutase-G93A transgenic mice. J. Neurosci. 2005;25:7805–7812. doi: 10.1523/JNEUROSCI.2038-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crow JP, Calingasan NY, Chen J, Hill JL, Beal MF. Manganese porphyrin given at symptom onset markedly extends survival of ALS mice. Ann. Neurol. 2005;58:258–265. doi: 10.1002/ana.20552. [DOI] [PubMed] [Google Scholar]
- 36.Park JH, Hong YH, Kim HJ, et al. Pyruvate slows disease progression in a G93A SOD1 mutant transgenic mouse model. Neurosci. Lett. 2007;413(3):265–269. doi: 10.1016/j.neulet.2006.11.058. [DOI] [PubMed] [Google Scholar]
- 37.Bilic E, Rudan I, Kusec V, Zurak N, Delimar D, Zagar M. Comparison of the growth hormone, IGF-1 and insulin in cerebrospinal fluid and serum between patients with motor neuron disease and healthy controls. Eur. J. Neurol. 2006;13(12):1340–1345. doi: 10.1111/j.1468-1331.2006.01503.x. [DOI] [PubMed] [Google Scholar]
- 38.Moreau C, Devos D, Brunaud-Danel V, et al. Paradoxical response of VEGF expression to hypoxia in CSF of patients with ALS. J. Neurol. Neurosurg. Psychiatry. 2006;77(2):255–257. doi: 10.1136/jnnp.2005.070904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dodge JC, Treleaven CM, Fidler JA, et al. AAV4-mediated expression of IGF-1 and VEGF within cellular components of the ventricular system improves survival outcome in familial ALS mice. Mol. Ther. 2010;18(12):2075–2084. doi: 10.1038/mt.2010.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dodge JC, Haidet AM, Yang W, et al. Delivery of AAV-IGF-1 to the CNS extends survival in ALS mice through modification of aberrant glial cell activity. Mol. Ther. 2008;16(6):1056–1064. doi: 10.1038/mt.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lunn JS, Sakowski SA, Kim B, Rosenberg AA, Feldman EL. Vascular endothelial growth factor prevents G93ASOD1-induced motor neuron degeneration. Dev. Neurobiol. 2009;69(13):871–884. doi: 10.1002/dneu.20747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakowski SA, Heavener SB, Lunn JS, et al. Neuroprotection using gene therapy to induce vascular endothelial growth factor-A expression. Gene Ther. 2009;16(11):1292–1299. doi: 10.1038/gt.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakowski SA, Schuyler AD, Feldman EL. Insulin-like growth factor-I for the treatment of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2009;10(2):63–73. doi: 10.1080/17482960802160370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borasio GD, Robberecht W, Leigh PN, et al. A placebo-controlled trial of insulin-like growth factor-I in amyotrophic lateral sclerosis. European ALS/IGF-I Study Group. Neurology. 1998;51(2):583–586. doi: 10.1212/wnl.51.2.583. [DOI] [PubMed] [Google Scholar]
- 45.A controlled trial of recombinant methionyl human BDNF in ALS: The BDNF Study Group (Phase III) Neurology. 1999;52(7):1427–1433. doi: 10.1212/wnl.52.7.1427. [DOI] [PubMed] [Google Scholar]
- 46.Miller RG, Petajan JH, Bryan WW, et al. A placebo-controlled trial of recombinant human ciliary neurotrophic (rhCNTF) factor in amyotrophic lateral sclerosis. rhCNTF ALS Study Group. Ann. Neurol. 1996;39(2):256–260. doi: 10.1002/ana.410390215. [DOI] [PubMed] [Google Scholar]
- 47.Lai EC, Felice KJ, Festoff BW, et al. Effect of recombinant human insulin-like growth factor-I on progression of ALS. A placebo-controlled study. The North America ALS/IGF-I Study Group. Neurology. 1997;49(6):1621–1630. doi: 10.1212/wnl.49.6.1621. [DOI] [PubMed] [Google Scholar]
- 48.Sorenson EJ, Windbank AJ, Mandrekar JN, et al. Subcutaneous IGF-1 is not beneficial in 2-year ALS trial. Neurology. 2008;71(22):1770–1775. doi: 10.1212/01.wnl.0000335970.78664.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wee CD, Kong L, Sumner CJ. The genetics of spinal muscular atrophies. Curr. Opin. Neurol. 2010;23(5):450–458. doi: 10.1097/WCO.0b013e32833e1765. [DOI] [PubMed] [Google Scholar]
