Abstract
The muscularis mucosae, a type of smooth muscle located between the urothelium and the urinary bladder detrusor, has been described, although its properties and role in bladder function have not been characterized. Here, using mucosal tissue strips isolated from guinea pig urinary bladders, we identified spontaneous phasic contractions (SPCs) that appear to originate in the muscularis mucosae. This smooth muscle layer exhibited Ca2+ waves and flashes, but localized Ca2+ events (Ca2+ sparks, purinergic receptor-mediated transients) were not detected. Ca2+ flashes, often in bursts, occurred with a frequency (∼5.7/min) similar to that of SPCs (∼4/min), suggesting that SPCs are triggered by bursts of Ca2+ flashes. The force generated by a single mucosal SPC represented the maximal force of the strip, whereas a single detrusor SPC was ∼3% of maximal force of the detrusor strip. Electrical field stimulation (0.5–50 Hz) evoked force transients in isolated detrusor and mucosal strips. Inhibition of cholinergic receptors significantly decreased force in detrusor and mucosal strips (at higher frequencies). Concurrent inhibition of purinergic and cholinergic receptors nearly abolished evoked responses in detrusor and mucosae. Mucosal SPCs were unaffected by blocking small-conductance Ca2+-activated K+ (SK) channels with apamin and were unchanged by blocking large-conductance Ca2+-activated K+ (BK) channels with iberiotoxin (IbTX), indicating that SK and BK channels play a much smaller role in regulating muscularis mucosae SPCs than they do in regulating detrusor SPCs. Consistent with this, BK channel current density in myocytes from muscularis mucosae was ∼20% of that in detrusor myocytes. These findings indicate that the muscularis mucosae in guinea pig represents a second smooth muscle compartment that is physiologically and pharmacologically distinct from the detrusor and may contribute to the overall contractile properties of the urinary bladder.
Keywords: calcium imaging, large-conductance calcium-activated potassium channel, potassium channels, calcium flashes, spontaneous phasic contractions
the urinary bladder is a hollow, muscular organ that serves two functions: urine storage and elimination. The bladder wall is composed of detrusor smooth muscle, which accounts for a large fraction of the bladder mass, and a layer of transitional epithelial cells known as the urothelium, which lines the luminal surface of the urinary bladder. The detrusor smooth muscle has been extensively characterized and is clearly responsible for the majority of the contractile properties of the urinary bladder (1, 69). The urothelium, which was originally thought to simply act as a passive, impermeable barrier that prevents the movement of solutes from the urine into the bloodstream, is now recognized to possess a broad range of sensory and signal transduction functions that greatly influence bladder physiology (for a review, see Ref. 5).
The detrusor exhibits two primary modes of contractile behavior: nerve-evoked contractions and spontaneous phasic contractions (SPCs). Nerve-evoked contractions of the detrusor are caused by release of ATP and acetylcholine (ACh) from parasympathetic nerve varicosities onto detrusor myocytes. ATP and ACh bind to purinergic and muscarinic receptors, respectively, triggering action potentials that increase intracellular Ca2+ and initiate contraction (31, 43, 53). In contrast, SPCs are induced by spontaneous action potentials in detrusor myocytes. Ca2+ entry through L-type voltage-dependent Ca2+ channels (L-VDCCs) mediates the upstroke of the detrusor smooth muscle action potential and provides Ca2+ for SPCs and nerve-evoked contractions (26, 29, 33, 53, 74). K+ efflux through large-conductance Ca2+-activated K+ (BK) channels and voltage-gated K+ (KV) channels is responsible for the repolarization phase of the action potential (29, 63). KV channels contribute to the afterhyperpolarization phase along with small-conductance Ca2+-activated K+ (SK) channels (12, 21, 25). Because BK and SK channels play such a central role in mediating action potential repolarization, blocking them with toxins such as iberiotoxin (IbTX) or apamin, respectively, potentiates the detrusor smooth muscle action potential and greatly increases the force of the resulting contractions (32, 44, 69). Activating BK channels with novel compounds such as NS11021 (43) or SK channels with NS309 decreases contraction force (unpublished observations).
The region between the basement layer of the urothelium and the luminal surface of the detrusor contains numerous sensory and effector nerve fibers (4, 14) and a dense network of blood vessels and capillaries (39, 40, 50, 51), all of which are embedded in a connective tissue matrix. A diverse group of cells reside in this area, including cells described as myofibroblasts, now known as interstitial cells of Cajal (ICCs) (nomenclature designated at the Fifth International Symposium on Interstitial Cells of Cajal, Ireland, 2007). The physiological role of these cells is unclear, but they may contribute to sensory processing and serve a role in mediating communication between the urothelium and detrusor (for review, see Ref. 46). A type of smooth muscle—the muscularis mucosae—has also been identified between the basement layer of the urothelium and the muscularis of the detrusor (10, 42, 54, 59, 67, 68). Although the existence of the muscularis mucosae is well established in the medical literature, particularly with respect to its role in the development of urinary bladder cancer, its functional role in normal urinary bladder contractility and physiology has not been characterized. In tissues that possess a similar layer, such as the gastrointestinal tract (for review, see Ref. 65) and esophagus (13, 17, 38), the muscularis mucosae has been shown to exhibit spontaneous phasic contractility and also to respond to nerve-induced stimulation. The extent and nature of spontaneous and nerve-evoked contractions of the muscularis mucosae in the esophagus exhibit large species-specific variability (65), highlighting potential uncertainties about their functional role in this tissue. In the colon, spontaneous phasic activity of the muscularis mucosae has been suggested to play a role in modulating mucosal secretion (56, 57, 61), reducing mucosal surface area in response to noxious stimuli (45, 55), and facilitating the release of acids into the stomach (62).
The properties of detrusor smooth muscle have often been investigated in urinary bladder strips with the mucosal or urothelial layer removed. It has been assumed that the mucosal layer is devoid of contractile behavior, but cells in this layer may release factors that could affect detrusor smooth muscle function. Contrary to this presumption, we found that mucosal strips exhibited SPCs that can be attributed to a layer of smooth muscle located just abluminal to the basement layer of the urothelium—the muscularis mucosae. These SPCs likely result from bursts of Ca2+ (flashes) that elevate Ca2+ in the mucosal layer. The force of SPCs generated by the mucosal layer was equivalent to that of detrusor SPCs; however, the peak force of detrusor contractions evoked by 60 mM K+ was ∼40-fold greater than that of the muscularis mucosae. In contrast to the detrusor, the mucosal layer exhibited very little SK and BK channel activity. These findings suggest that the muscularis mucosae is functionally distinct from the detrusor and may contribute to bladder physiology.
MATERIALS AND METHODS
Animals.
Male guinea pigs (250–400 g) were euthanized with isoflurane and exsanguinated according to a protocol approved by the Institutional Animal Care and Use Committee of the University of Vermont. The urinary bladders were removed and placed into ice-cold dissection solution (DS) consisting of (in mM) 80 monosodium glutamate, 55 NaCl, 6 KCl, 10 glucose, 10 HEPES, and 2 MgCl2, adjusted to pH 7.3.
Contractility studies.
Each bladder was cut open, rinsed with DS to remove any residual urine, and pinned to a Sylgard-lined dissecting dish with the serosal surface facing down. The bladders were trimmed into a rectangle ∼16 × 5 mm, and the mucosal layer was separated from the detrusor by gently lifting up a corner of the mucosae along a natural plane of division and then snipping away the connective tissue linking it to the underlying detrusor. The resulting detrusor and mucosal sheets were then cut into eight equivalent strips, each ∼5 × 2 mm. Detrusor strips were composed of detrusor smooth muscle, whereas mucosal strips included urothelium and muscularis mucosae smooth muscle. Intact bladder strips were prepared as described above without separation of mucosal and detrusor layers.
The contractile activity of detrusor and mucosal strips was analyzed separately. Each strip was suspended from a force transducer in a temperature-controlled (37°C) jacketed water bath containing physiological saline solution (PSS) consisting of (in mM) 119 NaCl, 4.7 KCl, 24 NaHCO3, 1.2 KH2PO4, 2.5 CaCl2, 1.2 MgSO4, and 11 glucose. Physiological pH was maintained by bubbling PSS with a gas mixture of 95% O2-5% CO2. After a brief equilibration period in PSS, each strip was stretched to ∼10 mN of tension. Force was measured and recorded with a MyoMed myograph system (Med Associates, Georgia, VT). The contractile response to nerve-mediated contractions was characterized by plotting the response to electrical field stimulation (EFS) of different frequencies (0.5, 2, 3.5, 5, 7.5, 10, 12.5, 20, 30, 40, and 50 Hz), administered as 20-V, 0.2-ms pulses over a duration of 2 s. The responses of tissue strips to various agonists and antagonists, added directly to the bath, were characterized as detailed below. Contraction amplitudes and force integrals were measured with Mini Analysis software (Synaptosoft, Fort Lee, NJ). In mucosae, SPCs often occurred during EFS. At lower stimulation frequencies the amplitude of SPCs was often much larger than the evoked response, making it very difficult to accurately measure the evoked response. For these experiments we used peak amplitudes of SPCs before and after each stimulus as our baseline and measured the amplitude of the evoked response that was greater than this value. Evoked responses less than SPC amplitude were plotted as zero.
