Abstract
Thermotolerance and heat acclimation are key adaptation processes that have been hitherto viewed as separate phenomena. Here, we provide evidence that these processes may share a common basis, as both may potentially be governed by the heat shock response. We evaluated the effects of a heat shock response-inhibitor (quercetin; 2,000 mg/day) on established markers of thermotolerance [gastrointestinal barrier permeability, plasma TNF-α, IL-6, and IL-10 concentrations, and leukocyte heat shock protein 70 (HSP70) content]. Heat acclimation reduced body temperatures, heart rate, and physiological strain during exercise/heat stress) in male subjects (n = 8) completing a 7-day heat acclimation protocol. These same subjects completed an identical protocol under placebo supplementation (placebo). Gastrointestinal barrier permeability and TNF-α were increased on the 1st day of exercise/heat stress in quercetin; no differences in these variables were reported in placebo. Exercise HSP70 responses were increased, and plasma cytokines (IL-6, IL-10) were decreased on the 7th day of heat acclimation in placebo; with concomitant reductions in exercise body temperatures, heart rate, and physiological strain. In contrast, gastrointestinal barrier permeability remained elevated, HSP70 was not increased, and IL-6, IL-10, and exercise body temperatures were not reduced on the 7th day of heat acclimation in quercetin. While exercise heart rate and physiological strain were reduced in quercetin, this occurred later in exercise than with placebo. Consistent with the concept that thermotolerance and heat acclimation are related through the heat shock response, repeated exercise/heat stress increases cytoprotective HSP70 and reduces circulating cytokines, contributing to reductions in cellular and systemic markers of heat strain. Exercising under a heat shock response-inhibitor prevents both cellular and systemic heat adaptations.
Keywords: heat shock response, cytokine, gastrointestinal barrier permeability
prior exposure to a single preconditioning heat stress allows cells (35), tissues (48), and animals (69) to survive an otherwise lethal heat shock. This protection is transient and synchronous with the heat shock response. While the heat shock response is best known for its role in protein management (44), it has also been shown to enhance epithelial barrier resistance to heat exposure (13–15) and reduce inflammatory cytokine production in heat-stressed animals through inhibition of NF-κB (12). Together, these improvements are thought to limit stress-mediated inflammation by preventing endotoxin translocation into the portal circulation and limiting the systemic inflammatory response.
Repeated exposure to exercise, to heat stress, or to exercise-heat stress improves the capacity of animals and humans to dissipate heat (38, 61) and reduces incidence of exertional heat illness (5, 27). This protection, termed heat acclimation, is long lasting (weeks to months with continued exposure) and results from improvements in sweat sensitivity and rate, plasma volume expansion, and enhanced vasodilatory properties of the cutaneous vasculature (50). Heat acclimation also invokes the heat shock response (38, 43, 71). In fact, evidence across multiple animal phyla, including ants (21), lizards (67), hydra (2), and other ocean dwellers (4, 9), suggests the heat shock response is integral to development of natural heat acclimatization. However, because the heat shock response is considered to be an adaptation that primarily confers thermotolerance, until recently (43, 71), its role in human heat acclimation has largely been overlooked.
More than 15 years ago, we proposed that the cellular mechanisms that convey thermotolerance in animals and humans might also contribute to the systemic adaptations associated with the heat-acclimated phenotype (49). At that time, our ability to test this hypothesis was limited, as we were unable to obtain an agent capable of attenuating the heat shock response. However, experiments performed in our laboratory and others over the past decade have shown the flavanol quercetin to be a potent suppressor of the heat shock response. This inhibition has been shown at the level of phophorylation (16) and trimerization (34) in the cytosol, as well as nuclear entry (30), promoter binding (26), hyperphosphorylation (51), mRNA expression (25, 15), and protein accumulation (12–15). More specifically, quercetin has been reported to 1) block acquisition of cellular thermotolerance (26); 2) increase apoptotic cell death (29); 3) potentiate heat-induced permeability in naive monolayers (14); 4) block the expected reductions in heat-induced permeability in preconditioned monolayers (13, 14); 5) reduce the expected reductions in LPS-induced cytokine release and mortality in preconditioned rats (51); and 6) reverse the reductions in inflammation and glandular disruption induced by heat shock protein 70 (HSP70) overexpression in an atrophic-gastritis mouse model (36). It must be noted that while quercetin is a potent heat shock factor-1 (HSF-1) inhibitor, it also exerts antioxidant (63, 70), anti-inflammatory (65), and cell-stabilizing effects (54) in cell culture and animal models. Cumulatively, these actions may reduce cellular stress, which could potentially reduce signaling for the heat shock response. Thus, ingestion of quercetin during repeated exercise and heat exposures may attenuate the heat shock response, reduce thermotolerance, and allow us to test for an association between the processes of thermotolerance and heat acclimation.
In the present study, we sought to determine whether dietary quercetin supplementation would suppress the heat shock response in a human population. Our purpose was to obtain data to support the role of the heat shock response in eliciting the cellular and systemic adaptations that result in heat acclimation in an exercising human population. To parse out the role of the heat shock response in these adaptations, subjects completed a standardized heat acclimation protocol twice; once they exercised under normal physiological conditions (placebo), while another time, they exercised under the influence of quercetin. Gastrointestinal barrier, cytokine, and HSP70 responses to both single and repeated bouts of exercise/heat stress were measured in these subjects to determine the role of the heat shock response in cellular adaptations. Body temperature, heart rate, and sweat responses during matched exercise bouts performed before and after heat acclimation were measured to determine the role of the heat shock response in the systemic adaptations that convey the heat-acclimated phenotype.
MATERIALS AND METHODS
Subjects
Eight men [(means ± SE), age: 28 (± 1.6) yr, body mass: 77.0 (± 3.5) kg, height: 178.4 (± 2.5) cm, body fat: 5.9 (± 0.7) %, maximal oxygen uptake 55.6 (± 2.3) ml·kg−1·min−1] participated in this study. Participants were healthy and physically active, and they did not disclose history of heat illness or gastrointestinal barrier dysfunction. Individual subjects completed 7 days of heat acclimation exercise in either quercetin- or placebo-supplemented conditions, allowed 95 (± 9) days for condition washout, then repeated heat acclimation in the opposite condition. Condition order was counterbalanced, and supplementation was double-blind. Data were collected during winter and spring months (October–March) to minimize effects of natural heat acclimatization. Data were collected in the morning hours to minimize effects of diurnal variations in cardiovascular, thermoregulatory, heat shock protein, and cytokine data. The study was approved by the ethics committee of the University of New Mexico, Albuquerque, NM. The study design was registered at ClinicalTrials.gov registration number NCT01168739. All study participants gave written informed consent prior to study participation. Experiments were conducted according to the principles expressed in the Declaration of Helsinki.
Experimental Design
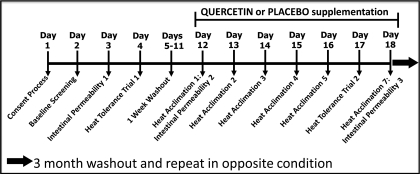
Subjects began the study by providing baseline measures of fitness [maximal oxygen consumption (V̇o2max) and body fat]. They next completed a baseline test of gastrointestinal barrier permeability, in nonexercise/heat stress conditions. On the following day, subjects performed a standardized heat tolerance test to provide baseline measurements of their capacity to tolerate exercise heat stress. After a 7-day washout, subjects began the heat acclimation protocol, which was performed under the influence of either 2 g/day quercetin or placebo (powdered food coloring) supplementation. Additional measures of gastrointestinal barrier permeability, cytokines, and HSP70 were performed on the 1st and 7th days of heat acclimation exercise, allowing researchers to examine subjects' acute and acquired thermotolerance responses. A second heat tolerance test was performed on the 6th day of heat acclimation exercise, allowing researchers to examine subjects' ability to acclimate to repeated exercise/heat exposure. Figure 1 provides a schematic of the study design. These measures are described in greater detail in the following sections.
