Abstract
During ventilatory acclimatization to hypoxia (VAH), time-dependent increases in ventilation lower Pco2 levels, and this persists on return to normoxia. We hypothesized that plasticity in the caudal nucleus tractus solitarii (NTS) contributes to VAH, as the NTS receives the first synapse from the carotid body chemoreceptor afferents and also contains CO2-sensitive neurons. We lesioned cells in the caudal NTS containing the neurokinin-1 receptor by microinjecting the neurotoxin saporin conjugated to substance P and measured ventilatory responses in awake, unrestrained rats 18 days later. Lesions did not affect hypoxic or hypercapnic ventilatory responses in normoxic control rats, in contrast to published reports for similar lesions in other central chemosensitive areas. Also, lesions did not affect the hypercapnic ventilatory response in chronically hypoxic rats (inspired Po2 = 90 Torr for 7 days). These results suggest functional differences between central chemoreceptor sites. However, lesions significantly increased ventilation in normoxia or acute hypoxia in chronically hypoxic rats. Hence, chronic hypoxia increases an inhibitory effect of neurokinin-1 receptor neurons in the NTS on ventilatory drive, indicating that these neurons contribute to plasticity during chronic hypoxia, although such plasticity does not explain VAH.
Keywords: chemosensitivity, substance P conjugated saporin, ventilatory acclimatization to hypoxia
multiple areas of the brain stem have been established as CO2 sensitive by both stimulation and lesion experiments (3–5, 11, 29, 36, 42–44, 47). The organization and significance of these multiple chemoreceptor sites is a major question in this field, and different sites may play different roles in controlling ventilation (V̇i) or the cardiovascular system during different states (19, 38, 41). We hypothesized that CO2-sensitive chemoreceptors in the nucleus tractus solitarii (NTS) play a role in ventilatory acclimatization to chronic hypoxia.
This hypothesis is based on several published observations. First, chronic hypoxia changes the ventilatory response to CO2 (60), and the NTS contains CO2-sensitive cells (11, 14, 43). Also, the NTS receives the first synapse from O2-sensitive afferents from the carotid bodies (15, 21, 22, 33, 59), and this could lead to hypoxic or activity-dependent plasticity that has been demonstrated in the central nervous system with chronic hypoxia (16, 49, 62). The NTS is, therefore, well suited to integrate the increased hypoxic drive to breathe from the arterial chemoreceptors with the decreased drive to breathe from hypocapnia in chronic hypoxia.
To test the role of CO2-sensitive chemoreceptors in the NTS to ventilatory acclimatization to hypoxia, we studied the effects of NTS lesions. Large lesions to the NTS are problematic because of the many sensory systems that synapse in the NTS, including not only the peripheral chemoreceptors, but also baroreceptors and pulmonary vagal afferents (15, 27). To quantify the importance of the NTS central chemoreceptor cells during chronic hypoxia, a lesion of only the CO2-sensitive cells would ideally be performed. No unequivocal marker of chemoreceptive cells has been identified yet, but other groups have used the neurokinin-1 receptor (NK1R) as a candidate marker to lesion central chemoreceptors in the brain stem. All of the putative central chemoreceptor areas contain cells expressing the NK1R, which is the receptor for substance P (SP) (37). The role of NK1R cells in CO2 sensitivity is not known, but lesions specifically targeting the NK1R cells in other chemosensitive areas of the brain stem decrease the hypercapnic ventilatory response (HCVR) in rats and goats (20, 45, 46). Moreover, it has been shown that NK1R cell lesions in the NTS do not kill the animal (52). We made lesions to the NK1R-expressing cells in the NTS of rats and measured the effects on ventilatory responses to O2 and CO2 in conscious rats before or after acclimatization to hypoxia.
METHODS
Experimental animals.
Male Sprague-Dawley rats (290–400 g; Charles River) were housed in standard rat cages in a vivarium and fed ad libitum a standard rat diet. A 12:12-h light-dark cycle was maintained within the vivarium. All experiments were approved by the University of California, San Diego, Animal Care and Use Committee. The experiments conformed to national standards for the care and use of experimental animals, as well as the American Physiological Society's “Guiding Principles in the Care and Use of Animals.”
Experimental groups.
Animals were placed into one of four groups, depending on whether they were chemically lesioned or unlesioned and maintained in normoxia (N) or chronic hypoxia (CH). A stable form of SP conjugated to the neurotoxin saporin (SP-SAP, Advanced Targeting Systems, San Diego, CA) was used to lesion NK1R-expressing cells in the NTS (61). An 11-amino acid nonsense peptide conjugated to saporin (Blank-SAP, Advanced Targeting Systems) was used as the control drug. For both N and CH, 11 Blank-SAP and 5 SP-SAP animals were used. A subset of these animals was measured in both N and CH (Blank-SAP, n = 4; SP-SAP, n = 2). No significant differences in body weights between the groups were observed (Blank-SAP = 346.0 ± 1.65 g; SP-SAP = 360.4 ± 12.9 g).
Exposure to chronic hypoxia.
Rats in the CH group were placed for 7 days in a hypobaric chamber maintained at 0.5 atm (380 mmHg), which approximates exposure to 10% inspired O2 in normobaric conditions at sea level and has been used extensively to study acclimatization in rats (e.g., Ref. 1). Animals, within individual cages, were placed into the hypobaric chamber, and the pressure was lowered from 1.0 to 0.5 atm over a 5-min period. The chamber was opened once daily for ∼10 min for regular cage maintenance, or when it was necessary to remove animals for experimentation. The hypobaric chamber was maintained in the same vivarium that housed the control animals.
Experimental drugs.
The NK1R is internalized following the binding of NK1R ligand (32), meaning that, if the neurotoxin saporin is bound to SP, it can enter and kill the cell (SP-SAP group). The Blank-SAP served as the control drug for these experiments, as the nonsense peptide should not bind to any receptors, and the saporin will be unable to enter the cells. All animals received 200-nl injections of drug (0.013 ng/nl), either SP-SAP or Blank-SAP, into the caudal NTS.
Surgical preparation.
Animals were initially anesthetized with 5% isoflurane in O2 and maintained under anesthesia with 2–2.5% isoflurane in O2. The skull was shaved, and the skin sterilized with betadine and alcohol. The head was placed into a Kopf stereotaxic holder. To visualize the dorsal brain stem, a midline incision was made, and the muscle retracted to expose the edge of the skull bone. The head was angled 45° nose down, and the dura was cut at the point it connects with the skull. The calamus scriptorius (which corresponds to −14.3 mm caudal from bregma) was then visible and used as a landmark for the injection sites (50). Microinjections were made (Nanopump, World Precision Instruments) with a glass micropipette with a 10-μm tip diameter. A total of eight injections (25 nl each) were made, with the patency of the micropipette tested after each injection. The first set of injections was made 0.1 mm rostral to the calamus scriptorius bilaterally, and the second set 0.4 mm rostral. Injections were made at two depths (0.2 and 0.4 mm deep) at every injection site.
