Abstract
Recent studies indicate that a substantial amount of metabolically active brown adipose tissue (BAT) exists in adult humans. Given the unique ability of BAT to convert calories to heat, there is intense interest in understanding the regulation of BAT metabolism in hopes that its manipulation might be an effective way of expending excess calories. Because of the established role of AMP-activated protein kinase (AMPK) as a “metabolic master switch” and its extremely high levels of activity in BAT, it was hypothesized that AMPK might play a central role in regulating BAT metabolism. To test this hypothesis, whole body α1-AMPK−/− (knockout) and wild-type mice were studied 1) under control (room temperature) conditions, 2) during chronic cold exposure (14 days at 4°C), and 3) during acute nonshivering thermogenesis (injection of a β3-adrenergic agonist). Under control conditions, loss of α1-AMPK resulted in downregulation of two important prothermogenic genes in BAT, thyrotropin-releasing hormone (−9.2-fold) and ciliary neurotrophic factor (−8.7-fold). Additionally, it caused significant upregulation of α2-AMPK activity in BAT, white adipose tissue, and liver, but not cardiac or skeletal muscle. During acute nonshivering thermogenesis and chronic cold exposure, body temperature was indistinguishable in the α1-AMPK−/− and wild-type mice. Similarly, the degree of cold-induced hyperphagia was identical in the two groups. We conclude that α1-AMPK does not play an obligatory role in these processes and that adaptations to chronic loss of α1-AMPK are able to compensate for its loss via several mechanisms.
Keywords: AMP-activated protein kinase, brown adipose tissue, thermoregulation
homeothermic animals have multiple mechanisms for maintaining body temperature when confronted with a cold environment, including shivering and redistributing blood flow away from the periphery (31). Additionally, it has long been recognized that human infants, as well as most types of rodents, have substantial amounts of brown adipose tissue (BAT), a mitochondria-rich and highly thermogenic type of adipose tissue that contains the thermogenic protein uncoupling protein 1 (UCP1) (3). The thermal output of BAT is regulated, in large part, by the noradrenergic activation of β3-adrenergic receptors on brown adipocytes. This results in a Gs-mediated activation of adenylate cyclase and protein kinase A (3).
While BAT was previously not thought to play an important physiological role in adult humans, recent studies have conclusively demonstrated that there is a considerable amount of BAT in adult humans and that it is metabolically active (6, 27, 34, 35). Given the unique ability of BAT to convert calories directly to heat, there is intense interest in understanding how BAT metabolism is regulated in hopes that manipulating the metabolic rate of BAT might be an effective way of expending excess calories and, possibly, decreasing obesity-related morbidity (4, 33).
Previously we reported that BAT in mice contains a higher level of AMP-activated protein kinase (AMPK) expression than any other tissue, including liver, muscle, heart, and white adipose tissue (WAT) (20). Given the established role of AMPK as a “metabolic master switch,” in that it helps regulate processes such as food intake, glucose uptake, fatty acid oxidation, mitochondrial biogenesis, protein synthesis, and appetite (12, 21, 32, 36), we hypothesized that AMPK might play a central role in regulating adaptations to chronic cold exposure, cold-induced hyperphagia, and acute nonshivering thermogenesis.
To test this hypothesis, whole body knockout mice lacking the α1-isoform of AMPK (α1-AMPK−/− mice) and wild-type controls were compared at baseline and under two conditions requiring thermogenesis. We examined 1) the role of α1-AMPK in chronic adaptations to cold exposure (14 days of 4°C) and 2) the role of α1-AMPK in sympathetic nervous system-induced activation of BAT and the accompanying acute induction of nonshivering thermogenesis, which we determined by measuring the thermogenic response to injection of the β3-adrenergic agonist CL-316243. A major goal of these studies was to characterize the enzymatic and transcriptional compensatory adaptations in BAT and other tissues in response to loss of α1-AMPK.
METHODS
Animals.
Wild-type and α1-AMPK−/− mice were derived from a breeding colony of C57Bl/6 α1-AMPK+/− mice (heterozygotes) at our institution (17). The exception to this were four wild-type mice purchased from Harlan Laboratories (data shown in Fig. 7). Mice were housed singly at a University of Wisconsin Animal Care Facility maintained at ∼24°C (referred to as “room temperature” or “control conditions”). The facilities and research protocols were approved by the University of Wisconsin Institutional Animal Care and Use Committee. Mice had unrestricted access to food and water. Mice were killed via cervical dislocation. Interscapular BAT was collected and cleaned of white fat on an aluminum plate on ice. Epididymal fat pads, liver, and skeletal muscle (gastrocnemius) were rapidly collected (in that order; ∼30 s each), frozen in liquid nitrogen, and stored at −80°C until analysis. The genotype of each mouse was confirmed by PCR.
Fig. 7.
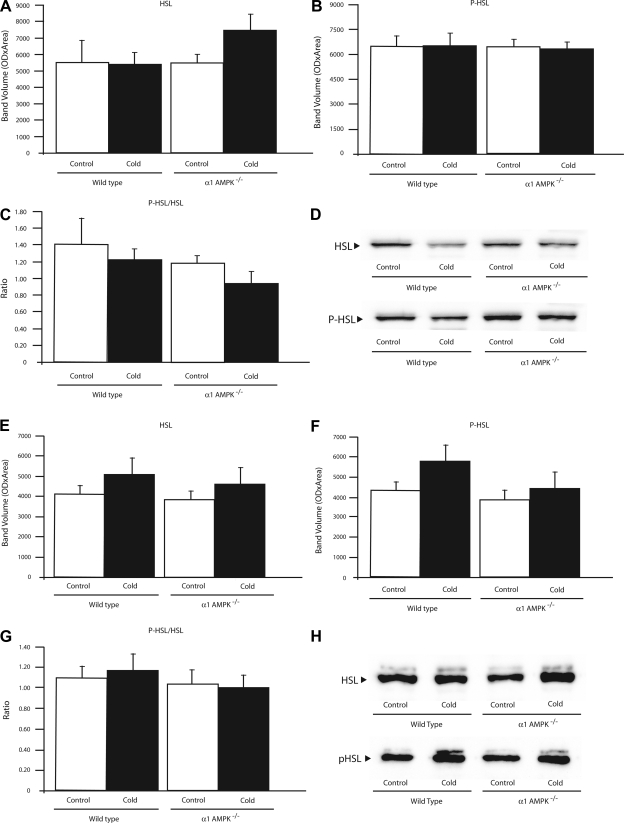
Effect of 14 days of cold exposure (4°C) on protein amounts of hormone-sensitive lipase (HSL), phosphorylated HSL (P-HSL), and ratio of P-HSL to HSL in BAT (A–C) and WAT (E–G) from wild-type and α1-AMPK−/− mice. Values are means ± SE (n = 3–5/group). D and H: representative Western blots from BAT and WAT, respectively. All samples were run in duplicate, and mean value for each mouse was used.