Fig. 1.
Study protocol schematic.
Baseline Measures of Fitness
Each subject performed a continuous graded treadmill test in a temperate room (22°C to 24°C, 30% to 40% relative humidity) to determine V̇o2max through open circuit spirometry. V̇o2max was defined as the highest 30-s value when two of the following criteria were met 1) a plateau (change in V̇o2 < 150 ml/min) with increase in workload, 2) a maximal respiratory exchange ratio greater than 1.1, and 3) heart rate (HR) greater than 95% of the age-predicted maximum. Percent body fat was calculated from the average of three skinfold sites (28).
Gastrointestinal Barrier Permeability
Following an overnight fast, subjects ingested 10 g lactulose (Kristalose, Cumberland Pharmaceuticals) dissolved in 50 ml dH2O. Subjects collected their urine into sterile containers for 8 h postingestion, from which 10 ml was aliquoted and stored at −80°C for subsequent analysis of urinary lactulose excretion. This procedure was completed three times in each study condition. The first was always performed at rest, in nonexercise heat stress conditions, and served as a baseline from which the second and third permeability tests were compared. The second and third permeability tests were performed on the first and last days of heat acclimation exercise, respectively. On these days subjects consumed the lactulose solution 2 h prior to exercise onset. This provided a 1-h window for absorption, which was followed by ingestion of either quercetin or placebo supplementation. Quercetin was consumed 1 h prior to exercise onset to ensure maximal plasma concentrations (41). The 8-h urine collection on these days represented a 120-min preexercise period, a 110-min exercise-rest-exercise period, and a 250-min postexercise period.
Study Diet and Supplementation Procedures
Quercetin is a dietary polyphenol found in high concentrations in apples, onions, and leafy green vegetables (41). Although American consumption is typically reported in the range of 25–30 mg/day (41), we were concerned with the possibility of overconsumption of dietary quercetin by individual subjects becoming a confounding variable. To minimize the likelihood that this would occur, registered dieticians constructed standardized diets for each subject and provided subjects with all food and beverages consumed during each study condition. The maximal daily intake of quercetin from dietary sources was set at 10 mg/day. As subjects ingested 2 g (2,000 mg) dietary quercetin supplementation each day in the quercetin-supplemented condition, this represents a 200-fold increase over possible dietary inclusion. Subjects also completed daily dietary recalls and returned all food containers and uneaten foodstuffs to study dieticians for weigh-back. Total energy intake, macronutrient ratios, and quercetin content of subject meals in both study conditions are presented in Table 1.
Table 1.
Study diet
| Condition | Intake, kcal/day | CHO, g/day | FAT, g/day | PRO, g/day | Q, g/day |
|---|---|---|---|---|---|
| Placebo | 3713.5 ± 44.3 | 466.5 ± 7.8 | 135.6 ± 1.7 | 174.3 ± 2.0 | 7.6 ± 0.2 |
| Quercetin | 3687.6 ± 37.7 | 458.8 ± 6.2 | 135.9 ± 1.6 | 175.4 ± 2.1 | 7.6 ± 0.2 |
Data are expressed as means ± SE.
Supplementation was accomplished by combining 1 g of quercetin powder (QU995; Quercegen Pharmaceuticals) or powdered food coloring with two servings of an orange breakfast beverage (Our Family, Nash Finch) and 16 oz of distilled water. This method of quercetin or placebo administration is similar to that performed in previous human experiments (42). The dose of quercetin used in this experiment (∼30 mg·kg−1·day−1) was chosen on the basis of quercetin's bioavailability and a similar study in which subjects exercised in the heat (7). Supplementation was administered in combination with each subjects' morning and evening meals. The macronutrient ratios of these meals were set at 30% fat, 30% carbohydrate, and 40% protein to increase quercetin bioavailability (6, 37).
Standardized Heat Tolerance Tests
Subjects began each heat tolerance test by providing a urine sample for hydration assessment via urine osmolality. They next inserted a calibrated rectal thermistor (YSI Precision 4400 Series; Yellow Springs, Yellow Springs, CO) 12 cm into the rectum. Following insertion, subjects provided a nude body mass to 0.1 kg, then donned athletic shorts, socks, and shoes and entered the environmental chamber. Subjects were next fitted with a telemetric heart rate (HR) transmitter strap and watch (S810i series; Polar). This was followed by fitting of uncovered skin thermistors (Grant Instruments) to the upper arm, upper thigh, chest, and calf with elastic straps. This equipment allowed for continuous monitoring of heart rate, core, and skin temperatures during exercise.
Each subject completed 45 min of exercise at a workload corresponding to 50% of his measured V̇o2max. Subjects drank ad libitum during exercise. After terminating exercise, subjects immediately left the environmental chamber, toweled dry, then provided a postexercise nude body mass for calculation of sweat losses and urine sample for hydration assessment. Whole body responses to exercise in the heat [core temperature, mean skin temperature [calculated as T̄sk = 0.3(Tchest) + 0.3(Tarm) + 0.2(Tthigh) + 0.2(Tcalf) (57)], mean body temperature [calculated as (T̄b) = 0.8(Tes) + 0.2(T̄sk)] (31), heart rate, physiological strain calculated as PSI = 5(cTes − iTes)·(39.5 − iTes)−1 + 5(cHR − iHR)·(180 − iHR)−1 (46)], plasma volume, and sweat rate were calculated, where c is current and i is initial. These procedures have been described previously (33, 71). Great care was taken to minimize the potential influence of extraneous variables (ambient environment, exercise characteristics, and subject hydration status) on experimental design (Table 2).
Table 2.
Equality of heat tolerance tests
| Urine Osmolality, mOsm/kg |
|||||||
|---|---|---|---|---|---|---|---|
| Dry Bulb Temperature, °C Postexercise | Relative Humidity, % | Oxygen Uptake, ml·kg−1·min−1 | Exercise Workload, m/s | H2O Intake, liters | Preexercise | Postexercise | |
| HT1 | 47.0 ± 0.4 | 19.7 ± 1.3 | 28.2 ± 1.0 | 2.11 ± 0.15 | 1.0 ± 0.13 | 487 ± 71 | 392 ± 87 |
| HT2 | 46.8 ± 0.4 | 21.0 ± 1.6 | 27.3 ± 1.5 | 2.11 ± 0.15 | 0.88 ± 0.12 | 440 ± 68 | 482 ± 89 |
| HT1 | 46.2 ± 0.4 | 22.3 ± 1.3 | 28.4 ± 1.3 | 2.11 ± 0.15 | 0.94 ± 0.11 | 475 ± 81 | 436 ± 85 |
| HT2 | 46.0 ± 0.4 | 21.2 ± 1.3 | 27.6 ± 1.6 | 2.11 ± 0.15 | 0.88 ± 0.23 | 511 ± 86 | 560 ± 77 |
Data are expressed as means ± SE. There were no differences (P > 0.05) in the ambient environment, subject exercise responses, or hydration status between the 1st (HT1) and 2nd (HT2) heat tolerance tests in either placebo-supplemented (in bold) or quercetin-supplemented (in roman) conditions.
Heat Acclimation Protocol
Subjects consumed either quercetin or placebo 1 h prior to the onset of each day's heat acclimation exercise. On each occasion, subjects exercised for 50 min in an environmental chamber (set at 46.5°C, 20% RH) to increase their core temperature to ≥39°C. They next rested for 10 min in the environmental chamber. This was followed immediately by performance of another 50-min exercise bout. This method of controlled hyperthermia allowed subjects to maintain core temperature above 39°C for the entire second 50-min exercise bout, providing for a greater and more sustained increase in core temperature over traditional protocols (38). Workload (speed/% grade) was recorded every 5 min, and oxygen consumption was recorded every 15 min during exercise. Additional measures of oxygen consumption were taken 5 min after each workload transition. Exercise termination criteria included 1) completing the full 100 min of exercise, 2) core temperature ≥39.5°C, 3) HR ≥ 98% of HRmax, or 4) subject request. Preparatory and exercise procedures were otherwise identical to those described previously for the heat tolerance tests. Additional measures of gastrointestinal barrier permeability and blood samples for thermotolerance variables were taken on days 1 and 7 of heat acclimation. Equality of mean core temperature (2nd 50-min exercise bout), peak core temperature, and mean exercise workloads are depicted in Table 3. Blood sampling and assay techniques are described in further detail in the following sections.