Electrophysiology.
To compare the electrophysiological properties of detrusor and mucosal smooth muscle cells, we first separated the mucosal layer from the detrusor as detailed above. The resulting tissue sheets were diced into small sections (∼2 × 2 mm), which were then placed into a 1 mg/ml papain solution (Worthington Biochemical, Freehold, NJ) prepared in DS containing 1 mg/ml dithioerythritol and incubated for ∼20 min at 37°C. After a brief rinse in ice-cold DS, tissue sections were transferred to a vial containing 1 mg/ml collagenase (type II) and 100 μM CaCl2 and incubated for ∼6 min at 37°C. After rinsing in ice-cold DS, the tissue sections were gently triturated by using a fire-polished Pasteur pipette to release smooth muscle cells.
The isolated cells in DS were placed in a glass-plated chamber for ∼20 min and then immersed in a bath solution containing (in mM) 134 NaCl, 6 KCl, 10 glucose, 1 MgCl2, 2 CaCl2, and 10 HEPES, adjusted to pH 7.3. Whole cell currents were recorded with the perforated-patch configuration of the patch-clamp technique. Pipettes with a resistance of 4–5 MΩ were filled with a solution consisting of (in mM) 110 potassium aspartate, 30 KCl, 10 NaCl, 1 MgCl2, 0.05 EGTA, and 10 HEPES with 200 μg/ml amphotericin, adjusted to pH 7.3. All reagents were from Sigma-Aldrich (St. Louis, MO) unless specifically indicated otherwise. Whole cell outward currents were evoked with 10-mV depolarizing steps (250 ms) from an initial holding potential of −60 mV up to +60 mV. Where applicable, IbTX (100 nM; Peptides International, Louisville, KY) was added to block BK channels, and all currents were normalized to cell capacitance (pA/pF). All patch-clamp experiments were performed at 22–23°C.
Ca2+ imaging.
Ca2+ events in the guinea pig urinary bladder were studied in mucosal and detrusor layers, prepared as described above. The resulting tissues were cut into thin strips (∼1 × 3 mm), mounted to a Sylgard block with small pins, and loaded with the Ca2+-sensitive dye fluo-4 by incubation for 60–75 min at room temperature with a solution of fluo-4 AM (10 μM) and pluronic acid (2.5 μg/ml) in (in mM) 134 NaCl, 6 KCl, 10 glucose, 1 MgCl2, 2 CaCl2, and 10 HEPES (pH 7.4). After loading, the tissue was placed in a chamber specialized for Ca2+ imaging and superfused with PSS at a rate of 1–2 ml/min at 37°C. Images were collected with a Noran Oz laser-scanning confocal microscope and Nikon ×20 dry (NA 0.75) or ×60 water-immersion (NA 1.2) fluorescent objectives with the aid of software developed by Prairie Technologies (Middleton, WI). Fluo-4 was excited with a krypton-argon laser at 488 nm, and the emitted fluorescence was collected at >500 nm. Each file was recorded for 30–60 s, and images were collected at a rate of 53 images/s. Imaging fields were 385 × 385 μm (256 × 256 pixels) for the ×20 objective and 131 × 131 μm (256 × 256 pixels) for the ×60 objective. The raw data files were converted to TIFF files and analyzed off-line with software developed in our laboratory by Dr. Adrian Bonev. Fluorescence changes in smooth muscle were measured by positioning boxes over regions of interest (ROIs), identified post hoc, and recording changes in fluorescence within these regions throughout the recording interval. The dimensions of ROIs were 15.2 × 15.2 μm (10 × 10 pixels) for the ×20 objective and 2.5 × 2.5 μm (10 × 10 pixels) for the ×60 objective.
Immunohistochemistry and histology.
Tissue strips (intact, detrusor, or mucosal), prepared as detailed above, were fixed in 4% paraformaldehyde in PSS and then embedded in paraffin blocks. Cross sections (10 μm thick) were cut from the blocks and mounted on glass slides. Some slides were stained with hematoxylin and eosin (H & E); others were analyzed by immunohistochemistry.
For immunohistochemistry, tissue cross sections were first permeabilized in 0.2% Triton X-100 in PSS at room temperature for 10 min and then blocked with 0.1% gelatin in PSS. Slide-mounted sections were then incubated overnight at 4°C with a mouse anti-smooth muscle α-actin antibody (Sigma-Aldrich; 1:200 dilution in 0.1% gelatin-PSS). After rinsing in PSS, slides were incubated with fluorescent secondary antibodies (Alexa Fluor 488, 1:500 in gelatin-PSS; Invitrogen, Carlsbad, CA) for 1 h at room temperature. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) before a coverslip was added.
Statistics.
GraphPad Software (San Diego, CA) and SigmaStat, version 2.0 (SYSTAT Software, Chicago, IL) were used for preparation of graphs and statistical analyses. Data are reported as means ± SE, where n represents the number of tissue strips (contractility experiments, Ca2+ imaging) or isolated myocytes (electrophysiology experiments). Comparisons between groups were made with a two-tailed Student's t-test; a P value < 0.05 was considered statistically significant.
RESULTS
Morphology of mucosal strips.
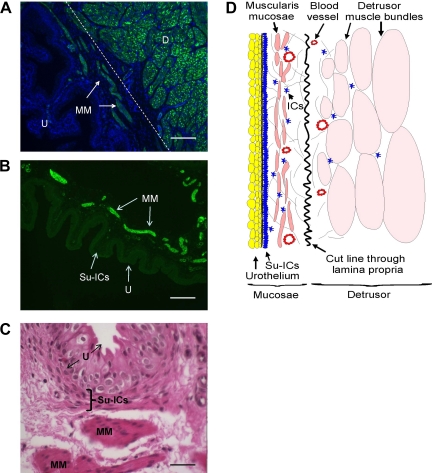
Figure 1A shows a fluorescence micrograph of a section of intact urinary bladder (detrusor + mucosae) immunostained for smooth muscle α-actin (green). Nuclei were stained with DAPI (blue). Smooth muscle α-actin was expressed throughout the detrusor smooth muscle and in the walls of the blood vessels. Notably, between the detrusor and urothelial layers there were discontinuous sheets of tissue that stained positive for α-actin. In addition to smooth muscle, some ICCs also express α-actin (37). Although this layer of positive α-actin expression may contain both ICCs and smooth muscle, we have termed this layer the muscularis mucosae by analogy to similar tissue in human urinary bladder (10, 42, 54, 59, 67, 68). Mucosal tissue strips, separated from the detrusor along the natural plane of division (shown in Fig. 1A) and immunostained for α-actin, contained the urothelium, blood vessels, and muscularis mucosae smooth muscle bundles (Fig. 1B). Located immediately below the basement membrane was a layer of cells that corresponds to the location of suburothelial ICCs (23). This micrograph has been overexposed to allow visualization of the urothelium and suburothelial ICC layer. It is clear that, even with overexposure, there is no evidence for smooth muscle α-actin expression in either the urothelium or suburothelial ICC layer. Figure 1C is a representative micrograph of a cross section of mucosal tissue stained with H & E. The suburothelial ICC layer is clearly visible as a band of cells, approximately three to five cells thick, lying immediately below the basement layer of the urothelium. Several mucosal smooth muscle bundles are also readily apparent. Figure 1D is a schematic of the tissue layers in the bladder wall showing the location of the muscularis mucosae. The guinea pig urinary bladder muscularis mucosae appears to be composed of a discontinuous band of smooth muscle sheets and bundles that are located in close proximity to the urothelium.
Fig. 1.