Assessment of acute thermogenic response to β3-adrenergic stimulation.
The selective agonist CL-316243 was injected intraperitoneally into lightly anesthetized mice (38). General anesthesia was induced with 1.0–1.5% isoflurane in oxygen via a nose cone. Each mouse (6 wild-type and 6 α1-AMPK−/− mice) received a vehicle trial and a CL-316243 trial separated by ≥2 days. For each trial, a mouse was placed on a water-filled heating pad while rectal (core) body temperature was recorded using a digital thermometer (model 450, Doric) and a temperature probe (model 452, Yellow Spring Instruments) for 5 min prior to the injection and for 30 min after the injection. When body temperature had stabilized, 0.1 ml of vehicle or 0.1 mg/kg CL-316243 was injected intraperitoneally in 0.1 ml of vehicle. The reproducibility and sensitivity of this system are indicated by the ∼0.1°C fall in body temperature that occurred as the room temperature drug or saline was first injected into each mouse.
Effects of chronic cold exposure.
Cold exposure studies were conducted in a controlled-temperature room at the University of Wisconsin-Madison Biotron facility. Seven- to 9-mo-old mice were singly housed without extra bedding. Temperature was reduced from ∼24°C to 4°C, where it remained for 14 days. Tissues were collected from these mice (8 wild-type and 7 α1-AMPK−/− mice) as well as from mice that had not undergone cold exposure (8 wild-type and 6 α1-AMPK−/− mice). To ensure that body weight, food intake, and body temperature were stable during this experimental protocol, seven additional mice were monitored for 14 days under room temperature conditions (data not shown).
AMPK activity assay.
AMPK activity was measured as previously described (20) and expressed as picomoles of phosphate incorporated per minute per milligram of homogenate protein. Briefly, tissue was homogenized in the presence of protease inhibitors and phosphatase inhibitors. One hundred micrograms of total protein (50 μg in WAT) from the resulting supernatant were immunoprecipitated with protein A/G agarose beads (Santa Cruz Biotechnology) and antibodies against the catalytic α1- or α2-subunit of AMPK (Upstate) overnight at 4°C. The beads containing the immunoprecipitated AMPK were washed, resuspended in reaction buffer with 0.2 mM SAMS peptide (8) and 0.2 mM [γ-32P]ATP, and incubated for 10 min at 37°C in a thermomixer in the presence of 200 mM AMP. After incubation, beads were quickly pelleted, and a portion of each supernatant was spotted on P-81 phosphocellulose paper washed in 1% phosphoric acid and then in acetone and air-dried. The incorporated radioactivity was counted in a TriCarb 3000 beta scintillation counter.
Citrate synthase activity assay.
Tissues were homogenized in the presence of protease inhibitors. Five microliters of appropriately diluted homogenate were incubated at 30°C in 250 μl of incubation medium containing 0.1 mM 5,5′-dithiobis-(2-nitrobenzoic acid), 0.5 mM oxaloacetate, 0.25% solution of Triton X-100, and 0.31 mM acetyl-CoA. The linear portion of the kinetics curve was used to calculate activity (19).
Western blotting.
Previously snap-frozen tissues were homogenized in 50 mM MOPS supplemented with 250 mM sucrose, 2 mM EDTA, 2 mM EGTA, and 5 μl/ml protease inhibitor cocktail (Sigma). For phosphorylated enzymes, 50 mM NaF, 5 mM NaPPi, and 1 mM Na3VO4 were added. Homogenates were centrifuged for 10 min at 600 g. Primary antibodies were as follows: UCP1 (catalog no. UCP11-A, Alpha Diagnostics), phosphorylated (Ser79) acetyl-CoA carboxylase (P-ACC; catalog no. 07-303, Upstate), phosphorylated (Thr172) AMPK (P-AMPK; catalog no. 07-626, Upstate), α1-AMPK (catalog no. 07-350, Upstate), α2-AMPK (raised in rabbit against the α2-AMPK peptide C-DDSAMHIPPGLKPHP), pan α-AMPK (catalog no. 07-181, Upstate), hormone-sensitive lipase (HSL; catalog no. 4107S, Cell Signaling), and phosphorylated (Ser565) HSL (P-HSL; catalog no. 4137S, Cell Signaling). Blots for total ACC were incubated with high-sensitivity streptavidin-horseradish peroxidase (catalog no. 21130, Pierce) in 5% BSA, 0.1% Tween 20, and 50 mM NaF in Tris-buffered saline. Immunoreactive bands were detected by SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific). All samples were run in duplicate, and the mean value for each mouse was used.
Quantitative real-time PCR array.
RNA was extracted from the BAT of four wild-type and four α1-AMPK−/− mice (RNA Mini Isolation kit 1283-018A, Invitrogen). RNA concentrations were determined using a spectrophotometer (model ND-1000, NanoDrop). RNA integrity number for each sample was determined with a RNA6000 PicoChip (catalog no. 5067-1513, Agilent) run on an Agilent 2100 BioAnalyzer. RNA was converted to cDNA using an RT2 Nano PreAMP cDNA synthesis kit (catalog no. C-06, SABiosciences). cDNA quality was tested by RT2 RNA QC PCR array (catalog no. PAMM-999, SABiosciences) and then run on the “Obesity” RT2 Profiler PCR array (catalog no. PAMM-17A, SABiosciences), which contains 84 genes of interest, which were normalized to the average of 5 housekeeping genes: Gusb, Hprt1, Hsp90ab1, Gapdh, and Actb. All kits were used according to the manufacturer's instructions, and software available at www.sabiosciences.com was used for analysis.
Statistics.
To assess the effects of genotype and cold exposure, group comparisons were made using a two-way ANOVA when possible. Student's t-test or one-way ANOVA with Tukey's post hoc test was also used. P < 0.05 was considered statistically significant. All data are expressed as means ± SE.