- 50.Rochette CF, Gilbert N, Simard LR. SMN gene duplication and the emergence of the SMN2 gene occurred in distinct hominids: SMN2 is unique to Homo sapiens. Hum. Genet. 2001;108(3):255–266. doi: 10.1007/s004390100473. [DOI] [PubMed] [Google Scholar]
- 51.Lorson CL, Rindt H, Shababi M. Spinal muscular atrophy: mechanisms and therapeutic strategies. Hum. Mol. Genet. 2010;19(R1):R111–R118. doi: 10.1093/hmg/ddq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coady TH, Shababi M, Tullis GE, Lorson CL. Restoration of SMN function: delivery of a trans-splicing RNA re-directs SMN2 pre-mRNA splicing. Mol. Ther. 2007;15(8):1471–1478. doi: 10.1038/sj.mt.6300222. [DOI] [PubMed] [Google Scholar]
- 53.Schrank B, Gotz R, Gunnersen JM, et al. Inactivation of the survival motor neuron gene, a candidate gene for human spinal muscular atrophy, leads to massive cell death in early mouse embryos. Proc. Natl Acad. Sci. USA. 1997;94(18):9920–9925. doi: 10.1073/pnas.94.18.9920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jablonka S, Schrank B, Kralewski M, Rossoll W, Sendtner M. Reduced survival motor neuron (smn) gene dose in mice leads to motor neuron degeneration: an animal model for spinal muscular atrophy type III. Hum. Mol. Genet. 2000;9(3):341–346. doi: 10.1093/hmg/9.3.341. [DOI] [PubMed] [Google Scholar]
- 55.Park GH, Kariya S, Monani UR. Spinal muscular atrophy: new and emerging insights from model mice. Curr. Neurol. Neurosci. Rep. 2010;10(2):108–117. doi: 10.1007/s11910-010-0095-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hsieh-Li HM, Chang JG, Jong YJ, et al. A mouse model for spinal muscular atrophy. Nat. Genet. 2000;24(1):66–70. doi: 10.1038/71709. [DOI] [PubMed] [Google Scholar]
- 57.Monani UR, Sendtner M, Coovert DD, et al. The human centromeric survival motor neuron gene (SMN2) rescues embryonic lethality in Smn−/− mice and results in a mouse with spinal muscular atrophy. Hum. Mol. Genet. 2000;9(3):333–339. doi: 10.1093/hmg/9.3.333. [DOI] [PubMed] [Google Scholar]
- 58.Gunaseeli I, Doss MX, Antzelevitch C, Hescheler J, Sachinidis A. Induced pluripotent stem cells as a model for accelerated patient-and disease-specific drug discovery. Curr. Med. Chem. 2010;17(8):759–766. doi: 10.2174/092986710790514480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Inoue H. Neurodegenerative disease-specific induced pluripotent stem cell research. Exp. Cell Res. 2010;316(16):2560–2564. doi: 10.1016/j.yexcr.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 60.Kiskinis E, Eggan K. Progress toward the clinical application of patient-specific pluripotent stem cells. J. Clin. Invest. 2010;120(1):51–59. doi: 10.1172/JCI40553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marchetto MC, Winner B, Gage FH. Pluripotent stem cells in neurodegenerative and neurodevelopmental diseases. Hum. Mol. Genet. 2010;19(R1):R71–R76. doi: 10.1093/hmg/ddq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 63.Yamanaka S, Blau HM. Nuclear reprogramming to a pluripotent state by three approaches. Nature. 2010;465(7299):704–712. doi: 10.1038/nature09229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007;1(1):39–49. doi: 10.1016/j.stem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 65. Yamanaka S. Induction of pluripotent stem cells from mouse fibroblasts by four transcription factors. Cell Prolif. 2008;41 Suppl. 1:51–56. doi: 10.1111/j.1365-2184.2008.00493.x. ▪▪ Describes the first generation of induced pluripotent stem cells utilizing the Yamanaka factors.