Following the injections, rats were maintained in normoxia for 11 days to allow for maximal cell killing, as determined by other groups (45) and confirmed by us in pilot studies. The CH group was then placed into the hypobaric chamber for an additional 7 days of hypoxia, whereas the N group remained in normoxia for 7 days. Most ventilatory measurements were taken as described below 18 days following the injection, but a subset of animals was measured in normoxia at 11 days before being placed in chronic hypoxia (Blank-SAP, n = 4; SP-SAP, n = 2).
Two days before ventilatory measurements, recovery surgery was performed under isoflurane to implant a temperature telemetry probe into the abdomen (G-2 Emitter, Respironics). Additionally, the femoral artery was cannulated with a custom catheter made of polyethylene tubing (PE-10 joined to PE-50). The catheter was tunneled under the skin to the shoulder area to allow access for blood-gas sampling and to prevent the rat from damaging the tubing.
Ventilatory and blood-gas measurements.
All ventilatory data were collected on awake, unrestrained animals using barometric plethysmography modified for continuous flow (1, 25). Briefly, barometric plethysmography records the pressure swings caused by the warming and expansion of air when it enters the lungs.
On the experimental day, rats were placed into a 7-liter sealed Plexiglas chamber. A thermometer and humidity probe were sealed in the box (Physitemp Thermalert TH-5). An electronic gas mixer (MFC-4, Sable) was used to regulate the inspired gas concentrations and provide high input impedance. The chamber gas concentrations were measured using a mass spectrometer (MGA 1100, Perkin-Elmer) with the gas exiting the chamber via a vacuum valve (m series, Nupro) to a vacuum pump. We did not use a reference chamber and compensated for changes in the pressure signal baseline by adjusting the vacuum valve to maintain a chamber pressure near atmospheric level, monitored by water manometer after each change of inspired gases. Respiratory frequency (fR) was calculated directly from the V̇i-induced pressure swings (Validyne, MP45). Tidal volume (Vt) was calculated using calibration pulses (1 ml) generated by using a gas-tight syringe and injecting air pulses into the chamber at a rate similar to the rats' frequency following the experimental measurements. V̇i was calculated as the product of fR and Vt and normalized for the animal's body weight.
The protocol commenced following an acclimation period to the box, which consisted of 35 min in either 21% O2 for N rats and 10% O2 for CH rats. Animals were exposed to 10 min of each experimental gas mixture before respiratory and arterial blood-gas measurements were taken. Each animal was challenged with hypoxia (inspired O2 fraction = 0.10) and two levels of hypercapnia (inspired CO2 fraction = 0.05 and 0.07), in addition to normoxia (inspired O2 fraction = 0.21). After each gas mixture, the animal was returned to its baseline inspired O2 concentration (21% O2 for N rats and 10% O2 for CH rats).
Arterial blood gases were measured immediately following 1-min collection of ventilatory data, ∼11 min into the gas exposure. Blood samples were taken no more than four times during the protocol, with a 0.2-ml sample taken each time. The samples were immediately analyzed on an Instrument Laboratory Synthesis GEM Premier 3000 blood-gas machine (San Jose, CA), which returned arterial O2 (PaO2), CO2 (PaCO2), pH, and hematocrit (Hct) values.
Lesion quantification.
Following the last experimental measure, rats were transcardially perfused with 4% paraformaldehyde, and the brain stems were removed, postfixed overnight in paraformaldehyde, and then transferred to 30% sucrose and cryoprotected overnight. The brain stems were frozen and sectioned at 30 μm using a cryostat (Reichert Jung, Cryocut 1800). The area containing the NTS was then stained using the floating sections method of immunohistochemistry for the NK1R. Primary antibody was applied overnight in a concentration of 1:1,000 (Advanced Targeting Systems), followed by 2 h of secondary antibody application (goat anti-rabbit conjugated to Cy-3; Jackson Immunoresearch). All incubations were done at room temperature on a laboratory shaker. The sections were then placed on slides and coverslipped using Vectashield mounting media plus 4,6-diamidino-2-phenylindole (DAPI) to counterstain the nuclei (Vector Laboratories).
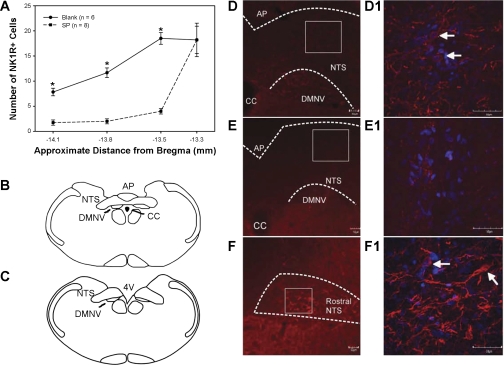
NK1R-positive cells were counted in the NTS at four levels (−14.08, −13.8, −13.5, and −13.3 mm referenced to bregma). The most rostral section was outside of the targeted caudal NTS area. A subset of the sections was counted using a confocal microscope (Olympus FV-1000), where a three-dimensional stack of images was collected and could be used to verify that NK1R immunoreactivity was surrounding a DAPI-stained cell nucleus. These sections were also counted using a nonconfocal fluorescent microscope (Nikon Eclipse E400). The cell counts were not significantly different between the two methods, so the nonconfocal microscope was used for quantification in the majority of the animals. To be considered an NK1R neuron, the cell had to be at least 10 μm large, have at least two processes, and the NK1 staining must surround a DAPI-stained nuclei (criteria modified from Ref. 52). Any rat that had <30% of the NK1R-positive neurons than the average control rat was included as a lesioned animal. No differences were observed between the number of NK1R-positive cells in the control N and CH rats, so all control rats were pooled for analysis. All pictures shown of the staining were taken using the Olympus FV-1000 confocal microscope.
Statistics.