RESULTS
Response to chronic cold exposure: body weight, temperature, food intake, and adipose tissue weights.
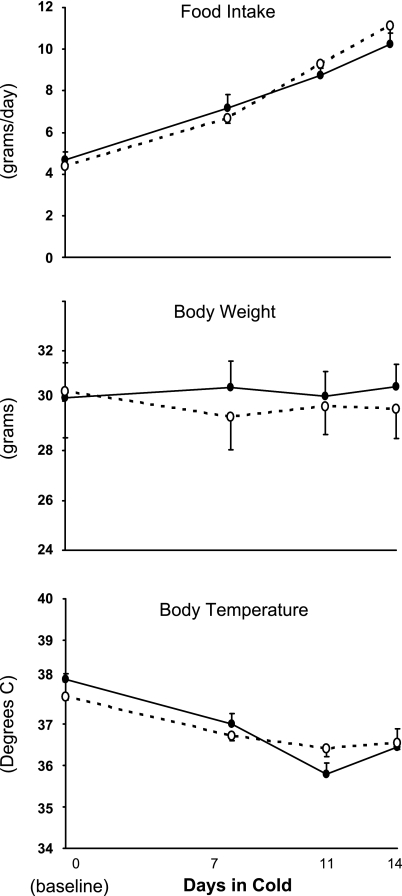
To determine if α1-AMPK is obligatory for the normal response to chronic cold exposure, wild-type and α1-AMPK−/− mice were studied under control conditions (∼24°C) and during 14 days in a cold environment (4°C). Prior to the cold exposure, there were no differences between the two groups in body weight, body temperature, or food consumption (Fig. 1). During cold exposure, food intake increased ∼2.5-fold in both groups, whereas body weight remained constant. Core body temperature decreased slightly in both groups during the 14 days of cold exposure, but this did not reach statistical significance and was not different between genotypes. These data demonstrate that α1-AMPK is not obligatory for the hyperphagic response to chronic cold exposure, nor is it obligatory for maintaining body temperature or weight during chronic cold exposure.
Fig. 1.
Effect of 14 days of cold exposure (4°C) on rate of food intake, body weight, and core body temperature in wild-type mice (●) and whole body knockout mice lacking the α1-isoform of AMP-activated protein kinase (α1-AMPK−/− mice, ○). There was no significant effect of genotype on any of the variables. Values are means ± SE (n = 7–8/group).
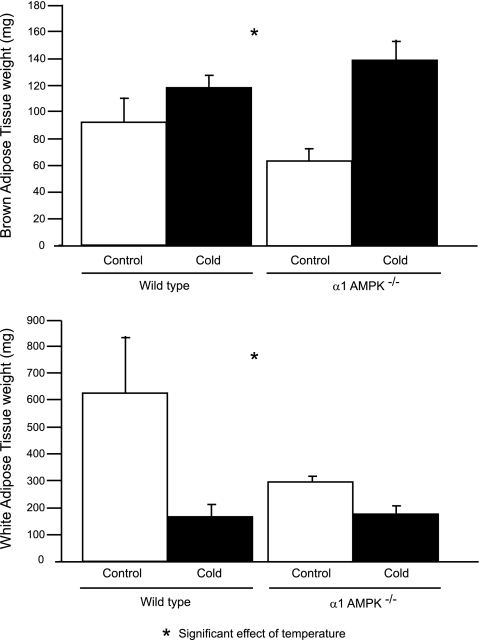
During cold exposure, BAT weight increased significantly more in AMPK−/− than wild-type mice (75 ± 15 vs. 27 ± 9 mg; Fig. 2A). Cold exposure caused a significantly larger decrease in WAT in wild-type than AMPK−/− mice (463 ± 45 vs. 118 ± 29 mg; Fig. 2B).
Fig. 2.
Effect of 14 days of cold exposure (4°C) on weight of brown adipose tissue (BAT) and white adipose tissue (WAT) in wild-type and α1-AMPK−/− mice. Values are means ± SE (n = 6–8/group).
Changes in tissue AMPK activities and proteins caused by loss of α1-AMPK and cold exposure.
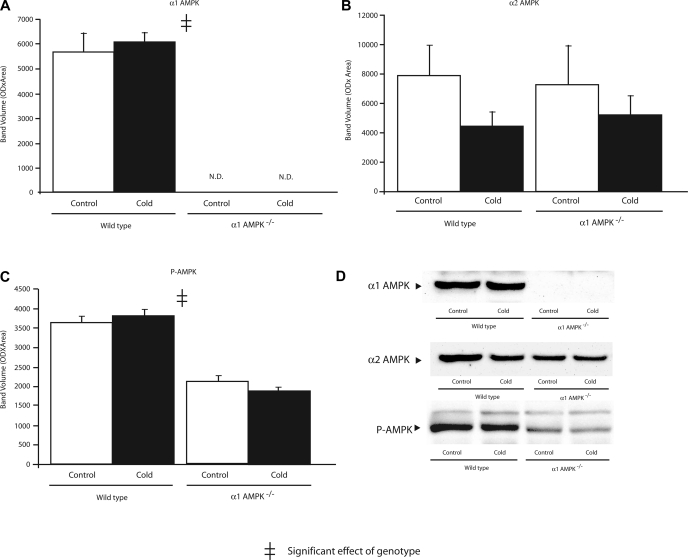
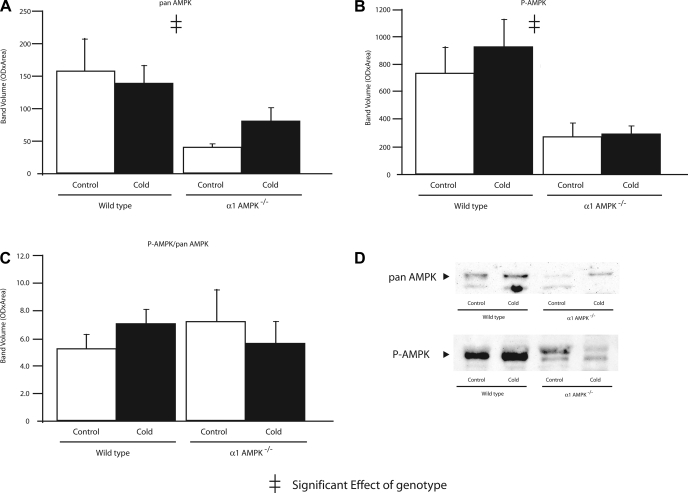
To determine if compensatory upregulation of α2-AMPK might occur in mice lacking α1-AMPK, activities of α1- and α2-AMPK were measured in BAT, WAT, liver, and skeletal muscle (Table 1). Under control conditions, α2-AMPK activity in BAT, WAT, and liver was significantly greater in α1-AMPK−/− than wild-type mice. α2-AMPK activity in skeletal muscle was not different in wild-type and α1-AMPK−/− mice. Thus, while there was no compensatory upregulation of α2-AMPK in muscle, all three nonmuscle tissues demonstrated a moderate upregulation of α2-AMPK in response to loss of α1-AMPK. As expected, the amount of P-AMPK was significantly lower in BAT and WAT of α1-AMPK−/− mice (Figs. 3 and 4). Expression of α2-AMPK protein was not different among groups (Fig. 3), possibly due to lower sensitivity of Western blots than AMPK activity assay.
