Abstract
β-Adrenergic receptor (AR) signaling is a regulator of skeletal muscle protein synthesis and mitochondrial biogenesis in mice. We hypothesized that β-AR blockade blunts postexercise skeletal muscle mitochondrial protein synthesis rates in adult humans. Six healthy men (mean ± SD: 26 ± 6 yr old, 39.9 ± 4.9 ml·kg−1·min−1 peak O2 uptake, 26.7 ± 2.0 kg/m2 body mass index) performed 1 h of stationary cycle ergometer exercise (60% peak O2 uptake) during 1) β-AR blockade (intravenous propranolol) and 2) administration of saline (control). Skeletal muscle mitochondrial, myofibrillar, and sarcoplasmic protein synthesis rates were assessed using [2H5]phenylalanine incorporation into skeletal muscle proteins after exercise. The mRNA content of signals for mitochondrial biogenesis was determined using real-time PCR. β-AR blockade decreased mitochondrial (from 0.217 ± 0.076 to 0.135 ± 0.031%/h, P < 0.05), but not myofibrillar or sarcoplasmic, protein synthesis rates. Peroxisome proliferator-activated receptor-γ coactivator-1α mRNA was increased ∼2.5-fold (P < 0.05) at 5 h compared with 1 h postexercise but was not influenced by β-AR blockade. We conclude that decreased β-AR signaling during cycling can blunt the postexercise increase in mitochondrial protein synthesis rates without affecting mRNA content.
Keywords: mitochondrial biogenesis, propranolol, stable isotope tracer, aerobic exercise
decreased mitochondrial content and function have been reported with aging and may contribute to chronic disease (30) and muscle wasting (37, 49). Because of their potential role in the pathogenesis of disease, mitochondria are a target of lifestyle and pharmacological therapies. For example, aerobic exercise stimulates mitochondrial proliferation (15) and likely contributes to the health benefits of exercise training (22). Conversely, certain drug classes cause mitochondrial dysfunction (9) and may contribute to skeletal muscle myopathies (10). Given the high prevalence of chronic disease and the widespread use of drug therapies and lifestyle recommendations for treatment of chronic diseases, it is necessary to understand how concurrent exercise and drug therapy affect mitochondrial biogenesis.
Recent evidence suggests that mitochondrial biogenesis following aerobic exercise is partly mediated through β-adrenergic receptor (AR) signaling (25). In mice, selective β2-AR stimulation under resting conditions increased the mRNA content of a regulator of mitochondrial biogenesis, peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) (24). Additionally, mice that were treated with the nonselective β-AR antagonist propranolol had a blunted increase of PGC-1α mRNA following aerobic exercise (25). Such findings are consistent with human aerobic training studies reporting blunted adaptations of select mitochondrial enzymes and maximal aerobic capacity during nonselective β-AR antagonist treatment (1, 46, 52). β-AR antagonists (β-blockers) are used to treat cardiovascular diseases and are commonly prescribed in conjunction with exercise (39). It is possible that β-blockers may impair mitochondrial adaptations and limit the beneficial effects of aerobic exercise (35). Such a negative relationship between β-blockers and exercise adaptations implies that drug prescription may be counterproductive to exercise recommendations.
Miura et al. (25) demonstrated that mitochondrial biogenesis may be regulated by β-AR signaling; however, the assessment was limited to changes in mRNA content for proteins that regulate mitochondrial biogenesis and was performed in mice. Changes in mRNA content may not lead to changes in mitochondrial protein content (28); therefore, it is necessary to use additional determinants, such as protein synthesis rates, to determine mitochondrial biogenesis. Another recent report using mice found that β2-AR agonist treatment increased mixed skeletal muscle and mitochondrial protein synthesis rates after 7 days of treatment (20). However, it is possible that there are interspecies differences in β-AR signaling between mice and humans.
Initially, to examine whether the findings of Miura et al. (25) were applicable to humans, we performed a 1-h infusion of the nonselective β-AR agonist isoproterenol (36). Contrary to the results reported by Miura et al., we did not increase mitochondrial biogenesis, as measured by mRNA signaling and incorporation of a stable isotope into mitochondrial proteins or whole body and skeletal muscle protein synthesis (36). Our dose of isoproterenol increased heart rate and blood pressure and was previously used to increase metabolic rate above resting values (5). The report of Koopman et al. (20) prompted us to examine further whether β-AR and changes in protein synthesis were unique to mice.
Our previous study was a gain-of-function design to evaluate if β-AR stimulation could induce mitochondrial biogenesis at rest (36). Here we conduct the loss-of-function experiment in which β-AR signaling is diminished during exercise. We hypothesized that blocking β1- and β2-ARs prior to and during a 1-h bout of cycling would decrease mitochondrial protein synthesis and mRNA content of genes related to mitochondrial biogenesis.
METHODS
Ethics approval.
The Institutional Review Board of Colorado State University approved the protocol. Each volunteer was informed of the potential risks, and written consent was obtained prior to enrollment in the study, which followed the guidelines set forth by the Declaration of Helsinki.
Study overview.
Six healthy adult men were studied during 2 or 3 days separated by ≥3 wk. First, a subset of three subjects was used to confirm the efficacy of our β-AR blockade protocol (see below). All subjects then performed 1 h of stationary cycle ergometer exercise [60% of unmedicated peak O2 uptake (V̇o2peak)] during 1) β-AR blockade (intravenous propranolol) and 2) administration of saline (control). The propranolol trial was always performed before the saline trial to make any adjustments in workload necessary for the subject to complete 1 h of exercise. An identical workload was repeated on the saline control day. After exercise on both days, the subjects rested for 5 h during infusion of a stable isotope and subsequent skeletal muscle sampling to determine protein synthesis rates and mRNA content of skeletal muscle.
Subject characteristics.
The subjects (Table 1) were healthy, on the basis of a medical history questionnaire, and free of any cardiac abnormalities, as determined by an exercising 12-lead electrocardiogram. The three subjects (Table 1) who volunteered for verification of β-AR blockade were a subset of the entire group. Exclusion criteria included a resting heart rate <40 beats/min because of the risk of hypotension during β-AR blockade. V̇o2peak was determined during incremental stationary cycle ergometer exercise (Velotron, RacerMate, Seattle, WA) via indirect calorimetry (ParvoMedics, Sandy, UT). Body composition was determined using dual-energy X-ray absorptiometry (Lunar Discovery W, GE Medical Systems, Madison, WI).