For each ventilatory (fR, Vt, and V̇i) and blood-gas (PaO2, PaCO2, arterial pH, Hct) variable, a mixed-factors ANOVA was performed. The between-subjects variables were the presence or absence of NK1R cell lesion and chronic hypoxia. When testing the effect on the HCVR, percent inspired CO2 (3 levels: 0, 5, and 7%) was the within-subjects variable, while percent inspired O2 (2 levels: 10 and 21%) was the within-subjects variable for the hypoxic ventilatory response (HVR). All averages are expressed as means ± SE, and P < 0.05 was considered significant. All statistical analysis was done using SPSS statistics software.
RESULTS
Lesion verification.
In total, very few cell bodies in the NTS were stained for the NK1R. Most of the immunoreactivity was located on cell processes, as illustrated in Fig. 1, D and E. In the control animals, there was an average of 38.0 ± 1.7 cells in the three sections encompassing the target area of the caudal NTS. N and CH Blank-SAP injected animals were pooled as no significant differences in NK1R cell number was observed. NK1R cell number at all levels of the caudal NTS decreased with SP-SAP, as shown in Fig. 1A. Only animals in which NK1R-positive cell counts were ≤30% of control counts, i.e., <11 cells total, were included into the SP-SAP group (mean of 7.3 ± 1.1 cells). Representative staining is shown in Fig. 1, D–F. Rostral to the obex, no difference in NK1R cell number was observed (Fig. 1F), indicating the lesion was localized to the caudal NTS, where SP-SAP was microinjected (Fig. 1, B, D, and E).
Fig. 1.
A: quantification of neurokinin-1 receptor (NK1R)-positive cell number in the nucleus tractus solitarii (NTS) in brain sections from four levels: the first three encompass the caudal NTS under the area postrema (AP), and the fourth section is in the rostral NTS. Only animals with at least a 70% decrease in NK1R-positive cells with substance P conjugated to saporin (SP-SAP) injected (dashed line) were considered lesioned. Values are means ± SE. *P < 0.05 from controls (solid line). B: location of caudal NTS as shown in D and E, approximately −13.8 mm from bregma. C: location of rostral NTS as shown in F and F1, approximately −13.3 mm from bregma. D: control animal injected with nonsense peptide conjugated to SAP (Blank-SAP). D1: higher magnification of caudal NTS of D. NK1R-positive cell bodies (arrows) and processes are present. E: animal injected with SP-SAP. E1: higher magnification of caudal NTS of E. No NK1R cell bodies were present; some processes were present. F: NTS rostral to SP-SAP injection site. F1: higher magnification of F. NK1R-positive cell bodies (arrows) and processes are present. For D and F, NTS is located inside white dashed line. Red staining denotes NK1R immunoreactivity, and blue the cell nucleus (4,6-diamidino-2-phenylindole). CC, central canal; DMNV, dorsal motor nucleus of the vagus; 4V, fourth ventricle.
Ventilatory acclimatization in chronically hypoxic rats.
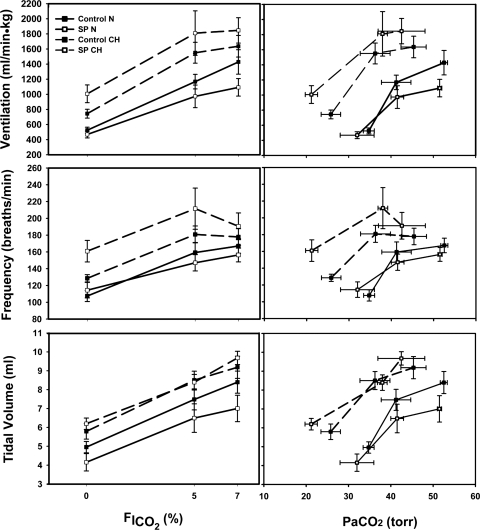
Ventilatory acclimatization was observed in the CH groups, as fR, Vt, and V̇i were all significantly increased in the CH groups (Figs. 2 and 3). Additionally, PaCO2 decreased significantly (Table 1), and Hct increased in the CH rats (N = 36.2 ± 4.4%; CH = 56.3 ± 3.1%), as reported previously (1).
Fig. 2.
Effect of the NK1R cell lesion on the hypercapnic ventilatory response (HCVR) in normoxic (N) and chronically hypoxic (CH) rats as a function of inspired fraction of CO2 (FiCO2; left) and arterial CO2 (PaCO2; right). SP-SAP lesions (dashed line) in the caudal NTS had no effect on any component of the HCVR in N or CH. Values are means ± SE.
Fig. 3.
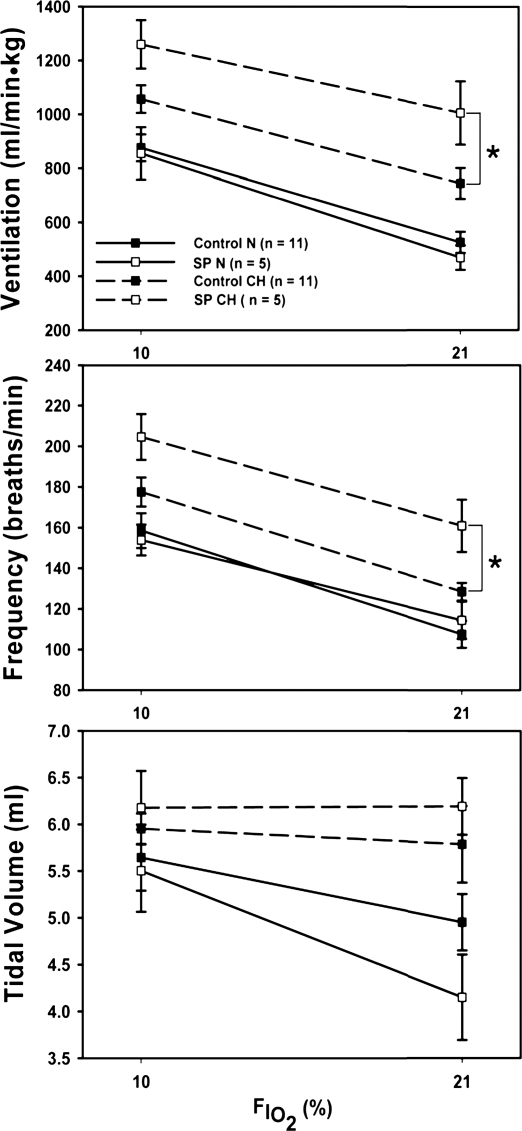
Effect of the NK1R cell lesion (dashed lines) on the HVR in N and CH rats. Lesions in the caudal NTS increased ventilation and frequency only in chronic hypoxia. FiO2, inspired fraction of O2. Values are means ± SE. *P < 0.05.
Table 1.