Table 1.
Effects of 14 days of cold exposure on isoform-specific AMPK activity
| Wild-type |
α1-AMPK−/− |
|||
|---|---|---|---|---|
| Control | Cold | Control | Cold | |
| BAT | ||||
| α1-AMPK* | 83.6 ± 13.8 | 133.5 ± 14.7 | −0.1 ± 0.1 | 0.0 ± 0.1 |
| α2-AMPK‡ | 9.7 ± 1.7 | 10.5 ± 1.1 | 14.2 ± 1.8 | 14.1 ± 2.3 |
| WAT | ||||
| α1-AMPK* | 62.3 ± 15.4 | 109.1 ± 12.9 | 0.1 ± 0.4 | 0.4 ± 0.3 |
| α2-AMPK†‡ | 3.3 ± 0.7 | 6.5 ± 0.9 | 5.5 ± 1.5 | 10.8 ± 1.5 |
| Liver | ||||
| α1-AMPK | 21.3 ± 3.6 | 28.0 ± 1.4 | −0.2 ± 0.1 | 0.0 ± 0.1 |
| α2-AMPK‡ | 11.2 ± 2.5 | 12.8 ± 1.7 | 22.0 ± 2.7 | 16.7 ± 2.1 |
| Skeletal muscle | ||||
| α1-AMPK | 5.4 ± 1.4 | 5.0 ± 1.0 | 0.0 ± 0.1 | 0.0 ± 0.1 |
| α2-AMPK‡ | 3.2 ± 0.3 | 4.5 ± 0.3 | 2.8 ± 0.3 | 5.0 ± 0.9 |
Values (means ± SE) are expressed in pmol 32P·min−1·mg protein−1 (n = 6/group). AMPK, AMP-activated protein kinase; BAT, brown adipose tissue; WAT, white adipose tissue.
Significant effect of temperature on α1-AMPK activity (t-test).
Significant effect of temperature on α2-AMPK activity (2-way ANOVA).
Significant effect of genotype on α2-AMPK activity (2-way ANOVA).
Fig. 3.
Effect of 14 days of cold exposure (4°C) on amount of α1-AMPK (A), α2-AMPK (B), and phosphorylated AMPK (P-AMPK, C) in BAT assessed by Western blot analysis in wild-type and α1-AMPK−/− mice. OD, optical density; ND, not detected. Values are means ± SE (n = 6–8/group).
Fig. 4.
Effect of 14 days of cold exposure (4°C) on amount of pan AMPK (A), P-AMPK (B), and P-AMPK-to-pan AMPK ratio (C) in WAT assessed by Western blot analysis in wild-type and α1-AMPK−/− mice. Values are means ± SE (n = 6–8/group). D: representative blots for pan AMPK and P-AMPK.
Chronic cold exposure significantly increased α1-AMPK activity in BAT and WAT and α2-AMPK activity in WAT. These increases in AMPK activity were not paralleled by increases in P-AMPK (Figs. 3 and 4).
While chronic cold exposure did not significantly affect α1-AMPK activity in skeletal muscle, α2-AMPK increased with cold exposure in skeletal muscle. In liver, cold exposure had no effect on the activity of either isoform of AMPK.
Response to chronic cold exposure: mitochondrial markers (UCP1 and CS) and downstream targets of AMPK (ACC and HSL).
Because AMPK activity has been linked to mitochondrial biogenesis (25, 39), we assessed key mitochondrial proteins in BAT from α1-AMPK−/− and wild-type mice. Additionally, two known substrates for AMPK that are important in free fatty acid oxidation, ACC and HSL, were studied.
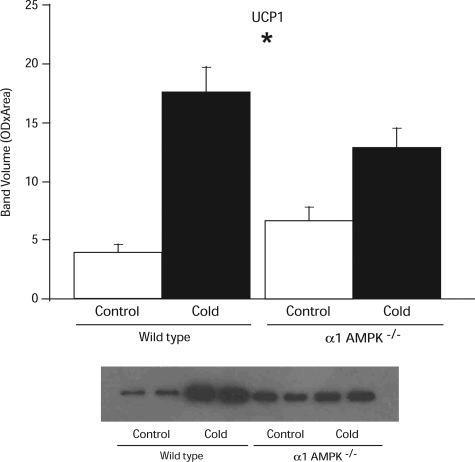
Under control conditions, UCP1 protein levels were 50% higher in α1-AMPK−/− than wild-type mice (P = 0.06; Fig. 5). After cold exposure, the amount of UCP1 protein in BAT increased 4.5-fold in wild-type mice but only doubled in α1-AMPK−/− mice (Fig. 5).
Fig. 5.
Effect of 14 days of cold exposure (4°C) on amount of uncoupling protein 1 (UCP1) in BAT assessed by Western blot analysis in wild-type and α1-AMPK−/− mice. Values are means ± SE (n = 5–6/group). *Significant effect of temperature.
CS activity, an index of mitochondrial mass, was measured in BAT, WAT, liver, and skeletal muscle (Table 2). Neither cold exposure nor genotype affected CS activity in BAT, liver, or skeletal muscle. However, chronic cold exposure caused significant increases and decreases in WAT CS activities.
Table 2.