Table 1.
Subject characteristics of subjects who volunteered for verification of β-blockade by isoproterenol + propranolol and propranolol
| Iso + Prop (n = 3) | Prop (n = 6) | |
|---|---|---|
| Age, yr | 28 ± 5 | 26 ± 6 |
| Height, m | 1.8 ± 0.1 | 1.78 ± 0.1 |
| Weight, kg | 86.5 ± 4.0 | 84.4 ± 6.0 |
| BMI, kg/m2 | 27.4 ± 0.3 | 26.7 ± 2.0 |
| Fat, % | 26.3 ± 5.1 | 25.1 ± 6.0 |
| Maximum O2 uptake | ||
| ml·kg−1·min−1 | 39.9 ± 2.4 | 39.9 ± 4.9 |
| l/min | 3.5 ± 0.3 | 3.35 ± 0.3 |
Values are means ± SD. The 3 subjects in the isoproterenol + propranolol (Iso + Prop) group were from the final Prop group.
BMI, body mass index.
Verification of β-AR blockade.
The efficacy of our β-AR blockade procedure was verified as previously described (26). Briefly, subjects arrived at the laboratory after an overnight fast and 24 h of abstention from vigorous exercise. Subjects were instrumented for the measurement of beat-by-beat heart rate (3-lead electrocardiogram) and blood pressure. An intravenous catheter was inserted into an antecubital vein, through which the nonselective β-AR agonist isoproterenol was administered in a continuous and incremental fashion. Dosing rates were increased every 5 min (9, 12, 15, 18, 21, and 24 ng·kg fat-free mass−1·min−1) until heart rate was increased 25 beats/min above resting levels. Next, the nonselective β-AR antagonist propranolol was intravenously infused, first as a priming dose (0.25 mg/kg at 1 mg/min) and then as a maintenance dose (0.006 mg·kg−1·min−1) throughout the 1 h of cycling at 60% V̇o2peak. After exercise, the infusion was stopped and the subject rested in bed. At 1 h postexercise, the isoproterenol dose that previously raised heart rate to 25 beats/min above the resting level was repeated to determine if heart rate and blood pressure increased. β-AR blockade was demonstrated by the absence of change in heart rate or blood pressure during β-AR stimulation with isoproterenol.
Study protocol.
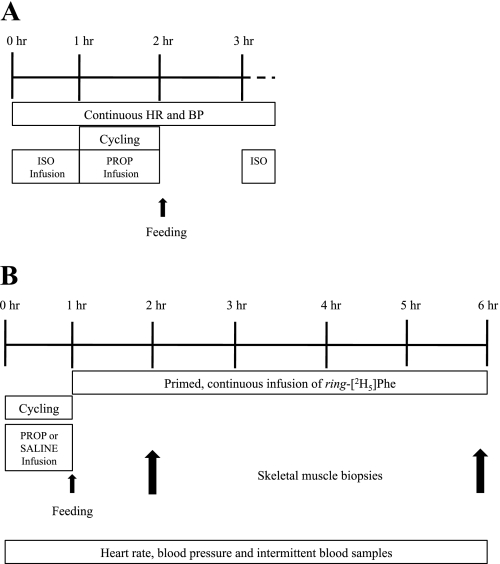
The study protocol is shown in Fig. 1. Subjects refrained from physical activity on the day before each trial, and an evening snack was provided for consumption on the night before each trial. The subjects reported to the laboratory following an overnight fast, and intravenous catheters were inserted into a dorsal hand vein for arterialized blood sampling and an antecubital vein for infusions. Beat-by-beat electrocardiograms were continuously recorded throughout the study day, and blood pressure was monitored with an automated cuff. On the first trial, propranolol was administered as a priming dose (0.25 mg/kg at 1 mg/min) and then as a maintenance dose (0.006 mg·kg−1·min−1) using a precision pump (Harvard Apparatus, Holliston, MA) following previously utilized protocols (2–4, 26). Immediately after the priming dose, the subjects began cycling for 1 h at 60% V̇o2peak during infusion of the maintenance dose. Expired air was collected every 15 min to confirm steady-state O2 consumption (V̇o2), as determined by indirect calorimetry. On day 2 of the trial, the subjects received an isovolumetric dose of saline, and the same absolute workload was performed. Immediately after exercise on both days, the subjects consumed a liquid meal (57% carbohydrate, 28% fat, 15% protein; Ross Laboratories, Abbott Park, IL) to replace the calories burned during exercise, as determined by average V̇o2 and respiratory exchange ratio. The beverage also contained sufficient protein (>20 g) to maximally stimulate protein synthesis following exercise (27). A primed continuous infusion of [2H5]phenylalanine (2 μmol/kg prime, 0.05 μmol·kg−1·min−1 continuous infusion) was performed for 5 h following exercise. Skeletal muscle samples (∼100–150 mg) were collected with a Bergstrom needle under local anesthesia (1% lidocaine) at 1 and 5 h following exercise. Intermittent arterialized-venous blood samples were collected from a heated dorsal hand vein. [2H5]phenylalanine (Cambridge Isotope, Cambridge, MA) was prepared in sterile isotonic saline by a pharmacy (Medical Center of the Rockies, Loveland, CO) and tested for pyrogenicity. As an additional precaution against infection, all solutions were passed through a 0.2-μm filter before being infused intravenously.
Fig. 1.
Study schematic. A: β-adrenergic receptor (AR) blockade was verified in 3 subjects using a graded isoproterenol (Iso) infusion to raise heart rate (HR) and blood pressure (BP); then propranolol (Prop) was infused during cycling, and isoproterenol infusion was repeated at 1 h postexercise. B: 6 subjects completed 2 trials with propranolol or saline infusion during cycling followed by stable isotope infusion and muscle biopsies.
Muscle protein synthesis.