Arterial blood-gas measurements during hypercapnia and hypoxia
| n | PaO2 | PaCO2 | pHa | |
|---|---|---|---|---|
| FiO2 = 0.10 | ||||
| Blank N | 6 | 38.17 ± 1.70 | 26.58 ± 1.14 | 7.59 ± 0.01 |
| SPN | 2 | 38.00 ± 2.00 | 23.50 ± 0.50† | 7.63 ± 0.00 |
| Blank CH | 5 | 44.80 ± 2.31 | 20.34 ± 1.76* | 7.51 ± 0.01* |
| SP CH | 5 | 39.40 ± 1.94 | 16.00 ± 0.71*† | 7.52 ± 0.01* |
| FiO2 = 0.21 | ||||
| Blank N | 6 | 96.50 ± 2.88 | 34.77 ± 1.30 | 7.47 ± 0.00 |
| SP N | 2 | 99.50 ± 0.50 | 32.00 ± 4.00† | 7.48 ± 0.00 |
| Blank CH | 5 | 107.25 ± 9.36 | 25.80 ± 2.30* | 7.44 ± 0.01* |
| SP CH | 5 | 101.40 ± 1.94 | 21.20 ± 1.46*† | 7.44 ± 0.01* |
| FiO2 = 0.21, FiCO2 = 0.05 | ||||
| Blank N | 5 | 123.40 ± 8.07 | 41.24 ± 3.48 | 7.38 ± 0.01 |
| SPN | 2 | 113.50 ± 2.50 | 41.50 ± 1.50 | 7.40 ± 0.01 |
| Blank CH | 5 | 132.40 ± 5.16 | 36.26 ± 3.54* | 7.30 ± 0.01* |
| SP CH | 5 | 120.67 ± 3.76 | 38.00 ± 1.15* | 7.27 ± 0.01* |
| FiO2 = 0.21, FiCO2 = 0.07 | ||||
| Blank N | 6 | 120.00 ± 6.10 | 52.53 ± 0.83 | 7.32 ± 0.01 |
| SP N | 2 | 126.00 ± 2.00 | 51.50 ± 0.50 | 7.33 ± 0.02 |
| Blank CH | 5 | 124.75 ± 7.57 | 45.35 ± 2.98* | 7.24 ± 0.02* |
| SP CH | 5 | 123.50 ± 3.23 | 42.50 ± 5.54* | 7.19 ± 0.01* |
Values are means ± SE; n, no. of rats. N, normoxic control; CH, chronically hypoxic; SP, stable substance P-conjugated saporin; Blank, blank-conjugated saporin; PaO2, arterial Po2; PaCO2, arterial Pco2; pHa, arterial pH; FiO2, fraction of inspired O2; FiCO2, fraction of inspired CO2.
P < 0.05 from normoxic value.
P < 0.05 from Blank value.
Effect of SP-SAP lesions on ventilatory sensitivity to CO2.
Contrary to our hypothesis, NTS lesions did not decrease ventilatory CO2 sensitivity (Fig. 2). This conclusion is the same if the data is analyzed in terms of inspired Pco2 or PaCO2, although there is a nonsignificant tendency for a decreased response after lesions in CH animals between 0% and 5% inhaled CO2 when the data is analyzed vs. PaCO2 (cf. slopes in Fig. 2). There was a tendency for NTS lesions to decrease V̇i at the highest level of CO2 in the N control rats, but, in chronically hypoxic rats, NTS lesions tended to increase V̇i at all CO2 levels (Fig. 2). The differences were primarily because of differences in fr. PaCO2 was significantly decreased with lesions after chronic hypoxia breathing room air, but differences were not significant with elevated CO2 (Table 1).
Effect of SP-SAP lesions on ventilatory sensitivity to O2.
There was no effect of NTS lesions on the HVR in N rats (Fig. 3). However, in chronically hypoxic rats, NTS lesions increased V̇i significantly in room air and 10% O2 (Fig. 3A). This difference was driven by an increased fR during chronic hypoxia in the lesioned animals (Fig. 3C), with no effect on Vt (Fig. 3B). The results are similar when ventilatory data is plotted against PaO2 instead of inspired Po2 also (not shown). PaCO2 decreased significantly in these conditions too (Table 1), as expected for hyperventilation, and considering that metabolic rate returns to control levels after 7 days of chronic hypoxia (1). PaO2 was not significantly different with NTS lesions, although it tended to be lower in the lesioned animals with significantly lower PaCO2 values (Table 1).
DISCUSSION
Summary of results.
NK1R cell lesions in the caudal NTS had no effect on V̇i in N animals in room air, hypercapnic, or hypoxic gases. In contrast, lesion of the NK1R cells in the caudal NTS in chronically hypoxic rats resulted in hyperventilation during normoxic and hypoxic conditions. This hyperventilation was caused by an increased fR, with no effect on Vt. There was a nonsignificant trend for V̇i in hypercapnia to be higher, with lesions in chronically hypoxic animals as well.
Critique of method.
The targeted cell killing method in this study has been used by several other laboratories (2, 18, 20, 41, 45, 46, 52, 57) and has been shown to specifically kill the NK1R-positive cells while not significantly decreasing the total number of cells in the injection site. We could not detect any differences in total cell number based on counts of DAPI-stained nuclei and have no evidence of any effects of SP-SAP other than destroying NK1R-positive cells.
We targeted the caudal NTS located under the area postrema as the location where the majority of CO2-sensitive neurons in the NTS complex have been identified in vitro (13, 14). However, ∼20% of chemoreceptors identified in medullary slices studied in vitro were located in the rostral NTS (13), and these would have been spared by our lesions. Hence, we cannot rule out that killing these other cells too would cause CO2 sensitivity to decrease in the N animals. On the other hand, NK1R immunoreactivity decreased in the dorsal motor nucleus of the vagus with SP-SAP lesions in the NTS of some animals, and this area contains CO2-sensitive neurons also (48). Hence, our results show the effects of killing NK1R neurons primarily in the caudal NTS, which includes both CO2-sensitive neurons and secondary neurons receiving afferent input from the arterial chemoreceptors that respond to O2 and CO2 (15, 21, 22).