Effects of 14 days of cold exposure on citrate synthase activity
| Wild-type |
α1-AMPK−/− |
|||
|---|---|---|---|---|
| Control | Cold | Control | Cold | |
| BAT | 1.47 ± 0.09 | 1.40 ± 0.05 | 1.43 ± 0.07 | 1.54 ± 0.10 |
| WAT* | 0.18 ± 0.05 | 0.39 ± 0.04 | 0.15 ± 0.03 | 0.40 ± 0.05 |
| Liver | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.06 ± 0.00 |
| Skeletal muscle | 0.37 ± 0.06 | 0.37 ± 0.02 | 0.29 ± 0.04 | 0.38 ± 0.06 |
Values (means ± SE) are expressed in μmol·min−1·mg−1 (n = 6/group).
Significant effect of temperature (2-way ANOVA).
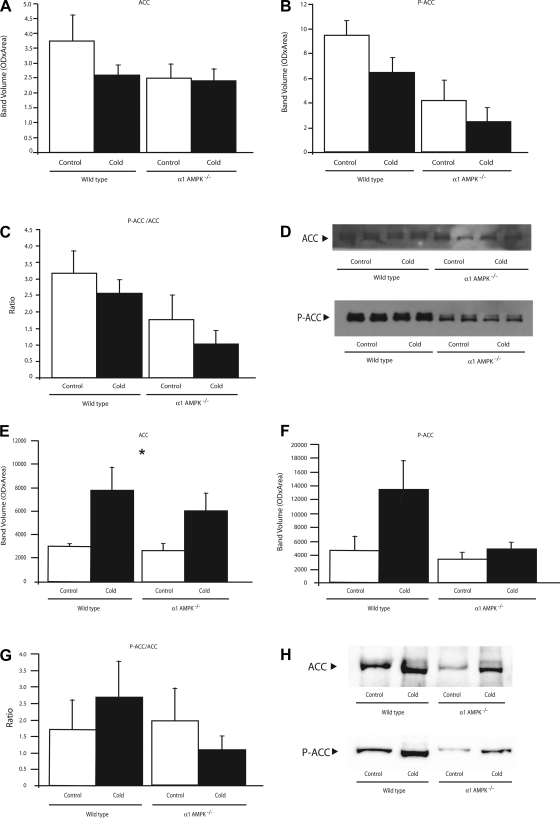
Under control conditions, BAT from α1-AMPK−/− mice had normal amounts of ACC but tended to have less P-ACC (P = 0.06) and a lower ratio of P-ACC to ACC (Fig. 6, A–D). Cold exposure did not significantly alter the amount of ACC or P-ACC or the ratio of P-ACC to ACC.
Fig. 6.
Effect of 14 days of cold exposure (4°C) on protein amounts of acetyl-CoA carboxylase (ACC, A), phosphorylated ACC (P-ACC, B), and ratio of phosphorylated to unphosphorylated ACC (C) in BAT (A–C) and WAT (E–G) from wild-type and α1-AMPK−/− mice. Values are means ± SE (n = 3–5/group). *Significant effect of temperature. D and H: representative Western blots from BAT and WAT, respectively. All samples were run in duplicate, and mean value for each mouse was used.
In WAT under control conditions, there was no effect of genotype on ACC, P-ACC, or the ratio of P-ACC to ACC (Fig. 6, E–H). Cold exposure caused a significant increase in ACC in both genotypes, as well as an approximately threefold increase in P-ACC in the wild-type mice compared with only a 30% increase in the α1-AMPK−/− mice.
Levels of HSL and P-HSL in BAT and WAT were not significantly altered by loss of α1-AMPK or chronic cold exposure (Fig. 7).
Gene expression in BAT: comparison of wild-type and α1-AMPK−/− mice.
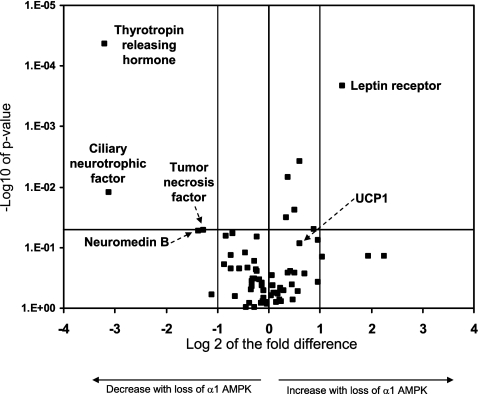
To evaluate the role of AMPK in regulating gene expression in BAT under room temperature conditions, we chose to study 84 genes related to physiological pathways relevant to thermoregulation, energy expenditure, adipokines, and diabetes (Fig. 8; see Supplemental Table S1 in Supplemental Material, available online at the Journal website). Of the 84 genes, 5 demonstrated differences that represented more than a doubling or halving (log 2 changes of >1.0 or −1.0, respectively) and were statistically significant at P ≤ 0.05. Of these genes, 4 were downregulated: thyrotropin-releasing hormone (Trh; −9.2 fold), ciliary neurotrophic factor (Cntf; −8.7 fold), tumor necrosis factor (Tnf; −2.6 fold), and neuromedin b (Nmb; −2.4 fold). The only gene that increased >1-fold in the α1-AMPK−/− mice was the leptin receptor gene (Lepr), which increased 2.7-fold, with the change being statistically significant. UCP1 was elevated 53% in the α1-AMPK−/− compared with wild-type mice (P = 0.08).
Fig. 8.
Effect of loss of α1-AMPK on gene expression in BAT under control conditions. Horizontal line indicates P = 0.05; center vertical line indicates no change in gene expression (0-fold change). Positive fold changes indicate increased gene expression in α1-AMPK−/− relative to wild-type mice (n = 4/group).
Response to acute β3-adrenergic stimulation.
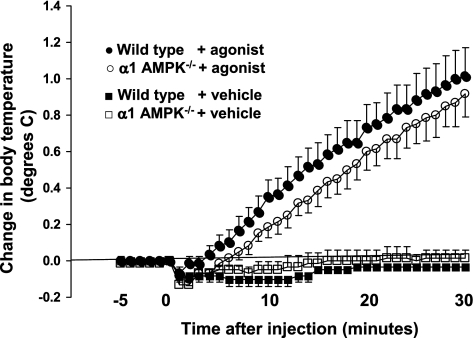
To determine if α1-AMPK is obligatory for the normal acute thermogenic response to β3-adrenergic stimulation, the effect of the β3-adrenergic-specific agonist CL-316243 on body temperature was measured in wild-type and α1-AMPK−/− mice (Fig. 9). Wild-type mice exhibited the expected response to intraperitoneal injection of CL-316243, increasing body temperature steadily by ∼1.0°C over the course of 30 min from a baseline of 36°C. The body temperature response to β3-adrenergic stimulation in α1-AMPK−/− mice was indistinguishable from that in wild-type mice. This finding demonstrates that α1-AMPK is not obligatory for the acute thermogenic response to β3-adrenergic stimulation.