Skeletal muscle protein synthesis rates were determined for subsarcolemmal mitochondrial, myofibrillar, and sarcoplasmic enriched fractions, as previously described (36). We used differential centrifugation to separate protein fractions that are not pure fractions but predominantly contain the proteins of interest. Briefly, ∼70 mg of muscle tissue were homogenized and centrifuged at 800 g to pellet a crude myofibrillar fraction. The supernatant was centrifuged at 9,000 g to separate the sarcoplasmic fraction (supernatant) and a mitochondrial fraction (pellet). The mitochondrial pellet was washed using a series of centrifugations, and the final mitochondrial, myofibrillar, and sarcoplasmic pellets were washed with 500 μl of 100% ethanol, centrifuged (1,000 g, 30 s, 4°C), and rinsed with water (repeated twice). Protein pellets were solubilized in 1 N NaOH (50°C, 15 min) and hydrolyzed into free amino acids (6 M HCl, 120°C, 24 h). Free amino acids were added to cation exchange columns and then derivatized to their t-butyldimethylsilyl derivatives, as previously described (12).
Samples were analyzed using gas chromatography-mass spectrometry (GC-MS; 7890A GC with 5975C MS, Agilent Technologies, Santa Clara, CA) in electron impact mode with selected ion monitoring at mass-to-charge ratios (m/z) of 234 (m + 0), 237 (m + 3), and 239 (m + 5), with m + 0 representing the parent ion. Samples were injected in duplicate, and the m + 0 peak area was kept within standardized limits to minimize differences in abundances due to concentration differences. Abundances were adjusted to standard curves that were derivatized from a single set of diluted standards and analyzed before and after each GC-MS analysis. Internal controls were included within each batch of samples to ensure consistency between GC-MS analyses. Tracer enrichments of muscle samples were determined as tracer-to-tracee ratio from a standard curve using the ratio of m + 5 to m + 3 (12). Plasma enrichments were determined from the ratio of m + 5 to m + 0. The fractional synthesis rate (FSR) was determined using the standard precursor product relationship: FSR = ΔEm/Ep, where ΔEm is change in enrichment of muscle proteins and EP is enrichment of the precursor. We adjusted for non-steady-state plasma tracer enrichments by calculating the precursor enrichment as the integral of the plasma phenylalanine enrichment over time (54). We used circulating plasma [2H5]phenylalanine as the precursor, as reported by others (8, 17, 18, 43), which may underestimate the true synthesis rates (see Ref. 44 for review). The low natural abundance of the infused tracer and washout period between separate trials allowed the assumption that background tracer enrichment is zero (45).
Real-time PCR.
Real-time PCR was used to determine changes in mRNA content for PGC-1α and downstream targets (Table 2). Total RNA was extracted from ∼10 mg of skeletal muscle using standard TRIzol methods (Invitrogen, Carlsbad, CA) and reverse-transcribed to cDNA, as previously described (36). Approximately 10 ng of cDNA were amplified using 20-μl reactions with a manufactured master mix (Thermo Fisher, Rockford, IL) in triplicate in clear 96-well plates using TaqMan probes and a real-time PCR system (model 7300, Applied Biosystems, Carlsbad, CA). PCR conditions were as follows: hot start (2 min at 50°C, 15 min at 95°C) followed by 40 cycles of denaturing and annealing (15 s at 95°C, 1 min at 60°C). The relative quantity of each gene target was normalized to a reference gene (β2-microglobulin), and fold changes were determined using the cycle threshold (2−ΔΔCt) method (31).
Table 2.
PCR sequences
| Target | Gene Symbol | Assay ID | Chromosome | Target Exon | Probe Sequence |
|---|---|---|---|---|---|
| PGC-1α | PPARGC1A | Hs00173304_m1 | 4 | 7 | TGGAACTGCAGGCCTAACTCCACCC |
| NRF1 | NRF1 | Hs00602161_m1 | 7 | 8 | TGATGGAGAGGTGGAACAAAATTGG |
| NRF2 | GABPA | Hs01022023_m1 | 21 | 9 | ACTCAGTCGTGCATTAAGATATTAT |
| TFAM | TFAM | Hs01082775_m1 | 10 | 5 | GATTCACCGCAGGAAAAGCTGAAGA |
| COX7a | COX7A1 | Hs00156989_m1 | 19 | 1 | GGCCCTTCGGGTGTCCCAGGCGCTG |
| mtND4 | MT-ND4 | Hs02596876_g1 | MT | CAAACTCCTGAGCCAACAACTTAAT |
PGC-1α, peroxisome proliferator-activated receptor-γ coactivator-1α; NRF1 and NRF2, nuclear respiratory factors 1 and 2; TFAM, transcription factor A, mitochondrial; COX7a, cytochrome c oxidase subunit VIIa; mtND4, mitochondrially encoded NADH dehydrogenase 4.
Statistics.
Heart rate, blood pressure, and V̇o2 were compared between trials using two-way (trial × time) ANOVA with repeated measures. Skeletal muscle FSR was compared using a one-way ANOVA. Changes in mRNA content were determined using two-way (trial × time) ANOVA with repeated measures on the ΔCt values (53) and are expressed as fold increase compared with the 1-h biopsy on the saline control day. Multiple comparisons were performed using Bonferroni's correction. Statistical significance was set at P = 0.05. Power analysis revealed that a sample size of six with standard deviation of 0.06, α at 0.05, and β ∼0.83 is able to detect a difference of 75% between treatments.
RESULTS
Verification of β-AR blockade.