The SP-SAP method of killing has been used by multiple laboratory groups specifically in the NTS, but, to date, only our group and one other (52) have quantified the number of NK1R-positive cells before and after lesion in this location. Potts et al. (52) found a greater absolute number of NK1R-positive cells than we did, but we used different antibodies, which may explain the differences. They had appropriate controls showing no fluorescence without the primary antibody, but the sensitivity of our primary antibodies could differ. Perhaps the most relevant observation is that they found significant physiological effects, with lesions sparing only 10–25% of the NK1R-positive neurons they identified (47). In contrast, we found no physiological effect with lesions sparing 19% of our neurons and have no evidence or reason to expect that the proportion of neurons killed by our SP-SAP lesions is any different than the proportion we quantified with NK1R immunoreactivity. Our method gave reproducible results for counting NK1R-positive neurons (cf. control animals had an average of 38.0 ± 1.7 cells) and was able to detect significant differences following our SP-SAP treatment (7.3 ± 1.1 cells, P > 0.05). Finally, we observed no correlations between the percent deletion of NK1R-positive neurons and changes in the HCVR when it was examined across individual animals.
Further evidence that the lesions we made should cause a significant decrease in the HCVR, if these NK1R neurons are necessary, is provided by studies using the same method in other areas of central CO2 chemosensitivity. Nattie and Li (45) counted a similar number of NK1R-positive neurons in the retrotrapezoid nucleus (RTN) (45 ± 12) and found significant effects with SP-SAP lesions that decreased NK1R-positive cells to 21% of control. In contrast, we saw no effect with lesions, leaving only 19% of control NK1R-positive neurons in the NTS.
Although PaO2 was not significantly different, it tended to be lower in the chronically hypoxic lesioned animals (Table 1). Also, PaCO2 was significantly lower in these animals, suggesting that the lesions in chronically hypoxic rats may impair gas exchange. We observed no other evidence of adverse effects from the lesions, such as abnormal body temperature, grooming and appearance, or stools. However, it is important to consider whether the lower PaO2 may have provided a significantly greater stimulus for acclimatization to chronic hypoxia that could explain results between the groups.
Based on predictions from previous studies quantifying V̇i as a function of PaO2 in conscious rats (1), the lower PaO2 we measured in chronically hypoxic lesioned rats (5–6 Torr) would not increase V̇i as much as we observed. Extrapolating between animals with and without lesions to make this prediction is reasonable, considering that we observed no effect of the lesions on the slopes of the HVR (Fig. 3). Additionally, we have no evidence that the lesions affect metabolic responses to hypoxia, which could also influence the level of V̇i. In N rats, there were no differences in V̇i or PaCO2 (Fig. 2 and Table 1, respectively), as expected if metabolic rate is similar with and without lesions. In chronically hypoxic rats, V̇i tends to be greater with lesions (Fig. 2), and PaCO2 is less (Table 1), consistent with the expected change in PaCO2 for increased V̇i and a constant metabolic rate.
Effect of the lesion on the ventilatory sensitivity to CO2.
The fact that NK1R cell destruction in the NTS does not diminish the HCVR in normoxia was unexpected. Lesions of the NK1R cells in both the RTN and medullary raphe, which are other sites of CO2 sensitivity, have been shown to decrease the HCVR (20, 41, 45). While the role of the NK1R cells in central CO2 sensitivity is unknown, the NK1R is present in every site of central chemosensitivity (37). The functional evidence for CO2 sensitivity in the NTS is the same as it is for other sites. CO2-sensitive cells have been identified in vitro (14), and stimulating the CO2-sensitive cells with either microinjected acetazolamide (11) or microdialized CO2 (43) increases V̇i in rats. The lack of effect of our lesion could indicate that the NK1R-positive cells are not involved in CO2 sensing in the NTS: they comprise only a portion of the chemoreceptor cells in the NTS (see Critique of method above), or they are redundant to CO2 sensitivity at other sites.
It is becoming increasingly clear that many cells in the brain stem have intrinsic chemosensitivity that can be modified by synaptic and/or gap junction connections with other cells. In fact, the number of cells excited by CO2 in the NTS increases in the presence of synaptic blockade, indicating the importance of synaptic input under normal conditions (17). Potentially, the NK1R-positive cells could still be chemoreceptors but play a more important role during a different state that was not tested in these experiments. We tested our animals only in the awake state, and there is some evidence for state dependence of chemoreception. For instance, micordialysis of CO2/H+ in the RTN and caudal ventrolateral medulla (Loeschcke's area) increases V̇i in unanesthetized rats during wakefulness, but not sleep (12, 30), while, in the medullary raphe, V̇i is increased only during sleep (44). We note that, in similar experiments, microdialysis of CO2/H+ into the NTS did increase V̇i in both sleep and wakefulness (43).
As we only targeted a small population of cells in the NTS, another possible explanation for the lack of effect of NK1R cell lesions in the NTS on the HCVR is that we may not have killed enough chemosensitive cells. Takakura et al. (57) found that 70% of the NK1R-Phox2B-positive cells in the RTN had to be destroyed to significantly decrease the HCVR. Other studies have found significant decreases in the HCVR with only 36–59% of the NK1R-positive cells in the RTN destroyed (45), but we used the more stringent criteria of 70% decreases in NK1R-positive cells for a successful lesion in the NTS (see Critique of method above). Focal inhibition with microinjections of muscimol in the NTS decreases the HCVR (40), and this treatment may be more effective because there are more GABAergic neurons that can be affected by this treatment compared with NK1R-positive cells being lesioned by our protocol. We do note that many processes were NK1R positive, and, if their cell bodies were in other brain stem areas, they may not have been affected by our treatment. Additionally, other central chemosensitive sites and/or neurotransmitter mechanisms in the arterial chemoreflex pathway may compensate for the effects of NK1R lesions in the caudal NTS on the HCVR.
In chronically hypoxic rats, NK1R lesions in the caudal NTS caused a significant increase in V̇i and decrease in PaCO2 while breathing room air. Also, there was a trend for these lesions to increase V̇i at all CO2 levels (Fig. 2). These results are opposite of our prediction that chronic hypoxia would increase ventilatory drive from NK1 cells in the NTS, but they do indicate a role for NK1R cells in the NTS in ventilatory acclimatization to hypoxia.
Effects of lesions on the HVR.
Lesions had no effect on the HVR in N rats (Fig. 3). This was surprising as the carotid sinus nerve afferents from carotid body chemoreceptors release SP in the caudal NTS in response to hypoxia (31, 56). However, after exposure to chronic hypoxia, NK1R lesions in the caudal NTS increased V̇i in both normoxia and hypoxia (Fig. 3). We note that the significant effects of the lesions in chronically hypoxic rats breathing room air are revealed in the HVR analysis using the very same normoxic control data (21% O2 and 0% CO2) that was used for the HCVR analysis. We observed this trend in the HCVR analysis, although it was not significant, because variability in the 5 and 7% CO2 data decreased the interaction in ANOVA between chronic O2 level and drug effects. The significant increase in V̇i in chronically hypoxic, lesioned rats breathing room air, relative to control rats, is opposite of what we predicted. As discussed above (Critique of method), we cannot completely rule out that the increased V̇i in the chronically hypoxic rats with lesions resulted from additional hypoxemia with gas exchange impairments from lesions, causing greater acclimatization to deeper hypoxia. However, we repeat that the magnitude of decrease in PaCO2 that we observed (Table 1) is not easily explained by what is known about acclimatization in rats and our data here (cf. Critique of method).