Fig. 9.
Effect of intraperitoneal injection of saline (vehicle) or the β3-adrenergic agonist CL-316243 on core body temperature in wild-type and α1-AMPK−/− mice with average baseline temperature of ∼36°C. There was no significant effect of genotype on body temperature. Values are means ± SE (n = 6/group).
DISCUSSION
We previously demonstrated a higher level of α1-AMPK activity in BAT than any other tissue in mice (20). In the present study, we confirm this finding and report that chronic loss of α1-AMPK from all tissues in the body, including BAT, results in adaptations allowing α1-AMPK−/− mice to have normal 1) tolerance to chronic cold, 2) hyperphagic response to chronic cold, and 3) acute activation of nonshivering thermogenesis. Thus, in contrast to what may have been expected, loss of α1-AMPK does not cause metabolic disarray manifest as impaired regulation of body temperature or body weight. Here we identify enzymatic and transcriptional adaptations to loss of α1-AMPK that likely contribute to the lack of a dramatic physiological phenotype under control conditions or during the imposed experimental stresses.
Adaptations to loss of α1-AMPK under control conditions.
The most direct form of adaptation to loss of α1-AMPK may be the upregulation of α2-AMPK activity, which occurred in BAT, WAT, and liver, but not in skeletal muscle (Table 1). The tissue specificity of this suggests that muscle lacks the signaling pathway linking α1- and α2-AMPK or that loss of α1-AMPK in muscle is not a sufficient perturbation to trigger compensatory upregulation of α2-AMPK.
Another potential adaptation in the α1-AMPK−/− mice relevant to regulation of body temperature and weight was the modest increase in levels of UCP1 protein (50%, P = 0.06) and mRNA (53%, P = 0.08) in the α1-AMPK−/− mice. Upregulation of this key mitochondrial protein could be a compensatory response to the downregulation of prothermogenic genes in the α1-AMPK−/− mice, as discussed below (Fig. 8).
Phosphorylation of ACC to P-ACC by AMPK is a key regulatory step in mitochondrial oxidation of free fatty acids (37). Consistent with the low total AMPK activity in the α1-AMPK−/− mice, BAT from these mice had somewhat reduced P-ACC levels (P = 0.06) and a significantly lower ratio of P-ACC to ACC (P = 0.02; Fig. 6, A–D). This demonstrates that compensatory upregulation of α2-AMPK in BAT is not sufficient to normalize P-ACC but that this lower level of P-ACC is adequate to prevent any obvious phenotype under our experimental conditions. In WAT, loss of α1-AMPK had no effect on P-ACC levels, suggesting that α2-AMPK was adequate to maintain P-ACC at normal levels.
A second mitochondrial enzyme critical for nonshivering thermogenesis is the tricarboxylic acid cycle enzyme CS. Additionally, CS has been used as an index of mitochondrial respiratory activity and mitochondrial biogenesis in WAT (9, 24). Loss of α1-AMPK had no effect on CS activity in BAT or other tissues (Table 2). HSL is a cytosolic enzyme phosphorylated and activated by AMPK as well as other kinases (7, 10). Adrenergic-induced activation of HSL is an important mechanism by which lipolysis is increased during elevated energy demand (15). We found that loss of α1-AMPK had no observable effect on HSL phosphorylation, likely due to the numerous factors besides AMPK activity that influence its degree of phosphorylation. Thus it appears that α1-AMPK is not obligatory for regulating CS or HSL in any of these tissues.
Effect of loss of α1-AMPK on gene expression in BAT under control conditions.
AMPK regulates gene expression via effects on transcription factors [i.e., peroxisome proliferator-activated receptor-γ (PPAR-γ)] and other kinases (i.e., mammalian target of rapamycin) and has been suggested as an anticancer target, since it inhibits protein synthesis and stimulates cellular catabolism (29, 30). Our data indicate that expression of 5 of the 84 genes studied in BAT was significantly altered by loss of α1-AMPK under room temperature conditions. A common factor in these five genes is that they have established effects on nonshivering thermogenesis. Specifically, Cntfr, Trh, and Lepr (via leptin signaling) are “prothermogenic” in brown adipocytes, either directly increasing nonshivering thermogenesis or facilitating sympathetically induced nonshivering thermogenesis (1, 14, 22, 31). In contrast, Tnf has been reported to inhibit nonshivering thermogenesis, and Nmb inhibits the prothermogenic action of Trh (23, 26). Therefore, two of the changes (decreases in Cntf and Trh) appear to be antithermogenic, and the other three changes favor thermogenesis. In a chronic knockout model, it is difficult to differentiate between the direct effects of the α1-AMPK gene deletion and compensatory secondary changes. We speculate that loss of α1-AMPK, itself a probable prothermogenic molecule, directly causes the decreases in prothermogenic genes and that changes in the other three genes are compensatory. This speculation is based on the literature demonstrating that AMPK increases substrate oxidation, an essential process during thermogenesis (13), and would also be consistent with our finding of modest increases in UCP1 gene expression and protein levels as a form of partial compensation for loss of prothermogenic molecules.
Adaptations to loss of α1-AMPK during chronic cold exposure.
Given AMPK's emerging role as a regulator of gene expression and cellular processes such as mitochondrial biogenesis (2, 25), we hypothesized that loss of α1-AMPK would prevent or attenuate some of the major physiological changes that occur during chronic cold exposure. We focus on mitochondria, because in skeletal muscle, AMPK has been reported to be a key element in a signaling pathway by which activation of AMPK caused phosphorylation and activation of PPAR-γ coactivator 1α, which in turn causes translocation of nuclear regulatory factors and increased synthesis of mitochondrial proteins (18). Instead, wild-type and α1-AMPK−/− mice were indistinguishable in their response to chronic cold with regard to their body weight, core temperature, and rate of food intake. This indicates that α1-AMPK is not obligatory in regulating these parameters and suggests that compensatory cellular and molecular responses to loss of α1-AMPK may mask any physiological phenotype in the whole animal. For example, α2-AMPK activity may be sufficient to accommodate the increased metabolic demands of chronic cold exposure. Interestingly, chronic cold exposure caused a further upregulation of α2-AMPK beyond that caused by loss of α1-AMPK in WAT and skeletal muscle, both of which are major contributors to body temperature maintenance during cold exposure (11, 28).