General histology of intact bladder and mucosal strips. A: low-magnification (×10) image of an intact bladder strip showing the detrusor smooth muscle layer (D) and mucosal smooth muscle layer (MM) immunostained for smooth muscle α-actin (green); nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; blue). The dotted line through the lamina propria indicates the approximate boundary separating the detrusor and the mucosae. This image has been overexposed to allow visualization of the urothelial (U) layer. B: low-magnification image (×10) of a mucosal strip immunostained for smooth muscle α-actin. Mucosal smooth muscle bundles (MM) are clearly evident, as are several blood vessels. The image has been overexposed to allow visualization of the urothelial lining (U) and the region where suburothelial ICCs (Su-ICs) are found. C: high-power magnification (×40) under oil immersion of a mucosal strip stained with hematoxylin and eosin (H & E). Although the Su-IC layer and mucosal smooth muscle bundles show similar H & E staining, the Su-IC layer was not positive for smooth muscle α-actin by immunostaining. D: schematic showing the muscularis mucosae located between the urothelium and the detrusor and the approximate boundary in the lamina propria separating the detrusor and mucosae. Scale bars, 100 μm (A and B) and 25 μm (C).
Mucosal tissue strips exhibit phasic contractions.
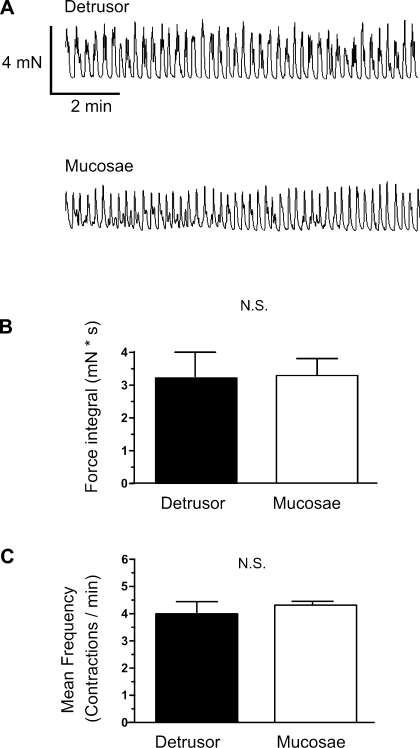
SPCs of urinary bladder (detrusor) smooth muscle strips from guinea pig have been extensively studied, but these studies have typically been performed with the mucosae removed so as to minimize possible influences from the urothelium (32, 33, 44). To address the potential functional properties of the mucosae and relate them to those of the detrusor, we examined the contractile properties of isolated mucosal and detrusor strips. Contrary to expectations, mucosal tissue strips exhibited robust SPCs (Fig. 2A). Interestingly, the SPCs of mucosal and detrusor strips taken from the same urinary bladder exhibited statistically similar force integrals and frequencies (Fig. 2, B and C). This similarity is striking given the much greater smooth muscle mass in the detrusor strips compared with the sparse distribution of smooth muscle in the mucosal strips (Fig. 1, A and B).
Fig. 2.
Comparison of spontaneous phasic contractions (SPCs) in detrusor and mucosal strips. A: representative myograph recordings obtained from detrusor (top) and mucosal (bottom) layers from the same urinary bladder strip. B: comparison of the force integral of SPCs from mucosal and detrusor strips. C: comparison of the frequency of SPCs from detrusor and mucosal strips. Data are expressed as means ± SE. N.S., not significant.
Mucosal SPCs are attributable to the muscularis mucosae.
One possible origin for the SPCs in mucosal tissue strips is the suburothelial ICC layer. However, in the guinea pig preparations, the suburothelial ICCs did not stain for smooth muscle α-actin (Fig. 1B) and thus seem unlikely to possess contractile properties. To determine whether SPCs in the guinea pig mucosae could be specifically linked to the muscularis mucosae, we measured Ca2+ activity in muscularis mucosae muscle bundles with the Ca2+ indicator dye fluo-4.
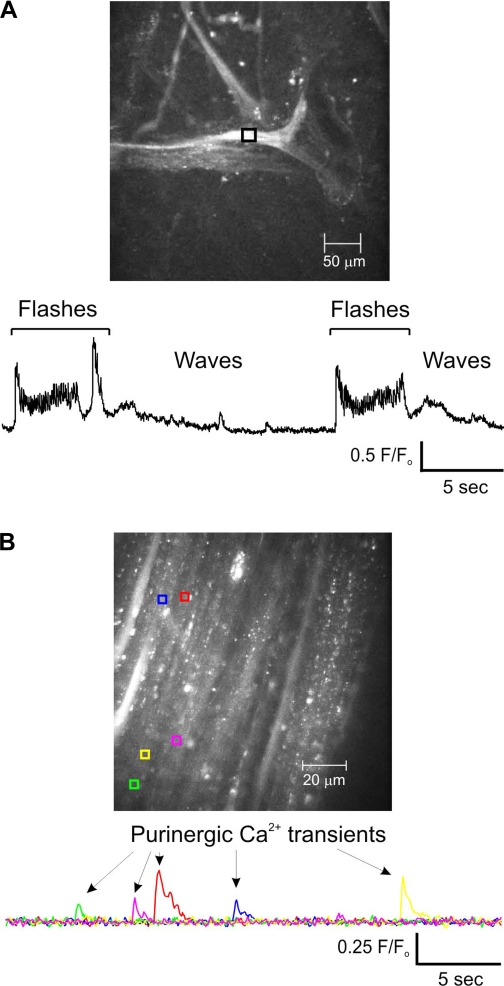
Detrusor smooth muscle cells exhibit four types of Ca2+ signals: 1) Ca2+ flashes, which are global events that represent extracellular Ca2+ entry during an action potential; 2) Ca2+ waves, which have a defined wave front that moves through the cell, are much slower than Ca2+ flashes, and result from inositol trisphosphate (IP3)-mediated release of Ca2+ from the sarcoplasmic reticulum (SR); 3) Ca2+ sparks, which are brief, localized events that represent the release of Ca2+ from the SR through ryanodine receptors; and 4) purinergic Ca2+ transients, which are brief, localized events that represent Ca2+ influx into the cell through P2X1 receptors in response to ATP release from parasympathetic nerve varicosities (30, 74). Representative video recordings of muscularis mucosae Ca2+ activity are presented in Supplemental Movies S1 and S2.1 In these recordings, Ca2+ waves (19.2 ± 2.4 Ca2+ waves/min; n = 15 cells) can be seen traveling along the muscle fibers that compose the muscularis mucosae muscle bundles. Periodically, a Ca2+ flash (or more often, a burst of Ca2+ flashes) occurs, followed by a quiescent period. The frequency of Ca2+ flashes/bursts was 5.7 ± 0.9/min (n = 15 mucosal muscle bundles; Fig. 3A), and each Ca2+ flash or burst rapidly elevated global Ca2+ levels in the muscle bundles. Importantly, each rise in global Ca2+ was followed by an SPC (compare Ca2+ flash/burst frequency in Fig. 3A with SPC frequency in Fig. 2C). Ca2+ waves were not observed during Ca2+ flash activity; however, as the tissue relaxed, Ca2+ levels dropped and the Ca2+ waves again became apparent, until the next burst of Ca2+ flashes. In some experiments (Supplemental Movie S2), separate muscle bundles bridged by smaller bundles exhibited concurrent global Ca2+ flashes, suggesting a high degree of coordination between the various sheets and bundles of muscle that constitute the muscularis mucosae.
Fig. 3.
Ca2+ events recorded from the muscularis mucosae and detrusor. A: bursts of Ca2+ flashes at regular intervals were followed by Ca2+ waves in muscularis mucosae (see Supplemental Movies S1 and S2). B: purinergic Ca2+ transients recorded from the detrusor. These purinergic Ca2+ transients were absent in recordings from the muscularis mucosae. F/F0 is the fractional fluorescense. F0 is the baseline fluorescense determinded by averaging 30 images with no Ca2+ events.
It is possible that the bundles of smooth muscle present in the mucosal region are simply loose bands of detrusor smooth muscle that have been dissected away with the mucosal strip. Although we cannot eliminate the possibility that some detrusor tissue might be present in mucosal tissue strips (and vice versa), our Ca2+ signaling data argue against this being a significant issue. Specifically, detrusor smooth muscle exhibits characteristic, localized Ca2+ transients that reflect activation of P2X1 receptors (30). In over 40 recordings of mucosal smooth muscle, such Ca2+ transients were never detected. They were, however, readily apparent in recordings from detrusor smooth muscle: 8 of 15 detrusor smooth muscle preparations exhibited spontaneous purinergic Ca2+ transients with a frequency of 3.9 ± 0.9 events·30 s−1·field−1 (Fig. 3B). Thus the Ca2+ activity of urinary bladder muscularis mucosae smooth muscle appears to be limited to Ca2+ waves and bursts of Ca2+ flashes, typified by the recording shown in Fig. 3A.