- 66.Zhou H, Ding S. Evolution of induced pluripotent stem cell technology. Curr. Opin. Hematol. 2010;17(4):276–280. doi: 10.1097/MOH.0b013e328339f2ee. [DOI] [PubMed] [Google Scholar]
- 67.Maherali N, Hochedlinger K. Guidelines and techniques for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;3(6):595–605. doi: 10.1016/j.stem.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 68.Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324(5928):797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu J, Thomson JA. Pluripotent stem cell lines. Genes Dev. 2008;22(15):1987–1997. doi: 10.1101/gad.1689808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim D, Kim CH, Moon JI, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4(6):472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang N, An MC, Montoro D, Ellerby LM. Characterization of human Huntington’s disease cell model from induced pluripotent stem cells. PLoS Curr. 2010;2 doi: 10.1371/currents.RRN1193. RRN1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soldner F, Hockemeyer D, Beard C, et al. Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136(5):964–977. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu J, Verma PJ, Evans-Galea MV, et al. Generation of induced pluripotent stem cell lines from Friedreich ataxia patients. Stem Cell Rev. 2010 doi: 10.1007/s12015-010-9210-x. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 74. Dimos JT, Rodolfa KT, Niakan KK, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321(5893):1218–1221. doi: 10.1126/science.1158799. ▪ Demonstrates the generation of induced pluripotent stem cells from an amyotrophic lateral sclerosis patient.
- 75. Ebert AD, Yu J, Rose FF, Jr, et al. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457(7227):277–280. doi: 10.1038/nature07677. ▪ Demonstrates the generation of induced pluripotent stem cells from a patient with spinal muscular atrophy.
- 76.Mikkelsen TS, Hanna J, Zhang X, et al. Dissecting direct reprogramming through integrative genomic analysis. Nature. 2008;454(7200):49–55. doi: 10.1038/nature07056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hu BY, Weick JP, Yu J, et al. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc. Natl Acad. Sci. USA. 2010;107(9):4335–4340. doi: 10.1073/pnas.0910012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kane NM, Nowrouzi A, Mukherjee S, et al. Lentivirus-mediated reprogramming of somatic cells in the absence of transgenic transcription factors. Mol. Ther. 2010;18(12):2139–2145. doi: 10.1038/mt.2010.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meissner A. Epigenetic modifications in pluripotent and differentiated cells. Nat. Biotechnol. 2010;28(10):1079–1088. doi: 10.1038/nbt.1684. [DOI] [PubMed] [Google Scholar]
- 80.Rolletschek A, Wobus AM. Induced human pluripotent stem cells: promises and open questions. Biol. Chem. 2009;390(9):845–849. doi: 10.1515/BC.2009.103. [DOI] [PubMed] [Google Scholar]
- 81.Stadtfeld M, Apostolou E, Akutsu H, et al. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature. 2010;465(7295):175–181. doi: 10.1038/nature09017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Polo JM, Liu S, Figueroa ME, et al. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat. Biotechnol. 2010;28(8):848–855. doi: 10.1038/nbt.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Corti S, Locatelli F, Donadoni C, et al. Wild-type bone marrow cells ameliorate the phenotype of SOD1-G93A ALS mice and contribute to CNS, heart and skeletal muscle tissues. Brain. 2004;127(Pt 11):2518–2532. doi: 10.1093/brain/awh273. [DOI] [PubMed] [Google Scholar]
- 84.Corti S, Nizzardo M, Nardini M, et al. Systemic transplantation of c-kit+ cells exerts a therapeutic effect in a model of amyotrophic lateral sclerosis. Hum. Mol. Genet. 2010;19(19):3782–3796. doi: 10.1093/hmg/ddq293. [DOI] [PubMed] [Google Scholar]
- 85.Mitrecic D, Nicaise C, Gajovic S, Pochet R. Distribution, differentiation, and survival of intravenously administered neural stem cells in a rat model of amyotrophic lateral sclerosis. Cell Transplant. 2010;19(5):537–548. doi: 10.3727/096368910X498269. [DOI] [PubMed] [Google Scholar]