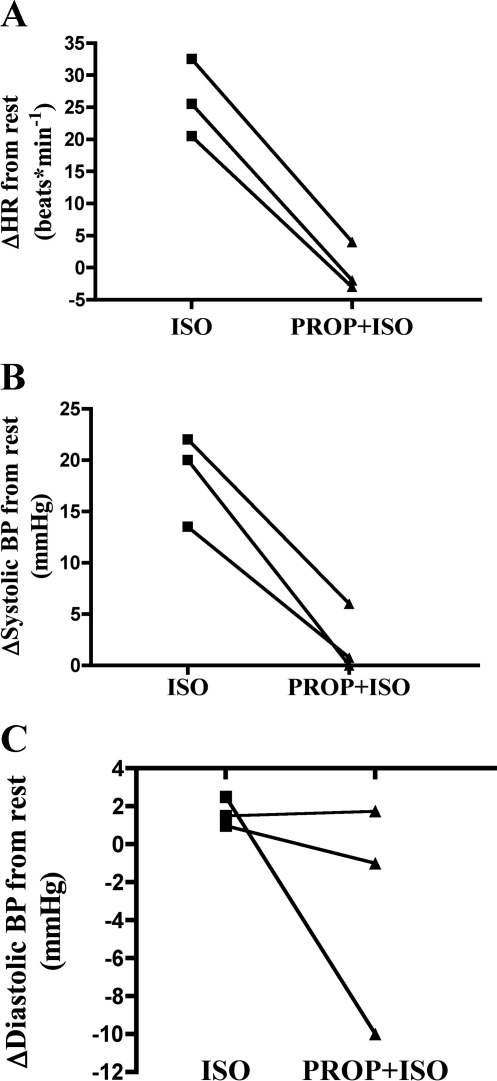
β-AR stimulation with isoproterenol increased heart rate and blood pressure above resting values. Repeating the β-AR stimulation following β-AR blockade (propranolol infusion) and 1 h of cycling had no effect on heart rate or blood pressure, indicating the efficacy of our β-AR blockade procedure (Fig. 2).
Fig. 2.
Efficacy of β-AR blockade protocol demonstrated in 3 subjects by lack of heart rate (A) or systolic (B) or diastolic (C) blood pressure response to β-AR stimulation with isoproterenol. Data are expressed as change from level at rest following isoproterenol infusion or propranolol followed by isoproterenol (Prop + Iso).
Response during exercise.
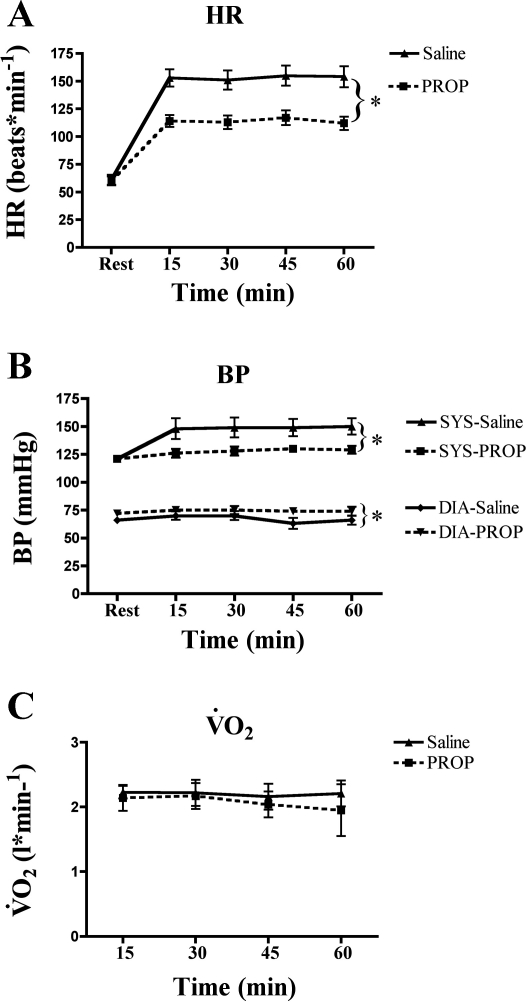
Compared with the control condition (saline administration), exercising heart rate and blood pressure were decreased during β-AR blockade (Fig. 3, A and B). Absolute workload was identical between conditions, resulting in a V̇o2 that was not different between trials (P > 0.05; Fig. 3C). Respiratory exchange ratio was not different between trials (data not shown).
Fig. 3.
Heart rate (A) and systolic (Sys) and diastolic (Dia) blood pressure (B) were lower during exercise with β-AR blockade (propranolol). Absolute workloads were identical, resulting in similar steady-state O2 consumption (V̇o2, C). *P < 0.01.
FSR.
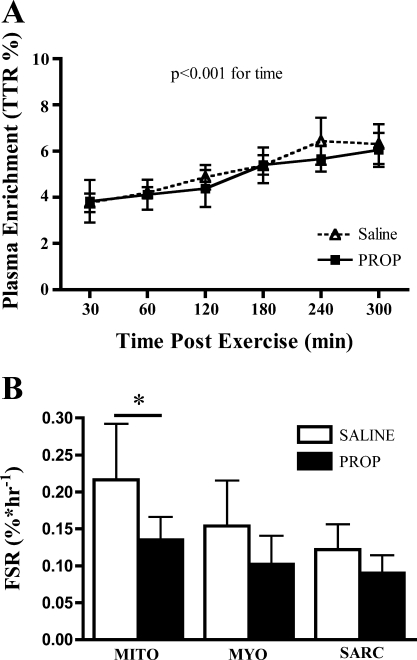
Plasma enrichment of [2H5]phenylalanine was not different between saline and propranolol trials (Fig. 4A). Skeletal muscle enrichments are reported in Table 3. Skeletal muscle FSR (Fig. 4B) of subsarcolemmal mitochondria was decreased with β-AR blockade (0.217 ± 0.076 and 0.135 ± 0.031%/h with saline and propranolol, respectively, P < 0.05). The FSR was not different between trials for myofibrillar (0.154 ± 0.061 and 0.102 ± 0.038%/h with saline and propranolol, respectively, P > 0.05) or sarcoplasmic (0.122 ± 0.034 and 0.0898 ± 0.025%/h with saline and propranolol, respectively, P > 0.05) proteins. The 95% confidence intervals for the difference with propranolol were −0.15 to −0.01%/h for mitochondrial proteins, −0.13 to 0.02%/h for myofibrillar proteins, and −0.10 to 0.04%/h for sarcoplasmic proteins.
Fig. 4.