The cold-induced increase in BAT mass was significantly larger in the α1-AMPK−/− than wild-type mice (75 vs. 27 mg). The finding that CS levels were the same in the two genotypes after cold exposure suggests that the α1-AMPK−/− mice have normal mitochondrial mass. Given that cold tolerance was normal in these mice, it appears that they may have undergone a larger degree of BAT hypertrophy during cold exposure to maintain nonshivering thermogenesis at levels comparable to the wild-type mice.
The difference between the amounts of epididymal fat in the control and cold-exposed mice was much less in the α1-AMPK−/− than wild-type mice (Fig. 2). Since body weight remained nearly identical in the two genotypes, it appears that the α1-AMPK−/− mice 1) preferentially used nonepididymal fat or 2) catabolized nonadipose tissue sources such as skeletal muscles to generate the heat needed to maintain body temperature (28). Our data indicate that failure of α1-AMPK−/− mice to use epididymal fat was not due to lower-than-normal levels of P-HSL. However, the failure of α1-AMPK−/− mice to robustly increase P-ACC during cold exposure may have played some role in the altered use of stored fat in these mice.
Effect of loss of α1-AMPK on UCP1, CS, ACC, and HSL during chronic cold exposure.
On the basis of studies indicating a link between AMPK and mitochondrial biogenesis, we hypothesized that loss of α1-AMPK activity would prevent (or attenuate) its upregulation in response to chronic cold (2). While mitochondrial biogenesis was not directly measured, we did find that the cold-induced increase in UCP1 protein was significantly less in the α1-AMPK−/− than wild-type mice (a doubling vs. a 4.5-fold increase, P = 0.003). Thus, while α1-AMPK−/− mice have slightly more UCP1 than wild-type mice under room temperature conditions, they do not upregulate UCP1 as robustly during cold exposure.
Cold exposure significantly increased CS activity in WAT, but not in the other tissues. This cold-induced increase of CS in WAT is consistent with previous reports that chronic cold can cause WAT to express genes, notably UCP1, more closely associated with BAT (5, 33).
In BAT, loss of α1-AMPK had no effect on the cold-induced decreases in P-ACC and the P-ACC-to-ACC ratio, demonstrating that these changes are not dependent on α1-AMPK and may be obligatory for normal cold tolerance. In WAT, both genotypes upregulated ACC protein levels in response to chronic cold. However, while wild-type mice demonstrated a robust increase in P-ACC, this response to cold exposure was essentially absent in the α1-AMPK−/− mice (Fig. 6F). This demonstrates that compensatory upregulation of α2-AMPK in WAT is not sufficient to normalize P-ACC but that this lower level of P-ACC does not induce cold intolerance. There was no effect of cold exposure or genotype on levels of HSL or P-HSL proteins.
Acute nonshivering thermogenesis in vivo.
We hypothesized that α1-AMPK plays an obligatory role in acute nonshivering thermogenesis induced by adrenergic stimulation. This notion was based on the recognized role of AMPK as a “fuel gauge” in some tissues and a report that acute in vitro stimulation of brown adipocytes with CL-316243 increases AMPK phosphorylation (16). Our in vivo findings demonstrate that α1-AMPK is not obligatory for adrenergic-induced nonshivering thermogenesis. As it was possible that a phenotype may be lacking during chronic cold exposure, there are several explanations. 1) α2-AMPK activity in α1-AMPK−/− mice may be adequate to phosphorylate AMPK's critical downstream targets, especially when one considers that loss of α1-AMPK causes a modest increase in α2-AMPK activity in BAT (Table 1). This possibility may seem unlikely, given the ∼10-times-greater α1- than α2-AMPK activity in BAT. However, what appears to be a quantitatively small amount of α2-AMPK activity might actually be localized in a way to compensate for loss of α1-AMPK. 2) AMPK-independent pathways could cause the rapid increase in oxidation of free fatty acids necessary during nonshivering thermogenesis.
Perspectives and Significance
BAT was previously thought to play a negligible role in regulating caloric output in adult humans. However, recent studies report considerable amounts of metabolically active BAT in adults (6, 27, 34, 35). Given the unique ability of BAT to convert calories directly to heat, there is intense interest in understanding how BAT metabolism is regulated, in hopes that manipulating the metabolic rate of BAT might be an effective way to expend excess calories (4, 33). Since AMPK has been called the metabolic master switch, we tested the hypothesis that loss of the abundant α1-isoform of AMPK would fundamentally alter the functioning of BAT in vivo. Instead, we found that loss of α1-AMPK from all tissues in the body, including BAT, does not cause observable in vivo changes in 1) chronic cold tolerance, 2) cold-induced hyperphagia, or 3) acute nonshivering thermogenesis. We speculate that enzymatic and transcriptional adaptations to loss of α1-AMPK (i.e., upregulation of α2-AMPK) contribute to the lack of a dramatic physiological phenotype regarding thermoregulation. Future studies attempting to understand the role of the abundance of α1-AMPK in BAT might focus on the acute inactivation of α1-AMPK to circumvent chronic compensation by α2-AMPK or alternate kinases. One approach could be use of α1-isoform-specific RNA interference on brown adipocytes in vitro.
GRANTS
These studies were supported by National Institutes of Health Grants R01 DK-080345 and T32 HL-007936.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Louise McCullough for essential help in these studies.