The results of our Ca2+ imaging indicate that the SPCs of the guinea pig mucosae most likely arise from bursts of Ca2+ flashes in smooth muscle sheets and bundles that compose the muscularis mucosae. In addition, despite the spatial separation of mucosal smooth muscle bundles and sheets, mechanisms that enable a high degree of coordination between the discrete islands of smooth muscle appear to exist. Finally, the unique Ca2+ signature of the muscularis mucosae indicates that it is physiologically distinct from the detrusor.
Mucosal phasic contractions are nonneurogenic and spontaneous.
The urothelium is known to release both ATP and ACh (19, 41, 73), and the submucosal space is richly innervated with nerve fibers that could also potentially release ATP and ACh (15, 24, 70). To determine whether mucosal phasic contractions were being driven by urothelial and/or neuronal release of ATP or ACh, we exposed mucosal tissue strips to atropine (20 μM) to block muscarinic receptors, α,β-methylene ATP (10 μM) to desensitize and block P2X purinergic receptors, or TTX (10 μM) to block neuronal transmission. None of these treatments significantly affected the frequency of mucosal SPCs (initial 5.6 ± 0.46/min, atropine 5.9 ± 0.50/min, α,β-methylene ATP 5.3 ± 0.41/min, TTX 5.8 ± 0.41/min; n = 7) or force, measured as peak amplitude (initial 0.88 ± 0.21 mN, atropine 0.85 ± 0.23 mN, α,β-methylene ATP 0.86 ± 0.16 mN, TTX 0.82 ± 0.23 mN; n = 7). The persistence of SPCs in the presence of these inhibitors indicates that they are most likely nonneurogenic in origin and are not driven by the release of ACh or ATP from submucosal nerve terminals or the urothelium.
Ca2+ requirements of mucosal SPCs.
The Ca2+ required for muscle contractions may be derived from intracellular or extracellular sources, or a combination of the two, depending on the nature of the muscle (1, 3). In the urinary bladder, treatment with selective L-VDCC inhibitors (e.g., nifedipine) strongly inhibits or abolishes spontaneous detrusor activity, indicating that detrusor contractility depends on the influx of extracellular Ca2+ (26, 29). Although SR Ca2+ stores are presumed to contribute to cholinergic detrusor contractions (3, 20), the role of this pathway is still incompletely understood (1, 18, 72). However, it is well established that SR Ca2+ release via ryanodine receptors in the form of Ca2+ sparks plays a role in opposing detrusor contraction (34, 35, 49).
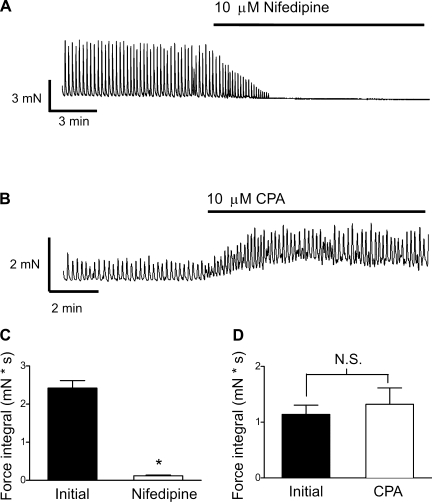
To determine the relative importance of intracellular and extracellular Ca2+ in mediating mucosal smooth muscle SPCs, we treated mucosal tissue strips with nifedipine (10 μM) to block L-VDCCs or cyclopiazonic acid (CPA, 10 μM) to deplete SR Ca2+ stores. Blocking L-VDCCs rapidly, and almost completely, eliminated mucosal SPCs (Fig. 4, A and C; P < 0.05, n = 7). In contrast, SR store depletion by CPA increased baseline tension (control 0.6 ± 0.2 mN, CPA 1.2 ± 0.2 mN; P < 0.05, n = 7), presumably reflecting elevated intracellular Ca2+ levels caused by blockade of the sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) pump, but did not significantly elevate the frequency (control 4.6 ± 0.4/min, CPA 6.3 ± 0.6/min; P > 0.05; n = 7) or force integral (P > 0.05; n = 7) of SPCs (Fig. 4, B and D). These results suggest that mucosal smooth muscle SPCs are dependent on the influx of extracellular Ca2+ through L-VDCCs but not on SR Ca2+ release.
Fig. 4.
Nifedipine, but not cyclopiazonic acid (CPA), abolished mucosal contractions. Inhibition of voltage-dependent Ca2+ channels (VDCCs) with nifedipine (10 μM) eliminated mucosal contractions (A), but inhibition of sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) with CPA (10 μM) did not inhibit mucosal smooth muscle SPCs (B). C and D: summary data showing that the force integral was abolished by nifedipine (C) but was not significantly altered by CPA (D). Data are expressed as means ± SE (*P < 0.05).
Force generation by mucosal smooth muscle.
The force generated by mucosal SPCs was similar to that of the matched detrusor strips (Fig. 2). This observation was intriguing because the smooth muscle content in the mucosal strips is clearly lower than that in the detrusor strips (Fig. 1, A and B). One possible explanation for this observation is that each spontaneous contraction of the detrusor only recruits a small fraction of the total muscle fibers present in the detrusor strip, whereas each SPC of the mucosal strip recruits a relatively large proportion of the available smooth muscle. If this is the case, then the total maximum force-generating capacity of the detrusor strips should be much greater than that of the mucosal strips, despite the similarity in spontaneous contraction force.
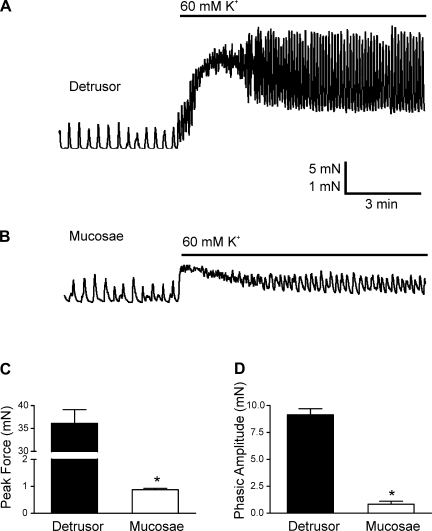
To investigate this possibility, we contracted mucosal and detrusor tissue strips by depolarizing with 60 mM K+. As shown in the original recordings (Fig. 5, A and B), depolarization with 60 mM K+ had a profoundly greater effect on the contractile response of detrusor strips, inducing approximately a 40-fold greater increase in peak force in these strips (36.12 ± 7.33 mN) than in mucosal strips (0.88 ± 0.21 mN; P < 0.05, n = 6; Fig. 5C). In addition, the steady-state force (tone) generated in response to 60 mM K+ (baseline in Fig. 5, A and B) was significantly greater for the detrusor strips (11.22 ± 4.53 mN) than the mucosal strips (0.67 ± 44 mN; P < 0.05, n = 6). Finally, the amplitude of the phasic contractions in the presence of 60 mM K+ was also significantly elevated in detrusor strips (9.15 ± 1.56 mN) compared with mucosal strips (0.81 ± 0.74 mN; Fig. 5D; P < 0.05, n = 8). These findings indicate that, although the force of mucosal SPCs may be equivalent to those of the detrusor, the detrusor has the potential to generate much more force than the mucosal smooth muscle. Interestingly, in the mucosal strips, the peak force amplitude generated by treatment with 60 mM K+ was not significantly greater (12.2% ± 2.2%; n = 6) than the amplitude of the SPCs that preceded it (Fig. 5B), suggesting that virtually all contractile cells available within the mucosal strip are recruited by mucosal SPCs.
Fig. 5.
Detrusor strips generate significantly greater force than mucosal strips in response to membrane depolarization with 60 mM K+. A and B: representative myograph recordings from a detrusor (A) and a mucosal (B) smooth muscle strip. C and D: comparison of the peak force of contractions (C) and the phasic amplitude (D) evoked by 60 mM K+. Data are expressed as means ± SE (*P < 0.05).
EFS evokes contraction of detrusor and mucosal strips.