Table 3.
Equality of stress exposure
| Mean Core Temperature, °C | Peak Core Temperature, °C | Mean Exercise Workload, m/s | |
|---|---|---|---|
| Day 1 | 39.02 ± 0.04 | 39.33 ± 0.12 | 1.75 ± 0.06 |
| Day 7 | 39.02 ± 0.04 | 39.35 ± 0.09 | 1.80 ± 0.06 |
| Day 1 | 38.93 ± 0.04 | 39.28 ± 0.14 | 1.77 ± 0.07 |
| Day 7 | 39.12 ± 0.03 | 39.42 ± 0.06 | 1.85 ± 0.07 |
Data are expressed as means ± SE. Mean and peak core temperatures, as well as mean exercise workloads, are depicted for the 1st and 7th days of heat acclimation in placebo-supplemented (in bold) and quercetin-supplemented (in roman) conditions.
Blood Sampling Procedure and Plasma Volume Expansion
Posture-controlled blood samples were collected, without stasis, from each subject before (pre), after (post), 2 h post (2 post), and 4 h post (4 post) exercise on the 1st and 7th days of heat acclimation exercise. These samples were used to assess endotoxin, cytokine, and HSP70 responses to exercise. Additional preexercise posture-controlled blood samples were collected prior to each heat tolerance test. These samples were used to assess changes in hematocrit and hemoglobin resulting from heat acclimation. Plasma volume expansion was calculated with a standardized formula (10).
Endotoxin
Plasma endotoxin was assessed with a limulus amebocyte lysate chromogenic endpoint assay from Cell Sciences (HIT302; Canton, MA) sensitive to 1.4 pg/ml. Samples were diluted 1:3 in endotoxin-free water and heated at 75°C for 10 min prior to assay. All samples and standards were measured in duplicate. Endotoxin concentration was taken as the average of sample absorbance after the control background had been removed.
Inflammatory/Anti-Inflammatory Cytokines
Serum TNF-α was assessed with a solid-phase chemiluminescent immunometric assay (Immulite 1000 TNF-α, Siemens Medical Solutions Diagnostics) sensitive to 1.7 pg/ml. Serum IL-6 was assessed with an ELISA (Quantikine HS, R&D Systems) sensitive to 0.039 pg/ml. Plasma IL-10 was assessed with an EIA (Titerzyme EIA, Assay Designs) sensitive to 3.75 pg/ml. All cytokines and standards were measured in duplicate, and all assays were performed according to the manufacturer's instructions.
HSP70
Peripheral blood mononuclear cell (PBMCs) were isolated from whole blood and analyzed for HSP70 protein content, as previously described (75). Minor modifications to this protocol included 1) the membrane was cut longitudinally after blocking; 2) primary monoclonal (SPA-812, Assay Designs) and secondary polyclonal (81–6120, Invitrogen) antibodies were applied to the upper half of the membrane for HSP70 detection; 3) the lower half of the membrane was treated with primary monoclonal (A5441, Sigma) and secondary polyclonal (61–0120, Invitrogen) antibodies for β-actin detection; and 4) HSP70 was quantified relative to β-actin to control for differences in gel loading.
Urinary Lactulose Excretion
Lactulose was quantified with an EIA (K-FRUGL, Megazyme), with some deviations from the manufacturer's instructions. The supplied glucose/fructose (0.2 mg/ml) standard was serially diluted 1:2. Fifty-five microliters of blank, standard, or urine were added to 96-well microtiter plates, followed by 55 μl of TAE, 10 μl of β-galactosidase, 10 μl imidizole buffer, and 10 μl β-NAD+/ATP solution. The plate was mixed and incubated for 3 min at 37°C, then read at 340 nm (A1). The plate was next incubated at 37°C for 2 h to allow β-galactosidase conversion of lactulose into free glucose and fructose. After incubation hexokinase + G-6-P dehydrogenase solution was diluted 1:5 in TAE buffer, and 10 μl was added to all occupied wells. The plate was then mixed and incubated for 5 min at 37°C, then read at 340 nm (A2). PGI was then diluted 1:5 in 0.5× TAE buffer, and 10 μl was added to the plate. The plate was then mixed, incubated at 37°C (5 min), and read at 340 nm (A3). Lactulose = (A3 − A2) − A1.
Statistical Analyses
All statistical analyses were performed with STATISTICA, ver. 7.1 (StatSoft). Variables were tested for normal distribution using the Kolmogorov-Smirnov test. Nonnormally distributed variables were log transformed to approximate a normal distribution before applying a t-test or repeated measures analysis. The repeated factors assumption of sphericity was tested with Mauchly's sphericity test. When necessary, a Greenhouse-Geisser correction was applied to the F ratio to correct for sphericity violations. All data represent mean ± SE for n = 8. Statistical significance was set at P ≤ 0.05.
Gastrointestinal Barrier Permeability
Alterations from basal gastrointestinal barrier function (lactulose and endotoxin) due to exercise/heat stress on day 1 and day 7 of heat acclimation were assessed via paired t-tests. The relationship between lactulose and endotoxin readings was examined via linear regression.
Molecular Responses to Heat Acclimation Exercise
Exercise alterations in TNF-α, IL-6, IL-10, and HSP70 were assessed via one-way repeated-measures ANOVA (time). Dunnett's test was used to assess significant differences from baseline.
Whole Body Responses to Heat Acclimation Exercise
Alterations in core temperature, mean skin temperature, mean body temperature, heart rate, and physiological strain responses to exercise on day 1 and day 7 of heat acclimation were assessed via two-way RM ANOVAs (day·time). Tukey's post hoc tests were used to assess significant main effects and interactions, where appropriate. Differences in plasma volume and whole body sweat rate from day 1 to day 7 of heat acclimation were examined via paired t-tests.
RESULTS
Heat Stress Day 1
Placebo supplementation.
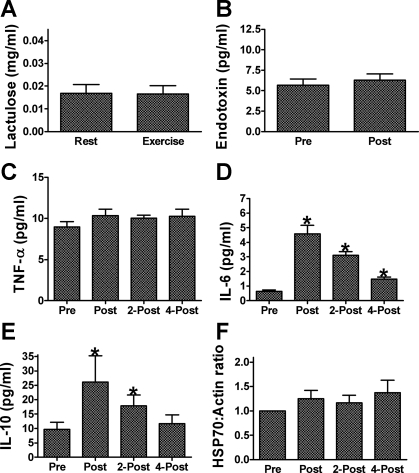
Urinary lactulose excretion and plasma endotoxin levels were not increased (P = 0.48 and 0.22, respectively) on the first day of exercise/heat stress in placebo. There was also no increase in subjects plasma TNF-α concentrations with exercise (P = 0.27). Plasma IL-6 concentrations showed the expected increases with exercise (P < 0.01), with IL-6 increasing from 0.64 ± 0.09 pg/ml before exercise to 4.58 ± 0.58 pg/ml immediately postexercise (P < 0.01), to 3.11 ± 0.24 pg/ml 2 h postexercise (P < 0.01), and to 1.49 ± 0.14 pg/ml 4 h postexercise (P < 0.01). Plasma IL-10 concentrations also increased with exercise (P < 0.01), from 9.69 ± 2.46 pg/ml before exercise to 26.13 ± 9.10 pg/ml immediately postexercise (P < 0.01) and to 17.81 ± 3.83 pg/ml 2 h postexercise (P < 0.01) but had returned to baseline levels by 4 h postexercise (11.71 ± 2.98 pg/ml; P > 0.05). We did not detect an increase in PBMC HSP70 content with exercise, (P = 0.33) (Fig. 2).