A: plasma enrichment of [2H5]phenylalanine increased over time (P < 0.001) but was not different between saline and propranolol. B: β-AR blockade (propranolol) during cycling decreased skeletal muscle fractional synthesis rate (FSR) for subsarcolemmal mitochondrial (Mito), but not myofibrillar (Myo) or sarcoplasmic (Sarc), proteins. *P < 0.05.
Table 3.
Muscle enrichments for mitochondrial, myofibrillar, and sarcoplasmic fractions between saline and propranolol trials
| Mito |
Myo |
Sarc |
||||
|---|---|---|---|---|---|---|
| 1 h | 5 h | 1 h | 5 h | 1 h | 5 h | |
| Saline | 0.037 ± 0.01 | 0.054 ± 0.02 | 0.032 ± 0.02 | 0.040 ± 0.01 | 0.023 ± 0.01 | 0.030 ± 0.01 |
| Prop | 0.022 ± 0.01 | 0.032 ± 0.01 | 0.017 ± 0.01 | 0.033 ± 0.02 | 0.012 ± 0.01 | 0.021 ± 0.01 |
Values (means ± SD) are expressed as tracer-to-tracee ratio.
Mito, mitochondrial; Myo, myofibrillar; Sarc, sarcoplasmic.
Real-time PCR.
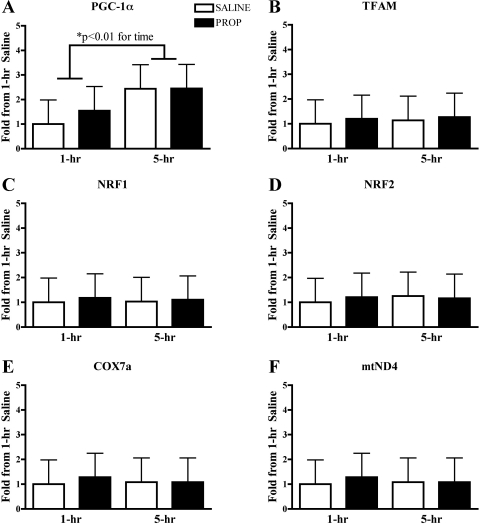
Real-time PCR revealed that PGC-1α mRNA was increased at 5 h compared with 1 h after cycling, with no differences between β-AR blockade and the control condition (P < 0.01 for time; Fig. 5A). Downstream mRNA signals for mitochondrial biogenesis were not different between time points or between conditions (Fig. 5, B–F).
Fig. 5.
A: real-time PCR revealed that peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) mRNA was increased at 5 h following cycling but was not different between saline and β-AR blockade (propranolol) trials. B–F: downstream mRNA signals for mitochondrial biogenesis were not different at 1 and 5 h following cycling between saline and β-AR blockade trials. Data are expressed as fold change compared with 1-h saline. TFAM, transcription factor A, mitochondrial; NRF1 and NRF2, nuclear respiratory factors 1 and 2; COX7a, cytochrome c oxidase subunit VIIa; mtND4, mitochondrially encoded NADH dehydrogenase 4.
DISCUSSION
Propranolol was infused during a 1-h bout of cycling to determine if nonselective β-AR blockade could alter skeletal muscle protein synthesis and mitochondrial biogenesis. Postexercise mitochondrial protein synthesis was greater during the saline than the propranolol trial. PGC-1α mRNA was increased at 5 h compared with 1 h postexercise but was not different between trials. Other downstream mRNA signals for mitochondrial biogenesis were not different between 1 and 5 h postexercise or between trials. These data imply that β-AR signaling is an important physiological determinant of postexercise mitochondrial biogenesis in adult humans.
Verification of β-AR blockade.
Nonselective β-AR blockade was confirmed in three subjects. The ability of isoproterenol to stimulate heart rate and blood pressure was determined before and after cycling with propranolol. The lack of cardiovascular stimulation at 1 h after cycling with propranolol indicates nonselective β-AR blockade during the acute period following cycling, which is when skeletal muscle protein synthesis rates and signals for mitochondrial biogenesis are elevated (14, 33, 51). Our biopsies were collected at 1 and 5 h after propranolol infusion, which is during the 3- to 6-h half-life of propranolol (34). We did not determine if there was complete blockade of all β-ARs on skeletal muscle. However, the demonstration of nonselective β-AR blockade combined with the half-life of propranolol indicates that β-AR signaling was decreased during our measurements of skeletal muscle protein synthesis and mRNA. As expected, the magnitude of increase in heart rate and blood pressure was decreased during cycling with β-AR blockade, suggesting that β-AR signaling was decreased in all subjects. Previous work has shown increased catecholamine signaling during exercise at 60% V̇o2peak (19). Thus our cycling workload was sufficient to increase β-AR signaling, and propranolol was able to blunt β-AR signaling.
Our study was designed as an integrative approach to assess mitochondrial biogenesis during recovery from aerobic exercise. PGC-1α and downstream mRNA content are increased by 2 h and have remained elevated for 8 h following exercise (32, 33). Additionally, skeletal muscle protein synthesis is increased in the hours following exercise and can remain elevated for 72 h (23, 51). We are confident that the timing of muscle sampling was adequate to capture any changes in mRNA content for mitochondrial biogenesis or changes in protein synthesis rates. We assessed mitochondrial biogenesis as changes in mRNA transcript content of select mitochondrial signaling proteins and mitochondrial protein synthesis. Previous studies of β-AR signaling and mitochondrial biogenesis have reported changes in mRNA content of mitochondrial signaling proteins (24, 25); however, such measurements do not take into account posttranscription or translational regulation. Our measurement of mitochondrial protein synthesis represents the cumulative regulation of signals for mitochondrial biogenesis.
FSR.
The synthesis rate of skeletal muscle subsarcolemmal mitochondrial proteins was blunted in the several hours following cycling with β-AR blockade. Our results provide short-term evidence that nonselective β-AR blockade during cycling can impair mitochondrial adaptations and support long-term studies that showed decreased mitochondrial enzyme activity during training with β-blocker drugs (1). A blunted response of mitochondrial protein synthesis to individual training sessions may lead to decreased adaptations over the long term.