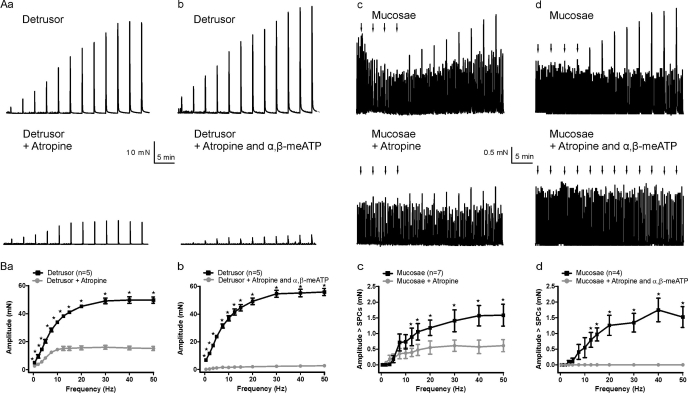
In addition to SPCs, the urinary bladder also contracts forcefully to EFS. To study the response of the detrusor and muscularis mucosae to EFS, we prepared tissue strips as described in materials and methods and placed them in tissue baths adjacent to stimulating electrodes. EFS (0.5–50 Hz) was used to stimulate contractions in detrusor and mucosal strips (Fig. 6). To measure the contribution of muscarinic and purinergic pathways to contractions, we applied atropine (10 μM) and/or α,β-methylene ATP (10 μM) to inhibit muscarinic and purinergic receptors, respectively. In detrusor strips, EFS caused a frequency-dependent increase in force. Atropine produced a frequency-dependent reduction in the amplitude of EFS-induced contractions, ranging from 47.4% at 0.5 Hz to 69.6% at 50 Hz (Fig. 6, Aa and Ba; n = 5 strips/group). In the presence of both atropine and α,β-methylene ATP, EFS-induced contractions were nearly abolished (mean reduction in amplitude for all frequencies 96.0%; n = 5; Fig. 6, Ab and Bb). In contrast, EFS evoked small increases in mucosal force that were only evident at higher frequencies. At lower stimulating frequencies, the evoked responses could not be accurately measured because of the presence of similar-sized SPCs. Inhibition of mucosal muscarinic receptors with atropine reduced force amplitude at higher stimulating frequencies (Fig. 6, Ac and Bc). In the presence of both atropine and the P2X1 receptor-desensitizing agent α,β-methylene ATP, evoked responses were not detected (Fig. 6, Ad and Bd). The maximum amplitude of the evoked contractions in detrusor strips was ∼10-fold greater than in mucosae, likely reflecting the much larger muscle mass of the detrusor.
Fig. 6.
Electrical field stimulation (EFS; 0.5–50 Hz) evoked frequency-dependent contractions in detrusor and mucosal strips. Aa–Ad: original recordings from detrusor and mucosae before and after the addition of atropine to block muscarinic receptors, and atropine + α,β-methylene ATP (α,β-meATP) to block muscarinic receptors and desensitize P2X1 receptors, respectively. Arrows indicate EFS applications in mucosal strips in which the evoked responses were smaller than the SPCs. Ba–Bd: detrusor (Ba, Bb) and mucosae (Bc, Bd) summary graphs showing amplitudes in control and after muscarinic and purinergic receptor inhibition. For mucosae, the amplitude of evoked responses was often less than that of SPCs and could not be accurately measured (see materials and methods). Responses less than the mean amplitude of SPCs are plotted as zero. Data are expressed as means ± SE (*P < 0.05).
BK channel expression and activity in mucosal smooth muscle.
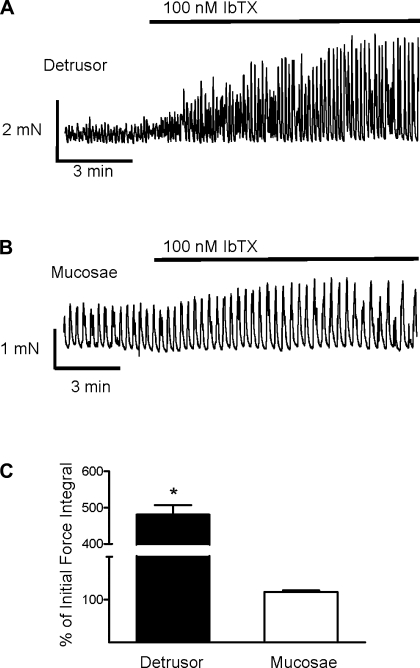
Given the prominent role that BK channels play in regulating detrusor contractility (9, 25, 29, 32, 33, 58, 69), we sought to determine their role in the regulation of mucosal smooth muscle contractility. To assess the relative importance of BK channels in the regulation of mucosal smooth muscle SPCs, we tested the effects of the BK channel blocker IbTX (100 nM) on the SPCs of mucosal and detrusor tissue strips. As expected, block of BK channels with IbTX greatly enhanced SPCs in detrusor strips (Fig. 7, A and C), increasing the force integral by almost fivefold (P < 0.05, n = 11 strips). In contrast, block of BK channels did not have a significant effect on mucosal SPCs (n = 16 strips; Fig. 7, B and C).
Fig. 7.
Large-conductance Ca2+-activated K+ (BK) channel block has a greater effect on the SPCs of detrusor than mucosal strips. A and B: representative myograph recordings obtained from a detrusor (A) and a mucosal (B) smooth muscle strip exposed to 100 nM IbTX. C: IbTX significantly increased the force integral of SPCs in detrusor but not in mucosae. Data are expressed as means ± SE (*P < 0.05).
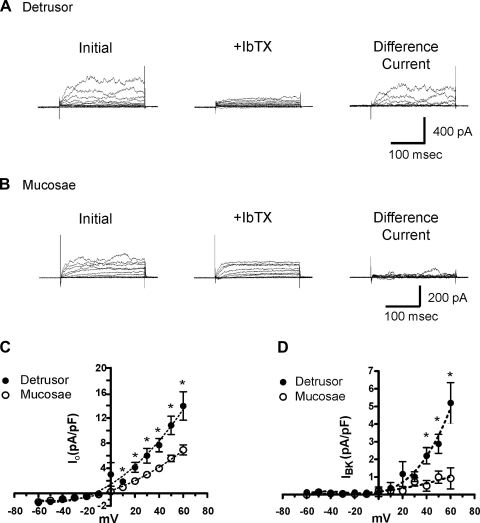
The feeble response of mucosal SPCs to IbTX suggests that the level of BK channel expression in mucosal smooth muscle is low relative to that in the detrusor. To investigate this possibility, we used the patch-clamp technique to compare IbTX-sensitive BK currents (IBK) in myocytes isolated from detrusor and mucosal smooth muscle. Whole cell currents were evoked from freshly isolated myocytes with 10-mV depolarizing steps from a holding potential of −60 mV up to +60 mV. With a capacitance of 32.3 ± 2.3 pF (n = 13), detrusor smooth muscle cells were significantly larger (P < 0.05) than mucosal smooth muscle cells (capacitance 25.9 ± 1.6 pF; n = 17). The peak outward current density (IO) of mucosal smooth muscle cells was significantly less than that of detrusor smooth muscle cells (Fig. 8, A and B, left). The resultant current-voltage (I-V) relationship of end-pulse currents (Fig. 8C) shows that the whole cell IO in mucosal smooth muscle cells was lower at all positive membrane potentials evaluated (P < 0.05). At +60 mV, IO was 13.9 ± 2.3 pA/pF (n = 13) in detrusor myocytes and 6.9 ± 0.8 pA/pF (n = 17) in mucosal myocytes—approximately a twofold difference.
Fig. 8.
BK channel current density is much larger in detrusor myocytes than in mucosal myocytes. A and B: representative outward current density (IO) (Initial; left) and IO after inhibition of BK channels (+IbTX; center); difference currents (right) obtained from a detrusor (A) and a mucosal (B) smooth muscle cell represent IbTX-sensitive BK current (IBK). C: comparison of the current-voltage (I-V) relationship for IO (Initial) evoked from −60 to +60 mV for isolated detrusor and mucosal smooth muscle cells. D: comparison of the I-V relationship for IBK evoked by 10-mV voltage steps from −60 to +60 mV for isolated detrusor and mucosal smooth muscle cells. Data are expressed as means ± SE (*P < 0.05).
The BK channel blocker IbTX (100 nM) was used to determine the IBK densities in isolated myocytes. IbTX had a greater effect on outward currents in detrusor cells than on those in mucosal smooth muscle cells (Fig. 8, A and B, center); thus the resulting IbTX difference current (Fig. 8, A and B, right) was much greater in the detrusor cells. The I-V curves for end-pulse currents (Fig. 8D) show that IBK was lower in mucosal smooth muscle cells than in detrusor smooth muscle cells, a difference that was significant at membrane potentials of +40 mV and above (P < 0.05, n = 13 cells each). At +60 mV, the current density was 5.20 ± 1.15 pA/pF in detrusor smooth muscle cells and only 0.92 ± 0.60 pA/pF in mucosal smooth muscle cells. Collectively, the results of these patch-clamp experiments suggest that the diminished contractile response to IbTX in mucosal tissue strips compared with that in detrusor strips (Fig. 7) may be due to a reduction in the density of IBK in mucosal smooth muscle myocytes.