Fig. 2.
Markers of thermotolerance in humans responding to an acute bout of exercise/heat stress under placebo supplementation. A: urinary lactulose excretion measured in nonexercise/heat stress conditions (Rest) and on the first day of exercise/heat stress. B: plasma endotoxin concentrations measured before (Pre) and after (Post) exercise. Plasma concentrations of TNF-α (C), IL-6 (D), and IL-10 (E) measured before (Pre), after (Post), 2 h after (2-Post) and 4 h after exercise (4-post). F: heat shock protein 70 (HSP70) protein content of peripheral blood mononuclear cells (PBMC) before (Pre), after (Post), 2 h after (2-Post), and 4 h after exercise (4-post). *Significant increase from preexercise value, P < 0.05. Data are expressed as means ± SE; n = 8.
Quercetin supplementation.
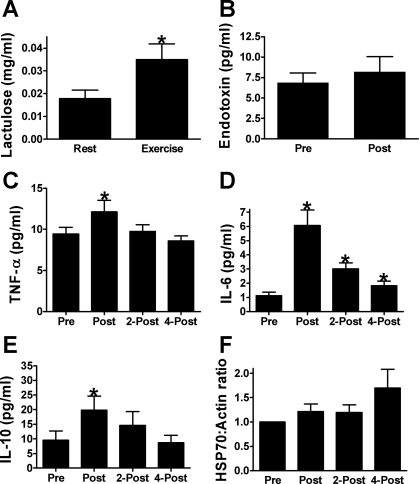
In contrast to that reported for the same subjects during placebo supplementation, urinary lactulose excretion was increased in quercetin [from 0.018 ± 0.004 mg/ml at baseline to 0.035 ± 0.007 mg/ml on the first day of exercise heat stress (P = 0.02)]. While plasma endotoxin levels tended (P = 0.12) to increase with exercise, this finding was nonsignificant. Subjects' plasma TNF-α concentrations were also increased from preexercise (9.43 ± 0.84 pg/ml) to immediately postexercise (12.15 ± 1.37 pg/ml; P = 0.02). Plasma IL-6 concentrations showed the expected increases with exercise (P < 0.01), increasing from 1.13 ± 0.24 pg/ml before exercise to 6.06 ± 1.10 pg/ml immediately after exercise (P < 0.01), to 3.00 ± 0.43 pg/ml 2 h postexercise (P < 0.01), and to 1.83 ± 0.33 pg/ml 4 h postexercise (P = 0.03). IL-10 was also increased (P < 0.01) from 9.56 ± 3.10 pg/ml before exercise to 19.80 ± 4.83 pg/ml immediately after exercise, (P < 0.01), but it was not different from baseline at 2 h (14.65 ± 4.66 pg/ml, P > 0.05) and 4 h (8.68 ± 2.48 pg/ml, P > 0.05) postexercise. We did not detect an increase in PBMC HSP70 content with exercise, (P = 0.14) (Fig. 3).
Fig. 3.
Markers of thermotolerance in humans responding to an acute bout of exercise/heat stress under quercetin supplementation. A: urinary lactulose excretion measured in nonexercise/heat stress conditions (Rest) and on the first day of exercise/heat stress. B: plasma endotoxin concentrations measured before (Pre) and after (Post) exercise. Plasma concentrations of TNF-α (C), IL-6 (D), and IL-10 (E) measured before (Pre), after (Post), 2 h after (2-Post), and 4 h after exercise (4-post). F: HSP70 protein content of PBMC before (Pre), after (Post), 2 h after (2-Post) and 4 h after exercise (4-post). *Significant increase from preexercise value, P < 0.05. Data are expressed as means ± SE; n = 8.
Heat Stress Day 7
Placebo supplementation.
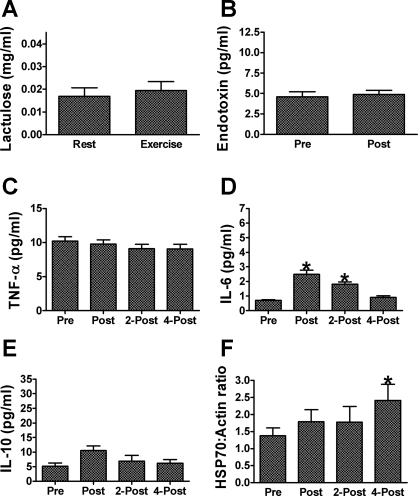
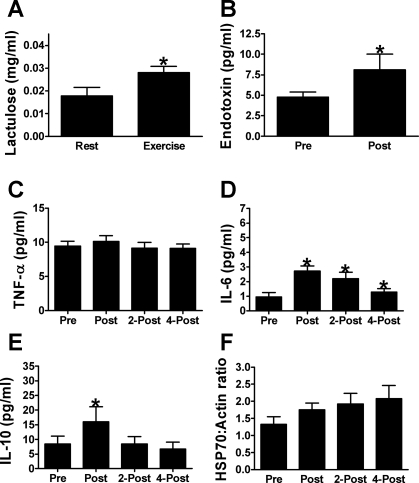
Urinary lactulose excretion (P = 0.23), plasma endotoxin (P = 0.30), and plasma TNF-α concentrations (P = 0.09) remained unaffected by 7 days of repeated exercise/heat stress under placebo conditions. The plasma IL-6 (P < 0.01) response to exercise also remained fairly consistent, with plasma IL-6 concentrations again increasing from 0.70 ± 0.06 pg/ml before exercise to 2.49 ± 0.28 pg/ml immediately postexercise (P < 0.01), and to 1.80 ± 0.17 pg/ml 2 h postexercise (P = 0.03). However, unlike that reported for acute exercise/heat exposure on day 1, IL-6 had returned to baseline by 4 h postexercise (P = 0.07). Also, in contrast to our findings on day 1, we did not detect an increase in IL-10 with exercise following repeated exercise/heat stress (P = 0.07). As expected, PBMC HSP70 content was increased (P = 0.05) from 1.38 ± 0.23 pixel units preexercise to 2.41 ± 0.48 pixel units by 4 h postexercise/heat stress (P = 0.01). This finding mirrors our previous observations in subjects completing a 10-day heat acclimation protocol (45) (Fig. 4).
Fig. 4.
Markers of thermotolerance in humans responding to 7 days of exercise/heat stress under placebo supplementation. A: urinary lactulose excretion measured in nonexercise/heat stress conditions (Rest) and on the first day of exercise/heat stress. B: plasma endotoxin concentrations measured before (Pre) and after (Post) exercise. Plasma concentrations of TNF-α (C), IL-6 (D), and IL-10 (E) measured before (Pre), after (Post), 2 h after (2-Post) and 4 h after exercise (4-post). F: HSP70 protein content of PBMC before (Pre), after (Post), 2 h after (2-Post) and 4 h after exercise (4-post). *Significant increase from preexercise value, P < 0.05. Data are expressed as mean ± SE; n = 8.
Quercetin supplementation.