In CH rats, lesions increased both V̇i and fR in room air and acute hypoxia. This is somewhat surprising, because microinjection of SP into the caudal NTS of rats and rabbits increases V̇i (9, 34, 35, 63, 64). Such an excitatory effect of SP is presumably acting by the carotid body chemoreflex pathway, as discussed above. Given the increased sensitivity to hypoxia of carotid bodies and afferent traffic in the carotid sinus nerve after acclimatization to hypoxia (reviewed by Ref. 55), one would have predicted a greater inhibitory effect on V̇i by blocking SP effects in chronically hypoxic rats. In contrast, we observed increased V̇i with NK1R lesions.
Mechanisms of lesion effects.
There is evidence for NK1R cells located in the caudal NTS being GABAergic inhibitory interneurons that might contribute to our results. NK1R and GABAA receptors colocalize in the NTS (8, 51, 53), and the inhibitory effect of somatosensory inputs on the baroreflex are blocked by GABAA or NK1R antagonists in the caudal NTS (6, 54). NK1R cell lesions in the caudal NTS also abolish the inhibitory effect of somatosensory input on the baroreflex, although lesions do not affect the cardiovascular response to arterial chemoreceptor stimulation by cyanide (52). This agrees with our observation that NK1R lesions in the caudal NTS have no effect on the ventilatory response to arterial chemoreceptor stimulation in N rats.
In contrast, NK1R lesions cause hyperventilation after acclimatization to hypoxia (Fig. 3), indicating a change in the effect of NK1R cells in the caudal NTS on ventilatory reflexes. While there is evidence for plasticity in GABA receptors during chronic hypoxia, it is not easy to explain the changes we observed with chronic hypoxia in terms of GABAergic neurotransmission. GABAA receptors in the pons are upregulated during chronic hypoxia (23), but dissociated NTS neurons from chronically hypoxic rats exhibit decreased sensitivity to GABAA receptor blockers (58). Also, inhibiting GABA neurotransmission in the caudal NTS decreases ventilatory drive in chronically hypoxic rats breathing normoxic gas (10). This is opposite the increase in ventilatory drive predicted by our results, if the NK1R lesions destroyed the inhibitory cells with GABA receptors too. However, not all GABA cells in the NTS contain the NK1R, and further experiments are necessary to determine whether the same population of interneurons with NK1R and GABAA receptors contribute to the effects observed in all of these studies.
Changes to other neurotransmitter systems on the NK1R-positive cells could also explain the effects of the lesion in chronic hypoxia. There is evidence in the dorsal horn of the spinal cord during chronic pain that persistent stimulation causes an upregulation of the NK1R and increased excitability of the NK1R-positive neurons (7, 24, 26). The number of cell bodies in the NTS positive for the NK1R was unchanged during chronic hypoxia, but that does not preclude a change in receptor number or function during chronic hypoxia, a condition that, similar to chronic pain, involves chronic SP release (31).
Because at least a subset of the NK1R cells in the NTS regulate cardiovascular reflexes, they might also play an important role in cardiovascular control during chronic hypoxia. Activation of the peripheral chemoreflex also activates sympathetic neural activity, which can cause vasoconstriction and increased blood pressure (28). Perhaps this population of NK1R cells is important in preventing sympathetic overactivation during chronic hypoxia. Blood pressure was not measured in our animals, so the effect of the lesion on blood pressure during chronic hypoxia is unknown, although lesion of these cells during normoxia does not change resting blood pressure (2).
Perspectives and Significance
We were surprised that NK1R lesion in the caudal NTS produced no effect on the HCVR in N control animals, in contrast to similar lesions in the RTN or raphe (20, 45, 46). Like other areas of central CO2 sensitivity, the NTS can clearly contribute to a central CO2-sensitive ventilatory response (38), but, unlike other areas, it is not necessary for a normal ventilatory response to CO2 in intact animals. This supports the idea that multiple sites of central chemosensitivity play different roles in different conditions and may be interdependent, even with peripheral chemoreceptors (19, 38, 39).
The significant increase in V̇i and decrease in PaCO2 with NK1R lesions in the caudal NTS after chronic hypoxia, but not before, demonstrates plasticity in the central nervous system mechanisms controlling breathing and regulating PaCO2. The direction of this effect is opposite from what we predicted, with lesions increasing instead of decreasing V̇i. The result is not easily explained by the loss of NK1R-positive neurons that function as CO2-sensitive central chemoreceptors or interneurons responding to SP release from carotid body chemoreceptor afferents. Further experiments are necessary to distinguish between plasticity in central CO2 sensitivity, the processing of afferent input from arterial chemoreceptors, or other integrative mechanisms. Also, the exact phenotype of the cells killed by the SP-SAP lesions and their role in ventilatory control circuits remains to be determined.
GRANTS
This study was supported by National Institutes of Health (NIH) Grant RO1 HL 081823 (F. L. Powell) and American Heart Association Grant 0615069Y (K. A. Wilkinson). Computer and statistical support was provided, in part, by Grant MO1-RR00827 from the National Center for Research Resources of the NIH for the University of California San Diego General Clinical Research Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGEMENTS
The authors thank Dr. Moh Mahlek for statistical assistance.