SK channel activity is minimal or absent in mucosal smooth muscle.
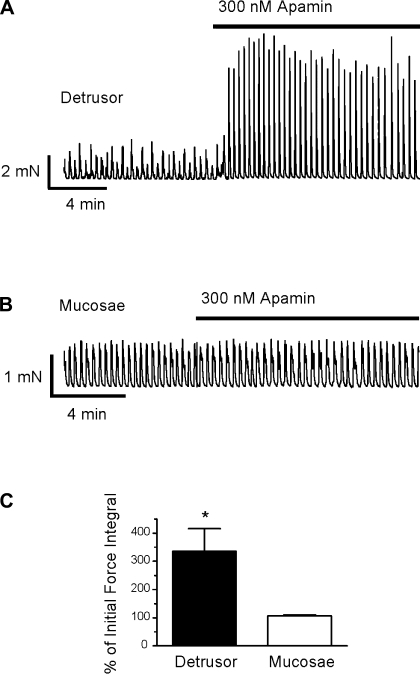
Urinary bladder contractility is also modulated by K+ efflux through SK channels (27, 33, 35), which contribute to the afterhyperpolarization of the urinary bladder smooth muscle action potential (12, 21); blocking SK channels results in an increase in detrusor SPCs (9, 27, 33, 35). To determine whether SK channel activity, like BK channel activity, was different between mucosal and detrusor myocytes, we first compared the effects of the SK channel blocker apamin (300 nM) on mucosal and detrusor smooth muscle SPCs. Representative myograph recordings of detrusor (Fig. 9A) and mucosal (Fig. 9B) smooth muscle strips clearly show a prominent effect of apamin on detrusor strips and essentially no effect on mucosal strips. Apamin increased the force integral of detrusor SPCs to 335.5 ± 79.7% of the initial force (P < 0.05, n = 9) but had no significant effect on mucosal SPCs (n = 17; Fig. 9C).
Fig. 9.
Small-conductance Ca2+-activated K+ (SK) channel block with apamin had no effect on mucosal strips. A and B: representative myograph recordings from detrusor (A) and mucosal (B) strips exposed to the SK channel blocker apamin (300 nM). C: apamin significantly increased the force integral of SPCs in detrusor but not in mucosae. Data are expressed as means ± SE (*P < 0.05).
DISCUSSION
In the present study, we provide the first characterization of the pharmacology and physiology of urinary bladder muscularis mucosae smooth muscle. Mucosae and detrusor strips exhibited SPCs of similar amplitudes and frequencies that depended on functional L-VDCCs. EFS induced a frequency-dependent increase in contraction amplitude in detrusor and mucosal strips that was nearly abolished by inhibition of both muscarinic and purinergic receptors. However, muscularis mucosae smooth muscle also exhibited distinctly different properties than detrusor smooth muscle. The maximal force-generating capacity (in response to 60 mM K+) of detrusor strips was ∼40-fold greater than that of mucosal strips. Indeed, the peak force of mucosal strip SPCs was similar to that induced by 60 mM K+, suggesting that mucosal SPCs engage all muscle bundles in a strip. The minimal effect of BK and SK channel blockers on mucosal SPCs is striking compared with the robust effects of these inhibitors on detrusor contractility.
Contractile properties of the muscularis mucosae.
The similarity of SPC amplitude and frequency between mucosal and detrusor strips from the same bladder is particularly striking. One explanation for this finding is that similar numbers of muscle bundles in the two strips are being driven by the same type of pacemaker. ICCs have been identified in both the mucosae and detrusor, although their function is still unclear (47, 71). Indeed, we observed Ca2+ signals in structures linking muscle bundles in the mucosae (see Fig. 3 and Supplemental Movies S1 and S2). Our data indicate that a SPC engages most of the smooth muscle in a mucosal strip. In contrast, based on our measurements, SPCs of the detrusor represent activation of a small fraction (∼3%) of the muscle in a strip.
Consistent with this, it has been shown that current injection into one detrusor myocyte cannot be detected at a distance of even a few cells from the microelectrode source (8). Similarly, Gillespie and colleagues (16) have shown that ex vivo whole bladder preparations exhibit “microcontractions” that are highly localized to discrete regions of the bladder and do not propagate across the whole bladder (these bladders also exhibited more complex, integrated contractions as well).
The force of evoked contractile responses in muscularis mucosae, whether induced by EFS or high K+, was comparable to that of SPCs. This was true even at higher stimulating frequencies, consistent with EFS engaging all of the smooth muscle bundles in the mucosal strip. There is little information on the innervation of the muscularis mucosae, although the suburothelial region surrounding the muscularis mucosae is richly innervated (4, 14). Studies of the digestive tract have shown that the innervation of the muscularis mucosae varies greatly among different regions and between species and includes cholinergic, peptidergic, and adrenergic nerve fibers (65).
We found that mucosal phasic contractions were absolutely dependent on Ca2+ influx through L-VDCCs, a property that is shared by detrusor smooth muscle (27, 29). Interestingly, it has been reported that gastrointestinal muscularis mucosae is insensitive to L-type channel antagonists (64, 66). Like SPCs in the detrusor (26), the SPCs of bladder mucosal smooth muscle do not appear to require SR Ca2+ stores. The muscularis mucosae exists in close proximity to the urothelium, nerve fibers, and ICCs, all of which could be potential sources of contractile modulators (2, 6, 28, 48, 52). However, the phasic contractions exhibited by mucosal strips appear to be nonneurogenic and spontaneous in origin, as they were not affected by inhibition of muscarinic receptors, purinergic receptors, or voltage-dependent sodium channels. Recently, Burcher and colleagues (60) reported that mucosal strips of pig urinary bladder, denuded of the detrusor smooth muscle layer, contract in response to carbachol and neurokinin A. The authors attributed this contractile response to the suburothelial ICCs rather than to the muscularis mucosae. They did, however, note that sparse smooth muscle was still present in these mucosal strips. Although we cannot definitively rule out the possibility that suburothelial ICCs may contribute to the SPCs of mucosal strips, we found that α-actin was only expressed in the discrete bundles of smooth muscle within the mucosae (Fig. 1). Furthermore, Ca2+ flashes, which reflect action potentials, were observed in cells resembling smooth muscle in the mucosal layer, and these flashes preceded each spontaneous contraction event (see Fig. 3A and Supplemental Movie S1). It is possible, however, that ICCs play some role in coordinating mucosal contractions.
The physiological role of SPCs in urinary bladder has been the focus of numerous studies; however, the precise role of SPCs remains unclear. These small, spontaneous nonvoiding contractions, which occur in parts of the bladder wall, have also been called micromotions (11) or microtransients (16). These events may be important during the filling phase, playing a role in adjusting the length of smooth muscle fibers in response to filling (7) or communicating information on bladder fullness (11, 16, 22). Because the contractile force of muscularis mucosae contractions is ∼40 times less than that of the detrusor, these events would be expected to contribute minimally to micturition.
BK and SK channels in muscularis mucosae.
The contractility of detrusor smooth muscle is profoundly influenced by BK and SK channels (27, 32, 33, 69). Given the major role that these two ion channel types play in modulating the excitability and contractility of detrusor smooth muscle, it was surprising that neither seemed to play a role in modulating the SPCs in mucosal smooth muscle. Neither blocking BK channels nor blocking SK channels had a significant effect on mucosal SPCs (Figs. 7 and 9). BK channel current density was also significantly lower in mucosal than in detrusor myocytes (Fig. 8). We have previously characterized SK currents in detrusor myocytes (36) and did not explore this issue in mucosal myocytes because the SK channel blocker apamin did not affect mucosal contractility (Fig. 9). These results indicate that mucosal smooth muscle has an ion channel composition distinctly different from that of detrusor myocytes. Taken together, the results of myography experiments (SK and BK channels) and electrophysiological data (BK channels) suggest that mucosal smooth muscle has properties that clearly distinguish it from detrusor smooth muscle.
Perspectives and Significance
In the present study we describe a separate compartment of smooth muscle—the muscularis mucosae—located just below the urothelium in guinea pig urinary bladder that underlies the SPCs in urothelial strips. The unique Ca2+ activity signature and pharmacological properties of the muscularis mucosae, such as its relative unresponsiveness to BK and SK channel blockers, indicate that this tissue layer is functionally distinct from the detrusor smooth muscle.