Quercetin supplementation resulted in persistent, exercise-induced increases in urinary lactulose excretion over baseline measurements (from 0.018 ± 0.004 mg/ml at baseline to 0.028 ± 0.003 mg/ml on the 7th day of exercise/heat stress; P = 0.04) (Fig. 5). This indicates heat acclimation under HSF-1 inhibition could not overcome this defect. Plasma endotoxin levels also rose from 4.76 ± 0.65 pg/ml before exercise to 8.12 ± 1.91 pg/ml immediately after exercise (P = 0.03) These data were further supported by a strong linear correlation (r2= 0.637) between plasma endotoxin levels and urinary lactulose excretion. When taken together, these results suggest that the impairment in gastrointestinal barrier function resulting from multiple days of exercise/heat stress may have exceeded the endotoxin-clearing capacity of the reticuloendothelial system. However, despite the increase in gastrointestinal permeability, we did not detect a persistent increase in plasma TNF-α at rest or with exercise (P = 0.57) in these subjects. Thus, there was a decline in postexercise TNF-α between days 1 and 7 of the exercise protocol. Similar to that reported for the same subjects under placebo supplementation, IL-6 (P < 0.01) remained affected by exercise, with plasma concentrations increasing from 0.95 ± 0.30 pg/ml before exercise to 2.71 ± 0.35 pg/ml immediately after exercise (P < 0.01) and to 2.19 ± 0.44 pg/ml 2 h postexercise (P < 0.01). However, unlike that reported on day 7 for placebo supplementation, plasma IL-6 concentrations at 4 h postexercise remained elevated (1.28 ± 0.24 pg/ml; P = 0.05). We also noted differences in plasma IL-10 responses to exercise/heat stress on day 7 of placebo and quercetin supplementation; in the former, there was no increase with exercise (P = 0.07), while in the latter, the increase (P = 0.04) from preexercise (8.42 ± 2.71 pg/ml) to immediately postexercise (15.94 ± 5.22 pg/ml) was retained (P = 0.05). In further contrast to what we reported for these subjects under placebo supplementation, we did not detect an increase in HSP70 with exercise (P = 0.19) (Fig. 5).
Fig. 5.
Markers of thermotolerance in humans responding to 7 days of exercise/heat stress under quercetin supplementation. A: urinary lactulose excretion measured in nonexercise/heat stress conditions (Rest) and on the first day of exercise/heat stress. B: plasma endotoxin concentrations measured before (Pre) and after (Post) exercise. Plasma concentrations of TNF-α (C), IL-6 (D), and IL-10 (E) measured before (Pre), after (Post), 2 h after (2-Post), and 4 h after exercise (4-post). F: HSP70 protein content of PBMC before (Pre), after (Post), 2 h after (2-Post) and 4 h after exercise (4-post). *Significant increase from preexercise value, P < 0.05. Data are expressed as means ± SE; n = 8.
Heat Acclimation
Placebo supplementation.
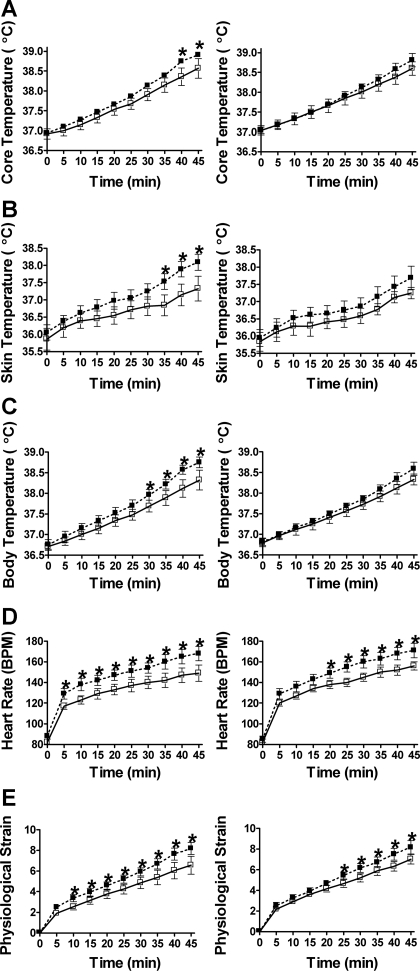
Core temperature (P = 0.02), mean skin temperature (P < 0.01), and mean body temperature (P < 0.01) were reduced after 7 days of heat acclimation under placebo supplementation. Specifically, core temperature was reduced at 40–45 min, mean skin temperature was reduced at 35–45 min, and mean body temperature was reduced at 30–45 min of exercise, respectively. Heart rate (P = 0.01) and physiological strain (P = 0.02) responses to exercise were also improved, with heart rate being reduced starting from 5 to 45 min of exercise, and physiological strain being reduced starting from 10 to 45 min of exercise (Fig. 6). We also noted significant increases in whole body sweat rate (from 31 ± 1.6 ml/min to 34.3 ± 2.2 ml/min; P = 0.04) and plasma volume expansion (16 ± 4%) (data not shown).
Fig. 6.
The HSF-1 inhibitor quercetin alters well-established systemic adaptations that characterize the heat-acclimated phenotype. Core temperature (A), mean skin temperature (B), mean body temperature (C), heart rate (D), and physiological strain (E) responses (scored 0–10, where 0 is equal to no strain and 10 is equal to high strain) to a heat tolerance test were performed preacclimation (■) and postacclimation (□) under placebo (left) and quercetin (right) conditions. Subjects were not under the influence of placebo or quercetin supplementation on the preacclimation test. *Significantly improved exercise response, P < 0.05. Data are expressed as means ± SE; n = 8 for all panels.
Quercetin supplementation.
Interestingly, core temperature (P = 0.13), mean skin temperature (P = 0.97), and mean body temperature (P = 0.11) responses to exercise/heat stress were not reduced after 7 days of heat acclimation under quercetin supplementation. Further, while overall improvements in heart rate (P = 0.02) and physiological strain (P = 0.02) responses to exercise/heat stress were detected in the quercetin condition, these reductions came much later in exercise. Compared with the placebo condition, where improvements in heart rate and physiological strain were evident at 5 and 10 min of exercise, respectively, under quercetin, improvements were not detected until 20 and 25 min of exercise had elapsed (Fig. 6). While thermoregulatory responses in subjects under quercetin supplementation were clearly compromised, these differences cannot be explained by differences in the subjects' ability to sweat (which increased from 30.3 ± 3.4 ml/min to 35.2 ± 3.7 ml/min; P = 0.002) or to expand their plasma volume (plasma volume expansion = 16 ± 5%), which were not different between conditions (P = 0.49 and 0.54, respectively).
DISCUSSION
In the present study, we examined the effect of a standard laboratory HSF-1 inhibitor, quercetin, on well-described markers of thermotolerance and heat acclimation in humans. We found that 2 g/day oral quercetin supplementation disrupted the normal cellular accumulation of HSP70 in PBMC. The blunted HSP70 response was associated with impairments in gastrointestinal barrier function and inflammatory/anti-inflammatory cytokine profiles. These findings in exercising humans mirror those in cells (25), tissues (13, 15), and animals (36, 53). While this is the first clear example of this effect in humans, the major finding of this study was the ability of quercetin ingestion to impede acquisition of the acclimated phenotype. Our findings are consistent with a possible role of HSF-1 in both thermotolerance and heat acclimation.
HSF-1 Inhibition and Reduced Acquisition of Heat-Acclimated Phenotype
The results from our study suggest a link between thermotolerance and heat acclimation through inhibition of the heat shock system in humans. An association between thermotolerance and heat adaptation has been suggested in prior reports, which took advantage of natural differences between insect and animal species. Perhaps, the best example of such adaptations is the Saharan ant Cataglyphis bombycina, which shows elevated levels of HSP70 at low ambient temperatures (25°C). This adaptation has been suggested to contribute to C. bombycina capacity to forage at midday, tolerating surface temperatures 15°C higher than all other desert ant species (21). This adaptation is vital to C. bombycina survival, as the associated increase in critical thermal maximum (53–55°C) gives it a slight competitive advantage over Acanthodactylus dumerili, a predatory lizard species with similar thermal adaptation (68). There is also evidence of elevated HSP70 levels at normal ambient temperatures in lizards (66) and multiple insect species (18, 58) that reside in environments prone to rapid temperature change. This high constitutive HSP70 expression has been suggested to allow these organisms to perform work at elevated temperatures, where de novo HSP70 synthesis would be too slow to confer any benefit (49). Benefits of this adaptation are apparent when one examines Hydra attenuate, a hydra species that has naturally lost the ability to accumulate HSP70 in response to thermal exposure. Acclimating this hydra species does not induce thermotolerance; rather, it increases mortality to subsequent thermal exposure (2). In all of these studies, the natural experiment suggested that improved heat tolerance, as measured by work in the heat, was associated with an elevated HSP response. Likewise, an evolved decrease in HSP response predicted less capacity to perform work in heat and heat intolerance.