REFERENCES
- 1. Aaron EA, Powell FL. Effect of chronic hypoxia on hypoxic ventilatory response in awake rats. J Appl Physiol 74: 1635–1640, 1993 [DOI] [PubMed] [Google Scholar]
- 2. Abdala AP, Schoorlemmer GH, Colombari E. Ablation of NK1 receptor bearing neurons in the nucleus of the solitary tract blunts cardiovascular reflexes in awake rats. Brain Res 1119: 165–173, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Akilesh MR, Kamper M, Li A, Nattie EE. Effects of unilateral lesions of retrotrapezoid nucleus on breathing in awake rats. J Appl Physiol 82: 469–479, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Bernard DG, Li A, Nattie EE. Evidence for central chemoreception in the midline raphe. J Appl Physiol 80: 108–115, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Biancardi V, Bicego KC, Almeida MC, Gargaglioni LH. Locus coeruleus noradrenergic neurons and CO2 drive to breathing. Pflügers Arch 455: 1119–1128, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Boscan P, Kasparov S, Paton JF. Somatic nociception activates NK1 receptors in the nucleus tractus solitarii to attenuate the baroreceptor cardiac reflex. Eur J Neurosci 16: 907–920, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Bradesi S, Kokkotou E, Simeonidis S, Patierno S, Ennes HS, Mittal Y, McRoberts JA, Ohning G, McLean P, Marvizon JC, Sternini C, Pothoulakis C, Mayer EA. The role of neurokinin 1 receptors in the maintenance of visceral hyperalgesia induced by repeated stress in rats. Gastroenterology 130: 1729–1742, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Chen CY, Bechtold AG, Tabor J, Bonham AC. Exercise reduces GABA synaptic input onto nucleus tractus solitarii baroreceptor second-order neurons via NK1 receptor internalization in spontaneously hypertensive rats. J Neurosci 29: 2754–2761, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen Z, Hedner J, Hedner T. Substance P in the ventrolateral medulla oblongata regulates ventilatory responses. J Appl Physiol 68: 2631–2639, 1990 [DOI] [PubMed] [Google Scholar]
- 10. Chung S, Ivy GO, Reid SG. GABA-mediated neurotransmission in the nucleus of the solitary tract alters resting ventilation following exposure to chronic hypoxia in conscious rats. Am J Physiol Regul Integr Comp Physiol 291: R1449–R1456, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Coates EL, Li A, Nattie EE. Widespread sites of brain stem ventilatory chemoreceptors. J Appl Physiol 75: 5–14, 1993 [DOI] [PubMed] [Google Scholar]
- 12. da Silva GS, Li A, Nattie E. High CO2/H+ dialysis in the caudal ventrolateral medulla (Loeschcke's area) increases ventilation in wakefulness. Respir Physiol Neurobiol 171: 46–53, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dean JB, Bayliss DA, Erickson JT, Lawing WL, Millhorn DE. Depolarization and stimulation of neurons in nucleus tractus solitarii by carbon dioxide does not require chemical synaptic input. Neuroscience 36: 207–216, 1990 [DOI] [PubMed] [Google Scholar]
- 14. Dean JB, Lawing WL, Millhorn DE. CO2 decreases membrane conductance and depolarizes neurons in the nucleus tractus solitarii. Exp Brain Res 76: 656–661, 1989 [DOI] [PubMed] [Google Scholar]
- 15. Donoghue S, Felder RB, Jordan D, Spyer KM. The central projections of carotid baroreceptors and chemoreceptors in the cat: a neurophysiological study. J Physiol 347: 397–409, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dwinell MR, Powell FL. Chronic hypoxia enhances the phrenic nerve response to arterial chemoreceptor stimulation in anesthetized rats. J Appl Physiol 87: 817–823, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Erlichman JS, Boyer AC, Reagan P, Putnam RW, Ritucci NA, Leiter JC. Chemosensory responses to CO2 in multiple brain stem nuclei determined using a voltage-sensitive dye in brain slices from rats. J Neurophysiol 102: 1577–1590, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBotzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci 4: 927–930, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guyenet PG. The 2008 Carl Ludwig Lecture: retrotrapezoid nucleus, CO2 homeostasis, and breathing automaticity. J Appl Physiol 105: 404–416, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hodges MR, Opansky C, Qian B, Davis S, Bonis J, Bastasic J, Leekley T, Pan LG, Forster HV. Transient attenuation of CO2 sensitivity after neurotoxic lesions in the medullary raphe area of awake goats. J Appl Physiol 97: 2236–2247, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Housley GD, Martin-Body RL, Dawson NJ, Sinclair JD. Brain stem projections of the glossopharyngeal nerve and its carotid sinus branch in the rat. Neuroscience 22: 237–250, 1987 [DOI] [PubMed] [Google Scholar]
- 22. Housley GD, Sinclair JD. Localization by kainic acid lesions of neurones transmitting the carotid chemoreceptor stimulus for respiration in rat. J Physiol 406: 99–114, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hsieh YH, Dick TE, Siegel RE. Adaptation to hypobaric hypoxia involves GABA A receptors in the pons. Am J Physiol Regul Integr Comp Physiol 294: R549–R557, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Ishigooka M, Zermann DH, Doggweiler R, Schmidt RA, Hashimoto T, Nakada T. Spinal NK1 receptor is upregulated after chronic bladder irritation. Pain 93: 43–50, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Jacky JP. A plethysmograph for long-term measurements of ventilation in unrestrained animals. J Appl Physiol 45: 644–647, 1978 [DOI] [PubMed] [Google Scholar]
- 26. Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci 26: 696–705, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Jordan D, Spyer KM. Brainstem integration of cardiovascular and pulmonary afferent activity. Prog Brain Res 67: 295–314, 1986 [DOI] [PubMed] [Google Scholar]
- 28. Kara T, Narkiewicz K, Somers VK. Chemoreflexes–physiology and clinical implications. Acta Physiol Scand 177: 377–384, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Li A, Nattie EE. Focal central chemoreceptor sensitivity in the RTN studied with a CO2 diffusion pipette in vivo. J Appl Physiol 83: 420–428, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Li A, Randall M, Nattie EE. CO2 microdialysis in retrotrapezoid nucleus of the rat increases breathing in wakefulness but not in sleep. J Appl Physiol 87: 910–919, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Lindefors N, Yamamoto Y, Pantaleo T, Lagercrantz H, Brodin E, Ungerstedt U. In vivo release of substance P in the nucleus tractus solitarii increases during hypoxia. Neurosci Lett 69: 94–97, 1986 [DOI] [PubMed] [Google Scholar]