- 86.Ohnishi S, Ito H, Suzuki Y, et al. Intra-bone marrow-bone marrow transplantation slows disease progression and prolongs survival in G93A mutant SOD1 transgenic mice, an animal model mouse for amyotrophic lateral sclerosis. Brain Res. 2009;1296:216–224. doi: 10.1016/j.brainres.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 87.Garbuzova-Davis S, Willing AE, Zigova T, et al. Intravenous administration of human umbilical cord blood cells in a mouse model of amyotrophic lateral sclerosis: distribution, migration, and differentiation. J. Hematother. Stem Cell Res. 2003;12(3):255–270. doi: 10.1089/152581603322022990. [DOI] [PubMed] [Google Scholar]
- 88.Garbuzova-Davis S, Sanberg CD, Kuzmin-Nichols N, et al. Human umbilical cord blood treatment in a mouse model of ALS: optimization of cell dose. PLoS ONE. 2008;3(6) doi: 10.1371/journal.pone.0002494. e2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chi L, Ke Y, Luo C, et al. Motor neuron degeneration promotes neural progenitor cell proliferation, migration, and neurogenesis in the spinal cords of amyotrophic lateral sclerosis mice. Stem Cells. 2006;24(1):34–43. doi: 10.1634/stemcells.2005-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Suzuki M, Mchugh J, Tork C, et al. GDNF secreting human neural progenitor cells protect dying motor neurons, but not their projection to muscle, in a rat model of familial ALS. PLoS ONE. 2007;2(1) doi: 10.1371/journal.pone.0000689. e689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu L, Ryugo DK, Pongstaporn T, Johe K, Koliatso VE. Human neural stem cell grafts in the spinal cord of SOD1 transgenic rats: differentiation and structural integration into the segmental motor circuitry. J. Comp. Neurol. 2009;514(4):297–309. doi: 10.1002/cne.22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Park S, Kim HT, Yun S, et al. Growth factor-expressing human neural progenitor cell grafts protect motor neurons but do not ameliorate motor performance and survival in ALS mice. Exp. Mol. Med. 2009;41(7):487–500. doi: 10.3858/emm.2009.41.7.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Suzuki M, Mchugh J, Tork C, et al. Direct muscle delivery of GDNF with human mesenchymal stem cells improves motor neuron survival and function in a rat model of familial ALS. Mol. Ther. 2008;16(12):2002–2010. doi: 10.1038/mt.2008.197. ▪▪ Describes the use of mesenchymal stem cells to deliver therapeutic growth factors in an amyotrophic lateral sclerosis model. It also demonstrates the efficacy of using stem cells in combination with growth factor therapy to protect both motor neurons and neuromuscular junctions.
- 94.Corti S, Nizzardo M, Nardini M, et al. Neural stem cell transplantation can ameliorate the phenotype of a mouse model of spinal muscular atrophy. J. Clin. Invest. 2008;118(10):3316–3330. doi: 10.1172/JCI35432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Corti S, Nizzardo M, Nardini M, et al. Embryonic stem cell-derived neural stem cells improve spinal muscular atrophy phenotype in mice. Brain. 2010;133(Pt 2):465–481. doi: 10.1093/brain/awp318. ▪▪ Demonstrates the use of neurons derived from embryonic stem cells to improve the lifespan of a spinal muscular atrophy mouse model.
- 96.Hung CW, Liou YJ, Lu SW, et al. Stem cell-based neuroprotective and neurorestorative strategies. Int. J. Mol. Sci. 2010;11(5):2039–2055. doi: 10.3390/ijms11052039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li JY, Christophersen NS, Hall V, Soulet D, Brundin P. Critical issues of clinical human embryonic stem cell therapy for brain repair. Trends Neurosci. 2008;31(3):146–153. doi: 10.1016/j.tins.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 98.Raore B, Federici T, Taub J, et al. Cervical multilevel intraspinal stem cell therapy: assessment of surgical risks in Gottingen minipigs. Spine. 2011;36(3):E164–E171. doi: 10.1097/BRS.0b013e3181d77a47. [DOI] [PubMed] [Google Scholar]
- 99.Cizkova D, Kakinohana O, Kucharova K, et al. Functional recovery in rats with ischemic paraplegia after spinal grafting of human spinal stem cells. Neuroscience. 2007;147(2):546–560. doi: 10.1016/j.neuroscience.2007.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yan J, Xu L, Welsh AM, et al. Extensive neuronal differentiation of human neural stem cell grafts in adult rat spinal cord. PLoS Med. 2007;4(2) doi: 10.1371/journal.pmed.0040039. e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Usvald D, Vodicka P, Hlucilova J, et al. Analysis of dosing regimen and reproducibility of intraspinal grafting of human spinal stem cells in immunosuppressed minipigs. Cell Transplant. 2010;19(9):1103–1122. doi: 10.3727/096368910X503406. [DOI] [PubMed] [Google Scholar]