Intriguingly, a similar repression of the heat shock response has been reported in heat-intolerant military personnel, and it has been suggested to explain the excessive metabolic heat storage and physiological strain these soldiers experience in response to exertional heat stress (45). It should also be noted that these soldiers are at significantly increased risk of developing exertional heat illness than the general population (17, 45). Reduced acclimatory capacity has also been reported in rats acclimatized under HSP70 blockade (39). Like that reported by heat-intolerant military personnel, and for otherwise healthy subjects in the present study, rats that were acclimated under HSP70 blockade exhibited decreased heat endurance and increased tissue damage in response to subsequent thermal challenge (23).
Critique of Approach: Alternative Actions of Quercetin that May Delay Heat Acclimation
Although quercetin is known to be a potent inhibitor of the heat shock response, it has other biological activities that may have influenced the results of this study. These areas are examined in the following sections.
Tight junctions.
Although we have consistently reported increased permeability in heat-stressed monolayers under quercetin supplementation (11–15), other authors have reported reductions in monolayer permeability following quercetin supplementation (1, 62). The increased permeability in our studies was associated with reduced expression and junctional localization of occludin, an integral tight junction protein. The reduced permeability reported by other authors was associated with upregulation of claudin-4, another tight junction protein. As such, it is possible that the increased gastrointestinal barrier permeability and plasma endotoxin concentrations reported in quercetin-supplemented subjects in the present study may reflect a complex interplay between barrier-supporting and barrier-reducing effects of dietary quercetin supplementation. However, it must be noted that both studies reporting positive barrier effects of quercetin were conducted in resting monolayers, in the absence of imposed stress, while our studies reporting negative barrier effects of quercetin utilized acute heat exposure to alter barrier properties.
Cytokines.
We suggest the results of this study reflect a quercetin-mediated suppression of the heat shock response during repeated exposure to exercise/heat stress, resulting in increased gastrointestinal barrier permeability, endotoxin translocation into the portal and systemic circulations, and disturbed proinflammatory and anti-inflammatory cytokine responses. These alterations are all characteristic of an overactive NF-κB system. However, quercetin is known to inhibit proinflammatory cytokine (TNF-α, IL-1β, IL-6, and IL-8) gene expression in LPS-stimulated macrophages, adipocytes, and PBMCs via modulation of the NF-κB system (52, 56). Quercetin has also been reported to improve inflammatory parameters in rodent models of intestinal inflammation (8, 32). As such, the results of this study likely reflect the outcome of a conflict between toxic (inhibition of the heat shock response) and beneficial (inhibition of the NF-κB system) effects of dietary quercetin supplementation. The net result may depend on the type (infection vs. exercise/heat) of stress.
Cellular stress.
Our previous cell and tissue culture experiments provide clear evidence of quercetin's ability to suppress HSF-1 activity. Inadequate stimulation of the heat shock response in these studies resulted in increased cellular stress and a loss of thermotolerance. While a similar mechanism may be expected in the present study, HSF-1 was not measured directly. As such, it is possible that our thermotolerance results may be explained by an alternative mechanism. One possibility is a quercetin-mediated reduction in cellular stress, resulting in reduced activation of the heat shock response. In fact, suppression of the unfolded protein response was recently reported in quercetin-supplemented intestinal epithelial cells (54). Reductions in misfolded protein aggregation during imposed stress would reduce competitive binding of heat shock proteins from HSF-1 in the cytosol (autoregulatory loop hypothesis of HSF-1 regulation; expertly reviewed in Ref. 47). Retention of HSF-1 in its monomer form would prevent its activation, nuclear entry, and ultimately propagation of the heat shock response. Although it is important to note this possibility, it must be noted that the net result would still be reduced HSF-1 activity and HSP70 accumulation.
Antioxidant capacity.
While quercetin's antioxidant capacity may have reduced cellular stress, and thus the impetus for activation of the heat shock response, it is also tenable that quercetin's antioxidant properties may have repressed formation of reactive oxygen species, which are integral mediators of cellular signaling responses. Although these effects have not been attributed to quercetin in a human model, suppressed leukocyte expression of HSF-1 and HSP70 following exhaustive treadmill exercise was shown in humans supplemented with α-tocopherol, a similar antioxidant (19, 55). More recently, oral antioxidant supplementation has been reported to suppress the improvements in insulin sensitivity that are normally associated with combined endurance/resistance exercise (59). Antioxidant ingestion has also been reported to block mitochondrial biogenesis in skeletal muscle, reducing the expected improvements in endurance capacity of rats and humans responding to 6–8 wk of exercise (22). In the present study, these effects may be linked to quercetin-mediated inhibition of hypoxia inducible factor 1α (HIF-1α) via impairments in MAPK-dependent phosphorylation (65). This is an intriguing concept, as HIF-1α pathways have been shown to be both activated by heat acclimation (40) and integral in acquisition of the heat-acclimated phenotype (64). This convergence of HSF-1 and HIF-1α transcription factors has been suggested to explain cross-tolerance, or the ability of prior exposure to one stressor to protect against subsequent exposure to an alternative stressor (24). As such, this may be the mechanism linking thermotolerance, heat acclimation, and the heat shock response in the present study.
Perspectives and Significance
This study adds to a growing body of literature that suggests antioxidant dietary supplementation may inhibit exercise-driven adaptations in otherwise healthy athletic populations. Our data suggest dietary quercetin supplementation impaired the cellular and systemic adaptations associated with both thermotolerance and heat acclimation in exercising humans. It is important to note that our study was limited by the fact that reductions in the heat shock response, as evidenced by reduced cellular accumulation of HSP70, may have been caused by quercetin-mediated reductions in cellular stress, and not by quercetin-mediated suppression of the heat shock response. Regardless of the mechanism, the fact remains that reductions in thermotolerance and heat acclimation reported in the present study occurred in concert with ingestion of a known HSF-1 blocker. More importantly, these effects were not shown in the same subjects under placebo supplementation. We suggest that more rigorous protocols utilizing a more specific HSF-1 blocker, longer heat acclimation protocols, and more severe core temperature perturbations are required to further parse out the effects of this dietary supplement on the heat shock response.
GRANTS
This research was supported by the University of New Mexico (UNM) General Clinical Research Center (National Institutes of Health, National Center for Research Resources, General Clinical Research Center Grant MO1-RR00997), the UNM Graduate and Professional Student Association, the UNM College of Education, and the Gatorade Sports Science Institute (all to M. Kuennen). Product donations were made by Quercegen Pharma and the Nash Finch.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank C. Calvin, J. Iverson, H. Lin, C. Mobarak, N. Morgan, B. Ramadass, and R. Wold for their excellent technical and professional assistance.