- 32. Mantyh PW, Allen CJ, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE. Rapid endocytosis of a G protein-coupled receptor: substance P evoked internalization of its receptor in the rat striatum in vivo. Proc Natl Acad Sci U S A 92: 2622–2626, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marchenko V, Sapru HN. Different patterns of respiratory and cardiovascular responses elicited by chemical stimulation of dorsal medulla in the rat. Brain Res 857: 99–109, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Mazzone SB, Geraghty DP. Respiratory actions of tachykinins in the nucleus of the solitary tract: characterization of receptors using selective agonists and antagonists. Br J Pharmacol 129: 1121–1131, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mazzone SB, Geraghty DP. Respiratory actions of tachykinins in the nucleus of the solitary tract: effect of neonatal capsaicin pretreatment. Br J Pharmacol 129: 1132–1139, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci 7: 1360–1369, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Nakaya Y, Kaneko T, Shigemoto R, Nakanishi S, Mizuno N. Immunohistochemical localization of substance P receptor in the central nervous system of the adult rat. J Comp Neurol 347: 249–274, 1994 [DOI] [PubMed] [Google Scholar]
- 38. Nattie E. Julius H. Comroe, Jr, Distinguished Lecture: Central chemoreception: then… and now. J Appl Physiol 110: 1–8, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nattie E, Li A. Central chemoreception in wakefulness and sleep: evidence for a distributed network and a role for orexin. J Appl Physiol 108: 1417–1424, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nattie E, Li A. Muscimol dialysis into the caudal aspect of the nucleus tractus solitarii of conscious rats inhibits chemoreception. Respir Physiol Neurobiol 164: 394–400, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nattie E, Li A. Neurokinin-1 receptor-expressing neurons in the ventral medulla are essential for normal central and peripheral chemoreception in the conscious rat. J Appl Physiol 101: 1596–1606, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Nattie EE. Chemoreception and tonic drive in the retrotrapezoid nucleus (RTN) region of the awake rat: bicuculline and muscimol dialysis in the RTN. Adv Exp Med Biol 499: 27–32, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Nattie EE, Li A. CO2 dialysis in nucleus tractus solitarius region of rat increases ventilation in sleep and wakefulness. J Appl Physiol 92: 2119–2130, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Nattie EE, Li A. CO2 dialysis in the medullary raphe of the rat increases ventilation in sleep. J Appl Physiol 90: 1247–1257, 2001 [DOI] [PubMed] [Google Scholar]
- 45. Nattie EE, Li A. Substance P-saporin lesion of neurons with NK1 receptors in one chemoreceptor site in rats decreases ventilation and chemosensitivity. J Physiol 544: 603–616, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nattie EE, Li A, Richerson GB, Lappi DA. Medullary serotonergic neurones and adjacent neurones that express neurokinin-1 receptors are both involved in chemoreception in vivo. J Physiol 556: 235–253, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nattie EE, Li AH, John WM. Lesions in retrotrapezoid nucleus decrease ventilatory output in anesthetized or decerebrate cats. J Appl Physiol 71: 1364–1375, 1991 [DOI] [PubMed] [Google Scholar]
- 48. Nichols NL, Mulkey DK, Wilkinson KA, Powell FL, Dean JB, Putnam RW. Characterization of the chemosensitive response of individual solitary complex neurons from adult rats. Am J Physiol Regul Integr Comp Physiol 296: R763–R773, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nichols NL, Wilkinson KA, Powell FL, Dean JB, Putnam RW. Chronic hypoxia suppresses the CO2 response of solitary complex (SC) neurons from rats. Respir Physiol Neurobiol 168: 272–280, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic, 1982 [DOI] [PubMed] [Google Scholar]
- 51. Potts JT. Inhibitory neurotransmission in the nucleus tractus solitarii: implications for baroreflex resetting during exercise. Exp Physiol 91: 59–72, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Potts JT, Fong AY, Anguelov PI, Lee S, McGovern D, Grias I. Targeted deletion of neurokinin-1 receptor expressing nucleus tractus solitarii neurons precludes somatosensory depression of arterial baroreceptor-heart rate reflex. Neuroscience 145: 1168–1181, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Potts JT, Fuchs IE, Li J, Leshnower B, Mitchell JH. Skeletal muscle afferent fibres release substance P in the nucleus tractus solitarii of anaesthetized cats. J Physiol 514: 829–841, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Potts JT, Paton JF, Mitchell JH, Garry MG, Kline G, Anguelov PT, Lee SM. Contraction-sensitive skeletal muscle afferents inhibit arterial baroreceptor signalling in the nucleus of the solitary tract: role of intrinsic GABA interneurons. Neuroscience 119: 201–214, 2003 [DOI] [PubMed] [Google Scholar]
- 55. Powell FL. The influence of chronic hypoxia upon chemoreception. Respir Physiol Neurobiol 157: 154–161, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Srinivasan M, Goiny M, Pantaleo T, Lagercrantz H, Brodin E, Runold M, Yamamoto Y. Enhanced in vivo release of substance P in the nucleus tractus solitarii during hypoxia in the rabbit: role of peripheral input. Brain Res 546: 211–216, 1991 [DOI] [PubMed] [Google Scholar]
- 57. Takakura AC, Moreira TS, Stornetta RL, West GH, Gwilt JM, Guyenet PG. Selective lesion of retrotrapezoid Phox2b-expressing neurons raises the apnoeic threshold in rats. J Physiol 586: 2975–2991, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tolstykh G, Belugin S, Mifflin S. Responses to GABA(A) receptor activation are altered in NTS neurons isolated from chronic hypoxic rats. Brain Res 1006: 107–113, 2004 [DOI] [PubMed] [Google Scholar]
- 59. Vardhan A, Kachroo A, Sapru HN. Excitatory amino acid receptors in commissural nucleus of the NTS mediate carotid chemoreceptor responses. Am J Physiol Regul Integr Comp Physiol 264: R41–R50, 1993 [DOI] [PubMed] [Google Scholar]
- 60. Weil JV. Ventilatory Control at High Altitude. In: Handbook of Physiology. The Respiratory System. Control of Breathing. Bethesda, MD: Am. Physiol. Soc., 1986, sect. 3, vol. II, pt. 2, chapt. 21, p. 703–728 [Google Scholar]
- 61. Wiley RG, Lappi DA. Destruction of neurokinin-1 receptor expressing cells in vitro and in vivo using substance P-saporin in rats. Neurosci Lett 230: 97–100, 1997 [DOI] [PubMed] [Google Scholar]
- 62. Wilkinson KA, Huey K, Dinger B, He L, Fidone S, Powell FL. Chronic hypoxia increases the gain of the hypoxic ventilatory response by a mechanism in the central nervous system. J Appl Physiol 109: 424–430, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yamamoto Y, Lagercrantz H. Some effects of substance P on central respiratory control in rabbit pups. Acta Physiol Scand 124: 449–455, 1985 [DOI] [PubMed] [Google Scholar]
- 64. Yamamoto Y, Lagercrantz H, von Euler C. Effects of substance P and TRH on ventilation and pattern of breathing in newborn rabbits. Acta Physiol Scand 113: 541–543, 1981 [DOI] [PubMed] [Google Scholar]