The regulation of β-AR signaling on mitochondrial protein synthesis appears to be mediated through β2-ARs. Previous reports showed that selective β2-AR agonists, but not β1-AR or α-AR agonists, increased PGC-1α mRNA at rest and selective β2-AR blockers abolished the postexercise increase of PGC-1α mRNA (25). Additionally, the blunted increase of mitochondrial enzymes during aerobic training was reported during treatment with nonselective β-AR blockers (e.g., atenolol or propranolol), but not selective β1-AR blockers (e.g., metoprolol or atenolol) (1, 16, 46). Others have reported no difference in V̇o2peak adaptations between selective and nonselective β-blocker treatments (42, 52). It is possible that different degrees of β-AR blockade due to various drug doses or binding preferences within selective or nonselective classes can explain variable training adaptations. It is important to note that studies with a greater focus on specific mitochondrial responses (e.g., mitochondrial enzyme activity) appear able to detect impairments with nonselective β-blockers that are not detected with whole body measures (e.g., V̇o2peak). Our approach was to measure acute changes in protein synthesis that may explain long-term differences in mitochondrial adaptations with nonselective β-AR blockade.
Myofibrillar protein synthesis was not different between the propranolol and saline trials. While β-AR antagonists are used to treat cardiovascular disease, β-AR agonists have been used for the treatment of muscle loss, such as with aging or disease (see Ref. 21 for review). In particular, selective β2-AR agonists stimulate skeletal muscle protein gains over several weeks in aging rats (41). We have shown that infusion of isoproterenol (a nonselective β-AR agonist) did not acutely stimulate skeletal muscle protein synthesis in humans (36). Recent data in mice showed that chronic treatment with a selective β2-AR agonist increased skeletal muscle protein synthesis of myofibrillar, mitochondrial, and sarcoplasmic fractions at 7 days, but not acutely or at 28 days of treatment (20). It is possible that our acute treatment with propranolol would result in significant decreases in myofibrillar and sarcoplasmic proteins if carried out for an extended period of time.
The primary outcome of our current study was mitochondrial protein synthesis, for which we demonstrate a difference in FSR. However, we were potentially underpowered to detect differences in the secondary outcomes of myofibrillar (−33%) and sarcoplasmic (−26%) FSR. The 95% confidence intervals for the difference between treatments are weighted toward a decrease in protein synthesis with propranolol treatment. It is possible that including additional subjects would reveal decreased protein synthesis in myofibrillar and sarcoplasmic fractions. Such a global regulation of protein synthesis rates is consistent with the increase in protein synthesis following selective β2-AR agonist treatment shown by Koopman et al. (20) and could be mediated through β-adrenergic regulation of the mammalian target of rapamycin pathway (11).
Our values of skeletal muscle FSR were determined following exercise in the fed state. The fractional synthesis of skeletal muscle mitochondria is higher than that of myofibrillar proteins at rest (36, 38) or following exercise (13) and is increased with intravenous amino acid infusion (6). Our measured mitochondrial protein synthesis rates are slightly higher than those reported by others because of the experimental conditions (i.e., postexercise and feeding) and use of a zero-background labeling to calculate rates (45). The high rate of mitochondrial protein synthesis reported here and by others indicates a high rate of mitochondrial protein turnover that can be stimulated by exercise (51) and nutrition (6).
We included postexercise nutritional supplementation to avoid a negative energy balance following exercise and to increase external validity. Because the subjects reported to the laboratory after an overnight fast and given the length of the protocol, the subjects would have been fasted for ∼18 h. It is known that negative energy balance decreases nitrogen balance (48). Furthermore, a prolonged fast and exercise would likely result in AMP-activated protein kinase activation and, therefore, PGC-1α activation. Even though our study did not include an exercise + fasting group, our results indicate that β-blockade can impair mitochondrial protein synthesis in the fed state following exercise.
Exercise V̇o2.
The 1-h cycling workload was adjusted to maintain 60% V̇o2peak during the first trial (propranolol), and the same absolute workload was repeated for the second trial (saline). The respiratory exchange ratios were not different between trials, indicating that substrate utilization was similar during the trials. Others have reported similar indirect calorimetry results during cycling exercise following propranolol treatment (50). Postexercise signals for mitochondrial biogenesis can be influenced by dietary habits that alter glycogen content (32) but are not related to circulating glucose or free fatty acid availability (40). Thus the potential influence of β-AR signaling on mitochondrial biogenesis is likely to be within skeletal muscle and not due to changes in circulating substrate metabolism during exercise.
PCR.
Contrary to our hypothesis and previous studies with mice (25), mRNA content of markers of mitochondrial biogenesis was not different between exercise with propranolol and with saline. PGC-1α mRNA was increased in both trials at 5 h compared with 1 h postexercise, which is similar to previous reports of human exercise trials (33). To minimize subject burden, we did not collect a muscle sample before exercise and cannot determine the change in mRNA content from resting values. However, our biopsies at 1 and 5 h after exercise provide two times points to compare between propranolol and saline trials and are within the time frame that PGC-1α and downstream mRNA are elevated (32).
Exercise-induced changes of PGC-1α mRNA content are dependent on exercise intensity (47) and appear to be regulated by the relative intensity, rather than the absolute intensity (29). Despite an acute blunting of heart rate response and increased perceived effort with propranolol, our exercise bouts were performed at the same relative intensities. Support for the same relative exercise intensity was that subjects were tested within 4 wk with no changes in exercise patterns in the intervening period, and equal respiratory exchange ratio, which scales to relative exercise intensity (7), between trials.
Perspectives and Significance
We previously reported that acute nonselective β-AR stimulation during rest did not increase mitochondrial protein synthesis or mRNA markers of mitochondrial biogenesis. Our current investigation indicates that β-AR signaling can modulate exercise-induced mitochondrial protein synthesis. The postexercise recovery period is a critical time for skeletal muscle remodeling, and decreasing sympathetic signals to skeletal muscle may attenuate adaptations. Further work should consider if chronic intake of β-AR blockers would decrease mitochondrial protein synthesis and contribute to impaired mitochondrial adaptations during exercise training.