REFERENCES
- 1. Amasheh M, Schlichter S, Amasheh S, Mankertz J, Zeitz M, Fromm M, Schulzke JD. Quercetin enhances epithelial barrier function and increases claudin-4 expression in Caco-2 cells. J Nutr 138: 1067–1073, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Bosch TC, Krylow SM, Bode HR, Steele RE. Thermotolerance and synthesis of heat shock proteins: These responses are present in Hydra attenuate but absent in Hydra oligactis. Proc Natl Acad Sci USA 85: 7927–7931, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bouchama A, al-Sedairy S, Siddiqui S, Shail E, Rezeig M. Elevated pyrogenic cytokines in heatstroke. Chest 104: 1498–1502, 1993 [DOI] [PubMed] [Google Scholar]
- 4. Buckley BA, Owen ME, Hofmann GE. Adjusting the thermostat: the threshold induction temperature for the heat-shock response in intertidal mussels (genus Mytilus) changes as a function of thermal history. J Exp Biol 204: 3571–3579, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Carter R, 3rd, Cheuvront SM, Williams JO, Kolka MA, Stephenson LA, Sawka MN, Amoroso PA. Epidemiology of hospitalizations and deaths from heat illness in soldiers. Med Sci Sports Exer 37: 1338–1344, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Cermak R, Landgraf S, Wolffram S. The bioavailability of quercetin in pigs depends on the glycoside moiety and on dietary factors. J Nutr 133: 2802–2807, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Cheuvront SN, Ely BR, Kenefick RW, Michniak-Kohn BB, Rood JC, Sawka MN. No effect of nutritional adenosine receptor antagonists on exercise performance in the heat. Am J Physiol Regul Integr Comp Physiol 296: R394–R401, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Comalada M, Camuesco D, Sierra S, Ballester I, Xaus J, Gálvez J, Zarzuelo A. In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-κB pathway. Eur J Immunol 35: 584–592, 2005 [DOI] [PubMed] [Google Scholar]
- 9. DiIorio PJ, Holsinger K, Schultz RJ, Hightower LE. Quantitative evidence that both Hsc70 and Hsp70 contribute to thermal adaptation in hybrids of the livebearing fishes Poeciliopsis. Cell Stress Chaperones 1: 139–147, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37: 247–248, 1974 [DOI] [PubMed] [Google Scholar]
- 11. Dokladny K, Kozak A, Wachulec M, Wallen ES, Menache MG, Kozak W, Kluger MK, Moseley PL. Effect of heat stress on LPS-induced febrile response in d-galactosamine-sensitized rats. Am J Physiol Regul Integr Comp Physiol 280: R338–R344, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Dokladny K, Lobb R, Wharton W, Ma TY, Moseley PL. LPS-induced cytokine levels are repressed by elevated expression of HSP70 in rats: possible role of NF-κB. Cell Stress Chaperones 15: 153–163, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dokladny K, Moseley PL, Ma TY. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. Am J Physiol Gastrointest Liver Physiol 290: G204–G212, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Dokladny K, Wharton W, Lobb R, Ma TY, Moseley PL. Induction of physiological thermotolerance in MDCK monolayers: contribution of heat shock protein 70. Cell Stress Chaperones 11: 268–275, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dokladny K, Ye D, Kennedy JC, Moseley PL, Ma TY. Cellular and molecular mechanisms of heat stress-induced up-regulation of occludin protein expression: Regulatory role of heat shock factor-1. Am J Pathol 172: 659–670, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. End DW, Look RA, Shaffer NL, Balles EA, Persico FJ. Non-selective inhibition of mammalian protein kinases by flavinoids in vitro. Res Commun Chem Pathol Pharmacol 56: 75–86, 1987 [PubMed] [Google Scholar]
- 17. Epstein Y. Heat intolerance: predisposing factor or residual injury? Med Sci Sports Exer 22: 29–35, 1990 [PubMed] [Google Scholar]
- 18. Evgen'ev MB, Garbuz DG, Shilova VY, Zatsepina OG. Molecular mechanisms underlying thermal adaptation of xeric animals. J Biosci 32: 489–499, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Fischer CP, Hiscock NJ, Basu S, Vessby B, Kallner A, Sjöberg LB, Febbraio MA, Pedersen BK. Vitamin E isoform-specific inhibition of the exercise-induced heat shock protein 72 expression in humans. J Appl Physiol 100: 1679–1687, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Fruth JM, Gisolfi CV. Work-heat tolerance in endurance- trained rats. J Appl Physiol 54: 249–253, 1983 [DOI] [PubMed] [Google Scholar]
- 21. Gehring WJ, Wehner R. Heat shock protein synthesis and thermotolerance in Cataglyphis, an ant from the Sahara desert. Proc Natl Acad Sci USA 92: 2994–2998, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gomez-Cabrera MC, Donemech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, Sastre J, Vina J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr 87: 142–9, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Horowitz M. Heat acclimation and cross-tolerance against novel stressors: genomic-physiological linkage. Prog Brain Res 162: 373–392, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Horowitz M, Assadi H. Heat acclimation-mediated cross-tolerance in cardioprotection: do HSP70 and HIF-1α play a role? Ann NY Acad Sci 1188: 199–206, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Hosokawa N, Hirayoshi K, Nikai A, Hosokawa Y, Marui N, Yoshida M, Sakai T, Nishino H, Aoike A, Kawai K, Nagata K. Flavonoids inhibit the expression of heat shock proteins. Cell Struct Funct 15: 393–401, 1990 [DOI] [PubMed] [Google Scholar]
- 26. Hosokawa N, Hirayoshi K, Kudo H, Takechi H, Aoike A, Kawai K, Nagata K. Inhibition of the activation of heat shock factor in vivo and in vitro by flavonoids. Mol Cell Biol 12: 3490–3498, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hung CH, Chang NC, Cheng BC, Lin MT. Progressive exercise preconditioning protects against circulatory shock during experimental heatstroke. Shock 23: 426–433, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr 40: 497–504, 1978 [DOI] [PubMed] [Google Scholar]
- 29. Jakubowicz-Gil J, Rzymowska J, Gawron A. Quercetin, apoptosis, heat shock. Biochem Pharmacol 64: 1591–1595, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Jakubowicz-Gil J, Pawlikowska-Pawlega B, Piersiak T, Pawelec J, Gawron A. Quercetin suppresses heat shock-induced nuclear translocation of Hsp72. Folia Histochem Cytobiol 43: 123–128, 2005 [PubMed] [Google Scholar]
- 31. Kenney WL. Heat flux and storage in hot environments. Int J Sports Med 19: s92–s95, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Kim H, Kong H, Choi B, Yang Y, Kim Y, Lim MJ, Neckers L, Jung Y. Metabolic and pharmacological properties of rutin, a dietary quercetin glycoside, for treatment of inflammatory bowel disease. Pharm Res 22: 1499–1509, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Kuennen MR, Gillum TL, Amorim FT, Kwon YS, Schneider SM. Palm cooling to reduce heat strain in subjects during simulated armoured vehicle transport. Eur J Appl Physiol 108: 1217–1223, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Li DP, Li Calzi S, Sánchez ER. Inhibition of heat shock factor activity prevents heat shock potentiation of glucocorticoid receptor-mediated gene expression. Cell Stress Chaperones 4: 223–234, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li GC. Elevated levels of 70,000 dalton heat shock protein in transiently thermotolerant Chinese hamster fibroblasts and in their stable heat-resistant variants. Int J Radiat Oncol Biol Phys 11: 165–177, 1985 [DOI] [PubMed] [Google Scholar]