REFERENCES
- 1. Al-Arabi A, Andrews JF. The effect of TRH and norepinephrine on the triglyceride droplets (TGD) in brown adipose tissue in warm acclimated rats. Biomed Sci Instrum 42: 507–512, 2006 [PubMed] [Google Scholar]
- 2. Bergeron R, Ren JM, Cadman KS, Moore IK, Perret P, Pypaert M, Young LH, Semenkovich CF, Shulman GI. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am J Physiol Endocrinol Metab 281: E1340–E1346, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Cinti S. The role of brown adipose tissue in human obesity. Nutr Metab Cardiovasc Dis 16: 569–574, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Commins SP, Watson PM, Padgett MA, Dudley A, Argyropoulos G, Gettys TW. Induction of uncoupling protein expression in brown and white adipose tissue by leptin. Endocrinology 140: 292–300, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360: 1509–1517, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Daval M, Diot-Dupuy F, Bazin R, Hainault I, Viollet B, Vaulont S, Hajduch E, Ferre P, Foufelle F. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J Biol Chem 280: 25250–25257, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Davies SP, Carling D, Hardie DG. Tissue distribution of the AMP-activated protein kinase, and lack of activation by cyclic-AMP-dependent protein kinase, studied using a specific and sensitive peptide assay. Eur J Biochem 186: 123–128, 1989 [DOI] [PubMed] [Google Scholar]
- 9. Deveaud C, Beauvoit B, Salin B, Schaeffer J, Rigoulet M. Regional differences in oxidative capacity of rat white adipose tissue are linked to the mitochondrial content of mature adipocytes. Mol Cell Biochem 267: 157–166, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Garton AJ, Campbell DG, Carling D, Hardie DG, Colbran RJ, Yeaman SJ. Phosphorylation of bovine hormone-sensitive lipase by the AMP-activated protein kinase. A possible antilipolytic mechanism. Eur J Biochem 179: 249–254, 1989 [DOI] [PubMed] [Google Scholar]
- 11. Granneman JG, Burnazi M, Zhu Z, Schwamb LA. White adipose tissue contributes to UCP1-independent thermogenesis. Am J Physiol Endocrinol Metab 285: E1230–E1236, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Hardie DG, Carling D. The AMP-activated protein kinase—fuel gauge of the mammalian cell? Eur J Biochem 246: 259–273, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Hardie DG, Pan DA. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem Soc Trans 30: 1064–1070, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Hermann GE, Barnes MJ, Rogers RC. Leptin and thyrotropin-releasing hormone: cooperative action in the hindbrain to activate brown adipose thermogenesis. Brain Res 1117: 118–124, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Holm C, Osterlund T, Laurell H, Contreras JA. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Annu Rev Nutr 20: 365–393, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Hutchinson DS, Chernogubova E, Dallner OS, Cannon B, Bengtsson T. β-Adrenoceptors, but not α-adrenoceptors, stimulate AMP-activated protein kinase in brown adipocytes independently of uncoupling protein-1. Diabetologia 48: 2386–2395, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Jorgensen SB, Viollet B, Andreelli F, Frosig C, Birk JB, Schjerling P, Vaulont S, Richter EA, Wojtaszewski JF. Knockout of the α2 but not α1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-β-4-ribofuranoside but not contraction-induced glucose uptake in skeletal muscle. J Biol Chem 279: 1070–1079, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Jornayvaz FR, Shulman GI. Regulation of mitochondrial biogenesis. Essays Biochem 47: 69–84, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuznetsov AV, Strobl D, Ruttmann E, Konigsrainer A, Margreiter R, Gnaiger E. Evaluation of mitochondrial respiratory function in small biopsies of liver. Anal Biochem 305: 186–194, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Mulligan JD, Gonzalez AA, Stewart AM, Carey HV, Saupe KW. Upregulation of AMPK during cold exposure occurs via distinct mechanisms in brown and white adipose tissue of the mouse. J Physiol 580: 677–684, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Namkoong C, Kim MS, Jang PG, Han SM, Park HS, Koh EH, Lee WJ, Kim JY, Park IS, Park JY, Lee KU. Enhanced hypothalamic AMP-activated protein kinase activity contributes to hyperphagia in diabetic rats. Diabetes 54: 63–68, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Ott V, Fasshauer M, Dalski A, Klein HH, Klein J. Direct effects of ciliary neurotrophic factor on brown adipocytes: evidence for a role in peripheral regulation of energy homeostasis. J Endocrinol 173: R1–R8, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Pazos-Moura CC, Ortiga-Carvalho TM, Gaspar de Moura E. The autocrine/paracrine regulation of thyrotropin secretion. Thyroid 13: 167–175, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Perrone CE, Mattocks DA, Jarvis-Morar M, Plummer JD, Orentreich N. Methionine restriction effects on mitochondrial biogenesis and aerobic capacity in white adipose tissue, liver, and skeletal muscle of F344 rats. Metabolism 59: 1000–1011, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Reznick RM, Shulman GI. The role of AMP-activated protein kinase in mitochondrial biogenesis. J Physiol 574: 33–39, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Romanatto T, Roman EA, Arruda AP, Denis RG, Solon C, Milanski M, Moraes JC, Bonfleur ML, Degasperi GR, Picardi PK, Hirabara S, Boschero AC, Curi R, Velloso LA. Deletion of tumor necrosis factor-α receptor 1 (TNFR1) protects against diet-induced obesity by means of increased thermogenesis. J Biol Chem 284: 36213–36222, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27. Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, Kawai Y, Tsujisaki M. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58: 1526–1531, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shabalina IG, Hoeks J, Kramarova TV, Schrauwen P, Cannon B, Nedergaard J. Cold tolerance of UCP1-ablated mice: a skeletal muscle mitochondria switch toward lipid oxidation with marked UCP3 up-regulation not associated with increased basal, fatty acid- or ROS-induced uncoupling or enhanced GDP effects. Biochim Biophys Acta 1797: 968–980, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer 9: 563–575, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shaw RJ, Bardeesy N, Manning BD, Lopez L, Kosmatka M, DePinho RA, Cantley LC. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell 6: 91–99, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Silva JE. Thermogenic mechanisms and their hormonal regulation. Physiol Rev 86: 435–464, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev 89: 1025–1078, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Tiraby C, Tavernier G, Lefort C, Larrouy D, Bouillaud F, Ricquier D, Langin D. Acquirement of brown fat cell features by human white adipocytes. J Biol Chem 278: 33370–33376, 2003 [DOI] [PubMed] [Google Scholar]
- 34. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med 360: 1500–1508, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med 360: 1518–1525, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol Endocrinol Metab 277: E1–E10, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Witters LA, Kemp BE. Insulin activation of acetyl-CoA carboxylase accompanied by inhibition of the 5′-AMP-activated protein kinase. J Biol Chem 267: 2864–2867, 1992 [PubMed] [Google Scholar]
- 38. Yoshida T, Umekawa T, Sakane N, Yoshimoto K, Kondo M. Effect of CL316,243, a highly specific β3-adrenoceptor agonist, on sympathetic nervous system activity in mice. Metabolism 45: 787–791, 1996 [DOI] [PubMed] [Google Scholar]
- 39. Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci USA 99: 15983–15987, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.