- 102.Riley JP, Raore B, Taub JS, Federici T, Boulis NM. Platform and cannula design improvements for spinal cord therapeutics delivery. Neurosurgery. 2011 doi: 10.1227/NEU.0b013e3182195680. (In Press) [DOI] [PubMed] [Google Scholar]
- 103.Chen L, Huang H, Zhang J, et al. Short-term outcome of olfactory ensheathing cells transplantation for treatment of amyotrophic lateral sclerosis. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2007;21(9):961–966. [PubMed] [Google Scholar]
- 104.Deda H, Inci MC, Kurekci AE, et al. Treatment of amyotrophic lateral sclerosis patients by autologous bone marrow-derived hematopoietic stem cell transplantation: a 1-year follow-up. Cytotherapy. 2009;11(1):18–25. doi: 10.1080/14653240802549470. [DOI] [PubMed] [Google Scholar]
- 105.Federici T, Riley J, Park J, Bain M, Boulis NM. Preclinical safety validation of a stabilized viral vector direct injection approach to the cervical spinal cord. Clin. Transl. Sci. 2009;2(2):165–167. doi: 10.1111/j.1752-8062.2008.00084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mazzini L, Ferrero I, Luparello V, et al. Mesenchymal stem cell transplantation in amyotrophic lateral sclerosis: a Phase I clinical trial. Exp. Neurol. 2010;223(1):229–237. doi: 10.1016/j.expneurol.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 107.Riley J, Butler J, Park J, et al. Targeted spinal cord therapeutics delivery: stabilized platform and MER guidance validation. Stereotact. Funct. Neurosurg. 2007;86(2):67–74. doi: 10.1159/000112426. [DOI] [PubMed] [Google Scholar]
- 108.Karussis D, Karageorgiou C, Vaknin-Dembinsky A, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch. Neurol. 2010;67(10):1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dantuma E, Merchant S, Sugaya K. Stem cells for the treatment of neurodegenerative diseases. Stem Cell Res. Ther. 2010;1(5):37. doi: 10.1186/scrt37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gogel S, Gubernator M, Minger SL. Progress and prospects: stem cells and neurological diseases. Gene Therapy. 2011;18(1):1–6. doi: 10.1038/gt.2010.130. [DOI] [PubMed] [Google Scholar]
- 111.Miller RH. The promise of stem cells for neural repair. Brain Res. 2006;1091(1):258–264. doi: 10.1016/j.brainres.2006.01.073. [DOI] [PubMed] [Google Scholar]
- 112.Webber DJ, Minger SL. Therapeutic potential of stem cells in central nervous system regeneration. Curr. Opin. Investig. Drugs. 2004;5(7):714–719. [PubMed] [Google Scholar]
- 113.Yu D, Silva GA. Stem cell sources and therapeutic approaches for central nervous system and neural retinal disorders. Neurosurg. Focus. 2008;24(3–4):E11. doi: 10.3171/FOC/2008/24/3-4/E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Geron Corporation: World’s first clinical trial of human embryonic stem cell therapy cleared. Regen. Med. 2009;4(2):161. [PubMed] [Google Scholar]
- 115.Lopez-Gonzalez R, Kunckles P, Velasco I. Transient recovery in a rat model of familial amyotrophic lateral sclerosis after transplantation of motor neurons derived from mouse embryonic stem cells. Cell Transplant. 2009;18(10):1171–1181. doi: 10.3727/096368909X12483162197123. [DOI] [PubMed] [Google Scholar]
- 116.Corti S, Locatelli F, Papadimitriou D, et al. Neural stem cells LewisX+ CXCR4+ modify disease progression in an amyotrophic lateral sclerosis model. Brain. 2007;130(Pt 5):1289–1305. doi: 10.1093/brain/awm043. [DOI] [PubMed] [Google Scholar]
- 117.Rizvanov AA, Guseva DS, Salafutdinov II, et al. Genetically modified human umbilical cord blood cells expressing vascular endothelial growth factor and fibroblast growth factor 2 differentiate into glial cells after transplantation into amyotrophic lateral sclerosis transgenic mice. Exp. Biol. Med. (Maywood) 2011;236(1):91–98. doi: 10.1258/ebm.2010.010172. [DOI] [PubMed] [Google Scholar]
- 118.Kim H, Kim HY, Choi MR, et al. Dose-dependent efficacy of ALS-human mesenchymal stem cells transplantation into cisterna magna in SOD1-G93A ALS mice. Neurosci. Lett. 2010;468(3):190–194. doi: 10.1016/j.neulet.2009.10.074. [DOI] [PubMed] [Google Scholar]
- 119.Hwang DH, Lee HJ, Park IH, et al. Intrathecal transplantation of human neural stem cells overexpressing VEGF provide behavioral improvement, disease onset delay and survival extension in transgenic ALS mice. Gene Ther. 2009;16(10):1234–1244. doi: 10.1038/gt.2009.80. [DOI] [PubMed] [Google Scholar]
- 120.Klein SM, Behrstock S, Mchugh J, et al. GDNF delivery using human neural progenitor cells in a rat model of ALS. Hum. Gene Ther. 2005;16(4):509–521. doi: 10.1089/hum.2005.16.509. [DOI] [PubMed] [Google Scholar]