SPCs are found in the urinary bladder of many species, including humans, yet their role is not well understood. They occur normally during bladder filling and may contribute to maintaining blood flow during periods of increased intravesicle pressure as would occur during bladder filling; alternatively, they may provide information on bladder fullness (22). Histological stains and Ca2+ imaging show a thin layer of smooth muscle—the muscularis mucosae—composed of connecting sheets and bundles that suggests synchronization over a large area. The muscularis mucosae is nonneurogenic and contracts in rhythmic bursts, suggesting local regulation, possibly through a network of ICC-type cells. Rhythmic bursts or SPCs with frequency and amplitude similar to those in the mucosae are also found in isolated strips of detrusor. The muscularis mucosae may contribute to the increase in nonvoiding contractions found in certain bladder pathologies, such as overactive bladder, and would provide a novel target for the development of pharmaceuticals to alleviate this condition.
GRANTS
This work was supported in part by the Totman Trust for Medical Research, National Institutes of Health (NIH) Grants DK-053832 and DK-065947 to M. T. Nelson, a Physiology Training grant to J. J. Layne (PHS T32-HL-007944), and NIH Grant 2-P20-RR-016435-06 from the COBRE Program of the National Center for Research Resources.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. David Hill-Eubanks and Bernhard Nausch for critical reading of this manuscript.
Footnotes
Supplemental Material for this article is available online at the Journal website.
REFERENCES
- 1. Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev 84: 935–986, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Andersson MC, Tobin G, Giglio D. Cholinergic nitric oxide release from the urinary bladder mucosa in cyclophosphamide-induced cystitis of the anaesthetized rat. Br J Pharmacol 153: 1438–1444, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berridge MJ. Smooth muscle cell calcium activation mechanisms. J Physiol 586: 5047–5061, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Birder LA. Involvement of the urinary bladder urothelium in signaling in the lower urinary tract. Proc West Pharmacol Soc 44: 85–86, 2001 [PubMed] [Google Scholar]
- 5. Birder LA. Urothelial signaling. Auton Neurosci 153: 33–40, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Birder LA, de Groat WC. Mechanisms of disease: involvement of the urothelium in bladder dysfunction. Nat Clin Pract 4: 46–54, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brading AF. Spontaneous activity of lower urinary tract smooth muscles: correlation between ion channels and tissue function. J Physiol 570: 13–22, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bramich NJ, Brading AF. Electrical properties of smooth muscle in the guinea-pig urinary bladder. J Physiol 492: 185–198, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buckner SA, Milicic I, Daza AV, Coghlan MJ, Gopalakrishnan M. Spontaneous phasic activity of the pig urinary bladder smooth muscle: characteristics and sensitivity to potassium channel modulators. Br J Pharmacol 135: 639–648, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chaimuangraj S, Dissaranan C, Leenanupunth C, Prathombutr P, Chalermsanyakorn P. Significance of muscularis mucosae in metastasis involvement of urinary bladder transitional cell carcinoma. J Med Assoc Thailand 89: 1447–1453, 2006 [PubMed] [Google Scholar]
- 11. Coolsaet BL, Van Duyl WA, Van Os-Bossagh P, De Bakker HV. New concepts in relation to urge and detrusor activity. Neurourol Urodyn 12: 463–471, 1993 [DOI] [PubMed] [Google Scholar]
- 12. Creed KE, Ishikawa S, Ito Y. Electrical and mechanical activity recorded from rabbit urinary bladder in response to nerve stimulation. J Physiol 338: 149–164, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Daniel EE, Jury J, Bowker P. Muscarinic receptors on nerves and muscles in opossum esophagus muscularis mucosa. Can J Physiol Pharmacol 65: 1903–1907, 1987 [DOI] [PubMed] [Google Scholar]
- 14. Dickson A, Avelino A, Cruz F, Ribeiro-da-Silva A. Peptidergic sensory and parasympathetic fiber sprouting in the mucosa of the rat urinary bladder in a chronic model of cyclophosphamide-induced cystitis. Neuroscience 141: 1633–1647, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Dixon JS, Gilpin CJ. Presumptive sensory axons of the human urinary bladder: a fine structural study. J Anat 151: 199–207, 1987 [PMC free article] [PubMed] [Google Scholar]
- 16. Drake MJ, Harvey IJ, Gillespie JI. Autonomous activity in the isolated guinea pig bladder. Exp Physiol 88: 19–30, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Eglen RM, Peelle B, Pulido-Rios MT, Leung E. Functional interactions between muscarinic M2 receptors and 5-hydroxytryptamine (5-HT)4 receptors and beta3-adrenoceptors in isolated oesophageal muscularis mucosae of the rat. Br J Pharmacol 119: 595–601, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ekman M, Andersson KE, Arner A. Signal transduction pathways of muscarinic receptor mediated activation in the newborn and adult mouse urinary bladder. BJU Int 103: 90–97, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes—a possible sensory mechanism? J Physiol 505: 503–511, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fry CH, Meng E, Young JS. The physiological function of lower urinary tract smooth muscle. Auton Neurosci 154: 3–13 [DOI] [PubMed] [Google Scholar]
- 21. Fujii K, Foster CD, Brading AF, Parekh AB. Potassium channel blockers and the effects of cromakalim on the smooth muscle of the guinea-pig bladder. Br J Pharmacol 99: 779–785, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gillespie JI. The autonomous bladder: a view of the origin of bladder overactivity and sensory urge. BJU Int 93: 478–483, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Gillespie JI, Markerink-van Ittersum M, De Vente J. Endogenous nitric oxide/cGMP signalling in the guinea pig bladder: evidence for distinct populations of sub-urothelial interstitial cells. Cell Tissue Res 325: 325–332, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Gillespie JI, Markerink-van Ittersum M, de Vente J. Sensory collaterals, intramural ganglia and motor nerves in the guinea-pig bladder: evidence for intramural neural circuits. Cell Tissue Res 325: 33–45, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Hashitani H, Brading AF. Electrical properties of detrusor smooth muscles from the pig and human urinary bladder. Br J Pharmacol 140: 146–158, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hashitani H, Brading AF. Ionic basis for the regulation of spontaneous excitation in detrusor smooth muscle cells of the guinea-pig urinary bladder. Br J Pharmacol 140: 159–169, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hashitani H, Brading AF, Suzuki H. Correlation between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the guinea-pig bladder. Br J Pharmacol 141: 183–193, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hawthorn MH, Chapple CR, Cock M, Chess-Williams R. Urothelium-derived inhibitory factor(s) influences on detrusor muscle contractility in vitro. Br J Pharmacol 129: 416–419, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heppner TJ, Bonev AD, Nelson MT. Ca2+-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am J Physiol Cell Physiol 273: C110–C117, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Heppner TJ, Bonev AD, Nelson MT. Elementary purinergic Ca2+ transients evoked by nerve stimulation in rat urinary bladder smooth muscle. J Physiol 564: 201–212, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heppner TJ, Werner ME, Nausch B, Vial C, Evans RJ, Nelson MT. Nerve-evoked purinergic signalling suppresses action potentials, Ca2+ flashes and contractility evoked by muscarinic receptor activation in mouse urinary bladder smooth muscle. J Physiol 587: 5275–5288, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Herrera GM, Etherton B, Nausch B, Nelson MT. Negative feedback regulation of nerve-mediated contractions by KCa channels in mouse urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol 289: R402–R409, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Herrera GM, Heppner TJ, Nelson MT. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am J Physiol Regul Integr Comp Physiol 279: R60–R68, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Herrera GM, Heppner TJ, Nelson MT. Voltage dependence of the coupling of Ca2+ sparks to BKCa channels in urinary bladder smooth muscle. Am J Physiol Cell Physiol 280: C481–C490, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Herrera GM, Nelson MT. Differential regulation of SK and BK channels by Ca2+ signals from Ca2+ channels and ryanodine receptors in guinea-pig urinary bladder myocytes. J Physiol 541: 483–492, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Herrera GM, Pozo MJ, Zvara P, Petkov GV, Bond CT, Adelman JP, Nelson MT. Urinary bladder instability induced by selective suppression of the murine small conductance calcium-activated potassium (SK3) channel. J Physiol 551: 893–903, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hinz B. Masters and servants of the force: the role of matrix adhesions in myofibroblast force perception and transmission. Eur J Cell Biol 85: 175–181, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Horinouchi T, Koshikawa H, Koike K. Effect of bupranolol for BRL37344 and noradrenaline-induced relaxations mediating atypical beta/beta3-adrenoceptor in rat oesophageal muscularis mucosae. Gen Pharmacol 33: 173–178, 1999 [DOI] [PubMed] [Google Scholar]