We conclude that nonselective β-AR blockade can blunt the postexercise increase in mitochondrial protein synthesis rates. Nonselective β-AR blockade did not alter the mRNA content for signals of mitochondrial biogenesis, suggesting that the impaired protein synthesis response is independent of changes to mRNA transcripts. Our results indicate that nonselective β-AR antagonists can impair mitochondrial adaptations to acute aerobic exercise and may lead to decreased training adaptations.
GRANTS
Funding was provided in part by the Gatorade Sport Science Institute (to M. M. Robinson). B. F. Miller is supported by National Institute on Aging Grant 1-K01-AG-031829-01A1.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank the subjects for their enthusiasm to complete the exercise protocols. We also thank Gary Luckasen and Dennis Larson for medical oversight and Joshua Drake for study assistance.
REFERENCES
- 1. Ades PA, Gunther PG, Meyer WL, Gibson TC, Maddalena J, Orfeo T. Cardiac and skeletal muscle adaptations to training in systemic hypertension and effect of β-blockade (metoprolol or propranolol). Am J Cardiol 66: 591–596, 1990 [DOI] [PubMed] [Google Scholar]
- 2. Bell C, Day DS, Jones PP, Christou DD, Petitt DS, Osterberg K, Melby CL, Seals DR. High energy flux mediates the tonically augmented β-adrenergic support of resting metabolic rate in habitually exercising older adults. J Clin Endocrinol Metab 89: 3573–3578, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Bell C, Petitt DS, Jones PP, Seals DR. Influence of adiposity on tonic sympathetic support of resting metabolism in healthy adults. Int J Obes Relat Metab Disord 27: 1315–1318, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Bell C, Seals DR, Monroe MB, Day DS, Shapiro LF, Johnson DG, Jones PP. Tonic sympathetic support of metabolic rate is attenuated with age, sedentary lifestyle, and female sex in healthy adults. J Clin Endocrinol Metab 86: 4440–4444, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Bell C, Stob NR, Seals DR. Thermogenic responsiveness to β-adrenergic stimulation is augmented in exercising versus sedentary adults: role of oxidative stress. J Physiol 570: 629–635, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bohe J, Low A, Wolfe RR, Rennie MJ. Human muscle protein synthesis is modulated by extracellular, not intramuscular, amino acid availability: a dose-response study. J Physiol 552: 315–324, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brooks GA, Mercier J. Balance of carbohydrate and lipid utilization during exercise: the “crossover” concept. J Appl Physiol 76: 2253–2261, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Caso G, Garlick PJ, Ballou LM, Vosswinkel JA, Gelato MC, McNurlan MA. The increase in human muscle protein synthesis induced by food intake is similar when assessed with the constant infusion and flooding techniques. J Nutr 136: 1504–1510, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Dykens JA, Will Y. The significance of mitochondrial toxicity testing in drug development. Drug Discov Today 12: 777–785, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Gambelli S, Dotti MT, Malandrini A, Mondelli M, Stromillo ML, Gaudiano C, Federico A. Mitochondrial alterations in muscle biopsies of patients on statin therapy. J Submicrosc Cytol Pathol 36: 85–89, 2004 [PubMed] [Google Scholar]
- 11. Gelinas JN, Banko JL, Hou L, Sonenberg N, Weeber EJ, Klann E, Nguyen PV. ERK and mTOR signaling couple β-adrenergic receptors to translation initiation machinery to gate induction of protein synthesis-dependent long-term potentiation. J Biol Chem 282: 27527–27535, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Harber MP, Konopka AR, Jemiolo B, Trappe SW, Trappe TA, Reidy PT. Muscle protein synthesis and gene expression during recovery from aerobic exercise in the fasted and fed states. Am J Physiol Regul Integr Comp Physiol 299: R1254–R1262, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Hartman JW, Tang JE, Wilkinson SB, Tarnopolsky MA, Lawrence RL, Fullerton AV, Phillips SM. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am J Clin Nutr 86: 373–381, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Haus JM, Miller BF, Carroll CC, Weinheimer EM, Trappe TA. The effect of strenuous aerobic exercise on skeletal muscle myofibrillar proteolysis in humans. Scand J Med Sci Sports 17: 260–266, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem 242: 2278–2282, 1967 [PubMed] [Google Scholar]
- 16. Ji LL, Lennon DL, Kochan RG, Nagle FJ, Lardy HA. Enzymatic adaptation to physical training under β-blockade in the rat. Evidence of a β2-adrenergic mechanism in skeletal muscle. J Clin Invest 78: 771–778, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Katsanos CS, Aarsland A, Cree MG, Wolfe RR. Muscle protein synthesis and balance responsiveness to essential amino acids ingestion in the presence of elevated plasma free fatty acid concentrations. J Clin Endocrinol Metab 94: 2984–2990, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab 291: E381–E387, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Kjaer M, Farrell PA, Christensen NJ, Galbo H. Increased epinephrine response and inaccurate glucoregulation in exercising athletes. J Appl Physiol 61: 1693–1700, 1986 [DOI] [PubMed] [Google Scholar]
- 20. Koopman R, Gehrig SM, Leger B, Trieu J, Walrand S, Murphy KT, Lynch GS. Cellular mechanisms underlying temporal changes in skeletal muscle protein synthesis and breakdown during chronic β-adrenoceptor stimulation in mice. J Physiol 588: 4811–4823, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lynch GS, Ryall JG. Role of β-adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiol Rev 88: 729–767, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol 61: 534–540, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj JA, Smith K, Rennie MJ. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol 567: 1021–1033, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miura S, Kai Y, Kamei Y, Ezaki O. Isoform-specific increases in murine skeletal muscle peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) mRNA in response to β2-adrenergic receptor activation and exercise. Endocrinology 149: 4527–4533, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Miura S, Kawanaka K, Kai Y, Tamura M, Goto M, Shiuchi T, Minokoshi Y, Ezaki O. An increase in murine skeletal muscle peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) mRNA in response to exercise is mediated by β-adrenergic receptor activation. Endocrinology 148: 3441–3448, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Monroe MB, Seals DR, Shapiro LF, Bell C, Johnson D, Parker Jones P. Direct evidence for tonic sympathetic support of resting metabolic rate in healthy adult humans. Am J Physiol Endocrinol Metab 280: E740–E744, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr 89: 161–168, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Mootha VK, Bunkenborg J, Olsen JV, Hjerrild M, Wisniewski JR, Stahl E, Bolouri MS, Ray HN, Sihag S, Kamal M, Patterson N, Lander ES, Mann M. Integrated analysis of protein composition, tissue diversity, and gene regulation in mouse mitochondria. Cell 115: 629–640, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Nordsborg NB, Lundby C, Leick L, Pilegaard H. Relative workload determines exercise-induced increases in PGC-1α mRNA. Med Sci Sports Exerc 42: 1477–1484, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300: 1140–1142, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pilegaard H, Osada T, Andersen LT, Helge JW, Saltin B, Neufer PD. Substrate availability and transcriptional regulation of metabolic genes in human skeletal muscle during recovery from exercise. Metabolism 54: 1048–1055, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1α gene in human skeletal muscle. J Physiol 546: 851–858, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reiter MJ. Cardiovascular drug class specificity: β-blockers. Prog Cardiovasc Dis 47: 11–33, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Robinson MM, Hamilton KL, Miller BF. The interactions of some commonly consumed drugs with mitochondrial adaptations to exercise. J Appl Physiol 107: 8–16, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Robinson MM, Richards JC, Hickey MS, Moore DR, Phillips SM, Bell C, Miller BF. Acute β-adrenergic stimulation does not alter mitochondrial protein synthesis or markers of mitochondrial biogenesis in adult men. Am J Physiol Regul Integr Comp Physiol 298: R25–R33, 2010 [DOI] [PubMed] [Google Scholar]
- 37. Romanello V, Guadagnin E, Gomes L, Roder I, Sandri C, Petersen Y, Milan G, Masiero E, Del Piccolo P, Foretz M, Scorrano L, Rudolf R, Sandri M. Mitochondrial fission and remodelling contributes to muscle atrophy. EMBO J 29: 1774–1785, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rooyackers OE, Adey DB, Ades PA, Nair KS. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci USA 93: 15364–15369, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rosendorff C, Black HR, Cannon CP, Gersh BJ, Gore J, Izzo JL, Jr, Kaplan NM, O'Connor CM, O'Gara PT, Oparil S. Treatment of hypertension in the prevention and management of ischemic heart disease: a scientific statement from the American Heart Association Council for High Blood Pressure Research and the Councils on Clinical Cardiology and Epidemiology and Prevention. Circulation 115: 2761–2788, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Russell AP, Hesselink MK, Lo SK, Schrauwen P. Regulation of metabolic transcriptional co-activators and transcription factors with acute exercise. FASEB J 19: 986–988, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Ryall JG, Plant DR, Gregorevic P, Sillence MN, Lynch GS. β2-Agonist administration reverses muscle wasting and improves muscle function in aged rats. J Physiol 555: 175–188, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Savin WM, Gordon EP, Kaplan SM, Hewitt BF, Harrison DC, Haskell WL. Exercise training during long-term β-blockade treatment in healthy subjects. Am J Cardiol 55: 101D–109D, 1985 [DOI] [PubMed] [Google Scholar]
- 43. Smith GI, Atherton P, Reeds DN, Mohammed BS, Jaffery H, Rankin D, Rennie MJ, Mittendorfer B. No major sex differences in muscle protein synthesis rates in the postabsorptive state and during hyperinsulinemia-hyperaminoacidemia in middle-aged adults. J Appl Physiol 107: 1308–1315, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Smith GI, Patterson BW, Mittendorfer B. Human muscle protein turnover—why is it so variable? J Appl Physiol 110: 480–491, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Smith GI, Villareal DT, Lambert CP, Reeds DN, Mohammed BS, Mittendorfer B. Timing of the initial muscle biopsy does not affect the measured muscle protein fractional synthesis rate during basal, postabsorptive conditions. J Appl Physiol 108: 363–368, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Svedenhag J, Henriksson J, Juhlin-Dannfelt A. β-Adrenergic blockade and training in human subjects: effects on muscle metabolic capacity. Am J Physiol Endocrinol Metab 247: E305–E311, 1984 [DOI] [PubMed] [Google Scholar]
- 47. Tadaishi M, Miura S, Kai Y, Kawasaki E, Koshinaka K, Kawanaka K, Nagata J, Oishi Y, Ezaki O. Effect of exercise intensity and AICAR on isoform-specific expressions of murine skeletal muscle PGC-1α mRNA: a role of β-adrenergic receptor activation. Am J Physiol Endocrinol Metab 300: E341–E349, 2011 [DOI] [PubMed] [Google Scholar]
- 48. Todd KS, Butterfield GE, Calloway DH. Nitrogen balance in men with adequate and deficient energy intake at three levels of work. J Nutr 114: 2107–2118, 1984 [DOI] [PubMed] [Google Scholar]
- 49. Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly YM, Gidlof S, Oldfors A, Wibom R, Tornell J, Jacobs HT, Larsson NG. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429: 417–423, 2004 [DOI] [PubMed] [Google Scholar]
- 50. van Baak MA, de Haan A, Saris WH, van Kordelaar E, Kuipers H, van der Vusse GJ. β-Adrenoceptor blockade and skeletal muscle energy metabolism during endurance exercise. J Appl Physiol 78: 307–313, 1995 [DOI] [PubMed] [Google Scholar]
- 51. Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA, Rennie MJ. Differential effects of resistance and endurance exercise in the fed state on signaling molecule phosphorylation and protein synthesis in human muscle. J Physiol 586: 3701–3717, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wolfel EE, Hiatt WR, Brammell HL, Carry MR, Ringel SP, Travis V, Horwitz LD. Effects of selective and nonselective β-adrenergic blockade on mechanisms of exercise conditioning. Circulation 74: 664–674, 1986 [DOI] [PubMed] [Google Scholar]
- 53. Yuan JS, Reed A, Chen F, Stewart CN., Jr Statistical analysis of real-time PCR data. BMC Bioinformatics 7: 85, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zilversmit DB. The design and analysis of isotope experiments. Am J Med 29: 832–848, 1960 [DOI] [PubMed] [Google Scholar]