- 36. Liu WL, Chen SJ, Chen Y, Sun LM, Zhang W, Zeng YM, Zhou TH, Si JM. Protective effects of heat shock protein70 induced by geranylgeranylacetone in atrophic gastritis in rats. Acta Pharmacol Sin 28: 1001–1006, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Lesser S, Cermak R, Wolffram S. Bioavailability of quercetin in pigs is influenced by the dietary fat content. J Nutr 134: 1508–1511, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Magalhães FD, Amorim FT, Freitas Passos RL, Fonseca MA, Moreira Oliveira KP, MalheirosLima MR, Guimarães JB, Ferreira-Junior JB, Martini ARP, Lima NRV, Soares DD, Oliveira ME, Carneiro Rodrigues LO. Heat and exercise acclimation increases intracellular levels of Hsp72 and inhibits exercise-induced increase in intracellular and plasma Hsp72 in humans. Cell Stress Chaperones 15: 885–895, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maloyan A, Horowitz M. β-Adrenergic signaling and thyroid hormones affect HSP72 expression during heat acclimation. J Appl Physiol 93: 107–115, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Maloyan A, Eli-Berchoer L, Semenza GL, Gerstenblith G, Stern MD, Horowitz M. HIF-1α-targeted pathways are activated by heat acclimation and contribute to acclimation-ischemic cross-tolerance in the heart. Physiol Genomics 23: 79–88, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Manach CG, Williamson C, Morand A, Scalbert A, Remesy C. Bioavailability and bioefficiency of polyphenols in humans. Review of 97 bioavailability studies. Am J Clin Nutr 81 Suppl: 230S–242S, 2005 [DOI] [PubMed] [Google Scholar]
- 42. McAnulty SR, McAnulty LS, Nieman DC, Quindry JC, Hosick PA, Hudson MH, Still L, Henson DA, Milne GL, Morrow JD, Dumke CL, Utter AC, Triplett NT, Dibarnardi A. Chronic quercetin ingestion and exercise-induced oxidative damage and inflammation. Appl Physiol Nutr Metab 33: 254–262, 2008 [DOI] [PubMed] [Google Scholar]
- 43. McClung JP, Hasday JD, He J, Montain SJ, Cheuvront SN, Sawka MN, Singh I. Exercise-heat acclimation in humans alters baseline levels and ex vivo heat inducibility of HSP72 and HSP90 in peripheral blood mononuclear cells. Am J Physiol Regul Integr Comp Physiol 294: R185–R191, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Mizzen LA, Welch WJ. Characterization of the thermotolerant cell. I. Effects on protein synthesis activity and the regulation of heat-shock protein 70 expression. J Cell Biol 106: 1105–1116, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moran DS, Eli-Berchoer L, Heled Y, Mendel L, Schocina M, Horowicz M. Heat intolerance: does gene transcription contribute? J Appl Physiol 100: 1370–1376, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Moran DS, Shitzer A, Pandolf KB. A physiological strain index to evaluate heat stress. Am J Physiol Regul Integr Comp Physiol 275: R129–R134, 1998 [DOI] [PubMed] [Google Scholar]
- 47. Morimoto RI. Cells in stress: transcriptional activation of heat shock genes. Science 259: 1409–1410, 1993 [DOI] [PubMed] [Google Scholar]
- 48. Moseley PL, Gapen C, Wallen ES, Walter ME, Peterson MW. Thermal stress induces epithelial permeability. Am J Physiol Cell Physiol 267: C425–C434, 1994 [DOI] [PubMed] [Google Scholar]
- 49. Moseley PL. Heat shock proteins and heat adaptation of the whole organism. J Appl Physiol 83: 1413–1417, 1997 [DOI] [PubMed] [Google Scholar]
- 50. Nadel ER, Pandolf KB, Roberts MF, Stolwijk JAJ. Mechanisms of thermal acclimation to exercise and heat. J Appl Physiol 37: 515–520, 1974 [DOI] [PubMed] [Google Scholar]
- 51. Nagai N, Nakai A, Nagata K. Quercetin suppresses heat shock response by down regulation of HSF1. Biochem Biophys Res Commun 208: 1099–1105, 1995 [DOI] [PubMed] [Google Scholar]
- 52. Nair MP, Mahajan S, Reynolds JL, Aalinkeel R, Nair H, Schwartz SA, Kandaswami C. The flavonoid quercetin inhibits proinflammatory cytokine (tumor necrosis factor alpha) gene expression in normal peripheral blood mononuclear cells via modulation of the NF-kappa beta system. Clin Vaccine Immunol 13: 319–328, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nakada J, Matsura T, Okazaki N, Nishida T, Togawa A, Minami Y, Inagaki Y, Ito H, Yamada K, Ishibe Y. Oral administration of geranylgeranylacetone improves survival rate in a rat endotoxin shock model: administration timing and heat shock protein 70 induction. Shock 24: 482–487, 2005 [DOI] [PubMed] [Google Scholar]
- 54. Natsume Y, Ito S, Satsu H, Shimizu M. Protective effect of quercetin on ER stress caused by calcium dynamics dysregulation in intestinal epithelial cells. Toxicology 258: 164–175, 2009 [DOI] [PubMed] [Google Scholar]
- 55. Niess AM, Fehrenbach E, Schlotz E, Sommer M, Angres C, Tschositsch K, Battenfeld N, Golly IC, Biesalski HK, Northoff H, Dickhuth HH. Effects of RRR-alpha-tocopherol on leukocyte expression of HSP72 in response to exhaustive treadmill exercise. Int J Sports Med 23: 445–452, 2002 [DOI] [PubMed] [Google Scholar]
- 56. Overman A, Bumrungpert A, Kennedy A, Martinez K, Chuang C, West T, Dawson B, Jia W, McIntosh M. Polyphenol-rich grape powder extract (GPE) attenuates inflammation in human macrophages and in human adipocytes exposed to macrophage-conditioned media. Int J Obes 34: 800–808, 2010 [DOI] [PubMed] [Google Scholar]
- 57. Ramanathan NL. A new weighting system for mean surface temperature of the human body. J Appl Physiol 19: 531–533, 1964 [DOI] [PubMed] [Google Scholar]
- 58. Rinehart JP, Hayward SAL, Elnitsky LA, Sandro LH, Lee RE, Jr, Denlinger DL. Continuous up-regulation of heat shock proteins in larvae, but not adults, of a polar insect. Proc Natl Acad Sci USA 103: 14223–14227, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ristow M, Zarse K, Oberbach A, Klotting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn R, Bluher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA 106: 8665–8870, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Romier B, Schneider YJ, Larondelle Y, During A. Dietary polyphenols can modulate the intestinal inflammatory response. Nutr Rev 67: 363–378, 2009 [DOI] [PubMed] [Google Scholar]
- 61. Sakurada S, Hales JR. A role for gastrointestinal endotoxins in enhancement of heat tolerance by physical fitness. J Appl Physiol 84: 207–214, 1998 [DOI] [PubMed] [Google Scholar]
- 62. Suzuki T, Hara H. Quercetin enhances intestinal barrier function through the assembly of zonula [corrected] occludens-2, occludin, and claudin-1 and the expression of claudin-4 in Caco-2 cells. J Nutr 139: 965–974, 2009 [DOI] [PubMed] [Google Scholar]
- 63. Terao J. Dietary flavonoids as antioxidants. Forum Nutr 61: 87–94, 2009 [DOI] [PubMed] [Google Scholar]
- 64. Treinin M, Shliar J, Jiang H, Powell-Coffman JA, Bromberg Z, Horowitz M. HIF-1 is required for heat acclimation in the nematode Caenorhabditis elegans. Physiol Genomics 14: 17–24, 2003 [DOI] [PubMed] [Google Scholar]
- 65. Triantafyllou A, Mylonis I, Simos G, Bonanou S, Tsakalof A. Flavonoids induce HIF-1α but impair its nuclear accumulation and activity. Free Radic Biol Med 44: 657–670, 2008 [DOI] [PubMed] [Google Scholar]
- 66. Ulmasov HA, Karaev KK, Lyashko VN, Evgen'ev MB. Heat-shock response in camel (Camelus dromedarius) blood cells and adaptation to hyperthermia. Comp Biochem Physiol B 106: 867–872, 1993 [DOI] [PubMed] [Google Scholar]
- 67. Ulmasov KA, Shammakov S, Karaev K, Evgen'Ev MB. Heat shock proteins and thermoresistance in lizards. Proc Natl Acad Sci USA 89: 1666–1670, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wehner R, Marsh AC, Wehner S. Desert ants on a thermal tightrope. Nature 357: 586–587, 1992 [Google Scholar]
- 69. Weshler Z, Kapp DS, Lord PF, Hayes T. Development and decay of systemic thermotolerance in rats. Cancer Res 44: 1347–1351, 1984 [PubMed] [Google Scholar]
- 70. Wilson WJ, Poellinger L. The dietary flavonoid quercetin modulates HIF-1 alpha activity in endothelial cells. Biochem Biophys Res Commun 293: 446–450, 2002 [DOI] [PubMed] [Google Scholar]
- 71. Yamada PM, Amorim FT, Moseley P, Robergs R, Schneider SM. Effect of heat acclimation on heat shock protein 72 and interleukin-10 in humans. J Appl Physiol 103: 1196–1204, 2007 [DOI] [PubMed] [Google Scholar]