- 39. Hossler FE, Kao RL. Microvasculature of the urinary bladder of the dog: a study using vascular corrosion casting. Microsc Microanal 13: 220–227, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Hossler FE, Monson FC. Microvasculature of the rabbit urinary bladder. Anat Rec 243: 438–448, 1995 [DOI] [PubMed] [Google Scholar]
- 41. Kanai A, Roppolo J, Ikeda Y, Zabbarova I, Tai C, Birder L, Griffiths D, de Groat W, Fry C. Origin of spontaneous activity in neonatal and adult rat bladders and its enhancement by stretch and muscarinic agonists. Am J Physiol Renal Physiol 292: F1065–F1072, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Keep JC, Piehl M, Miller A, Oyasu R. Invasive carcinomas of the urinary bladder. Evaluation of tunica muscularis mucosae involvement. Am J Clin Pathol 91: 575–579, 1989 [DOI] [PubMed] [Google Scholar]
- 43. Layne JJ, Nausch B, Olesen SP, Nelson MT. BK channel activation by NS11021 decreases excitability and contractility of urinary bladder smooth muscle. Am J Physiol Regul Integr Comp Physiol 298: R378–R384, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Layne JJ, Werner ME, Hill-Eubanks DC, Nelson MT. NFATc3 regulates BK channel function in murine urinary bladder smooth muscle. Am J Physiol Cell Physiol 295: C611–C623, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mailman D, Tso P, Granger DN. Effects of oleic acid and bile salts on canine villous motility. Life Sci 45: 455–461, 1989 [DOI] [PubMed] [Google Scholar]
- 46. McCloskey KD. Interstitial cells in the urinary bladder—localization and function. Neurourol Urodyn 29: 82–87, 2010 [DOI] [PubMed] [Google Scholar]
- 47. McCloskey KD, Anderson UA, Davidson RA, Bayguinov YR, Sanders KM, Ward SM. Comparison of mechanical and electrical activity and interstitial cells of Cajal in urinary bladders from wild-type and W/Wv mice. Br J Pharmacol 156: 273–283, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Meng E, Young JS, Brading AF. Spontaneous activity of mouse detrusor smooth muscle and the effects of the urothelium. Neurourol Urodyn 27: 79–87, 2008 [DOI] [PubMed] [Google Scholar]
- 49. Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem 279: 36746–36752, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Miodonski AJ, Litwin JA. Microvascular architecture of the human urinary bladder wall: a corrosion casting study. Anat Rec 254: 375–381, 1999 [DOI] [PubMed] [Google Scholar]
- 51. Miodonski AJ, Litwin JA, Nowogrodzka-Zagorska M, Gorczyca J. Vascular architecture of normal human urinary bladder and its remodeling in cancer, as revealed by corrosion casting. Ital J Anat Embryol 106: 221–228, 2001 [PubMed] [Google Scholar]
- 52. Murakami S, Chapple CR, Akino H, Sellers DJ, Chess-Williams R. The role of the urothelium in mediating bladder responses to isoprenaline. BJU Int 99: 669–673, 2007 [DOI] [PubMed] [Google Scholar]
- 53. Nausch B, Heppner TJ, Nelson MT. Nerve-released acetylcholine contracts urinary bladder smooth muscle by inducing action potentials independently of IP3-mediated calcium release. Am J Physiol Regul Integr Comp Physiol 299: R878–R888, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Paner GP, Ro JY, Wojcik EM, Venkataraman G, Datta MW, Amin MB. Further characterization of the muscle layers and lamina propria of the urinary bladder by systematic histologic mapping: implications for pathologic staging of invasive urothelial carcinoma. Am J Surg Pathol 31: 1420–1429, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Percy WH, Brunz JT, Burgers RE, Fromm TH, Merkwan CL, van Dis J. Interrelationship between colonic muscularis mucosae activity and changes in transmucosal potential difference. Am J Physiol Gastrointest Liver Physiol 281: G479–G489, 2001 [DOI] [PubMed] [Google Scholar]
- 56. Percy WH, Fromm TH, Wangsness CE. Muscularis mucosae contraction evokes colonic secretion via prostaglandin synthesis and nerve stimulation. Am J Physiol Gastrointest Liver Physiol 284: G213–G220, 2003 [DOI] [PubMed] [Google Scholar]
- 57. Percy WH, Warren JM, Brunz JT. Characteristics of the muscularis mucosae in the acid-secreting region of the rabbit stomach. Am J Physiol Gastrointest Liver Physiol 276: G1213–G1220, 1999 [DOI] [PubMed] [Google Scholar]
- 58. Petkov GV, Bonev AD, Heppner TJ, Brenner R, Aldrich RW, Nelson MT. Beta1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J Physiol 537: 443–452, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ro JY, Ayala AG, el-Naggar A. Muscularis mucosa of urinary bladder. Importance for staging and treatment. Am J Surg Pathol 11: 668–673, 1987 [DOI] [PubMed] [Google Scholar]
- 60. Sadananda P, Chess-Williams R, Burcher E. Contractile properties of the pig bladder mucosa in response to neurokinin A: a role for myofibroblasts? Br J Pharmacol 153: 1465–1473, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Seelig LL, Jr, Schlusselberg DS, Smith WK, Woodward DJ. Mucosal nerves and smooth muscle relationships with gastric glands of the opossum: an ultrastructural and three-dimensional reconstruction study. Am J Anat 174: 15–26, 1985 [DOI] [PubMed] [Google Scholar]
- 62. Synnerstad I, Ekblad E, Sundler F, Holm L. Gastric mucosal smooth muscles may explain oscillations in glandular pressure: role of vasoactive intestinal peptide. Gastroenterology 114: 284–294, 1998 [DOI] [PubMed] [Google Scholar]
- 63. Thorneloe KS, Nelson MT. Properties and molecular basis of the mouse urinary bladder voltage-gated K+ current. J Physiol 549: 65–74, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Triggle DJ. Calcium channel antagonists: clinical uses—past, present and future. Biochem Pharmacol 74: 1–9, 2007 [DOI] [PubMed] [Google Scholar]
- 65. Uchida K, Kamikawa Y. Muscularis mucosae—the forgotten sibling. J Smooth Muscle Res 43: 157–177, 2007 [DOI] [PubMed] [Google Scholar]
- 66. Uchida K, Yuzuki R, Kamikawa Y. Ba2+ selectively inhibits receptor-mediated contraction of the esophageal muscularis mucosae. Eur J Pharmacol 362: 83–86, 1998 [DOI] [PubMed] [Google Scholar]
- 67. Vakar-Lopez F, Shen SS, Zhang S, Tamboli P, Ayala AG, Ro JY. Muscularis mucosae of the urinary bladder revisited with emphasis on its hyperplastic patterns: a study of a large series of cystectomy specimens. Ann Diagn Pathol 11: 395–401, 2007 [DOI] [PubMed] [Google Scholar]
- 68. Weaver MG, Abdul-Karim FW. The prevalence and character of the muscularis mucosae of the human urinary bladder. Histopathology 17: 563–566, 1990 [DOI] [PubMed] [Google Scholar]
- 69. Werner ME, Knorn AM, Meredith AL, Aldrich RW, Nelson MT. Frequency encoding of cholinergic- and purinergic-mediated signaling to mouse urinary bladder smooth muscle: modulation by BK channels. Am J Physiol Regul Integr Comp Physiol 292: R616–R624, 2007 [DOI] [PubMed] [Google Scholar]
- 70. Wiseman OJ, Brady CM, Hussain IF, Dasgupta P, Watt H, Fowler CJ, Landon DN. The ultrastructure of bladder lamina propria nerves in healthy subjects and patients with detrusor hyperreflexia. J Urol 168: 2040–2045, 2002 [DOI] [PubMed] [Google Scholar]
- 71. Wu C, Sui GP, Fry CH. Purinergic regulation of guinea pig suburothelial myofibroblasts. J Physiol 559: 231–243, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wuest M, Hiller N, Braeter M, Hakenberg OW, Wirth MP, Ravens U. Contribution of Ca2+ influx to carbachol-induced detrusor contraction is different in human urinary bladder compared to pig and mouse. Eur J Pharmacol 565: 180–189, 2007 [DOI] [PubMed] [Google Scholar]
- 73. Yoshida M, Inadome A, Maeda Y, Satoji Y, Masunaga K, Sugiyama Y, Murakami S. Non-neuronal cholinergic system in human bladder urothelium. Urology 67: 425–430, 2006 [DOI] [PubMed] [Google Scholar]
- 74. Young JS, Meng E, Cunnane TC, Brain KL. Spontaneous purinergic neurotransmission in the mouse urinary bladder. J Physiol 586: 5743–5755, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.